Abstract
Background
Sudden cardiac arrest (SCA) may be caused by an acute and reversible myocardial injury, a chronic and irreversible myocardial damage, or a primary ventricular arrhythmia. Cardiac magnetic resonance imaging may identify myocardial edema (ME), which denotes acute and reversible myocardial damage. We evaluated the arrhythmic outcome of SCA survivors during follow‐up and tested the prognostic role of ME.
Methods and Results
We included a consecutive series of 101 (71% men, median age 47 years) SCA survivors from 9 collaborative centers who underwent early (<1 month) cardiac magnetic resonance imaging and received an implantable cardioverter‐defibrillator (ICD). On T2‐weighted sequences, ME was found in 18 of 101 (18%) patients. According to cardiac magnetic resonance imaging findings, the arrhythmic SCA was ascribed to acute myocardial injury (either ischemic [n=10] or inflammatory [n=8]), to chronic structural heart diseases (ischemic heart disease [n=11], cardiomyopathy [n=20], or other [n=23]), or to primarily arrhythmic syndrome (n=29). During a follow‐up of 47 months (28 to 67 months), 24 of 101 (24%) patients received an appropriate ICD intervention. ME was associated with a significantly higher survival free from both any ICD interventions (log‐rank=0.04) and ICD shocks (log‐rank=0.03) and remained an independent predictor of better arrhythmic outcome after adjustment for left ventricular ejection fraction and late gadolinium enhancement. The risk of appropriate ICD intervention was unrelated to the type of underlying heart disease.
Conclusions
ME on early cardiac magnetic resonance imaging, which denotes an acute and transient arrhythmogenic substrate, predicted a favorable long‐term arrhythmic outcome of SCA survivors. These findings may have a substantial impact on future guidelines on the management of SCA survivors.
Keywords: cardiac magnetic resonance, implantable cardioverter‐defibrillator, out‐of‐hospital cardiac arrest, prognosis, ventricular arrhythmia
Subject Categories: Sudden Cardiac Death, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- CA
cardiac arrest
- LGE
late gadolinium enhancement
- ME
myocardial edema
- SCA
sudden cardiac arrest
Clinical Perspective
What Is New?
Previous studies demonstrated that cardiac magnetic resonance (CMR) is a key imaging test for identifying the myocardial substrate of arrhythmic sudden cardiac arrest. Besides postcontrast sequences for late gadolinium enhancement/myocardial fibrosis, tissue characterization by CMR includes T2‐weighted sequences for detection of myocardial edema, which represents the imaging hallmark of an acute and reversible myocardial injury, either ischemic or nonischemic.
In the present multicenter study we found that demonstration of myocardial edema by CMR early (<1 month) after sudden cardiac arrest was associated with a significantly lower rate of appropriate implantable cardioverter‐defibrillator interventions during a median follow‐up of 4 years, independently from left ventricular ejection fraction and myocardial late gadolinium enhancement.
What Are the Clinical Implications?
Considering its important diagnostic and prognostic value, early CMR should be incorporated in the clinical flow chart for evaluation of survivors of sudden cardiac arrest, and demonstration of myocardial edema by early CMR may influence the decision‐making of implantable cardioverter‐defibrillator implantation after a sudden cardiac arrest, given its prediction of good arrhythmic outcome.
Arrhythmic sudden cardiac arrest (SCA) may be caused by a variety of substrates and mechanisms, which mainly include: (1) acute and transient ventricular electrical instability such as that occurring in the setting of a reversible myocardial injury, either ischemic (acute myocardial infarction) or inflammatory (acute myocarditis); (2) chronic and irreversible myocardial substrates such as ischemic heart disease, cardiomyopathy, or other structural abnormalities; and (3) ventricular arrhythmias in the absence of structural heart disease, caused by genetically determined cardiac ion channel diseases, idiopathic ventricular fibrillation (VF), drug toxicity, or electrolyte imbalance. 1 The identification of the mechanism plays a key role in the management of SCA, including the implant of an implantable cardioverter‐defibrillator (ICD). 2 , 3
Cardiovascular evaluation after SCA based on ECG, echocardiogram, and coronary angiogram may be inconclusive. 4 , 5 , 6 , 7 Cardiac magnetic resonance (CMR) imaging has become the gold standard imaging technique not only for assessment of morphofunctional ventricular abnormalities but also for detection of myocardial lesions, owing to its unique ability to noninvasively characterize tissue composition. 8 Previous studies demonstrated that CMR is a key imaging test for diagnosing the substrate of SCA. 9 , 10 , 11 , 12 , 13 Besides postcontrast sequences for late gadolinium enhancement (LGE)/myocardial fibrosis, tissue characterization by CMR includes T2‐weighted sequences for detection of myocardial edema (ME), which represents the imaging hallmark of an acute and reversible myocardial injury. 8 The diagnosis of SCA secondary to a reversible cause is particularly relevant as current guidelines do not suggest ICD implantation. 2 , 3 However, whether identification of ME by CMR provides prognostic information after SCA and may influence ICD implantation remains to be established.
The aim of the present multicenter study was to test the hypothesis that ME as evidenced by early CMR imaging may predict a favorable prognosis of SCA survivors, in relation to the reversibility of the myocardial substrate. To this purpose, we evaluated the long‐term arrhythmic outcome of a cohort of consecutive patients admitted for SCA who underwent early CMR imaging and received an ICD for secondary prevention, and tested the prognostic role of ME.
METHODS
This multicenter study retrospectively evaluated a cohort of consecutive patients surviving an arrhythmic SCA who were admitted to 9 Italian medical centers from 2009 to 2019. To be included in the study, SCA survivors needed to undergo early CMR imaging within 1 month after the acute event, with a comprehensive tissue characterization protocol including T2‐weighted sequences for ME, to receive an ICD for secondary prevention and to have a follow‐up of at least 1 year. Early CMR study was performed during hospitalization in patients who recovered from neurological impairment, who were weaned from mechanical ventilation, and who had no other contraindications such as renal insufficiency with creatinine clearance <30 mL/min or no magnetic resonance imaging–compatible devices. The study was approved by the local ethical committees. Given the retrospective and observational nature of the study, consent from patients was waived. Data supporting the results are available from the corresponding author upon reasonable request.
Clinical Evaluation and Coronary Angiography
Clinical evaluation included personal and family history, ECG, and echocardiography in all patients. Coronary angiography was performed during hospitalization in 91 of 101 (90%) patients; the remaining 10 patients were young individuals without coronary risk factors and showed clear clinical evidence of nonischemic cause of the SCA such as cardiomyopathy or channelopathy.
CMR Imaging
The CMR study was performed on 1.5 Tesla scanners by all collaborative centers (Siemens, n=4; Philips, n=4; General Electric, n=1) using different software approved for clinical and research use for postprocessing analysis. The study protocol included long‐ and short‐axis cine sequences, T2‐weighted short‐tau inversion recovery sequences for evaluation of ME, and postcontrast images (2‐dimensional segmented breath‐held fast low‐angle shot inversion recovery) for assessment of LGE.
Biventricular function and volumes, myocardial thickness, and regional wall motion abnormalities were assessed using dedicated software by tracing endocardial and epicardial borders on each short‐axis cine slice in end diastole and systole. End‐systolic and ‐diastolic volumes were indexed on the surface area.
The presence and distribution of ME was established by measurement of myocardial signal intensity normalized to skeletal muscle in the same slice. For postcontrast imaging, short‐ and long‐axis images were acquired 10 minutes after the administration of the gadolinium‐based contrast agent. LGE was automatically quantified using a threshold of 5 SDs above the mean signal intensity of the remote myocardium. The presence, extent, distribution, and pattern of ME and LGE were confirmed on 2 orthogonal planes. The distribution patterns of ME and LGE were considered ischemic if the subendocardium was involved. The ischemic patterns were defined as subendocardial or transmural if involving <50% or >50% of the myocardial wall, respectively. When the subendocardium was spared, the pattern was considered nonischemic and classified as subepicardial or midmyocardial. 14 , 15
All CMR studies were analyzed by 2 independent investigators for each collaborative medical center, blinded to the clinical, angiographic, and CMR findings and outcomes. In case of disagreement, the CMR images were sent to the central core laboratory of the University of Padova for a third opinion.
Definitions
At angiographic examination, obgstructive coronary artery disease (CAD) was defined in the presence of a stenosis ≥70%. A culprit coronary artery was defined as an obstructive coronary artery with thrombolysis in myocardial infarction 0 or 1 flow with abrupt closure, or thrombolysis in myocardial infarction 2 or 3 flow with features suggestive of thrombus or ulcerated plaques. Additional findings supporting the diagnosis included ST‐segment elevation and dynamic T‐wave changes in the corresponding ECG location and evidence of matching regional wall motion abnormality on left ventriculogram or echocardiogram. 16
In patients with obstructive (≥70%) CAD, the presence of ME on T2 images with an ischemic pattern and distribution in the territory tributary to the coronary artery was considered diagnostic for acute ischemic myocardial injury. The presence of isolated LGE with an ischemic pattern without evidence of ME was considered diagnostic of postmyocardial infarction scar. 15
The diagnosis of cardiomyopathies and channelopathies was made according to current criteria. 2 , 3 The presence of ME, in association with signs of LGE on postcontrast images, was considered suggestive of an acute inflammatory injury and diagnosed as a clinically suspected acute myocarditis.
Isolated, nonischemic left ventricular (LV) scar was defined as the presence of LGE on CMR postcontrast sequences involving ≥1 LV segments and mostly involving outer wall layers without reaching the subendocardium, in the absence of other structural ventricular abnormalities. 17 Small or poorly defined patches of LGE were considered of uncertain significance in the absence of other abnormalities. Focal and isolated LGE at the right ventricular insertion points onto the left ventricle (“junctional” LGE) was considered a nonpathological finding because of its relatively high prevalence in normal individuals and its benign clinical meaning. 17
Arrhythmic mitral valve prolapse was defined as a myxomatous mitral valve with annular disjunction and thickening/elongation of both leaflets in association with postcontrast evidence of LGE/myocardial fibrosis affecting the inferobasal LV free wall (under the posterior leaflet) and/or the papillary muscle. 18
Follow‐Up
Patients were followed at 3‐ to 6‐month intervals after ICD placement via clinic visits or phone calls. Clinical and imaging data were collected over follow‐up to determine the patients' alive status and whether they reached the primary arrhythmic end point consisting of appropriate ICD intervention, defined as: (1) shock for sustained ventricular tachycardia above the programmed rate cutoff of the ICD (generally >180 beats per minute) or VF; or (2) antitachycardia pacing.
Appropriate ICD intervention was defined as an ICD shock delivered in response to a ventricular tachyarrhythmia as documented by stored intracardiac ECG data. The distinction between appropriate and inappropriate ICD intervention was based on morphologic features, tachycardia onset, rate stability, and atrial electrograms (when available). Death categories were categorized as cardiovascular and noncardiovascular. Lead‐ and device‐related complications such as inappropriate shocks and pocket revision/lead extraction were also recorded. No patient was lost to follow‐up.
Statistical Analysis
Categorical variables are expressed as number (percentage). As normality distribution was not assumed, continuous variables are expressed as median (first through third quartiles). Categorical variables were compared using χ2 or Fisher exact test, as appropriate. Continuous data were compared using the Mann‐Whitney U test. Differences in event‐free survival was expressed by Kaplan‐Meyer survival curves and evaluated with the log‐rank test. A univariate and multivariable Cox regression analysis (adjusted for LV ejection fraction [LVEF] and LGE) was performed to test the independent predictive value of ME for appropriate ICD interventions. The LVEF and LGE covariates were chosen on a clinical basis. P<0.05 was considered significant. Data were analyzed using SPSS version 23 (IBM).
RESULTS
Among a total of 684 consecutive patients admitted for out‐of‐hospital SCA in the 9 collaborative medical centers, 194 died during the acute phase. Among the remaining 490 patients, 286 patients were excluded from the study because they did not undergo CMR imaging and/or did not receive an ICD, as the cause of SCA was clearly related to an acute myocardial infarction, other reversible causes, or a previously diagnosed disease; 87 were not able to undergo early CMR study because of neurological impairment and/or mechanical ventilation; 7 had severe kidney failure with creatinine clearance <30 mL/min; and 4 had magnetic resonance imaging–noncompatible devices. Finally, 5 patients were not included because of poor CMR quality images.
The remaining 101 patients (median age, 47 years [31–59 years]; 70% men) constituted the study population, whose clinical characteristics are reported in Table 1. CMR was performed a median of 11 days (8 to 22 days) after SCA.
Table 1.
Baseline Characteristics
| Men | 71 (70) |
| Age (range), y | 47 (31–59) |
| Competitive sports activity | |
| Current | 8 (8) |
| Previous | 4 (4) |
| Family history | |
| Sudden death | 6 (6) |
| Ischemic heart disease | 16 (16) |
| Cardiomyopathy | 8 (8) |
| Ion channel disease | 4 (4) |
| Personal history | |
| Ischemic heart disease | 8 (8) |
| Valvular heart disease ≥moderate | 2 (2) |
| Cardiomyopathy | 5 (5) |
| Ion channel disease | 2 (2) |
| Symptoms | |
| Syncope | 7 (7) |
| Presyncope | 6 (6) |
| Palpitations | 5 (5) |
| Chest pain | 8 (8) |
| Cardiac arrest characteristics | |
| VT/VF at presentation | 94 (94) |
| During sports activity or within 1 h | 16 (16) |
| Bystander CPR | 75 (74) |
| Postresuscitation ST‐segment elevation | 8 (8) |
| In‐hospital management | |
| Therapeutic hypothermia* | 49 (49) |
| Coronary angiography | |
| At admission | 40 (40) |
| During hospitalization | 51 (50) |
| No | 10 (10) |
| Coronary arteries | |
| Coronary artery disease (n=91) | |
| Chronic occlusion | 6 (7) |
| Stenosis 70% – 99% | 11 (12) |
| Stenosis <70% | 13 (14) |
| No stenosis | 61 (67) |
| No. of vessels with stenosis >70% (n=17) | |
| 1 | 4 (24) |
| 2 | 8 (47) |
| 3 | 5 (29) |
Values are expressed as number (percentage) unless otherwise indicated. CPR indicates cardiopulmonary resuscitation; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Performed according to the comatose status after resuscitation and the availability of the treatment at the participating center at the time of the event.
CMR Imaging Findings
The final diagnoses are summarized in Table 2.
Table 2.
Final Diagnosis
| Ischemic heart disease | 21 (21) |
| Acute coronary syndrome | 9 (9) |
| Chronic ischemic disease | 11 (11) |
| Coronary spasm | 1 (1) |
| Structural, nonischemic heart disease | 51 (50) |
| Dilated cardiomyopathy | 13 (13) |
| Arrhythmogenic cardiomyopathy | 4 (4) |
| Hypertrophic cardiomyopathy | 3 (3) |
| Mitral valve prolapse | 7 (7) |
| Acute myocarditis | 7 (7) |
| Takotsubo syndrome | 1 (1) |
| Isolated (idiopathic) nonischemic LV LGE | 16 (16) |
| Structurally normal heart | 29 (29) |
| Long‐QT syndrome | 5 (5) |
| Brugada syndrome | 5 (5) |
| Idiopathic ventricular fibrillation | 19 (19) |
Values are expressed as number (percentage). LV LGE indicates left ventricular late gadolinium enhancement.
ME With an Ischemic Pattern
Evidence of ME with a pattern consistent with an acute ischemic myocardial injury was found in 11 patients, 6 with obstructive coronary stenosis (4 with and 2 without angiographic evidence of acute plaque complication), 3 with nonobstructive coronary stenosis at coronary angiography, and 2 with normal coronary arteries. In the 9 cases with coronary stenosis, either obstructive or nonobstructive, ME had an ischemic pattern, and showed a regional distribution that was concordant with the myocardial territory tributary of the stenotic coronary artery. Of these 9 patients, 6 with obstructive coronary stenosis and 1 with moderate stenosis but abnormal invasive fractional flow reserve, underwent percutaneous coronary revascularization. Of the remaining 2 patients with normal coronary arteries, 1 showed transmural ME of the midapical anteroseptal LV segments and received a diagnosis of variant angina based on recurrent transient chest pain with anterior ST‐segment elevation and documentation of coronary spasm of the left anterior descending artery during coronary angiography. The other showed transmural ME that circumferentially involved the midapical LV segments and received a diagnosis of acute myocardial injury attributable to Takotsubo syndrome, which was confirmed by transient akinesia of the midapical LV segments.
In all patients with ME except the one with Takotsubo syndrome, postcontrast sequences also showed LGE. The location of LGE corresponded to the edematous LV segments in 8 of 10 patients, which was consistent with an acute myocardial ischemic injury without previous myocardial infarction (Figure 1A and 1B). In the other 2 patients, the LGE also affected other remote nonedematous segments, which was consistent with an acute myocardial ischemic injury superimposed on a concomitant previous postmyocardial infarction scar.
Figure 1. Representative examples of patients experiencing sudden cardiac arrest secondary to ischemic heart disease.
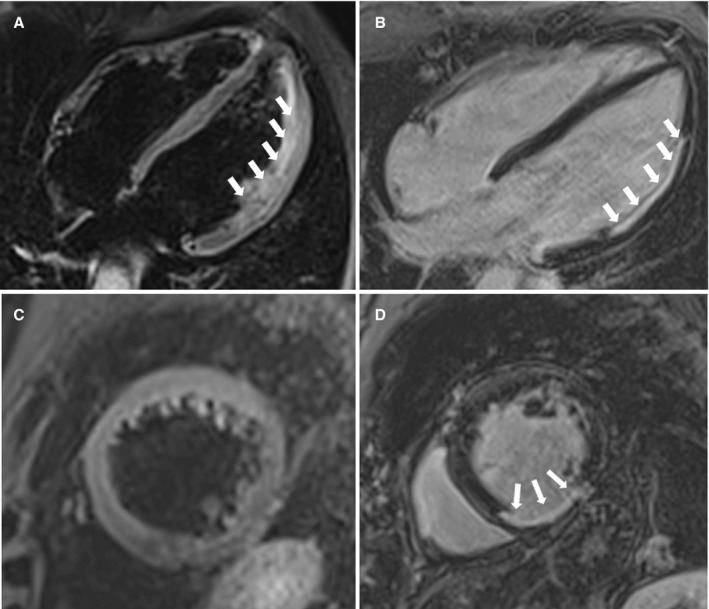
Cardiac magnetic resonance (CMR) showing transmural myocardial edema (ME) of the lateral left ventricular wall (4 chambers, long‐axis view; T2‐weighted sequences) (A) and subendocardial late gadolinium enhancement (LGE) involving the same region (4‐chamber, long‐axis view; T1‐weighted inversion recovery postcontrast sequences) (B) in a patient with obstructive stenosis of the circumflex artery, suggesting acute myocardial infarction. CMR showing no ME (short‐axis view; T2‐weighted sequences) (C) and transmural LGE involving the inferior left ventricular wall (short‐axis view; T1‐weighted inversion recovery postcontrast sequences) (D) in a patient with chronic right coronary artery occlusion suggesting chronic ischemic heart disease.
Ischemic Myocardial Scar Without ME
In all 11 SCA survivors with obstructive CAD and no evidence of ME, early CMR showed isolated LV LGE with a subendocardial or transmural distribution, which was consistent with a chronic postinfarction myocardial scar in the absence of acute myocardial ischemic injury (Figure 1B and 1C).
Nonischemic ME
Seven patients showed subepicardial or midmyocardial ME involving the inferolateral LV wall associated with LGE, consistent with an inflammatory acute myocardial injury (clinically suspected acute myocarditis) (Figure 2A and 2B). In 4 cases, endomyocardial biopsy was performed that confirmed the diagnosis.
Figure 2. Representative examples of patients experiencing sudden cardiac arrest secondary to nonischemic heart disease.
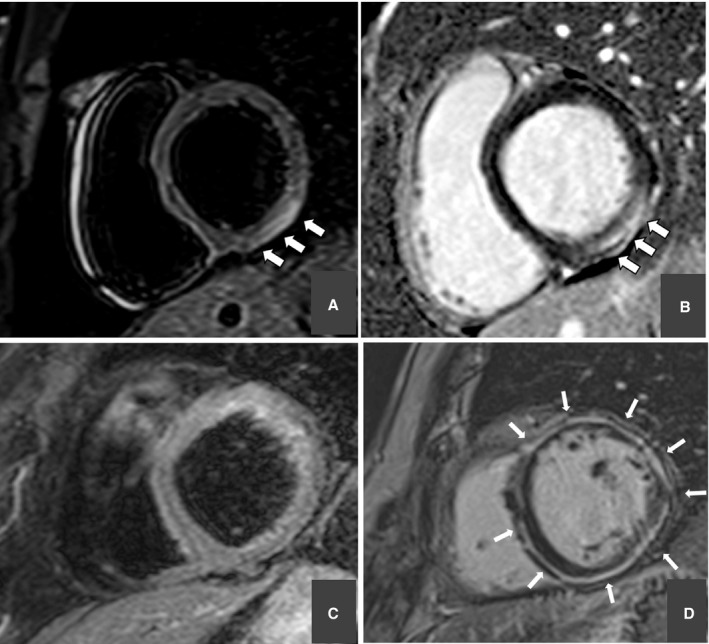
Cardiac magnetic resonance (CMR) showing subepicardial myocardial edema (ME) of the inferolateral left ventricular wall (short‐axis view; T2‐weighted sequences) (A) and subepicardial late gadolinium enhancement (LGE) involving the same region (short‐axis view; T1‐weighted inversion recovery postcontrast sequences) (B) in a patient with normal coronary artery, suggesting acute myocarditis. CMR showing no ME (short‐axis view; T2‐weighted sequences) (C) and circumferential subepicardial‐midmyocardial LGE (short‐axis view; T1‐weighted inversion recovery postcontrast sequences) (D) in a patient with left‐dominant arrhythmogenic cardiomyopathy.
Nonischemic Structural Heart Disease Without ME
In 43 SCA survivors, CMR identified the myocardial substrate of SCA in the following chronic structural heart muscle diseases: dilated cardiomyopathy in 13; arrhythmogenic cardiomyopathy in 4; hypertrophic cardiomyopathy in 3; isolated (idiopathic) nonischemic LV scar in 16; and “arrhythmic” mitral valve prolapse in 7 (Figure 2C and 2D).
Normal CMR
In 29 SCA survivors there was no clinical or CMR evidence of acute or chronic structural myocardial abnormalities, and the mechanism of cardiac arrest (CA) was considered the consequence of a primarily electrical syndrome. Ten of 29 SCA survivors had genetic, clinical, and ECG findings diagnostic for cardiac ion channel disease, ie, long QT syndrome in 5 patients and Brugada syndrome in 5 patients. In the remaining 19 SCA survivors, the arrhythmic CA was ascribed to idiopathic VF.
ICD Implantation and Outcome
Type of ICD, antiarrhythmic therapy at discharge, and outcome data are shown in Table 3. In 42% of patients, the ICD was programmed with 2 detection zones (both ventricular tachycardia and VF zone) and in 58% with 1 zone (VF only). Ventricular tachycardia detection threshold was set at a median value of 190 beats per minute (range, 176–200 beats per minute), and VF detection was set at a threshold of 222 beats per minute (range, 200–240 bpm). The minimum threshold for ICD intervention did not significantly differ between patients with and without ME, with and without LGE. or among the 3 different disease categories.
Table 3.
Type of ICD, Antiarrhythmic Therapy at Discharge, and Outcome Data
| Follow‐up (range), mo | 47 (26–67) |
| Type of ICD | |
| Single‐chamber | 54 (53) |
| Dual‐chamber | 18 (18) |
| CRT‐D | 8 (8) |
| Subcutaneous | 21 (21) |
| Antiarrhythmic drug therapy at discharge | |
| β‐Blockers | 57 (56) |
| Calcium channel blockers | 4 (4) |
| Amiodarone | 10 (10) |
| ICD intervention | |
| ≥1 ATP | 11 (11) |
| ≥1 shock | 18 (18) |
| ≥1 ATP or shock | 24 (24) |
| ≥ inappropriate shock | 14 (14) |
| Complications requiring system revision | |
| Pocket hematoma | 1 (1) |
| Lead failure | 2 (2) |
| Infection | 2 (2) |
| Death | |
| Cardiovascular death | 2 (2) |
| Noncardiovascular death | 2 (2) |
Values are expressed as number (percentage) unless otherwise indicated. ATP indicates antitachycardia pacing; CRT‐D, cardiac resynchronisation therapy; and ICD, implantable cardioverter‐defibrillator.
During a median follow‐up of 47 months (28 to 67 months), 24 of 101 (24%) SCA survivors received appropriate ICD therapy, which consisted of antitachycardia pacing therapy only in 6 patients and ≥1 ICD shocks in 18 patients.
Fourteen SCA survivors experienced ≥1 inappropriate ICD shocks. Complications requiring revision occurred in 5 patients and included pocket hematoma in 1 patient, lead fracture in 2 patients, and infective endocarditis in 2 patients. Two patients died of cardiovascular causes during the follow‐up and other 2 for noncardiovascular events.
Correlation of Results
Correlation between findings of early CMR imaging and arrhythmic outcome during follow‐up are shown in Table 4.
Table 4.
CMR Findings According to Arrhythmic Events During Follow‐Up
| Overall (N=101) | ICD therapy + (n=24) |
ICD therapy − (n=77) |
P log‐rank | |
|---|---|---|---|---|
| Left ventricle | ||||
| Ejection fraction | 56 (44–61) | 55 (48–61) | 56 (41–60) | 0.49 |
| Ejection fraction <50% | 37 (37) | 9 (38) | 28 (36) | |
| Dilation (EDV >75 mL/mq) | 38 (38) | 11 (46) | 27 (35) | 0.84 |
| Regional WMA | 28 (28) | 5 (21) | 23 (30) | 0.94 |
| Right ventricle | ||||
| Dysfunction (EF <50%) | 11 (11) | 2 (8) | 9 (12) | 0.28 |
| Dilation (EDV >75 mL/mq) | 5 (5) | 2 (8) | 3 (4) | 0.11 |
| Regional WMA | 7 (7) | 1 (4) | 6 (8) | 0.78 |
| Tissue characterization | ||||
| Edema | 18 (18) | 2 (8) | 16 (21) | 0.04 |
| Subendocardial/transmural | 11 (11) | 2 (8) | 9 (12) | |
| Subepicardial/midmural | 7 (7) | 0 | 7 (19) | |
| Late enhancement | 58 (58) | 14 (58) | 44 (57) | 0.49 |
| Subendocardial/transmural | 21 (21) | 4 (17) | 17 (22) | |
| Subepicardial/midmural | 37 (37) | 10 (42) | 27 (35) | |
| Type of disease | ||||
| Ischemic heart disease | 21 (21) | 4 (17) | 17 (22) | 0.42 |
| Nonischemic heart disease | 51 (50) | 12 (50) | 39 (49) | |
| Primary arrhythmia syndrome | 29 (29) | 8 (33) | 21 (27) | |
Values are expressed as number (percentage) unless otherwise indicated. CMR indicates cardiac magnetic resonance; EDV, end‐diastolic volume; EF, ejection fraction; ICD, implantable cardioverter‐defibrillator; and WMA, wall motion abnormality.
Findings from Kaplan‐Meier analysis showed that demonstration of ME in the T2‐weighted sequences on early CMR imaging was associated with a significantly better overall event‐free survival: 11% (2 of 18) of patients with ME experienced any appropriate ICD intervention versus 27% (22 of 83) of those without ME (log‐rank=0.04) (Figure 3A). ME continued to predict a significantly more favorable outcome by Kaplan‐Meier analysis when the arrhythmic end point was limited to ICD shock only: 6% (1 of 18) of patients with ME reached this end point versus 20% (17 of 83) of those without ME (log‐rank=0.03) (Figure 3B). The survival free from either any appropriate ICD intervention or ICD shock did not differ among survivors of arrhythmic CA depending on the underlying disease category (ischemic heart disease, nonischemic heart disease, or primarily arrhythmic syndrome) (Figure 4).
Figure 3. Survival free from appropriate implantable cardioverter‐defibrillator (ICD) intervention according to the presence of myocardial edema (ME).
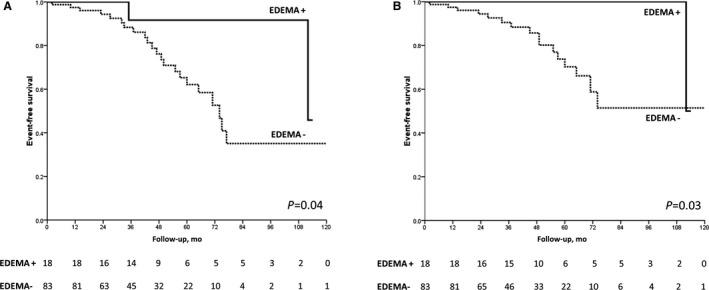
Kaplan‐Meier analysis of survival free from appropriate ICD interventions (antitachycardia pacing or shock [A] or shock only [B] according to the presence of ME on cardiac magnetic resonance).
Figure 4. Survival free from appropriate implantable cardioverter‐defibrillator (ICD) intervention according to the type of disease.
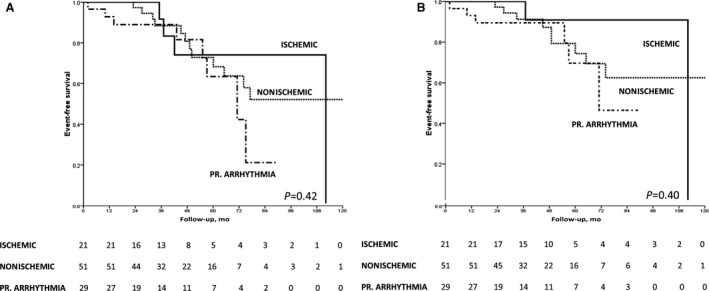
Kaplan‐Meier analysis of survival free from appropriate ICD interventions (antitachycardia pacing or shock [A] or shock only [B] according to the type of cardiovascular disease).
The 2 patients with ME who underwent early CMR imaging and experienced appropriate ICD interventions during the follow‐up had survived SCA precipitated by acute myocardial ischemia, which was superimposed on chronic ischemic cardiomyopathy, multivessel coronary obstruction with no indication for revascularization, severely reduced LVEF (25% and 21%, respectively), and multiregional LGE.
On multivariate analysis, ME remained an independent predictor of arrhythmic outcome after adjustment for LVEF and LGE (Table 5).
Table 5.
Univariate and Multivariable Cox Regression Analysis for Predictors of Appropriate ICD Intervention
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| ME | 0.26 | 0.06–0.92 | 0.038 | 0.22 | 0.05–0.94 | 0.041 |
| LVEF, % | 0.99 | 0.97–1.02 | 0.58 | 0.98 | 0.95–1.01 | 0.19 |
| LGE | 1.33 | 0.59–3.01 | 0.49 | 1.46 | 0.60–3.54 | 0.41 |
HR indicates hazard ratio; ICD, implantable cardioverter‐defibrillator; and ME, myocardial edema.
Left ventricular ejection fraction (LVEF) and late gadolinium enhancement (LGE) were included in the multivariable analysis as potential confounders based on their clinical relevance despite the result of the univariate analysis.
Figure 5 summarizes the study protocol and the main study findings.
Figure 5. Summary of study protocol and main results.
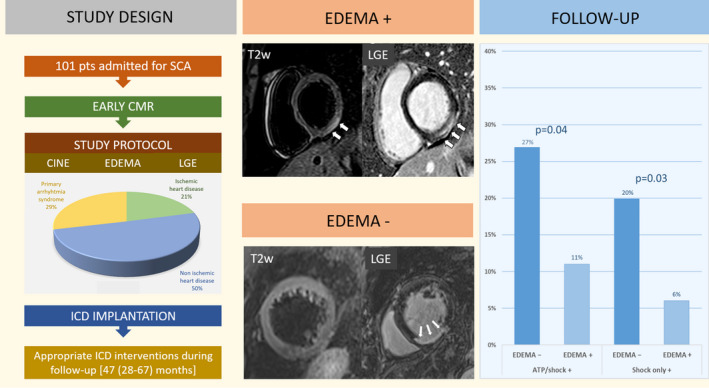
ATP indicates antitachycardia pacing; CMR, cardiac magnetic resonance; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; and SCA, sudden cardiac arrest.
DISCUSSION
This multicenter study aimed to evaluate the long‐term arrhythmic outcome in a cohort of SCA survivors and to test the prognostic role of ME as evidenced by early CMR in predicting appropriate ICD intervention. The main findings were that: (1) early CMR imaging demonstrated on T2 sequences the presence of ME, attributable to either ischemic or nonischemic heart disease, in 18% of SCA survivors; (2) over a median follow‐up of nearly 4 years, 89% of SCA survivors with ME had an uneventful long‐term arrhythmic outcome; (3) ME was associated with a significantly lower risk of ICD discharge for life‐threatening arrhythmic events during follow‐up and remained an independent predictor of better arrhythmic outcome after adjustment for LVEF and LGE; and (4) the risk of appropriate ICD intervention during follow‐up was unrelated to the underlying heart disease, whether ischemic, nonischemic, or primarily arrhythmic.
Previous Studies
Prior studies in patients who survived SCA showed that CMR tissue characterization provides incremental diagnostic and prognostic value. 9 , 10 , 11 , 12 , 13 It was demonstrated that imaging myocardial LGE/fibrosis is a key test for diagnosing the underlying cause of SCA and provides added value for arrhythmic risk stratification in both ischemic and nonischemic heart diseases, regardless of ventricular ejection fraction or severity of CAD. Data on the clinical meaning and prognostic role of ME as evidenced by early CMR imaging in SCA survivors are limited because in the majority of previous studies scans were performed late after the episode of SCA, preventing detection of ME by T2‐weighted sequences. All study patients underwent early CMR imaging, ie, within 1 month after SCA, a necessary prerequisite for demonstration of ME, which is a transient phenomenon. 8 , 9 A previous study primarily addressed the diagnostic value of ME identified by early CMR in a small group of patients who survived SCA. Short‐term follow‐up data were secondarily reported and only half of the small patient sample received an ICD. 9 The present multicenter prognostic study primarily focused on the value of ME by early CMR imaging to predict the occurrence of appropriate ICD intervention in a larger group of patients, collected from 9 Italian centers, all treated with an ICD and followed up for a longer period. This allowed systematically correlating the presence of ME by early CMR imaging and the occurrence of ICD interventions during follow‐up. The findings confirm and extend previous preliminary observations on the favorable and independent predictive value of ME.
Pathophysiology of ME
ME is the expression of early tissue changes, which occur during an acute ischemic or inflammatory injury and consist of accumulation of intercellular water (cytogenic edema) followed by interstitial water (vasogenic edema). 19 T2‐weighted imaging is sensitive to myocardial water content and allows for the visualization by CMR of ME in a variety of acute heart diseases, including acute coronary syndromes, acute myocarditis, Takotsubo syndrome, and acute graft rejection. In patients with arrhythmic SCA, T2‐weighted images provide an added diagnostic value as ME represents the tissue marker for an acute and reversible myocardial injury, either ischemic or inflammatory, underlying VF. 15 , 20 , 21 , 22 , 23 , 24 Whether ME represents a bystander hallmark of an arrhythmogenic myocardial insult underlying VF (ie, acute myocardial ischemia) or it is directly involved in the genesis of the ventricular electrical instability through the induction of a depolarization/repolarization inhomogeneity of the edematous myocardium remains to be established.
Identifying a Reversible Cause of SCA
The knowledge of the acuity and reversibility of the myocardial substrate underlying arrhythmic SCA may be crucial in clinical decision‐making. The SCA survivors with an underlying identifiable chronic irreversible or noncorrectable cause are at high risk for recurrence of malignant ventricular arrhythmias and subsequent death. On the contrary, patients with arrhythmic SCA attributable to an identifiable acute transient or correctable cause have a low risk of arrhythmic death during follow‐up. Accordingly, current guidelines recommend ICD implantation for secondary prevention in survivors of SCA, unless the cause is deemed reversible and/or correctable. 2 , 3 Putative transient causes of SCA include acute ischemic events or acute myocarditis. Correctable causes consist of electrolyte imbalance, antiarrhythmic drug reaction, cocaine or other illicit drug use, or other. 1 Despite these recommendations, ICDs are implanted in up to 40% of SCA survivors with a potentially reversible cause of SCA, without clear evidence to guide this decision. 25 , 26 , 27
Indeed, determining the exact cause of SCA and its reversibility is a clinical challenge. Unless a clear culprit lesion is identified by coronary angiography, differentiation between acute myocardial infarction and chronic CAD may be difficult. 6 In fact, SCA may not be preceded by ischemic symptoms, and troponin increase after SCA may not be specific. Diagnosing acute myocarditis in a patient who had SCA also represents a clinical puzzle. Symptoms, dynamic ECG changes, and troponin elevation may lack specificity after SCA because they may be secondary to an anoxic injury (postresuscitation syndrome). 4 , 5 Other presumed reversible causes of CA, such as electrolyte abnormalities, may be either the cause or the consequence of SCA and associated resuscitation efforts.
Role of ME in SCA Survivors
The results of our study showed that myocardial tissue characterization by early CMR offers the advantage to interpret the mechanism responsible for the arrhythmic SCA on an objective pathophysiologic basis. In combination with postcontrast sequences for LGE/fibrosis, CMR sequences for ME offered the potential to evaluate whether the arrhythmic event responsible for SCA occurred in association with an acute and reversible myocardial injury or with chronic and irreversible structural myocardial disease.
In our study, T2‐weighted sequences allowed us to identify acute myocardial ischemia as the arrhythmic substrate in 30% of SCA survivors with coronary artery stenosis by demonstrating the presence of ME in the territory tributary of an obstructed coronary artery. The detection of ME led to the identification or confirmation of the culprit coronary artery in patients with multiple vessel disease and equivocal coronary angiography findings. ME was also demonstrated in 13% of SCA survivors without obstructive CAD: in these cases, acute myocardial injury was ascribed to acute myocarditis, coronary artery spasm, or Takotsubo syndrome.
Among the remaining 83 patients without evidence of ME, SCA was caused by chronic structural heart disease or primarily arrhythmic syndrome in the presence of a structurally normal heart. Structural heart diseases included cardiomyopathies, isolated nonischemic myocardial scar, or arrhythmic mitral valve prolapse.
Clinical Outcome
Twenty‐four percent of our patients experienced ≥1 appropriate ICD interventions over a median follow‐up of nearly 4 years, a figure in keeping with the previously reported high risk of recurrences among SCA survivors 2 , 3 ; however, only 2% died of cardiovascular causes. Indeed, our cohort with SCA included relatively young patients (median age, 47 years) with predominant nonischemic heart disease and mild systolic ventricular dysfunction. This may be explained by their higher acute survival rate and the rapid recovery from neurological, respiratory, or other complications allowing early CMR imaging.
Our follow‐up results confirmed the study hypothesis that SCA survivors with evidence of ME, which is an objective marker of an acute and transient arrhythmogenic myocardial substrate, had a more favorable arrhythmic outcome. 9 We found that all but 2 patients with ME had an uneventful arrhythmic outcome. Although SCA in both patients was associated with acute ischemic ME, they also had a concomitant chronic ischemic cardiomyopathy with a severely reduced LVEF (25% and 21%, respectively), which is a recognized permanent condition associated with a high risk of SCD. Of note, ME regardless of its cause remained an independent predictor of better arrhythmic outcome after adjustment for LVEF and LGE.
Furthermore, the risk of appropriate ICD intervention during follow‐up did not differ with regard to the type of underlying heart disease, whether ischemic, nonischemic, or primarily arrhythmic.
Study Limitations
The study population reflects the inclusion criteria and the difficulty in performing early CMR imaging after SCA. Accordingly, the results may not be generalized to all patients with SCA. However, it should be recognized that a sizeable proportion of patients admitted for SCA is not eligible for early CMR imaging because of death, mechanical ventilation, kidney injury, or neurological impairment. Parametric T2 mapping technique was not available during the study period at the majority of participating centers, and we elected not to report the findings of a few patients for lack of clinical relevance. Moreover, the study was retrospective, but data quality were assured by the inclusion only of patients who received an ICD and were regularly followed up.
Conclusions
The results of this multicenter study on patients with SCA who received an ICD for secondary prevention suggest that demonstration by early CMR of ME, which is a marker of acute and reversible arrhythmogenic myocardial injury, may predict a favorable outcome with a lower incidence of appropriate shocks for relapses of life‐threatening ventricular tachyarrhythmias irrespective of its cause and potential confounders such as LVEF and LGE. As expected, the arrhythmic outcome may be influenced by concomitant chronic heart diseases, which act as a permanent substrate for VF. The observational study design and the relatively limited number of SCA survivors undergoing early CMR imaging and ICD implantation do not allow definitive conclusions on the relationship between ME and arrhythmic outcome. Hence, the results of the present study should be considered hypothesis‐generating and are expected to stimulate future prospective investigations on large patient populations over a longer follow‐up, hopefully using the parametric T2 mapping technique for evaluation of ME. Validation by further studies of our preliminary results on the favorable prognostic value of ME may have a substantial impact on future guidelines for the management of SCA survivors.
Sources of Funding
Dr Baldi was supported by grant 733381 from the European Union Horizon 2020 Research and Innovation Program of ESCAPE‐NET.
Disclosures
None.
Acknowledgments
We would like to thank the Italian Association of Cardiac Arrhythmias (AIAC) for the technical and networking support during the preparation of this study.
For Sources of Funding and Disclosures, see page 12.
See Editorial by Clark and Hughes
REFERENCES
- 1. Sutherland GR. Sudden cardiac death: the pro‐arrhythmic interaction of an acute loading with an underlying substrate. Eur Heart J. 2017;38:2986–2994. doi: 10.1093/eurheartj/ehw449 [DOI] [PubMed] [Google Scholar]
- 2. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e211–e269. doi: 10.1161/CIR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 3. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 4. Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 5. Price S, Uddin S, Quinn T. Echocardiography in cardiac arrest. Curr Opin Crit Care. 2010;16:211–215. doi: 10.1097/MCC.0b013e3283399d4c [DOI] [PubMed] [Google Scholar]
- 6. McFadden P, Reynolds JC, Madder RD, Brown M. Diagnostic test accuracy of the initial electrocardiogram after resuscitation from cardiac arrest to indicate invasive coronary angiographic findings and attempted revascularization: a systematic review and meta‐analysis. Resuscitation. 2021;160:20–36. doi: 10.1016/j.resuscitation.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 7. Deif B, Roberts JD. Diagnostic evaluation and arrhythmia mechanisms in survivors of unexplained cardiac arrest. Pacing Clin Electrophysiol. 2019;42:1320–1330. doi: 10.1111/pace.13780 [DOI] [PubMed] [Google Scholar]
- 8. Treibel TA, White SK, Moon JC. Myocardial tissue characterization: histological and pathophysiological correlation. Curr Cardiovasc Imaging Rep. 2014;7:9254. doi: 10.1007/s12410-013-9254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zorzi A, Susana A, De Lazzari M, Migliore F, Vescovo G, Scarpa D, Baritussio A, Tarantini G, Cacciavillani L, Giorgi B, et al. Diagnostic value and prognostic implications of early cardiac magnetic resonance in survivors of out‐of‐hospital cardiac arrest. Heart Rhythm. 2018;15:1031–1041. doi: 10.1016/j.hrthm.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 10. Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA, Danik SB, Verdini DJ, Tokuda M, Tedrow UB, et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging. 2015;8:414–423. doi: 10.1016/j.jcmg.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodrigues P, Joshi A, Williams H, Westwood M, Petersen SE, Zemrak F, Schilling RJ, Kirkby C, Wragg A, Manisty C, et al. Diagnosis and prognosis in sudden cardiac arrest survivors without coronary artery disease: utility of a clinical approach using cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006709. doi: 10.1161/CIRCIMAGING.117.006709 [DOI] [PubMed] [Google Scholar]
- 12. Baritussio A, Zorzi A, Ghosh Dastidar A, Susana A, Mattesi G, Rodrigues J, Biglino G, Scatteia A, De Garate E, Strange J, et al. Out of hospital cardiac arrest survivors with inconclusive coronary angiogram: impact of cardiovascular magnetic resonance on clinical management and decision‐making. Resuscitation. 2017;116:91–97. doi: 10.1016/j.resuscitation.2017.03.039 [DOI] [PubMed] [Google Scholar]
- 13. Zaremba T, Brøndberg AK, Jensen HK, Kim WY. Cardiac magnetic resonance characteristics in young survivors of aborted sudden cardiac death. Eur J Radiol. 2018;105:141–147. doi: 10.1016/j.ejrad.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 14. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non‐ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258 [DOI] [PubMed] [Google Scholar]
- 15. Perazzolo Marra M, Lima JAC, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32:284–293. doi: 10.1093/eurheartj/ehq409 [DOI] [PubMed] [Google Scholar]
- 16. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 17. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, et al. Nonischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. doi: 10.1161/CIRCEP.116.004229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basso C, Iliceto S, Thiene G, Perazzolo MM. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140:952–964. doi: 10.1161/CIRCULATIONAHA.118.034075 [DOI] [PubMed] [Google Scholar]
- 19. Garcia‐Dorado D, Andres‐Villarreal M, Ruiz‐Meana M, Inserte J, Barba I. Myocardial edema: a translational view. J Mol Cell Cardiol. 2012;52:931–939. doi: 10.1016/j.yjmcc.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 20. Carbone I, Friedrich MG. Myocardial edema imaging by cardiovascular magnetic resonance: current status and future potential. Curr Cardiol Rep. 2012;14:1–6. doi: 10.1007/s11886-011-0235-9 [DOI] [PubMed] [Google Scholar]
- 21. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 22. Marie PY, Angioï M, Carteaux JP, Escanye JM, Mattei S, Tzvetanov K, Claudon O, Hassan N, Danchin N, Karcher G, et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black‐blood magnetic resonance imaging sequence. J Am Coll Cardiol. 2001;37:825–831. doi: 10.1016/S0735-1097(00)01196-7 [DOI] [PubMed] [Google Scholar]
- 23. Eitel I, von Knobelsdorff‐Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992 [DOI] [PubMed] [Google Scholar]
- 24. Migliore F, Zorzi A, Perazzolo Marra M, Iliceto S, Corrado D. Myocardial edema as a substrate of electrocardiographic abnormalities and life‐threatening arrhythmias in reversible ventricular dysfunction of takotsubo cardiomyopathy: imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm. 2015;12:1867–1877. doi: 10.1016/j.hrthm.2015.04.041 [DOI] [PubMed] [Google Scholar]
- 25. De Lazzari M, Zorzi A, Baritussio A, Siciliano M, Migliore F, Susana A, Giorgi B, Lacognata C, Iliceto S, Perazzolo Marra M, et al. Relationship between T‐wave inversion and transmural myocardial edema as evidenced by cardiac magnetic resonance in patients with clinically suspected acute myocarditis: clinical and prognostic implications. J Electrocardiol. 2016;49:587–595. doi: 10.1016/j.jelectrocard.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 26. Madhavan M, Friedman PA, Lennon RJ, Prasad A, White RD, Sriram CS, Gulati R, Gersh BJ. Implantable cardioverter‐defibrillator therapy in patients with ventricular fibrillation out of hospital cardiac arrest secondary to acute coronary syndrome. J Am Heart Assoc. 2015;4:e001255. doi: 10.1161/JAHA.114.001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ladejobi A, Pasupula DK, Adhikari S, Javed A, Durrani AF, Patil S, Qin D, Ahmad S, Munir MB, Rijal S, et al. Implantable defibrillator therapy in cardiac arrest survivors with a reversible cause. Circ Arrhythm Electrophysiol. 2018;11:e005940. doi: 10.1161/CIRCEP.117.005940 [DOI] [PubMed] [Google Scholar]


