Abstract
Background
Patients with single‐ventricle physiology who undergo the Fontan procedure are at risk for thrombotic events associated with significant morbidity and mortality. The UNIVERSE Study evaluated the efficacy and safety of a novel liquid rivaroxaban formulation, using a body weight–adjusted dosing regimen, versus acetylsalicylic acid (ASA) in children post‐Fontan.
Methods and Results
The UNIVERSE Study was a randomized, multicenter, 2‐part, open‐label study of rivaroxaban, in children who had undergone a Fontan procedure, to evaluate its dosing regimen, safety, and efficacy. Part A was the single‐arm part of the study that determined the pharmacokinetics/pharmacodynamics and safety of rivaroxaban in 12 participants before proceeding to part B, whereby 100 participants were randomized 2:1 to open‐label rivaroxaban versus ASA. The study period was 12 months. A total of 112 participants were enrolled across 35 sites in 10 countries. In part B, for safety outcomes, major bleeding occurred in one participant on rivaroxaban (epistaxis that required transfusion). Clinically relevant nonmajor bleeding occurred in 6% of participants on rivaroxaban versus 9% on ASA. Trivial bleeding occurred in 33% of participants on rivaroxaban versus 35% on ASA. For efficacy outcomes, 1 participant on rivaroxaban in part B had a pulmonary embolism (2% overall event rate); and for ASA, 1 participant had ischemic stroke and 2 had venous thrombosis (9% overall event rate).
Conclusions
In this study, participants who received rivaroxaban for thromboprophylaxis had a similar safety profile and fewer thrombotic events, albeit not statistically significant, compared with those in the ASA group.
Registration
URL: https://www.clinicaltrials.gov. Identifier: NCT02846532.
Keywords: children, Fontan, major bleeding, rivaroxaban, thrombotic events
Subject Categories: Clinical Studies
Nonstandard Abbreviations and Acronyms
- ASA
acetylsalicylic acid
Clinical Perspective
What Is New?
Patient care providers are aware that the standard of care for thromboprophylaxis in children post‐Fontan procedure lacks evidence‐based recommendations.
This study shows data on an anticoagulant that has adequate safety and efficacy profiles and a new oral formulation that is age appropriate, with evidence‐based and body weight–adjusted dosing regimens for children after they undergo the Fontan procedure.
What Are the Clinical Implications?
The study highlights a potential new use in pediatrics of an oral anticoagulant that does not require regular laboratory monitoring or parenteral administration.
Single ventricle is a complex cardiac disorder representing 7.7% of congenital heart disease diagnosed in childhood. 1 The Fontan procedure separates pulmonary and systemic circulations by directing all systemic venous return to the pulmonary vascular bed while the functional single ventricle is committed to supporting the systemic circulation. This results in venous hypertension and passive pulmonary blood flow, predisposing to stasis and endothelial dysfunction, which, together with developmental and acquired coagulation system abnormalities, may predispose to both bleeding and thrombotic events. 2 Thromboembolism is associated with an increased risk of morbidity and mortality. In 2 retrospective series published, the prevalence of thromboembolism in patients following the Fontan procedure was estimated to be between 17% and 33%. 3 , 4 Thromboembolic risk appears to be highest within the first 3 to 12 months after the operation, yet it may persist over the following years. 5 There has been considerable debate about the optimal thromboprophylaxis duration and strategy. There are limited evidence‐based guidelines, 6 , 7 given the rarity of both single‐ventricle patients and thrombotic events that have precluded large‐scale definitive clinical trials. Observational data have suggested that some thromboprophylaxis is better than none, 8 , 9 but studies have suggested equipoise on antiplatelet versus anticoagulant therapies. 10 , 11 However, with the use of either anticoagulant or antiplatelet agents, an important residual risk of thrombotic events remains. Anticoagulant use is problematic given the difficulties, especially in children, associated with vitamin K antagonists (most commonly warfarin), which require dietary modifications, and low‐molecular‐weight heparins, which require subcutaneous injections; both forms of anticoagulation also require relatively frequent phlebotomy for monitoring of anticoagulation effect. For aspirin (acetylsalicylic acid, hereafter referred to as ASA), there are no data on ASA resistance or the optimal dose for thromboprophylaxis in children.
ASA inhibits platelet aggregation. The mechanism of action of ASA is suppression of thromboxane A2 production by irreversible inactivation of the prostaglandin cyclo‐oxygenase enzyme required for thromboxane synthesis. Because it inhibits platelet aggregation at lower doses, ASA is indicated as part of the standard treatment of acute angina pectoris, acute myocardial infarction, stroke, and other thrombosis. 12 Antiplatelet therapy for long‐term antithrombotic therapy is recommended after the Fontan procedure. However, long‐term therapy with warfarin may be indicated after the Fontan procedure for higher‐risk patients (eg, patients with anatomic or hemodynamic risk factors). 6 Adverse reactions associated with the use of ASA include allergic reactions and increased risk of gastrointestinal bleeding. 12
Rivaroxaban is an oral, highly selective, direct factor Xa inhibitor. Inhibition of factor Xa interrupts the intrinsic and extrinsic pathways of the blood coagulation cascade, inhibiting both thrombin formation and the development of thrombi. It has been widely used in adults for thromboprophylaxis and for the treatment and the reduction in the risk of recurrence of deep vein thrombosis and pulmonary embolism. 13 , 14 , 15 Rivaroxaban has the advantage over conventional anticoagulants, like warfarin, of being an oral medication with no food‐drug interactions, limited drug‐drug interactions, and no requirement for venipunctures for monitoring, as well as possibly a more favorable safety profile. 16 Use of rivaroxaban may lead to greater acceptability, compliance, and a more stable anticoagulation profile in the young population of patients who have had the Fontan procedure, thereby making rivaroxaban a good candidate for long‐term use in this population.
In the UNIVERSE Study, we sought to determine the optimal dosing, and comparative efficacy and safety of rivaroxaban versus ASA for the prevention of thrombotic events in pediatric patients with single‐ventricle physiology who had recently undergone the Fontan procedure, using a novel, age‐appropriate, liquid formulation.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Yale University Open Data Access Project. Information on how to request data can be found at https://yoda.yale.edu/how‐request‐data.
Trial Design
The UNIVERSE Study was a prospective, multicenter, open‐label, active controlled, 2‐part study of rivaroxaban in children with single‐ventricle physiology who had the Fontan procedure within 4 months before enrollment. The pediatric dose regimen in the trial was designed to match the exposure range in adults treated with rivaroxaban, 10 mg once daily, which is an effective dose for the prevention of thrombotic events in adults. The study rationale and trial design have been published previously. 17 The protocol was approved by the Institutional Review Board or Ethics Committee for each participating center. Part A evaluated the single‐ and multiple‐dose pharmacokinetic and pharmacodynamic properties of rivaroxaban to confirm the dose and dosing regimen selected. Part B evaluated the comparative safety and efficacy of rivaroxaban versus ASA when used for thromboprophylaxis for 12 months. Participants in part B were randomized 2:1 to receive body weight–adjusted rivaroxaban administered twice daily or ASA, given once daily (≈5 mg/kg) (Figure 1).
Figure 1. Trial design.
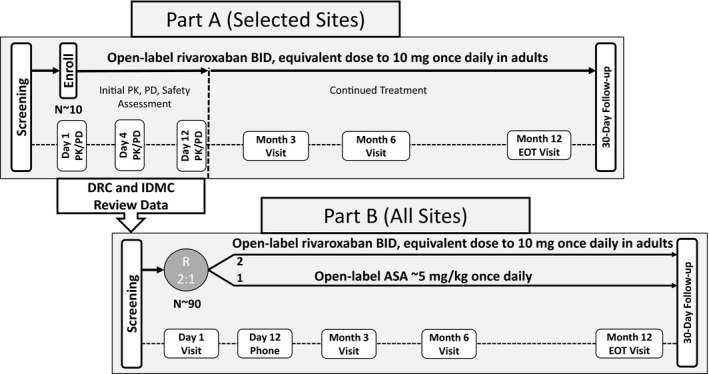
ASA indicates acetylsalicylic acid; BID, twice daily; DRC, Data Review Committee; EOT, end of treatment; IDMC, independent data monitoring committee; PD, pharmacodynamics; PK, pharmacokinetics; and R, randomization.
Oversight
An independent data and safety monitoring board evaluated all pharmacokinetic, pharmacodynamic, safety, and efficacy data to ensure participant safety throughout the study. In part A only, the independent data and safety monitoring board reviewed cumulative data from the initial pharmacokinetic, pharmacodynamic, and safety assessment period, providing a recommendation to the executive committee to stop enrollment in part A and start enrollment into part B.
The central independent adjudication committee was composed of specialist physicians. Its members did not enroll patients, were not involved in study monitoring, and did not have operational responsibilities for the conduct of the study. Members reviewed and adjudicated all safety (bleeding) and efficacy (thrombotic) outcomes that occurred post enrollment (Table S2).
Patients
Pediatric patients were eligible for participation in the trial if they were between 2 and 8 years of age with single‐ventricle congenital heart disease and had completed an initial Fontan procedure within 4 months before enrollment. Eligible patients were required to be clinically stable and able to tolerate oral or nasogastric feedings. Because the objective of the study was to evaluate rivaroxaban for primary thromboprophylaxis, all patients were required to have an initial post‐Fontan transthoracic echocardiographic screening without any evidence of thrombosis.
Patients were excluded from participation if they had thrombosis, a history of gastrointestinal disease or surgery associated with impaired absorption, active bleeding or high risk of bleeding contraindicating antiplatelet or anticoagulant therapy, including history of intracranial hemorrhage, or contraindications to ASA or rivaroxaban. A full listing of the inclusion and exclusion criteria is in Table S1. Written informed consent for trial participation was obtained from the parent or guardian of each participant; a child’s assent was also obtained if required per local guidelines.
Trial Regimen and Follow‐Up
Participants in part A had a 12‐day initial pharmacokinetic, pharmacodynamic, and safety assessment period, a 12‐month open‐label treatment period, and a 30‐day follow‐up telephone contact. Pharmacokinetic and pharmacodynamic blood samples were collected on day 1 and day 4 of rivaroxaban administration, and the results were assessed by day 12 by the independent data monitoring committee before the participant continued in the study to complete the planned 12‐month treatment period. Safety and efficacy were evaluated throughout the study. Participants had additional pharmacokinetic and pharmacodynamic samples collected at month 3 and month 12. Pharmacokinetic/pharmacodynamic results will be published separately.
Participants in part B were randomized on day 1 to receive either rivaroxaban oral suspension or ASA for 12 months. Pharmacokinetic and pharmacodynamic samples for those participants randomized to rivaroxaban in part B were obtained at day 1, month 3, and month 12. Safety and efficacy were evaluated throughout the study for all participants.
The rivaroxaban dose used in UNIVERSE Study was determined via an adapted physiologically based pharmacokinetics modeling approach previously established in healthy children. 18 , 19 This model was reparameterized to reflect the special physiology of the pediatric Fontan population (lower body weight, smaller body height, and reduced cardiac output). The dose‐exposure relation of rivaroxaban in these post‐Fontan patients was comparable to that in healthy children if liver function is not impaired. 20
Rivaroxaban was administered twice daily in an open‐label manner using a novel, 0.1% (1 mg/mL) oral suspension (body weight–adjusted dosing [Table 1]) that targeted exposure (Area under the plasma concentration time curve from 0 to 24 hours at steady state) matching the exposure range of rivaroxaban, 10 mg total daily dose, effectively used in adults for thromboprophylaxis. ASA was provided as 81‐ or 100‐mg tablets, according to local practice. Participants who were randomized to ASA received approximately 5 mg/kg of ASA as a single daily dose. Dose adjustments were made at month 6 on the basis of body weight changes for all subjects in both part A and part B as needed.
Table 1.
Dosing Table for Rivaroxaban Administration
| Body weight, kg | BID dose, mg or mL* | Total daily dose, mg† |
|---|---|---|
| 7–<8 | 1.1 | 2.2 |
| 8–<10 | 1.6 | 3.2 |
| 10–<12 | 1.7 | 3.4 |
| 12–<20 | 2.0 | 4.0 |
| 20–<30 | 2.5 | 5.0 |
BID indicates twice daily.
Oral suspension 0.1% (1 mg/mL).
Equivalent to exposure of 10 mg once daily in adults.
Outcome Measures
The primary safety outcome was major bleeding events, as defined by the International Society on Thrombosis and Haemostasis. 21 The secondary safety outcomes were clinically relevant nonmajor bleeding and trivial (minimal) bleeding events.
The primary efficacy outcome was any thrombotic event (venous or arterial), defined as the appearance of a new thrombotic burden within the cardiovascular system noted on either routine surveillance or clinically indicated imaging, or the occurrence of a clinical event known to be strongly associated with thrombus (eg, stroke or pulmonary embolism).
All safety and thrombotic events were adjudicated by the central independent adjudication committee.
Statistical Analysis
Safety and efficacy results were summarized using appropriate descriptive statistics. The central independent adjudication committee–adjudicated results were used in the final analysis for all subjects. Because of the limited availability of the study population and the expected low event rates, the study was not powered to test formal hypotheses for efficacy. A total of at least 100 pediatric participants were planned to be enrolled in this study based on regulatory feedback to obtain sufficient pharmacokinetics/pharmacodynamic and safety data in this unique pediatric population.
Results
Baseline Participant Characteristics
A total of 129 children with single‐ventricle congenital heart disease were screened at 36 sites in 10 countries (Argentina, Belgium, Brazil, Canada, Japan, Malaysia, Mexico, the Netherlands, Spain, and the United States). Of these, 112 participants met the eligibility criteria and were enrolled in the UNIVERSE Study (12 in the rivaroxaban part A group, 66 in the rivaroxaban part B group, and 34 in the ASA part B group) (Figure 2).
Figure 2. UNIVERSE Study flow.
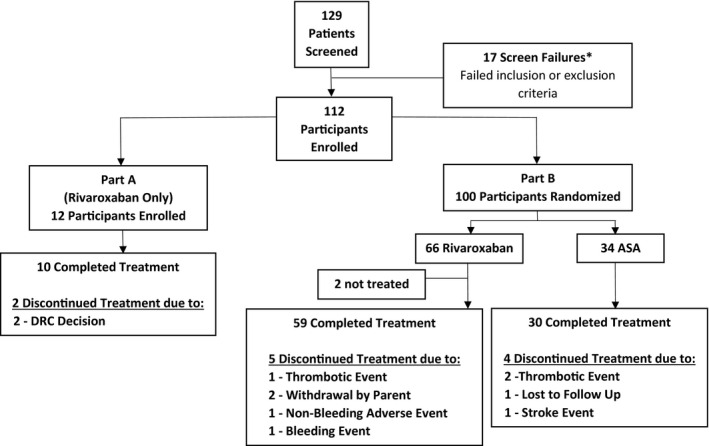
*Reasons for screen failures: of the 17 subjects, 8 did not meet inclusion criteria (2 did not have the Fontan within 4 months of enrollment, 4 did not tolerate oral feedings, and 2 had nonsatisfactory screening echocardiogram) and 11 met at least 1 exclusion criterion (3 had evidence of thrombosis on screening echocardiogram, 4 were on prohibited medications, 1 had a known contraindication to acetylsalicylic acid (ASA), and 3 were not considered appropriate candidates for the study by their physicians). DRC indicates Data Review Committee.
The demographic and baseline characteristics were generally balanced between the rivaroxaban and the ASA groups in part B (Table 2); however, there were slightly more male patients in the ASA group (68%) versus the rivaroxaban group (55%). There was a slightly shorter duration between the Fontan procedure and first study drug dose in the ASA group (mean, 37 days) than in the rivaroxaban group (mean, 45 days). The rivaroxaban part A group had a younger mean age (2.5±0.7 years), a lower mean weight, and a shorter mean duration (mean, 12 days) between the Fontan procedure and first dose than both groups in part B. The shorter duration in days between the Fontan procedure and the first dose of study drug in part A was probably attributable to the study design, which required frequent blood draws on day 1 and day 4 for pharmacokinetic/pharmacodynamic testing in part A. Most investigators planned to have day 1 and day 4 blood work conveniently performed while the children were still hospitalized postoperatively from the Fontan procedure and with an intravenous catheter still in place, to avoid multiple venipunctures.
Table 2.
Baseline Characteristics
| Characteristics | Rivaroxaban | ASA | |
|---|---|---|---|
|
Part A (N=12) |
Part B (N=66) |
Part B (N=34) |
|
| Age at screening, mean (SD), y | 2.5 (0.7) | 4.1 (1.7) | 4.2 (1.8) |
| Male sex, n (%) | 7 (58) | 36 (55) | 23 (68) |
| Weight, mean (SD), kg | 13.8 (2.4) | 15.8 (3.7) | 15.7 (3.1) |
| Height, mean (SD), cm | 90 (7) | 101 (13) | 103 (12) |
| Heart rate, mean (SD), beats/min | 111 (13) | 109 (16) | 107 (15) |
| Systolic blood pressure, mean (SD), mm Hg | 109 (9) | 99 (13) | 102 (11) |
| Diastolic blood pressure, mean (SD), mm Hg | 63 (11) | 59 (11) | 63 (9) |
| Race, n (%) | |||
| Asian | 0 | 14 (21) | 7 (21) |
| Black | 3 (25) | 8 (12) | 1 (3) |
| White | 8 (67) | 40 (61) | 20 (59) |
| Other | 1 (8) | 2 (3) | 3 (9) |
| Not reported | 0 | 2 (3) | 3 (9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1 (8) | 22 (33) | 11 (32) |
| Not Hispanic or Latino | 11 (92) | 42 (64) | 19 (56) |
| Not reported | 0 | 2 (3) | 4 (12) |
| Duration between Fontan procedure and first dose of study drug, d* | |||
| Mean (SD) | 12 (17) | 45 (41) | 37 (35) |
| Median | 4 | 34 | 24 |
| Range | 2–61 | 2–124 | 2–117 |
Age is defined as a participant’s age at screening. Enrolled: all participants in part A who received at least 1 dose of study drug and all participants in part B who were randomized. ASA indicates acetylsalicylic acid.
Duration between Fontan procedure and first dose of study drug is calculated as date of first dose of study drug–date of Fontan procedure.
The pharmacokinetic/pharmacodynamic results of part A of the UNIVERSE Study showed that exposure of rivaroxaban in children after the Fontan procedure receiving a body weight–adjusted dosing regimen was similar to the exposure in adults who received 10‐mg total daily dose for thromboprophylaxis. The UNIVERSE Study pharmacokinetic/pharmacodynamic work will be reported separately. The same body weight–adjusted dosing regimen was used in part B of the study.
Safety Outcomes
Major Bleeds
There was 1 participant with a major bleeding event adjudicated in the rivaroxaban part B group (2%). This single event of major bleeding, epistaxis, reported in the rivaroxaban group was in a noncritical site, which had stopped by the time the child arrived at the hospital. The child was not evaluated by an ear, nose, and throat specialist. However, it was considered a major bleeding event because the participant required a blood transfusion. There were no participants with major bleeding events reported in the ASA part B or in the rivaroxaban part A group (Table 3).
Table 3.
Summary of Bleeding Events
| Bleeding events | Rivaroxaban | ASA | |
|---|---|---|---|
|
Part A (N=12) |
Part B (N=64) |
Part B (N=34) |
|
| Participant with ≥1 on‐treatment bleeding events | 4 (33) | 23 (36) | 14 (41) |
| Major bleeding | 0 | 1 (2) | 0 |
| Clinically relevant nonmajor bleeding | 1 (8) | 4 (6) | 3 (9) |
| Gastrointestinal | 0 | 2 (3) | 1 (3) |
| Lower gastrointestinal | 0 | 2 (3) | 1 (3) |
| Gingival | 0 | 1 (2) | 0 |
| Hematoma | 0 | 0 | 1 (3) |
| Skin | 1 (8) | 1 (2) | 1 (3) |
| Subconjunctival | 0 | 0 | 1 (3) |
| Trivial bleeding | 3 (25) | 21 (33) | 12 (35) |
| Epistaxis | 0 | 7 (11) | 3 (9) |
| Gastrointestinal | 0 | 1 (2) | 1 (3) |
| Lower gastrointestinal | 0 | 0 | 1 (3) |
| Upper gastrointestinal | 0 | 1 (2) | 1 (3) |
| Gingival | 1 (8) | 3 (5) | 1 (3) |
| Hematoma | 2 (17) | 7 (11) | 2 (6) |
| Skin | 0 | 14 (22) | 8 (24) |
| Vascular access site | 0 | 2 (3) | 0 |
Data are given as number (percentage). Percentages were calculated with the number of participants in each group as denominator. Incidence is based on the number of subjects, not the number of events. A participant may appear in different sites/categories. Safety analysis set: all participants in part A who received at least 1 dose of study drug and all participants in part B who were randomized and received at least 1 dose of study drug. The primary safety outcome is major bleed that meets the International Society on Thrombosis and Haemostasis definition: overt bleeding and: (1) associated with a decrease in hemoglobin of ≥2 g/dL; (2) leading to a transfusion of the equivalent of ≥2 units of packed red blood cells or whole blood in adults; (3) occurring in a critical site: intracranial, intraspinal, intraocular, pericardial, intra‐articular, intramuscular with compartment syndrome, or retroperitoneal; or (4) contributing to death. ASA indicates acetylsalicylic acid.
Clinically Relevant Nonmajor Bleeds
In part B, the proportion of participants with clinically relevant nonmajor bleeding events was less in the rivaroxaban (4 [6%]) than in the ASA group (3 [9%]). In the rivaroxaban group, the bleeding events occurred in the lower gastrointestinal tract (2 [3%]), gingival tissue (1 [2%]), and the skin (1 [2%]). In the ASA group, these events occurred in the lower gastrointestinal tract (1 [3%]), the skin (1 [3%]), hematoma (1 [3%]), and subconjunctival (1 [3%]). In the rivaroxaban part A group, there was 1 (8%) participant reported with a clinically relevant nonmajor bleeding event in the skin (Table 3).
Trivial Bleeds
In part B, the proportion of participants with trivial bleeding was similar in the rivaroxaban and the ASA groups (21 [33%] versus 12 [35%], respectively). The most frequent site of trivial bleeding was the skin in both groups (14 [22%] in the rivaroxaban group and 8 [24%] in the ASA group). In the rivaroxaban part A group, there were 3 (25%) participants reported with trivial bleeds (2 skin hematomas and 1 gingival) (Table 3).
Any Bleeding
In part B, the proportion of participants with any bleeding events was similar in the rivaroxaban group than in the ASA group (36% versus 41%) (Table 3).
Adverse Events, Including Bleeds
In part B, the proportion of participants who reported at least one adverse event or serious adverse event was similar in both groups, with adverse events of 86% versus 85% and serious adverse events of 28% versus 24% in the rivaroxaban group versus the ASA group, respectively (Table 4). Adverse events and serious adverse events were generally balanced between the rivaroxaban and the ASA groups. In the respiratory organ class, however, pleural effusions (nonhemorrhagic) were more frequently reported as adverse events or serious adverse events in the rivaroxaban group than in the ASA group (Table 4). Pleural effusions are expected events after the Fontan procedure and were considered nonrelated to the study drug by the investigators. In part B, the adverse events more frequently reported were infections in both groups (40 [63%] in the rivaroxaban group and 22 [65%] in the ASA group). In part A, there were 11 (92%) participants who reported adverse events and 6 (50%) who reported serious adverse events (Table 4). The most frequently reported adverse events in all treatment groups were infections (eg, nasopharyngitis, upper respiratory tract infections, and bronchitis).
Table 4.
Summary of Adverse Events
| Adverse events | Rivaroxaban | ASA | |
|---|---|---|---|
|
Part A (N=12) |
Part B (N=64) |
Part B (N=34) |
|
| Participants with ≥1 adverse events | 11 (92) | 55 (86) | 29 (85) |
| Infections | 8 (67) | 40 (63) | 22 (65) |
| Respiratory, thoracic, and mediastinal disorders | 5 (42) | 29 (45) | 9 (26) |
| Pleural effusion | 3 (25) | 12 (19) | 2 (6) |
| Gastrointestinal disorders | 6 (50) | 19 (30) | 9 (26) |
| Injury, poisoning, and procedural complications | 4 (33) | 18 (28) | 10 (29) |
| Skin and subcutaneous tissue disorders | 3 (25) | 19 (30) | 9 (26) |
| General disorders and administration site conditions | 1 (8) | 17 (27) | 8 (24) |
| Vascular disorders | 2 (17) | 3 (5) | 1 (3) |
| Participants with ≥1 serious adverse events | 6 (50) | 18 (28) | 8 (24) |
| Infections | 3 (25) | 5 (8) | 4 (12) |
| Respiratory, thoracic, and mediastinal disorders | 2 (17) | 9 (14) | 3 (9) |
| Pleural effusion | 2 (17) | 9 (14) | 2 (6) |
Data are given as number (percentage). Percentages were calculated with the number of participants in each group as denominator. All adverse events were treatment emergent. Treatment emergent is defined as an adverse event or serious adverse event that occurs after the first dose and up to 2 days after the last dose of study drug. Participants are counted only once for any given event, regardless of the number of times they experienced the event. The organ classes are sorted in descending order of incidence >10% based on rivaroxaban. ASA indicates acetylsalicylic acid.
Efficacy Outcomes
In the rivaroxaban part B group, 1 participant (2%) was reported with pulmonary embolism on day 84 of study treatment (121 days post‐Fontan procedure). In the ASA group, 3 participants (9%) were reported with thrombotic events (2 participants [6%] with venous thrombotic events reported on day 177 and day 179 of treatment [191 and 183 days post‐Fontan procedure, respectively]; and 1 participant (3%) who had an ischemic stroke on day 122 of treatment [133 days post‐Fontan procedure]). In the rivaroxaban part A group, 1 participant (8%) had a venous thrombotic event on day 362 of treatment (364 days post‐Fontan procedure) (Table 5). This study was not powered for efficacy hypothesis testing (post hoc log‐rank test P=0.095). Overall, the rivaroxaban group had a proportion of thrombotic events of 3% versus 9% in the ASA group (Table 5).
Table 5.
Efficacy Outcomes
| Efficacy outcomes | Rivaroxaban | ASA | ||
|---|---|---|---|---|
|
Part A (N=12) |
Part B (N=64) |
Total (N=76) |
Part B (N=34) |
|
|
Primary efficacy outcome: Any thrombotic event |
1 (8) | 1 (2) | 2 (3) | 3 (9) |
| Ischemic stroke | 0 | 0 | 0 | 1 (3) |
| Pulmonary embolism | 0 | 1 (2) | 1 (1) | 0 |
| Venous thrombosis | 1 (8) | 0 | 1 (1) | 2 (6) |
| Arterial/intracardiac thrombosis | 0 | 0 | 0 | 0 |
Data are given as number (percentage). Percentages were calculated with the number of participants in each group as denominator. Full analysis set: all participants in part A who received at least 1 dose of study drug and all participants in part B who were randomized and received at least 1 dose of study drug. ASA indicates acetylsalicylic acid.
Discussion
The UNIVERSE Study specifically targeted a pediatric cardiac population at high risk for thrombosis at a vulnerable age. The study showed a low prevalence of both thrombotic and bleeding events in the rivaroxaban and the ASA groups during a high‐risk period early after Fontan procedure. In the rivaroxaban group, there was only one major bleeding episode (epistaxis) and a slightly lower prevalence of clinically relevant and trivial bleeding compared with ASA. The prevalence and pattern of adverse events were comparable. Although the study was not powered for efficacy outcomes, there was a lower proportion of thrombotic events in the rivaroxaban group relative to the ASA group, although this did not reach statistical significance. Together, this suggests a trend toward a favorable risk/benefit profile for rivaroxaban in this clinical scenario.
The Fontan physiology for a patient with a single functional ventricle is characterized by the lack of an underlying subpulmonic ventricle and, hence, passive pulmonary blood flow. This sets the stage for systemic venous hypertension and sluggish flow, which, together with both developmental and acquired imbalances of prothrombotic, antithrombotic, and fibrinolytic circulating factors, predisposes to thrombosis. 2 Although there is an increased prevalence of thrombosis in the stages of single‐ventricle management preceding the Fontan procedure, 8 the 3‐to 12‐month period immediately after Fontan has been identified as a particularly high‐risk period. 5 The UNIVERSE Study targeted this specific high‐risk period.
The reported prevalence of thrombosis post‐Fontan procedure in the literature varies widely depending on the study design. There is a high degree of variability about the presence and type of surveillance strategies, the time since Fontan, the duration of time period covered, and the inclusion of asymptomatic events. 3 A systematic review and meta‐analysis by Marrone et al that included 1075 patients (20% on ASA and 80% on warfarin) showed an overall thromboembolism rate of 5%, with no significant difference between warfarin and ASA. 10 This is similar to the overall event rate reported herein. A further meta‐analysis by Alsaied et al of 1200 patients showed that although some thromboprophylaxis was better than none, warfarin was no more effective than ASA (odds ratio, 0.94). 9 A randomized trial by Monagle et al of ASA versus warfarin during the 2 year period immediately after Fontan procedure showed no significant difference, with an overall prevalence of thrombosis events of 19%, a higher prevalence than in other studies because of greater surveillance with transesophageal echocardiography. 11 Iyengar et al studied the time‐related freedom from a thrombotic event for patients >1 year after Fontan. Of 475 patients included, the 10‐year freedom from thrombosis event was 91%, with no significant difference between those treated with ASA versus warfarin after both propensity score adjustment and propensity score matching. 22 Despite the lack of difference between an antiplatelet agent, ASA, and an anticoagulant, warfarin, an important residual risk of thrombosis event remains.
Given the probable equivalency on thromboprophylaxis efficacy between ASA and warfarin, other considerations, particularly safety, may drive preference for a given therapy. Warfarin is a challenging medication to manage, particularly in young patients. It has significant food‐drug and drug‐drug interactions, and dose titration can be difficult in a child experiencing rapid somatic growth. Management with warfarin requires close monitoring with inconvenient blood draws to maintain anticoagulation within a narrow target range. This was evident in the randomized trial of ASA versus warfarin described above, 11 whereby a secondary analysis of the data showed that an important proportion of those on warfarin frequently failed to maintain an international normalized ratio within the target range. 5 Compared with those on ASA or on well‐maintained warfarin, those on poorly controlled warfarin (<30% of international normalized ratios within target range) had a significantly increased risk of thrombotic events, with a hazard ratio of 3.53. A retrospective review by Faircloth et al showed that for those on warfarin, a greater time spent in the therapeutic range was associated with reduced thrombosis and bleeding events. 23 Because of these challenges with warfarin, the appeal of a direct oral anticoagulant, such as rivaroxaban, is evident, given the limited food and drug interactions and the lack of need for monitoring.
Recommendations for thromboprophylaxis after the Fontan procedure are limited by the suboptimal quality of the evidence. Guidelines from the American College of Chest Physicians in 2012 recommended ASA or unfractionated heparin followed by a vitamin K antagonist for thromboprophylaxis in children after the Fontan procedure. 7 A Scientific Statement from the American Heart Association in 2013 indicated that a range of strategies might be considered, including using warfarin or low‐molecular‐weight heparin for the first 3 to 12 months after Fontan, using antiplatelet therapy for long‐term thromboprophylaxis, and using warfarin for long‐term thromboprophylaxis for those patients with risk factors for thrombotic events. 6 As patients undergoing the Fontan procedure age, their thromboembolic risk increases because of a higher incidence of atrial arrhythmias and ventricular dysfunction during adulthood. A longitudinal real‐world data study from Germany showed an increasing use of novel oral anticoagulants, including rivaroxaban, in adults with congenital heart disease over time. 24 A recent systematic review demonstrated an annual thromboembolic and major bleeding event rate of 3.13% (95% CI, 1.18%–8.03%) and 3.17% (95% CI, 0.15%–41.39%), respectively, in adult patients undergoing the Fontan procedure and concluded that non–vitamin K oral anticoagulants appear safe and effective in adults with congenital heart disease without mechanical prostheses. 25 Further prospective studies are needed to define the benefit/risk profile for rivaroxaban in the population undergoing the Fontan procedure, but the current study suggests a favorable comparison to ASA. Given the advantages of rivaroxaban over warfarin, if efficacy and safety prove to be similar to well‐managed warfarin, rivaroxaban may be preferred in scenarios where long‐term anticoagulation is recommended.
The current randomized study shows a comparable prevalence and pattern of bleeding events between rivaroxaban and ASA. Comparisons of the risk of bleeding events with different thromboprophylactic strategies in nonrandomized studies are fraught by differing periods of observation, differing classifications of bleeding events, and different clinical scenarios. Randomized clinical trials, such as the present UNIVERSE Study, are rare, and pediatric experience with direct oral anticoagulants is limited. A clinical trial of rivaroxaban versus standard anticoagulants for short‐term treatment of venous thromboembolism in children (EINSTEIN‐Jr Phase 3 Trial) showed a similar prevalence of major or clinically relevant nonmajor bleeding (3% with rivaroxaban and 2% with standard anticoagulation). 26 In the randomized trial of ASA versus warfarin after the Fontan procedure described above, major bleeding occurred in 2% on ASA and 2% on warfarin, but minor bleeding was significantly more frequent with warfarin at all bleeding sites (33% with warfarin and 14% with ASA, overall). 11
This study must be considered in light of some potential limitations. Although the study was open label and not powered to test a formal hypothesis for efficacy because of recognized unique challenges in this pediatric population, 27 the study does suggest a benefit/risk ratio with rivaroxaban that is likely similar, and possibly favorable when compared with ASA. The observation period was limited to up to 12 months after the Fontan procedure, and although this does cover the high‐risk period for thrombotic events, it would be expected that thromboprophylaxis might need to be extended. The limited systematic surveillance for thrombosis (ie, transthoracic echocardiogram) likely restricted the detection of asymptomatic thromboses, which do have clinical relevance, particularly in increasing the risk of postthrombotic syndrome and of subsequent thrombotic events. 28 Given that the single‐ventricle population is limited and that thrombotic events are relatively infrequent, time related, and somewhat unpredictable, there are considerable challenges to performing a definitive study. 27
Conclusions
Although this randomized study of thromboprophylaxis in children with single‐ventricle physiology after the Fontan procedure was not powered for efficacy, the participants who received rivaroxaban had a lower prevalence of thrombotic events, albeit not achieving statistical significance, and similar safety profile compared with those in the ASA group.
Further studies are necessary to support definitive recommendations, although these studies are expected to be exceedingly challenging.
Sources of Funding
This study was funded by Bayer AG and Janssen Research & Development, LLC.
Disclosures
Dr McCrindle has served on the scientific advisory board for Janssen R&D, LLC, Chiesi, and Esperion; was the Data and Safety Monitoring Board Chair for Amryt Pharma; and was an investigator for Janssen R&D, LLC. Dr Michelson has served on the scientific advisory board for Janssen R&D, LLC, AstraZeneca, Chiesi, and Stasys. Dr Justino has served on the scientific advisory board for Janssen R&D, LLC, and Pediastent; has been a consultant for Abiomed, Edwards Lifesciences, and Medtronic; has intellectual property for PolyVascular; and has a grant from W.L. Gore. Dr Harris received consultant/honoraria fees from Janssen R&D, LLC. Dr Pina is an employee with equity ownership of Janssen R&D, LLC. C. Peluso is an employee with equity ownership of Janssen R&D, LLC. K. Nessel is an employee with equity ownership of Janssen R&D, LLC. W. Lu is an employee with equity ownership of Janssen R&D, LLC. Dr Li has served on the scientific advisory board for Janssen R&D, LLC. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
UNIVERSE Study Investigators
Guillermo Chantada, Alejandro Peirone (Argentina), Marc Gewillig, Daniel De Wolf, Thierry Sluysmans (Belgium), Andrea Wirich Lenzi, Maria Angelica Binotto, Estela Suzana Horowitz (Brazil), Brian McCrindle, Kevin Harris, Nagib Dahdah (Canada), Keisuke Sato, Hiroshi Ono, Koichi Sagawa, Jun Muneuchi (Japan), Hasri Samion (Malaysia), Sergio Ruiz, Juan Sandoval, Alexis Palacios (Mexico), J.M.P.J Breur (the Netherlands), Federico Gutierrez‐Iarraya, Teresa Alvarez, Dimpna Albert, Fernando Rueda (Spain), Joseph Rossano, Kevin Hill, Haleh Heydarian, Robert Niebler, Howard Weber, Biagio Pietra, Andrew Van Bergen, Juan Alejos, Shaji Menon, Marie Steiner, Jonathan Cramer, Ben Reinking (United States).
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.021765
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Brian W. McCrindle, Email: brian.mccrindle@sickkids.ca.
for the UNIVERSE Study Investigators:
Guillermo Chantada, Alejandro Peirone, Andrea Wirich Lenzi, Maria Angelica Binotto, Nagib Dahdah, Keisuke Sato, Hiroshi Ono, Koichi Sagawa, Jun Muneuchi, Hasri Samion, Sergio Ruiz, Alexis Palacios, J.M.P.J Breur, Federico Gutierrez‐Iarraya, Teresa Alvarez, Dimpna Albert, Fernando Rueda, Joseph Rossano, Kevin Hill, Haleh Heydarian, Robert Niebler, Howard Weber, Biagio Pietra, Andrew Van Bergen, Juan Alejos, Shaji Menon, Marie Steiner, Jonathan Cramer, and Ben Reinking
References
- 1. O'Leary PW. Prevalence, clinical presentation and natural history of patients with single ventricle. Progr Pediatr Cardio. 2002;16:31–38. DOI: 10.1016/S1058-9813(02)00042-5. DOI: 10.1016/S1058-9813(02)00042-5. [DOI] [Google Scholar]
- 2. Attard C, Huang J, Monagle P, Ignjatovic V. Pathophysiology of thrombosis and anticoagulation post Fontan surgery. Thromb Res. 2018;172:204–213. DOI: 10.1016/j.thromres.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 3. Monagle P, Cochrane AB, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after Fontan procedures ‐ ‐ the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. 1998;115:493–498. [DOI] [PubMed] [Google Scholar]
- 4. Balling G, Vogt M, Kaemmerer H, Eicken A, Meisner H, Hess J. Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg. 2000;119:745–752. DOI: 10.1016/S0022-5223(00)70010-9. [DOI] [PubMed] [Google Scholar]
- 5. McCrindle BW, Manlhiot C, Cochrane A, Roberts R, Hughes M, Szechtman B, Weintraub R, Andrew M, Monagle P; Fontan Anticoagulation Study Group. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346–353. DOI: 10.1016/j.jacc.2012.08.1023. [DOI] [PubMed] [Google Scholar]
- 6. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, Feltes TF, Foster E, Hinoki K, Ichord RN, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;128:2622–2703. DOI: 10.1161/01.cir.0000436140.77832.7a. [DOI] [PubMed] [Google Scholar]
- 7. Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak‐Göttl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S. DOI: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manlhiot C, Brandão LR, Kwok J, Kegel S, Menjak IB, Carew CL, Chan AK, Schwartz SM, Sivarajan VB, Caldarone CA, et al. Thrombotic complications and thromboprophylaxis across all three stages of single ventricle heart palliation. J Pediatr. 2012;161:513–519. DOI: 10.1016/j.jpeds.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 9. Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta‐analysis. Heart. 2015;101:1731–1737. DOI: 10.1136/heartjnl-2015-307930. [DOI] [PubMed] [Google Scholar]
- 10. Marrone C, Galasso G, Piccolo R, de Leva F, Paladini R, Piscione F, Santoro G. Antiplatelet versus anticoagulation therapy after extracardiac conduit Fontan: a systematic review and meta‐analysis. Pediatr Cardiol. 2011;32:32–39. DOI: 10.1007/s00246-010-9808-4. [DOI] [PubMed] [Google Scholar]
- 11. Monagle P, Cochrane A, Roberts R, Manlhiot C, Weintraub R, Szechtman B, Hughes M, Andrew M, McCrindle BW; Fontan Anticoagulation Study Group. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58:645–651. DOI: 10.1016/j.jacc.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 12. Bayer AG. Aspirin low dose tablet summary of product characteristics. 2017. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=075b103e‐0bb4‐4b7a‐ac0e‐5645bcbd0a07. Accessed March 18, 2021.
- 13. Prins MH, Lensing AWA, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, Raskob GE, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thrombosis J. 2013;11:21. DOI: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turpie AGG, Lassen MR, Eriksson BI, Gent M, Berkowitz SD, Misselwitz F, Bandel TJ, Homering M, Westermeier T, Kakkar AK. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty: pooled analysis of four studies. Thromb Haemost. 2011;105:444–453. DOI: 10.1160/TH10-09-0601. [DOI] [PubMed] [Google Scholar]
- 15. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. DOI: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 16. Chan N, Sobieraj‐Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. 2020;396:1767–1776. DOI: 10.1016/S0140-6736(20)32439-9. [DOI] [PubMed] [Google Scholar]
- 17. Pina LM, Dong X, Zhang L, Samtani MN, Michelson AD, Justino H, Bonnet D, Harris KC, Jefferies J, McCrindle BW, et al. Rivaroxaban, a direct factor Xa inhibitor, versus acetylsalicylic acid as thromboprophylaxis in children post‐Fontan procedure: rationale and design of a prospective, randomized trial (the UNIVERSE study). Am Heart J. 2019;213:97–104. DOI: 10.1016/j.ahj.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 18. Wilmann S, Thelen K, Kubitza D, Lensing AWA, Frede M, Coboeken K, Stampfuss J, Burghaus R, Mück W, Lippert J. Pharmacokinetics of rivaroxaban in children using physiologically based and population pharmacokinetic modelling: an EINSTEIN‐Jr phase 1 study. Thrombosis J. 2018;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilmann S, Becker C, Burghaus R, Coboeken K, Edginton A, Lippert J, Siegmund H, Thelen K, Mück W. Development of a paediatric population‐based model of the pharmacokinetics of rivaroxaban. Clin Pharmacokinet. 2014;53:89–102. DOI: 10.1007/s40262-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schnizler K. PH‐38483: physiology‐based prediction of rivaroxaban pharmacokinetics in children with congenital heart disease after Fontan procedure. Data on file February 24, 2015.
- 21. Schulman S, Kearon C; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thrombosis Haemostasis. 2005;3:692–694. DOI: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22. Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Attard C, Weintraub RG, et al. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardiothorac Surg. 2016;50:980–987. DOI: 10.1093/ejcts/ezw159. [DOI] [PubMed] [Google Scholar]
- 23. Faircloth JM, Miner KM, Alsaied T, Nelson N, Ciambarella J, Mizuno T, Palumbo JS, Vinks AA, Veldtman GR. Time in therapeutic range as a marker for thrombotic and bleeding outcomes in Fontan patients. J Thromb Thrombolysis. 2017;44:38–47. DOI: 10.1007/s11239-017-1499-8. [DOI] [PubMed] [Google Scholar]
- 24. Freisinger E, Gerß J, Makowski L, Marschall U, Reinecke H, Baumgartner H, Koeppe J, Diller GP. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients. Eur Heart J. 2020;41:4168–4177. DOI: 10.1093/eurheartj/ehaa844. [DOI] [PubMed] [Google Scholar]
- 25. Stalikas N, Doundoulakis I, Karagiannidis E, Bouras E, Kartas A, Frogoudaki A, Karvounis H, Dimopoulos K, Giannakoulas G. Non‐vitamin K oral anticoagulants in adults with congenital heart disease: a systemic review. J Clin Med. 2020;9:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K, Bonnet D, Connor P, Hooimeijer HL, Torres M, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7:e18–e27. [DOI] [PubMed] [Google Scholar]
- 27. McCrindle BW, Li JS, Manlhiot C, Tweddell JS, Giglia TM, Massicotte MP, Monagle P, Krishnamurthy R, Mahaffey KW, Michelson AD, et al. Challenges and priorities for research: a report from the National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH) Working Group on thrombosis in pediatric cardiology and congenital heart disease. Circulation. 2014;130:1192–1203. DOI: 10.1161/CIRCULATIONAHA.113.008428. [DOI] [PubMed] [Google Scholar]
- 28. Manlhiot C, McCrindle BW, Williams S, Menjak IB, O’Shea S, Chan AK, Brandão LR. Characterization of post‐thrombotic syndrome in children with cardiac disease. J Pediatr. 2019;207:42–48. DOI: 10.1016/j.jpeds.2018.10.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


