Abstract
Eighteen cases of Acanthamoeba-associated keratitis among contact lens wearers seen at the Department of Ophthalmology, Karl-Franzens-University, Graz, Austria, between 1996 and 1999 are reviewed. The amoebae were proven to be the causative agents in three patients. The aim of our study was to discriminate between clinically relevant and nonrelevant isolates and to assess the relatedness of the isolates to published strains. Altogether, 20 strains of free-living amoebae, including 15 Acanthamoeba strains, 3 Vahlkampfia strains, and 2 Hartmannella strains, were isolated from clinical specimens. The virulent Acanthamoeba strains were identified as A. polyphaga and two strains of A. hatchetti. To our knowledge this is the first determination of keratitis-causing Acanthamoeba strains in Austria. Clinically relevant isolates differed markedly from nonrelevant isolates with respect to their physiological properties. 18S ribosomal DNA sequence types were determined for the three physiologically most-divergent strains including one of the keratitis-causing strains. This highly virulent strain exhibited sequence type T6, a sequence type not previously associated with keratitis. Sequence data indicate that Acanthamoeba strains causing keratitis as well as nonpathogenic strains of Acanthamoeba in Austria are most closely related to published strains from other parts of the world. Moreover, the results of our study support the assumption that pathogenicity in Acanthamoeba is a distinct capability of certain strains and not dependent on appropriate conditions for the establishment of an infection.
Within the past few years free-living amoebae of the genus Acanthamoeba have gained increasing clinical relevance mainly as causative agents of a very often seriously progressing keratitis. The first ocular infections with acanthamoebae were diagnosed in 1974 (21). Since then the occurrence of keratitis due to Acanthamoeba (Acanthamoeba keratitis) has been escalating in correlation with the increasing number of contact lens wearers. Contaminated contact lens care systems usually are the first step in Acanthamoeba keratitis pathogenesis. The most prevalent risk factors are contact lens wear, poor hygiene, and a compromised corneal barrier. Users of extended-wear lenses are at special risk. Nevertheless, about 10 to 15% of cases of Acanthamoeba keratitis occur in persons who do not wear contact lenses (15).
The prognosis for Acanthamoeba keratitis, when the disease is diagnosed at an early stage and treated adequately, is rather good, but fast and reliable diagnosis is of crucial importance. Clinical signs and symptoms of Acanthamoeba keratitis are easily confused with fungal or viral keratitis. Initial improvement or stabilization in response to topical antibacterial, antiviral, antifungal, or corticosteroid therapy can occur, altering the clinical picture and thus complicating diagnosis. Clinical diagnosis should be based on the presence of keratitis with severe pain and photophobia, stromal infiltrates, radial keratoneuritis, and sometimes pseudodendriform epithelial lesions. Cysts or trophozoites, found in corneal scrapings, on contact lenses, and inside of lens storage cases, are confirmatory. Agar culture is the mainstay for laboratory detection of Acanthamoeba (12).
Various Acanthamoeba species have been reported to be able to cause keratitis: A. castellanii, A. polyphaga, A. hatchetti, A. culbertsoni, A. rhysodes, A. lugdunensis, A. quina, and A. griffini (26). Although isolates can easily be recognized as belonging to the genus Acanthamoeba by their polygonal cysts, accurate species determination is still problematic. An important step forward in the differentiation of acanthamoebae was the division of the genus into three morphological groups by Pussard and Pons (24). Since then a number of attempts to enable a more precise identification have been made. A very promising method for identification and phylogenetic studies is the analysis of the 18S rRNA gene sequence. Recently Stothard et al. (27) identified 12 Acanthamoeba sequence types, the vast majority of keratitis-causing strains belonging to sequence type T4. However, a final system does not yet exist, and, moreover, representatives from same species differ with respect to their pathogenicities.
We report on 18 cases of Acanthamoeba-associated keratitis seen at the Department of Ophthalmology, Karl-Franzens-University, Graz, Austria, between 1996 and 1999; in three cases the amoebae were the disease-causing agents. The aim of our study was to discriminate between clinically relevant and nonrelevant isolates and to assess the relatedness of our isolates to published strains by 18S ribosomal DNA (rDNA) sequence similarities.
MATERIALS AND METHODS
Patients.
Clinical specimens of keratitis patients presenting at the Department of Ophthalmology were on a routine basis investigated for Acanthamoeba spp. During 1996 to 1999 specimens from 18 keratitis patients, 10 women (55.6%) and 8 men (44.4%), yielded Acanthamoeba. The definitive diagnosis of Acanthamoeba keratitis on the basis of typical clinical signs, no response to antibacterial or antiviral treatment, and detection of acanthamoebae in the corneal epithelium was verified for three patients (16.7%).
All 18 patients were contact lens wearers, and for all patients cysts were detectable in contact lens cases by lactophenol cotton blue staining (Table 1). Thirteen patients (72.2%) wore soft contact lenses, 3 (16.7%) wore rigid gas-permeable lenses, and 2 (11.1%) wore both types. Patients' ages ranged from 15 to 54 years (mean, 29 years). The most prevalent clinical signs we observed were chronic keratitis and keratoconjunctivitis; clinical signs for Acanthamoeba keratitis, namely, presence of keratitis with severe pain and photophobia, stromal infiltrates, radial keratoneuritis, and sometimes pseudodendriform epithelial lesions, were seen in seven patients (38.9%). In three of these patients the definitive diagnosis of Acanthamoeba keratitis was verified. Of the 18 patients, only these 3 showed no response to antibacterial or antiviral treatment. Moreover, these were the only three cases with acanthamoebae detectable in corneal scrapings.
TABLE 1.
Clinical data of patients with Acanthamoeba-associated keratitisa
| Patient code | Sexb | Age (yr) | Contact lens type | Clinical signs |
|---|---|---|---|---|
| 1OAP | F | 37 | Soft | Keratoconjunctivitis RE |
| 2HAP | F | 15 | Soft | Keratitis LE |
| 3PYP | M | 20 | RE, soft; LE, rigid gas permeable | Keratitis LE |
| 4REP | M | 29 | Soft | Keratoconjunctivitis LE |
| 5STP | F | 25 | Soft | Keratitis dentritica RE |
| 6DOP | M | 23 | Soft | Keratitis |
| 7TOP | F | 54 | Soft | Keratoconjunctivitis |
| 8PRP | M | 15 | Soft | Keratitis LE |
| 9GUP | F | 26 | Soft | Corneal infiltration RE |
| 10DRP | F | 18 | Soft | Keratitis superficialis RE |
| 11DSP | M | 41 | RE, soft; LE, rigid gas permeable | Keratitis superficialis LE |
| 12JOP | M | 43 | Rigid gas permeable | Ulcus corneae LE |
| 13PTP | F | 43 | Soft | Keratitis superficialis RE |
| 14SRP | M | 26 | Soft | Keratitis RE |
| 15SOP | M | 39 | Rigid gas permeable | Keratitis RE |
| 16KVP | F | 20 | Soft | Keratoconjunctivitis LE |
| 17SHP | F | 19 | Rigid gas permeable | Corneal infiltrates LE |
| 18MAP | F | 30 | Soft | Keratitis RE |
Cases in which acanthamoebae were confirmed as causative agents are boldface. In all other cases, acanthamoebae were detected only in lens storage cases. RE, right eye; LE, left eye.
M, male; F, female.
The Acanthamoeba keratitis patients were a 15-year-old female wearer of soft daily-wear lenses (2HAP), a 41-year-old male with rigid gas-permeable lenses in the afflicted left eye (11DSP), and a 39-year-old male wearer of rigid gas-permeable lenses in both eyes (15SOP). In two of these cases the initial diagnosis had been herpes simplex virus keratitis, which did not improve despite antiviral therapy. The time between clinical onset and correct diagnosis ranged from 1 to 5 weeks. Antiamoebic treatment consisted of local application of propamidine isethionate (Brolene eyedrops), hexamidine isethionate (Desomedine eyedrops), and bacitracin plus neomycin (Nebacetin ointment). No perforating keratoplasty was necessary. The first patient recovered, with a best corrected visual acuity of 20/20; the visual acuity of the second patient was 20/60 in the affected eye; the third patient still had corneal erosions, maybe caused by the toxicity of the medication.
Isolation and culture.
Contact lenses, corneal scrapings, and swabs from contact lens cases were transferred to nonnutrient agar plates covered with 100 μl of a 24-h-old culture of Escherichia coli in brain heart infusion medium. The plates were sealed and incubated at 30°C for 14 days and examined every 48 h for amoebal growth. Positive cultures were diluted in order to eliminate coexisting ciliates, flagellates, bacteria, and fungi by harvesting amoebae at a noncontaminated site of the plate with a sterile cotton-tipped applicator and transferring the amoebae to a fresh plate. All isolates were cloned with the use of a micromanipulator and incubated at various temperatures (30, 34, 37, and 42°C). They were examined daily by phase-contrast microscopy, and amoebal growth and temperature tolerances were recorded.
Identification and characterization.
Amoebae were identified as belonging to one of the cyst morphological groups (Acanthamoeba sp. groups I to III) established by Pussard and Pons (24), and species determination was performed according to the identification key of Page (23). Differentiation was achieved mainly on the basis of cyst size, number of opercula, and temperature tolerance.
Moreover, all isolates were examined for their cytopathic effects to a human cell line (HEp-2). Amoebae were axenized by harvesting cysts from the plate cultures, incubating them in 3% HCl overnight in order to eliminate the bacteria, and transferring the amoebae into liquid culture. As a liquid medium we used proteose peptone-yeast extract-glucose (23). We cultured the amoebae in 150-cm2 tissue culture flasks (Corning Costar, Bodenheim, Germany) at 30°C. Trophozoites were harvested from the axenic cultures by centrifugation (500 × g for 7 min) and transferred onto a monolayer of HEp-2 cells in an amoeba/cell ratio of 1/10. The amoebae were designated as highly cytopathic when the monolayer was completely lysed after 24 to 48 h.
Molecular biology analysis.
18S rDNA sequence analysis was performed for three isolates most divergent with respect to their physiological properties (strains 4RE, 9GU, and 11DS) in order to determine the differences of these strains from and their relatedness to the published strains from other parts of the world. The 4RE and the 9GU strains were derived from contact lens cases of non-Acanthamoeba keratitis patients, while the 11DS strain was isolated from the corneal scraping of Acanthamoeba keratitis patient 11DSP.
For molecular biology investigations amoebae (∼106 cells) were harvested from actively growing axenic cultures by centrifugation at 500 × g for 7 min. Whole-cell DNA was isolated by a modified UNSET procedure (14). Briefly, the pellet was resuspended in 500 μl of UNSET lysis buffer, overlaid with 500 μl of phenol-chloroform-isoamylalcohol (PCI), and shaken gently for 5 h. DNA was extracted by multiple PCI extraction, precipitated in alcohol, air dried and resuspended in 30 μl of sterile double-distilled water. The 18S rRNA gene was amplified using the SSU1 and SSU2 primers (9), complementary to the 5′ and 3′ ends of the gene, respectively, and a standard amplification program (30 cycles; 95°C for 1 min, 50°C for 2 min, 72°C for 3 min). Amplification of the 18S rRNA gene was visualized with ethidium bromide in an agarose gel electrophoresis. The amplified gene was sequenced stepwise by direct sequencing from the PCR product using the Thermo Sequenase II sequencing kit (Amersham Pharmacia Biotech GmbH, Vienna, Austria) and subsequent construction of complementary internal primers. Sequences were obtained from both strands. Sequencing was carried out in a 310 ABI PRISM automated sequencer (PE Applied Biosystems, Langen, Germany).
Sequence data were processed with the GeneDoc (22) sequence editor, and sequences were compared to the ones of published strains using a BLAST search (2). ClustalX (29) was used for pairwise alignment and calculation of the percentage of sequence dissimilarity.
Nucleotide sequence accession numbers.
Sequence data reported in this paper were deposited in GenBank and are available under the following reference numbers: strain 4RE, AF251937; strain 9GU, AF251938; strain 11DS, AF251939.
RESULTS
Identification and characterization of isolates.
In all, 20 strains of free-living amoebae including 15 Acanthamoeba strains, 3 Vahlkampfia strains, and 2 Hartmannella strains were isolated from clinical specimens (Table 2). Four specimens revealed two variant strains of free-living amoebae each (2HAP, 6DOP, 7TOP, and 8PRP), and in two cases culture was unsuccessful (12JOP and 13PTP). Fourteen of the 15 Acanthamoeba isolates were identified as belonging to Acanthamoeba sp. group II. However, several strains exhibited rather varied cyst morphologies with respect to size and number of opercula although they were derived from a clone. These strains were classified according to the average cyst morphology. One strain (18MA) was designated A. lenticulata (morphological group III), the cysts being rather small and round. No isolate exhibited a group I morphology. In all, A. hatchetti was identified six times, A. rhysodes and A. polyphaga were each identified three times, A. triangularis was identified twice, and A. lenticulata was identified once.
TABLE 2.
Parasitological data for patients with Acanthamoeba-associated keratitisa
| Patient code | LPCBb stain | Culture | Morphological identification | Strain code | Temperature tolerance (°C) | Cytopathic effectd |
|---|---|---|---|---|---|---|
| 1OAP | P | P | Vahlkampfia sp. | 1OA | 30 | − |
| 2HAP | P | P | A. rhysodes | 2HAA | 37 | − |
| A. hatchetti | 2HAB | 42 | +++ | |||
| 3PYP | P | P | V. debilis | 3PY | 30 | − |
| 4REP | P | P | A. hatchetti | 4RE | 37 | − |
| 5STP | P | P | A. hatchetti | 5ST | 37 | − |
| 6DOP | P | P | A. rhysodes | 6DOA | 34 | − |
| Hartmannella sp. | 6DOB | 30 | − | |||
| 7TOP | P | P | A. hatchetti | 7TOA | 37 | − |
| V. enterica | 7TOB | 30 | − | |||
| 8PRP | P | P | A. triangularis | 8PRA | 30 | − |
| H. vermiformis | 8PRB | 30 | − | |||
| 9GUP | P | P | A. polyphaga | 9GU | 37 | ++ |
| 10DRP | P | P | A. triangularis | 10DR | 30 | − |
| 11DSP | P | P | A. hatchetti | 11DS | 42 | +++ |
| 12JOP | P | N | No growth | 12JO | NDc | ND |
| 13PTP | P | N | No growth | 13PT | ND | ND |
| 14SRP | P | P | A. hatchetti | 14SR | 37 | − |
| 15SOP | P | P | A. polyphaga | 15SO | 37 | ++ |
| 16KVP | P | P | A. polyphaga | 16KV | 37 | ++ |
| 17SHP | P | P | A. rhysodes | 17SH | 34 | − |
| 18MAP | P | P | A. lenticulata | 18MA | 37 | − |
Cases in which acanthamoebae were confirmed as causative agents are boldface. In all other cases, acanthamoebae were detected only in lens storage cases. P, positive; N, negative.
LPCB, lactophenol cotton blue.
ND, no data.
The number of plus signs is a measure of the cytopathic effect. −, no cytopathic effect.
Acanthamoeba spp. had been proven to be of clinical relevance in three cases (2HAP, 11DSP, and 15SOP). All of these isolates showed an Acanthamoeba group II cyst morphology. In one case (2HAP) two different strains of Acanthamoeba, A. rhysodes and A. hatchetti, were detected. The other keratitis-causing isolates were identified as A. hatchetti (11DS) and A. polyphaga (15SO).
Temperature tolerance tests revealed that all 20 isolates of free-living amoebae grew at 30°C; 13 of them also grew at 34°C, 11 strains grew at 37°C, and 2 strains grew at 42°C. All of the thermophilic strains were among the Acanthamoeba isolates. The two Vahlkampfia strains and the Hartmannella strain showed no growth above 30°C. Five isolates (2HAB, 9GU, 11DS, 15SO, and 16KV) showed cytopathic effects against monolayers of HEp-2 cells. Strains 2HAB, 11DS, and 15SO had been isolated from Acanthamoeba keratitis patients, yet two strains (9GU and 16KV) isolated from patients without Acanthamoeba keratitis also exhibited moderate cytopathic effects (monolayer lysis after 3 to 4 days). From the two different Acanthamoeba strains isolated from patient 2HAP only one (2HAB) showed pathogenicity-related physiological characteristics. Eventually only this strain was responsible for the infection, while the other strain (2HAA), with no pathogenicity-associated characteristics, was only a concontaminant.
The clinically relevant strains 2HAB, 11DS, and 15SO not only showed high-temperature tolerance and high cytopathic effects but also generally exhibited far higher growth rates than the other isolates.
Molecular biology analysis.
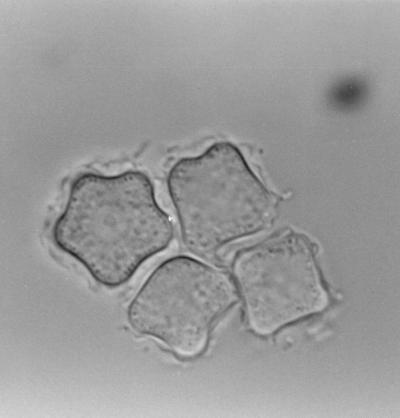
All strains investigated revealed more than 97% sequence identity to classified published strains (Table 3) and could thus be assigned to one of the 12 published sequence types, as sequence types differ from one another by at least 5% (27). Nonpathogenic strain 4RE (GenBank accession no. AF251937), having been identified as A. hatchetti, displayed sequence type T11, with 99.3% identity to the BH-2 A. hatchetti strain isolated in brackish water in the United States (GenBank accession no. AF019068). The 9GU strain (GenBank accession no. AF251938), which did not cause disease but which is pathogenic to tissue culture, showed 98.6% identity to the Castellani strain of A. castellanii (GenBank accession no. U07413), which has sequence type T4. This strain of A. castellanii has been isolated from a yeast culture in the United Kingdom and is the type strain for the species A. castellanii. We thus reclassified our isolate, initially classified as A. polyphaga, as A. castellanii. Most of the published A. polyphaga isolates show sequence type T4, as do most of the A. castellanii isolates. Stothard et al. (27) proposed to reclassify all sequence type T4 isolates as A. castellanii. Strain 11DS (GenBank accession no. AF251939), isolated from the contact lens case of a patient with serious Acanthamoeba keratitis, showed an 18S rDNA sequence with highest identity (97.7%) to the 2802 strain of A. palestiniensis with sequence type T6, isolated in a swimming pool in France (GenBank accession no. AF019063). However, the 11DS strain exhibits a typical A. hatchetti morphology, with most of the cysts being rectangular (Fig. 1), and the isolate showed the ability to grow at 42°C, while A. palestiniensis is described as showing no growth even at 37°C (23). Moreover, because A. hatchetti and A. palestiniensis are described as polyphyletic (27), we prefer not to reclassify this isolate.
TABLE 3.
Sequence identity of isolates to published strains
| Isolate | % Identity | Strain | Habitat | GenBank accession no. | Sequence type | Morphological group |
|---|---|---|---|---|---|---|
| 4RE | 99.3 | A. hatchetti BH-2 | Brackish water, United States | AF019068 | T11 | II |
| 9GU | 98.6 | A. castellanii Castellani | Yeast culture, United Kingdom | U07413 | T4 | II |
| 11DS | 97.7 | A. palestiniensis 2802 | Swimming pool, France | AF019063 | T6 | III |
FIG. 1.
Cysts of the 11DS isolate with typical A. hatchetti morphology, as shown by phase-contrast microscopy. Magnification, ×1,000.
The sequences of our strains clearly diverged from one another, all exhibiting different sequence types, namely, T4, T6, and T11. The 4RE and the 9GU strains were more closely related to one another (5.57% dissimilarity) than either was to the 11DS strain. The 4RE and 9GU strains showed 9.68 and 9.40% sequence dissimilarities, respectively, to the 11DS strain.
DISCUSSION
Identification and characterization of isolates.
Altogether 15 strains of Acanthamoeba, 3 strains of Vahlkampfia, and 2 strains of Hartmannella were isolated. During the last few years it has become apparent that Vahlkampfia and Naegleria (11) as well as Hartmannella (1, 16) can cause keratitis or at least be associated with keratitis. However, neither the Hartmannella strain nor the Vahlkampfia strains were of clinical relevancy in our study.
In two cases the amoebae could not be grown in vitro. This may partially be due to the fact that patients had already been treated for bacteria, and antibacterial and antifungal treatment is at least partly effective against free-living amoebae. On the other hand, contact lens disinfectants, even if not used properly, compromise amoebal viability. Moreover, amoebae penetrate the cornea during the course of infection, protruding up to Descemet's membrane. It might therefore in some cases be impossible to isolate viable amoebae by scraping. Several studies report on unsuccessful attempts to culture amoebae from clinical specimens (15, 26).
In general most of the acanthamoebae isolated were identified as belonging to Acanthamoeba sp. group II, which also is reported to be the most prevalent group of these microorganisms (23). In a former study we could demonstrate that in the area of Vienna Naegleria seems to be the predominant genus in environmental habitats, whereas probing of shower heads and tap water mainly revealed amoebae of the genera Acanthamoeba and Hartmannella (our unpublished data). This supports the assumption that, in Acanthamoeba keratitis, infection is primarily acquired via contaminated contact lenses and lens cases, the amoebae deriving mainly from tap water, rather than by outdoor swimming. Domestic tap water as source of Acanthamoeba sp. in Acanthamoeba keratitis was described for the first time in 1990 (17). Interestingly two of the Acanthamoeba keratitis patients (n = 3) discussed here wore rigid gas-permeable contact lenses in the afflicted eyes. Usually wearers of soft lenses are more likely to acquire Acanthamoeba keratitis, as the hydrophilic material seems to support attachment and survival of cysts. However, several cases of Acanthamoeba keratitis in wearers of rigid gas-permeable contact lenses have been reported (4, 25). A case of Acanthamoeba keratitis occurring in a wearer of daily-disposable contact lenses has also been documented recently (31). We did not observe any correlation to age or sex, and in none of the three patients did surgical treatment become necessary. In a study from the United Kingdom severe visual loss was seen in about 15% of the patients (25).
Morphological determination was rather difficult in some cases as cysts, although all deriving from one clone, had varied morphologies, and generally intraspecific polymorphism is rather common among acanthamoebae (3). The four Acanthamoeba strains isolated from the contact lens cases of the three Acanthamoeba keratitis patients were identified by cyst morphology as A. rhysodes, A. polyphaga, and two strains of A. hatchetti. All of these species are known to cause keratitis (26). To our knowledge this is the first determination of keratitis-causing strains in Austria. In 1989, Huber-Spitzy et al. described a case of Acanthamoeba keratitis in a 37-year-old woman in Austria (13). They detected cysts of Acanthamoeba in the corneal epithelium, but the amoeba was neither isolated nor identified to the species level.
Clinical relevance of strains.
Remarkably, in the majority of keratitis cases we investigated, the acanthamoebae were of no clinical relevance. Although Acanthamoeba keratitis has become of increasing importance within the last 10 years, correlating to the greater number of contact lens wearers, it is still a rare disease. The annualized incidence of Acanthamoeba keratitis is estimated as 0.14 per 100,000 individuals (25).
Also other studies revealed that the majority of people do not develop a keratitis in spite of coming into contact with acanthamoebae; the prevalence of acanthamoebae and other free-living amoebae in asymptomatic contact lens wearers has been reported frequently (8, 18). Even from the nasal mucosae of healthy individuals different strains of Acanthamoeba spp. could be isolated (20). Apart from such widely accepted risk factors as extended contact lens wear and microlesions in the cornea, certainly the immune status of the patient may play a fundamental role in the course of infection and may result in enhanced susceptibility to developing an Acanthamoeba keratitis. It was shown that nearly 50% of healthy individuals carry antibodies against Acanthamoeba, probably due to the ubiquity of this microorganism (28).
Moreover, apart from differences in predisposition of patients, one can assume that amoeba strains vary in pathogenicity. Mazur et al. demonstrated in an animal model a clear correlation between the occurrence of eye infections and the degree of virulence of the strains after installation into the brain (19), which suggests that pathogenicity is not so much dependent on environmental conditions but rather represents a distinct characteristic of certain strains. Nevertheless the initial infective dose is certainly of considerable importance. The factors which prime amoebae for pathogenicity are poorly understood. Temperature tolerance (10) and cell culture pathogenicity (3, 5, 6) seem to be related to pathogenicity potential. Temperature tolerance is widely accepted to be a prerequisite for pathogenicity in granulomatous amoebic encephalitis caused by Acanthamoeba, as the human body temperature is 37°C. However, the human eye has a mean temperature of only around 34°C. Yet data presented here indicate that clinically relevant strains not only show higher temperature tolerances but also generally yield far higher growth rates, which certainly is of major importance for the establishment of infection. Temperature tolerance among keratitis-causing Acanthamoeba strains is rather conspicuous (3, 7). Moreover, the keratitis-causing acanthamoebae described here were highly cytopathic to HEp-2 cells, producing complete destruction of the monolayer in 1 to 2 days. Altogether, there seems to be a correlation between clinical relevance and pathogenicity-related physiological characteristics.
The occurrence of free-living amoebae of nonpathogenic strains in contact lens cases, however, is still of medical interest, as they can harbor bacteria inside their cysts, protecting them from disinfectants, and thus function as vectors. In a recent study we showed that viable Pseudomonas aeruginosa, one of the major ocular pathogens, can survive in and be reisolated from cysts of Acanthamoeba (30).
Molecular biology analysis.
Interestingly, the three investigated strains showing the most-diverse physiological capacities also differed with respect to their 18S rDNA sequences, exhibiting three different sequence types. The two strains with no clinical relevancy, both classified as group II acanthamoebae, showed sequence type T4 (strain 9GU) and sequence type T11 (strain 4RE). Sequence types T3, T4, and T11 are most closely related, and all represent morphological group II (27). T4 is reported to be the sequence type isolated most frequently. Moreover, the majority of keratitis-causing Acanthamoeba strains are reported to belong to sequence type T4 (27).
The keratitis-causing and highly cytopathic strain 11DS, identified as A. hatchetti, morphological group II, exhibited sequence type T6. Sequence type T6 is represented by a single strain, which had been identified morphologically as A. palestiniensis belonging to morphological group III. Stothard et al. also report on a strain presumed to be group II but with a group III sequence. The authors assume that A. palestiniensis and A. hatchetti are polyphyletic (27). However, a final system does not yet exist, and most probably diversification will still be necessary. T6 isolates have not previously been reported to cause keratitis. Also no keratitis-causing strain is known to exhibit sequence type T2, which is the sequence type most closely related to sequence type T6.
Altogether the results of our study support the assumption that pathogenicity in Acanthamoeba seems to be a distinct capability of certain strains and not so much dependent on appropriate conditions for the establishment of an infection. Moreover, data presented here indicate that Acanthamoeba keratitis-causing strains as well as nonpathogenic strains of Acanthamoeba in Austria are most closely related to published strains from other parts of the world.
REFERENCES
- 1.Aitken D, Hay J, Kinnear F B, Kirkness S M, Lee W R, Seal D V. Amebic keratitis in a wearer of disposable contact lenses due to a mixed Vahlkampfia and Hartmannella infection. Ophthalmology. 1996;103:485–494. doi: 10.1016/s0161-6420(96)30667-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Badenoch P R, Adams M, Coster D J. Corneal virulence, cytopathic effect on human keratocytes and genetic characterisation of Acanthamoeba. Int J Parasitol. 1995;25:229–239. doi: 10.1016/0020-7519(94)00075-y. [DOI] [PubMed] [Google Scholar]
- 4.Cohen E J, Fulton J C, Hoffman C J, Rapuano C J, Liabson P R. Trends in contact lens-associated corneal ulcers. Cornea. 1996;15:566–570. [PubMed] [Google Scholar]
- 5.Cursons R T M, Brown T J. Use of cell cultures as an indicator of pathogenicity of free-living amoebae. J Clin Pathol. 1978;31:1–11. doi: 10.1136/jcp.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonckheere J F. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol. 1980;39:681–685. doi: 10.1128/aem.39.4.681-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dini L A, Cockinos C, Frean J A, Niszl I A, Markus M B. Unusual case of Acanthamoeba polyphaga and Pseudomonas aeruginosa keratitis in a contact lens wearer from Gauteng, South Africa. J Clin Microbiol. 2000;38:826–829. doi: 10.1128/jcm.38.2.826-829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donzis P B, Mondino B J, Weissman B A, Bruckner D A. Microbial contamination of contact lens care systems. Am J Ophthalmol. 1987;104:325–333. doi: 10.1016/0002-9394(87)90219-4. [DOI] [PubMed] [Google Scholar]
- 9.Gast R J, Ledee D R, Fuerst P A, Byers T. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J Eukaryot Microbiol. 1996;43:498–504. doi: 10.1111/j.1550-7408.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 10.Griffin J L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebae. Science. 1972;178:869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- 11.Harminder S D, Azurara-Blanco A, Hossain M, Lloyd J. Non-Acanthamoeba amebic keratitis. Cornea. 1998;17:675–677. doi: 10.1097/00003226-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hay J, Kinnear F B, Kirkness C M, Seal D V. Acanthamoeba keratitis: laboratory diagnosis, characterisation of protozoa and treatment. Scieh Weekly Rep. 1995;29:90–91. [Google Scholar]
- 13.Huber-Spitzy V, Grabner G, Arocker-Mettinger E, Baumgartner I, Skorpik F, Rappersberger C, Haddad R. Acanthamoeba keratitis. An underdiagnosed entity? Klin Monatsbl Augenheilkd. 1989;194:454–457. doi: 10.1055/s-2008-1046400. [DOI] [PubMed] [Google Scholar]
- 14.Hugo E R, Stewart V J, Gast R J, Byers T J. Purification of amoeba mtDNA using the UNSET procedure. In: Soldo A T, Lee J J, editors. Protocols in protozoology. Lawrence, Kans: Allen; 1992. pp. D7.1–7.2. [Google Scholar]
- 15.Illingworth C D, Cook S D. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508. doi: 10.1016/s0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy S M, Devine P, Hurley C, Ooi Y S, Collum L M T. Corneal infection associated with Hartmannella vermiformis in contact lens wearer. Lancet. 1995;346:637–638. [PubMed] [Google Scholar]
- 17.Kilvington S, Larkin D F, White D G, Beeching J R. Laboratory investigation of Acanthamoeba keratitis. J Clin Microbiol. 1990;28:2711–2725. doi: 10.1128/jcm.28.12.2722-2725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin D F P, Kilvington S, Easty D L. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol. 1990;74:133–135. doi: 10.1136/bjo.74.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazur T, Hadas E, Gustowska L, Winiecka-Krusnell J, Linder E. Secondary amoebic eye infections in mice due to Acanthamoeba sp. Parasitol Res. 1999;85:776–778. doi: 10.1007/s004360050630. [DOI] [PubMed] [Google Scholar]
- 20.Michel R, Röhl R, Schneider H. Isolation of free-living amoebae from nasal mucosa of healthy individuals. Zentbl Bakteriol Hyg. 1982;176:155–159. [PubMed] [Google Scholar]
- 21.Nagington F, Watson P G, Playfair T J, McGill J, Hones B R, Steele A D M. Amoebic infection of the eye. Lancet. 1974;2:1537–1540. doi: 10.1016/s0140-6736(74)90285-2. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas K B, Nicholas H B, Jr, Deerfield D W., II GeneDoc: analysis and visualization of genetic variation. Embnew News. 1997;4:14. [Google Scholar]
- 23.Page F C. Nackte Rhizopoda. In: Page F C, Siemensma F J, editors. Nackte Rhizopoda und Heliozoa. G. Stuttgart, Germany: Fischer; 1991. pp. 3–170. [Google Scholar]
- 24.Pussard M, Pons R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida) Protistologica. 1977;8:557–598. [Google Scholar]
- 25.Radford C F, Lehmann O J, Dart J K G. Acanthamoeba keratitis: multicentre survey in England 1992–6. Br J Ophthalmol. 1998;82:1387–1392. doi: 10.1136/bjo.82.12.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaumberg D A, Snow K K, Dana M R. The epidemic of Acanthamoeba keratitis: where do we stand? Cornea. 1998;17:3–10. doi: 10.1097/00003226-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Stothard D R, Schroeder-Dietrich J M, Awwad M H, Gast R J, Ledee D R, Rodriguez-Zaragoza S, Dean C L, Fuerst P A, Byers T. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rDNA gene sequence types. J Eukaryot Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka Y, Suguri S, Harada M, Hayabara T, Suzumori K, Otha N. Acanthamoeba-specific human T-cell clones isolated from healthy individuals. Parasitol Res. 1994;80:549–553. doi: 10.1007/BF00933001. [DOI] [PubMed] [Google Scholar]
- 29.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walochnik J, Picher O, Aspöck C, Ullmann M, Sommer R, Aspöck H. Interactions of “Limax amoebae” and gram-negative bacteria: experimental studies and review of current problems. Tokai J Exp Clin Med. 1999;23:273–278. [PubMed] [Google Scholar]
- 31.Woodruff S A, Dart J K G. Acanthamoeba keratitis occurring with daily disposable contact lens wear. Br J Ophthalmol. 1999;83:1088–1089. doi: 10.1136/bjo.83.9.1088a. [DOI] [PMC free article] [PubMed] [Google Scholar]