Abstract
Background
Gestational weight gain (GWG) and prepregnancy obesity are garnering more attention as determining factors of pregnancy outcomes when it comes to the wellbeing of both the mother and her baby. This study was conducted to describe the pattern of GWG among participants of Riyadh Mother and Baby Multicenter Cohort Study (RAHMA) and to investigate the detrimental effects of excessive GWG and prepregnancy obesity on pregnancy outcomes.
Methods
RAHMA is a multicentre cohort study conducted in three hospitals in Riyadh, Saudi Arabia. Participants were categorized according to the Institute of Medicine into inadequate, adequate, and excessive GWG, and stratified by body mass index (BMI) into under/normal weight, overweight, and obese. To examine the independent effect of maternal prepregnancy obesity and GWG, a multivariate regression model was used and adjusted odds ratio (AOR) and 95% Confidence Interval (CI) for each outcome were calculated.
Results
A total of 7029 participants were included in this study; 31.8% had adequate GWG, 25.9% had excessive GWG and 42.3% had inadequate GWG, while 29.7% had normal BMI, 33.3% were overweight, 34.8% were obese, and 2.2% were underweight. Excessive GWG was independently associated with increased risk of hypertensive events, (AOR = 1.77, 95% CI 1.20–2.63). Obesity was associated with higher risk of gestational diabetes (AOR 2.11, 95% CI 1.76–2.53), hypertensive events (AOR 2.06, 95% CI 1.48–3.01), and delivery by emergency caesarean section (AOR = 1.63, 95% CI 1.35–1.97). Infants of obese women had increased odds of macrosomia (AOR 3.11, 95% CI 1.94–4.99) and lower odds of low birth weight (AOR = 0.68, 95% CI 0.53–0.88).
Conclusion
In comparison to excessive GWG, which increases the risk of hypertensive events during pregnancy, prepregnancy obesity is associated with more adverse outcomes including GDM, hypertensive events in pregnancy and emergency CS.
Introduction
Gestational weight gain (GWG) and prepregnancy obesity are garnering more attention as determining factors of pregnancy outcomes when it comes to the wellbeing of both the mother and her baby [1–3]. GWG is the amount of maternal weight gained between conception and the time of birth to accommodate the growing fetus. In 1990, the Institute of Medicine (IOM) of the United States of America released formulated recommendations for healthy GWG during pregnancy [4]. Since that time many aspects of the health of women of childbearing age have changed. In 2009, the IOM modified the 1990 guidelines based on findings from research performed over almost two decades following their initial recommendations. The updated IOM guidelines included recommendations for GWG in twin pregnancy, changes to the recommendations for obese women, and a recommendation that all women should strive to be within the normal body mass index (BMI) range when they conceive [5].
The IOM concluded that prepregnancy body mass index (BMI) was an important predictor of birth weight, independent of maternal GWG, and that prepregnancy BMI should be used to guide recommendations for GWG. Thus, determining BMI became an integral part of the physical examination of pregnant women [3].
Physiologic weight gain during pregnancy can be attributed primarily to the weight of the developing fetus and to increases in maternal body water, blood volume and fat. Physiologic changes related to pregnancy result in optimum weight gain for women with a normal BMI from 11 to 16 kg [6].
In recent years, adverse pregnancy outcomes have been linked to both inadequate and excessive GWG. Excessive GWG (above the IOM target range) is associated with increased risk for postpartum weight retention, obesity, and the related co-morbidities such as chronic hypertension (HTN), pregnancy-related HTN (gestational HTN, preeclampsia), gestational diabetes (GDM) or type 2 diabetes mellitus. In addition to the increased risk of obstetric complications including caesarean section delivery (CS), and the birth of a macrosomic infant [6–9].
GWG during pregnancy is also associated with offspring health, as the risk for childhood obesity increases when GWG is excessive [10].
Nevertheless, insufficient GWG (below the IOM target range) is associated with increased incidence of preterm delivery, birth of a small-for-gestational age infant, and low birthweight (LBW) [6, 9].
Maternal obesity carries an increased risk of many adverse pregnancy outcomes. A recent systematic review of cohort studies showed that almost 24% of pregnancy complications were related to maternal obesity [11]. Such adverse effects included increased rate of caesarean section (CS) delivery, HTN, GDM, large for gestational age infants and stillbirths [12, 13]. Furthermore, infants born to obese mothers are at increased risk of cardiac and metabolic diseases in addition to childhood obesity [14].
There is high prevalence of prepregnancy obesity and overweight among Saudi women with documented association with GDM, induction of labour, pregnancy associated HTN, CS delivery and macrosomia [15–17].
Despite the various pregnancy outcomes being dependent on GWG, it is seldom discussed as an independent entity from prepregnancy obesity. This is particularly true in Saudi Arabia with literature addressing GWG being outdated or including a relatively small sample size [18, 19]. The aim of this study is to describe the pattern of GWG among participants of Riyadh Mother and Baby Multicenter Cohort Study (RAHMA), to predict the associated risk factor, and to analyse the independent impact of excessive GWG and prepregnancy obesity on the incidence of adverse maternal and fetal outcomes.
Materials and methods
Study design and participants
This study was approved by the Institutional Review Boards at each participating hospital and followed Helsinki’s declaration guidelines for research on human subjects. King Abdullah International Medical Research Centre, approval letter 11/062; King Fahad Medical City Research Centre, approval letter 013–017; and King Saud University, approval letter 13–985.
Further to Institution Review Approval from all participating hospitals, participating mothers gave written informed consent.
RAHMA is a multicentre cohort study conducted in three hospitals in Riyadh, Saudi Arabia which are King Khalid University Hospital, King Fahad Medical City which is run by the Ministry of Health (MOH), and King Abdul-Aziz Medical City, which is affiliated to the National Guards. Recruitment of the cohort commenced in November 2013 and data collection concluded in March 2015. A stratified cluster random sampling approach was used to choose the participating hospitals. The kind of hospital, which comprised (MOH), military, and university hospitals, was used as a stratifying variable [17].
All mothers who gave birth in one of the participating hospitals, as well as their babies, were eligible to be enrolled in the study. Before signing a written informed consent, the moms were given written information about the study in Arabic. Non-Saudi women were not included in the study [17].
A standardised data collection sheet was used. Data were obtained from both the maternal and infant medical records in addition to an in-person post-delivery maternal self-administrated questionnaire (within 2 days after delivery), which provided data on the mothers’ sociodemographic conditions (S1 File). Further details of the study methodology are available in the cohort profile report [17].
In this sub-cohort we excluded pregnancies with multiple gestations and women with missing data on prepregnancy BMI. Comparison of the main outcome determining factors of excluded and included population is provided in S1 Table.
The study population were grouped into three groups of GWG (inadequate, adequate and excessive GWG) according to Institute of Medicine (IOM) classification [5], and stratified into three groups according to prepregnancy BMI (under/normal weight, overweight, obesity).
Outcome variables
Gestational Diabetes (GDM) and pre-gestational Diabetes based on the cut-off values used by the World Health Organization (WHO) [20].
Hypertensive disorders of pregnancy based on the report of the American national working group on high blood pressure in pregnancy [21]. As the incidence of pre-eclampsia and eclampsia were low, all hypertensive events were aggregated in one group for analysis.
Maternal prepregnancy body mass index (BMI) was calculated from weight prior to pregnancy and height measured during the first antenatal clinic. Participants were classified according to the WHO BMI definitions as follows: underweight ≤18.4 kg/m2, healthy weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (≥ 30 kg/m2) [22].
Gestational Weight gain (GWG) was calculated as the difference between the term weight and the prepregnancy weight.
- Classification of GWG was adopted according to Institute of Medicine (IOM) [5]. The IOM guidelines recommend the following cut-off values for adequate GWG:
- ➢ a total weight gain of 12.5–18 Kg for underweight BMI women (≤18.5 kg/m2)
- ➢ a total weight gain of 11.5–16 kg for normal BMI women (18.5–24.9 kg/m2)
- ➢ a total weight gain of 7–11.5 kg for overweight women (BMI of 25–29.9 kg/m2)
- ➢ a total weight gain of 5–9 kg for all obese women (BMI of 30 kg/m2or greater)
Macrosomia is defined as a birth weight at term of ≥ 4.0 kg [23].
Low birth weight (LBW) is defined as a birth weight < 2.5 kg at term [24].
Preterm birth is defined as birth that takes place before 37 weeks gestation [25].
Statistical analysis
Description of all study variables in form of mean ±standard deviation (for quantitative variables) or frequency and percentages (for qualitative variables) was done. Comparison of continuous variables among the three GWG groups was done using Analysis of Variance (ANOVA) after testing of normality distribution. Association between GWG and various categorical variables was tested using Chi-Square tests. For the independent effect of obesity and GWG on the pregnancy outcome, we examined effect modification of GWG categories stratified by prepregnancy BMI categories for each pregnancy outcome using Chi-square and crude Odds Ratio.
Multivariable logistic regression was used to examine the independent association between pregnancy outcomes and GWG. The covariates included in the model were those of proven influence on GWG. The models were adjusted for maternal age, parity and gestational age as continuous variables. GWG and prepregnancy BMI were analysed as categorical variables where adequate GWG was used as the reference group for GWG and normal prepregnancy BMI group was considered as the reference group for prepregnancy obesity.
We tested multiplicative interaction by including cross-product terms for GWG categories and prepregnancy BMI in the multivariable model. Adjusted odds ratio (AOR), with confidence intervals (CIs) at 95% were presented, and statistical significance was set at two tailed with p-value < 0.05. Statistical analyses were performed with IBM SPSS version 21 [26] and Stata 16.0 [27].
Results
A total of 7029 participants were included in this study. According to the IOM guideline [5], 31.8% had adequate GWG during pregnancy, while 25.9% had excessive GWG and 42.3% had inadequate GWG. Only 157 women (2.2%) were classified as underweight while 2338 (33.3%) were overweight and 2447 (34.8%) were obese.
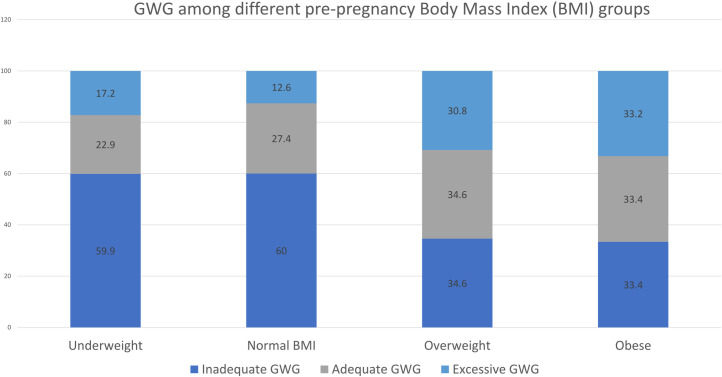
Participants in different IOM groups were of comparable age, exposure to SHS as well as history of pre-gestational diabetes and HTN (Table 1). Women with excessive GWG have significantly higher prepregnancy BMI and gestational age at delivery compared to women with inadequate GWG (P<0.05) (Table 1). Nevertheless, participants with inadequate GWG were of significantly higher parity (Table 1). Inadequate GWG was more common among underweight and normal prepregnancy BMI while excessive GWG was more prevalent among overweight and obese women (Fig 1).
Table 1. Participant characteristics by institute of medicine gestational weight gain categories.
| Characteristics | Inadequate (N = 2972) | Adequate (N = 2236) | Excessive (N = 1821) | p-value |
|---|---|---|---|---|
| Age (years) | 29.7±5.9 | 29.7±5.9 | 29.8±5.7 | 0.75 |
| <20 | 78(2.6) | 49(2.2) | 40(2.2) | 0.69 |
| 20–29 | 1432(48.2) | 1126(50.4) | 898(49.3) | |
| 30–34 | 789(26.5) | 587(26.3) | 476(26.1) | |
| 35+ | 673(22.6) | 474(21.2) | 407(22.4) | |
| Prepregnancy BMI (kg/m 2 ) | 27.1±5.8 | 28.6±5.7 | 29.4±5.4 | <0.001 |
| Underweight/normal BMI | 1346 (45.3) | 607 (27.2) | 289 (15.9) | <0.01 |
| Overweight | 808 (27.2) | 810 (36.2) | 720 (39.5) | |
| Obese | 817 (27.5) | 818 (36.6) | 812 (44.6) | |
| Parity | 2.46±2.22 | 2.31±2.14 | 2.11±2.04 | |
| 0 | 630(21.2) | 520(23.3) | 475(26.1) | 0.003 |
| 1–4 | 1461(49.2) | 1061(47.5) | 852(46.8) | |
| 5+ | 881(29.6) | 654(29.3) | 492(27.0) | |
| Second-hand smoker | 615(24.9) | 482(26.6) | 357(24.2) | 0.24 |
| Pregestational Diabetes | 78(3.7) | 55(3.6) | 64(5.1) | 0.09 |
| Pregestational hypertension | 34(1.1) | 24(1.1) | 32(1.8) | 0.102 |
| Gestational age at delivery (weeks) | 38.7±2.0 | 38.9±1.9 | 38.9±1.7 | <0.001 |
Data are expressed in frequency (percentage) or mean± Standard deviation, BMI = Body Mass Index.
Fig 1. Distribution of gestational weight gain according to institute of medicine among various prepregnancy body mass index groups.
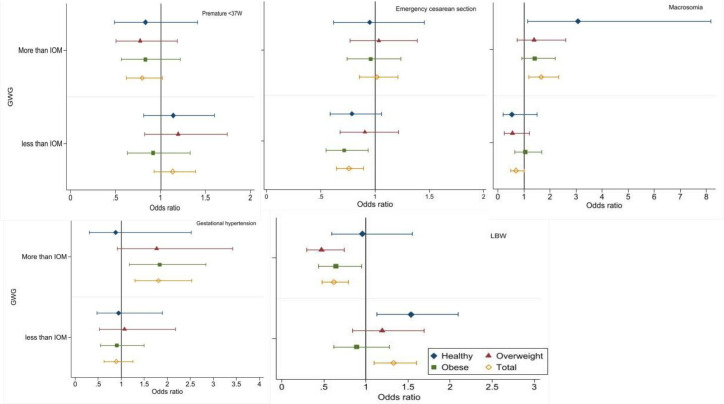
Compared to women with adequate GWG, women who gained excess weight had significantly higher probability of developing HTN during pregnancy (4.8% versus 2.7%), deliver a macrosomic baby (4.9% versus 3.0%), and they were less likely to have an LBW baby (5.4% versus 8.5%) (Table 2). Additionally, they had a non-significant greater odds of emergency CS delivery and GDM. Women who gained inadequate weight were less likely to deliver by CS (11.6% versus 14.8%) and more probably to have LBW (11.0% versus 8.5%) (Fig 2 and Table 2).
Table 2. Association of pregnancy outcomes with gestational weight gain among participants according to the prepregnancy body mass index.
| Outcome | Inadequate (N = 2972) | Adequate (N = 2236) | Excessive (N = 1821) | P-value |
|---|---|---|---|---|
| BMI category | Event/Total (%) | Event/Total (%) | Event/Total (%) | |
| Emergency Caesarean section | ||||
| All women | 346 (11.6) | 330 (14.8) | 273(15.0) | <0.001 |
| Underweight/normal BMI | 138 (10.2) | 77 (12.7) | 35 (12.1) | 0.25 |
| Overweight | 98 (12.1) | 107 (13.2) | 98 (13.6) | 0.665 |
| Obese | 110 (13.5) | 146 (17.8) | 140 (17.2) | <0.001 |
| HTN | ||||
| All women | 71(2.4) | 60(2.7) | 86 (4.8) | <0.001 |
| Underweight/normal BMI | 25 (1.9) | 12 (2.0) | 5 (1.7) | 0.969 |
| Overweight | 16 (2.0) | 15 (1.9) | 23 (3.2) | 0.150 |
| Obese | 30 (3.7) | 33 (4.1) | 58 (7.2) | 0.002 |
| Gestational Diabetes | ||||
| All women | 497 (24.6) | 371 (25.0) | 324 (27.4) | 0.183 |
| Underweight/normal BMI | 161 (18.0) | 70 (17.3) | 34 (16.7) | 0.891 |
| Overweight | 125 (22.3) | 105 (20.3) | 104 (24.2) | 0.357 |
| Obese | 211 (37.1) | 196 (34.8) | 186 (33.9) | 0.509 |
| Preterm birth | ||||
| All women | 254 (8.7) | 169 (7.8) | 111(6.3) | 0.010 |
| Underweight/normal BMI | 132 (10.0) | 52 (8.8) | 21 (7.5) | 0.67 |
| Overweight | 64 (8.1) | 54 (6.8) | 38 (5.4) | 0.119 |
| Obese | 58 (7.3) | 63 (7.9) | 52 (6.6) | 0.644 |
| Macrosomia | ||||
| All women | 55 (2.1) | 61 (3.0) | 84 (4.9) | <0.001 |
| Underweight/normal BMI | 8 (0.7) | 7 (1.3) | 10 (3.8) | 0.002 |
| Overweight | 10 (1.4) | 18 (2.4) | 23 (3.3) | 0.045 |
| Obese | 38 (5.0) | 36 (4.8) | 51 (6.6) | 0.225 |
| Low Birth Weight | ||||
| All women | 326 (11.0) | 190 (8.5) | 99 (5.4) | <0.01 |
| Underweight/normal BMI | 191 (14.2) | 59 (9.7) | 27 (9.3) | 0.005 |
| Overweight | 75 (9.3) | 64 (7.9) | 28 (3.9) | <0.001 |
| Obese | 60 (7.3) | 67 (8.2) | 44 (5.4) | 0.079 |
Data are expressed in frequency (percentage) or mean± Standard deviation, BMI = Body Mass Index.
Fig 2. Association of pregnancy outcomes with gestational weight gain among participants according to the prepregnancy body mass index.
Women with obesity were less likely to deliver by CS if they restricted their GWG (13.5% versus 17.2%). Compared to women with normal BMI, obese women, had less probability to deliver an LBW baby; (5.4% versus 9.3%) and nearly six-times the possibility to develop HTN during pregnancy; (7.2% versus 1.7%) if they had excessive GWG (Fig 2 and Table 2).
The effect of GWG on the different pregnancy outcomes was attenuated after adjusting of all confounders and its significant effect maintained only for CS, HTN and birth weight, whilst the independent effect of prepregnancy obesity was significantly associated with all pregnancy outcomes except the preterm birth (Table 3). Excessive GWG significantly doubled the HTN risk (AOR = 1.77, 95% CI 1.20–2.63) and diminished the risk of LBW by 39% (AOR = 0.61, 95% CI 0.41–0.90) (Table 3). Restricting the GWG significantly lowered the risk of CS by 30% (AOR = 0.70, CI 0.54–0.90) and the risk of macrosomia by 61% (AOR = 0.39, 95% CI, 0.20–0.76). Compared to women with normal prepregnancy BMI, women with prepregnancy obesity had an increase of the risk of emergency CS; (AOR = 1.63, 95% CI 1.35–1.97,), nearly double the risk of HTN (AOR = 2.06, 95% CI 1.48–3.03) and GDM (AOR = 2.11, 95% CI 1.76–2.53), and increased risk of delivering a macrosomic baby by 1.59 times (95% CI 1.11–2.27), meanwhile, they had lower risk of LBW by 32% (AOR = 0.68, 95% CI 0.53–0.88) (Table 3).
Table 3. Independent effect of gestational wight gain and prepregnancy body mass index on different pregnancy outcomes.
| Pregnancy outcome | Inadequate GWG | Excessive GWG | Prepregnancy Obesity |
|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Emergency Caesarean section¶ | 0.70 (0.54–0.90) * | 1.07 (0.85–1.12) | 1.63 (1.35–1.97) * |
| Any hypertensive event in pregnancy§ | 0.93(0.59–1.47) | 1.77 (1.20–2.63) * | 2.06 (1.48–3.03) * |
| Gestational Diabetes§ | 1.06 (0.87–1.31) | 1.10 (0.90–1.35) | 2.11 (1.76–2.53) * |
| Preterm birth^ | 1.16 (0.90–1.48) | 0.83 (0.63–1.05) | 0.90 (0.67–1.22) |
| Macrosomia¶ | 0.39 (0.20–0.76) * | 1.07 (0.65–1.76) | 3.11 (1.94–4.99) * |
| Low Birth Weight¶ | 1.31 (0.95–1.81) | 0.61 (0.41–0.90) * | 0.68 (0.53–0.88) * |
Abbreviations: GWG; gestational Weight Gain, AOR; adjusted odds ratio, CI; confidence interval, BMI; Body Mass Index.
Inadequate GWG and Excessive GWG are compared to women within IOM GWG recommendation.
Prepregnancy Obesity is compared to women with normal prepregnancy Body Mass Index.
¶Adjustment of maternal age, parity, Gestational Diabetes, Gestational Hypertension, gestational age at delivery.
§ adjusted for of maternal age, parity, gestational age at delivery.
^ Adjustment of maternal age, parity, Gestational Diabetes, Gestational Hypertension.
*p-value less than 0.05.
Testing the interaction between GWG and prepregnancy BMI was not statistically significant when considered in the regression models.
Discussion
The results of this study showed that a larger proportion of obese and overweight women had excessive GWG compared to women with normal prepregnancy weight or those who were underweight. The study showed that maternal obesity was associated with GDM, hypertensive events in pregnancy and emergency CS, while excessive GWG was associated only with hypertensive events in pregnancy. Neonates of obese mothers in this sub-cohort were more likely to be macrosomic and less likely to be of LBW. Conversely, women with inadequate GWG, irrespective of their BMI, were less likely to deliver by emergency CS or to deliver a macrosomic baby. This study showed no association between prepregnancy weight or GWG and preterm birth among Saudi women.
The greater influence of prepregnancy obesity on the adverse outcome of pregnancy compared to excessive GWG was documented by previous studies, however obese women with excessive GWG are at the greatest risk of adverse pregnancy outcomes [11, 28, 29].
Previous studies showed that obesity is associated with hypertensive disorders of pregnancy and GDM which is in line with our results [30, 31]. Published reports showed that obesity increases the risk of pre-eclampsia by 3 to10-fold compared to normal weight women with an additional 1.4 to 5-fold increase in the risk of venous thromboembolism in pregnancy and the postpartum period [32, 33]. Moreover, obesity is reported to increase the risk of GDM by 4–9 times compared to women with normal prepregnancy weight which agrees with findings in this study [34]. It has been suggested that in countries with a high prevalence of maternal obesity, such as in the case of Saudi Arabia, control of obesity prior to pregnancy may result in 14–35% fewer women with GDM and hypertensive disorders in pregnancy [35]. Thus, control of obesity among Saudi women in the reproductive age group will have a major impact on maternal and child health considering the high prevalence of GDM of 24% which we have observed and reported previously [17]. In this study, excessive GWG was associated with hypertensive disorders in pregnancy. Previous studies reported conflicting results for the effect of excess GWG on hypertensive events in pregnancy with some studies suggesting the association [36, 37] and other studies rejecting such association [28, 38]. However, a recently published study confirmed the association between excessive body fluid which was associated with excessive GWG as the main factor responsible for preeclampsia [39].
It is not surprising that obese women in this study had almost twofold the risk of CS delivery compared to normal weight women considering the high prevalence of obstetric complications such as preeclampsia and GDM in addition to the increased risk of fetal macrosomia among them. Similar findings were reported in other cohorts [40, 41]. The risk of CS delivery was found to increase by 1.4-fold in overweight women compared to normal weight women and almost three times more in morbidly obese women [41]. This observation suggests a linear relationship between the risk of CS delivery and maternal obesity. We did not observe an association between excessive GWG and CS delivery in this cohort, albeit, such association was recently reported in a systematic review of 23 observational studies [8].
High birthweight is more common in pregnancies complicated by maternal obesity than in pregnancies of normal weight women [34]. Our results showed that obesity increased the risk of macrosomia by almost threefold. The birth of a big baby is associated with multiple maternal and neonatal complications including postpartum haemorrhage, birth canal injuries, shoulder dystocia and birth fracture [42].
Contrary to the findings of other investigators [8], we did not find an association between inadequate GWG and LBW or PTB, however, we have documented the association of prepregnancy underweight and LBW in the same cohort in a recent publication [15].
Our findings on the effects of low GWG in decreasing the odds for macrosomia and CS delivery have practical application in women with prepregnancy overweight and obesity in reducing the rate of these adverse outcomes. Similar findings were reported by other investigators [43, 44] with the recommendations of tailoring weight gain for individual woman based on the degree of obesity and the balance between avoidance of adverse effects of excessive weight gain and inadequate weight gain [45, 46].
It is noteworthy that differences in the prevalence of obesity, overweight, and excessive weight gain were observed between different ethnic groups [47, 48], independent of socioeconomic and lifestyle characteristics. This observation supports further investigation into the optimum range of GWG in Saudi women with a high BMI to achieve the least pregnancy adverse outcomes, guided by the recommendation of the IOM.
We found conflicting reports about the effects of weight loss in obese women during pregnancy. Some reports showed a significant reduction in the adverse outcomes associated with weight loss with no significant increase in the rate of PTB, or LBW, while other reported an increased rate of small for gestational age infants [49, 50].
In this study, prepregnancy obesity was observed to have a greater effect on adverse pregnancy outcomes than excessive GWG, which may be explained by the effects of the maternal insulin resistance, hyperinsulinemia and inflammation mediators, which seem to contribute to placental and fetal dysfunction in early pregnancy before the effect of GWG is established [51]. This premise is further supported by the limited success of different lifestyle and dietary interventions during pregnancy in improving fetal overgrowth, preeclampsia or GDM, which are adverse effects of prepregnancy obesity rather than excessive GWG [52–54].
Implications of the study findings to practice and research
Effective interventions including various modalities of diet and exercise should be employed to reduce weight gain during pregnancy and prepregnancy obesity as well as weight retention during the postpartum period [55, 56].
Targeting Saudi women in the reproductive age group with health education about the possible serious adverse effects of obesity on their reproductive life. Such health education should be part of the school and university curricula as over 90% of the women are attending schools or universities.
Further research should be directed to the investigation of community specific interventions to reduce the burden of obesity among reproductive age group women and to the investigation of effects of maternal obesity on Intrauterine programming and the neonate, child, and adult future health [57].
Strength and limitations
This is the first study from Saudi Arabia to investigate the effect of prepregnancy weight and GWG on different maternal and perinatal outcomes. The large cohort of over 7000 participants included in the study provided an accurate and specific account of the effect of prepregnancy weight and GWG on the outcome of pregnancy among Saudi mothers with consideration of all covariates in the analysis. The study will offer important results for health services planning for intervention to reduce prepregnancy obesity and overweight as well as for targeting high risk pregnancy in women with high BMI with specific healthcare. We are aware of the limitation of this study including the observation nature of the investigation and the lack of data on some outcomes such as postpartum weight retention (PPWR), nevertheless, we have investigated PPWR in a follow-up study of the same cohort [58]. In addition, we had to exclude a considerable number of the participants due to incomplete data on the main determinants of the investigation, however, we find no significant differences between the population included in this study and the one excluded (S1 File).
Conclusion
In comparison to excessive GWG, which increases the risk of hypertensive events during pregnancy, prepregnancy obesity is associated with more adverse outcomes including GDM, hypertensive events in pregnancy and emergency CS.
Supporting information
(XLSX)
(DOCX)
Acknowledgments
Our gratitude is extended to all the mothers who participated in this study and the lab technicians who performed the lab analysis of the blood samples.
Data Availability
Data are available from the Ethics Committee at King Saud university, who can be contacted at irb@ksu.edu.sa for researchers who meet the criteria for access to confidential data. Data is restricted to protect participant privacy.
Funding Statement
The authors would like to thank the Center for Promising Research in Social Research and Women’s Studies, Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University for funding this Project in 2020. Award/Grant number is not applicable. The funder did not play any role in collection of data, decision to publish or preparation of the manuscript.
References
- 1.Li C, Liu Y, Zhang W. Joint and Independent Associations of Gestational Weight Gain and Pre-Pregnancy Body Mass Index with Outcomes of Pregnancy in Chinese Women: A Retrospective Cohort Study. PLoS One. 2015;10(8):e0136850. Epub 2015/08/28. doi: 10.1371/journal.pone.0136850 ; PubMed Central PMCID: PMC4552294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mastroeni MF, Czarnobay SA, Kroll C, Figueiredo KB, Mastroeni SS, Silva JC, et al. The Independent Importance of Pre-pregnancy Weight and Gestational Weight Gain for the Prevention of Large-for Gestational Age Brazilian Newborns. Maternal and child health journal. 2017;21(4):705–14. Epub 2016/07/28. doi: 10.1007/s10995-016-2156-0 . [DOI] [PubMed] [Google Scholar]
- 3.Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, et al. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. Jama. 2019;321(17):1702–15. Epub 2019/05/08. doi: 10.1001/jama.2019.3820 ; PubMed Central PMCID: PMC6506886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine Committee on Nutritional Status During P, Lactation. Nutrition During Pregnancy: Part I Weight Gain: Part II Nutrient Supplements. Washington (DC): National Academies Press (US) Copyright © 1990 by the National Academy of Sciences.; 1990. [Google Scholar]
- 5.Institute of M, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US) Copyright © 2009, National Academy of Sciences.; 2009. [PubMed] [Google Scholar]
- 6.Kominiarek MA, Peaceman AM. Gestational weight gain. American journal of obstetrics and gynecology. 2017;217(6):642–51. Epub 2017/05/28. doi: 10.1016/j.ajog.2017.05.040 ; PubMed Central PMCID: PMC5701873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durst JK, Sutton AL, Cliver SP, Tita AT, Biggio JR. Impact of Gestational Weight Gain on Perinatal Outcomes in Obese Women. American journal of perinatology. 2016;33(9):849–55. Epub 2016/03/11. doi: 10.1055/s-0036-1579650 . [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. Jama. 2017;317(21):2207–25. Epub 2017/06/07. doi: 10.1001/jama.2017.3635 ; PubMed Central PMCID: PMC5815056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kominiarek MA, Saade G, Mele L, Bailit J, Reddy UM, Wapner RJ, et al. Association Between Gestational Weight Gain and Perinatal Outcomes. Obstetrics and gynecology. 2018;132(4):875–81. Epub 2018/09/12. doi: 10.1097/AOG.0000000000002854 ; PubMed Central PMCID: PMC6153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstetrics and gynecology. 2015;125(4):773–81. Epub 2015/03/10. doi: 10.1097/AOG.0000000000000739 ; PubMed Central PMCID: PMC4425284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG: an international journal of obstetrics and gynaecology. 2019;126(8):984–95. Epub 2019/02/21. doi: 10.1111/1471-0528.15661 ; PubMed Central PMCID: PMC6554069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. Jama. 2014;311(15):1536–46. Epub 2014/04/17. doi: 10.1001/jama.2014.2269 . [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi M, Maroufizadeh S, Omani-Samani R, Almasi-Hashiani A, Amini P. The effect of prepregnancy body mass index on birth weight, preterm birth, cesarean section, and preeclampsia in pregnant women. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2019;32(22):3818–23. Epub 2018/05/18. doi: 10.1080/14767058.2018.1473366 . [DOI] [PubMed] [Google Scholar]
- 14.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction (Cambridge, England). 2010;140(3):387–98. Epub 2010/06/22. doi: 10.1530/REP-10-0077 . [DOI] [PubMed] [Google Scholar]
- 15.Wahabi H, Esmaeil S, Fayed A. Maternal Prepregnancy Weight and Pregnancy Outcomes in Saudi Women: Subgroup Analysis from Riyadh Mother and Baby Cohort Study (RAHMA). BioMed Research International. 2021;2021:6655942. doi: 10.1155/2021/6655942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Gilany AH, El-Wehady A. Prevalence of obesity in a Saudi obstetric population. Obes Facts. 2009;2(4):217–20. Epub 2010/01/08. doi: 10.1159/000226597 ; PubMed Central PMCID: PMC6515936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahabi H, Fayed A, Esmaeil S, Alzeidan R, Elawad M, Tabassum R, et al. Riyadh Mother and Baby Multicenter Cohort Study: The Cohort Profile. PloS one. 2016;11(3):e0150297. Epub 2016/03/05. doi: 10.1371/journal.pone.0150297 ; PubMed Central PMCID: PMC4777404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallatah AM, Babatin HM, Nassibi KM, Banweer MK, Fayoumi MN, Oraif AM. Maternal and Neonatal Outcomes among Obese Pregnant Women in King Abdulaziz University Hospital: A Retrospective Single-Center Medical Record Review. Medical archives (Sarajevo, Bosnia and Herzegovina). 2019;73(6):425–32. Epub 2020/02/23. doi: 10.5455/medarh.2019.73.425-432 ; PubMed Central PMCID: PMC7007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meher Un N. Impact of obesity on fetomaternal outcome in pregnant saudi females. International journal of health sciences. 2011;5(2 Suppl 1):40–1. Epub 2011/07/01. ; PubMed Central PMCID: PMC3533340. [PMC free article] [PubMed] [Google Scholar]
- 20.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341–63. Epub 2014/05/23. doi: 10.1016/j.diabres.2013.10.012 . [DOI] [PubMed] [Google Scholar]
- 21.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American journal of obstetrics and gynecology. 2000;183(1):S1–s22. Epub 2000/08/02. . [PubMed] [Google Scholar]
- 22.WHO. Health topics/ Obesity. Available from: https://www.who.int/health-topics/obesity#tab=tab_1.
- 23.Macrosomia: ACOG Practice Bulletin Summary, Number 216. Obstetrics and gynecology. 2020;135(1):246–8. Epub 2019/12/20. doi: 10.1097/AOG.0000000000003607 . [DOI] [PubMed] [Google Scholar]
- 24.Cutland CL, Lackritz EM, Mallett-Moore T, Bardají A, Chandrasekaran R, Lahariya C, et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48 Pt A):6492–500. doi: 10.1016/j.vaccine.2017.01.049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Practice Bulletin No. 171: Management of Preterm Labor. Obstetrics and gynecology. 2016;128(4):e155–64. Epub 2016/09/24. doi: 10.1097/AOG.0000000000001711 . [DOI] [PubMed] [Google Scholar]
- 26.IBM. How to cite IBM SPSS Statistics or earlier versions of SPSS [Tenth October 2021]. Available from: https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions-spss.
- 27.STATA. Stata Corp. 2016, Stata Statistical Software: Release 16; Stata Corp LP, College Station, TX [Tenth October 2021]. Available from: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/.
- 28.Gaillard R, Durmuş B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring, Md). 2013;21(5):1046–55. Epub 2013/06/21. doi: 10.1002/oby.20088 . [DOI] [PubMed] [Google Scholar]
- 29.Chen CN, Chen HS, Hsu HC. Maternal Prepregnancy Body Mass Index, Gestational Weight Gain, and Risk of Adverse Perinatal Outcomes in Taiwan: A Population-Based Birth Cohort Study. International journal of environmental research and public health. 2020;17(4). Epub 2020/02/23. doi: 10.3390/ijerph17041221 ; PubMed Central PMCID: PMC7068269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. The lancet Diabetes & endocrinology. 2016;4(12):1025–36. Epub 2016/10/17. doi: 10.1016/S2213-8587(16)30217-0 . [DOI] [PubMed] [Google Scholar]
- 31.Heude B, Thiébaugeorges O, Goua V, Forhan A, Kaminski M, Foliguet B, et al. Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Maternal and child health journal. 2012;16(2):355–63. Epub 2011/01/25. doi: 10.1007/s10995-011-0741-9 ; PubMed Central PMCID: PMC3472402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alsheef MA, Alabbad AM, Albassam RA, Alarfaj RM, Zaidi ARZ, Al-Arfaj O, et al. Pregnancy and Venous Thromboembolism: Risk Factors, Trends, Management, and Mortality. BioMed Research International. 2020;2020:4071892. doi: 10.1155/2020/4071892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan ES, Wilson E, Watkins T, Gao F, Hunt BJ. Maternal obesity and venous thromboembolism. International journal of obstetric anesthesia. 2012;21(3):253–63. Epub 2012/06/01. doi: 10.1016/j.ijoa.2012.01.002 . [DOI] [PubMed] [Google Scholar]
- 34.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2015;16(8):621–38. Epub 2015/05/29. doi: 10.1111/obr.12288 . [DOI] [PubMed] [Google Scholar]
- 35.Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, Bilano V, et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2015;16(9):758–70. Epub 2015/06/23. doi: 10.1111/obr.12293 . [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Hong Z, Zhang L. Associations of prepregnancy body mass index and gestational weight gain with pregnancy outcomes in nulliparous women delivering single live babies. Scientific reports. 2015;5:12863. Epub 2015/08/06. doi: 10.1038/srep12863 ; PubMed Central PMCID: PMC4525357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugen M, Brantsæter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy and Childbirth. 2014;14(1):201. doi: 10.1186/1471-2393-14-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Dwyer V, O’Toole F, Darcy S, Farah N, Kennelly MM, Turner MJ. Maternal obesity and gestational weight gain. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology. 2013;33(7):671–4. Epub 2013/10/17. doi: 10.3109/01443615.2013.821461 . [DOI] [PubMed] [Google Scholar]
- 39.Hillesund ER, Seland S, Bere E, Sagedal LR, Torstveit MK, Lohne-Seiler H, et al. Preeclampsia and gestational weight gain in the Norwegian Fit for Delivery trial. BMC Res Notes. 2018;11(1):282-. doi: 10.1186/s13104-018-3396-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frolova AI, Wang JJ, Conner SN, Tuuli MG, Macones GA, Woolfolk CL, et al. Spontaneous labor onset and outcomes in obese women at term. American journal of perinatology. 2018;35(1):59. doi: 10.1055/s-0037-1605574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2007;8(5):385–94. Epub 2007/08/25. doi: 10.1111/j.1467-789X.2007.00397.x . [DOI] [PubMed] [Google Scholar]
- 42.Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019;54(3):308–18. Epub 2019/04/03. doi: 10.1002/uog.20279 . [DOI] [PubMed] [Google Scholar]
- 43.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. The American journal of clinical nutrition. 2010;91(6):1642–8. Epub 2010/04/02. doi: 10.3945/ajcn.2009.29008 ; PubMed Central PMCID: PMC2869513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstetrics and gynecology. 2015;125(3):566–75. Epub 2015/03/03. doi: 10.1097/AOG.0000000000000677 . [DOI] [PubMed] [Google Scholar]
- 45.Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. The American journal of clinical nutrition. 2009;90(6):1552–8. Epub 2009/10/09. doi: 10.3945/ajcn.2009.28026 . [DOI] [PubMed] [Google Scholar]
- 46.Beyerlein A, Lack N, von Kries R. Within-population average ranges compared with Institute of Medicine recommendations for gestational weight gain. Obstetrics and gynecology. 2010;116(5):1111–8. Epub 2010/10/23. doi: 10.1097/AOG.0b013e3181f1ad8b . [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Miao Q, Huang T, Fell DB, Harvey ALJ, Wen SW, et al. Racial/ethnic variations in gestational weight gain: a population-based study in Ontario. Can J Public Health. 2019;110(5):657–67. doi: 10.17269/s41997-019-00250-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bahadoer S, Gaillard R, Felix JF, Raat H, Renders CM, Hofman A, et al. Ethnic disparities in maternal obesity and weight gain during pregnancy. The Generation R Study. Eur J Obstet Gynecol Reprod Biol. 2015;193:51–60. Epub 07/08. doi: 10.1016/j.ejogrb.2015.06.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. American journal of obstetrics and gynecology. 2013;208(3):205.e1-7. Epub 2012/12/19. doi: 10.1016/j.ajog.2012.12.018 . [DOI] [PubMed] [Google Scholar]
- 50.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstetrics and gynecology. 2011;117(5):1065–70. Epub 2011/04/22. doi: 10.1097/AOG.0b013e318214f1d1 . [DOI] [PubMed] [Google Scholar]
- 51.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ (Clinical research ed). 2017;356:j1. Epub 2017/02/10. doi: 10.1136/bmj.j1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ (Clinical research ed). 2014;348:g1285. Epub 2014/02/12. doi: 10.1136/bmj.g1285 ; PubMed Central PMCID: PMC3919179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes care. 2011;34(12):2502–7. Epub 2011/10/06. doi: 10.2337/dc11-1150 ; PubMed Central PMCID: PMC3220844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tieu J, Shepherd E, Middleton P, Crowther CA. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. The Cochrane database of systematic reviews. 2017;1(1):Cd006674. Epub 2017/01/04. doi: 10.1002/14651858.CD006674.pub3 ; PubMed Central PMCID: PMC6464792 Caroline A Crowther: none known. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim S, O’Reilly S, Behrens H, Skinner T, Ellis I, Dunbar JA. Effective strategies for weight loss in post-partum women: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2015;16(11):972–87. Epub 2015/08/28. doi: 10.1111/obr.12312 . [DOI] [PubMed] [Google Scholar]
- 56.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. The Cochrane database of systematic reviews. 2015;(6):Cd007145. Epub 2015/06/13. doi: 10.1002/14651858.CD007145.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62(10):1789–801. Epub 2019/08/28. doi: 10.1007/s00125-019-4951-9 ; PubMed Central PMCID: PMC6731191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahabi HA, Fayed AA, Tharkar S, Esmaeil SA, Bakhsh H. Postpartum Weight Retention and Cardiometabolic Risk among Saudi Women: A Follow-Up Study of RAHMA Subcohort. Biomed Res Int. 2019;2019:2957429. Epub 2019/07/30. doi: 10.1155/2019/2957429 ; PubMed Central PMCID: PMC6634075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
Data are available from the Ethics Committee at King Saud university, who can be contacted at irb@ksu.edu.sa for researchers who meet the criteria for access to confidential data. Data is restricted to protect participant privacy.