Abstract
Background
Sodium‐glucose cotransporter 2 inhibitors improve cardiovascular outcomes in patients with diabetes with and without heart failure (HF). However, their influence on sympathetic nerve activity (SNA) remains unclear. The purpose of this study was to evaluate the effect of sodium‐glucose cotransporter 2 inhibitors on SNA and compare the responses of SNA to sodium‐glucose cotransporter 2 inhibitors in patients with type 2 diabetes with and without HF.
Methods and Results
Eighteen patients with type 2 diabetes, 10 with HF (65.4±3.68 years) and 8 without HF (63.3±3.62 years), were included. Muscle SNA (MSNA), heart rate, and blood pressure were recorded before and 12 weeks after administration of dapagliflozin (5 mg/day). Sympathetic and cardiovagal baroreflex sensitivity were simultaneously calculated. Brain natriuretic peptide level increased significantly at baseline in patients with HF than those without HF, while MSNA, blood pressure, and hemoglobin A1c did not differ between the 2 groups. Fasting blood glucose and homeostatic model assessment of insulin resistance did not change in either group after administering dapagliflozin. MSNA decreased significantly in both groups. However, the reduction in MSNA was significantly higher in patients with HF than patients with non‐HF (−20.2±3.46 versus −9.38±3.65 bursts/100 heartbeats; P=0.049), which was concordant with the decrease in brain natriuretic peptide.
Conclusions
Dapagliflozin significantly decreased MSNA in patients with type 2 diabetes regardless of its blood glucose‐lowering effect. Moreover, the reduction in MSNA was more prominent in patients with HF than in patients with non‐HF. These results indicate that the cardioprotective effects of sodium‐glucose cotransporter 2 inhibitors may, in part, be attributed to improved SNA.
Keywords: baroreflex sensitivity, diabetes mellitus, heart failure, muscle sympathetic nerve activity
Subject Categories: Heart Failure, Autonomic Nervous System
Nonstandard Abbreviations and Acronyms
- BF
burst frequency
- BI
burst incidence
- BRS
baroreflex sensitivity
- MSNA
muscle sympathetic nerve activity
- SGLT2i
sodium‐glucose cotransporter 2 inhibitor
- SNA
sympathetic nerve activity
Clinical Perspective
What Is New?
Twelve weeks of a sodium‐glucose cotransporter 2 inhibitor (dapagliflozin 5 mg/day) administration significantly decreased muscle sympathetic nerve activity in patients with type 2 diabetes.
The decrease in muscle sympathetic nerve activity in patients with diabetes with heart failure was more pronounced than in patients with diabetes without heart failure.
The decrease in muscle sympathetic nerve activity was correlated with the decrease in brain natriuretic peptide but not with diabetic profiles.
What Are the Clinical Implications?
Dapagliflozin showed a significant sympatho‐inhibition which could be a part of cardioprotective effects.
Our finding supports the current evidence that sodium‐glucose cotransporter 2 inhibitors are effective in patients with heart failure regardless of the presence or absence of diabetes and might help to expand the indications for other sympatho‐exited diseases in the future.
Augmented sympathetic nerve activity (SNA) is related to worsening prognosis in patients with heart failure (HF). 1 Type 2 diabetes is also reported to increase SNA. 2 , 3 Diabetes increases the incidence of cardiovascular complications, including coronary artery disease and HF. 4 Further augmented SNA in patients with type 2 diabetes with HF presumably results in adverse outcomes. The goals of diabetes treatments are to prevent these complications and improve cardiovascular mortality. Sodium‐glucose cotransporter 2 inhibitors (SGLT2i) reduce the mortality rate from cardiovascular complications in patients with type 2 diabetes. 5 , 6 , 7 Moreover, SGLT2i reduces the rate of worsening HF and death in patients with HF regardless of the presence or absence of diabetes 8 ; hence, these results do not account for solely a glucose‐lowering effect. 9 Osmotic diuresis is a favorable mechanism in which SGLT2i reduces blood pressure and improves HF, 10 but SGLT2i is reported to lower blood pressure (BP) without increasing heart rate (HR) which cannot be explained by the diuretic effect. 11
Several studies have demonstrated that SGLT2i reduces SNA. 12 , 13 SGLT2i‐reduced SNA is attributed to improved baroreflex sensitivity (BRS) in the streptozotocin‐induced diabetic rat model. 14 In humans, muscle SNA (MSNA) recordings are recognized as the gold standard method for assessing SNA outflow to peripheral vascular beds. 15 We previously demonstrated that pioglitazone decreases MSNA associated with improved insulin resistance. 16 However, it is still not clearly understood whether SGLT2i reduces SNA in patients with type 2 diabetes, and efficacy in patients with and without HF is unclear.
In this study, we examined the effect of an SGLT2i on MSNA in patients with type 2 diabetes and evaluated whether the responses are related to insulin resistance and/or BRS. We also investigated whether the response of MSNA to SGLT2i differed between patients with type 2 diabetes with and without HF.
METHODS
Subjects
Patients with type 2 diabetes whose hemoglobin A1c (HbA1c) was >7.0% and were aged 20 to 80 years with at least 2 atherosclerotic risk factors were included in this study. Patients with an estimated glomerular filtration rate <45 mL/min per 1.73 m2, decompensating HF, or HbA1c >10% were excluded from this study. The diabetes diagnosis was confirmed as recommended by the Japan Diabetic Society. 17 Briefly, diabetes was diagnosed when the patient’s HbA1c was >6.5% and fasting blood glucose (FBG) was ≥126 mg/dL (or casual blood glucose ≥200 mg/dL). A re‐examination was performed within 1 month if only HbA1c or blood glucose was within the diabetic range. 17 Patients were divided into 2 groups by the presence (HF group) or absence (non‐HF group) of HF. HF was diagnosed according to the guideline of the Japanese Circulation Society. 18 HF was diagnosed when patients had New York Heart Association class Ⅱ or Ⅲ symptoms and/or their brain natriuretic peptide (BNP) was ≥100 pg/mL, and cardiac dysfunction was confirmed by echocardiography (eg, reduced left ventricular ejection fraction, significant valvular disease, diastolic dysfunction, or signs of elevated right and/or left ventricular pressure). This study protocol was approved by the Research Ethics Board of Kanazawa University (Kanazawa, Japan). This study was registered at the University Hospital Medical Information Network Center (UMIN, Tokyo, Japan) Clinical Trials Registration System (UMIN000026335). All participants provided written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Protocol
Baseline MSNA were recorded after anthropometric and laboratory data were collected, and 5 mg/day dapagliflozin was prescribed. Follow‐up measurements were performed 12 weeks after drug administration began. No restrictions were placed on the patients’ medications before beginning the study; however, changes in drugs used to treat HF and diabetes were prohibited during the study except when the disease condition worsened.
Measurements
All experiments were performed in a quiet, electrically shielded room in the morning with the subject in the supine position. All participants were asked to abstain from alcohol, caffeine, and strenuous exercise for 24 hours. HR was determined from a continuous ECG. beat‐to‐beat BP was recorded continuously from the radial artery using a non‐invasive tonometry monitoring system (JENTOW‐7700; Nihon Colin, Komaki, Japan). Postganglionic MSNA was recorded from the left peroneal nerve, as described previously. 19 , 20 , 21 Briefly, with the subject in a comfortable supine position, the common peroneal nerve was located by palpation and stimulated electrically via the skin surface. A tungsten microelectrode was inserted percutaneously into a motor fascicle of the peroneal nerve. The electrode was adjusted until spontaneous pulse‐synchronous sympathetic burst activities could be recorded. The electrodes were connected to a preamplifier at a gain of 1000 and an amplifier at a gain of 70. The signal was fed through a band‐pass filter (500–3000 kHz) and a resistance‐capacitance integrated circuit with a time constant of 0.1 seconds to produce a mean voltage neurogram using a PowerLab recording system (Model ML 785/85P; ADInstruments, Bella Vista, Australia). The raw nerve signal was obtained at 12 000 Hz; other signals were obtained at 1000 Hz. Multi‐unit MSNA was identified offline based on its relationship to cardiac activity in the integrated nerve recording in a blinded fashion by an experienced investigator. Burst activity was identified as a >3 signal to noise ratio on mean voltage neurograms. For each subject, multi‐unit MSNA was expressed as the number per minute (burst frequency [BF]) (bursts/minute) and the number per 100 heartbeats (burst incidence [BI]) (bursts/100 heartbeats). Data were acquired for at least 10 minutes. Multi‐unit MSNA has high reproducibility. 21 , 22
Echocardiography (Aplio; Toshiba, Tokyo, Japan) was performed on the same day or within 1 week before the MSNA measurement. Left ventricular ejection fraction was measured using Simpson method. E/e′ was calculated by the E wave derived from transmitral flow per e′ measured by tissue Doppler imaging of the left ventricular basal septal wall. FBG, immunoreactive insulin, HbA1c, BNP, and lipids were measured on the same day. The homeostasis model assessment of the insulin resistance index was calculated as HOMA‐IR=fasting glucose (mg/dL)×fasting insulin (μU/mL)/405. 23
BRS Analysis
Spontaneous arterial baroreflex modulation of HR (cardiovagal BRS [CBRS]) was estimated using the sequence method. 24 The beat‐to‐beat time series of systolic BP (SBP) and the RR interval were analyzed using HemoLab software (Harald Stauss Scientific, Iowa City, IA) as reported previously. 25 , 26 Briefly, sequences of ≥3 beats, during which SBP and the RR interval of the following beat changed in parallel (ie, positive correlation), were detected and divided into 2 groups (up sequences, rising SBP; down sequences, decreasing SBP). The mean value of the slope calculated from the relationship between the change in SBP and RR interval (ms/mm Hg) was regarded as CBRS. The mean slope of all sequences (ie, a combination of the up and down sequences) was calculated as the CBRS slope. A least‐square linear regression analysis was applied to each sequence and only sequences in which R 2>0.80 were accepted. Arterial baroreflex modulation of MSNA (sympathetic BRS [SBRS]) was assessed by examining the relationship between the occurrence of MSNA and diastolic BP (DBP). 20 , 27 , 28 Over a 5‐minute resting period, the DBP of individual heartbeats was grouped into 2 mm Hg interval bins, and the percentage of diastolic cycles associated with a sympathetic burst for each interval was plotted against the mean pressure interval. The reflex gain was defined as the slope of the regression line and expressed as bursts/100 heartbeats/mm Hg. If the regression line possessed an R≥0.5, the slope of the line was taken as the SBRS slope.
Statistical Analysis
Values are expressed as mean±SE. The statistical analysis was performed using SPSS software (version 25.0, IBM Corp., Armonk, NY). The paired t test was used to compare differences in the variables in all patients between baseline and 12 weeks after dapagliflozin administration. The unpaired t test was used to compare differences between the HF and non‐HF groups. Welch t test was applied if the variance was heteroscedastic. The Chi‐square test was adopted to compare differences in the population rate between groups. Linear regression analyses were performed to determine the relationship between change in MSNA and other variables (HbA1c, FBG, HOMA‐IR, insulin, HR, BMI, estimated glomerular filtration rate, CBRS, and SBRS). Linear regression analyses were also performed between changes in BNP and others (MSNA, HR, SBP, DBP, BMI, HbA1c, age, and baseline medications). A P<0.05 (2‐sided) was considered significant.
RESULTS
Twenty‐two patients were submitted to this study between March 2017 and March 2020. Among them, the baseline MSNA of 3 patients could not be adequately recorded, and they rejected attending the remainder of the study. One patient withdrew his agreement, and rejected follow‐up measurements; however, baseline MSNA was recorded. Finally, 18 patients with type 2 diabetes (10 patients, in the HF group; and 8 patients, in the non‐HF group) were included in the study and completed the follow‐up. No acute worsening of HF or diabetes requiring an urgent visit and/or hospitalization was observed, and all subjects continued the fixed dose of dapagliflozin during the study.
The baseline characteristics of the participants categorized by the HF group are presented in Table 1. No differences in the physical findings were observed between the non‐HF and HF groups (all, P>0.05). BNP was significantly higher in the HF group than the non‐HF group (161±58.6 versus 23.9±9.37 pg/mL, P=0.045), but other blood results including the diabetic parameters were not different between the groups (all, P>0.05). As expected, ejection fraction was significantly lower in the HF group than the non‐HF group (43.7±3.95% versus 65.3±2.18%, P<0.001). The mean values of the MSNA parameters (BF and BI) in both groups increased but tended to be higher in the HF group than the non‐HF group (50.1±1.88 versus 44.5±2.35 bursts/min, P=0.078). The rate of β‐blocker use was significantly higher in the HF group than the non‐HF group (90% versus 37.5%, P=0.006). The use of diuretics tended to be higher in the HF group than the non‐HF group (25% versus 70%, P=0.058). No differences were observed in the rates of use of other antihypertensive drugs or hypoglycemic agents between the groups (all, P>0.05).
Table 1.
Baseline Characteristics in Each Group
| Non‐HF (n=8) | HF (n=10) | P value | |
|---|---|---|---|
| Age, y | 65.4±3.68 | 63.3±3.62 | 0.696 |
| Men, n (%) | 6 (75.0) | 8 (80.0) | 0.800 |
| Body weight, kg | 72.1±3.92 | 68.8±4.94 | 0.633 |
| BMI, kg/m2 | 26.3±1.07 | 24.9±0.99 | 0.361 |
| Heart rate, /min | 76.9±4.64 | 76.5±2.14 | 0.895 |
| SBP, mm Hg | 119±4.84 | 121±3.73 | 0.841 |
| DBP, mm Hg | 72.1±3.82 | 73.5±3.20 | 0.785 |
| Hypertension, n (%) | 7 (87.5) | 10 (100) | 0.250 |
| Dyslipidemia, n (%) | 8 (100) | 10 (100) | 1.000 |
| Blood test | |||
| LDL, mg/dL | 72.3±6.54 | 82.7±9.44 | 0.389 |
| FBG, mg/dL | 137±7.57 | 163±15.9 | 0.159 |
| HbA1c, % | 7.49±0.09 | 7.98±0.21 | 0.057 |
| Insulin, μU/mL | 12.7±3.53 | 16.2±4.30 | 0.552 |
| HOMA‐IR | 4.14±1.27 | 6.68±2.45 | 0.176 |
| eGFR, mL/min per 1.73 m2 | 61.4±7.68 | 60.6±6.18 | 0.940 |
| BNP, pg/mL | 23.9±9.37 | 161±58.6 | 0.045 |
| UCG | |||
| LVEF, % | 65.3±2.18 | 43.7±3.95 | <0.001 |
| LVDd, mm | 45.9±1.18 | 54.2±5.11 | 0.145 |
| LVDs, mm | 29.6±1.25 | 42.4±4.98 | 0.033 |
| LAD, mm | 39.6±1.47 | 39.0±4.44 | 0.890 |
| E/e′ | 13.7±1.14 | 12.6±2.82 | 0.728 |
| Sympathetic nerve activity | |||
| BF, bursts/min | 44.5±2.35 | 50.1±1.88 | 0.078 |
| BI (bursts/100 heartbeats) | 61.2±4.31 | 69.0±1.77 | 0.127 |
| CBRS, ms/mm Hg | 6.06±0.93 | 11.2±4.21 | 0.318 |
| SBRS (bursts·100 heartbeats−1 mm Hg−1) | −1.24±0.33 | −2.17±1.06 | 0.427 |
| Medication, n (%) | |||
| β‐blocker | 3 (37.5) | 9 (90) | 0.006 |
| Calcium channel blocker | 5 (62.5) | 3 (30) | 0.168 |
| Statin | 8 (100) | 10 (100) | 1.000 |
| ARB or ACE‐I | 6 (75.0) | 10 (100) | 0.094 |
| Diuretics | 2 (25.0) | 7 (70.0) | 0.058 |
| Sulfonyl urea | 1 (12.5) | 1 (10.0) | 0.866 |
| DPP‐4 inhibitor | 2 (25.0) | 4 (40.0) | 0.502 |
| Glinide | 0 (0) | 2 (20.0) | 0.180 |
| α‐GI | 1 (12.5) | 2 (20.0) | 0.671 |
| GLP‐1 agonist | 2 (25.0) | 2 (20.0) | 0.800 |
| Biguanide | 3 (37.5) | 1 (10.0) | 0.163 |
| Thiazolidine | 1 (12.5) | 0 (0.0) | 0.250 |
| Insulin | 2 (25.0) | 4 (40.0) | 0.502 |
Unpaired‐t test was performed to compare differences in each parameter between groups. Welch t test was applied if the variance was heteroscedastic. The Chi‐square test was adopted to compare differences in the population rate of diseases and medications between groups. Insulin and HOMA‐IR were compared among patients without insulin treatment (All, N=12; non‐CHF, n=6; CHF, n=6). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin Ⅱ receptor blocker; BF, burst frequency; BI, burst incidence; BMI, body mass index; BNP, brain natriuretic peptide; CBRS, cardiovagal baroreflex sensitivity; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GLP‐1, glucagon like peptide; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis assessment of insulin resistance; LAD, left atrial dimension; LDL, low‐density lipoprotein; LVDd, left ventricular end diastolic diameter; LVDs, left ventricular end systolic diameter; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SBRS, sympathetic baroreflex sensitivity; UCG, ultrasound cardiography; and α‐GI, α‐glucosidase inhibitor.
Effects of SGLT2i in Patients With Diabetes With and Without HF
Typical MSNA recordings of 1 patient with type 2 diabetes and HF are shown in Figure 1. MSNA bursts decreased after dapagliflozin was administered. To investigate the difference in the effect of dapagliflozin between patients with type 2 diabetes with and without HF, its responses were compared between groups (Table 2). MSNA (BF and BI) decreased significantly in the HF group compared with baseline (BF, −15.2±2.73 bursts/min, P<0.001; BI, −20.2±3.36 bursts/100 heartbeats, P<0.001), while BMI was unchanged (−0.02±0.20 kg/m2, P=0.910). BNP and HR also significantly decreased in the HF group (BNP, −47.7±20.7, P=0.047; HR, −4.60±1.24 beats/min, P=0.005). In the non‐HF group, BMI, HbA1c, and MSNA (BI) decreased significantly compared with baseline (BMI, −0.87±0.22 kg/m2, P=0.008; HbA1c, −0.56±0.19%, P=0.021; BI, −9.38±3.65 bursts/100 heartbeats, P=0.037). The reduction in BMI was significantly lower in the non‐HF group than in the HF group (P=0.014). No differences in other parameters, including BP, diabetic profile, renal function, cardiac function, or BRS were observed between the groups (all, P>0.05). SBRS and CBRS were not different between baseline and 12 weeks administration in both groups (SBRS of HF, −2.58±2.42, P=0.459; SBRS of non‐HF, 0.27±0.35, P=0.334; CBRS of HF, 1.44±2.33, P=0.245; CBRS of non‐HF, 2.18±1.72, P=0.58).
Figure 1. Representative recording of muscle sympathetic nerve activity in a patient with type 2 diabetes with heart failure.
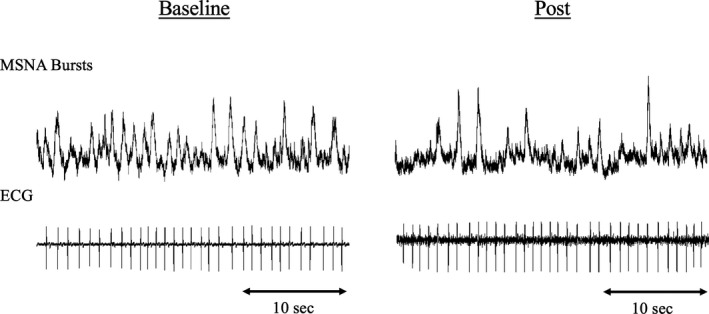
Muscle sympathetic nerve activity was high at baseline and apparently decreased by 12 weeks administration of dapagliflozin. MSNA indicates muscle sympathetic nerve activity.
Table 2.
Changes in Parameters by Dapagliflozin in Each Group
| Non‐HF | HF | P value | |
|---|---|---|---|
| ΔSBP, mm Hg | 1.75±5.34 | −8.30±5.05 | 0.193 |
| ΔDBP, mm Hg | 1.63±4.44 | −2.60±2.91 | 0.422 |
| ΔHeart rate, /min | −1.63±2.88 | −4.60±1.24* | 0.322 |
| ΔBody mass index, kg/m2 | −0.87±0.22* | −0.02±0.20 | 0.014 |
| Blood test | |||
| ΔHbA1c, % | −0.56±0.19* | −0.17±0.14 | 0.113 |
| ΔFBG, mg/dL | −11.5±7.34 | −23.9±14.8 | 0.474 |
| ΔHOMA‐IR | 0.04±0.37 | 0.22±0.09 | 0.668 |
| ΔInsulin, μU/mL | −1.83±1.71 | 0.53±0.37 | 0.235 |
| ΔLDL, mg/dL | −10.2±6.21 | −1.13±5.59 | 0.300 |
| ΔBNP, pg/mL | 4.5±3.67 | −47.7±20.7* | 0.033 |
| ΔeGFR, mL/min per 1.73 m2 | −1.47±2.66 | −2.75±2.97 | 0.753 |
| UCG | |||
| ΔEF, % | 0.67±2.83 | 2.00±2.88 | 0.753 |
| ΔLAD, mm | −3.28±1.94 | −1.65±1.38 | 0.496 |
| ΔLVDd, mm | −3.50±2.62 | 0.13±1.46 | 0.221 |
| ΔLVDs, mm | −2.5±1.88 | 0.00±1.18 | 0.259 |
| ΔE/e′ | −1.15±1.16 | 0.68±0.89 | 0.227 |
| Sympathetic nerve activity | |||
| ΔBF, bursts/min | −4.95±3.10 | −15.2±2.73* | 0.025 |
| ΔBI (bursts/100 heartbeats) | −9.38±3.65* | −20.2±3.46* | 0.049 |
| ΔCBRS, ms/mm Hg | 2.18±1.72 | 1.44±2.33 | 0.806 |
| ΔSBRS (bursts·100 heartbeats−1 mm Hg−1) | 0.27±0.35 | −2.58±2.42 | 0.295 |
Paired‐t test was performed to compare within group differences in each parameter between before and 12 weeks after treatment. Unpaired‐t test was performed to compare differences in each parameter change between groups. Welch t test was applied if the variance was heteroscedastic. BF indicates burst frequency; BI, burst incidence; BNP, brain natriuretic peptide; CBRS, cardiovagal baroreflex sensitivity; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis assessment of insulin resistance; LAD, left atrial dimension; LDL, low‐density lipoprotein; LVDd, left ventricular end diastolic diameter; LVDs, left ventricular end systolic diameter; P value, P value between non‐CHF group and CHF group; SBP, systolic blood pressure; SBRS, sympathetic baroreflex sensitivity; and UCG, ultrasound cardiography.
P<0.05 compared with the baseline of the same group.
As shown in Figure 2, the reductions in MSNA were significantly greater in the HF group than the non‐HF group (both in absolute value and percentage reduction) (ΔBF%, −29.4±4.46 versus −9.24±7.96%, P=0.034; ΔBI%, −28.5±4.37 versus −12.6±6.37%, P=0.049).
Figure 2. Difference in muscle sympathetic nerve activity (MSNA) response to sodium glucose cotransporter 2 inhibitors between patients with diabetes with and without heart failure.
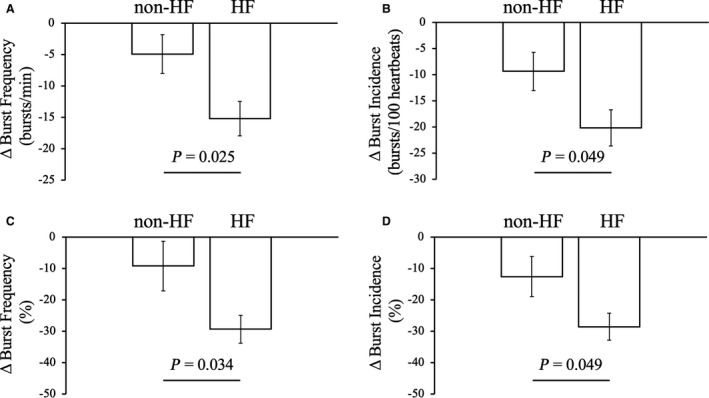
Change in MSNA (burst frequency and burst incidence) absolute value and percentage reduction by 12 weeks administration of dapagliflozin was significantly higher in patients with diabetes and heart failure than patients with diabetes without heart failure. A, Changes in absolute MSNA (burst frequency) value by dapagliflozin. B, Changes in absolute MSNA (burst incidence) value by dapagliflozin. C, Percentage reduction in MSNA (burst frequency) after 12 weeks administration of dapagliflozin. D, Percentage reduction in MSNA (burst incidence) after 12 weeks administration of dapagliflozin. HF indicates heart failure. Unpaired‐t test were performed to compare differences between groups.
Relationship Between MSNA and Other Parameters in Patients With Type 2 Diabetes Treated With an SGLT2i
A linear regression analysis was performed to evaluate the relationship between the change in MSNA and other parameters (Table 3, Figure 3) among all subjects. The amount of change in MSNA (BF) was significantly correlated with that of BNP (ΔBF, R=0.50, P=0.034; ΔBI, R=0.54, P=0.022) (Figure 3). However, no relationship was detected between reduced MSNA and the other parameters, including the diabetes parameters (ΔHbA1c, R=−0.27, P=0.313; ΔFBG, R=0.09, P=0.780; ΔHOMA‐IR, R=0.36, P=0.481; ΔInsulin, R=−0.53, P=0.280), HR (R=0.22, P=0.389) and BRS (CBRS, R=−0.29, P=0.384; SBRS, R=0.16, P=0.593) (Table 3). To scrutinize the effect of MSNA reduction on BNP, univariate and multivariate linear regression analyses were performed (Table 4). In addition to MSNA change (ΔBF), ΔHR, ΔSBP, ΔDBP were selected as independent variables. HbA1c was also selected because HbA1c was significantly decreased by SGLT2i in the non‐HF group (Table 2). Baseline medications that were commonly used for HF treatment and may affect BNP (β‐blocker, ARB/ACE‐I, diuretics) 18 were also included in the analysis. In univariate analysis, no variables other than ΔBF were significantly correlated with ΔBNP (all, P>0.05) (Table 4). However statistically not significant, ΔHR and ΔBP were positively correlated with ΔBF (ΔHR, R=0.22, P=0.389; ΔSBP, R=0.13, P=0.605; ΔDBP, 0.16, P=0.517) and favorable effect of HR and BP reduction on cardiac workload/BNP are clinically reasonable (the decrease in HR and BP can improve myocardial stress via decreasing myocardial oxygen consumption). 29 , 30 Moreover, average HR was significantly decreased by SGLT2i in HF group (Table 2). So, multivariate analysis was performed by using these factors as independent variables (Table 4). As a result, ΔBF was significantly correlated with ΔBNP (model 1, standardized coefficients [β]=0.57, P=0.018; model 2, β=0.52, P=0.045; model 3, β=0.62, P=0.037) independent of HR (β=−0.31, P=0.174) and BP change (ΔSBP, β=−0.06, P=0.809; ΔDBP, β=−0.20, P=0.466).
Table 3.
Relationship Between Changes in MSNA and Changes in Other Parameters
| ΔBF (bursts/min) | ||
|---|---|---|
| R | P value | |
| ΔHbA1c, % | −0.27 | 0.313 |
| ΔFBG, mg/dL | 0.09 | 0.780 |
| ΔHOMA‐IR | 0.36 | 0.481 |
| ΔInsulin, μU/mL | −0.53 | 0.280 |
| ΔHeart rate, /min | 0.22 | 0.389 |
| ΔBMI, kg/m2 | −0.32 | 0.207 |
| ΔeGFR, mL/min per 1.73 m2 | 0.26 | 0.322 |
| ΔCBRS, ms/mm Hg | −0.29 | 0.384 |
| ΔSBRS (bursts·100 heartbeats−1 mm Hg−1) | 0.16 | 0.593 |
Univariate linear regression analusis between ΔBF and change in other parameters were performed. BMI indicates body mass index; CBRS, cardiovagal baroreflex sensitivity; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis assessment of insulin resistance; HR, heart rate; and SBRS, sympathetic baroreflex sensitivity.
Figure 3. Relationship between changes in muscle sympathetic nerve activity and changes in brain natriuretic peptide by 12 weeks administration of dapagliflozin.
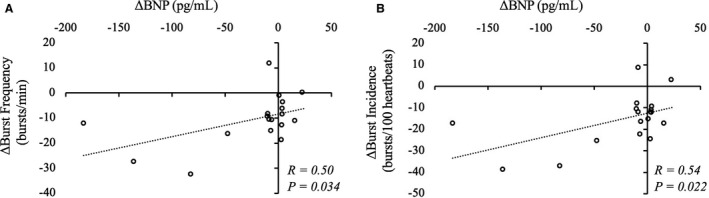
The changes in muscle sympathetic nerve activity (burst frequency and burst incidence) were significantly related to the change in brain natriuretic peptide. A, A linear relationship between the change in burst frequency and the change in brain natriuretic peptide after 12 weeks administration of dapagliflozin; (B) a linear relationship between the change in burst incidence and the change in brain natriuretic peptide after 12 weeks administration of dapagliflozin. BNP indicates brain natriuretic peptide; and R, correlation coefficient by univariate linear regression analysis.
Table 4.
Relationship Between Changes in BNP and Changes in Other Parameters
| ΔBNP (pg/mL) | |||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| R | P value | β | P value | VIF | |
| ΔBF (bursts/min) (model 1) | 0.50 | 0.034 | 0.57 | 0.018 | 1.05 |
| ΔBF (bursts/min) (model 2) | 0.52 | 0.045 | 1.15 | ||
| ΔBF (bursts/min) (model 3) | 0.62 | 0.037 | 1.54 | ||
| ΔHeart rate, /min | 0.22 | 0.389 | −0.31 | 0.174 | 1.05 |
| ΔSBP, mm Hg | 0.13 | 0.605 | −0.06 | 0.809 | 1.15 |
| ΔDBP, mm Hg | 0.16 | 0.517 | −0.20 | 0.466 | 1.54 |
| ΔBMI, kg/m2 | −0.20 | 0.453 | |||
| ΔHbA1c, % | −0.24 | 0.380 | |||
| Age | 0.07 | 0.787 | |||
| β‐blocker | −0.39 | 0.107 | |||
| ARB or ACE‐I | −0.15 | 0.564 | |||
| Diuretics | −0.13 | 0.612 | |||
Univeriate linear regression analysis was performed between ΔBNP and other parameters. Multivariate linear regression analysis was performed using 3 models below. Model 1: Dependent variable, ΔBNP; independent variables, ΔBF and ΔHR. Model 2: dependent variable, ΔBNP; independent variables, ΔBF and ΔSBP. Model 3: dependent variable, ΔBNP; Independent variables, ΔBF and ΔDBP. ACE‐I indicates angiotensin coverting enzyme inhibitor; ARB, angiotensin Ⅱ receptor blocker; BF, burst frequency; BMI, body mass index; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; MSNA, muscle sympathetic nerve activity; R, correlation coefficient value in univariate analysis; SBP, systolic blood pressure; VIF, variance inflation factor; and β, standardized coefficients value in multivariate analysis.
DISCUSSION
The novel findings of the present study are as follows: 12 weeks of 5 mg/day dapagliflozin significantly decreased MSNA in patients with type 2 diabetes with and without HF; dapagliflozin reduced MSNA more markedly in patients with HF than in patients without HF; and the reduction in MSNA was significantly correlated with the change in BNP but not with the change in BRS or the diabetic profiles, such as insulin resistance. These results indicate that the cardioprotective effects of SGLT2i are, in part, attributed to improved SNA.
Benefits of SGLT2i Beyond the Glucose‐Lowering Effect
A variety of favorable effects of SGLT2i have been reported regardless of the drug type. 31 SGLT2i reduce body weight 32 and improve non‐alcoholic fatty liver disease 33 and renal function. 34 , 35 Furthermore, SGLT2i successfully improve cardiovascular outcomes regardless of the presence or absence of diabetes. 8 , 9 Increased glycosuria and natriuresis attributable to the inhibitory effect of SGLT2 at the renal proximal tubule is regarded as the main pathway of SGLT2i to lower glucose. 36 Natriuresis reduces plasma volume and blood pressure; hence, both cardiac preload and afterload decrease. 37 In the present study, BMI was significantly decreased in non‐HF group, which is consistent with previous reports. The reduction in plasma volume unloads the cardiopulmonary baroreceptor, 38 and lowering BP unloads the arterial baroreceptor. 39 Unloading of these baroreceptors decreases vagal nerve activity and increases SNA, resulting in a higher HR. 40 However, in previous studies, SGLT2i did not increase HR despite a significant reduction in BP. 5 , 6 , 7 Moreover, in some studies including the present study, HR decreased in response to SGLT2i. 41 An effect of SGLT2i on SNA is suggested from these results, in addition to the evidence of improving cardiovascular outcomes. 12 , 13
An osmotic diuretic effect is a key mechanism of how SGLT2 beneficially affects patients with type 2 diabetes with and without HF. 37 In our study, the reduction in BMI was larger in the non‐HF group than the HF group and the change was only significant in the non‐HF group. The baseline diuretics usage rate tended to be higher in the HF group than the non‐HF group. Although not compared in this study, the amount of the baseline diuretic dose is expected to be larger in the HF group than the non‐HF group, which could attenuate the diuretic effect of SGLT2i. This difference in the volume change by SGLT2i between groups might affect the difference in the BMI and HbA1c change. The reduction in HbA1c was significant in the non‐HF group but not in the HF group, and although statistically not significant, the change in low‐density lipoprotein in the non‐HF group seems higher than in the HF group. Same with BMI change, these might be explained by the difference in the diuretic effect between groups since the hypoglycemic effect of SGLT2i depends on urination. 37 We did not measure urine volume change in this study, so we could not confirm the difference between groups. Further studies are needed to determine the differences in diuretic and hypoglycemic effects of SGLT2i in the presence and absence of HF. On the contrary, the changes in MSNA and BNP in the HF group were significantly greater than in the non‐HF group. These results indicate that the beneficial effects of SGLT2i on patients with HF were not caused only by the diuretic effect, but other mechanisms also played a crucial role.
Possible Mechanisms for the Reduction in MSNA and the Difference Between Groups
An animal study using a nondiabetic rat model of chronic kidney disease revealed that SGLT2i reduce the low‐frequency component of heart rate variability, indicating reduced SNA. 42 Another study using streptozotocin‐induced diabetic rats showed that a non‐depressor dose of SGLT2i ameliorates arterial pressure lability associated with sympatho‐inhibition and improves the BRS. 14 A recent study demonstrated that MSNA does not increase in response to SGLT2i despite a numerical increase in urine volume and significant reductions in BP and body weight. 43 By contrast, in our study, MSNA decreased significantly in response to dapagliflozin. A possible reason for this dissociation is thought to be the timing of measurements. In the previous study, MSNA was measured 4 days after administration had begun. The increase in urine volume reaches its peak during the first 24 hours of SGLT2i administration and gradually decreases after a few days. 44 , 45 Thus, 4 days after administration was near the peak of volume loss. Therefore, the reduction of SNA may have been buffered by significant volume loss and lowering of blood pressure that can cause the arterial baroreflex and cardiopulmonary baroreceptor unloading, as the authors reported. 43 In our study, MSNA was measured 12 weeks after administration, which was far from the peak of volume loss. A recent study reported that 24 weeks of administration of SGLT2i significantly reduces the ratio of the low frequency (LF) component to the high frequency (HF) component (LF/HF ratio) of heart rate variability in patients with diabetes with acute myocardial infarction. 46 The LF/HF ratio is an indirect parameter of SNA. This result is consistent with our results on long‐term treatment with SGLT2i.
We measured insulin resistance, SBRS, and CBRS to clarify the potential mechanism that SGLT2i reduces MSNA. However, contrary to our hypothesis, the reduction in MSNA was not related to BRS or the diabetic profile. We could not clarify the underlying mechanism for the reduction of MSNA in this cohort; however, our results indicate that other potential effects of SGLT2i contributed to reducing MSNA. One plausible mechanism is that an improvement of renal afferent SNA has been suggested as a pathway to reduce SNA. 13 , 47 Another mechanism is a direct effect of SGLT2i on the central nervous system. SGLT2i can cross the blood‐brain barrier and has been the focus as a new treatment method for brain diseases, including seizures 48 and Alzheimer disease. 49 Therefore, it might be possible that SGLT2i reduces SNA by directly reducing the central discharge of SNA. To verify these issues, future studies assessing the effect of SGLT2i, particularly on renal SNA and/or central commands, are expected.
ΔBNP was significantly correlated with ΔMSNA in the regression analysis. The univariate linear regression analyses showed that no baseline factors and parameters change by SGLT2i other than ΔMSNA associated with ΔBNP, and the multivariate analysis showed that ΔMSNA significantly correlated to ΔBNP independent of SBP, DBP, and HR change. It has been reported that BNP is significantly related to MSNA and plasma norepinephrine concentrations in patients with HF. 50 It is likely that improvements in MSNA reduced cardiac stress, which contributed to the BNP reduction in our study. This result supports the hypothesis that SGLT2i improves HF by reducing SNA. Despite that MSNA decreased significantly in response to SGLT2i, most ultrasound cardiography parameters remained unchanged. Studies applying a longer and more detailed follow‐up are needed to evaluate the change in cardiac function by SGLT2i. However, it is conceivable that unloading of cardiac stress, which could be caused by reducing SNA, was more beneficial for impaired hearts than normal hearts, resulting in improved MSNA and BNP more prominently in patients with diabetes with HF than those without HF.
Clinical Implications for Adding SGLT2i to Conventional HF Treatment and/or Other Cardiovascular Diseases
β‐blocker administration is a well‐established treatment for inhibiting SNA and improving the prognosis in patients with HF. 51 β‐blockers diminish the SNA effect by inhibiting β adrenoreceptors at the effector organs (eg, heart), but not by directly reducing central efferent firing of SNA itself. 52 As a result, withdrawal of a β‐blocker can cause significant sympatho‐excitation and worsen cardiovascular outcomes in concert with the upregulation of β adrenoreceptors. 53 , 54 , 55 In our study, SGLT2i reduced MSNA in patients with HF treated with a β‐blocker (90%), which reflects the reduction of central sympathetic efferent neuronal discharge to skeletal muscle. This character of SGLT2i also provides an additional sympatho‐inhibitory effect on HF, which contributes to the improvement in cardiovascular mortality in conventionally treated patients with HF. Increased SNA is associated with many cardiovascular diseases including cardiac ischemia, 56 , 57 resistant hypertension, 58 and pulmonary hypertension. 59 While some diseases as obstructive sleep apnea were reported to develop cardiovascular diseases via sympatho‐excitation. 60 , 61 Therefore, the sympatho‐inhibitory effect of SGLT2i observed in our study might be applicable to prevent/treat these diseases regardless of the presence or absence of diabetes. Future studies are expected to verify the effect of SGLT2i on patients with various cardiovascular diseases.
Limitations
This study had some limitations. First, the result that average BP was unchanged in all groups and HR decreased in HF group are inconsistent with the results of previous randomized trials in which BP decreased significantly and HR was unchanged. 5 , 6 , 7 In this study, baseline treatments for HF and HT were already adopted. Although it is difficult to compare statistically, baseline average BP in our study was well controlled, and tended to be lower than that of previous trials. 5 , 6 , 7 This difference might affect the dissociation in the BP change by SGLT2i between our study and previous trials. It has been shown that HR in patients with resting HR >70 bpm decreased, but resting HR in patients with resting HR ≤70 remained unchanged by SGLT2i. This result indicates that SGLT2i may reduce HR only in patients with augmented SNA. 41 In our study, baseline average HR was >70 bpm in both groups and MSNA tended to be higher in HF group than non‐HF group. Therefore, the result that HR was significantly decreased in HF group is consistent with the previous report. 41 Diabetic profiles were also unchanged except HbA1c in the non‐HF group even though SGLT2i significantly decreased HbA1c/FBG and improved insulin sensitivity in previous studies. 62 In the previous studies, baseline HbA1c before SGLT2i treatment was prone to be higher than our study (>8%). 5 , 7 In our study, the subjects had already received the treatment for diabetes, and the baseline value of HbA1c was <8% in both HF and non‐HF groups. So, the impact of SGLT2i on glycemic control might have been smaller than in previous studies. Also, a previous study, which was consistent with our results, demonstrated that HbA1c was not significantly decreased by SGLT2i in patients with type 2 diabetes with well‐controlled HbA1c. 46 Because our primary outcome was the change in MSNA by SGLT2i, we can’t exclude that the results for the glycemic control were statistically underpowered given the relatively small sample size. Noteworthy, dapagliflozin significantly reduced MSNA in our study without significant improvement in the diabetic profiles. Second, in other studies, SGLT2i significantly decreased other parameters including serum uric acid and erythropoietin, 37 but these were not measured in our study. These parameters may have improved in our study, yet our main purpose was to evaluate the effect of SGLT2i on SNA. Measuring these parameters was unnecessary for our primary outcome. Third, we measured HOMA‐IR from serum FBG and insulin as parameters of insulin resistance. To analyze the actual insulin resistance of each organ, other detailed methods, such as a glucose clamp, might be better. 63 However, HOMA‐IR, calculated from FBG and insulin, is a reliable marker of systemic insulin resistance and is an independent predictor of cardiovascular disease in patients with type 2 diabetes. 23 We believe the result that the significant decrease of MSNA regardless of the HOMA‐IR value supports a recent randomized clinical trial showing a favorable effect of SGLT2i on cardiovascular outcome regardless of the presence or absence of diabetes. 8 Fourth, the sample size was relatively small and only Asians were included, also most subjects were men (78%). So, it is not clear that these results can apply to a larger population of diverse races. Finally, no control group was used in this study. A control group is important to confirm a cause‐and‐effect relationship in a study. Since there is no control group in our study, the influence of other modifying factors, including the placebo effect, on the results cannot be completely ruled out. To strengthen our findings, future studies designed to compare the differences in the effects on SNA between SGLT2i and placebo or other drugs with larger numbers and more diverse races are needed.
CONCLUSIONS
Twelve weeks of dapagliflozin 5 mg/day administration significantly decreased MSNA regardless of the diabetic profile or BRS change in patients with type 2 diabetes. The change of MSNA in patients with HF was more remarkable than that of patients with non‐HF. These results indicate that SGLT2i reduced SNA, which could be beneficial in patients with diabetes complicated by HF.
Sources of Funding
This work was supported by JSPS KAKENHI Grant Number JP19K16289.
Disclosures
None.
Acknowledgments
We would like to thank Dr Cui Jian (Pennsylvania State University College of Medicine) for support related to the analyses of baroreflex function.
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, de Matos LNJ, Braga AMW, Middlekauff HR, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135:302–307. doi: 10.1016/j.ijcard.2008.03.056 [DOI] [PubMed] [Google Scholar]
- 2. Hoffman RP, Sinkey CA, Anderson EA. Muscle sympathetic nerve activity is higher in intensively versus conventionally treated IDDM subjects. Diabetes Care. 1995;18:287–291. doi: 10.2337/diacare.18.3.287 [DOI] [PubMed] [Google Scholar]
- 3. Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE [DOI] [PubMed] [Google Scholar]
- 4. van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(suppl 1):S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 6. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Desai M, Li Q, Deng H, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–1550. doi: 10.1161/CIRCULATIONAHA.118.035901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Kosiborod M, Fitchett D, Wanner C, Hehnke U, Kaspers S, George JT, Zinman B. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138:1904–1907. doi: 10.1161/CIRCULATIONAHA.118.035759 [DOI] [PubMed] [Google Scholar]
- 10. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose cotransporter‐2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. doi: 10.1111/dom.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wan N, Rahman A, Hitomi H, Nishiyama A. The effects of sodium‐glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol (Lausanne). 2018;9:421. doi: 10.3389/fendo.2018.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 14. Yoshikawa T, Kishi T, Shinohara K, Takesue K, Shibata R, Sonoda N, Inoguchi T, Sunagawa K, Tsutsui H, Hirooka Y. Arterial pressure lability is improved by sodium‐glucose cotransporter 2 inhibitor in streptozotocin‐induced diabetic rats. Hypertens Res. 2017;40:646–651. doi: 10.1038/hr.2017.14 [DOI] [PubMed] [Google Scholar]
- 15. Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919 [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi D, Takamura M, Murai H, Usui S, Ikeda T, Inomata J, Takashima S, Kato T, Furusho H, Takeshita Y, et al. Effect of pioglitazone on muscle sympathetic nerve activity in type 2 diabetes mellitus with α‐glucosidase inhibitor. Auton Neurosci. 2010;158:86–91. doi: 10.1016/j.autneu.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 17. Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. 2018;9:1–45. doi: 10.1007/s13340-018-0345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—digest version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342 [DOI] [PubMed] [Google Scholar]
- 19. Murai H, Takamura M, Maruyama M, Nakano M, Ikeda T, Kobayashi D, Otowa K‐I, Ootsuji H, Okajima M, Furusho H, et al. Altered firing pattern of single‐unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J Physiol. 2009;587:2613–2622. doi: 10.1113/jphysiol.2009.172627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inomata J‐I, Murai H, Kaneko S, Hamaoka T, Ikeda T, Kobayashi D, Usui S, Furusho H, Sugiyama YU, Takata S, et al. Differential effects of azelnidipine and amlodipine on sympathetic nerve activity in patients with primary hypertension. J Hypertens. 2014;32:1898–1904. doi: 10.1097/HJH.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 21. Hamaoka T, Murai H, Kaneko S, Usui S, Okabe Y, Tokuhisa H, Kato T, Furusho H, Sugiyama YU, Nakatsumi Y, et al. Single‐unit muscle sympathetic nerve activity reflects sleep apnea severity, especially in severe obstructive sleep apnea patients. Front Physiol. 2016;7:66. doi: 10.3389/fphys.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fagius J, Wallin BG. Long‐term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234 [DOI] [PubMed] [Google Scholar]
- 23. Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, et al. HOMA‐estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135 [DOI] [PubMed] [Google Scholar]
- 24. Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor‐heart rate reflex by 24‐hour intra‐arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–222. doi: 10.1161/01.HYP.12.2.214 [DOI] [PubMed] [Google Scholar]
- 25. Iellamo F, Hughson RL, Castrucci F, Legramante JM, Raimondi G, Peruzzi G, Tallarida G. Evaluation of spontaneous baroreflex modulation of sinus node during isometric exercise in healthy humans. Am J Physiol Heart Circ Physiol. 1994;267:H994–H1001. doi: 10.1152/ajpheart.1994.267.3.H994 [DOI] [PubMed] [Google Scholar]
- 26. Cui J, Boehmer J, Blaha C, Sinoway LI. Muscle sympathetic nerve activity response to heat stress is attenuated in chronic heart failure patients. Am J Physiol Regul Integr Comp Physiol. 2017;312:R873–R882. doi: 10.1152/ajpregu.00355.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tokuhisa H, Murai H, Okabe Y, Hamaoka T, Sugimoto H, Mukai Y, Inoue O, Takashima S‐I, Kato T, Usui S, et al. Differential effects of lipophilic and hydrophilic statins on muscle sympathetic nerve activity in heart failure with preserved left ventricular ejection fraction. Auton Neurosci. 2018;213:8–14. doi: 10.1016/j.autneu.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 28. Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsen AH, Jessen N, Nørrelund H, Tolbod LP, Harms HJ, Feddersen S, Nielsen F, Brøsen K, Hansson NH, Frøkiær J, et al. A randomised, double‐blind, placebo‐controlled trial of metformin on myocardial efficiency in insulin‐resistant chronic heart failure patients without diabetes. Eur J Heart Fail. 2020;22:1628–1637. doi: 10.1002/ejhf.1656 [DOI] [PubMed] [Google Scholar]
- 30. Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–927. doi: 10.1161/CIRCULATIONAHA.106.660639 [DOI] [PubMed] [Google Scholar]
- 31. Kluger AY, Tecson KM, Lee AY, Lerma EV, Rangaswami J, Lepor NE, Cobble ME, McCullough PA. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18:99. doi: 10.1186/s12933-019-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium‐glucose cotransporter‐2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19:1630–1641. doi: 10.1111/obr.12755 [DOI] [PubMed] [Google Scholar]
- 33. Gharaibeh NE, Rahhal MN, Rahimi L, Ismail‐Beigi F. SGLT‐2 inhibitors as promising therapeutics for non‐alcoholic fatty liver disease: pathophysiology, clinical outcomes, and future directions. Diabetes Metab Syndr Obes. 2019;12:1001–1012. doi: 10.2147/DMSO.S212715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 35. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 36. Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–2086. doi: 10.1007/s00125-018-4654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 38. Ferguson DW, Hayes DW. Nifedipine potentiates cardiopulmonary baroreflex control of sympathetic nerve activity in healthy humans. Direct evidence from microneurographic studies. Circulation. 1989;80:285–298. doi: 10.1161/01.CIR.80.2.285 [DOI] [PubMed] [Google Scholar]
- 39. La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol. 2012;97:39–50. doi: 10.1113/expphysiol.2011.057554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: subanalysis of placebo‐controlled, double‐blind clinical trials. J Diabetes Investig. 2018;9:638–641. doi: 10.1111/jdi.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wan N, Fujisawa Y, Kobara H, Masaki T, Nakano D, Rahman A, Nishiyama A. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens Res. 2020;43:492–499. doi: 10.1038/s41440-020-0410-8 [DOI] [PubMed] [Google Scholar]
- 43. Jordan J, Tank J, Heusser K, Heise T, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:604–612. doi: 10.1016/j.jash.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, Arakawa K, Togo M, Inagaki N, Kaku K. Factors affecting canagliflozin‐induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–451. doi: 10.1007/s12325-016-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sasaki T, Seino Y, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Pharmacokinetics, pharmacodynamics, and safety of luseogliflozin in japanese patients with type 2 diabetes mellitus: a randomized, single‐blind, placebo‐controlled trial. Adv Ther. 2015;32:319–340. doi: 10.1007/s12325-015-0200-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimizu W, Kubota Y, Hoshika YU, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki Y‐K, Yamamoto T, Takano H, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148. doi: 10.1186/s12933-020-01127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gilbert RE. SGLT2 inhibitors: β blockers for the kidney? Lancet Diabetes Endocrinol. 2016;4:814. doi: 10.1016/S2213-8587(16)30237-6 [DOI] [PubMed] [Google Scholar]
- 48. Erdogan MA, Yusuf D, Christy J, Solmaz V, Erdogan A, Taskiran E, Erbas O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol‐induced murine model of epilepsy. BMC Neurol. 2018;18:81. doi: 10.1186/s12883-018-1086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Esterline R, Oscarsson J, Burns J. A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer's disease? Int Rev Neurobiol. 2020;155:113–140. [DOI] [PubMed] [Google Scholar]
- 50. Seravalle G, Quarti‐Trevano F, Dell'Oro R, Gronda E, Spaziani D, Facchetti R, Cuspidi C, Mancia G, Grassi G. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens. 2019;37:443–448. doi: 10.1097/HJH.0000000000001856 [DOI] [PubMed] [Google Scholar]
- 51. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101 [DOI] [PubMed] [Google Scholar]
- 52. Azevedo ER, Kubo T, Mak S, Al‐Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD. Nonselective versus selective beta‐adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation. 2001;104:2194–2199. doi: 10.1161/hc4301.098282 [DOI] [PubMed] [Google Scholar]
- 53. Gilbert EM, Olsen SL, Renlund DG, Bristow MR. beta‐adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71:23c–29c. doi: 10.1016/0002-9149(93)90083-O [DOI] [PubMed] [Google Scholar]
- 54. Tygesen H, Andersson B, Di Lenarda A, Rundqvist B, Sinagra G, Hjalmarson A, Waagstein F, Wennerblom B. Potential risk of beta‐blockade withdrawal in congestive heart failure due to abrupt autonomic changes. Int J Cardiol. 1999;68:171–177. [DOI] [PubMed] [Google Scholar]
- 55. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta‐blocker withdrawal in acute decompensated heart failure: a systematic review and meta‐analysis. JACC Heart Fail. 2015;3:647–653. doi: 10.1016/j.jchf.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary DA. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation. 2002;106:793–797. doi: 10.1161/01.CIR.0000025610.14665.21 [DOI] [PubMed] [Google Scholar]
- 57. Notarius CF, Spaak J, Morris BL, Floras JS. Comparison of muscle sympathetic activity in ischemic and nonischemic heart failure. J Card Fail. 2007;13:470–475. doi: 10.1016/j.cardfail.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 58. Grassi G, Seravalle G, Brambilla G, Pini C, Alimento M, Facchetti R, Spaziani D, Cuspidi C, Mancia G. Marked sympathetic activation and baroreflex dysfunction in true resistant hypertension. Int J Cardiol. 2014;177:1020–1025. doi: 10.1016/j.ijcard.2014.09.138 [DOI] [PubMed] [Google Scholar]
- 59. Chen SL, Zhang FF, Xu J, Xie DJ, Zhou L, Nguyen T, Stone GW. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single‐center, prospective, first‐in‐man PADN‐1 study (first‐in‐man pulmonary artery denervation for treatment of pulmonary artery hypertension). J Am Coll Cardiol. 2013;62:1092–1100. doi: 10.1016/j.jacc.2013.05.075 [DOI] [PubMed] [Google Scholar]
- 60. Hamaoka T, Murai H, Takata S, Hirai T, Sugimoto H, Mukai Y, Okabe Y, Tokuhisa H, Takashima S‐I, Usui S, et al. Different prognosis between severe and very severe obstructive sleep apnea patients; five year outcomes. J Cardiol. 2020;76:573–579. doi: 10.1016/j.jjcc.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 61. Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. doi: 10.1161/01.HYP.32.6.1039 [DOI] [PubMed] [Google Scholar]
- 62. DeFronzo RA, Norton L, Abdul‐Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170 [DOI] [PubMed] [Google Scholar]
- 63. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]


