Abstract
The anomalous aortic origin of the right coronary artery (AAORCA) from the left sinus is a congenital anomaly affecting both the origin and course of the right coronary artery. AAORCA is nowadays easily and increasingly recognized by several cardiac imaging modalities. In most cases, patients remain asymptomatic; however, in some, and especially in young athletes, symptoms start to appear following exertion. A literature review was conducted on the surgical management of AAORCA by searching the Pubmed and Google Scholar databases. The inclusion criteria included manuscripts reporting surgical outcomes of AAORCA for ≥1 of the 3 techniques of interest (unroofing, reimplantation, and coronary artery bypass grafting) and manuscripts written in English and that were published between 2010 and 2020. The surgical management of AAORCA can be done through several techniques, most commonly the unroofing of the intramural segment of the AAORCA, the reimplantation of the native right coronary artery onto the right sinus of the aortic root, and coronary artery bypass grafting with either arterial or venous graft conduits with or without ligation of the proximal right coronary artery. Superiority of one surgical technique has not yet been formally proven because of the rare nature of this condition and the lack of any prospective randomized controlled trial or robust prospective observational studies.
Keywords: anomalous right coronary artery from left sinus, congenital heart disease, coronary artery bypass graft surgery, RCA translocation and re‐implantation, unroofing
Subject Categories: Vascular Disease, Coronary Artery Disease, Cardiovascular Surgery, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- AAOCA
anomalous aortic origin of the coronary arteries
- AAORCA
anomalous aortic origin of the right coronary artery
- CTA
computed tomography angiography
- POD
postoperative day
- SCD
sudden cardiac death
Anomalous aortic origin of the coronary arteries (AAOCA) is a congenital anomaly with a prevalence of 0.1% to 0.3%, defined by an abnormal origin, course, number, or size of either the left or the right coronary artery (RCA). 1 Although most patients are asymptomatic, AAOCA has been associated with increased risks of sudden cardiac death (SCD), myocardial ischemia, syncope, and arrhythmias. 2 This risk is most pronounced in young competitive athletes. 3 In this population, surgical repair is recommended, even if the patient is asymptomatic, especially when the coronary artery takes an intramural course. Anomalous aortic origin of the right coronary artery (AAORCA) was reported to be more common than its left‐sided counterpart, with an estimated incidence of 0.1% to 0.9%. 4
Many surgical techniques have been employed for the treatment of AAORCA, with unroofing, coronary artery bypass grafting (CABG), and RCA translocation and reimplantation being the most used procedures. Surgical unroofing has emerged as the surgical procedure of choice for treatment of AAOCA, followed by CABG of the anomalous segment when unroofing is not possible (Figure 1). However, these techniques present many limitations, which prompted the emergence of the RCA translocation and reimplantation technique to address the challenges with unroofing and CABG (Figure 2).
Figure 1. Unroofing and CABG techniques.
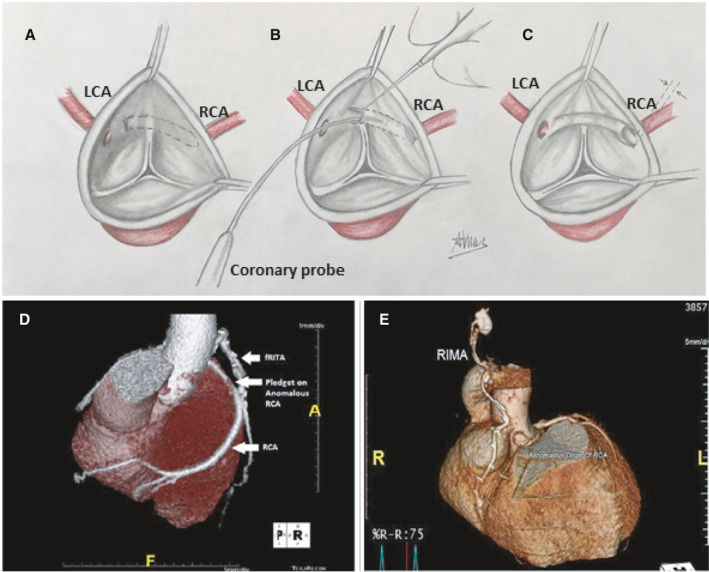
A, Surgical view of an anomalous right coronary artery originating from the left coronary sinus with an intramural segment. B, Unroofing procedure during which the intramural segment is released. C, Final result after unroofing. D and E, Coronary artery bypass grafting of the anomalous right coronary artery with a free (D) and in situ (E) right internal mammary artery. A, anterior; fRITA, Free right internal thoracic artery; L, left; LCA, left coronary artery; R, right; RCA, right coronary artery; RIMA, right internal mammary artery. D, was reprinted with permission from Gaudino et al. 26 Copyright ©2020, Elsevier. E, was reprinted with permission from Ibraheem et al. 24 Copyright ©2019, Blackwell Publishing, Inc.
Figure 2. Right coronary artery translocation and reimplantation technique.
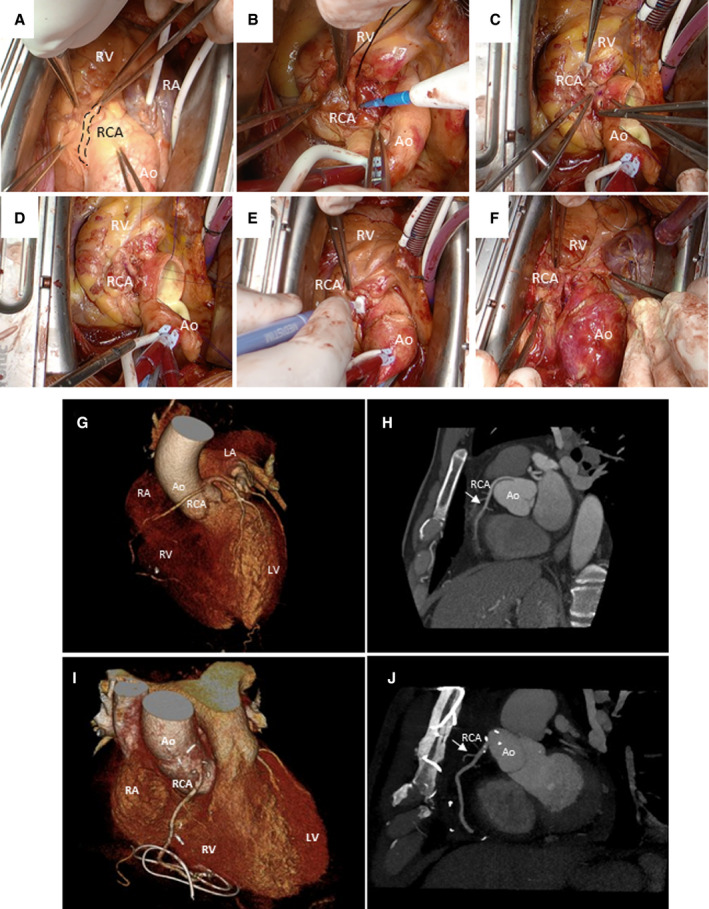
A, Identification of the RCA showing its anomalous origin and trajectory (dashed line). It originates in the left coronary sinus and courses anteriorly between the aorta and pulmonary arteries. B, Completed mobilization of the RCA over ≈1.5 to 2 cm. C, Orientation of RCA anastomosis with heel directed toward the atrioventricular groove. D, Completed anastomosis seen from the epicardial. E, Doppler flow measurement with cardioplegia administration. F, Evaluation of anastomoses off cardiopulmonary bypass. G, Preoperative CT angiogram showing a 3‐dimensional reconstruction of an anomalous RCA origination from the left coronary sinus. H, Sagittal view of a preoperative cardiac CT demonstrating the abnormal course (arrow) of the RCA exiting from the left sinus. I, Postoperative CT angiogram showing a 3‐dimensional reconstruction of the reimplanted RCA. J, Sagittal view of a postoperative cardiac CT demonstrating the corrected course (arrow) of the anomalous RCA. Ao indicates aorta; CT, computed tomography; LA, left atrium; LV, left ventricle; RA, right atrium; RCA, right coronary artery; and RV, right ventricle. A through F, were reprinted with permission from by Grau et al. 57 Copyright ©2021, Elsevier.
This review will focus on AAORCA arising from the left sinus of Valsalva that take an intramural course between the aorta and pulmonary artery, with 2 main objectives: (1) reviewing the literature on AAORCA pathophysiology, clinical presentation, and diagnosis; and (2) comparing the most common surgical approaches employed, highlighting their differences in surgical technique and postoperative outcomes.
Methods
We performed a literature review on the surgical management of AAORCA by searching the PubMed and Google Scholar databases. Our inclusion criteria were the following: (1) The manuscript must report surgical outcomes of AAORCA for ≥1 of the 3 techniques of interest (unroofing, reimplantation, and CABG); (2) it must be written in English; and (3) it must have been published between 2010 and 2020. Two different authors (K.R. and J.H.) reviewed the manuscripts independently. The reference lists of each eligible manuscript were scanned for additional publications that met our inclusion criteria but that were not identified on our initial search.
Results
A total of 30 manuscripts were included in our review. Of those publications, 11, 12, and 14 papers reported clinical outcomes for the unroofing, CABG, and reimplantation techniques, respectively. The unroofing manuscripts collectively describe the outcomes of 209 patients with AAORCA (Table 1). The mean age for this cohort was 23.4 years (range, 1 week to 70 years; median, 16 years). There was no 30‐day mortality among the unroofing group. For the CABG technique, the total number of patients reported was 38 (Table 2). Their mean age was 44.5 years (range, 18–75 years; median, 39 years). There was 1 early and 1 late postoperative mortality reported. As for the reimplantation group, there was a total of 78 patients, with a mean age of 29.0 years (range, 4–72 years; median, 22 years; Table 3). There were no cardiac deaths among the reimplantation group.
Table 1.
Outcomes of the Unroofing Procedure for AAORCA in Cohorts Reported Between 2010 and 2020
| First author, PubMed ID | Year | Cohort size, n | Mean age, y (range) | Mean follow‐up, mo (range) | Mean cross‐clamp time/mean CPB time, min | Mortality, n | Complications | Comments |
|---|---|---|---|---|---|---|---|---|
| Frommelt, 21439578 5 | 2011 | 20* | 12 (4–16) | 22 (0–98) | NR | 0 | 3 patients had aborted SCD | … |
| Mumtaz, 21353004 6 | 2011 | 15* | 15 (5–54) | 17 (1–63) | 53/80 | 0 | … | … |
| Sharma, 25038010 7 | 2014 | 69* | 39.6 (13–70) | 18 (1–84) | 33/43 | 0 | 2 patients required CABG (RIMA to RCA) because of flow acceleration at the RCA ostium | 2 patients previously had CABG (with RIMA) but were reoperated for persistent symptoms |
| Habibi, 24403368 8 | 2014 | 1 | 49 | 45 | NR | 0 | 1 patient had nonsustained VT | … |
| Cho, 24911900 9 | 2015 | 3* | 36.3 (23–46) | 43 (38–49) | 66/89 | 0 | … | … |
| Dekel, 26050848 10 | 2015 | 3 | 32.6 (17–49) | NR | NR | 0 | … | 2/3 patients had concomitant reimplantation |
| Hirono, 26908358 11 | 2016 | 3 | 14.3 (13–15) | 36 | NR | 0 | … | |
| Mainwaring, 27142404 12 | 2016 | 63* | 15 (0–65) | 72 (1–168) | NR | 0 | 2 patients required reoperation for persistent symptoms: 1 underwent reoperation with RCA reimplantation, and 1 had myocardial bridge release | … |
| Palmieri, 29146296 13 | 2018 | 2 | 12.5 (12–13) | 4.5 (3–6) | NR | 0 | … | All patients were athletes |
| Mery, 29074047 14 | 2018 | 29* | 13 (8–18) | 24 (0.75–48) | 77/115 | 0 | 2 patients with residual persistent symptoms | … |
| Pradhan, 32321432 15 | 2020 | 1 | 52 | 10 | NR | 0 | … |
AAORCA indicates anomalous aortic origin of the right coronary artery; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; NR, not reported; RCA, right coronary artery; RIMA, right internal mammary artery; SCD, sudden cardiac death; and VT, ventricular tachycardia.
Subgroup from a larger cohort.
Table 2.
Outcomes of Coronary Artery Bypass Grafting for AAORCA in Cohorts Reported Between 2010 and 2020
| First author, PubMed ID | Year | Cohort size, n | Mean age, y (range) | Mean follow‐up, mo (range) | Mean cross‐clamp time/mean CPB time, min | Mortality, n | Complications | Proximal RCA ligation Yes/No | Type of graft | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Yanagawa, 21039851 16 | 2011 | 1 | 27 | 3 | NR | 0 | … | NR | SVG | Cardioverter‐defibrillator was implanted |
| Reddy, 21795060 17 | 2012 | 4 | 55 (50–61) | 14 (5–37) | NR | 0 | 1 hemothorax requiring thoracoscopic drainage; 1 conversion to sternotomy | Yes | RIMA | Through right anterior mini‐thoracotomy, off‐pump |
| Balghith, 24174845 18 | 2013 | 1 | 75 | 60 | NR | 0 | … | NR | SVG | Patient had CABG×4 (LIMA to LAD, SVG to RCA, SVG to OM1 to OM2) |
| Izgi, 25484559 19 | 2014 | 1 | 25 | 2 | NR | 0 | … | Yes | RIMA | Robotic CABG through mini‐thoracotomy |
| Heo, 24450442 20 | 2014 | 2* | 52.5 (44–61) | 13 (9–17) | NR | 0 | … |
1 Yes 1 No |
1 RGEA | |
| Cho, 24911900 9 | 2015 | 4* | 66.75 (62–74) | 39.75 (32–45) | N/A (Off‐pump) | 0 | … | NR |
2 RIMA 2 RA |
Off‐pump CABG |
| Fuglsang, 26255002 21 | 2015 | 1 | 39 | 1.5 | NR | 0 | … | NR | RIMA | … |
| Cronin, 26961556 22 | 2016 | 1 | 18 | 2 | NR | 0 | … | NR | RIMA | … |
| Refatllari, 27275346 23 | 2016 | 1 | 59 | NR | NR | 0 | … | NR | SVG | Concomitant MVR. |
| Palmieri, 29146296 13 | 2018 | 1* | 25 | 15 | NR | 0 | … | NR | RIMA | … |
| Ibraheem, 31475409 24 | 2019 | 16 | 35 (30–45) | 63.4 (0–108) | NR | 2 |
1 patient died on POD4 from severe heart failure; 1 patient had RCA graft occlusion at 1‐year follow‐up (he was reoperated 1 year later for persistent symptoms but died from severe RV failure); 1 patient had persistent symptoms at follow‐up and was treated medically. |
14 Yes 2 No |
RIMA | Off‐pump CABG for all pts. The 2 patients who passed away were the patients who did not have RCA ligation |
| Saleem, 32310847 25 | 2020 | 5* | 54 (38–72) | 66 (28–100) | NR | 0 |
1 pericardial tamponade; 1 postpericardiotomy syndrome |
Yes |
2 SVG 3 RIMA |
… |
| Gaudino, 31987826 26 | 2020 | 3 | 13.5 | 66 | 0 | … | Yes | RIMA |
AAORCA indicates anomalous aortic origin of the right coronary artery; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; LIMA, left internal mammary artery; MVR, mitral valve replacement; N/A, not applicable; NR, not reported; OM, obtuse marginal; POD, postoperative day; RA, radial artery; RCA, right coronary artery; RGEA, right gastroepiploic artery; RIMA, right internal mammary artery; RV, right ventricle; and SVG, saphenous vein graft.
Subgroup from a larger cohort.
Table 3.
Outcomes of RCA Reimplantation for AAORCA in Cohorts Reported Between 2010 and 2020
| First author, PubMed ID | Year | Cohort size, n | Mean age, y (range) | Mean follow‐up, mo (range) | Mean cross‐clamp time/mean CPB time, min | Mortality, n | Complications | Comments |
|---|---|---|---|---|---|---|---|---|
| Goda, 21606050 27 | 2011 | 4 | 30 (13–38) | 5.3 (1.6–7.5) | 34/55 | 0 | … | On‐pump dissection with aortotomy |
| Izumi, 25087812 28 | 2014 | 2 | 35.5 (22–49) | NR | 25/98 | 0 | … | No aortotomy |
| Mainwaring, 27142404 12 | 2016 | 9* | 15 | 72 (1–168) | NR | 0 | … | … |
| Law, 27112655 14 | 2016 | 16 | 46.6 (17–70) | 60.5 (12–132) | NR | 1 (malignancy) |
1 SVG bypass because of failure to wean from CPB; 1 patient remained symptomatic |
… |
| Cubero, 28520537 29 | 2017 | 13 | 39 (11–72) | 65 (upper limit: 144) | 32/53 | 0 | 1 PCI because of concomitant atherosclerotic CAD | … |
| Saleem, 32310847 25 | 2020 | 2* | 28.5 (19–38) | 20.5 (11–30) | NR | 0 |
1 pericardial tamponade; 1 postpericardiotomy syndrome |
… |
| Gaillard, 32572445 30 | 2020 | 18* | 14 (4–49) | 38 (1–180) | 47/60 | 0 |
1 PCI because of hypoplastic RCA followed by CABG at 2 years because of in‐stent stenosis; 1 patient remained symptomatic |
13 of the patients had concomitant ostial enlargement |
| Mery, 29074047 14 | 2018 | 6* | 13 (8–18) | 24 (0.75–48) | 77/115 | 0 |
1 patient required CABG (SVG) to RCA on POD1 because of ischemia; 1 patient with nonspecific chest pain |
… |
| Jo, 22163129 31 | 2011 | 1 | 24 | 24 | NR | 0 | … | |
| Han, 22517666 32 | 2013 | 1 | 21 | 8 | NR | 0 | Acute LCx coronary artery thrombosis followed by proximal LAD thrombosis 3 mo later | |
| Jadoon, 23257642 33 | 2012 | 1 | 48 | 12 | 73/100 | 0 | Failure to wean from CPB requiring RVAD, IABP and a prophylactic RIMA to RCA graft | RV failure was likely caused by poor myocardial protection |
| Inoue, 24296224 34 | 2013 | 2 | 62 (61–63) | 9.5 (4–15) | NR | 0 | … | Under CPB with beating heart |
| Heo, 24450442 20 | 2014 | 1* | 29 | 25 | NR | 0 | … | Under CPB with cardioplegic arrest |
| Dekel, 26050848 10 | 2015 | 2* | 24.5 (17–32) | NR | NR | 0 | … | Patients had concomitant unroofing of the RCA |
AAORCA indicates anomalous aortic origin of the right coronary artery; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CPB, cardiopulmonary bypass; IABP, intra‐aortic balloon pump; LAD, left anterior descending; LCx, left circumflex; NR, not reported; PCI, percutaneous coronary intervention; POD, postoperative day; RA, radial artery, RCA, right coronary artery; RIMA, right internal mammary artery; RV, right ventricle; RVAD, right ventricular assist device; and SVG, saphenous vein graft.
Subgroup from a larger cohort.
In the unroofing cohort, 2 patients required intraoperative CABG with a right internal mammary artery graft because of flow acceleration at the RCA ostium detected on the intraoperative transesophageal echocardiography, after unroofing. 7 Additionally, 2 other patients underwent reoperation because of residual symptoms or radiographic evidence of myocardial ischemia. 12 The first patient was a 17‐year‐old girl with chest pain and reversible ischemia in the RCA territory on functional magnetic resonance imaging (MRI). She subsequently underwent RCA translocation and reimplantation. However, her symptoms did not subside and were thought to be caused by a myocardial bridge that was only discovered after her second operation. The second patient was a 17‐year‐old girl who remained symptomatic after her unroofing procedure. Further investigations revealed a myocardial bridge over her left anterior descending, which was released during a second operation, after which her symptoms were successfully resolved. Two additional patients remained symptomatic with nonspecific chest pain after the unroofing of their abnormal RCA. 14 Moreover, 3 patients had cardiac arrest requiring resuscitation postoperatively, 5 and another patient developed high‐risk ventricular arrhythmias. 8
Among the CABG group, 1 patient developed pericardial tamponade, 25 another patient required thoracoscopic drainage of a large hemothorax, 17 and 1 patient suffered from a postpericardiotomy syndrome. 25 In addition, there were 2 operative deaths in the patients undergoing CABG. 24 The first patient was a 30‐year‐old man with 3‐vessel disease who had undergone an off‐pump CABG (left internal mammary artery to left anterior descending, saphenous vein graft to obtuse marginal artery, and right internal mammary artery to RCA) without ligation of his anomalous RCA. He developed cardiogenic shock and passed away on postoperative day (POD) 4. The second patient was a 35‐year‐old man who underwent a single‐vessel off‐pump CABG with a right internal mammary artery graft to his RCA. His immediate postoperative course was unremarkable; however, his symptoms recurred 1 year later, and he underwent a repeat on‐pump CABG. He passed away on POD2 from severe right ventricular dysfunction.
As for the reimplantation cohort, 2 patients required intraoperative CABG to the RCA attributable to failure to wean from cardiopulmonary bypass. 4 , 33 One of these 2 patients also required insertion of an intra‐aortic balloon pump as well as implantation of a right ventricular assist device. 33 For this patient, the poor right ventricular function was thought to be caused by inadequate myocardial protection because of poor flow of the cardioplegia solution through the anomalous RCA. A third patient required a CABG on POD1 because of severe ischemia, 14 and 2 other patients had percutaneous coronary intervention in the early postoperative period because of concomitant atherosclerotic coronary artery disease (CAD), 29 and to hypoplasia of the reimplanted RCA. 30 Moreover, 1 patient developed pericardial tamponade and another developed postpericardiotomy syndrome. 25 Another patient had acute thrombotic occlusion of his left circumflex artery, which was attributed to inadequate coronary flow reserve and endothelial dysfunction. 32 Interestingly, this patient developed thrombosis of his left anterior descending artery 3 months later. In addition, 3 patients remained symptomatic with chest pain at long‐term follow‐up. 4 , 14 , 30
Discussion
Anatomy and Pathophysiology
In AAORCA, the anomalous RCA can either originate from the pulmonary artery, the ascending aorta, the left ventricle, the left anterior descending artery, the left circumflex artery, the posterior sinus of Valsalva, or from the left sinus of Valsalva. 35 The RCA arising from the left sinus of Valsalva is one of the most common subtypes. A report by Roberts et al 36 in 2005 looking at 10 necropsy cases with patients displaying this phenotype revealed an association between the abnormal RCA arising from the left sinus and SCD. In AAORCA arising from the left coronary sinus, the anomalous artery may originate from a separate ostium or share the orifice with the other coronary artery and, in most cases, takes an intramural course within the wall of the aorta, adjacent to the pulmonary artery (interarterial course). AAORCA can then undergo 3 different courses: (1) a high interarterial course between the aorta and pulmonary artery, (2) a hypoplastic anomalous orifice with shorter interarterial course, or (3) a low interarterial course between the aorta and right ventricular outflow tract. 37 Angina, SCD, and major adverse cardiac events, were found to be significantly higher in patients with a high interarterial course. 37
The underlying mechanisms leading to myocardial ischemia and SCD are still poorly understood in AAORCA. One theory proposes that during exercise, as the aorta expands because of the increased flow, compression of the coronary artery between the aorta and pulmonary arteries can occur, obstructing blood flow. 38 This is observed in cases with or without an intramural course of the RCA. 38 Additionally, an acute angle take‐off and the resulting slitlike orifice, can contribute to the kinking of the coronary artery, and lead to coronary occlusion especially during exertion. 39 Another theory supports coronary vasospasm, which is possibly caused by endothelial injury and ischemia as a consequence of the long distance that the coronary artery has to travel from one side to another. 35 Moreover, it has been suggested that an abnormal origin and course of the coronary arteries renders these conduits more predisposed to premature atherosclerosis development. In fact, it has been shown that coronary atherosclerosis requiring intervention, whether through a percutaneous or surgical revascularization, has been observed in 40% of total AAOCA reported cases. 40
Clinical Presentation and Diagnosis
Patients with coronary anomalies can present with chest pain, arrhythmias, syncope, dyspnea, myocardial infarction, and SCD. However, in most cases, they are asymptomatic, and their diagnosis is commonly made incidentally on echocardiogram, cardiac catheterization, or provocative testing for other cardiovascular diseases. 41 In fact, coronary artery anomalies have been identified in 1% of all routine autopsy examinations; in patients undergoing diagnostic coronary angiography, it is found in 0.6% to 5.6% of the cases. 35 Echocardiography examination of 3504 elite competitive athletes revealed AAOCA in 0.09% of asymptomatic patients. 42 The presence of the anomalous coronary in these young athletes can lead to ventricular arrhythmias, and, if left undiagnosed, can result in SCD. 43 Hence, early patient diagnosis is crucial when myocardial ischemia has been documented through different testing modalities to allow repair and prevent premature death.
The diagnosis of AAOCA is commonly made using transthoracic echocardiography, especially in children, which is an accepted screening modality for this condition. 44 It is a noninvasive procedure that can be performed without patient sedation or use of radiation. It is a rapid and widely available test, with a low cost, that allows clear visualization of the origin of the vessels. It also helps in the visualization of the commonly missed intramural course when color Doppler is employed. 45 However, transthoracic echocardiography presents with some limitations. First, it requires experienced operators for accurate identification of the coronary ostia and proper diagnosis. 46 Second, it has a poor spatial resolution, therefore limiting the possibility of getting a detailed description of the AAOCA features and surrounding structures. 47
The choice of using complementary techniques for AAOCA diagnosis and characterization depends highly on the centers’ availability for equipment and expertise. Computed tomography angiography (CTA) and MRI coronary angiography have been the diagnostic tools of choice, as they provide superior resolution images compared to transthoracic echocardiography. CTA helps identify AAOCA with a high spatial resolution with a rapid scan time. It also enables the detection of CAD. 48 Nonetheless, this technique requires exposure to contrast agents and radiation. On the other hand, MRI was shown to be a great tool that overcomes the CTA limitations regarding radiation and contrast agents. It enables the identification of the AAOCA and their anatomic course, especially in patients previously reported to have a normal angiogram. 49 However, it presents its own limitations of having a lower anatomic resolution than that of CTA and requiring a longer acquisition time at a higher cost. Invasive procedures, such as transesophageal echocardiography, have been employed to better visualize the AAOCA perioperatively. 50 However, transesophageal echocardiography remains a nonroutine procedure, and patients diagnosed through transesophageal echocardiography are still referred for CTA or MRI for higher resolution imaging. Multiple imaging modalities are often required to highlight specific features of the anatomic anomaly and aid in the planning of an individualized surgical approach.
Indications for Surgical Intervention
The AAOCA management strategy, including surgical referral, depends mainly on patient symptoms and presence of high‐risk features in coronary imaging. Surgical intervention in RCA anomalies is a source of controversy, given that patients do not always present with obvious clinical and radiographic evidence of ischemia. 1 , 12 , 14 The American Association of Thoracic Surgery expert consensus guidelines published in 2017 support that individuals with AAOCA with symptoms (ischemic chest pain, syncope secondary to ventricular arrhythmia, or history of aborted SCD) should be activity‐restricted and offered surgery (Class I, Level of Evidence B). 51 In contrast, individuals with AAORCA may participate in competitive sports if they are asymptomatic and have a negative stress test (exercise stress test with additional imaging such as echocardiography and nuclear perfusion imaging), and after counseling regarding their risk of SCD (Class IIa, Level of Evidence C). 51 When surgery is indicated, the repair should aim to eliminate the intramural course and any associated ostial narrowing of the anomalous artery by unroofing, ostioplasty, or reimplantation (Class I, Level of Evidence B). 51
The best management strategy of AAORCA remains a highly controversial topic, especially for asymptomatic patients. 1 , 2 , 12 , 26 In fact, the 2017 American Association of Thoracic Surgery expert consensus guidelines recommend surgical treatment for anomalous left coronary artery and for symptomatic AAORCA with a positive inducible ischemia test. 51 However, even for asymptomatic patients with AAORCA, SCD has been reported. 30 Surgical mortality following AAOCA repair is very rare, which is well depicted in our findings. 52 A recent survey from the Congenital Heart Surgeons Society involving 113 participants reported only 2 deaths following surgical repair (1.77%). 53
Careful patient counseling should therefore be offered to asymptomatic patients with AAORCA, and surgery should be offered to competitive athletes and to patients who prefer surgical management. 30 , 51 Once eligibility for surgery has been established, another source of controversy arises: the choice of the optimal operation. In fact, although several techniques have been described, none of them has been proven to be superior, and they each have their own limitations (Table 4). 4 , 5 , 30 , 51
Table 4.
Summary of the Advantages and Disadvantages of the Unroofing, Coronary Artery Bypass Grafting, and Translocation and Reimplantation Techniques for AAORCA
| Technique | Advantages | Disadvantages |
|---|---|---|
| Unroofing |
|
|
| CABG |
|
|
| Translocation and reimplantation |
|
|
AAOCA indicates anomalous aortic origin of the right coronary artery; AI, aortic insufficiency; CABG, coronary artery bypass grafting; CAD, coronary artery disease; and CPB, cardiopulmonary bypass.
Surgical Management of AAORCA
Unroofing
In 1981, Mustafa et al 54 were the first to report the unroofing technique (Figure 1) as a strategy to repair the intramural segment of an anomalous coronary artery. Unroofing is a procedure that involves performing an aortotomy to free the intramural coronary segment, by making a longitudinal incision just above it, and creating a neo‐ostium. Coronary unroofing remains the most commonly performed procedure for AAOCA, 1 as it is fairly simple and low risk. 14 , 30 The popularity of the unroofing strategy among surgeons is well illustrated by our findings: Of the 325 patients reported in our literature review, 209 (64.3%) underwent this procedure, compared with 38 (11.7%) for CABG and 78 (24.0%) for RCA reimplantation. In addition, patients who had unroofing of their RCA were on average younger than the patients from the 2 other techniques (23.4 years versus 44.5 years for CABG and 29.0 years for RCA reimplantation). The unroofing technique is indeed the easiest procedure to perform in very young and small patients. It is also most suitable for anomalies with a long intramural course, but it is likely inappropriate in patients with a short intramural course. In a recent prospective cohort study by Mery et al, 14 the unroofing procedure was associated with postoperative ischemia in patients with short intramural segments attributable to compression of the coronary artery at the level of the intracoronary pillar, defined as a soft‐tissue structure of variable thickness that extends from the commissure up to the aortic sinotubular junction. 14 This group advocates that the unroofing procedure should be reserved for patients with long intramural segments but that, in patients with short intramural segments, and those in which the anomalous coronary artery crosses the intracoronary pillar, coronary artery transfer and reimplantation is likely a better surgical option.
Unroofing should also be avoided in cases where the anomalous coronary courses below the aortic valve. 14 In fact, when unroofing is performed in the context of a subvalvular intramural course, commissural resuspension is warranted, and even with this additional step, the risk of causing iatrogenic aortic insufficiency persists. 14 , 55 Postoperative aortic regurgitation requiring reoperation for either an aortic valve repair, replacement, or a Ross procedure has been previously reported. 52 , 55 Another downside of the unroofing procedure is that, while correcting the intramural course, it does not address other concomitant anatomic anomalies, such as a slitlike ostium, an acute angle take‐off, and an interarterial course. In fact, several groups have described cohorts of patients who remained symptomatic after an unroofing procedure or patients who, after resolution of their AAORCA symptoms, still had asymptomatic myocardial ischemia detectable when performing a stress test. 4 , 12 , 14 , 56 Although more technically challenging, alternative approaches such as coronary reimplantation in the correct sinus may represent a better long‐term solution when several high‐risk features are present.
Coronary Artery Bypass Grafting
Another proposed surgical strategy for AAORCA is to bypass the anomalous coronary segment with either an arterial or a venous graft (Figure 1). This technique is usually reserved for older patients with concomitant acquired CAD because, in the absence of sufficient coronary artery stenosis, there is a substantial risk of competitive flow from the native vessel, which can in turn lead to early graft failure. A solution to this challenge is to ligate the proximal anomalous coronary artery to prevent native flow altogether. However, coronary ligation is not always well tolerated. This was described by Ibraheem and colleagues in 2 patients who developed intraoperative right ventricular dysfunction following the snaring of their AAORCA. 24 Interestingly, these 2 young patients passed away from operative complications: 1 developed severe heart failure and passed on POD4; 1 had bypass graft failure at 1 year and was reoperated but died from right ventricular failure 2 days later. Some of these complications may be related to the inability of the graft to provide adequate flow in the setting of a normal RCA that has been acutely ligated proximally.
Some advantages of the CABG technique include its avoidance of an aortotomy and the need to manipulate the intercoronary commissure. Moreover, it is the most widely performed cardiac operation, and therefore, all surgeons are well familiar with this procedure. It can also be achieved with or without cardiopulmonary bypass and has been described through minimally invasive approaches. 17 When CABG is performed for AAORCA, it often requires complete occlusion of a healthy RCA distal to the intramural segment in most patients to prevent competitive flow between the native RCA and the bypass graft. This is imperative when arterial grafts are used to bypass these vessels.
Translocation and Reimplantation
RCA translocation with reimplantation involves repositioning the coronary artery orifice to its appropriate anatomic location (Figure 2). It requires mobilization of the native vessel up to the intramural segment and its reimplantation in either the right or the noncoronary sinus of Valsalva or in the anterior ascending aorta. This technique restores normal anatomic and physiological conditions and therefore provides a solution for most morphological anomalies found in AAORCA, including slitlike ostium, acute angle take‐off, and intramural and interarterial course. We have recently published a technique paper with more details on the step‐by‐step surgical procedure. 57 One of the main advantages of reimplantation when compared with other strategies is that it does not require extensive manipulation of the aortic wall, valve, and root. Moreover, RCA translocation with reimplantation uses the same logic as arterial revascularization surgery. In fact, the proximal aorto‐coronary anastomosis is very similar to the one performed in a CABG with an arterial graft. However, unlike in CABG, it uses the best possible conduit for the myocardial perfusion, as long as there is no evidence of atherosclerosis or hypoplasia of the native RCA causing limitation of blood flow.
However, RCA reimplantation remains challenging and has its own limitations. An important consideration when performing reimplantation is the location of the new ostium, to avoid tension on the anastomosis and kinking. 30 A common mistake is to perform insufficient dissection of the anomalous coronary, which in turn limits complete mobilization and translocation of the vessel. Also, this technique is particularly difficult in young patients because of the size of their coronaries, making the aorto‐coronary anastomosis challenging.
Limitations
There are some limitations to our review. First, some of the papers reviewed did not report all the data that we aimed to collect, including intraoperative and follow‐up data. Second, because we have included case reports in our review, there is an incremental risk for publication bias, since cases are usually reported when associated with an adverse event or with unique features. In other words, patients with AORCA who underwent an uneventful surgical repair with 1 of the 3 techniques we assessed may be underrepresented in our cohort. Finally, we decided to study only 3 surgical techniques, although other operations have been described in the literature, such as pulmonary artery translocation. In fact, with the aim of remaining succinct but comprehensive, we chose to focus on only the 3 most commonly performed techniques.
Conclusions
In summary, anomalous RCA can be managed with several surgical techniques, most commonly unroofing, CABG, and RCA translocation and reimplantation. When choosing which operation to perform on a patient, all the anatomic abnormalities should be addressed, and the surgical correction should be individualized to every patient to maximize the chances of success. To this day, superiority of one surgical technique has not yet been formally proven. Given the extremely rare nature of AAOCA, good‐quality randomized controlled trials are difficult to conduct. However, we need further prospective observational data to better assess the long‐term implications of each surgical technique.
Sources of Funding
Lara Gharibeh is the recipient of a Strategic Endowed Fellowship from the University of Ottawa Heart Institute.
Disclosures
None.
Acknowledgments
The authors thank Ms Paloma Segura for her help in drawing panels A through C in Figure 1.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Cheezum MK, Liberthson RR, Shah NR, Villines TC, O’Gara PT, Landzberg MJ, Blankstein R. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69:1592‐1608. doi: 10.1016/j.jacc.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 2. Rahmouni K, Bernier P‐L. Current management of anomalous aortic origin of a coronary artery: a Pan‐Canadian survey. World J Pediat Congenit Heart Surg. 2021;12(3):387–393. doi: 10.1177/2150135121999030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benson PA. Anomalous aortic origin of coronary artery with sudden death: case report and review. Am Heart J. 1970;79:254–257. doi: 10.1016/0002-8703(70)90316-9 [DOI] [PubMed] [Google Scholar]
- 4. Law T, Dunne B, Stamp N, Ho KM, Andrews D. Surgical results and outcomes after reimplantation for the management of anomalous aortic origin of the right coronary artery. Ann Thorac Surg. 2016;102:192–198. doi: 10.1016/j.athoracsur.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 5. Frommelt PC, Sheridan DC, Berger S, Frommelt MA, Tweddell JS. Ten‐year experience with surgical unroofing of anomalous aortic origin of a coronary artery from the opposite sinus with an interarterial course. J Thorac Cardiovasc Surg. 2011;142:1046–1051. doi: 10.1016/j.jtcvs.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 6. Mumtaz MA, Lorber RE, Arruda J, Pettersson GB, Mavroudis C. Surgery for anomalous aortic origin of the coronary artery. Ann Thorac Surg. 2011;91(3):811–815; discussion 814–815. doi: 10.1016/j.athoracsur.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 7. Sharma V, Burkhart HM, Dearani JA, Suri RM, Daly RC, Park SJ, Horner JM, Phillips SD, Schaff HV. Surgical unroofing of anomalous aortic origin of a coronary artery: a single‐center experience. Ann Thorac Surg. 2014;98(3):941–945. doi: 10.1016/j.athoracsur.2014.04.114 [DOI] [PubMed] [Google Scholar]
- 8. Habibi SE, Shafi S, Ali A, Khan R, Kaszala K, Sumption KF, Szentpetery S, Jovin IS. Chest pain and ventricular tachycardia in a patient with surgically corrected anomalous right coronary artery from the left sinus of Valsalva. World J Pediatr Congenit Heart Surg. 2014;5:114–117. doi: 10.1177/2150135113497973 [DOI] [PubMed] [Google Scholar]
- 9. Cho S‐H, Joo H‐C, Yoo K‐J, Youn Y‐N. Anomalous origin of right coronary artery from left coronary sinus: surgical management and clinical result. Thorac Cardiovasc Surg. 2015;63:360–366. [DOI] [PubMed] [Google Scholar]
- 10. Dekel H, Hickey EJ, Wallen J, Caldarone CA. Repair of anomalous aortic origin of coronary arteries with combined unroofing and unflooring technique. J Thorac Cardiovasc Surg. 2015;150:422–424. doi: 10.1016/j.jtcvs.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 11. Hirono K, Hata Y, Miyao N, Nakaoka H, Saito K, Ibuki K, Watanabe K, Ozawa S, Higuma T, Yoshimura N, et al. Anomalous origin of the right coronary artery evaluated with multidetector computed tomography and its clinical relevance. J Cardiol. 2016;68:196–201. doi: 10.1016/j.jjcc.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Mainwaring RD, Murphy DJ, Rogers IS, Chan FP, Petrossian E, Palmon M, Hanley FL. Surgical repair of 115 patients with anomalous aortic origin of a coronary artery from a single institution. World J Pediatr Congenit Heart Surg. 2016;7:353–359. doi: 10.1177/2150135116641892 [DOI] [PubMed] [Google Scholar]
- 13. Palmieri V, Gervasi S, Bianco M, Cogliani R, Poscolieri B, Cuccaro F, Marano R, Mazzari M, Basso C, Zeppilli P. Anomalous origin of coronary arteries from the “wrong” sinus in athletes: diagnosis and management strategies. Int J Cardiol. 2018;252:13–20. doi: 10.1016/j.ijcard.2017.10.117 [DOI] [PubMed] [Google Scholar]
- 14. Mery CM, De León LE, Molossi S, Sexson‐Tejtel SK, Agrawal H, Krishnamurthy R, Masand P, Qureshi AM, McKenzie ED, Fraser CD. Outcomes of surgical intervention for anomalous aortic origin of a coronary artery: a large contemporary prospective cohort study. J Thorac Cardiovasc Surg. 2018;155(1):305–319.e4. doi: 10.1016/j.jtcvs.2017.08.116 [DOI] [PubMed] [Google Scholar]
- 15. Pradhan S, Gresa K, Trappe H‐J. Anomalous right coronary artery with interarterial course depicting an unusual case of an electrical storm: a case presentation. BMC Cardiovasc Disord. 2020;20:192. doi: 10.1186/s12872-020-01486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yanagawa B, Alghamdi AA, Chen RB, Amankwaa A, Verma S. Coronary artery bypass graft for anomalous right coronary artery. J Card Surg. 2011;26:44–46. doi: 10.1111/j.1540-8191.2010.01116.x [DOI] [PubMed] [Google Scholar]
- 17. Reddy RC, Takahashi M, Beckles Dl, Filsoufi F. Anomalous right coronary artery from the left sinus: a minimally invasive approach. Eur J Card‐Thorac Surg. 2012;41:287–290. doi: 10.1016/j.ejcts.2011.06.026 [DOI] [PubMed] [Google Scholar]
- 18. Balghith M. Anomalous origin of the right coronary artery from the proximal left anterior descending artery and a single coronary artery anomaly: three case reports. J Saudi Heart Assoc. 2013;25:43–46. doi: 10.1016/j.jsha.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izgi C, Feray H, Erdem G, Kaya Z. Anomalous origin and interarterial course of right coronary artery associated with angina and proven ischemia. Int J Angiol. 2014;23:271–274. doi: 10.1055/s-0033-1349165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heo W, Min H‐K, Kang DK, Jun HJ, Hwang Y‐H, Lee HC. Three different situations and approaches in the management for anomalous origin of the right coronary artery from the left coronary sinus: case report. J Cardiothorac Surg. 2014;9:21. doi: 10.1186/1749-8090-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuglsang S, Heiberg J, Byg J, Hjortdal VE. Anomalous origin of the right coronary artery with an interarterial course and intramural part. Int J Surg Case Rep. 2015;14:92–94. doi: 10.1016/j.ijscr.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cronin H, Curtin R. Cardiac arrest in an 18‐year‐old man caused by anomalous right coronary artery origin. BMJ Case Rep. 2016;bcr2015213629. doi: 10.1136/bcr-2015-213629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Refatllari A, Likaj E, Dumani S, Hasimi E, Goda A. Surgical treatment of anomalous origin of right coronary artery in a patient with mitral stenosis. Open Access Maced J Med Sci. 2016;4:131–134. doi: 10.3889/oamjms.2016.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibraheem WI, Abass OA, Toema AM, Yehia AM. Coronary artery bypass grafting experience in the setting of an anomalous origin of the right coronary artery from the left sinus of Valsalva: midterm results. J Card Surg. 2019;34:1162–1171. doi: 10.1111/jocs.14234 [DOI] [PubMed] [Google Scholar]
- 25. Saleem S, Syed M, Elzanaty AM, Nazir S, Changal K, Gul S, Sheikh M. Interarterial course of anomalous right coronary artery: role of symptoms and surgical outcomes. Coron Artery Dis. 2020;31:538–544. doi: 10.1097/MCA.0000000000000893 [DOI] [PubMed] [Google Scholar]
- 26. Gaudino M, Robinson NB, Hameed I, Girardi LN. Coronary bypass with the free internal thoracic artery to treat anomalous right coronary artery. Ann Thorac Surg. 2020;109:E371–E373. doi: 10.1016/j.athoracsur.2019.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goda M, Meuris B, Meyns B. Right coronary translocation for anomalous origin of right coronary artery from the left coronary sinus. Interact Cardiovasc Thorac Surg. 2011;13:201–202. doi: 10.1510/icvts.2011.268888 [DOI] [PubMed] [Google Scholar]
- 28. Izumi K, Wilbring M, Stumpf J, Matschke K, Kappert U. Direct reimplantation as an alternative approach for treatment of anomalous aortic origin of the right coronary artery. Ann Thorac Surg. 2014;98:740–742. doi: 10.1016/j.athoracsur.2013.12.075 [DOI] [PubMed] [Google Scholar]
- 29. Cubero A, Crespo A, Hamzeh G, Cortes A, Rivas D, Aramendi JI. Anomalous origin of right coronary artery from left coronary sinus‐13 cases treated with the reimplantation technique. World J Pediatr Congenit Heart Surg. 2017;8:315–320. doi: 10.1177/2150135116688172 [DOI] [PubMed] [Google Scholar]
- 30. Gaillard M, Pontailler M, Danial P, Moreau de Bellaing A, Gaudin R, du Puy‐Montbrun L, Murtuza B, Haydar A, Malekzadeh‐Milani S, Bonnet D, et al. Anomalous aortic origin of coronary arteries: an alternative to the unroofing strategy. Eur J Cardio‐Thorac Surg. 2020;58(5):975–982. doi: 10.1093/ejcts/ezaa129 [DOI] [PubMed] [Google Scholar]
- 31. Jo Y, Uranaka Y, Iwaki H, Matsumoto J, Koura T, Negishi K. Sudden cardiac arrest: associated with anomalous origin of the right coronary artery from the left main coronary artery. Tex Heart Inst J. 2011;38:539–543. [PMC free article] [PubMed] [Google Scholar]
- 32. Han SY, Heitner JF, Brener SJ. Recurrent coronary artery thrombosis after anomalous right coronary artery re‐implantation to the aorta. Catheter Cardiovasc Interv. 2013;82:163–167. doi: 10.1002/ccd.24446 [DOI] [PubMed] [Google Scholar]
- 33. Jadoon MA, Graham ANJ. Alternative myocardial protection strategies are necessary in patients with intramural aortic course of anomalous coronary artery. Case Rep. 2012;2012: bcr2012007448. doi: 10.1136/bcr-2012-007448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inoue Y, Kawajiri H, Suzuki S, Tamura T. Novel beating heart repair for anomalous origin of right coronary artery. Ann Thorac Surg. 2013;96:e141–e143. doi: 10.1016/j.athoracsur.2013.06.124 [DOI] [PubMed] [Google Scholar]
- 35. Yurtdaş M, Gülen O. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Cardiol J. 2012;19:122–129. doi: 10.5603/CJ.2012.0023 [DOI] [PubMed] [Google Scholar]
- 36. Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982;49(4):863–868. doi: 10.1016/0002-9149(82)91970-1 [DOI] [PubMed] [Google Scholar]
- 37. Lee H‐J, Hong YJ, Kim HY, Lee J, Hur J, Choi BW, Chang H‐J, Nam JE, Choe KO, Kim YJ. Anomalous origin of the right coronary artery from the left coronary sinus with an interarterial course: subtypes and clinical importance. Radiology. 2012;262(1):101–108. doi: 10.1148/radiol.11110823 [DOI] [PubMed] [Google Scholar]
- 38. Cheitlin MD, De castro CM, Mcallister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva, a not‐so‐minor congenital anomaly. Circulation. 1974;50:780–787. doi: 10.1161/01.CIR.50.4.780 [DOI] [PubMed] [Google Scholar]
- 39. Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20(3):640–647. doi: 10.1016/0735-1097(92)90019-J [DOI] [PubMed] [Google Scholar]
- 40. Calabrò P, Bianchi R, Palmieri R, Sordelli C, Bigazzi MC, Calabrò R. Evidence of right coronary from mid‐left anterior descending coronary: a rare case of coronary anomalous origin. Eur Heart J. 2009;30(5):565. doi: 10.1093/eurheartj/ehn414 [DOI] [PubMed] [Google Scholar]
- 41. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110 [DOI] [PubMed] [Google Scholar]
- 42. Zeppilli P, dello Russo A, Santini C, Palmieri V, Natale L, Giordano A, Frustaci A. In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest. 1998;114:89–93. doi: 10.1378/chest.114.1.89 [DOI] [PubMed] [Google Scholar]
- 43. Corrado D, Thiene G, Cocco P, Frescura C. Non‐atherosclerotic coronary artery disease and sudden death in the young. Br Heart J. 1992;68:601–607. doi: 10.1136/hrt.68.12.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37(2):593–597. doi: 10.1016/S0735-1097(00)01136-0 [DOI] [PubMed] [Google Scholar]
- 45. Frommelt PC, Berger S, Pelech AN, Bergstrom S, Williamson JG. Prospective identification of anomalous origin of left coronary artery from the right sinus of Valsalva using transthoracic echocardiography: importance of color Doppler flow mapping. Pediatr Cardiol. 2001;22:327–332. doi: 10.1007/s002460010239 [DOI] [PubMed] [Google Scholar]
- 46. Pelliccia A, Spataro A, Maron BJ. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am J Cardiol. 1993;72:978–979. doi: 10.1016/0002-9149(93)91120-7 [DOI] [PubMed] [Google Scholar]
- 47. Brothers JA, Whitehead KK, Keller MS, Fogel MA, Paridon SM, Weinberg PM, Harris MA. Cardiac MRI and CT: differentiation of normal ostium and intraseptal course from slitlike ostium and interarterial course in anomalous left coronary artery in children. Am J Roentgenol. 2015;204:W104–W109. doi: 10.2214/AJR.14.12953 [DOI] [PubMed] [Google Scholar]
- 48. Wang A, Pulsipher MW, Jaggers J, Peterson GE, O'Laughlin MP, Bashore TM, Harrison JK. Simultaneous biplane coronary and pulmonary arteriography: a novel technique for defining the course of an anomalous left main coronary artery originating from the right sinus of Valsalva. Catheter Cardiovasc Diagn. 1997;42:73–78. doi: [DOI] [PubMed] [Google Scholar]
- 49. McConnell MV, Ganz P, Selwyn AP, Li W, Edelman RR, Manning WJ. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation. 1995;92:3158–3162. doi: 10.1161/01.CIR.92.11.3158 [DOI] [PubMed] [Google Scholar]
- 50. Sasson Z, Grande P, Lorette I, Mcewan P. Proximal narrowing of anomalous right coronary artery from the left coronary sinus: delineation by omniplane transesophageal echocardiogram. Can J Cardiol. 1996;12:529–531. [PubMed] [Google Scholar]
- 51. Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD, Tweddell JS. Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153(6):1440–1457. doi: 10.1016/j.jtcvs.2016.06.066 [DOI] [PubMed] [Google Scholar]
- 52. Nees SN, Flyer JN, Chelliah A, Dayton JD, Touchette L, Kalfa D, Chai PJ, Bacha EA, Anderson BR. Patients with anomalous aortic origin of the coronary artery remain at risk after surgical repair. J Thorac Cardiovasc Surg. 2018;155(6):2554–2564.e3. doi: 10.1016/j.jtcvs.2017.12.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brothers J, Gaynor JW, Paridon S, Lorber R, Jacobs M. Anomalous aortic origin of a coronary artery with an interarterial course: understanding current management strategies in children and young adults. Pediatr Cardiol. 2009;30:911–921. doi: 10.1007/s00246-009-9461-y [DOI] [PubMed] [Google Scholar]
- 54. Mustafa I, Gula G, Radley‐Smith R, Durrer S, Yacoub M. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg. 1981;82:297–300. doi: 10.1016/S0022-5223(19)39371-7 [DOI] [PubMed] [Google Scholar]
- 55. Romp RL, Herlong JR, Landolfo CK, Sanders SP, Miller CE, Ungerleider RM, Jaggers J. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg. 2003;76(2):589–596. doi: 10.1016/S0003-4975(03)00436-3 [DOI] [PubMed] [Google Scholar]
- 56. Brothers JA, Mcbride MG, Marino BS, Tomlinson RS, Seliem MA, Pampaloni MH, Gaynor JW, Spray Tl, Paridon SM. Exercise performance and quality of life following surgical repair of anomalous aortic origin of a coronary artery in the pediatric population. J Thorac Cardiovasc Surg. 2009;137:380–384. doi: 10.1016/j.jtcvs.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grau JB, Rahmouni K, Castillo J, Ruel M, Maharajh G. Reimplantation for anomalous right coronary artery. JTCVS Techniques. 2021;7:226–228. doi: 10.1016/j.xjtc.2021.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]


