Abstract
Background
Although safety and tolerability of vericiguat were established in the VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) trial in patients with heart failure with reduced ejection fraction, some subgroups may be more susceptible to symptomatic hypotension, such as older patients, those with lower baseline systolic blood pressure (SBP), or those concurrently taking angiotensin receptor neprilysin inhibitors. We described the SBP trajectories over time and compared the occurrence of symptomatic hypotension or syncope by treatment arm in potentially vulnerable subgroups in VICTORIA. We also evaluated the relation between the efficacy of vericiguat and baseline SBP.
Methods and Results
Among patients receiving at least 1 dose of the study drug (n=5034), potentially vulnerable subgroups were those >75 years old (n=1395), those with baseline SBP 100–110 mm Hg (n=1344), and those taking angiotensin receptor neprilysin inhibitors (n=730). SBP trajectory was plotted as mean change from baseline over time. The treatment effect on time to symptomatic hypotension or syncope was evaluated overall and by subgroup, and the primary efficacy composite outcome (heart failure hospitalization or cardiovascular death) across baseline SBP was examined using Cox proportional hazards models. SBP trajectories showed a small initial decline in SBP with vericiguat in those >75 years old (versus younger patients), as well as those receiving angiotensin receptor neprilysin inhibitors (versus none), with SBP returning to baseline thereafter. Patients with SBP <110 mm Hg at baseline showed a trend to increasing SBP over time, which was similar in both treatment arms. Safety event rates were generally low and similar between treatment arms within each subgroup. In Cox proportional hazards analysis, there were similar numbers of safety events with vericiguat versus placebo (adjusted hazard ratio [HR], 1.18; 95% CI, 0.99–1.39; P=0.059).
No difference existed between treatment arms in landmark analysis beginning after the titration phase (ie, post 4 weeks) (adjusted HR, 1.14; 95% CI, 0.93–1.38; P=0.20). The benefit of vericiguat compared with placebo on the primary composite efficacy outcome was similar across the spectrum of baseline SBP (P for interaction=0.32).
Conclusions
These data demonstrate the safety of vericiguat in a broad population of patients with worsening heart failure with reduced ejection fraction, even among those predisposed to hypotension. Vericiguat’s efficacy persisted regardless of baseline SBP.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02861534.
Keywords: blood pressure, heart failure, heart failure with reduced ejection fraction, safety events, vericiguat
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- ARNI
angiotensin receptor neprilysin inhibitors
- CHAMP‐HF
Change the Management of Patients with Heart Failure
- HFrEF
heart failure with reduced ejection fraction
- SBP
systolic blood pressure
- SGLT2i
sodium glucose cotransporter‐2 inhibitors
- VICTORIA
Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction
Clinical Perspective
What Is New?
Consistent with the safety and tolerability of vericiguat observed in the overall VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) trial, there were no excessive reductions in blood pressure with vericiguat across subgroups representing potentially vulnerable patients predisposed to blood pressure decreases, such as older patients, those with lower baseline blood pressure, and patients receiving concurrent angiotensin receptor neprilysin inhibitors.
The benefit of vericiguat was consistent across baseline systolic blood pressure (P for interaction=0.32).
What Are the Clinical Implications?
Along with prior evidence of benefit with vericiguat regardless of age and background therapy, our findings indicate the favorable benefit‐to‐risk ratio of vericiguat extends to patients potentially predisposed to blood pressure decreases.
Among patients with heart failure with reduced ejection fraction (HFrEF), concerns about hypotension often limit the use of potentially life‐saving medications. In the CHAMP‐HF (Change the Management of Patients with Heart Failure) registry, lower systolic blood pressure (SBP) was an independent predictor of underuse or under‐dosing of neurohormonal antagonists in eligible patients. 1 Although hypotensive patients should be evaluated for dehydration/overdiuresis with consideration of reducing or removing medications causing hypotension without survival benefit, it should also be emphasized that application of guideline‐directed medical therapies has been shown to improve clinical outcomes in HFrEF despite lowering SBP. Physicians must therefore balance the risk of hypotension with the expected longer‐term therapeutic benefits because withholding effective therapies for this reason may contribute to poor prognosis among patients with HFrEF. Indeed, even when SBP is not limiting, further data from CHAMP‐HF suggest a reluctance of physicians to prescribe or up‐titrate beneficial, but potentially hypotensive‐inducing agents, in the absence of other contraindications. 2 Newer effective therapies in HFrEF that may lower SBP, such as angiotensin receptor‐neprilysin inhibitors (ARNI), 3 further complicate management of patients with HFrEF given their advanced age, frequent comorbidities, and multiple concomitant medications.
Recently, the VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) trial randomized 5050 patients within 6 months of worsening HFrEF to the soluble guanylate cyclase stimulator vericiguat or placebo on top of high use of contemporary guideline‐directed medical therapy and showed that vericiguat significantly reduced the risk for the primary outcome of HF hospitalization or cardiovascular death over a median follow‐up of 10.8 months (hazard ratio [HR], 0.90; 95% CI, 0.82–0.98). 4 Prespecified adverse events of interest were symptomatic hypotension and syncope, which occurred in 9.1% versus 7.9% (P=0.12), and 4.0% versus 3.5% (P=0.30) of the vericiguat versus placebo groups, respectively. Although these safety and tolerability results were reassuring for the use of vericiguat in the overall studied population, it seems likely that there may be some patients who are more prone than others to blood pressure decreases, symptomatic hypotension, or syncope. Potentially vulnerable subgroups may be older patients, those with lower baseline SBP, or those concurrently taking vasoactive medications. Specifically, because neprilysin inhibition may also augment the guanylate cyclase‐cyclic guanosine monophosphate pathway, the effect of the combined use of ARNI and vericiguat on SBP is of particular interest. 5 , 6 Accordingly, we aimed to describe the SBP trajectories during treatment and compared the occurrence of symptomatic hypotension or syncope in patients receiving vericiguat compared with placebo, with particular emphasis on potentially vulnerable subgroups. We also evaluated the relation between the efficacy of vericiguat and baseline SBP.
Methods
Data will be made available as outlined in the VICTORIA data sharing charter (https://thecvc.ca/victoria/data‐sharing/).
Trial Overview
Briefly, VICTORIA (NCT02861534) was a multicenter, international, randomized, placebo‐controlled trial that investigated the efficacy and safety of vericiguat, on top of evidence‐based therapy, in patients with chronic HFrEF (EF <45%, New York Heart Association class II–IV) and a recent (<6 months) worsening HF event. 7 Patients with SBP <100 mm Hg and those on long‐acting nitrates or phosphodiesterase type 5 inhibitors were excluded and the use of any intravenous treatment within 24 hours before randomization was prohibited. After a screening phase of up to 30 days, eligible patients were randomized 1:1 to start on a 2.5 mg dose of vericiguat or matching placebo, then up‐titrated to 5 mg and subsequently to the target dose of 10 mg once daily of vericiguat or matching placebo in a blinded fashion. There was no run‐in phase in VICTORIA. The up‐titration protocol was based on mean SBP and clinical symptoms were assessed at 2‐week intervals, with a 4‐week total titration phase. Patients were then evaluated every 4 months until study completion. At every subsequent visit, additional efforts were taken at the discretion of the investigator to maximize the likelihood of titration to the 10 mg target dose, based on mean SBP measurement and safety considerations. Prespecified adverse events of clinical interest were symptomatic hypotension and syncope (both nonserious and serious adverse events), for which patients were monitored through the course of the study, with all events of hypotension captured via electronic or paper reporting. The primary outcome was a composite of HF hospitalization or cardiovascular death; the secondary end points were HF hospitalization alone, cardiovascular death alone, a composite of all‐cause mortality or HF hospitalization, and all‐cause death alone. The trial protocol was approved by institutional review boards or ethics committees at the participating sites and all the patients provided informed consent.
Potentially Vulnerable Subgroups
Potentially vulnerable subgroups were defined as follows: (1) age >75 years old, (2) SBP 100 to 110 mm Hg, and (3) baseline therapy with ARNI. Our selected age cutoff of 75 years and SBP cutoff of 110 mm Hg corresponded to the oldest age and lowest SBP categories in prior analyses of newer HFrEF therapies. 8 , 9 , 10 Given potential synergistic effects of sacubitril and vericiguat on particulate and soluble guanylate cyclase, respectively, the subgroup comparison by baseline ARNI use was prespecified and enrollment of patients receiving baseline ARNI was actively encouraged throughout the trial. 7 Although the efficacy outcomes by age group (above and below 75 years old) and baseline ARNI use have been reported, 4 the subgroup analyses of safety events have not.
Further subgroups of interest included patients with NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide above and below 4000 pg/mL and 8000 pg/mL, because treatment heterogeneity by baseline NT‐proBNP had been previously observed in VICTORIA, 4 with cut points from the treatment effect across the spectrum of (log‐transformed) NT‐proBNP at 4000 pg/mL and 8000 pg/mL. 11 Finally, recognizing that sodium glucose cotransporter‐2 inhibitors (SGLT2i) will be increasingly used in HFrEF, we also examined the small subgroup of patients receiving SGLT2i at baseline (n=122 [2.4%]). Patients with nonmissing information for each criterion were included in these subgroup analyses.
Statistical Analysis
Patients were included in these analyses if they received at least 1 dose of the study drug (n=5034). Baseline characteristics were summarized for each vulnerable subgroup and compared with the rest of the population not meeting the individual subgroup criteria, with continuous variables presented as medians with 25th and 75th percentiles and categorical variables presented as counts (percentages). Characteristics were compared between groups using 2‐sample t tests or Wilcoxon rank sum tests for continuous variables and chi‐square or Fisher’s exact test for categorical variables. SBP trajectory for each subgroup was plotted as mean change from baseline over time with the corresponding standard errors. SBP measurements obtained before the date of the first dose and 14 days after the last dose of study medication were included.
The treatment effect on time to symptomatic hypotension or syncope was evaluated overall and by subgroup. The number of events and incidence rates are presented within each treatment arm and subgroup for the composite safety outcome and symptomatic hypotension and syncope individually. Incidence rates are calculated as the number of events per 100 patient‐years of follow‐up. Evaluation of the treatment effect and modification of the treatment effect by subgroup were performed using Cox proportional hazards models for the 3 outcomes (composite of symptomatic hypotension and syncope and individual components). Adjusted models were evaluated using the following list of covariates: age, sex, race, SBP, ARNI use, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker use, New York Heart Association class, estimated glomerular filtration rate, and NT‐proBNP. Continuous covariates used for determining subgroups were included categorically when necessary. The proportional hazards assumption was assessed using weighted Schoenfeld residuals, with no major violations found. Hazard ratios (HRs) with 95% CIs comparing vericiguat with placebo, overall, and within subgroups, are presented. For models assessing the interaction between treatment and subgroup, the P value for the interaction is also presented.
To assess the incidence of symptomatic hypotension or syncope after dose titration by randomized treatment, a landmark analysis was performed for patients who reached the 4‐week (post‐titration) visit without an event of symptomatic hypotension or syncope. Day 0 for assessing events began at 4 weeks post randomization. Treatment effect was assessed using a Cox proportional hazards model. In order to reduce the potential for any bias incurred in performing a landmark analysis, the model was adjusted for potential confounders (urate, region/race, and implantable cardiac defibrillator for syncope; SBP, QT interval, chloride, calcium, region/race, implantable cardiac defibrillator, randomized while hospitalized, and history of diabetes, atrial flutter, and hyperlipidemia for symptomatic hypotension). We used backward selection with a 0.05 cutoff, starting with a list of ≈65 baseline characteristics, for each of the 3 end points (composite safety end point and the components). The proportional hazards assumption was assessed, with no major violations found. The HRs (95% CI) comparing vericiguat with placebo and associated P value for each outcome are presented.
Finally, the effect of vericiguat compared with placebo on the primary efficacy composite outcome (HF hospitalization or cardiovascular death) was examined across the spectrum of baseline SBP using a Cox proportional hazards model. The relation between baseline SBP and HF hospitalization/cardiovascular death was assessed for nonlinearity using natural cubic splines, and the relation was found to be nonlinear. The final model included randomized treatment, SBP using natural cubic splines, and the interaction between treatment and SBP. HRs (95% CI) for the treatment effect were estimated at 1 mm Hg intervals of SBP from 100 to 160 mm Hg.
All analyses were conducted by the Duke Clinical Research Institute (Durham, NC) using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Baseline Characteristics by Vulnerable Subgroups
Among a total of 5034 patients with recent worsening HF who received at least 1 dose of study drug, mean age was 67.3±12.2 years and mean SBP was 121.4±15.7 mm Hg, with distribution as shown in Figure S1. There were 1395 (27.6%) patients >75 years old and 1344 (26.6%) with SBP 100 to 110 mm Hg.
Baseline characteristics according to the location of patients in the 3 aforementioned vulnerable subgroups are shown in Table 1. Compared with younger patients, those >75 years old were more likely to be female and White with comorbidities (atrial fibrillation, hypertension, hyperlipidemia, anemia) and a history of cardiovascular disease (coronary artery disease, stroke, peripheral artery disease); have higher baseline SBP, lower glomerular filtration rate, and higher NT‐proBNP; and were less likely to be on guideline‐directed triple therapy at baseline. Compared with patients with higher baseline SBP, those with SBP <110 mm Hg were slightly younger, more often Asian, more likely to be within 3 months of HF hospitalization, and more likely to be receiving guideline‐directed triple therapy, with higher baseline NT‐proBNP. Guideline‐directed triple therapy was commonly used, with 730 (14.5%) patients receiving ARNI at baseline. Compared with patients not receiving ARNI, those receiving ARNI at baseline were younger, more likely to be male and Asian with a similar comorbidity burden but lower baseline SBP, and more likely to have received triple therapy and devices.
Table 1.
Baseline Characteristics by Age, Baseline SBP, and Use of ARNI
| Age >75 y | SBP <110 mm Hg | Use of ARNI at baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic |
Yes (N=1395) |
No (N=3639) |
P value |
Yes (N=1344) |
No (N=3690) |
P value |
Yes (N=730) |
No (N=4303) |
P value |
| Age, y | 80.0 (78.0, 84.0) | 64.0 (57.0, 70.0) | <0.001 | 68.0 (58.0, 76.0) | 69.0 (61.0, 77.0) | <0.001 | 67.0 (58.0, 75.0) | 69.0 (60.0, 77.0) | <0.001 |
| Female sex | 398 (28.5%) | 808 (22.2%) | <0.001 | 314 (23.4%) | 892 (24.2%) | 0.55 | 152 (20.8%) | 1053 (24.5%) | 0.033 |
| Race * | <0.001 | <0.001 | <0.001 | ||||||
| Asian | 273 (19.6%) | 858 (23.6%) | 381 (28.4%) | 750 (20.3%) | 204 (27.9%) | 927 (21.5%) | |||
| Black | 21 (1.5%) | 228 (6.3%) | 80 (6.0%) | 169 (4.6%) | 39 (5.3%) | 210 (4.9%) | |||
| Other | 97 (7.0%) | 332 (9.1%) | 122 (9.1%) | 307 (8.3%) | 23 (3.2%) | 406 (9.4%) | |||
| White | 1004 (72.0%) | 2220 (61.0%) | 760 (56.6%) | 2464 (66.8%) | 464 (63.6%) | 2759 (64.1%) | |||
| Region | <0.001 | <0.001 | <0.001 | ||||||
| Asia Pacific | 316 (22.7%) | 865 (23.8%) | 402 (29.9%) | 779 (21.1%) | 212 (29.0%) | 969 (22.5%) | |||
| Eastern Europe | 362 (25.9%) | 1330 (36.5%) | 341 (25.4%) | 1351 (36.6%) | 87 (11.9%) | 1605 (37.3%) | |||
| Latin and South America | 212 (15.2%) | 512 (14.1%) | 179 (13.3%) | 545 (14.8%) | 64 (8.8%) | 660 (15.3%) | |||
| North America | 131 (9.4%) | 422 (11.6%) | 179 (13.3%) | 374 (10.1%) | 147 (20.1%) | 406 (9.4%) | |||
| Western Europe | 374 (26.8%) | 510 (14.0%) | 243 (18.1%) | 641 (17.4%) | 220 (30.1%) | 663 (15.4%) | |||
| Index event | <0.001 | <0.001 | 0.27 | ||||||
| HF hospitalization 3–6 mo | 258 (18.5%) | 604 (16.6%) | 208 (15.5%) | 654 (17.7%) | 138 (18.9%) | 724 (16.8%) | |||
| HF hospitalization within 3 mo | 880 (63.1%) | 2491 (68.5%) | 955 (71.1%) | 2416 (65.5%) | 486 (66.6%) | 2885 (67.0%) | |||
| Intravenous diuretic for HF (without hospitalization) within 3 mo | 257 (18.4%) | 544 (14.9%) | 181 (13.5%) | 620 (16.8%) | 106 (14.5%) | 694 (16.1%) | |||
| Body mass index, kg/m2 | 25.2 (22.6–28.6) | 27.5 (24.2–31.8) | <0.001 | 26.0 (23.0–29.7) | 27.2 (24.0– 31.3) | <0.001 | 27.4 (24.3– 31.8) | 26.8 (23.6–30.7) | <0.001 |
| Ejection fraction, % | 31.0 (25.0–38.0) | 28.0 (21.0–35.0) | <0.001 | 25.0 (20.0–32.0) | 30.0 (25.0–36.0) | <0.001 | 27.0 (20.0–33.5) | 30.0 (23.0–35.0) | <0.001 |
| New York Heart Association class | 0.27 | 0.20 | 0.60 | ||||||
| I | 1 (0.1%) | 1 (0.0%) | 0 (0.0%) | 2 (0.1%) | 0 (0.0%) | 2 (0.0%) | |||
| II | 805 (57.7%) | 2160 (59.4%) | 773 (57.5%) | 2192 (59.4%) | 427 (58.6%) | 2538 (59.0%) | |||
| III | 575 (41.2%) | 1424 (39.2%) | 547 (40.7%) | 1452 (39.4%) | 296 (40.6%) | 1702 (39.6%) | |||
| IV | 14 (1.0%) | 52 (1.4%) | 24 (1.8%) | 42 (1.1%) | 6 (0.8%) | 60 (1.4%) | |||
| Medical history | |||||||||
| Atrial fibrillation | 817 (58.6%) | 1445 (39.7%) | <0.001 | 619 (46.1%) | 1643 (44.5%) | 0.33 | 327 (44.8%) | 1934 (44.9%) | 0.94 |
| Diabetes | 592 (42.4%) | 1772 (48.7%) | <0.001 | 590 (43.9%) | 1774 (48.1%) | 0.009 | 348 (47.7%) | 2016 (46.9%) | 0.68 |
| Hypertension | 1192 (85.4%) | 2795 (76.8%) | <0.001 | 893 (66.4%) | 3094 (83.8%) | <0.001 | 567 (77.7%) | 3419 (79.5%) | 0.27 |
| Hyperlipidemia | 860 (61.6%) | 2027 (55.7%) | <0.001 | 720 (53.6%) | 2167 (58.7%) | 0.001 | 435 (59.6%) | 2451 (57.0%) | 0.18 |
| Anemia | 421 (30.2%) | 647 (17.8%) | <0.001 | 282 (21.0%) | 786 (21.3%) | 0.81 | 156 (21.4%) | 912 (21.2%) | 0.92 |
| History of coronary artery disease | 896 (64.2%) | 1959 (53.8%) | <0.001 | 707 (52.6%) | 2148 (58.2%) | <0.001 | 397 (54.4%) | 2458 (57.1%) | 0.17 |
| History of Stroke | 186 (13.3%) | 391 (10.7%) | 0.010 | 144 (10.7%) | 433 (11.7%) | 0.32 | 89 (12.2%) | 488 (11.3%) | 0.50 |
| History of peripheral artery disease | 226 (16.2%) | 404 (11.1%) | <0.001 | 124 (9.2%) | 506 (13.7%) | <0.001 | 91 (12.5%) | 539 (12.5%) | 0.96 |
| Vitals | |||||||||
| SBP, mm Hg | 120.0 (110.0–133.0) | 118.0 (108.0–130.0) | <0.001 | 105.0 (102.0–107.0) | 124.0 (117.0–136.0) | <0.001 | 116.0 (107.0–126.0) | 119.0 (109.0–132.0) | <0.001 |
| Diastolic blood pressure, mm Hg | 69.0 (61.0–76.0) | 74.0 (67.0–81.0) | <0.001 | 65.5 (60.0–71.0) | 75.0 (68.0–82.0) | <0.001 | 71.0 (64.0–79.0) | 73.0 (65.0–80.0) | 0.002 |
| Heart rate, beats per minute | 70.0 (62.0–79.0) | 72.2 (64.0–82.0) | <0.001 | 72.0 (64.0–81.0) | 72.0 (64.0–81.0) | 0.52 | 70.0 (63.0–79.0) | 72.0 (64.0–81.0) | <0.001 |
| Standard of care medications and devices | |||||||||
| Angiotensin‐converting enzyme or angiotensin receptor blocker | 998 (71.6%) | 2698 (74.1%) | 0.07 | 942 (70.1%) | 2754 (74.6%) | 0.001 | 27 (3.7%) | 3669 (85.3%) | <0.001 |
| Beta blocker | 1281 (91.9%) | 3404 (93.5%) | 0.039 | 1249 (93.0%) | 3436 (93.1%) | 0.89 | 664 (91.0%) | 4021 (93.4%) | 0.014 |
| ARNI | 172 (12.3%) | 558 (15.3%) | 0.007 | 239 (17.8%) | 491 (13.3%) | <0.001 | 730 (100.0%) | 0 (0.0%) | <0.001 |
| Mineralocorticoid receptor antagonist | 786 (56.4%) | 2755 (75.7%) | <0.001 | 1047 (78.0%) | 2494 (67.6%) | <0.001 | 525 (71.9%) | 3016 (70.1%) | 0.32 |
| Triple therapy | 638 (45.7%) | 2367 (65.0%) | <0.001 | 862 (64.1%) | 2143 (58.1%) | <0.001 | 482 (66.0%) | 2523 (58.6%) | <0.001 |
| Biventricular pacemaker | 263 (18.9%) | 475 (13.1%) | <0.001 | 232 (17.3%) | 506 (13.7%) | 0.002 | 131 (17.9%) | 607 (14.1%) | 0.007 |
| Implantable cardioverter defibrillator | 359 (25.8%) | 1039 (28.6%) | 0.047 | 445 (33.1%) | 953 (25.8%) | <0.001 | 309 (42.3%) | 1089 (25.3%) | <0.001 |
| Laboratory results | |||||||||
| Creatinine, mg/dL | 1.4 (1.1–1.8) | 1.1 (0.9–1.5) | <0.001 | 1.2 (0.9–1.6) | 1.2 (0.9–1.6) | 0.57 | 1.2 (1.0–1.6) | 1.2 (0.9–1.6) | 0.009 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 47.0 (33.9–60.6) | 62.8 (45.7–83.2) | <0.001 | 58.6 (40.7–78.5) | 58.3 (41.4–76.7) | 0.74 | 55.6 (41.4–74.5) | 58.7 (41.2–77.7) | 0.10 |
| N‐terminal pro‐B‐type natriuretic peptide, pg/mL | 3460 (1914–6234) | 2611 (1452–5001) | <0.001 | 3228 (1824–5587) | 2708 (1470–5233) | <0.001 | 2718 (1554–5033) | 2833 (1555–5358) | 0.28 |
Data presented as median (25th, 75th) or number (%), unless otherwise indicated. ARNI indicates angiotensin receptor neprilysin inhibitors; ; HF, heart failure; and SBP, systolic blood pressure.
Race was reported by the patient.
Dose Achieved and Treatment Adherence
Treatment dose achieved after standardized up‐titration by protocol (based on mean SBP and clinical symptoms) was as follows. Among patients >75 years old, the proportion of patients achieving the 10 mg target dose of study drug was similar for vericiguat (78%) and placebo (78%); this was also similar to the proportion of younger patients achieving the target dose of vericiguat (79%). Among patients with baseline SBP 100 to 110 mm Hg, the proportion of patients achieving the 10 mg target dose of study drug was similar for vericiguat (60%) and placebo (62%); however, a higher proportion of patients with higher baseline SBP (≥110 mm Hg) achieved the target dose of vericiguat (86%) and placebo (87%). Among patients receiving ARNI, the proportion of patients achieving the 10 mg target dose of study drug was slightly lower for vericiguat (69%) than placebo (73%); however, a higher proportion of patients not receiving ARNI achieved the target 10 mg dose of vericiguat (81%) and placebo (82%).
Treatment adherence rates were similarly high in all subgroups over the duration of the study: 95.6% for patients >75 years old (versus 94.9% in younger patients), 94.2% in those with baseline SBP <110 mm Hg (versus 95.4% in those with higher SBP), and 94.6% in those receiving baseline ARNI therapy (versus 95.2% in those not receiving ARNI).
SBP Trajectory by Vulnerable Subgroups
As previously reported in the overall VICTORIA cohort, 4 SBP declined slightly more in the vericiguat than placebo group over the first 16 weeks before returning to baseline (Figure 1A). Compared with younger patients, the initial decline in SBP with vericiguat was more pronounced in those >75 years old, with SBP still returning to baseline thereafter (Figure 1B and 1C). A similar SBP trajectory was observed in patients >85 years old, compared with those above and below 65 years of age, whereas a smaller difference between treatment arms was observed (Figure S2A).
Figure 1. SBP trajectory (mean ±1 SE) over time in the entire VICTORIA population (A), and by subgroups of age below (B) or above (C) 75 years; SBP above (D) or below (E) 110 mm Hg; and those not receiving (F) or receiving (G) ARNI at baseline.
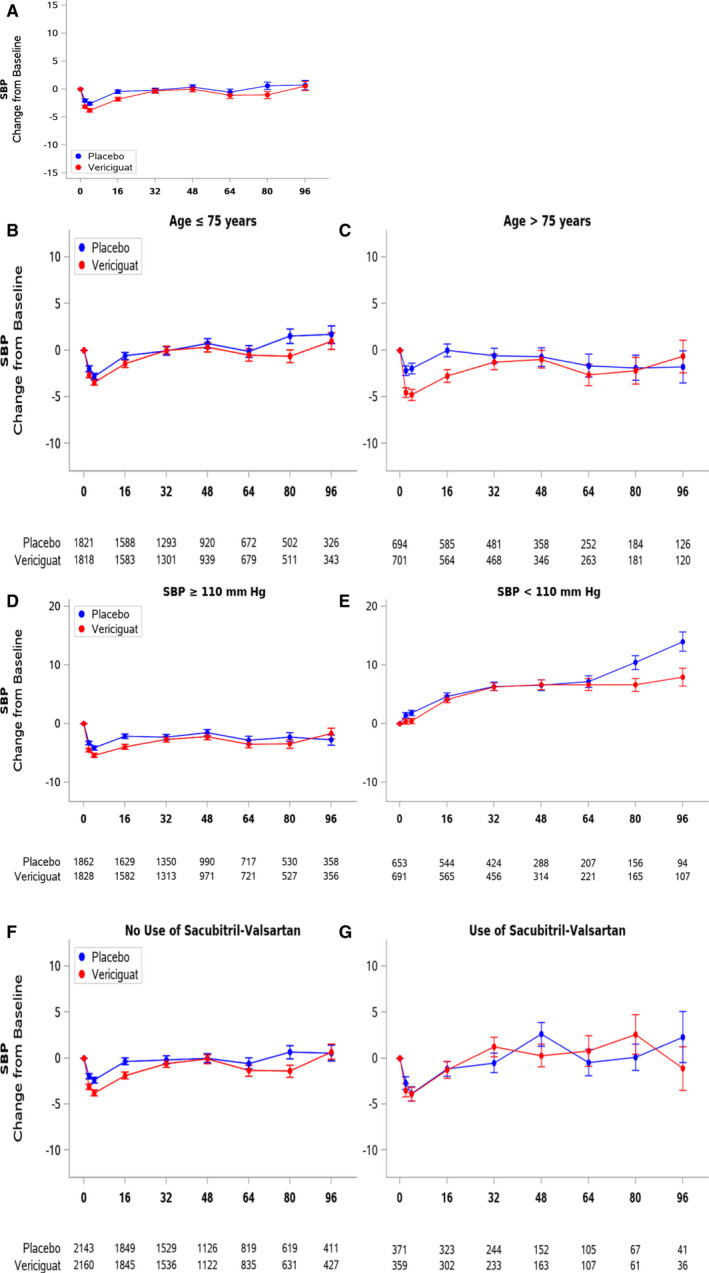
ARNI indicates angiotensin receptor neprilysin inhibitors; SBP: systolic blood pressure; and VICTORIA: Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction.
Compared with patients with higher baseline SBP, those with SBP <110 mm Hg at baseline showed a trend to increasing SBP over time, which was similar in both treatment arms (Figure 1D and 1E). Compared with patients not receiving ARNI, those receiving ARNI at baseline showed a similar SBP trajectory, with no difference between treatment arms (Figure 1F and 1G). Likewise, the SBP trajectories were similar between patients receiving (versus not receiving) any renin‐angiotensin blockade, maximal doses of renin‐angiotensin system inhibitors, and guideline‐directed triple therapy (Figure S3).
Occurrence of Symptomatic Hypotension and Syncope
In adjusted Cox proportional hazards analysis for time to the composite of symptomatic hypotension or syncope in the overall cohort (Figure 2A through 2C) there was no significant difference in hazard rates for vericiguat compared with placebo (adjusted HR, 1.18; 95% CI, 0.99–1.39; P=0.059) (Figure 2A). There was no significant difference in the rates of symptomatic hypotension alone (adjusted HR, 1.17; 95% CI, 0.96–1.42; P=0.13) (Figure 2B) or syncope alone (adjusted HR, 1.16; 95% CI, 0.86–1.55; P=0.33) (Figure 2C) between treatment arms. Treatment discontinuation rates were also similar between treatment arms, regardless of occurrence of syncope or symptomatic hypotension (Table S1). Patients experiencing symptomatic hypotension or syncope were at increased risk of the primary outcome (adjusted HR, 1.55; 95% CI, 1.31–1.82). In landmark analysis beginning after the titration phase (ie, post 4 weeks) (Figure 2D through 2F), there was no difference in the occurrence of symptomatic hypotension or syncope between vericiguat‐ and placebo‐treated patients, either before (unadjusted HR, 1.13; 95% CI, 0.93–1.37; P=0.22) or after multivariable adjustment (adjusted HR, 1.14; 95% CI, 0.93–1.38; P=0.20) (Figure 2D). Similarly, there was no difference in the rates of symptomatic hypotension alone (unadjusted HR, 1.09; 95% CI, 0.86–1.38, P=0.47; adjusted HR, 1.13; 95% CI, 0.89–1.43; P=0.32) (Figure 2E) or syncope alone (unadjusted HR, 1.17; 95% CI, 0.86–1.59; P=0.32; adjusted HR, 1.20; 95% CI, 0.88–1.64; P=0.26) (Figure 2F) between treatment arms.
Figure 2. Kaplan‐Meier event curves for time from randomization to (A) symptomatic hypotension or syncope, (B) symptomatic hypotension alone, and (C) syncope alone, and (D) landmark analysis in the post‐titration phase (ie, after 4 weeks) of time to symptomatic hypotension or syncope, (E) symptomatic hypotension alone, (F) and syncope alone. aHR indicates adjusted hazard ratio.
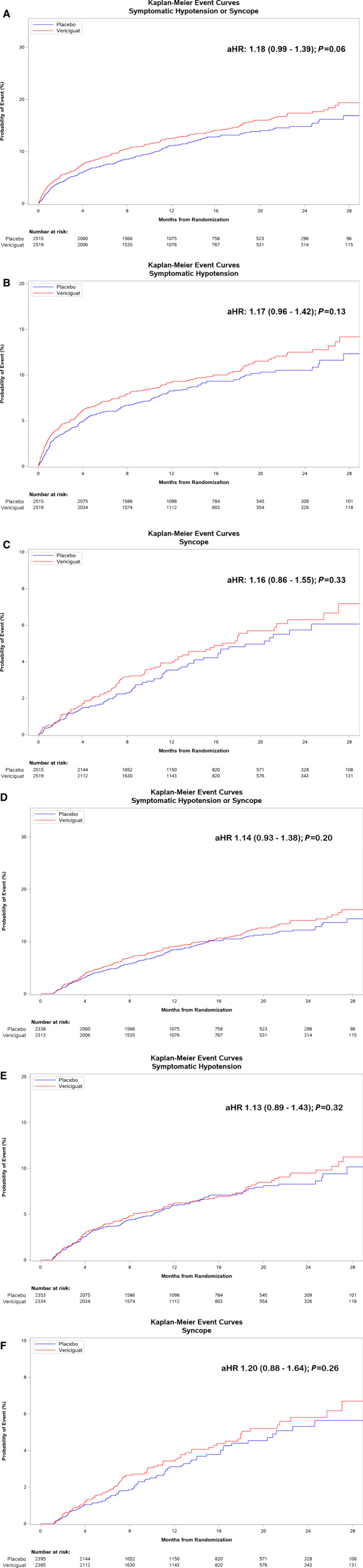
When analyzed by subgroups (Table 2), event rates were similar between treatment arms within each subgroup.
Table 2.
Treatment Effect on Time to Symptomatic Hypotension or Syncope by Vulnerable Subgroups
|
Vericiguat Rate (Events)* |
Placebo Rate (Events)* |
Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Symptomatic hypotension or syncope | ||||||
| Age ≤75 y | 12.03 (222) | 9.61 (179) | 1.26 (1.03–1.53) | 0.28 | 1.23 (1.01–1.51) | 0.42 |
| Age >75 y | 13.35 (90) | 12.99 (88) | 1.03 (0.77–1.39) | 1.06 (0.78–1.44) | ||
| SBP ≥110 mm Hg | 10.08 (193) | 9.13 (178) | 1.11 (0.91–1.36) | 0.36 | 1.11 (0.90–1.37) | 0.33 |
| SBP <110 mm Hg | 19.66 (119) | 15.05 (89) | 1.30 (0.99–1.71) | 1.32 (0.99–1.75) | ||
| No use of ARNI | 11.61 (258) | 9.55 (212) | 1.22 (1.02–1.46) | 0.48 | 1.23 (1.02–1.49) | 0.24 |
| Use of ARNI | 18.09 (54) | 17.23 (55) | 1.05 (0.72–1.53) | 0.95 (0.64–1.41) | ||
| Symptomatic hypotension | ||||||
| Age ≤75 y | 8.88 (168) | 6.84 (130) | 1.30 (1.04–1.64) | 0.08 | 1.27 (1.00–1.61) | 0.20 |
| Age >75 y | 8.85 (61) | 9.96 (68) | 0.90 (0.64–1.27) | 0.96 (0.67–1.37) | ||
| SBP ≥110 mm Hg | 7.14 (140) | 6.34 (126) | 1.14 (0.89–1.44) | 0.82 | 1.13 (0.88–1.45) | 0.70 |
| SBP <110 mm Hg | 14.36 (89) | 12.06 (72) | 1.19 (0.87–1.62) | 1.23 (0.89–1.69) | ||
| No use of ARNI | 8.12 (185) | 6.87 (155) | 1.19 (0.96–1.47) | 0.73 | 1.21 (0.97–1.51) | 0.48 |
| Use of ARNI | 14.53 (44) | 13.20 (43) | 1.10 (0.72–1.67) | 1.01 (0.65–1.57) | ||
| Syncope | ||||||
| Age ≤75 y | 3.53 (69) | 3.13 (61) | 1.13 (0.80–1.60) | 0.72 | 1.15 (0.81–1.63) | 0.94 |
| Age >75 y | 4.50 (32) | 3.55 (26) | 1.27 (0.76–2.13) | 1.18 (0.69–2.02) | ||
| SBP ≥110 mm Hg | 3.30 (66) | 3.08 (63) | 1.08 (0.76–1.52) | 0.41 | 1.09 (0.77–1.55) | 0.52 |
| SBP <110 mm Hg | 5.26 (35) | 3.78 (24) | 1.40 (0.83–2.35) | 1.35 (0.79–2.30) | ||
| No use of ARNI | 3.77 (88) | 3.00 (70) | 1.26 (0.92–1.73) | 0.25 | 1.29 (0.93–1.77) | 0.10 |
| Use of ARNI | 3.91 (13) | 4.93 (17) | 0.79 (0.39–1.63) | 0.65 (0.30–1.38) | ||
ARNI indicates angiotensin receptor neprilysin inhibitors; HR, hazard ratio; and SBP, systolic blood pressure.
Number of events per 100 patient‐years of follow‐up.
Efficacy of Vericiguat According to Baseline SBP
Although the benefit of vericiguat compared with placebo on the primary composite outcome (HF hospitalization or cardiovascular death) appeared more pronounced in younger patients, 4 when we examined the effect of vericiguat compared with placebo on the primary composite outcome across the spectrum of baseline SBP (Figure 3), there was no evidence of treatment heterogeneity (P for interaction=0.32).
Figure 3. Treatment effect of vericiguat compared with placebo on the primary composite end point (first heart failure hospitalization or cardiovascular death) according to baseline systolic blood pressure.
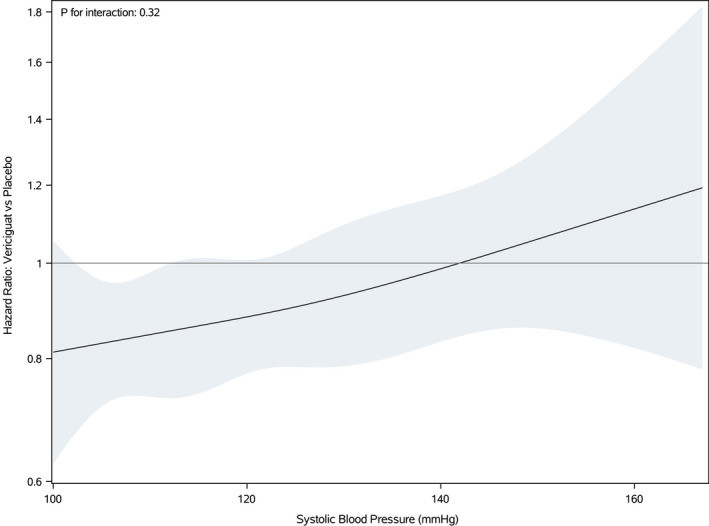
Further Subgroup Analyses
We also examined SBP trajectory, as well as occurrence of symptomatic hypotension and syncope according to NT‐proBNP cut points above and below 4000 pg/mL and 8000 pg/mL (Figure S4, Table S2). The rates of symptomatic hypotension and syncope were similar between treatment arms in each NT‐proBNP strata, with no difference between NT‐proBNP subgroups.
Patients receiving SGLT2i at baseline (n=122) were younger (median [25th, 75th percentile] age 64 [58, 74] versus 69 [60, 77] years), more likely to be male (86% versus 76%), more likely to have a history of diabetes (98% versus 46%), had lower baseline SBP (115 [106–131] versus 119 [109–131] mm Hg), and more concurrent ARNI use (21% versus 14%) but similar overall use of triple therapy (63% versus 60%) compared with patients not receiving SGLT2i. In the small group of patients receiving SGLT2i at baseline, the proportion of patients achieving the 10 mg target dose of study drug was slightly lower for vericiguat (74%) than placebo (85%) but similar to the proportion of patients not receiving SGLT2i who achieved the target 10 mg dose of vericiguat (79%) and placebo (81%). SBP trajectories showed that patients receiving SGLT2i at baseline appeared to have lower SBP compared with those who were not, although CIs were wide (Figure S5). Symptomatic hypotension and syncope event rates were numerically higher in those receiving SGLT2i at baseline compared with those who were not; however, numbers of events were small and the differences were not statistically significant (Table S3).
Discussion
Consistent with the safety and tolerability of vericiguat observed in the overall VICTORIA trial, we did not observe excessive blood pressure decreases with vericiguat across a large number of subgroups representing potentially vulnerable patients predisposed to blood pressure decreases. These subgroups included older patients, those with lower baseline SBP, as well as patients receiving concurrent ARNI therapy. There was a small numerical increase in occurrence of symptomatic hypotension or syncope early during dose titration but no difference in rates of symptomatic hypotension or syncope from 4 weeks onwards. The rates of symptomatic hypotension and rates of syncope were generally low and similar between randomized treatment arms across specified subgroups. Along with prior evidence of benefit with vericiguat regardless of age and background therapy, as well as this new evidence of the consistency of benefit across baseline SBP, our findings indicate the favorable benefit to risk ratio of vericiguat extends to patients potentially predisposed to blood pressure decreases.
These data provide important reassurance to physicians who may be reluctant to prescribe medications likely to cause hypotension, even if they are known to be beneficial. The SBP trajectories in the current report showed that despite an initial small dip in SBP in all subgroups—also seen in the placebo arm—continued treatment with vericiguat was associated with recovery and stabilization of SBP. Such patterns of SBP change have also been previously reported with initiation of renin‐angiotensin system inhibitors, 12 , 13 ARNI, 9 and SGLT2i 10 in HFrEF and have been attributed to a potential improvement in perfusion with beneficial afterload lowering therapies, offsetting any treatment‐induced reduction in SBP. Furthermore, the small nonsignificant increase in symptomatic hypotension or syncope during dose titration did not prevent the majority (≥60%) of patients in each subgroup from achieving the 10 mg target dose of vericiguat, and there was reassuringly no further excess safety events with continued treatment.
The relation between baseline SBP, outcomes, and treatment effect in HFrEF is known to be complex. A low SBP is associated with worse outcomes in patients with HFrEF, indicative of more advanced cardiac disease with worse haemodynamic status, or because low SBP reflects underuse of (or intolerance to) effective therapies. 14 Indeed in VICTORIA, the occurrence of syncope or symptomatic hypotension identified patients at higher risk of the primary outcome. It is particularly noteworthy that the efficacy of vericiguat was similar across baseline SBP, even down to a baseline SBP of 100 mm Hg. Vericiguat could be safely initiated and up‐titrated, even in patients with baseline SBP <110 mm Hg, following a fixed protocol (based on SBP) that applied to both treatment arms. Among patients with baseline SBP <110 mm Hg, the similar proportion of patients achieving the target 10 mg dose in both vericiguat and placebo sham titration indicates that the dose was limited by the titration protocol rather than by intolerance to vericiguat. The lower baseline SBP in patients receiving ARNI and SGLT2i may similarly explain the lower proportion of patients achieving the target dose of vericiguat in these subgroups. Nonetheless, the consistent beneficial effect on the primary efficacy composite (HF hospitalization or cardiovascular death), coupled with lack of excess safety events (symptomatic hypotension and syncope) in patients with low baseline SBP, suggests that vericiguat is an important therapeutic option in these patients.
We acknowledge that these are post hoc analyses, using arbitrary cut‐offs for assignment of subgroups, although supplementary analyses were performed to examine other cutoffs for age. VICTORIA excluded patients with SBP <100 mm Hg and included only relatively small subgroups with concurrent ARNI and SGLT2i treatment. The landmark analysis using post‐titration data to support tolerability may bias our data by removing patients lost during titration and should therefore be interpreted to refer to patients who tolerated the up‐titration. Nonetheless, results were consistent with the overall analysis of all available timepoints and were adjusted for an extensive list of baseline characteristics to minimize bias.
Conclusions
In conclusion, vericiguat is safe and hemodynamically tolerated in a broad population of patients with worsening HFrEF, even in patients at older age, with lower SBP, and on other HF therapies with the potential to interact in the cyclic guanosine monophosphate pathway or decrease blood pressure (such as ARNI).
Sources of Funding
The VICTORIA trial was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and Bayer AG, Wuppertal, Germany.
Disclosures
Lam reports grants and personal fees from Merck and Bayer during the conduct of the study; grants from AstraZeneca, Bayer, Boston Scientific, and Roche Diagnostics and personal fees from Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, and WebMD Global LLC outside the submitted work; in addition, Dr Lam has a patent to PCT/SG2016/050217 pending and a patent to 16/216,929 issued; and is co‐founder and non‐executive director of Us2.ai. Lopatin reports personal fees from MSD during the conduct of the study. Ezekowitz reports grants and personal fees from Bayer and Merck during the conduct of the study; and grants and personal fees from Amgen, AstraZeneca, and gBoehringer‐Ingelheim outside the submitted work. Pieske reports personal fees from Merck and Bayer Healthcare during the conduct of the study; and personal fees from Novartis, Astra Zeneca, BMS, and Servier outside the submitted work. O’Connor reports research funding from Merck and consulting fees from Bayer, Dey LP, and Bristol‐Myers Squibb Foundation. Roessig is an employee of Bayer AG. Patel is an employee of Merck Inc., Co. Anstrom reports research grants from Merck and the National Institutes of Health. Hernandez reports grants and personal fees from Merck and personal fees from Bayer during the conduct of the study; grants and personal fees from AstraZeneca, Novartis, and Boehringer Ingelheim; grants from American Regent, and personal fees from Amgen and Boston Scientific outside the submitted work. Armstrong reports grants and personal fees from Merck during the conduct of the study; and grants and personal fees from Bayer, grants from Sanofi‐aventis Recherche & Developpement, Boehringer Ingelheim, and CSL Limited; and personal fees from AstraZeneca and Novartis outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S3
Figures S1–S5
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021094
For Sources of Funding and Disclosures, see page 11.
References
- 1. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 2. Peri‐Okonny PA, Mi X, Khariton Y, Patel KK, Thomas L, Fonarow GC, Sharma PP, Duffy CI, Albert NM, Butler J, et al. Target doses of heart failure medical therapy and blood pressure: insights from the CHAMP‐HF registry. JACC Heart Fail. 2019;7:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 5. Lang NN, Dobbin SJH, Petrie MC. Vericiguat in worsening heart failure: agonising over, or celebrating, agonism in the VICTORIA trial. Cardiovasc Res. 2020;116:e152–e155. doi: 10.1093/cvr/cvaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boerrigter G, Burnett JC Jr. Nitric oxide‐independent stimulation of soluble guanylate cyclase with BAY 41–2272 in cardiovascular disease. Cardiovasc Drug Rev. 2007;25:30–45. doi: 10.1111/j.1527-3466.2007.00003.x [DOI] [PubMed] [Google Scholar]
- 7. Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA Trial. JACC Heart Fail. 2018;6:96–104. [DOI] [PubMed] [Google Scholar]
- 8. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation. 2020;141:100–111. doi: 10.1161/CIRCULATIONAHA.119.044133 [DOI] [PubMed] [Google Scholar]
- 9. Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J. 2017;38:1132–1143. doi: 10.1093/eurheartj/ehw570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA‐HF). Eur Heart J. 2020;41:3402–3418. doi: 10.1093/eurheartj/ehaa496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ezekowitz JA, O’Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, Butler J, Lam CSP, Ponikowski P, Emdin M, et al. N‐terminal Pro‐B‐type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8:931–939. doi: 10.1016/j.jchf.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 12. Anand IS, Rector TS, Kuskowski M, Thomas S, Holwerda NJ, Cohn JN. Effect of baseline and changes in systolic blood pressure over time on the effectiveness of valsartan in the Valsartan Heart Failure Trial. Circ Heart Fail. 2008;1:34–42. doi: 10.1161/CIRCHEARTFAILURE.107.736975 [DOI] [PubMed] [Google Scholar]
- 13. Meredith PA, Östergren J, Anand I, Puu M, Solomon SD, Michelson EL, Olofsson B, Granger CB, Yusuf S, Swedberg K, et al. Clinical outcomes according to baseline blood pressure in patients with a low ejection fraction in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) Program. J Am Coll Cardiol. 2008;52:2000–2007. doi: 10.1016/j.jacc.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 14. Gheorghiade M, Vaduganathan M, Ambrosy A, Böhm M, Campia U, Cleland JGF, Fedele F, Fonarow GC, Maggioni AP, Mebazaa A, et al. Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev. 2013;18:107–122. doi: 10.1007/s10741-012-9315-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S5


