Summary
Chronic disease self-management is the establishment and maintenance of behaviors needed to be an active participant in one’s health care and experience the best health outcomes. Kidney disease self-management behaviors to slow disease progression include engaging in exercise or physical activity; adhering to a diet low in sodium, potassium, and phosphorus; monitoring laboratory parameters; managing complex medication regimens; coping with disease-related emotional distress; and communicating effectively with providers. Durable behavior change has been difficult to achieve in kidney disease, in part because of an incomplete understanding of the multilevel factors determining chronic disease self-management in this patient group. The biopsychosocial model of chronic illness care posits that an individual’s health outcomes result from biological, psychological, social, and environmental factors as part of a multilevel systems hierarchy. Although this theoretical model has been used to comprehensively identify factors driving self-management in other chronic conditions, it has been applied infrequently to behavioral interventions in kidney disease. In this scoping review, we apply the biopsychosocial model of health to identify individual, interpersonal, and systems-level drivers of kidney disease self-management behaviors. We further highlight factors that may serve as novel, impactful targets of theory-based behavioral interventions to understand and sustain behavior change in kidney disease.
Keywords: Kidney disease self-management behaviors, biopsychosocial
Successful disease self-management requires patients to acquire and maintain the knowledge, skills, and confidence to manage the symptoms, treatments, and psychological consequences of a chronic health condition.1 Supporting effective self-management behaviors is prioritized by patients living with chronic disease as well as by research and policy organizations.2 The Institute of Medicine’s “New Report on Living Well with Chronic Illness” emphasizes self-management programs as a means to foster partnerships between patients, providers, and health systems to empower patients to become active participants in their care.3
As evidenced by both qualitative and quantitative analyses, kidney disease self-management is burdensome. Adults living with kidney disease must adhere to complex dietary restrictions, are prescribed an average of nine daily medications, manage numerous comorbid medical conditions, attend frequent medical visits with multiple providers, and manage emotional distress. This occurs commonly in the context of age-accelerated cognitive and functional decline.4–9 Self-management interventions in kidney disease have shown mixed efficacy on clinically meaningful outcomes, including blood pressure and hemoglobin A1c control, urinary sodium excretion, and health care utilization.10–18
Factors contributing to the unclear benefits of current kidney disease self-management interventions include not being informed by an underlying theoretical model, not recognizing or adapting to biological factors such as cognitive and functional decline, and not accounting for social and environmental drivers of self-management. Thus, facilitating promotion of favorable kidney disease self-management behaviors to reduce disease progression and improve health outcomes across settings has been difficult to achieve. Because Medicare spending related to management of chronic kidney disease (CKD) and kidney failure exceeded $120 billion in 2017, developing effective programs to empower patients to self-manage their disease remains of utmost importance.19 Research in this field calls for a paired personalized and systems-based approach to both general health and kidney disease self-management.20,21 Thus, there is an unmet need to contextualize self-management within an individual’s social and economic environment.22,23
The biopsychosocial model of chronic illness care acknowledges that an individual’s health outcomes result from an intricate blend of biological, psychological, social, and environmental factors as part of a systems hierarchy.24 Although this model has been applied to understand more comprehensively the drivers of chronic disease self-management in chronic conditions such as human immunodeficiency virus and diabetes mellitus, the biopsychosocial model of health has not been used routinely to form the basis of behavioral interventions in kidney disease populations.25,26 In this scoping review, we identify individual, interpersonal, and social/environmental factors associated with disease self-management behaviors in kidney disease populations organized according to the biopsychosocial model. We propose that using this model to identify drivers of self-management will allow for a person-centered, comprehensive understanding of self-management in this patient population, and identify impactful, underinvestigated factors and effect modifiers that need to be addressed as part of interventions to foster durable behavior change in kidney disease.
METHODS
We adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews checklist to conduct this scoping review.27 We conducted an online search from November 2020 to February 2021 using MEDLINE, EMBASE, PsycINFO, PubMed, Ovid, and CINAHL to determine factors associated with any of the following kidney disease self-management behaviors: exercise or physical activity, medication adherence, visit adherence, diet control, emotion management, laboratory self-monitoring, communication with providers, or a self-reported summary scale of any of the aforementioned behaviors. Key words were as follows: “‘self-management’ AND ‘kidney,’” “kidney adherence,” “kidney exercise,” “kidney depressive OR mood,” “kidney medication management,” “kidney diet adherence,” “kidney coping,” and “kidney behaviors.” Studies were considered eligible from any date range and if written in English and conducted among individuals age 18 years or older. Both quantitative and qualitative analyses were included, and studies across all kidney disease subpopulations (CKD, hemodialysis, peritoneal dialysis, and transplantation) were included. We organized determinants of each self-management behavior using a biopsychosocial model and separated results into biological, psychological, and social and environmental factors. We also collected information regarding the investigation’s study design, sample size, type of kidney disease subpopulation examined, self-management behavior or behaviors studied, and effect sizes of each biopsychosocial factor on self-management, if reported.
RESULTS
Tables 1, 2, and 3 summarize the quantitative and qualitative evidence related to individual, interpersonal, and systems-level determinants of kidney disease self-management organized using a biopsychosocial framework. Figure 1 outlines the hypothesized associations between each determinant and kidney disease self-management, and a summary and narrative overview of the findings is described later.
Table 1.
Biological Drivers of Kidney Disease Self-Management Behaviors
| Patient-Reported Self-Management Behavior(s) | Study | Study Population, Sample Size (N), Study Design | Additional Notes and Details of Effect Size if Reported |
|---|---|---|---|
| Age | |||
| Summary of self-management behaviors | Kugler et al, 200533 | Hemodialysis (N = 916) Multicenter cross-sectional; quantitative |
|
| Chan et al, 201040 | Peritoneal dialysis (N = 153) Cross-sectional; quantitative cluster analysis |
Cluster analysis showed two clusters (76 versus 77) of patients with unique self-management profiles More patients in the adherent cluster were older |
|
| Lin et al, 201128 | Kidney transplant (N = 101) Cross-sectional; quantitative |
Standardized β = 0.22 R2 = 0.37 for age, post-kidney transplant time, health care provider support, financial satisfaction |
|
| Chen et al, 201837 | CKD (N = 410) Cross-sectional; quantitative |
Standardized β = 0.22 R2 = 0.52 for age, social support, health literacy, and marital status |
|
| Khezerloo et al, 201936 | Kidney transplant (N = 360) Cross-sectional; quantitative |
Adjusted β = −0.31 (95% CI, −0.39 to −0.24) Adjusted for sex, marital status, educational status, past dialysis type, past dialysis vintage, transplant duration, type of organ (live versus cadaveric) |
|
| Exercise and physical activity | Da Costa Rosa et al, 201738 | Hemodialysis (N = 79) Cross-sectional; quantitative |
OR, 5.8 (95% CI, 1.11–30.19) Participants wore an accelerometer; lower physical activity was associated with older age |
| Vallance et al, 201939 | Kidney transplant (N = 1,284) Cross-sectional; quantitative |
Unstandardized β = −0.48 min/d (95% CI, −0.75 to −0.22) Each additional year older was associated with 0.48 fewer min/d (~30 seconds) of moderate-to-vigorous physical activity |
|
| Medication adherence | Chan et al, 201231 | Hemodialysis (N = 188) Cross-sectional; quantitative |
Standardized β = 0.18 R2 = 0.22 for age and dialysis vintage |
| Scheel et al, 201829 | Kidney transplant (N = 330) Cross-sectional; quantitative |
Standardized β = −0.022 OR, 0.98 (95% CI, 0.96–0.99) |
|
| Fluid adherence | Kugler et al, 200533 | Hemodialysis (N = 916) Multicenter cross-sectional; quantitative |
r = −0.07 for association between age and frequency of fluid nonadherence r = −0.11 for association between age and degree of fluid nonadherence |
| Chan et al, 201231 | Hemodialysis (N = 188) Cross-sectional; quantitative |
Standardized β = 0.21 R2 = 0.39 for age, sex, dialysis vintage, employment |
|
| Mellon et al, 201332 | Hemodialysis (N = 50) Cross-sectional; quantitative |
Standardized β = −0.40 R2 = 0.23 for age, serum phosphorus, physical symptom burden, depressive symptoms, anxious mood |
|
| Clark-Cutaia et al, 201430 | Hemodialysis (N = 122) Cross-sectional; quantitative |
Unstandardized β = −0.94 OR, 0.39 (95% CI, 0.18–0.87) |
|
| Washington et al, 201835 | Hemodialysis (N = 107) Cross-sectional; quantitative |
OR, 1.08 (95% CI, 1.01–1.14) | |
| Diet adherence | Khalil et al, 201134 | Hemodialysis (N = 100) Cross-sectional; quantitative |
Unstandardized β = −0.05 OR, 0.95 (95% CI, 0.92–0.98) Objective dietary nonadherence measured by serum potassium level >5.5 mg/dL, serum phosphorus level >5.5 mg/dL, or blood urea nitrogen level >100 mg/dL |
| Chan et al, 201231 | Hemodialysis (N = 188) Cross-sectional; quantitative |
Standardized β = 0.16 R2 = 0.34 for age, sex, employment |
|
| Clark-Cutaia et al, 201430 | Hemodialysis (N = 122) Cross-sectional; quantitative |
Unstandardized β = −0.93 OR, 0.39 (95% CI, 0.18–0.86) |
|
| Physical fatigue, frailty | |||
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
50% reported fatigue |
| Kontos et al, 200744 | Hemodialysis (N = 17) Cross-sectional; qualitative |
NA | |
| Byrne et al, 201114 | Hemodialysis (N = 78) Cross-sectional; quantitative |
40% reported fatigue | |
| Delgado et al, 201215 | Hemodialysis (N = 100) Cross-sectional; quantitative |
67% reported fatigue on dialysis days; 40% reported fatigue on nondialysis days | |
| Darawad et al, 201317 | Hemodialysis (N = 190) Cross-sectional; quantitative |
84% reported fatigue; did not specify relationship to dialysis treatment | |
| Painter et al, 201447 | Hemodialysis (N = 15 staff; 6 patients) Cross-sectional; qualitative |
NA | |
| Fiaccadori et al, 201418 | Hemodialysis (N = 104) Cross-sectional; quantitative |
57% reported fatigue on dialysis days | |
| Bossola et al, 201416 | Hemodialysis (N = 105) Cross-sectional; quantitative |
67% reported fatigue on dialysis days | |
| Jhamb et al, 201643 | Hemodialysis (N = 16) Cross-sectional; qualitative |
NA | |
| Hornik et al, 201950 | Hemodialysis (N = 72) Prospective (2 wk); quantitative |
OR, 0.28 (95% CI, 0.21–0.69) | |
| Tarca et al, 202051 | Hemodialysis Systematic review and meta-analysis of 21 quantitative studies |
NA | |
| Cognitive impairment | |||
| Medication adherence | Gordon et al, 200756 | Kidney transplant (N = 20) Prospective (1 mo); qualitative |
NA |
| Orr et al, 200757 | Kidney transplant (N = 26) Cross-sectional; qualitative |
NA | |
| Ruppar et al, 200959 | Kidney transplant (N = 19) Cross-sectional; qualitative |
NA | |
| Gordon et al, 200960 | Kidney transplant (N = 82) Cross-sectional; qualitative |
NA | |
| Tielen et al, 201158 | Kidney transplant (N = 26) Cross-sectional; qualitative |
NA | |
| Chan et al, 201231 | Hemodialysis (N = 188) Cross-sectional; quantitative |
81% reported forgetfulness as the primary barrier to medication adherence | |
| Kefale et al, 201855 | CKD (N = 256) Cross-sectional; quantitative |
79% reported forgetfulness as the primary barrier to medication adherence |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; NA, not applicable; OR, odds ratio.
Table 2.
Psychological Drivers of Kidney Disease Self-Management Behaviors
| Patient-Reported Self-Management Behavior(s) | Study | Study Population,Sample Size (N), Study Design | Additional Notes andDetails of Effect Size if Reported |
|---|---|---|---|
| Subjective health literacy | |||
| Summary of self-management behaviors | Chen et al, 201837 | CKD (N = 410) Cross-sectional; quantitative |
Standardized β = 0.26 R2 = 0.50 R2 = 0.52 for health literacy, age, social support, and marital status |
| Medication adherence | Patzer et al, 201662 | Kidney transplant (N = 99) Cross-sectional; quantitative |
OR, 2.93 (95% CI, 1.13–7.56) for association between health literacy and tacrolimus adherence, measured by either self-report or serologic level |
| Demian et al, 201663 | Kidney transplant (N = 96) Cross-sectional; quantitative |
Unstandardized β = −0.31 R2 = 0.08 for health literacy, age, sex, employment, depressive symptoms |
|
| Diet adherence | Thomas et al, 200174 | Hemodialysis (N = 276) Cross-sectional; quantitative |
OR, 1.09 (95% CI, 1.00–1.19) for association between higher dietary knowledge and higher dietary adherence Patients all age >50 |
| Visit adherence | Green et al, 201364 | Hemodialysis (N = 260) Prospective (2y); quantitative (study was part of a larger randomized controlled trial) |
Missed, 0.6% versus 0.3%; adjusted incidence rate ratio, 2.14 (95% CI, 1.10–4.17) adjusted for age, sex, self-reported race, employment, income, comorbid conditions, dialysis vintage in years, dialysis schedule, type of vascular access, dialysis unit, randomization group |
| Perceived knowledge | |||
| Summary of self-management behaviors | Martin et al, 201072 | Kidney transplant (N = 38) Cross-sectional; qualitative |
NA |
| Schmidt-Mohler et al, 201473 | Kidney transplant (N = 12) Cross-sectional; mixed-methods |
NA | |
| Kahn et al, 201569 | CKD (N = 34) Cross-sectional; qualitative |
NA Many participants were uninsured and underinsured |
|
| Baay et al, 201967 | CKD (N = 37) Cross-sectional; qualitative |
NA | |
| Hwang et al, 202068 | CKD (N = 20) Cross-sectional; qualitative |
NA Knowledge related to diet was a barrier to self-management |
|
| Ranahan et al, 202071 | Kidney transplant (N = 20) Cross-sectional; qualitative |
NA | |
| Schrauben et al, 202075 | CKD (N = 401) Cross-sectional; quantitative |
Standardized β = 1.07 (95% CI, 0.50–1.63) adjusted for sex, self-reported race, education, income, estimated glomerular filtration rate, diabetes, urine protein-creatinine ratio, hypertension awareness of CKD diagnosis, body mass index, number of times seen by nephrologist in 1 y | |
| Nair et al, 20217 | CKD (N = 50) Cross-sectional; qualitative |
NA | |
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
16% reported a lack of knowledge regarding benefits of exercise |
| Painter et al, 201447 | Hemodialysis (N = 15 staff; 6 patients) Cross-sectional; qualitative |
NA | |
| Jayaseelan et al, 201876 | Hemodialysis (N = 274) Cross-sectional; quantitative |
30%−50% scored incorrectly on various questions related to benefits of exercise | |
| Medication adherence | Sontakke et al, 201570 | CKD (N = 150) Cross-sectional; qualitative |
68% reported a lack of knowledge related to the importance of medication adherence |
| Dietary adherence | Thomas et al, 200174 | Hemodialysis (N = 276) Cross-sectional; quantitative |
OR, 1.09 (95% CI, 1.00–1.19) for association between higher dietary knowledge and higher dietary adherence patients all age >50 |
| Depressive symptoms | |||
| Summary of self-management behaviors | Sakraida et al, 201679 | CKD (N = 29) Cross-sectional; quantitative |
r = −0.42 for association between depressive symptoms and blood sugar checks, agitation and fruit/vegetable intake (−0.38), sadness and foot-washing or exercise frequency (−0.38, −0.40) r = −0.53 for association between total depressive symptom score and foot checks |
| Xie et al, 201978 | Kidney transplant (N = 483) Cross-sectional; quantitative |
r = −0.16 | |
| Ranahan et al, 202071 | Kidney transplant (N = 20) Cross-sectional; qualitative |
NA | |
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
34% reported depressive symptoms; 26% reported anxious mood |
| Painter et al, 201447 | Hemodialysis (N = 15 staff; 6 patients) Cross-sectional; qualitative |
NA | |
| Fiaccadori et al, 201418 | Hemodialysis (N = 104) Cross-sectional; quantitative |
50% reported sadness OR, 2.59 (95% CI, 1.10–6.09) for physical inactivity adjusted for age and sex |
|
| Medication adherence | Cukor et al, 200977 | Hemodialysis (N = 65) Cross-sectional; quantitative |
Standardized β = −0.28 R2 = 0.51 for depressive symptoms, sex, hospitalizations, education, age, ethnicity, use of dialysis versus transplant |
| Diet adherence | Khalil et al, 201134 | Hemodialysis (N = 100) Cross-sectional; quantitative |
Unstandardized β = 0.84 OR, 2.3 (95% CI, 1.07–4.95) |
| Fluid adherence | Washington et al, 201835 | Hemodialysis (N = 107) Cross-sectional; quantitative |
Unstandardized β = 0.94 OR, 2.6 (95% CI, 1.16–5.7) |
| Spiritual well-being | |||
| Summary of kidney disease self-management behaviors | Ranahan et al, 202071 | Kidney transplant (N = 20) Cross-sectional; qualitative |
NA |
| Nair et al, 20217 | CKD (N = 50) Cross-sectional; qualitative |
NA | |
| Exercise and physical activity | Sieverdes et al, 201582 | Hemodialysis (N = 22) Cross-sectional; qualitative |
OR, 0.82 (95% CI, 0.67–0.99) |
| Self-efficacy | |||
| Summary of self-management behaviors | Wu et al, 201690 | CKD (N = 247) Cross-sectional; quantitative |
r = 0.44 for association between self-efficacy and self-management The association between perceived kidney disease knowledge and self-management was fully mediated by self-efficacy (Z = 4.82) and the effect was 50% |
| Jamieson et al, 201685 | Kidney transplant Systematic review and thematic analysis of 50 qualitative studies |
NA | |
| Donald et al, 20194 | CKD(N = 37 + 15 caregivers) Cross-sectional; qualitative |
NA | |
| Chuang et al, 202189 | Hemodialysis (N = 130) Cross-sectional; quantitative |
Unstandardized β = 0.29 SE, 0.01 | |
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
60% reported a lack of motivation r = −0.31 for association between motivation and exercise r = 0.40 for association between self-efficacy and exercise |
| Medication adherence | Curtin et al, 200886 | CKD (N = 174) Cross-sectional; quantitative |
Unstandardized β = 0.13 (SE, 0.036) R2 = 0.13 for self-efficacy, age, education, hypertension, diabetes, serum creatinine, mental component summary and physical component summary of the 36-item Short Form Survey85 |
| Paterson et al, 201888 | Kidney transplant (N = 211) Cross-sectional; quantitative |
Self-efficacy had direct effects on adherence via structural equation modeling Regression coefficient, 0.93; effect size, 4.59 Adherence was measured via self-report, immunosuppressant level, and pill refills |
|
| Motivation | |||
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
60% reported a lack of motivation r = −0.31 for association between motivation and exercise r = 0.40 for association between self-efficacy and exercise |
| Kontos et al, 200744 | Hemodialysis (N = 17) Cross-sectional; qualitative |
NA | |
| Fiaccadori et al, 201418 | Hemodialysis (N = 104) Cross-sectional; quantitative |
42% reported a lack of motivation | |
| Painter et al, 201447 | Hemodialysis (N = 15 staff; 6 patients) Cross-sectional; qualitative |
NA | |
| Jhamb et al, 201643 | Hemodialysis (N = 16) Cross-sectional; qualitative |
NA | |
| Song et al, 201992 | Hemodialysis (N = 44) Cross-sectional; qualitative |
NA | |
| Liu et al, 202091 | Hemodialysis (N = 10) Cross-sectional; qualitative |
NA | |
| Visit adherence | Goldade et al, 201194 | Kidney transplant (N = 39) Cross-sectional; qualitative |
NA Motivation was a barrier to visit adherence |
| Chenitz et al, 201493 | Hemodialysis (N = 30) Cross-sectional; qualitative |
NA Motivation was a barrier to visit adherence |
|
| Patient activation | |||
| Summary of self-management behaviors | Zimbudzi et al, 201797 | CKD (N = 317) Cross-sectional; quantitative |
Patient activation was associated with a summary of self-management behaviors as well as the specific behaviors of diet and blood sugar checking |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; NA, not applicable; OR, odds ratio.
Table 3.
Social and Environmental Drivers of Kidney Disease Self-Management Behaviors
| Patient-Reported Self-Management Behavior(s) Studied | Study | Study Population, Sample Size (N), Study Design | Additional Notes and Details of Effect Size if Reported |
|---|---|---|---|
| Social support | |||
| Summary of self-management behaviors | Kahn et al, 201569 | CKD (N = 34) Cross-sectional; qualitative |
NA Many participants were uninsured and underinsured |
| Washington et al, 2016106 | Hemodialysis (N = 107) Cross-sectional; mixed-methods |
NA | |
| Chen et al, 201837 | CKD (N = 410) Cross-sectional; quantitative |
Standardized β = 0.59 R2 = 0.52 for social support, age, health literacy, and marital status |
|
| Xie et al, 201978 | Kidney transplant (N = 483) Cross-sectional; quantitative |
r = 0.30 for association between social support and self-management | |
| Exercise and physical activity | Williams et al, 1991103 | Hemodialysis (N = 40) Cross-sectional; quantitative |
75% who engaged in a structured physical activity program reported having encouraging support groups |
| Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
12% reported a lack of social support | |
| Clarke et al, 2015104 | CKD (N = 36) Cross-sectional; qualitative |
NA | |
| Kendrick et al, 2019105 | CKD (N = 41) Cross-sectional; qualitative |
NA Social support from family was a barrier in this study |
|
| Liu et al, 202091 | Hemodialysis (N = 10) Cross-sectional; qualitative |
NA | |
| Medication adherence | Van Camp et al, 2014101 | Hemodialysis (N = 135) Prospective (2 mo); quantitative |
OR, 2.94 (95% CI, 1.23–7.03) for association between living with a partner and phosphate binder adherence |
| Scheel et al, 201829 | Kidney transplant (N = 330) Cross-sectional; quantitative |
Standardized β = −0.035 OR, 0.96 (95% CI, 0.93–1.00) |
|
| Visit adherence | Goldade et al, 201194 | Kidney transplant (N = 39) Cross-sectional; qualitative |
NA Motivation was a barrier to visit adherence |
| Been-Dahmen et al, 2018102 | Kidney transplant (N = 41) Cross-sectional; qualitative |
NA | |
| Fluid control adherence | Christensen et al, 1992100 | Hemodialysis (N = 78) Cross-sectional; quantitative |
Using multivariate analysis of covariance, main effects were found for family support (6.16) on intradialytic weight gain after adjustment for diabetic status |
| Pang et al, 200199 | Hemodialysis (N = 92) Cross-sectional; quantitative |
Unstandardized β = −0.54 R2 = 0.38 for social support, comorbidity, income |
|
| Health care provider support | |||
| Summary of self-management behaviors | Lin et al, 201128 | Kidney transplant (N = 101) Cross-sectional; quantitative |
Standardized β = 0.22 R2 = 0.37 for age, post-kidney transplant time, health care provider support, financial satisfaction |
| Hwang et al, 202068 | CKD (N = 20) Cross-sectional; qualitative |
Therapeutic alliance between patients and providers were facilitators of self-management | |
| Exercise and physical activity | Clarke et al, 2015104 | CKD (N = 36) Cross-sectional; qualitative |
NA |
| Young et al, 2015109 | Hemodialysis (N = 24 patients and 9 dialysis unit staff) Cross-sectional; Qualitative |
NA | |
| Thompson et al, 2016108 | Hemodialysis (N = 25 patients and 11 dialysis unit staff) Cross-sectional; Qualitative |
NA | |
| Visit adherence | Saran et al, 2003110 | Hemodialysis (N = 3,359) Prospective cohort; quantitative |
OR, 0.75 (95% CI, not reported) for association between kidney dietician and lower intradialytic weight gain |
| Fluid control adherence | Saran et al, 2003110 | Hemodialysis (N = 3,359) Prospective cohort; quantitative |
OR, 0.94 (95% CI, not reported) for association between trained staff and fewer skipped treatments |
| Yokoyama et al, 2009111 | Hemodialysis (N = 71) Cross-sectional; quantitative |
OR, 2.51 (95% CI, 0.99–6.34) after adjustments for age, sex, diuretic use, education, dialysis duration, dialysis adequacy (Kt/V), body mass index, diabetes status | |
| Environment | |||
| Summary of self-management behaviors | Lin et al, 201128 | Kidney transplant (N = 101) Cross-sectional; quantitative |
Standardized β = 0.22 R2 = 0.37 for age, post-kidney transplant time, health care provider support, financial satisfaction |
| Exercise and physical activity | Goodman et al, 200446 | Hemodialysis (N = 50) Cross-sectional; quantitative |
50% reported weather-related concerns; 12% reported a lack of sidewalks |
| Kendrick et al, 2019105 | CKD (N = 41) Cross-sectional; qualitative |
NA | |
| Song et al, 201992 | Hemodialysis (N = 44) Cross-sectional; qualitative |
NA | |
| Medication adherence | Kadowaki et al, 2014122 | Kidney transplant (N = 315) Cross-sectional; quantitative |
16% had difficulties continuing oral immunosuppressants due to supply delay and drugs being lost in the tsunami |
| Visit adherence | Chenitz et al, 201493 | Hemodialysis (N = 30) Cross-sectional; qualitative |
NA |
| Brar et al, 2014113 | Hemodialysis (N = 79 centers) Cross-sectional; quantitative |
Providers at 77% of centers (consisting of nephrologists, surgeons, social workers) reported transportation as the primary barrier to dialysis nonadherence | |
| Chan et al, 2014114 | Hemodialysis (182,536) Prospective (5-y); quantitative |
Using a van for transport: OR, 1.21 (95% CI, 1.16–1.25) for missed visit Travel time >17 min: OR, 1.10 (95% CI, 1.07–1.15) for missed visit Snowfall: OR, 2.68 (95% CI, 2.60–2.77) for missed visit |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; NA, not applicable; OR, odds ratio.
Figure 1.
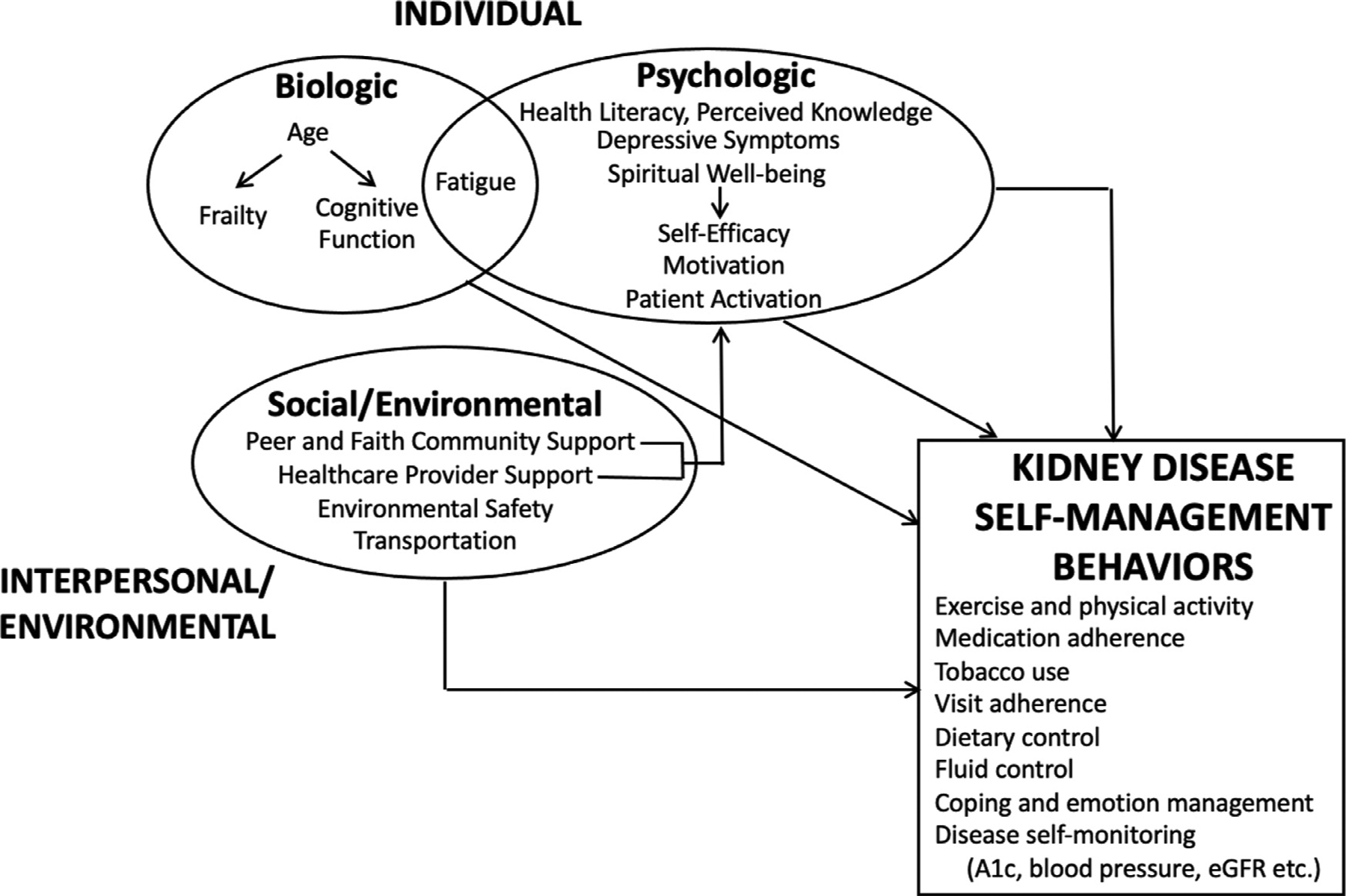
A biopsychosocial model of kidney disease self-management behaviors. Arrows indicate hypothesized associations between constructs. Abbreviation: eGFR, estimated glomerular filtration rate.
BIOLOGICAL DRIVERS OF KIDNEY DISEASE SELF-MANAGEMENT BEHAVIORS
Age
Age is a biological factor that has demonstrated associations with frequency of successful health behaviors in kidney disease. Older adults with kidney disease face significant self-management challenges owing to multi-morbidity and its associated polypharmacy.4–9 Despite this, most evidence supports that older adults engage more frequently and successfully in kidney disease self-management behaviors. In a cross-sectional study of 101 patients in Taiwan who were within 5 years of a kidney transplant, older age had moderate, positive correlations with the frequency of monitoring for transplant rejection, medication adherence, and exercise (r = 0.39, 0.27, and 0.46, respectively).28 According to the investigators, participants’ proximity to receipt of their kidney transplants and higher educational attainment may have partially explained these observations. Similarly, among 410 Taiwanese patients living with CKD, those older than age 65 years (compared with those who were age 44 years) reported an increased frequency of self-reported kidney disease self-management behaviors.29
Just as older adults have been found to engage more frequently in health behaviors in kidney disease, so have younger adults shown a decreased frequency of health engagement, particularly with regard to fluid management in hemodialysis. Among 122 patients across 13 dialysis centers in the northeastern United States, younger participants were found to have a higher interdialytic weight gain as well as a higher median sodium intake.30 Among 188 participants receiving hemodialysis in Malaysia, younger age was associated with self-reported fluid, dietary, and medication nonadherence.31 Similarly, among 50 patients receiving hemodialysis in Ireland, younger age was associated independently with greater intradialytic weight gain after adjustments for depressive symptoms, anxious mood, serum phosphorus, and physical symptom burden.32 Younger age correlated with both the frequency and degree of dietary and fluid nonadherence in a multicenter study across Belgium and Germany.33 Among 100 patients receiving hemodialysis in the United States, every 1-year increase in age increased the likelihood of serologic evidence of kidney diet adherence (measured by increased blood urea nitrogen, potassium, or phosphorus) by 5%.34 Age also was associated with improved adherence to fluid restriction in a study of 107 patients receiving hemodialysis (odds ratio [OR], 1.08; 95% CI, 1.01–1.14).35
A smaller body of evidence supports that age has an inverse relationship with successful health behavior engagement in kidney disease. In a cross-sectional analyses of kidney transplant recipients, older age was associated independently with lower scores on a summary scale of frequency of kidney disease self-management and self-reported immunosuppressant adherence, respectively.36,37 In two studies using wearable devices to measure physical activity, older age was associated with less time spent doing moderate-to-vigorous physical activity among patients who were receiving hemodialysis or who had received a kidney transplant (OR, 5.80; 95% confidence interval [CI], 1.11–30.19; unstandardized β, −0.48 min/d; 95% CI −0.75–0.22, respectively).38,39 Finally, in one of the few studies to examine self-management in peritoneal dialysis, participants who were older reported less overall participation in self-management behaviors.40
The mechanisms that explain the aforementioned age-related differences in the frequency of kidney disease self-management behavior frequency are underinvestigated. Aging in kidney disease is associated with such physiologic changes as increased cerebrovascular burden and alterations in muscle composition.8,9 Furthermore, the length of time that an individual has lived with a disease has been shown to be associated with a greater frequency of behavioral engagement in chronic illness.41 The psychological adjustments that accompany living with a chronic illness over time may in part explain why older adults with kidney disease seem to engage more successfully in disease management behaviors.
Physical fatigue and frailty
Fatigue, a complex construct caused by a combination of biological and psychological factors, has been identified as a barrier to kidney disease self-management behaviors in multiple studies.42 The most robust evidence for the effects of fatigue on kidney disease self-management behaviors is within the context of exercise frequency and physical activity. In two qualitative analyses among patients receiving hemodialysis, physical weakness and feelings of tiredness were major barriers to engagement in low-impact physical activity.43,44 In a survey-based ranking study of 78 patients receiving hemodialysis, feeling “too tired” was the most predominant barrier to physical activity (40%).45 In a study of 100 patients receiving hemodialysis, most participants reported fatigue on both dialysis and nondialysis days (67% and 40%, respectively) as the primary barriers to physical activity.15 Similar frequencies of fatigue were reported as barriers to physical activity in studies of patients receiving hemodialysis in Italy, Jordan, and the United States.16–18,46,47
We were unable to identify studies that examined associations between objective physiologic assessments such as shrinkage and grip strength (such as is included in the Fried Frailty Index) and kidney disease self-management behaviors, despite the prevalence and prognostic significance of frailty in kidney disease.48,49 Evidence does support, however, that patient-reported functional impairment may affect physical activity levels in kidney disease. A prospective study of 72 patients receiving dialysis found that frailty, as measured by the Canadian Study of Health and Aging Scale, was associated by decreased adherence to physical activity.50 Finally, in a systematic review and meta-analysis of 21 studies among patients receiving hemodialysis, patient-reported muscle strength and physical fatigue were correlated moderately with physical activity levels.51 Notably, patients’ median ages in these studies ranged from 48 to 75 years, suggesting that patient perceptions of physical weakness may be independent of age-related functional decline among those with end-stage kidney disease receiving hemodialysis. Future research is needed to determine whether patient-reported perceptions of fatigue and functional decline are associated more strongly with kidney disease self-management behaviors compared with physiologic performance measures.
Cognitive impairment
In addition to experiences of fatigue, patients with kidney disease also face age-accelerated cognitive decline. Cognitive impairment has been shown to worsen with estimated glomerular filtration rate decline and after hemodialysis initiation.52–54 The effect of specific cognitive deficits such as inattention, memory impairment, and an inability to plan and problem solve on kidney disease self-management behaviors remains underinvestigated. Some qualitative and quantitative studies identify patient reports of forgetfulness and memory problems as barriers to medication adherence.
Among 256 patients with CKD in Ethiopia and 188 patients receiving hemodialysis in Malaysia, forgetfulness was the primary barrier to medication adherence.31,55 In three qualitative studies among kidney transplant recipients of all ages, patients described forgetfulness as one of the main reason for missed medication doses.56–58 Among 19 patients who successfully maintained a kidney transplant for 25 years, using reminder messages, establishing daily routines, and cultivating problem-solving skills were key facilitators of medication adherence.59 In another study of 82 individuals who were within 2 months of receiving a kidney transplant, participants described organizing medications into pill boxes, maintaining a routine schedule of taking medication, and relying on friends and family to provide reminders.60 Given the prevalence of age-accelerated cognitive declines in kidney disease populations, whether impairments in attention, planning, or problem solving affect specific kidney disease self-management behaviors or kidney outcomes warrants further rigorous investigation.
PSYCHOLOGICAL DRIVERS OF KIDNEY DISEASE SELF-MANAGEMENT BEHAVIORS
Health literacy and perceived kidney knowledge
Health literacy generally is defined as the personal, cognitive, and social skills needed for an individual to gain access to, understand, and use information for knowledge, health promotion, and maintenance of well-being.61 Some evidence supports that health literacy, measured subjectively using patient-reported scales, has modest effects on kidney disease self-management behaviors related to adherence. In two studies of kidney transplant recipients, health literacy was associated with better adherence to immunosuppressants, measured by either self-report or serologic level.62,63 Among 260 patients receiving hemodialysis, lower health literacy was associated independently with a greater frequency of missed dialysis treatments (missed treatment, 0.6% versus 0.3%; adjusted incidence rate ratio, 2.14; 95% CI, 1.10–4.17) at the 2-year follow-up evaluation.64 However, among Taiwanese patients with CKD, health literacy only explained 5.5% of the variance in a summary scale of kidney disease self-management behaviors, suggesting that other factors may affect self-management tasks more significantly that are associated with higher logistical demands.37
Knowledge refers to having a theoretical and practical understanding of a subject through experience and education.65 Having the ability to read, understand, and use alphabetic and numeric information are key components of objective knowledge. Perceived knowledge refers to one’s self-assessment or feeling of knowing information.66 Evidence supports that perceived kidney disease–related knowledge is associated with self-management behaviors specific to kidney health. A lack of understanding of the meaning and impact of estimations of glomerular filtration rate, a lack of knowledge related to the purpose of specific medications, and misunderstanding dietary requirements each have been identified qualitatively as barriers to kidney disease self-management in CKD populations.67–70 In an interview study of 20 patients with a kidney transplant and eight transplant nephrologists, both knowledge of importance of medications to preserve kidney function and prevent transplant rejection emerged as facilitators of self-management behaviors.71 A lack of knowledge related to prognosis emerged as a strong barrier to emotion management and adaptive coping among patients living with CKD as well as those who had received kidney transplants.7,72,73 Among 276 patients, with more than 50 of them receiving hemodialysis, those who reported dietary adherence also reported higher knowledge about foods specific to a kidney diet (OR, 1.09; 95% CI, 1.00–1.19).74 Among 401 participants with CKD, perceived kidney disease knowledge, although not health literacy, was associated with kidney disease self-management behaviors.75
A lack of knowledge related to benefits of exercise has emerged qualitatively and quantitively as a key barrier to kidney disease self-management. In a study of 50 patients receiving hemodialysis, 16% reported a lack of knowledge related to the benefits of exercise.43 In a qualitative study of 15 dialysis unit staff, allied health professionals perceived that a lack of knowledge related to exercise was a major barrier to patient engagement in physical activity.47 In a study of 274 patients from 10 different dialysis units, 30% to 50% of participants answered incorrectly when responding to questions related to the benefits of exercise on kidney health.76 In addition to examining the effects of health literacy on other kidney disease self-management behaviors, such as laboratory disease self-monitoring, more research is needed to determine which behavior is more impacted by more accurate and robust knowledge acquisition.
Depressive symptoms
Depressive symptoms are the most frequently studied form of psychopathology in patients living with kidney disease. Prevalence estimates of depressive symptoms among individuals with kidney disease range from 10% to 30%. Qualitative and quantitative evidence supports that depressive symptoms moderately correlate with self-management behaviors across kidney disease subpopulations.71,77 In a cross-sectional analysis of 483 patients in China who received kidney transplants, depressive symptoms had small but statistically significant negative correlations with patients’ report of frequency of self-management behaviors (r2 = −0.16).78 That is to say, patients who reported more frequent depressive symptoms also reported fewer successful self-management behaviors. In a study of 100 patients receiving hemodialysis, a one-unit increase in depressive symptoms on a scale was associated with more than double the risk for nonadherence to fluid restriction and dietary control.33 A decreased frequency of depressive symptoms also was associated with improved adherence to fluid restriction in a study of 107 patients receiving hemodialysis (OR, 0.82; 95% CI, 0.67–0.99), although this association weakened when self-efficacy was added to the model.35 In 65 patients receiving dialysis, depressive symptoms accounted for an additional 12% (beyond gender and use of dialysis versus transplant) of the variance in medication adherence.77 Among 29 individuals with CKD and diabetes, depressive symptom severity measured by the Beck Depression Inventory (BDI) correlated moderately with various self-management behaviors.79 Feeling worthless, as reported on the BDI, correlated with a decreased frequency of blood sugar checks (r = −0.42), agitation correlated with a decreased frequency of fruit and vegetable intake (r = −0.38), and reports of sadness correlated with a decreased frequency of foot-washing and exercise (r = −0.38, r = −0.40, respectively). Notably, those self-management behaviors that could have been affected by fatigue had the strongest correlations with the overall BDI score. Effects of depressive symptoms on patient experiences of fatigue may further explain the results of studies examining barriers to physical activity. Depressed mood has been reported by dialysis unit staff as a likely barrier to patient engagement in physical activity.47 Depressive symptoms also were associated significantly with higher odds of physical inactivity in another study on hemodialysis after adjusting for age and sex (OR, 2.59; 95% CI, 1.10–6.09).18 Future investigations may benefit from studies that identify the differential effects of depressive symptoms and patients’ perceptions of fatigue on kidney disease self-management behaviors.
Spiritual well-being
Spiritual well-being, or the ability to integrate meaning and purpose in life through connectedness with the self or a power external to oneself, is an understudied but potentially impactful driver of kidney disease self-management.80 In a study of 76,443 adults in the Southern Community Cohort Study, spirituality among black participants resulted in a 20% decreased risk of end-stage kidney disease despite adjustments for differences in age, income, sex, frequency of depressive symptoms, and size of social network.81 Belief in a higher power facilitated self-management in a study of patients who had received kidney transplants, and turning to spirituality to cope frequently was emphasized as a facilitator of emotion management among older adults living with CKD.7,71 Among patients receiving hemodialysis who were awaiting a kidney transplant, spirituality was reported as a source of confidence when engaging in physical activity.82 Because evidence supports adults in the United States who are older, multimorbid, and who report limited economic resources frequently turn to spirituality to cope, examining the salutatory effects of spirituality on kidney disease self-management may be an impactful component of the biopsychosocial model of chronic illness care that has yet to be assiduously investigated in kidney disease self-management research.83
Self-Efficacy, Motivation, and Patient Activation
Self-efficacy, or one’s confidence in the ability to execute a task, is one of the most impactful drivers of self-management behaviors across kidney disease populations.84 Patients with kidney disease and their caregivers consistently report that an individual’s belief in their own abilities is requisite to successful self-management.4 In a systematic review and thematic synthesis of 50 qualitative studies conducted among individuals who had received a kidney transplant, self-efficacy was identified as a key driver of numerous disease self-management behaviors.85 These included mastering complex tasks, cultivating problem-solving skills, and fostering adaptive coping behaviors. In a cross-sectional analysis of 174 patients with CKD, self-efficacy was associated significantly with improved patient perceptions of a therapeutic alliance with providers and medication adherence, even after adjusting for demographics and clinical characteristics.86 Self-efficacy correlated positively with self-reported engagement in physical activity among 50 patients receiving hemodialysis (r = 0.40).46
The significance of self-efficacy on kidney disease self-management has been shown further in two studies that used structural equation modeling, a multivariate statistical technique that uses factor analysis and multiple regression to analyze structural associations between latent and measured variables.87 When using structural equation modeling to characterize the direction and strength of associations between self-efficacy, neurocognitive deficits, and depressive symptoms on medication adherence among patients with a kidney transplant, self-efficacy continued to have direct effects on medication adherence. Neurocognitive abilities and depressive symptom effects on medication adherence were indirect.88 Self-efficacy also has been shown to mediate the relationship between kidney knowledge and self-management behaviors in patients living with CKD receiving hemodialysis.89,90 Given this evidence, self-efficacy and related constructs likely need to be requisite targets of behavioral interventions in kidney disease.
In addition to self-efficacy, motivation also has been found to facilitate certain kidney disease self-management behaviors. In a small study of older adults receiving hemodialysis, level of motivation emerged as a strong determinant of engaging in exercise.91 Similar themes have emerged in other studies, among both adults receiving hemodialysis and kidney transplant recipients.18,43,44,46,92,93 Motivation also has been shown to quantitatively correlate inversely with engagement in physical activity on hemodialysis (r = −0.31).45 Interestingly, higher levels of motivation paradoxically may be a barrier to regular visit adherence. Among 39 patients with a kidney transplant who belonged to under-represented groups with limited economic resources, those who characterized themselves as highly motivated perceived visits with their nephrologist as not essential to their self-management.94
Many studies examining self-efficacy, motivation, and its association with kidney disease self-management behaviors include it in conjunction with measures of kidney disease–related knowledge and skills.11 Patient activation, the knowledge, confidence, and ability to be an engaged, empowered participant in one’s health, combines several of the aforementioned psychological constructs.95 A 13-item instrument that assesses patient activation, the Patient-Activation Measure, is now a CKD quality metric, despite limited existing evidence supporting its association with clinically meaningful outcomes in kidney disease.96 One study has shown cross-sectional associations between activation levels and frequency of select diabetes-related self-care activities among individuals with CKD.97 It is possible that the Patient-Activation Measure may be a parsimonious and effective way to identify an individual’s degree of knowledge, confidence, and ability to self-manage kidney disease. Further studies examining this association are needed given its introduction to kidney health policy for performance evaluation in the United States.
SOCIAL AND ENVIRONMENTAL DRIVERS OF KIDNEY DISEASE SELF-MANAGEMENT BEHAVIORS
Social support from peers, family, and community
There is increasing acknowledgment that successful disease self-management is not only a product of individual factors but also his or her social and environmental context. Social support, the perception that one is cared for and is part of a supportive social network, is a significant driver of kidney disease self-management across kidney disease populations.98 In the cross-sectional analysis by Xie et al78 of 483 patients who had received a kidney transplant, social support correlated moderately with frequency of successful self-management behaviors (r = 0.30). In the analysis by Chen et al37 of 410 patients living with CKD, social support explained 32% of the variance in kidney disease–management behaviors. Among patients receiving hemodialysis, satisfaction with social support (compared with the frequency of depressive symptoms, health-related locus of control, comorbidity scores, and income) had the largest association with lower intradialytic weight gain.99 In a study of 78 patients receiving hemodialysis, family support had a significant effect on intradialytic weight gain after adjusting for participant diabetes status.100
Social support also has been shown to associate with adherence to medications and provider visits in kidney disease populations. Living with a partner and physical symptoms related to quality of life explained 26% of the variance in phosphate-binder adherence among adults receiving hemodialysis, and social support was associated significantly with immunosuppressant adherence among 330 kidney transplant recipients.31,101 Peer support was cited as a facilitator of visit adherence in two qualitative studies of patients living with a kidney transplant.94,102
A supportive social environment is particularly helpful in driving engagement in exercise across kidney disease populations. Social support was a facilitator to engaging in exercise behaviors in three cross-sectional studies of adults receiving hemodialysis and in one qualitative study in CKD.46,91,103,104 It is important to note that social support from an individual’s environment may not always facilitate successful kidney disease self-management. In a qualitative study of 41 patients with advanced CKD, patients described family relationships as barriers to engaging in frequent and adequate exercise.105
In addition to receiving social support from peers, patients living with kidney disease also may seek support from their religious communities. In a mixed-methods study of patients receiving hemodialysis, having a social network and adhering to a religious faith were identified by patients as strong drivers of successful self-management.106 In a separate qualitative study of 34 patients with CKD who reported low annual incomes, support from patients’ faith communities facilitated self-management.69 Given the large effect size of social support on the frequency of successful engagement in health behaviors across kidney disease subpopulations, delineating the quality and effects of specific sources and types of social support, including instrumental support and the size of a patient’s social network, must be pursued.
Health care provider support
In addition to gaining strength and empowerment from peers, family, and community-based organizations and faith groups, individuals feel empowered to self-manage their chronic condition when they feel aligned with and trusting of their health care providers and other members of their health care team.107 Patients with CKD and their caregivers have emphasized the need for social relationships and support from their health care environment, including nephrology organizations and nephrologists.4 In two qualitative studies among patients with advanced CKD, participants reported not engaging in regular exercise because of a lack of communication and counseling by their nephrologists regarding the benefits of this behavior on reducing kidney disease progression.104–105 Twenty-five patients interviewed as part of a randomized controlled trial of intradialytic exercise showed that support from a kinesiologist enhanced their perceptions of confidence and capability to engage in exercise.108 Similar themes arose in two qualitative studies in hemodialysis and CKD.68,109
Support from within a dialysis facility frequently has been cited as critical to many aspects of kidney disease self-management behaviors. In a prospective analysis of 3,359 patients in the Dialysis Outcomes and Practice Patterns Study, centers that reported more hours of having trained staff had patients with lower odds of skipping treatments (OR, 0.94; 95% CI was not reported), and those who reported having a kidney dietician had patients with lower intradialytic weight gain (OR, 0.75; 95% CI was not reported).110 Less-frequent dialysis staff encouragement was associated independently with an increased odds of nonadherence to fluid restriction (OR, 2.51; 95% CI, 0.99–6.34).111
Perceptions of support from health care providers may act synergistically with other factors to facilitate self-management behaviors. Among 101 Taiwanese patients who recently received kidney transplants, health care provider support in conjunction with age, post–kidney transplant time, and financial satisfaction accounted for 37.2% of the variance in self-monitoring frequency for transplant infection and rejection.30 Further investigations are needed to identify the frequency of health care provider-level support that is needed to achieve durable behavior change in kidney disease.112
The built environment
In addition to acknowledging the effect of interpersonal relationships on chronic illness disease management, the biopsychosocial model of health also accounts for an individual’s environmental context. Barriers and facilitators to disease self-management may exist within an individual’s home or external environment. Patients living with CKD and receiving hemodialysis have cited restrictions within and outside their home as barriers to exercise and physical activity.91,92,105 These restrictions included concerns about physical safety, housing insecurity, and transportation. Difficulties with transportation, perhaps the most frequently described environmental barrier to self-management in hemodialysis, are known to prevent patients from attending in-center appointments.93,113 In an observational study of 182,536 adults receiving in-center hemodialysis in the United States (accounting for 44 million dialysis treatments), traveling to dialysis via a transportation van and having a travel time of more than 17 minutes each were associated with an increased odds of a missed treatment (OR, 1.21; 95% CI, 1.16–1.25; OR, 1.10; 95% CI, 1.07–1.15, respectively). In that analysis, missed treatments also were more frequent during periods of heavy snowfall (OR, 2.68; 95% CI, 2.60–2.77).114 A lack of transportation also has been shown to prevent patients who receive hemodialysis from engaging in exercise and physical activity.43,45 Given the increasing age and prevalence of functional deficits in adults living with kidney disease, interventions that target home and environmental barriers to disease self-management as well are worthy of further development and implementation.49,115
DISCUSSION
We applied the biopsychosocial model of chronic illness care to comprehensively identify individual, interpersonal, and social/environmental determinants of engagement in health behaviors across kidney disease subpopulations. Our scoping review includes known determinants of kidney disease self-management that show small to moderate effect sizes on behavioral outcomes, and it also pinpoints areas for future investigation to advance the rigor of behavioral intervention research in kidney disease. Only one study included adults receiving home dialysis modalities and only 22 studies included those with nondialysis CKD, emphasizing the need to identify drivers of behavioral engagement in these increasing populations. Of the nearly 100 studies included in this scoping review, most were cross-sectional; only six applied a prospective study design. Thus, whether certain factors (such as depressive symptoms) cause or are impacted by poor disease management behaviors remains unable to be determined and the long-term impact of certain potentially impactful factors (such as self-efficacy) on kidney disease management remains underinvestigated. Most studies only collected determinants of interest at baseline. Although this likely is valid for biologic constructs such as age, cognitive function, and frailty, psychological constructs are known to vary with time and clinical context.116 Thus, longitudinal studies using a repeated-measures design of psychological factors may identify more accurately when patients would receive the greatest support from a behavioral intervention.
Far more research is needed to identify the direct and indirect effects of biological factors that are prevalent in kidney disease populations on the frequency of successful behavioral engagement. Given the physical frailty and cognitive decline known to be prevalent in the rapidly aging kidney disease population, and the known effects of these factors on behavioral engagement in other chronic illnesses, identifying whether and to what extent age, physical, and cognitive function affect behavioral engagement in kidney disease is of utmost primacy.117,118 Furthermore, the impact of environmental factors, including physical barriers in the home, that may interact with these biologic factors to affect the frequency of successful disease self-management remains unknown.115
Key psychological and social factors (self-efficacy and social support) in the biopsychosocial model of chronic illness care have shown strong effect sizes on the frequency of behavioral engagement in kidney disease. However, these important variables do not fully explain kidney disease behavior patterns, warranting investigation of other potentially impactful psychological factors that are not represented, including anxiety, health-related locus of control, and affect.119,120 Furthermore, the effects of environmental factors (such as transportation barriers, access to peer or faith communities) that may interact with self-efficacy and social support to differentially affect the frequency of behavioral engagement are underinvestigated. Finally, patient activation, driven by perceived knowledge, self-efficacy, and personal skills (including perceptions of self-efficacy in communication and physical ability to perform self-management tasks), combines several factors included in a biopsychosocial approach.95 Thus, patient activation may serve as a comprehensive and impactful intervention target to ignite health behavior improvements.
Nearly all studies included in this review used a self-reported scale to measure the kidney disease self-management behavior of interest. Although evidence supports the validity of patient-reported measures to assess patients’ moods and experiences, cross-validating these scales with biobehavioral performance measures will increase internal validity and promote reproducibility.121 Furthermore, most studies of kidney disease self-management focus on adherence, whether related to medication management, diet, or health care visits. Factors that drive the frequency of engagement in other kidney disease self-management behaviors, such as laboratory self-monitoring (measuring blood pressure, blood glucose level, and so forth), tobacco use, emotion management (coping), and communication with providers remain relatively understudied. More research is needed to determine the differential effects of disease self-monitoring, tobacco use, emotion management, and patient–provider communication on clinically meaningful outcomes in kidney disease such that interventions can be developed to specifically target these behaviors.
Kidney disease self-management is dynamic and multifaceted, thus optimally informed by considering its alignment with the biopsychosocial model. Evaluating self-management behaviors in kidney disease using the biopsychosocial model of chronic illness care highlights known and understudied individual and systems-level drivers of behavioral engagement in this population. Applying the biopsychosocial model to comprehensively understand drivers of kidney disease self-management will identify the type and amount of specific behavioral intervention components, including the need for educational modules, the inclusion of subspecialist or caregiver-delivered psychosocial support, and the dismantling of structural environmental barriers. Subsequent steps to advance the rigor of behavioral research in kidney disease will require longitudinal, mixed-methods (quantitative and qualitative) analyses that include CKD and home dialysis populations, use biobehavioral measures to track behavioral engagement, account for biologic factors prevalent in the rapidly aging kidney disease population, and acknowledge an individual’s home, social, and community environment. The nephrology community must apply a biopsychosocial approach to durably sustain health behavior change for all adults living with kidney disease.
Financial support:
Supported by Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute grants K12HS026395 (D.N. and K.L.C.) and National Institute of Diabetes and Digestive and Kidney Diseases P30DK114809 (K.C.).
Footnotes
Conflict of interest statement: none.
REFERENCES
- 1.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh. 2011;43:255–64. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality. Self-management support. [cited 2020 December 21]. Available from: https://www.ahrq.gov/ncepcr/tools/self-mgmt/self.html.
- 3.Harris JR, Wallace RB. The Institute of Medicine’s new report on living well with chronic illness. Prev Chronic Dis. 2012;9: E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donald M, Beanlands H, Straus S, Ronksley P, Tam-Tham H, Finlay J, et al. Identifying needs for self-management interventions for adults with CKD and their caregivers: a qualitative study. Am J Kidney Dis. 2019;74:474–82. [DOI] [PubMed] [Google Scholar]
- 5.Roux-Marson C, Baranski JB, Fafin C, Exterman G, Vigneau C, Couchoud C, et al. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr. 2020;20:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowling CB, Wandenberg AE, Phillips LS, McClellan WM, Johnson TM, Echt KV. Older patients’ perspectives on managing complexity in CKD self-management. Clin J Am Soc Nephrol. 2017;12:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair D, Bonnet K, Wild MG, Umeukeje EM, Fissell RB, Faulkner ML, et al. Psychological adaptation to serious illness: a qualitative study among patients with advanced chronic kidney disease. J Pain Symptom Manage. 2021;61:32–41. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–9. [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG, Stehman-Breen C, Freid LF, Song X, Siscovick D, Fried LP, et al. The presents of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–7. [DOI] [PubMed] [Google Scholar]
- 10.Reid C, Hall J, Boys J, Lewis S, Chang A. Self-management of hemodialysis for end stage renal disease: a systematic review. JBI Libr Syst Rev. 2011;9:69–103. [DOI] [PubMed] [Google Scholar]
- 11.Donald M, Kahlon B, Beanlands H, Straus S, Ronksley P, Herrington G, et al. Self-management interventions for adults with chronic kidney disease: a scoping review. BMJ Open. 2018;8: e019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams A, Manias E, Walker R, Gorelik A. A multifactorial intervention to improve blood pressure control in co-existing diabetes and kidney disease: a feasibility randomized controlled trial. J Adv Nurs. 2012;68:2515–25. [DOI] [PubMed] [Google Scholar]
- 13.Ishani A, Christopher J, Palmer D, Otterness S, Clothier B, Nugent S, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68:41–9. [DOI] [PubMed] [Google Scholar]
- 14.Byrne J, Khunti K, Stone M, Farooqi A, Carr S. Feasibility of a structured group education session to improve self-management of blood pressure in people with chronic kidney disease: an open randomized pilot trial. BMJ Open. 2011;1:e000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012; 27:1152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossola M, Pellu V, Di Stasio E, Tazza L, Giungi S, Nebiolo PE. Self-reported physical activity in patients on chronic hemodialysis: correlates and barriers. Blood Purif. 2014;38:24–9. [DOI] [PubMed] [Google Scholar]
- 17.Darawad MW, Khalil AA. Jordanian dialysis patients’ perceived exercise benefits and barriers: a correlation study. Rehabil Nurs. 2013;38:315–22. [DOI] [PubMed] [Google Scholar]
- 18.Fiaccadori E, Sabatino A, Schito F, Angella F, Malagoli M, Tucci M, et al. Barriers to physical activity in chronic hemodialysis patients: a single-center pilot study in an Italian dialysis facility. Kidney Blood Press Res. 2014;39:169–75. [DOI] [PubMed] [Google Scholar]
- 19.United States Renal Data System. Annual data report. [cited 2021 January 10]. Available from: https://adr.usrds.org/2020/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd
- 20.Mills SL, Pumarino J, Clark N, Carroll S, Dennis S, Koehn S, et al. Understanding how self-management interventions work for disadvantaged populations living with chronic conditions: protocol for a realist synthesis. BMJ Open. 2014;4:e005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy A, Rogers A, Chew-Graham C, Blakeman T, Bowen R, Gardner C, et al. Implementation of a self-management support approach (WISE) across a health system: a process evaluation explaining what did and did not work for organizations, clinicians, and patients. Implement Sci. 2014;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabanayagam C, Lim SC. Risk behavior patterns and outcomes of CKD – potential for individualizing behavioral interventions. Kidney Int Rep. 2019;4:11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbane S, Hazzan AD, Halinski C, Mathew AT. Challenges and opportunities in late-stage chronic kidney disease. Clin Kidney J. 2015;8:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusnanto H, Agustin D, Hilmanto D. Biopsychosocial model of illness in primary care: a hermeneutic literature review. J Family Med Prim Care. 2018;7:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21:1321–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peyrot M, Rubin RR. Behavioral and psychosocial interventions in diabetes: a conceptual review. Diabetes Care. 2007;30:2433–40. [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 28.Lin SY, Fetzer SJ, Lee PC, Chen CH. Predicting adherence to health care recommendations using health promotion behaviors in kidney transplant recipients within 1–5 years post-transplant. J Clin Nurs. 2011;20:3313–21. [DOI] [PubMed] [Google Scholar]
- 29.Scheel JF, Schieber K, Reber S, Stoessel L, Waldmann E, Jank S, et al. Psychosocial variables associated with immunosuppressive medication nonadherence after renal transplantation. Front Psychiatry. 2018;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark-Cutaia MN, Ren D, Hoffman LA, Burke LE, Sevick MA. Adherence to hemodialysis dietary sodium recommendations: influence of patient characteristics, self-efficacy, and perceived barriers. J Ren Nutr. 2014;24:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan YM, Zalilah MS, Hii SZ. Determinants of compliance behaviors among patients undergoing hemodialysis in Malaysia. PLoS One. 2012;7:e41362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellon L, Regan D, Curtis R. Factors influencing adherence among Irish hemodialysis patients. Patient Educ Couns. 2013;92:88–93. [DOI] [PubMed] [Google Scholar]
- 33.Kugler C, Vlaminck H, Haverich A, Maes B. Nonadherence with diet and fluid restrictions among adults having hemodialysis. J Nurs Scholarsh. 2005;37:25–9. [DOI] [PubMed] [Google Scholar]
- 34.Khalil AA, Frazier SK, Lennie TA, Sawaya BP. Depressive symptoms and dietary adherence in patients with end-stage renal disease. J Ren Care. 2011;37:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Washington T, Hain DJ, Zimmerman S, Carlton-LaNey I. Identification of potential mediators between depression and fluid adherence in older adults undergoing hemodialysis treatment. Nephrol Nurs J. 2018;45:251–9. [PubMed] [Google Scholar]
- 36.Khezerloo S, Mahmoudi H, Nia HS, Vafadar Z. Predictors of self-management among kidney transplant recipients. Urol J. 2019;16:366–70. [DOI] [PubMed] [Google Scholar]
- 37.Chen YC, Chang LC, Liu CY, Ho YF, Went SC, Tsai TI. The roles of social support and health literacy in self-management among patients with chronic kidney disease. J Nurs Scholarsh. 2018;50:265–75. [DOI] [PubMed] [Google Scholar]
- 38.da Costa Rosa SC, Nishimoto DY, Freitas Junior IF, Ciolac EG, Monteiro HL. Factors associated with levels of physical activity in chronic kidney disease patients undergoing hemodialysis: the role of dialysis versus nondialysis day. Phys Act Health. 2017;14:726–32. [DOI] [PubMed] [Google Scholar]
- 39.Vallance JK, Johnson ST, Thompson ST, Wen K, Lam NN, Boyle T, et al. Prevalence and correlates of accelerometer-based physical activity and sedentary time among kidney transplant recipients. Can J Kidney Health Dis. 2019;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan MF, Wong FKY, Chow SKY. Investigating the health profile of patients with end-stage renal failure receiving peritoneal dialysis: a cluster analysis. J Clin Nurs. 2010;19:649–57. [DOI] [PubMed] [Google Scholar]
- 41.Auduly A The over time development of chronic illness self-management patterns: a longitudinal qualitative study. BMC Public Health. 2013;13:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda F, Straus SE, Hickie I, Sharpe MC, Dobbins JC, Komar-off A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. [DOI] [PubMed] [Google Scholar]
- 43.Jhamb M, Mcnulty ML, Ingalsbe G, Childers JW, Schell J, Conroy MB, et al. Knowledge, barriers, and facilitators of exercise in dialysis patients: a qualitative study of patients, staff, and nephrologists. BMC Nephrol. 2016;17:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontos PC, Miller KL, Brooks D, Jassal SV, Spanjevic L, Devins GM, et al. Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol. 2007;39:1303–11. [DOI] [PubMed] [Google Scholar]
- 45.Byrne K, Russell M. Physical activity levels of patients with chronic disease requiring dialysis. Physiother Ireland. 2011;32:29–33. [Google Scholar]
- 46.Goodman ED, Ballou MB. Perceived barriers and motivators to exercise in hemodialysis patients. Nephrol Nurs J. 2004;31:23–9. [PubMed] [Google Scholar]
- 47.Painter P, Clark L, Olausson J. Physical function and physical activity assessment and promotion in the hemodialysis clinic: a qualitative study. Am J Kidney Dis. 2014;64:425–33. [DOI] [PubMed] [Google Scholar]
- 48.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 49.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornik B, Frailty Dulawa J. quality of life, anxiety, and other factors affecting adherence to physical activity recommendations by hemodialysis patients. Int J Environ Res Public Health. 2019;16:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarca BD, Wycherley TP, Bennett P, Meade A, Ferrar KE. Modifiable physical factors associated with physical functioning for patients receiving dialysis: a systematic review. Phys Act Health. 2020;17:475–89. [DOI] [PubMed] [Google Scholar]
- 52.Chu NM, Gross AL, Shaffer AA, Haugen CE, Norman SP, Xue QL, et al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol. 2019;30:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2005;16:2127–33. [DOI] [PubMed] [Google Scholar]
- 54.Tamura MK, Vittinghoff E, Hsu CY, Tam K, Seliger SL, Sozio S, et al. Loss of executive function after dialysis initiation with chronic kidney disease. Kidney Int. 2017;91:948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kefale B, Tadesse Y, Alebachew M, Engidawork E. Management practice, and adherence and its contributing factors among patients with chronic kidney disease at Tikur Anbessa Specialized Hospital: a hospital based cross-sectional study. Int J Nephrol. 2018;2018::2903139. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Gordon EJ, Prohaska TR, Gallant MP, Siminoff LA. Adherence to immunosuppression: a prospective diary study. Transplant Proc. 2007;39:3081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orr A, Orr D, Willis S, Holmes M, Britton P. Patient perceptions of factors influencing adherence to medication following kidney transplant. Psychol Health Med. 2007;12:509–17. [DOI] [PubMed] [Google Scholar]
- 58.Tielen M, van Exel JA, van Buren MC, Maasdam L, Weimar W. Attitudes towards medication non-adherence in elderly kidney transplant patients: a Q methodology study. Nephrol Dial Transplant. 2011;26:1723–8. [DOI] [PubMed] [Google Scholar]
- 59.Ruppar TM, Russell CL. Medication adherence in successful kidney transplant recipients. Transplant. 2009;19:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon EJ, Gallant M, Sehgal AR, Conti D, Siminoff LA. Medication-taking among adult renal transplant recipients: barriers and strategies. Transpl Int. 2009;22:534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sorensen K, Van den Broucke S, Fullam J, Doyle G, Pelikan J, Slonska Z, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health. 2012;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patzer RE, Serper M, Reese PP, Przytula K, Koval R, Ladner DP, et al. Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant. 2016;30:1294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demian MN, Shapiro RJ, Thornton WL. An observational study of health literacy and medication adherence in adult kidney transplant recipients. Clin Kidney J. 2016;9:858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green JA, Mor MK, Shields AM, Sevick MA, Arnold RM, Palevsky PM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. Am J Kidney Dis. 2013;62:73–80. [DOI] [PubMed] [Google Scholar]
- 65.Definition of ‘knowledge.’ Merriam-Webster Dictionary. [cited 2021 January 10]. Available from: https://www.merriam-webster.com/dictionary/knowledge.
- 66.Radecki CM, Jaccard J. Perceptions of knowledge, actual knowledge, and information search behavior. J Exp Soc Psychol. 1995;31:107–38. [Google Scholar]
- 67.Baay S, Hemmelgarn B, Tam-Tham H, Finlay J, Elliott MJ, Straus S, et al. Understanding adults with chronic kidney disease and their caregivers’ self-management experiences: a qualitative study using the theoretical domains framework. Can J Kidney Health Dis. 2019;6:2054358119848126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang SJ, Tan NC, Yoon S, Ramakrishnan C, Paulpandi M, Gun S, et al. Perceived barriers and facilitators to chronic kidney disease care among patients in Singapore: a qualitative study. BMJ Open. 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahn LS, Vest BM, Madurai N, Singh R, York TM, Cinnarone CW, et al. Chronic kidney disease treatment burden among low-income primary care patients. Chronic Illn. 2015;11:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sontakke S, Budania R, Bajait C, Jaiswal K, Pimpalkhute S. Evaluation of adherence to therapy in patients of chronic kidney disease. Indian J Pharmacol. 2015;47:668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranahan M, Visger JV, Kayler LK. Describing barriers and facilitators for medication adherence and self-management among kidney transplant recipients using the information-motivation-behavioral skills model. Clin Transplant. 2020;34:e13862. [DOI] [PubMed] [Google Scholar]
- 72.Martin SC, Stone AM, Scott AM, Brashers DE. Medical, personal, and social forms of uncertainty across the transplantation trajectory. Qual Health Res. 2010;20:182–96. [DOI] [PubMed] [Google Scholar]
- 73.Schmid-Mohler Schaffer-Keller P, Frei A, Fehr T, Spirig R. A mixed-method study to explore patients’ perspective of self-management tasks in the early phase after kidney transplant. Prog Transplant. 2014;24:8–18. [DOI] [PubMed] [Google Scholar]
- 74.Thomas LK, Sargent RG, Michels PC, Richter DL, Valois RF, Moore CG. Identification of the factors associated with compliance to therapeutic diets in older adults with end stage renal disease. J Ren Nutr. 2001;11:80–9. [DOI] [PubMed] [Google Scholar]
- 75.Schrauben SJ, Cavanaugh KL, Fagerlin A, Ikizler TA, Ricardo AC, Eneanya ND, et al. The relationship of disease-specific knowledge and health literacy with the uptake of self-care behaviors in CKD. Kidney Int Rep. 2020;5:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jayaseelan G, Bennett P, Bradshaw W, Wang W, Rawson H. Exercise benefits and barriers: the perceptions of people receiving hemodialysis. Nephrol Nurs J. 2018;45:185–219. [PubMed] [Google Scholar]
- 77.Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75:1223–9. [DOI] [PubMed] [Google Scholar]
- 78.Xie J, Liu J, Liu M, Yan J, Ding S, Ma K. Self-management and related psychosocial variables among renal transplant patients. Transplant Proc. 2019;51:734–41. [DOI] [PubMed] [Google Scholar]
- 79.Sakraida TJ, Weber MT. The relationship between depressive symptoms and self-management behaviors in patients with T2DM and Stage 3 CKD. Perspect Psychiatr Care. 2016;52:273–82. [DOI] [PubMed] [Google Scholar]
- 80.Ellison CW. Spiritual well-being: conceptualization and measurement. J Psychol Theol. 1983;;11:330–40. [Google Scholar]
- 81.Nair D, Cavanaugh KL, Wallston KA, Mason O, Stewart TG, Blot AJ, et al. Religion, spirituality, and risk of end-stage kidney disease among adults of low socioeconomic status in the Southeastern United States. J Health Care Poor Underserved. 2020;31:1727–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sieverdes JC, Raynor PA, Armstrong T, Jenkins CH, Sox LR, Treiber FA. Attitudes and perceptions of patients on the kidney transplant waiting list toward mobile health-delivered physical activity programs. Prog Transplant. 2015;25:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Religious Landscape Study. Pew Research Forum. [cited 2020 December 20]. Available from: https://www.pewforum.org/religious-landscape-study.
- 84.Bandura A Self-efficacy mechanism in human agency. Am Psychol. 1982;;37:122–47. [Google Scholar]
- 85.Jamieson NJ, Hanson CS, Josephson MA, Gordon EJ, Craig JC, Halleck F, et al. Motivations, challenges, and attitudes to self-management in kidney transplant recipients: a systematic review of qualitative studies. Am J Kidney Dis. 2016;67:461–78. [DOI] [PubMed] [Google Scholar]
- 86.Curtin RB, Walters BA, Schatell D, Pennell P, Wise M, Klicko K. Self-efficacy and self-management behaviors in patients with chronic kidney disease. Adv Chronic Kidney Dis. 2008;15:191–205. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan D Structural equation modeling: foundations and extensions. 2nd ed. California: Thousand Oaks; 2008. [Google Scholar]
- 88.Paterson TSE, O’Rourke N, Shapiro RJ, Thornton WL. Medication adherence in renal transplant recipients: a latent variable model of psychosocial and neurocognitive predictors. PLoS One. 2018;13:e0204219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chuang LM, Wu SFV, Lee MC, Lin LJ, Liang SY, Lai PC, et al. The effects of knowledge and self-management of patients with early-stage chronic kidney disease: self-efficacy is a mediator. Jpn J Nurs Sci. 2021;18:e12388. [DOI] [PubMed] [Google Scholar]
- 90.Wu SFV, Hsieh NC, Lin LJ, Tsai JM. Prediction of self-care behavior on the basis of knowledge about chronic kidney disease using self-efficacy as a mediator. J Clin Nurs. 2016;25:2609–18. [DOI] [PubMed] [Google Scholar]
- 91.Liu CK, Afezoli D, Seo J, Syeda H, Zheng S, Folta SC. Perceptions of physical activity in African American older adults on hemodialysis: themes from key informant interviews. Arch Rehabil Res Clin Transl. 2020;2:100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song Y, Wang J, Chen X, Guo Y, Wang X, Liang W. Facilitators and barriers to exercise influenced by traditional Chinese culture: a qualitative study of Chinese patients undergoing hemodialysis. Transcult Nurs. 2019;30:558–68. [DOI] [PubMed] [Google Scholar]
- 93.Chenitz KB, Fernando M, Shea JA. In-center hemodialysis attendance: patient perceptions of risks, barriers, and recommendations. Hemodial Int. 2014;18:364–73. [DOI] [PubMed] [Google Scholar]
- 94.Goldade K, Sidhwani S, Patel S, Brendt L, Vigliaturo J, Kasiske B, et al. Kidney transplant patients’ perceptions, beliefs, and barriers related to regular nephrology outpatient visits. Am J Kidney Dis. 2011;;57:11–20. [DOI] [PubMed] [Google Scholar]
- 95.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nair D, Cavanaugh KL. Measuring patient activation as part of kidney disease policy: are we there yet? J Am Soc Nephrol. 2020;31:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zimbudzi E, Lo C, Ranasinha S, Kerr PG, Polkinghorne KR, Teede H, et al. The association between patient activation and self-care practices: a cross-sectional study of an Australian population with comorbid diabetes and chronic kidney disease. Health Expect. 2017;20:1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearson JE. The definition and measurement of social support. J Couns Dev. 1986;;64:390–5. [Google Scholar]
- 99.Pang SK, Ip WY, Chang AM. Psychosocial correlates of fluid compliance among Chinese hemodialysis patients. Adv Nurs. 2001;35:691–8. [DOI] [PubMed] [Google Scholar]
- 100.Christensen AJ, Smith TW, Turner CW, Holman JM, Gregory MC, Rich MA. Family support, physical impairment, and adherence in hemodialysis: an investigation of main and buffering effects. J Behav Med. 1992;15:313–25. [DOI] [PubMed] [Google Scholar]
- 101.Van Camp YPM, Vrijens B, Adraham I, Rompaey BV, Elseviers MM. Adherence to phosphate binders in hemodialysis patients: prevalence and determinants. J Nephrol. 2014;;27:673–9. [DOI] [PubMed] [Google Scholar]
- 102.Been-Dahmen JMJ, Grijpma JW, Ista E, Dwarswaard J, Maasdam L, Weimar W, et al. Self-management challenges and support needs among kidney transplant recipients: a qualitative study. J Adv Nurs. 2018;74:2393–405. [DOI] [PubMed] [Google Scholar]
- 103.Williams A, Stephens R, Mcknight T, Dodd S. Factors affecting adherence of end-stage renal disease patients to an exercise programme. Br J Sp Med. 1991;;25:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke AL, Young HM, Hull KL, Hudson N, Burton JO, Smith AC. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015; 30:1885–92. [DOI] [PubMed] [Google Scholar]
- 105.Kendrick J, Ritchie M, Andrews E. Exercise in individuals with CKD: a focus group study exploring patient attitudes, motivations, and barriers to exercise. Kidney Med. 2019;1:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Washington T, Zimmerman S, Browne T. Factors associated with chronic kidney disease self-management. Soc Work Public Health. 2016;31:58–69. [DOI] [PubMed] [Google Scholar]
- 107.Dineen-Griffin S, Garcia-Cardenas V, Williams K, Benrimoi SI. Helping patients help themselves: a systematic review of self-management support strategies in primary healthcare practice. PLoS One. 2019;14:e0220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thompson S, Tonelli M, Klarenbach S, Molzahn A. A qualitative study to explore patient and staff perceptions of intradialytic exercise. Clin J Am Soc Nephrol. 2016;11:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Young HML, Hudson N, Clarke AL, Dungey M, Feehally J, Burton JO, et al. Patient and staff perceptions of intradialytic exercise before and after implementation: a qualitative study. PLoS One. 2015;10:e0128995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saran R, Bragg-Greshman JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–62. [DOI] [PubMed] [Google Scholar]
- 111.Yokoyama Y, Suzukamo Y, Hotta O, Yamazaki S, Kawaguchi T, Hasegawa T, et al. Dialysis staff encouragement and fluid control adherence in patients on hemodialysis. Nephrol Nurs J. 2009;36:289–97. [PubMed] [Google Scholar]
- 112.Umeukeje EM, Osman R, Nettles AL, Wallston KA, Cavanaugh KL. Provider attitudes and support of patients’ autonomy for phosphate binder medication adherence. J Patient Exp. 2020;7:708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brar A, Babakhani A, Salifu MO, Jindal RM. Evaluation of non-adherence in patients undergoing dialysis and kidney transplantation: United States transplantation practice patterns survey. Transplant Proc. 2014;46:1340–6. [DOI] [PubMed] [Google Scholar]
- 114.Chan KE, Thadhani RI, Maddux FW. Adherence barriers to chronic dialysis in the United States. J Am Soc Nephrol. 2014;25:2642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crews DC, Delaney AM, Taylor JLW, Cudjoe TKM, Nkimbeng M, Roberts L, et al. Pilot intervention addressing social support and functioning of low socioeconomic status older adults with ESRD: the Seniors Optimizing Community Integration to Advance Better Living with ESRD (SOCIABLE) Study. Kidney Med. 2019;1:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Geiser C, Keller BT, Lockhart G, Eid M, Cole DA, Koch T. Distinguishing state variability from trait change in longitudinal data: the role of measurement (non)variance in latent state-trait analyses. Behav Res Methods. 2015;47:172–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stevens LA, Viswanathan GV, Weiner DE. CKD and ESRD in the elderly: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faul AC, Yankeelov PA, Rowan NL, Gillette P, Nicholas LD, Borders KW, et al. Impact of geriatric assessment and self-management support on community-dwelling older adults with chronic illnesses. J Gerontol Soc Work. 2009;52:230–49. [DOI] [PubMed] [Google Scholar]
- 119.Silva AN, Moratelli L, Tavares PL, Marsicano EDO, Pinhati RR, Colugnati FAB, et al. Self-efficacy beliefs, locus of control, religiosity, and non-adherence to immunosuppressive medications in kidney transplant patients. Nephrology (Carlton). 2016;21:938–43. [DOI] [PubMed] [Google Scholar]
- 120.Glover LM, Butler-Williams C, Cain0Shields L, Forde AT, Pur-nell TS, Young B, et al. Optimism is associated with chronic kidney disease and rapid kidney function decline among African Americans in the Jackson Heart Study. Psychosom Res. 2020;139:110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turner RR, Quittner AL, Parasuraman BM, Kallich JD, Cleeland CS. Patient-reported outcomes: instrument development and selection issues. Value Health. 2007;10(Suppl 2):S86–93. [DOI] [PubMed] [Google Scholar]
- 122.Kadowaki M, Saito M, Amada N, Haga I, Nakamura A, Tokodai K. Medication compliance in renal transplant patients during the Great East Japan Earthquake. Transplantation Proceedings. 2014;46 (2):610–2. 10.1016/j.transproceed.2013.11.039. [DOI] [PubMed] [Google Scholar]


