Abstract
Background
Patients with tetralogy of Fallot (ToF) are considered at high risk of infective endocarditis (IE) as a result of altered hemodynamics and multiple invasive procedures, including pulmonary valve replacement (PVR). Data on the long‐term risk of IE are sparse.
Methods and Results
In this observational cohort study, all patients with ToF born from 1977 to 2018 were identified using Danish nationwide registries and followed from date of birth until occurrence of first‐time IE, emigration, death, or end of study (December 31, 2018). The comparative risk of IE among patients with ToF versus age‐ and sex‐matched controls from the background population was assessed. Because of rules on anonymity, exact numbers cannot be reported if the number of patients is <4. A total of 1164 patients with ToF were identified and matched with 4656 controls. Among patients with ToF, 851 (73.1%) underwent early surgical intracardiac repair and 276 (23.7%) underwent PVR during follow‐up. During a median follow‐up of 20.3 years, 41 (3.5%) patients with ToF (comprising 24 [8.7%] with PVR and 17 [1.9%] without PVR) and <4 (<0.8%) controls were admitted with IE. The incidence rates of IE per 10 000 person‐years were 22.4 (95% CI, 16.5–30.4) and 0.1 (95% CI, 0.01–0.7) among patients and controls, respectively. Moreover, PVR was associated with a further increased incidence of IE among patients with ToF (incidence rates per 10 000 person‐years with and without PVR were 46.7 [95% CI, 25.1–86.6] and 2.8 [95% CI 2.0–4.0], respectively).
Conclusions
Patients with ToF are associated with a substantially higher incidence of IE than the background population. In particular, PVR was associated with an increased incidence of IE. With an increasing life expectancy of these patients, intensified awareness, preventive measures, and surveillance of this patient group are decisive.
Keywords: congenital heart disease, epidemiology, infective endocarditis, tetralogy of Fallot
Subject Categories: Epidemiology, Congenital Heart Disease, Infectious Endocarditis
Nonstandard Abbreviations and Acronyms
- IE
infective endocarditis
- NCSP
Nordic Medico‐Statistical Committee Classification of Surgical Procedures
- PVR
pulmonary valve replacement
- ToF
tetralogy of Fallot
Clinical Perspective
What Is New?
The long‐term incidence of infective endocarditis among patients with tetralogy of Fallot and in particular among those undergoing pulmonary valve replacement is substantial and significantly higher compared with the background population.
What Are the Clinical Implications?
With an increasing life‐expectancy of these patients, intensified control and surveillance as well as implementation of relevant precautionary measures are advisable in order to increase the quality of life among these patients.
Patients with congenital heart diseases (CHDs) are considered to be at a lifelong high risk of infective endocarditis (IE), an infection associated with high morbidity and mortality. 1 , 2 , 3 , 4 , 5 , 6 Studies suggest that patients with tetralogy of Fallot (ToF) are at high risk of IE; however, data are sparse and limited by lack of long‐term follow‐up and small number of patients. 7 , 8 , 9 Most traditional risk factors for IE are strongly associated with age and are exogenous (heart valve protheses, cardiac implantable devices, etc), and patients are exposed to these late in life. 9 , 10 CHD stands out by the fact that patients with CHD are at risk for IE from birth. Hence, the relative short‐term risk of IE associated with CHD may be lower than the associated IE risk of other risk factors, but CHD may still be associated with a substantial lifetime risk of IE. Yet data on the long‐term risk of IE in an important CHD such as ToF are limited.
The overall survival for patients with CHD has improved during recent decades, and ToF is one of the most common complex CHDs with a high survival rate into adulthood. 11 , 12 , 13 However, the survival rate among patients >40 years of age with ToF is low, and the morbidity as well as the socioeconomic burden are high. 13 , 14 , 15 , 16 , 17 Patients with ToF often undergo multiple surgical procedures during early childhood and may also undergo pulmonary valve replacement (PVR) during early adulthood. These factors also influence risk of IE by providing a local point of attack for bacteria and the foundation for acquiring IE. To guide the clinical decision making and patient surveillance and also to improve life span and clinical outcomes of these patients, investigation of morbidity and adverse outcomes including IE is of great interest. The objective of this study was to examine the risk of IE among patients with ToF in a Danish nationwide cohort and to compare the risk of IE among patients with ToF with the background population in Denmark.
METHODS
Data Sources
Anonymized data were made available from Statistics Denmark, and permission from Statistics Denmark is necessary to access data. All Danish residents are assigned a unique and permanent civil registration number allowing accurate linkage of nationwide administrative registries at an individual level. For this study, data from the following 2 nationwide administrative registries were obtained: (1) the Danish National Patient Registry, which holds information on all hospital admissions and outpatient contacts from 1977 according to the International Classification of Diseases, Eighth Revision (ICD‐8) and Tenth Revision (ICD‐10) and surgical procedures according to the Nordic Medico‐Statistical Committee Classification of Surgical Procedures (NCSP) 18 ; and (2) the Danish National Population Registry, which contains information on birth date, sex, and vital status (ie, whether a person is alive and resident in Denmark, disappeared [persons whose residence is unknown to Danish authorities], emigrated, or dead—along with the date of these events). 19 The Danish registries are validated and of high quality as described in detail previously. 18 , 19 , 20 , 21 , 22
Study Population
All Danish citizens born in the 42‐year period from January 1, 1977, to December 31, 2018, and diagnosed with ToF were included in the study. Patients with ToF were identified using the following ICD‐8, ICD‐10, and NCSP codes: 74629, 30970, 31500, 31580, DQ213, DQ213A, DQ218C, KFHE00, KFHE10, KFHE20, KFHE30, and KFHE96. The diagnosis was validated by the authors by examination of 150 random records of patients with ToF with a positive predictive value of 98%. Moreover, 103 surgical records of patients with ToF born and dead in the study period and who underwent surgery at Copenhagen University Hospital, Rigshospitalet, were examined, and the diagnosis was validated with a positive predictive value of 95%. Patients with concomitant diagnosis or surgery codes not corresponding to ToF were excluded from this study to further increase the validity of the diagnosis. Using risk‐set matching, the study population was matched with controls from the background population in a 1:4 ratio based on sex and year of birth.
Covariates
Frequency of specific NCSP codes for palliative surgery, intracardiac repair, and PVR are shown in Table S1. Associated malformations among patients with ToF are shown in Table S2. Comorbidities and concomitant congenital malformations were obtained by inpatient and outpatient diagnoses and were updated day by day (see Table S3 for ICD‐8 codes, ICD‐10 codes, and NCSP codes). The presence of a cardiac implantable electronic device was defined as an implantation of a pacemaker or implantable cardioverter and were also updated day by day (see Table S3 for NCSP codes). Associated anomalies, comorbidities, and presence of cardiac implantable electronic devices were assessed at 1, 10, and 20 years of age among patients and controls who were alive and had follow‐up to these time points (Table 1).
Table 1.
Baseline Characteristics Among Patients With ToF and Controls Alive and With Follow‐Up at 1, 10, and 20 Years of Age, Respectively
| 1 y | 10 y | 20 y | ||||
|---|---|---|---|---|---|---|
| Patients (n=997) | Controls (n=4561) | Patients (n=733) | Controls (n=3817) | Patients (n=450) | Controls (n=2555) | |
| Comorbidities, n (%) | ||||||
| Ischemic heart disease | 7 (0.7) | <4 (<0.1) | 8 (1.1) | <4 (<0.1) | 5 (1.1) | <4 (<0.2) |
| Chronic heart failure | 76 (7.6) | <4 (<0.1) | 54 (7.4) | <4 (<0.1) | 27 (6.0) | <4 (<0.2) |
| Arrhythmia | 8 (0.8) | <4 (<0.1) | 11 (1.5) | <4 (<0.1) | 27 (6.0) | 5 (0.2) |
| Stroke and transient ischemic attack | <4 (<0.4) | <4 (<0.1) | 6 (0.8) | <4 (<0.1) | 7 (1.6) | <4 (<0.2) |
| Coagulopathy | <4 (<0.4) | <4 (<0.1) | 8 (1.1) | 11 (0.3) | 8 (1.8) | 15 (0.6) |
| Bleeding | 5 (0.5) | 4 (0.1) | 17 (2.3) | 17 (0.5) | 13 (2.9) | 10 (0.4) |
| Chronic renal failure | 8 (0.8) | <4 (<0.1) | 7 (1.0) | 5 (0.1) | 5 (1.1) | 8 (0.3) |
| Septic and cardiogenic shock | 45 (4.5) | 17 (0.4) | 31 (4.2) | 27 (0.7) | 24 (5.3) | 29 (1.1) |
| Mitral, aortic, or tricuspid valve surgery | 8 (0.8) | <4 (<0.1) | 8 (1.1) | <4 (<0.1) | 7 (1.5) | <4 (<0.2) |
| Cardiac implantable electronic devices | <4 (<0.4) | <4 (<0.1) | <4 (<0.5) | <4 (<0.1) | <4 (<0.9) | <4 (<0.2) |
| Congenital malformations, n (%) | ||||||
| Eyes, eyelid, lacrimal apparatus, or orbit | 17 (1.7) | 10 (0.2) | 19 (2.6) | 17 (0.5) | 12 (2.7) | 9 (0.4) |
| Ears | 11 (1.1) | <4 (<0.1) | 20 (2.7) | 17 (0.5) | 20 (4.4) | 23 (0.9) |
| Nose, pharynx, larynx, trachea, or lungs | 11 (1.1) | 12 (0.3) | 16 (2.2) | 11 (0.3) | 7 (1.6) | 8 (0.3) |
| Palate, tongue, or lips | 30 (3.0) | 17 (0.34) | 36 (4.9) | 14 (0.4) | 24 (5.3) | 7 (0.3) |
| Digestion system | 28 (2.8) | 19 (0.4) | 24 (3.3) | 24 (0.6) | 14 (3.1) | 18 (0.7) |
| Reproductive system | 9 (0.7) | 9 (0.2) | 10 (1.4) | 20 (0.5) | 15 (3.3) | 21 (0.8) |
| Urinary system | 20 (2.0) | 7 (0.2) | 18 (2.5) | 5 (0.1) | 14 (3.1) | <4 (<0.2) |
| Extremities | 44 (4.4) | 44 (1.0) | 53 (7.2) | 66 (1.7) | 43 (9.6) | 48 (1.9) |
| Congenital syndromes | 94 (9.4) | 7 (0.2) | 96 (13.1) | 9 (0.2) | 59 (13.3) | <4 (<0.2) |
| DiGeorge syndrome | 15 (1.5) | <4 (<0.1) | 22 (3.0) | <4 (<0.1) | 15 (3.3) | <4 (<0.2) |
| Cardiac malformations, n (%) | ||||||
| Heart chambers | 96 (9.6) | <4 (<0.1) | 88 (12.0) | <4 (<0.1) | 53 (11.8) | <4 (<0.2) |
| Atrial septal defect | 134 (13.1) | 10 (0.2) | 130 (17.7) | 11 (0.3) | 75 (16.7) | 4 (0.2) |
| Pulmonary or tricuspid valve | 135 (13.5) | <4 (<0.1) | 166 (22.7) | <4 (<0.1) | 113 (25.1) | <4 (<0.2) |
| Aortic or mitral valve | 34 (3.4) | <4 (<0.1) | 48 (6.6) | <4 (<0.1) | 44 (9.8) | <4 (<0.2) |
| The great arteries | 132 (13.2) | 4 (0.1) | 158 (21.6) | <4 (<0.1) | 100 (22.2) | <4 (<0.2) |
| Persisting ductus arteriosus | 50 (5.0) | <4 (<0.1) | 56 (7.6) | <4 (<0.1) | 32 (7.1) | <4 (<0.2) |
| Coarctatio aortae | 19 (1.9) | <4 (<0.1) | 16 (2.2) | <4 (<0.1) | 10 (2.2) | <4 (<0.2) |
| The great veins | 10 (1.0) | <4 (<0.1) | 12 (1.6) | <4 (<0.1) | 7 (1.6) | <4 (<0.2) |
| The peripheral circulation | 4 (0.4) | <4 (<0.1) | 6 (0.8) | <4 (<0.1) | 6 (1.3) | <4 (<0.2) |
ToF indicates tetralogy of Fallot.
Outcomes
The primary outcome was incident IE. IE was defined from the following ICD‐8 and ICD‐10 codes: 42100 (acute endocarditis), 42101 (subacute endocarditis), 42108 (acute and subacute endocarditis), 42109 (acute and subacute endocarditis), 42199 (acute and subacute endocarditis), DI33 (acute and subacute endocarditis), DI38 (endocarditis, valve unspecified), and DI398 (endocarditis unspecified). The diagnosis of IE in the Danish National Patient Registry has previously been validated with a positive predictive value of 90%, and we applied the same definition of IE for this study. 23 Mortality was a secondary outcome. Patients were followed from birth until occurrence of IE, emigration, death, or end of the study (December 31, 2018), whichever came first.
Statistical Analysis
Descriptive characteristics of patients with ToF and controls from the background population were reported as frequencies with percentages for categorical variables and medians with 25th to 75th percentiles for continuous variables.
The cumulative incidences of IE according to groups were estimated using the Aalen‐Johansen estimator incorporating the competing risk of death, and differences between groups were assessed using Gray’s test. 24 The cumulative incidences of all‐cause mortality according to groups were estimated using the Kaplan‐Meier estimator, and differences between groups were assessed using the log‐rank test. Crude incidence rates of IE and all‐cause mortality were calculated as number of events per 10 000 person‐years. Further, in cause‐specific Cox regression models, the rates of IE and all‐cause mortality among patients and controls were calculated as hazard ratios (HRs) with 95% CIs. These models were adjusted for PVR and relevant comorbidities (ie, chronic renal failure, diabetes, presence of cardiac implantable electronic devices, other valve surgery) and PVR as well as all covariates were included as time‐varying coefficients.
In a supplementary analysis, the comparative risks of IE among patients with versus without pulmonary valve prostheses were analyzed using Cox regression models in PVR, and the above‐mentioned comorbidities were included as time‐varying coefficients.
Because of rules on anonymity by Statistics Denmark, exact numbers cannot be reported if the number of patients is <4. The proportional hazards assumption was tested and found valid in the adjusted time‐dependent Cox regression analysis, and similar HRs were found in a landmark analysis dividing follow‐up into 2 periods from 0 to 1 and >1 year of age. Relevant interactions including sex and year of birth and comorbidities were tested and found insignificant, unless otherwise stated. All statistical analyses were performed with SAS statistical software (version 9.4, SAS Institute, Cary, NC). A 2‐sided P<0.05 was considered statistically significant.
Sensitivity Analysis
To test the robustness of our findings, a sensitivity analysis excluding patients who did not undergo intracardiac repair was conducted. Index was defined as the day of intracardiac repair among patients. Among controls, index was defined as the day of surgery for their matched patient with ToF making sure that the control was born and alive on that day.
Ethics
In Denmark, registry‐based studies that are conducted for the sole purpose of statistics and scientific research do not require ethical approval or informed consent by law. However, the study is approved by the data responsible institute (the Capital Region of Denmark [approval number: P‐2019‐523]) in accordance with the General Data Protection Regulation.
RESULTS
Baseline Characteristics
In the period from January 1, 1977, to December 31, 2018, 1164 patients (56.4% men) were diagnosed with ToF in Denmark with a median age at time of diagnosis of 2.6 years (25th–75th percentile, 0.3–15.1) (Figure 1). Median time of follow‐up was 20.3 years (15.5 [25th–75th percentile, 4.4–25.2] and 21.1 [25th–75th percentile, 12.5–28.5] years among patients and controls, respectively). Among patients with ToF, 265 (22.8%) underwent palliative surgery (median age, 0.3 years [25th–75th percentile, 0.1–1.1]), 851 (73.1%) underwent intracardiac repair (median age of 1.1 years [25th–75th percentile 0.5–2.5]), and 276 (23.7%) underwent PVR (median age at first‐time PVR, 12.6 years [25th–75th percentile, 3.5–19.3]). Among those who underwent palliative surgery, 187 underwent intracardiac repair later on. Among those who underwent repair, 258 underwent PVR later on, whereas it concerned 18 of those who did not undergo repair in Denmark. All 1164 patients with ToF were matched with 4656 controls from the background population. Table 1 summarizes characteristics among patients and controls at 1, 10, and 20 years of age. Overall, patients with ToF had higher prevalence of congenital syndromes and concomitant congenital malformations, including malformations of the extremities and digestive system as well as various cardiac malformations compared with controls. Moreover, compared with controls, patients with ToF had higher prevalence of comorbidities, including chronic heart failure, arrhythmias, and septic/cardiogenic shock. Table S4 shows baseline characteristics among patients with ToF undergoing intracardiac repair and their matched controls at index.
Figure 1. Flowchart of the selection of the study population.
IE During Follow‐Up
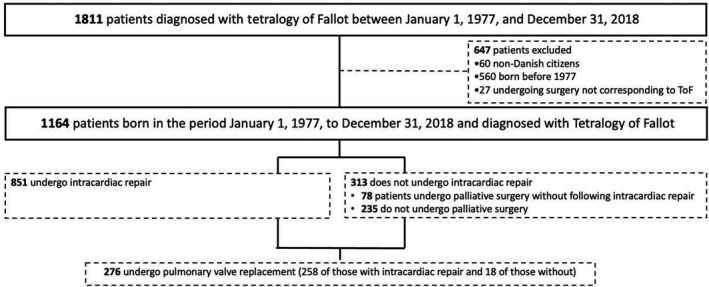
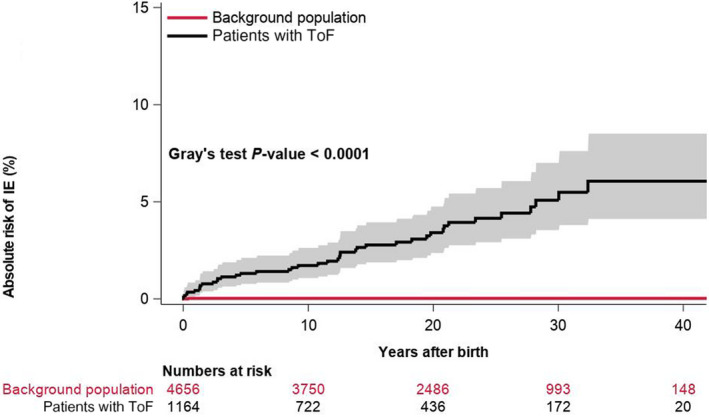
In total, 41 (3.5%) patients had IE during follow‐up. Median time from date of birth to IE was 11.6 years (25th–75th percentile, 2.8–20.9). Table 2 shows characteristics among patients with ToF with versus without IE. Overall, patients experiencing IE had a higher burden of concomitant cardiac malformations and comorbidities, including chronic heart failure and arrhythmias compared with those who did not develop IE. Among controls, <4 incidents of IE occurred during follow‐up. Figure 2 depicts the unadjusted cumulative incidences of IE among patients with ToF and matched controls from the background population. The incidence of IE has remained stable during the past decades (Figure 3). The incidence rates of IE per 10 000 person‐years were 22.4 (95% CI, 16.5–30.4) and 0.1 (95% CI, 0.01–0.7) among patients and controls, respectively. In a time‐dependent multivariable Cox regression model, the incidence of IE was higher among patients compared with controls (HR, 177.8; 95% CI, 24.2–1305.1).
Table 2.
Characteristics Among Patients With ToF Among Those With vs Without IE at Admission for IE and a Corresponding Date, Respectively
| Patients with IE (n=41) | Patients without IE (n=1123) | |
|---|---|---|
| Demographics | ||
| Age, y, median (IQR) | 11.6 (2.8–20.9) | 14.5 (3.4–24.8) |
| Male, n (%) | 26 (63.4) | 630 (56.1) |
| Comorbidities, n (%) | ||
| Ischemic heart disease | 4 (9.8) | 22 (2.0) |
| Chronic heart failure | 8 (19.5) | 105 (9.4) |
| Arrhythmia | 4 (9.8) | 56 (5.0) |
| Stroke and transient ischemic attack | <4 (<9.8) | 13 (1.2) |
| Coagulopathy | <4 (<9.8) | 14 (1.3) |
| Bleeding | <4 (<9.8) | 40 (3.6) |
| Chronic renal failure | <4 (<9.8) | 24 (2.1) |
| Septic and cardiogenic shock | 12 (29.3) | 69 (6.1) |
| Hypertension | <4 (<9.8) | 12 (1.1 |
| Mitral, aortic, or tricuspid valve surgery | <4 (<9.8) | <4 (<0.3) |
| Cardiac implantable electronic devices | <4 (<9.8) | <4 (<0.3) |
| Malformations, n (%) | ||
| Eyes, eyelid, the lacrimal apparatus, or the orbit | <4 (<9.8) | 34 (3.0) |
| Ears | <4 (<9.8) | 36 (3.2) |
| Nose, pharynx, larynx, trachea, or lungs | <4 (<9.8) | 28 (2.5) |
| Palate, tongue, or lips | <4 (<9.8) | 66 (5.9) |
| Digestion system | 4 (9.8) | 56 (5.0) |
| Reproductive system | <4 (<9.8) | 25 (2.2) |
| Urinary system | <4 (<9.8) | 40 (3.6) |
| Extremities | <4 (<9.8) | 100 (8.9) |
| Congenital syndromes | 5 (12.2) | 182 (16.2) |
| DiGeorge syndrome | <4 (<9.8) | 40 (3.6) |
| Heart chambers | 7 (17.1) | 140 (12.5) |
| Atrial septal defect | 8 (19.5) | 191 (17.0) |
| Pulmonary or tricuspid valve | 16 (39.0) | 261 (23.2) |
| Aortic or mitral valve | 5 (12.2) | 73 (6.5) |
| The great arteries | 17 (41.5) | 228 (20.3) |
| Persisting ductus arteriosus | 7 (17.1) | 81 (7.2) |
| Coarctatio aortae | <4 (<9.8) | 24 (2.1) |
| The great veins | <4 (<9.8) | 21 (1.9) |
| The peripheral circulation | <4 (<9.8) | 19 (1.7) |
IE indicates infective endocarditis; and ToF, tetralogy of Fallot.
Figure 2. Cumulative incidence of IE among patients with ToF and matched controls from the background population.
IE indicates infective endocarditis; and ToF, tetralogy of Fallot.
Figure 3. Incidence of IE among patients with ToF divided into 4 time periods (1977–1985, 1985–1995, 1995–2005, and 2005–1918).
IE indicates infective endocarditis; and ToF, tetralogy of Fallot.
In the sensitivity analysis excluding patients who did not undergo intracardiac repair, similar results were found: 32 (3.8%) patients yet no controls developed IE during follow‐up, corresponding to an incidence rate of IE per 10 000 person‐years of 26.3 (95% CI, 18.6–37.3) among patients. Figure S1 shows the unadjusted cumulative incidences of IE among patients with ToF and matched controls from the background population.
All‐Cause Mortality During Follow‐Up
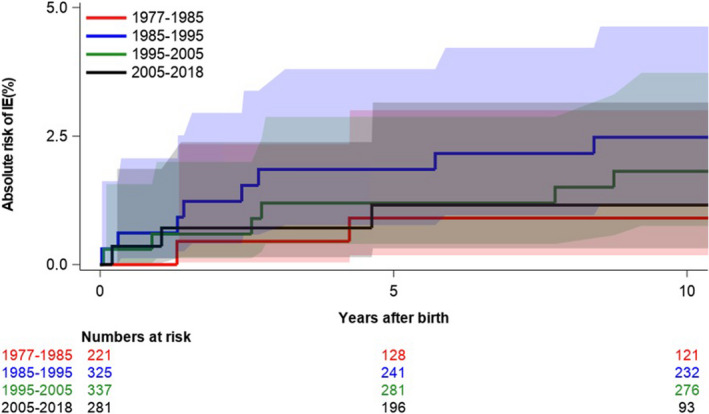
In total, 272 (23.4%) patients and 23 (0.5%) matched controls from the background population died during follow‐up. Figure 4 shows Kaplan‐Meier curves for all‐cause mortality among patients with ToF and controls. All‐cause mortality among patients with ToF has improved in recent decades (Figure 5). Mortality rates per 10 000 person‐years were 145.4 (95% CI, 129.1–163.7) and 2.4 (95% CI, 1.6–3.6) among patients and controls, respectively. In multivariable Cox regression analysis, patients had a higher rate of death (HR, 53.7; 95% CI, 35.1–82.5).
Figure 4. Cumulative risk of all‐cause mortality among patients with ToF and matched controls from the background population.
ToF indicates tetralogy of Fallot.
Figure 5. Incidence of all‐cause mortality among patients with ToF divided into 4 time periods (1977–1985, 1985–1995, 1995–2005, and 2005–1918).
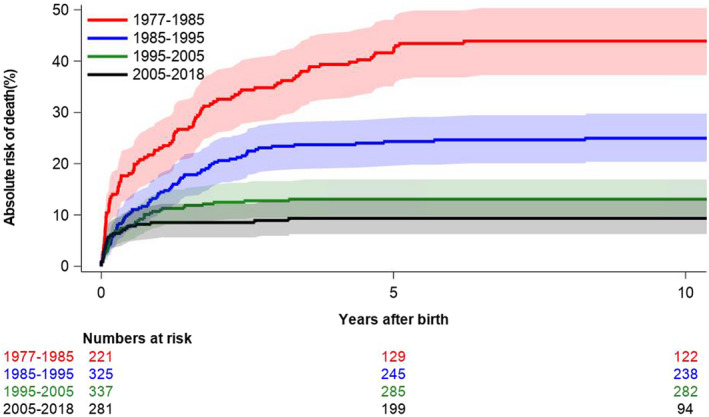
ToF indicates tetralogy of Fallot.
In the sensitivity analysis excluding patients who did not undergo intracardiac repair, similar results were found but with an expected lower mortality: 118 (13.9%) patients and 14 (0.4%) controls died during follow‐up, corresponding to mortality rates per 10 000 person‐years of 94.5 (95% CI, 78.9–113.2) and 2.4 (95% CI, 1.4–4.1), respectively, and an HR of 33.6 (95% CI, 19.2–59.0). Figure S2 shows the unadjusted cumulative incidences of all‐cause mortality among patients with ToF and matched controls from the background population.
Supplementary Analysis
During follow‐up, 24 (8.7%) patients with PVR experienced IE, while 17 (1.9%) of patients without PVR experienced IE. Median time from PVR to IE was 5.4 years (95% CI, 3.4–10.4 years). In time‐dependent analysis, the incidence rates of IE per 10 000 person‐years in patients with and without PVR were 46.7 (95% CI, 25.1–86.6) and 2.8 (95% CI, 2.0–4.0), respectively. In time‐dependent multivariable Cox regression analysis, PVR was associated with a significant higher rate of IE (HR, 2.9; 95% CI, 1.3–6.9), but a nonsignificant difference in rate of death (HR, 1.0; 95% CI, 0.6–1.6).
DISCUSSION
In this nationwide study, we examined the long‐term risk of IE in a birth cohort of Danish residents diagnosed with ToF during a 42‐year follow‐up period from 1977 to 2018. The study yielded the following 3 major findings: First, the risk of IE among patients with ToF was substantial and significantly higher than that of age‐ and sex‐matched controls from the background population. Second, among patients with ToF, the absolute risk of IE from day of birth and during follow‐up was 3.5%. Third, among ToF‐patients, PVR was associated with a higher risk of IE.
Incidence of IE
It is well known that patients with CHD are at high risk of IE. 9 ToF is one of the most common complex CHDs with survival to adulthood. With an increased life expectancy of patients with ToF, the cumulative lifetime risk of IE is expected to increase as well. A few studies have examined the risk of IE among patients with ToF; however, data are sparse and limited by lack of long‐term follow‐up and small number of patients. Moller and Anderson evaluated case records from children with cardiac malformations, including 86 patients with ToF during a study period of 11 years. 7 In total, 10 patients with ToF experienced IE corresponding to a risk of 11.6% during a mean follow‐up time of 30 years. Further, Morris et al 8 examined the risk of IE following primary surgery for CHD including 497 patients with ToF and found a 1.3% risk of IE among patients with ToF 25 years after surgery. Finally, Dennis et al 13 followed 168 patients with ToF during a mean follow‐up period of 8 years and reported that 8 (5%) experienced IE. Thus, the risk of IE among patients with ToF has been found to be high, but also highly varying between studies. Possible explanations for this discrepancy are differences in timing and duration of the follow‐up period, differences in surgical and interventional procedures, and differences in the number of patients undergoing PVR.
As patients with ToF are born with a high risk of IE because of the nature of their CHD—the exact pathophysiological mechanism remains speculative—it is paramount to examine the risk of IE from the day of birth. To the best of our knowledge, no prior studies have examined the risk of IE since birth nor in a follow‐up period as long as up to 42 years on a national scale; this study, therefore, adds valuable information. Also, no prior studies have compared the risk of IE in patients with ToF with matched controls from the background population.
In our study, 3.5% of patients with ToF had IE during a median follow‐up time of 15.5 years, whereas <0.1% of the age‐ and sex‐matched controls developed IE. This substantially increased risk could be attributable to the multiple surgical and invasive procedures, including pulmonary valve replacement (PVR), which changes the underlying substrates predisposing to endocardial infection. Moreover, the high prevalence of associated syndromes including DiGeorge syndrome and multiple cardiac malformations in patients with ToF may contribute to the substantial risk of IE. Furthermore, patients who undergo neonatal thymectomy attributable to cardiac surgery are also at increased risk of infections similar to the clinical features observed in DiGeorge syndrome. 25 It is important to emphasize that these patients have an increased risk of IE from the day of birth and that the cumulative risk is proportionately increasing with increasing age. In our study, patients with ToF are followed from the day of birth in follow‐up period up to 42 years and the risk of IE is substantial, but the actual lifetime risk of IE may be even higher if the follow‐up period was even longer. Although the exact pathophysiology underlying this considerable increased risk remains to be explored, these findings underline the importance of appropriate management and follow‐up on patients with ToF as well as preventive efforts.
Pulmonary Valve Replacement
Identifying patients with ToF at particular high risk of IE may have important implications for purposes of prevention. With increasing pulmonary regurgitation or stenosis, PVR is indicated on the basis of symptoms, arrhythmia as well as the size and function of the right ventricle to prevent adverse outcomes. Although PVR relieves the right ventricle from the volume overload, the presence of prosthetic heart valves is known to be a high‐risk factor for IE. It is therefore likely that PVR may confer a higher risk of IE. In line with this, we found an almost 3 times higher risk of IE among patients with versus without PVR. Similar results have previously been found by Dobbels et al, 26 who found an increased risk of IE among patients with versus without PVR in 273 patients with ToF. Whether this increased risk of IE is attributable to the presence of prosthetic heart valves or to the fact that these patients are more prone to IE remains to be investigated. In a recent study, Gröning et al 27 investigated the impact of the conduit type on the incidence of IE and found that bovine pulmonary conduits were more prone to IE, with Melody valves being the most frequently affected type of conduit. In conclusion, patients with ToF with PVR represent a subgroup of patients in further increased risk of IE. These findings warrant intensified control and surveillance as well as implementation of relevant precautionary measures and intensified patient education to increase the quality of life among patients with ToF with increasing life expectancy.
Strengths and Limitations
The main strength of this study is the completeness of data in a large nationwide cohort of all patients diagnosed with ToF in a 42‐year follow‐up period. The Danish health care system, funded by taxes, provides equal access to health care services for all residents regardless of socioeconomic or insurance status. However, the findings in this study should be viewed in the context of a number of limitations. Data on procedural and surgical characteristics and complications in relation to these procedures were not available. The accuracy of the data relies upon the coding in nationwide administrative registries, though these have been validated previously. 23 , 28 Patients with ToF were identified through the Danish National Patient Registry, which is based on ICD and NCSP codes and was validated with a positive predictive value of 98%. This entails a possible lesser misclassification bias, but with highly significant results this is presumably of low impact. Moreover, the diagnosis of IE and prosthetic valve IE have previously been validated with high positive predictive values. 23 Unfortunately, the diagnosis of DiGeorge syndrome has not been validated, and no data exist before 1994. We were not able to differentiate between the location of IE (ie, prosthetic valve, native valve, or cardiac implantable electronic devices). In addition, data on microbiology, use of antibiotic therapy, echocardiographic findings, and other imaging modalities (eg, computed tomography) in patients with IE were not available. Further, we were not able to compare surgical versus transcatheter PVR meaningfully because of few patients in the transcatheter group and thus too little power. The observational nature of this study precludes the assessment of cause‐effect relationships, and the influence of potential confounders and thus residual confounding cannot be omitted despite adjustment for potential confounders in the Cox regression models. Patients with ToF are significantly different from the background population with respect to concomitant congenital malformations and comorbidities. Thus, the substantially increased risk of IE could be a result of multiple associated anomalies and comorbidities among patients with ToF but presumably with the cardiac tetralogy of paramount importance.
CONCLUSIONS
The long‐term incidence of IE among patients with ToF is substantial and significantly higher compared with the background population. In particular, this IE incidence was high among patients who underwent PVR. With an increasing life expectancy of these patients, intensified control and surveillance as well as implementation of relevant precautionary measures are advisable to increase the quality of life among these patients.
Sources of Funding
None.
Disclosures
Dr Gislason reports research grants from Bristol‐Myers Squibb, Pfizer, and Boehringer Ingelheim. Dr Torp‐Pedersen reports a grant for randomized study from Bayer and a grant for epidemiological study from Novo Nordisk. Dr Køber serves as a consultant for Boehringer Ingelheim and has received other support from AstraZeneca, Novartis, and Novo Nordisk. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S2
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022445
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Østergaard L, Valeur N, Bundgaard H, Butt JH, Ihlemann N, Køber L, Fosbøl EL. Temporal changes in infective endocarditis guidelines during the last 12 years: high‐level evidence needed. Am Heart J. 2017;193:70–75. doi: 10.1016/j.ahj.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 2. Erichsen P, Gislason GH, Bruun NE. The increasing incidence of infective endocarditis in Denmark, 1994–2011. Eur J Intern Med. 2016;35:95–99. doi: 10.1016/j.ejim.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 3. Moreillon P, Que Y‐A. Infective endocarditis. Lancet. 2004;363:139–149. doi: 10.1016/S0140-6736(03)15266-X [DOI] [PubMed] [Google Scholar]
- 4. Butt JH, Kragholm K, Dalager‐Pedersen M, Rørth R, Kristensen SL, Chaudry MS, Valeur N, Østergaard L, Torp‐Pedersen C, Gislason GH, et al. Return to the workforce following infective endocarditis—a nationwide cohort study. Am Heart J. 2018;195:130–138. doi: 10.1016/j.ahj.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 5. Ferro JM, Fonseca AC. Infective endocarditis. Handb Clin Neurol. 2014;119:75–91. doi: 10.1016/B978-0-7020-4086-3.00007-2 [DOI] [PubMed] [Google Scholar]
- 6. Havers‐Borgersen E, Fosbøl EL, Rørth R, Kragholm K, Kristensen SL, Bundgaard H, Bruun NE, Østergaard L, Aslam M, Valeur N, et al. Nursing home admission and initiation of domiciliary care following infective endocarditis. Glob Heart. 2019;14:41–46.e2. doi: 10.1016/j.gheart.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 7. Moller JH, Anderson RC. 1,000 consecutive children with a cardiac malformation with 26‐ to 37‐year follow‐up. Am J Cardiol. 1992;70:661–667. doi: 10.1016/0002-9149(92)90209-H [DOI] [PubMed] [Google Scholar]
- 8. Morris CD, Reller MD, Menashe VD. Thirty‐year incidence of infective endocarditis after surgery for congenital heart defect. J Am Med Assoc. 1998;279:599–603. doi: 10.1001/jama.279.8.599 [DOI] [PubMed] [Google Scholar]
- 9. Østergaard L, Valeur N, Ihlemann N, Bundgaard H, Gislason G, Torp‐Pedersen C, Bruun NE, Søndergaard L, Køber L, Fosbøl EL. Incidence of infective endocarditis among patients considered at high risk. Eur Heart J. 2018;39:623–629. doi: 10.1093/eurheartj/ehx682 [DOI] [PubMed] [Google Scholar]
- 10. Østergaard L, Valeur N, Wang A, Bundgaard H, Aslam M, Gislason G, Torp‐Pedersen C, Bruun NE, Søndergaard L, Køber L, et al. Incidence of infective endocarditis in patients considered at moderate risk. Eur Heart J. 2019;40:1355–1361. doi: 10.1093/eurheartj/ehy629 [DOI] [PubMed] [Google Scholar]
- 11. Choudhury A, Narula J, Jain P, Kapoor P. Infective endocarditis of main pulmonary artery in tetralogy of Fallot: “transesophageal echocardiography adds lease of life”. Ann Card Anaesth. 2016;19:551. doi: 10.4103/0971-9784.185563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madsen NL, Marino BS, Woo JG, Thomsen RW, Videbœk J, Laursen HB, Olsen M. Congenital heart disease with and without cyanotic potential and the long‐term risk of diabetes mellitus: a population‐based follow‐up study. J Am Heart Assoc. 2016;5:e003076. doi: 10.1161/JAHA.115.003076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dennis M, Moore B, Kotchetkova I, Pressley L, Cordina R, Celermajer DS. Adults with repaired tetralogy: low mortality but high morbidity up to middle age. Open Heart. 2017;4:1–6. doi: 10.1136/openhrt-2016-000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiu S‐N, Wang J‐K, Chen H‐C, Lin M‐T, Wu E‐T, Chen C‐A, Huang S‐C, Chang C‐I, Chen Y‐S, Chiu I‐S, et al. Long‐term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–125. doi: 10.1161/CIRCOUTCOMES.111.963603 [DOI] [PubMed] [Google Scholar]
- 15. Cuypers JAAE, Menting ME, Konings EEM, Opić P, Utens EMWJ, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, et al. Unnatural history of tetralogy of Fallot. Circulation. 2014;130:1944–1953. doi: 10.1161/CIRCULATIONAHA.114.009454 [DOI] [PubMed] [Google Scholar]
- 16. Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8 [DOI] [PubMed] [Google Scholar]
- 17. Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG, Webb GD, McCrindle BW. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009;35:156–164. doi: 10.1016/j.ejcts.2008.06.050 [DOI] [PubMed] [Google Scholar]
- 18. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 19. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 20. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 21. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103–105. doi: 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- 22. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39:91–94. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 23. Østergaard L, Adelborg K, Sundbøll J, Pedersen L, Fosbøl EL, Schmidt M. Positive predictive value of infective endocarditis in the Danish National Patient Registry: a validation study. BMJ Open. 2018;6:e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin G, So Y, Johnston G, Nc C. Analyzing survival data with competing risks using SAS ® software. SAS Glob Forum. 2012;344:1–8. [Google Scholar]
- 25. Sullivan PG, Wallach JD, Ioannidis JPA. Meta‐analysis comparing established risk prediction models (EuroSCORE II, STS Score, and ACEF Score) for perioperative mortality during cardiac surgery. Am J Cardiol. 2016;118:1574–1582. doi: 10.1016/j.amjcard.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 26. Dobbels B, Herregods M‐C, Troost E, Van De Bruaene A, Rega F, Budts W, De Meester P. Early versus late pulmonary valve replacement in patients with transannular patch‐repaired tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2017;25:427–433. doi: 10.1093/icvts/ivx118 [DOI] [PubMed] [Google Scholar]
- 27. Gröning M, Tahri NB, Søndergaard L, Helvind M, Ersbøll MK, Ørbæk AH. Infective endocarditis in right ventricular outflow tract conduits: a register‐based comparison of homografts, Contegra grafts and Melody transcatheter valves. Eur J Cardiothorac Surg. 2019;0:1–7. doi: 10.1093/ejcts/ezy478 [DOI] [PubMed] [Google Scholar]
- 28. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S2


