Abstract
Objective
Environmental toxicants are suspected to play a part in the pathogenesis of idiopathic Parkinson’s disease (PD) and may underlie its increasing incidence. Mercury exposure in humans is common and is increasing due to accelerating levels of atmospheric mercury, and mercury damages cells via oxidative stress, cell membrane damage, and autoimmunity, mechanisms suspected in the pathogenesis of PD. We therefore compared the cellular distribution of mercury in the tissues of people with and without PD who had evidence of previous mercury exposure by mercury being present in their locus ceruleus neurons.
Materials and methods
Paraffin sections from the brain and general organs of two people with PD, two people without PD with a history of mercury exposure, and ten people without PD or known mercury exposure, were stained for inorganic mercury using autometallography, combined with immunostaining for a-synuclein and glial cells. All had mercury-containing neurons in locus ceruleus neurons. Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) was used to confirm the presence of mercury and to look for other potentially toxic elements. Autometallography-stained locus ceruleus paraffin sections were examined to compare the frequency of previous mercury exposure between 20 PD and 40 non-PD individuals.
Results
In PD brains, autometallography-detected mercury was seen in neurons affected by the disease, such as those in the substantia nigra, motor cortex, striatum, thalamus, and cerebellum. Mercury was seen in oligodendrocytes in white and grey matter. Mercury often co-localised with Lewy bodies and neurites. A more restricted distribution of brain mercury was seen in people without PD (both with or without known mercury exposure), with no mercury present in the substantia nigra, striatum, or thalamus. The presence of autometallography-detected mercury in PD was confirmed with LA-ICP-MS, which demonstrated other potentially toxic metals in the locus ceruleus and high iron levels in white matter. Autometallography-detected mercury was found in locus ceruleus neurons in a similar proportion of PD (65%) and non-PD (63%) individuals.
Conclusions
In people with PD, mercury was found in neurons and oligodendrocytes in regions of the brain that are affected by the disease, and often co-localised with aggregated a-synuclein. Mercury in the motor cortex, thalamus and striatum could result in bradykinesia and rigidity, and mercury in the cerebellum could cause tremor. People without PD had a restricted uptake of mercury into the brain. The similar frequency of mercury in the locus ceruleus of people with and without PD suggests these two groups have had comparable previous mercury exposures but that PD brains have a greater predisposition to take up circulating mercury. While this post mortem study does not provide a direct link between mercury and idiopathic PD, it adds to the body of evidence that metal toxicants such as mercury play a role in the disease. A precautionary approach would be to reduce rising mercury levels in the atmosphere by limiting the burning of fossil fuels, which may be contributing to the increasing incidence of PD.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative condition with a large socioeconomic burden [1]. Clinical and pathological features of PD include a world-wide distribution, male predominance, a variable clinical presentation and progress, motor, non-motor and multisystem clinical involvement, a long prodromal period during which constipation and sleep disorders are common, pathogenic pathways involving oxidative stress, inflammation, and damage to membrane systems such as mitochondria and lysosomes, and collections of aggregated a-synuclein in Lewy bodies and neurites [1]. The incidence and prevalence of PD have risen markedly in the past two decades [1]. This growth is not all attributable to population aging, and we appear to be facing a Parkinson’s pandemic [2].
Single gene abnormalities and genetic variants with low individual effect sizes are responsible for only a minority of later-onset PD cases [1]. The reported increased risk of PD in people with an affected first-degree relative could indicate a shared environment, with twin studies showing a similar concordance in monozygotic and dizygotic twins, and 90% of people with PD have no family history of PD [3]. Attention has therefore turned to environmental factors that could trigger PD, possibly via gene-environment interactions [3–6]. Epidemiological studies, some supported by animal and in vitro models of PD pathology, have implicated potentially toxic substances including pesticides, solvents, and metals such as iron and mercury as possible pathogenic agents [3]. However, it is difficult to estimate toxicant exposures from epidemiological data, especially for diseases that may start in early life. In addition, bulk chemical analyses of tissues affected by PD are not sensitive enough to detect toxicants that affect only a small proportion of cells. Therefore, elemental bioimaging of potentially toxic elements at the cellular level is needed to estimate the toxicant burden in the brains of people with neurodegenerative disorders [7].
Several indirect lines of evidence suggest that an environmental toxicant such as mercury could play a role in PD. The steadily increasing amount of mercury vapor in the atmosphere [8], and consequently in fish [9], could underlie the increased incidence of PD [2]. Atmospheric mercury is distributed world-wide via the atmosphere-water-soil cycle [10, 11], which accords with the global prevalence of PD [12]. Men are more likely to be exposed to mercury in industrial occupations, and men are more at risk of getting PD [1]. The uptake of mercury in humans is variable and depends on the chemical form of mercury and source of exposure [13, 14], which fits with the variation in PD symptoms between patients [1]. An early-life exposure to mercury, for example from eating mercury-contaminated fish, could seed the brain and other organs with methylmercury, with later cell damage due to the slow demethylation of methylmercury to inorganic mercury [15], which would result in PD motor symptoms manifesting only later in life [1]. The prevalence of mercury in human cells increases with aging [16], which could underlie the symptoms of PD increasing in later ages. Mercury has multiple toxic effects which include the generation of free radicals [17], autoimmune inflammation [18], and attachment to sulfhydryl-rich cell membranes in organelles such as mitochondria, lysosomes, and the Golgi apparatus [13, 19], all features implicated in the pathogenesis of PD [1]. Finally, mercury can induce a-synuclein aggregation [20] and so could be involved in the generation of Lewy bodies and neurites.
To look for evidence that mercury could underlie some pathogenetic aspects of PD, we used two elemental bioimaging techniques to study the distribution of mercury in people with and without PD. To look for differences in mercury uptake, we chose to study people with evidence of previous mercury exposure based on their having mercury in locus ceruleus neurons, where mercury persists for long periods of time [7]. We studied tissue from forensic/coronial autopsies, since people who die unexpectedly or from unnatural causes are more likely to have earlier stages of PD, which makes identifying causative toxicants more feasible. Furthermore, in forensic autopsies non-central nervous system (CNS) organs are sampled so any role mercury might play in these organs in PD [21–23] could be assessed.
Materials and methods
Ethics
This study (X14-029) was approved by the Human Research Committee, Sydney Local Health District (Royal Prince Alfred Hospital Zone). This institutional review board waived the need for written informed consent from relatives of individuals studied since this was a de-identified retrospective study of archived paraffin-embedded tissue. Data were fully anonymised on the research database after initial access to Department of Forensic Medicine records.
Sample collection
Multifocal brain samples
Paraffin-embedded tissue blocks from multifocal locations within the cerebrum, cerebellum and brain stem were obtained from the tissue archive of The New South Wales Department of Forensic Medicine from 14 people who had evidence of previous exposure to mercury because their locus ceruleus neurons in the rostral pons had been found in a previous study to contain mercury [7]. The samples studied were from: (1) Two women with a clinical diagnosis of idiopathic PD and neuropathological evidence of Lewy body-positive PD, aged 72 and 76 years (PD1 and PD2), (2) Two men with known sources of mercury exposure, one aged 24 years who had been exposed to self-injected metallic mercury for five months (ME1) [24], and one a professional fisherman aged 39 years who was probably exposed to methylmercury from seafood consumption (ME2) [25], and (3) Ten people without PD and without known sources of mercury exposure, aged 59–104 years, 3 male and 7 female.
Locus ceruleus-only samples
To compare the frequency of mercury exposure of people with and without PD, paraffin sections of rostral pons containing the locus ceruleus that had been stained with autometallography in a previous study [7] were re-examined. These were from (1) 20 PD patients (10 male, 10 female, mean age 74 years, SD 9 years, age range 59–95 years), including the two PD patients above, and (2) 40 non-PD individuals (20 male, 20 female, mean age 78 years, SD 14 years, age range 59–95 years, consisting of 20 with non-PD neurodegenerative disorders (15 with Alzheimer’s disease, 2 with multiple system atrophy, and one each with frontotemporal dementia, progressive supranuclear palsy, and myotonic dystrophy), 15 with no major pre-mortem conditions, 4 with a psychosis (2 with bipolar disorder, and 1 each with depression and schizophrenia), and 1 with cancer. The frequencies of the presence of autometallography-detected iHg in locus ceruleus neurons in these two groups were compared with contingency analysis and Fisher’s exact test using Prism 9 software.
Autometallography
Paraffin blocks were sectioned at 7 μm with a Feather S35 stainless steel disposable microtome blade, deparaffinised, and stained with silver nitrate autometallography, which represents the presence of inorganic mercury (iHg) as black silver grains surrounding the mercury [26]. Autometallography is a sensitive amplification technique that can detect as few as 10 mercury sulphide/selenide molecules in a cell [27]. Sections were placed in physical developer containing 50% gum arabic, citrate buffer, hydroquinone and silver nitrate at 26°C for 80 minutes in the dark, washed in 5% sodium thiosulphate to remove unbound silver, counterstained with mercury-free hematoxylin or Luxol-fast blue, and viewed with bright-field microscopy. Sections were stained with hematoxylin only to act as a control for the autometallography. Each staining run included a control section of mouse spinal cord where motor neuron cell bodies contained mercury following an intraperitoneal injection of mercuric chloride; these sections were from archived paraffin blocks of a previously-published experiment approved by the Animal Ethics Committee of the University of Sydney [28]. Representative sections were stained with autometallography and then immunostained for a-synuclein with 1:500 monoclonal mouse-anti-human alpha-synuclein (Invitrogen 328100), astrocytes with 1:2000 polyclonal rabbit-anti-human glial fibrillary acidic protein (Dako Z0334), microglia/macrophages with 1:400 monoclonal mouse-anti-human CD68 (Dako M0876), and endothelial cells with 1:100 monoclonal mouse-anti-human CD31 (Dako JC70A). Antibodies were visualised using diaminobenzidine tetrahydrochloride (DAB), or Magenta Substrate Chromogen System GV925 when the black autometallography staining was obscured by the brown DAB. Oligodendrocytes were identified by their characteristic GFAP-negativity, cleared cytoplasm and contrast-enhanced nuclei.
Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS)
To confirm which metal autometallography was demonstrating, since autometallography can also detect inorganic silver and bismuth [29, 30], and to look for the presence of other potentially toxic elements, 7 μm paraffin sections of representative sections from patient PD2 were deparaffinised and subjected to LA-ICP-MS for mercury, silver, bismuth, aluminium, gold, cadmium, chromium, iron, nickel, lead and potassium. Analyses were carried out on a New Wave Research NWR-193 laser and a Teledyne Cetac LSX-213 G2+ laser hyphenated to an Agilent Technologies 7700x ICP-MS, with argon used as the carrier gas. LA-ICP-MS conditions were optimised on NIST 612 Trace Element in Glass CRM and the sample was ablated with a 50 μm spot size and a scan speed of 100 μm/s at a frequency of 20 Hz. The data were collated into a single image file using in-house developed software [31] and visualised using FIJI.
Results
Multifocal brain autometallography
The presence of autometallography-detected iHg was compared between the brains of people with PD, people without PD who had a known source of mercury exposure, and people without PD or a known source of mercury exposure, all of whom had iHg detected in locus ceruleus neurons.
Parkinson’s disease patients
Neurons. In the two people with PD, neurons containing cytoplasmic iHg were confirmed to be present in the locus ceruleus, and were also seen in some of the few surviving neurons of the substantia nigra compacta (referred to here as the substantia nigra), the frontal motor cortex (mostly corticomotoneurons/Betz cells), the striatum (medium-sized neurons in the caudate and putamen), thalamus, cerebellar cortex (Purkinje and granule cells), cerebellar dentate nucleus, lateral geniculate nucleus [32], dorsal raphe nucleus, cranial nerve motor nuclei 5, 6 and 7, dorsal vagal nucleus, nucleus ambiguus, and inferior olivary nucleus (Figs 1 and 2 and Table 1). The distribution of mercury between the two PD patients mostly overlapped, but some variability was seen: PD1 did not have mercury in cranial nerve motor nuclei or amygdala neurons, and PD2 did not have cerebellar neuronal mercury. Mercury was not seen in either patient in neurons of the globus pallidus, the subthalamic nucleus, the substantia nigra reticulata, or the basal nucleus.
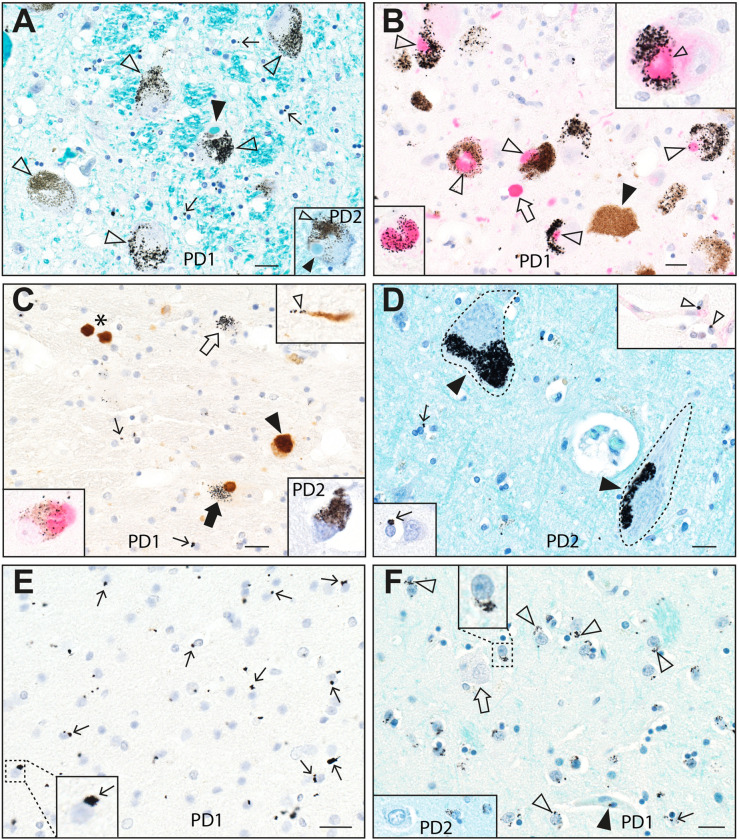
Fig 1. Mercury in the locus ceruleus, substantia nigra, cerebral cortex and striatum in Parkinson’s disease.
(A) Black mercury grains (eg, open arrowheads) are present in the cytoplasm of most locus ceruleus neurons in PD1 (main image) and PD2 (inset). Lewy bodies with haloes (filled arrowheads) are seen in some mercury-containing neurons. Numerous oligodendrocytes have small mercury deposits (eg, arrows) adjacent to their nuclei. Autometallography/Luxol fast blue. (B) Magenta immunostaining of a-synuclein shows co-localisation of Lewy bodies (open arrowheads) with black-staining mercury in locus ceruleus neurons of PD1. An extra-neuronal a-synuclein aggregate (open arrow) has no associated mercury. No Lewy bodies are present in a locus ceruleus neuron not containing mercury (filled arrowhead). The right upper inset shows a nearby locus ceruleus neuron at higher magnification with Lewy body/mercury co-localisation. The left lower inset shows mercury within a Lewy body. Autometallography/a-synuclein Magenta/hematoxylin. (C) Brown DAB immunostaining of a-synuclein shows co-localisation of a Lewy body with mercury (filled arrow) in a remaining substantia nigra neuron of PD1. A nearby Lewy neurite (right upper inset) shows associated mercury grains (open arrowhead). Some intraneuronal (filled arrowhead) and extraneuronal (*) Lewy bodies do not appear to contain mercury, possibly because of masking by the dense brown DAB staining. Scattered oligodendrocytes have small mercury deposits (eg, thin arrows). Autometallography/a-synuclein DAB/hematoxylin. Left lower inset: Magenta a-synuclein staining shows black-staining mercury co-localised with a Lewy body in a nearby substantia nigra neuron (autometallography/a-synuclein Magenta/hematoxylin). Right lower inset: black-staining mercury is also present in a few remaining substantia nigra neurons in PD2 (autometallography/Luxol fast blue). (D) Dense mercury grains (filled arrowheads) are present in two corticomotoneuron cell bodies (dashed outlines) of PD2. Scattered oligodendrocytes (arrows) have small mercury deposits (one magnified in the left lower inset). Autometallography/Luxol fast blue. Two nearby pericytes contain mercury deposits (open arrowheads, right upper inset, autometallography/CD31/hematoxylin). (E) Numerous oligodendrocytes in the parietal white matter of PD1 have mercury deposits (eg, arrows) either attached to the nuclear membrane or adjacent to the nucleus. A magnified view is shown in the left lower inset. Autometallography/a-synuclein/hematoxylin. (F) Mercury grains (eg, open arrowheads) are present in the paranuclear region of medium-sized neurons in the putamen (one enlarged in the upper inset) of PD1. A large neuron (open arrow) contains no mercury. Small mercury deposits are present adjacent to nuclei of scattered oligodendrocytes (thin arrow) and pericytes (closed arrowhead). In the left lower inset, a similar distribution of mercury is seen in PD2 in medium-sized (right) but not large (left) neurons in the putamen. Autometallography/Luxol fast blue. PD: case numbers. Bars = 20 μm.
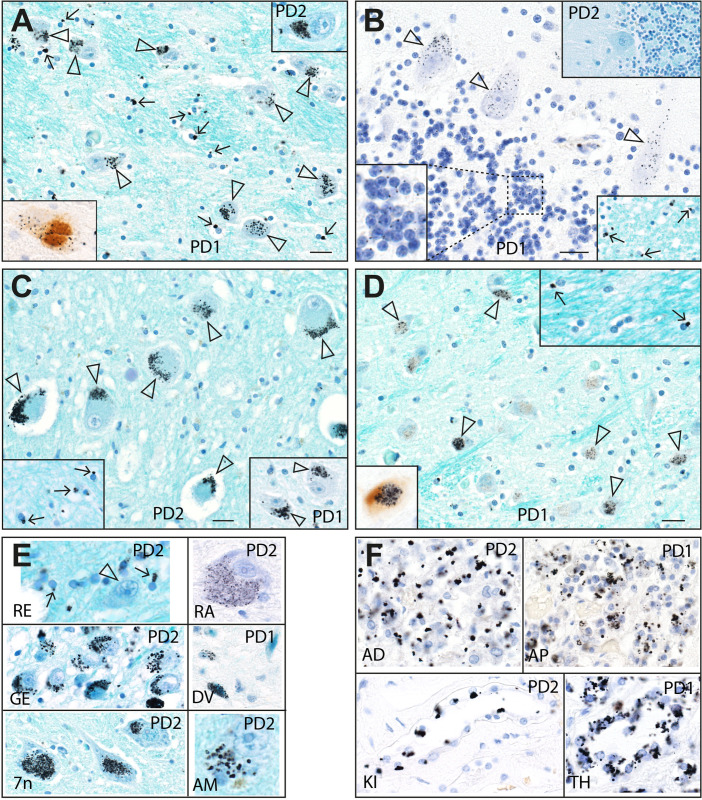
Fig 2. Mercury in the thalamus, cerebellum, other CNS regions, and non-CNS organs in Parkinson’s disease.
(A) Black mercury grains are present in the cytoplasm of most thalamic neurons (eg, open arrowheads), in both PD1 (main image) and PD2 (right upper inset). Numerous oligodendrocytes have small mercury deposits (eg, arrows) adjacent to their nuclei. Autometallography/Luxol fast blue. A PD1 thalamic neuron with mercury co-localised with aggregated a-synuclein is seen (left lower inset, autometallography/a-synuclein DAB/hematoxylin). (B) Cerebellar Purkinje cells of PD1 contain small scattered cytoplasmic mercury grains (arrowheads). Cerebellar granule cells contain single paranuclear mercury grains (enlarged in the left lower inset). Scattered oligodendrocytes in the adjacent cerebellar white matter contain paranuclear mercury grains (eg, arrows, right lower inset). No mercury is present in the cerebellar cortex of PD2 (right upper inset). Autometallography/Luxol fast blue. (C) Black mercury grains (arrowheads) are present in the cytoplasm of cerebellar dentate neurons of PD2 (main image) and PD1 (right lower inset). Numerous oligodendrocytes in the nearby dentate internal white matter have paranuclear mercury deposits (arrows, left lower inset). Autometallography/Luxol fast blue. (D) Scattered neurons in the inferior olivary nucleus in the medulla oblongata of PD1 contain mercury grains (arrowheads). White matter internal to the olive contains oligodendrocytes with paranuclear mercury deposits (arrows, right upper inset). Autometallography/Luxol fast blue. Left lower inset: one nearby neuron in the inferior olivary nucleus has co-localised black mercury grains and brown aggregated a-synuclein (autometallography/a-synuclein DAB/hematoxylin). (E) Mercury grains are present in the juxtanuclear region of oligodendrocytes (arrows), but not of neurons (arrowhead), in the red nucleus (RE). Black mercury deposits are seen in the cytoplasm of neurons in the dorsal raphe nucleus (RA), lateral geniculate nucleus (GE), dorsal vagal nucleus (DV), the facial motor nucleus (7n) and the amygdala (AM). Autometallography/Luxol fast blue. (F) Mercury grains are present in cells of the adrenal medulla (AD), anterior pituitary (AP), kidney Henle loop (KI), and thyroid follicles (TH). Autometallography/hematoxylin. PD: case numbers. Bars = 20 μm.
Table 1. Mercury in neurons of people (1) with PD, (2) with mercury exposure but without PD, and (3) without known mercury exposure or PD.
| PD | Hg exposure | No PD, no known Hg exposure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72F | 76F | 24M | 39M | 59F | 61M | 61F | 81F | 86F | 87M | 89F | 95F | 98M | 104F | |
| Locus ceruleus | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Substantia nigra | + | + | - | - | - | - | - | - | - | - | - | - | - | - |
| Cerebral cortex | + | + | + | - | - | - | - | - | - | - | - | - | - | - |
| Caudate/putamen | + | + | - | - | na | - | - | - | - | - | - | - | - | - |
| Thalamus | + | + | - | - | - | - | na | - | - | na | - | - | - | - |
| Subthalamic nucleus | - | - | - | - | na | - | - | - | - | - | na | - | - | - |
| Globus pallidus | - | - | - | - | na | - | - | - | - | - | - | - | - | - |
| Geniculate nucleus | + | + | - | + | - | + | - | - | - | - | - | - | - | - |
| Amygdala | - | + | - | - | - | - | - | - | - | - | - | - | - | na |
| Cerebellar cortex | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cerebellar dentate nucleus | + | + | + | + | - | + | - | - | - | - | - | - | - | - |
| Inferior olivary nucleus | + | na | - | + | - | - | - | - | - | - | - | - | - | - |
| Dorsal raphe nucleus | + | + | - | - | - | - | - | - | - | - | - | - | - | - |
| CN 5, 6, 7 motor nuclei | - | + | - | + | - | - | - | - | - | - | - | - | - | - |
| Dorsal vagal nucleus | + | + | - | - | - | - | - | - | - | - | - | - | + | - |
| Ambiguus nucleus | + | + | + | - | - | - | - | - | - | - | - | - | na | - |
+ mercury present,—no mercury present, CN: cranial nerve, Hg: mercury, na: section not available, M: male, F: female, PD: Parkinson’s disease.
Lewy bodies and neurites. Mercury was co-localised with a-synuclein aggregates within some neurons or in the neuropil, especially in the locus ceruleus and substantia nigra (Figs 1 and 2). This co-localisation was not invariable, since some neurons contained mercury without a-synuclein aggregates, and some a-synuclein aggregates (particularly those free in the neuropil) did not contain mercury. Small mercury deposits were seen within some a-synuclein-positive Lewy neurites (Fig 1).
Oligodendrocytes. Juxta-nuclear mercury deposits were seen in multiple oligodendrocytes in both grey and white matter regions of the CNS (Figs 1 and 2). In the cerebral neocortex and hippocampus, the white matter oligodendrocytes most consistently affected were those in subcortical regions. The outflow tracts of grey matter regions where neurons contained mercury, such as the cerebellar dentate nucleus, contained many mercury-containing oligodendrocytes (Fig 2).
Other CNS cells. Juxta-nuclear mercury deposits were seen in scattered pericytes (identified as cells adjacent to CD31-positive endothelial cells) throughout the CNS (Fig 1). No mercury was seen in astrocytes, microglia, or endothelial cells.
Non-CNS cells. In both PD patients, mercury was present in chromaffin cells of the adrenal medulla, kidney proximal tubules and thin Henle loops, and thyroid follicular cells (Fig 2). PD1 had mercury in anterior pituitary cells (the other PD case had no pituitary sample taken).
People with known sources of mercury exposure
In the man exposed to metallic mercury (ME1), in addition to the locus ceruleus, neuronal iHg was seen in corticomotoneurons in the frontal cortex, cranial nerve motor nucleus 3 and 4, the cerebellar dentate nucleus, and in a few neurons in the nucleus ambiguus (Fig 3). Scattered oligodendrocytes in the frontal and occipital cortices and in the putamen had mercury deposits. Some endothelial cells in all CNS regions contained mercury. In the frontal white matter, perivascular astrocyte cell bodies and their processes connecting to the perivascular space contained mercury. Most pinealocytes contained mercury (Fig 3) (the pineal was not removed from other cases). Mercury was seen in the same cells of the adrenal medulla and kidney as above.
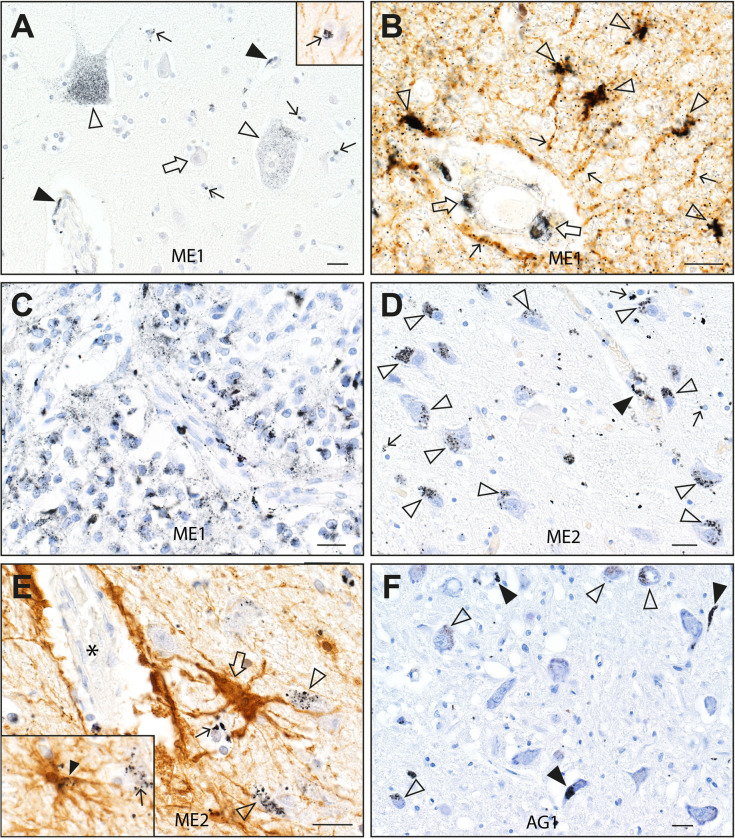
Fig 3. Mercury staining after exposure to mercury and in advanced age.
(A) In ME1, black mercury grains are seen in the frontal motor cortex in two corticomotoneurons (open arrowheads), in scattered oligodendrocytes (eg, thin arrows), in endothelial cells (eg, closed arrowheads), but not in smaller cortical neurons (eg, open arrow). Autometallography/hematoxylin. Inset: a mercury-containing oligodendrocyte (arrow) is surrounded by GFAP-positive processes of interlaminar astrocytes (autometallography/GFAP DAB/hematoxylin). (B) In the frontal lobe white matter of ME1, mercury is present in endothelial cells (open arrows), and perivascular astrocyte cells bodies (eg, arrowheads) and processes (eg, thin arrows). Autometallography/GFAP DAB/hematoxylin. (C) Mercury is present in numerous pinealocytes in the pineal gland of ME1. Autometallography/hematoxylin. (D) In ME2, mercury grains are present in the cytoplasm of numerous lateral geniculate nucleus neurons (open arrowheads), scattered oligodendrocytes (eg, arrows), and in endothelial cells (filled arrowhead). Autometallography/hematoxylin. (E) In the lateral geniculate nucleus of ME2 a perivascular astrocyte (open arrow) has processes connecting a blood vessel (*) with mercury-containing neurons (open arrowheads) and oligodendrocytes (thin arrow). Inset: a nearby astrocyte process (closed arrowhead) contains mercury grains and connects with a mercury-containing oligodendrocyte (thin arrow). Autometallography/GFAP DAB/hematoxylin. (F) In the 95 years-old non-PD male control, some neurons (open arrowheads) and small blood vessels (filled arrowheads) in the dorsal vagal nucleus contain mercury. Autometallography/hematoxylin. ME: case numbers. Bars = 20 μm.
In the professional fisherman (ME2), in addition to the locus ceruleus, neuronal iHg was seen in neurons of the lateral geniculate nucleus [32], cuneate and gracile nuclei, cranial nerve motor nucleus 4, inferior olivary nucleus, medullary reticular formation and cerebellar dentate nucleus (Fig 3). Particulate mercury was seen adjacent to Purkinje cell bodies, possibly in Bergmann glia. A few scattered oligodendrocytes in most CNS regions contained mercury. Perivascular astrocytes containing mercury were in contact with lateral geniculate neurons and oligodendrocytes which both contained mercury (Fig 3). Mercury was seen in the same cells of the adrenal medulla, kidney and thyroid as above.
People without PD or known sources of mercury exposure
Among these 10 people, one 61 years-old male had iHg in lateral geniculate and cerebellar dentate neurons, and one 98 years-old male had iHg in a few neurons of the dorsal vagal nucleus (Fig 3). In the remaining eight people no brain regions apart from the locus ceruleus contained iHg. No oligodendrocyte iHg was seen in any of these ten brains. In this control group, iHg was present in eight of the nine kidney samples, six of the eight thyroid samples, and in all five pituitary samples taken.
Locus ceruleus-only autometallography
Autometallographic-detected iHg was present in 13 of 20 (65%) PD locus ceruleus samples and in 15 of 40 (63%) of the non-PD control locus ceruleus samples, an insignificant difference on contingency testing.
LA-ICP-MS
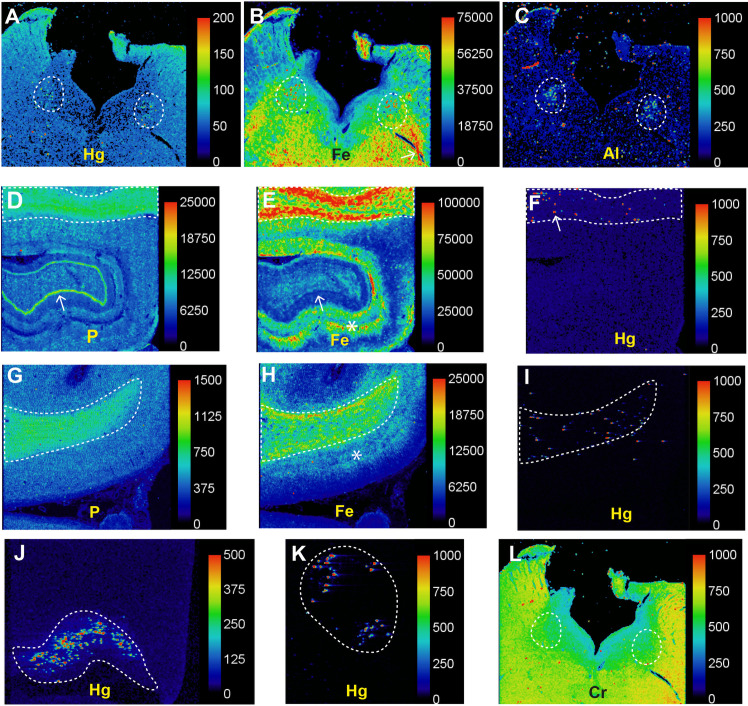
LA-ICP-MS of the posterior pons, lateral geniculate nucleus, facial motor nucleus, frontal lobe, hippocampus, and cerebellar hemisphere of PD2 showed mercury in regions where autometallography staining was positive, and did not detect mercury in autometallography-negative regions (Figs 4 and S1). Mercury was detected in regions containing large amounts of autometallography-detected intraneuronal iHg, such as the locus ceruleus, lateral geniculate neurons [32] and facial motor neurons, and in the frontal and hippocampal white matter where oligodendrocytes contained autometallography-detected iHg. The locus ceruleus also contained iron, aluminium and nickel, which were not seen in other regions. Iron was prominent in the hippocampal white matter and peri-dentate gyrus hippocampal grey matter, and present in the cerebral and pontine white matter, the deeper layers of the frontal cortex, and cerebellar subcortical white matter. Chromium was widespread in the posterior pons and hippocampus.
Fig 4. LA-ICP-MS in Parkinson’s disease (PD2).
(A) Particulate mercury is seen in both locus ceruleus nuclei (outlined) where numerous neurons were autometallography-positive. (B) Particulate iron deposits are seen in both locus ceruleus nuclei (outlined) in the posterior pons. Linear deposits of iron in the pontine white matter (eg, arrow) are probably from red blood cells in blood vessels. (C) Particulate aluminium is seen in both locus ceruleus nuclei (outlined). (D) A normal high nuclear density shown by the phosphorus image is present in the hippocampal white matter (top, outlined) as well as in the dentate gyrus (arrow). (E) A large amount of iron is present in the hippocampal white matter (outlined), and in grey matter adjacent to the dentate gyrus (arrow, dark blue line). (F) Particulate mercury is seen in the hippocampal white matter (eg, arrow) where oligodendrocytes were autometallography-positive. (G) The normal high nuclear density of the frontal white matter (outlined) is shown in the phosphorus image. (H) The frontal white matter contains more iron than the frontal cortex, where most iron is in the deeper cortical layers (*) adjacent to the white matter. (I) Particulate mercury is present in the frontal white matter where oligodendrocytes were autometallography-positive. (J) Speckled mercury is present in the lateral geniculate nucleus where neurons were autometallography-positive. (K) Mercury is seen in pontine facial motor neurons, which were autometallography-positive. (L) Chromium is widespread in the posterior pons, without particular accumulation in the locus ceruleus (outlined). Scale = counts per second (proportional to abundance).
Discussion
Key findings of this study are that people with PD who have been exposed to mercury (ie, with locus ceruleus mercury) had mercury within neurons and oligodendrocytes in regions of the brain known to be affected by PD, and had mercury associated with a-synuclein aggregates in Lewy bodies and neurites. This contrasts with people without PD (either those with or without known mercury exposure) who had previous mercury exposure (with mercury in locus ceruleus neurons) where mercury uptake was limited to a few brain sites and to non-CNS organs. People with and without PD appeared to have had similar exposures to mercury, judged by the presence of locus ceruleus mercury, suggesting that PD is more likely to result from a predisposition to take up toxic metals into the nervous system, rather than from environmental exposure to toxic metals such as mercury alone.
Synergistic interactions between metal toxins are increasingly being recognised [33, 34], so it is of interest that in a PD brain aluminium and nickel were seen together with mercury in the locus ceruleus. Mixtures of toxic metals, including mercury, cadmium, silver and lead, have previously been noted in the human locus ceruleus [7], indicating that uptake of multiple metal toxicants by the human brain is not unusual. Mercury itself can induce a-synuclein aggregation [20], but interactions between pesticides and metals can also accelerate the formation of a-synuclein fibrils in vitro [35] and so could be another factor underlying the formation of Lewy bodies and neurites in PD. Iron is present in large quantities in the human substantia nigra and locus ceruleus [36], and both mercury and iron were seen in white matter tracts in this PD case. The white matter iron, particularly high in the hippocampus, was probably within oligodendrocytes that are the predominant iron-containing cells in the brain [37, 38]. Iron has been suggested to play a role in PD [39] and in the synuclein disorder multiple system atrophy where oligodendrocytes contain a-synuclein inclusions [40]. Although interactions of brain iron and mercury are not described, the presence of both metals in PD oligodendrocytes, and in substantia nigra and locus ceruleus neurons, suggests synergy between these metals could make these cells susceptible to toxic damage.
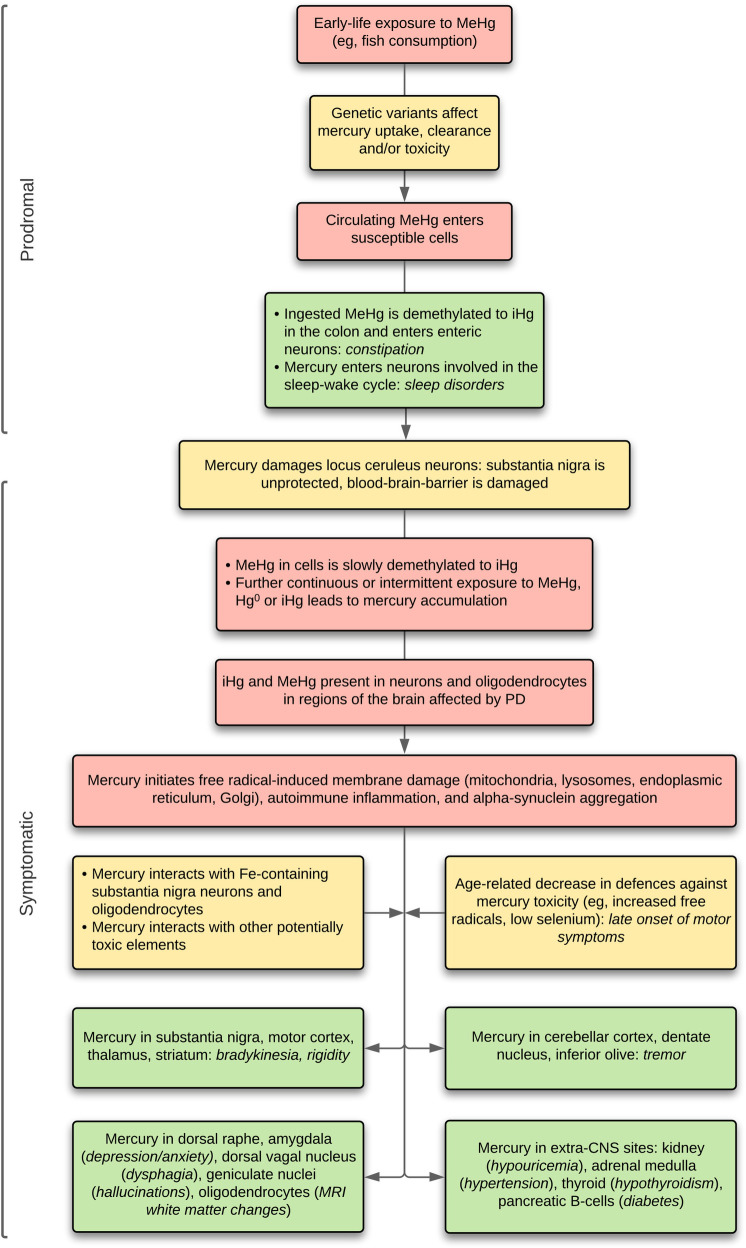
Based on the distribution of mercury within the brain, a hypothetical model of toxicant-induced PD can be constructed, with the metal playing a part in both the prodromal period and in the later motor and non-motor manifestations, as outlined in Fig 5 and detailed below.
Fig 5. Hypothetical model of mercury-induced Parkinson’s disease.
In this model, an early-life exposure to methylmercury (MeHg), aided by genetic susceptibility to brain mercury uptake, seeds susceptible cells, with later toxic effects modified by further genetic susceptibilities. During the prodromal period, mercury in colonic and sleep cycle-related neurons causes constipation and sleep disorders. Early uptake of mercury by the locus ceruleus places the substantia nigra at risk of toxicant damage, and permits toxicants to pass through the blood-brain-barrier. Inorganic mercury (iHg) slowly accumulates in neurons and oligodendrocytes, both from demethylation of methylmercury and from further exposures to mercury. When cellular mercury concentrations reach a critical level in neurons and oligodendrocytes, aided by synergistic effects in iron-containing cells, interactions with other toxicants, and decreasing aging-related natural defenses, mercury damages membranous organelles in these cells and promotes a-synuclein aggregation. This results in symptomatic PD, with motor, non-motor, and non-CNS associated symptoms. Hg°: mercury vapor.
Prodromal stage. Early life exposure to methylmercury, probably from fish consumption, could result in mercury entering susceptible cells via methionine receptors [13]. The American Academy of Pediatrics states that fish can be added to a baby’s food within a few months of starting solid foods, and the USA Food and Drug Administration recommends up to three servings of fish per week from the age of two years, with avoidance of high-mercury fish such as shark (www.FDA.gov/fishadvice). However, in many countries the labelling fish is inadequate and cheaper fish often contain shark meat. Two prominent PD prodromal symptoms, constipation and disordered sleep, could be related to mercury exposure (Fig 5). This is because ingested methylmercury is demethylated in the colon to inorganic mercury [13] which can then be absorbed and enter enteric neurons to reduce colon mobility and cause constipation (no colonic samples were available from our samples to look for mercury in enteric neurons). Second, mercury was found in pinealocytes in the man exposed to metallic mercury, suggesting that changes in melatonin secretion, important for the sleep-wake cycle, could be present after mercury exposure. In addition, neurons of the suprachiasmatic nucleus, which controls the timing of the sleep-wake cycle, selectively take up bismuth [41], which has the same tissue distribution as mercury [42]. The suprachiasmatic nucleus is often damaged during autopsy brain removal and could not be identified in our samples.
Progression to symptomatic PD. Several factors could increase the chance for mercury to promote progression of PD prodromal symptoms to later motor disorders (Fig 5). (1) The ability of the body to deal with the toxic effects of mercury depends on the actions of many genes [14, 43]. Polymorphisms in these genes may affect the ability of cells to control mercury entry, toxicity or elimination and make these cells more susceptible to mercury-induced damage. (2) The locus ceruleus is damaged early in most cases of PD [44, 45]. Mercury in locus ceruleus neurons would reduce noradrenaline output and impair the integrity of blood-brain-barrier [46], allowing easier access to the CNS to circulating toxicants. Furthermore, the locus ceruleus protects the substantia nigra from damage [44, 45, 47] so any toxicants within the substantia nigra would be more active if locus ceruleus neurons were not producing noradrenaline. Damage to the locus ceruleus could also contribute to non-motor PD symptoms such as autonomic disturbances, sleep disorders, depression, cognitive difficulties, and hyposmia [45, 48]. (3) Repeated or continuous exposure to environmental sources of mercury would increase the level of mercury within cells, until a tipping point was reach in later age. Common sources of mercury are from eating mercury-contaminated fish, certain occupations, and dental amalgam fillings [13, 14]. Of note, the prevalence of inorganic mercury in human cells has been found to increase during aging [16] which could contribute to the increasing PD symptoms on aging. (4) Decreased defences to mercury neurotoxicity because of aging, for example from an increased production of reactive oxygen species [49] or from reduced levels of selenium [50], could also contribute to the appearance of PD symptoms in later life. This is relevant to the frequent finding of Lewy bodies in normal elderly subjects, which may represent preclinical PD [51], since mercury can persist in substantia nigra neurons many years after initial exposure without causing symptoms [52].
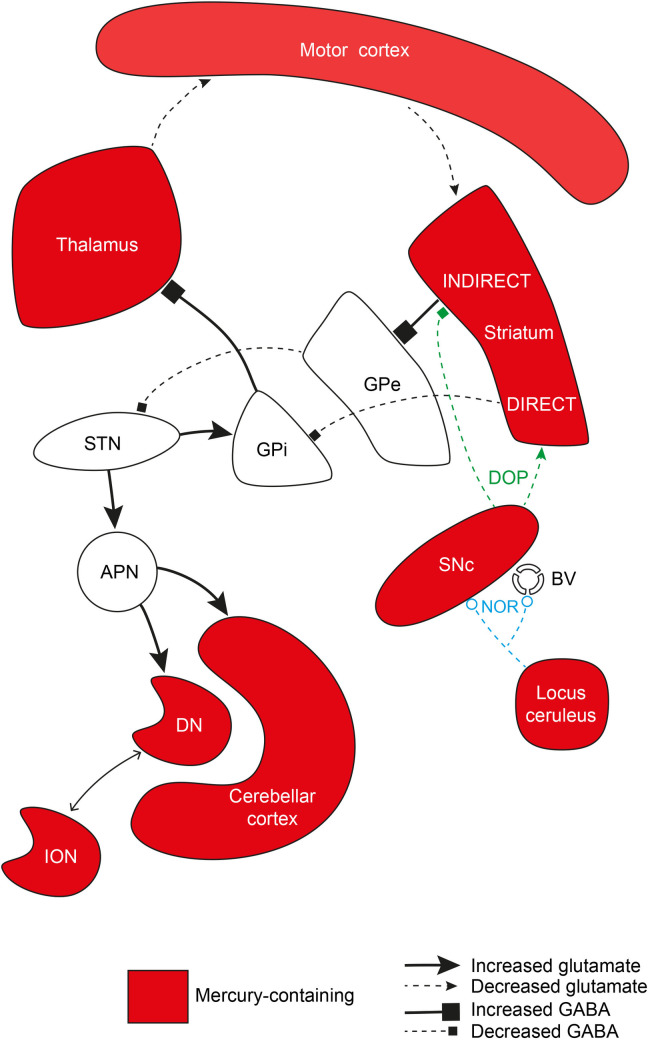
Bradykinesia and rigidity. Bradykinesia and rigidity are major motor features of PD [53]. In the classical model of motor circuit pathophysiology, bradykinesia and rigidity occur when decreased dopamine from the substantia nigra has opposing effects on the striatal direct and indirect pathways, leading to suppressed globus pallidus externa firing with consequently increased subthalamic nucleus activity, as well as increased globus pallidus interna-mediated thalamic inhibition [54] (Fig 6). In our PD patients, neurons containing mercury were prominent in the substantia nigra, motor cortex, and thalamus, all regions with a decreased neuronal output in the classical model. The mercury content of corticomotoneurons was high, which may be because of a trans-synaptic passage of mercury from the adrenal medulla [55] or kidney [56], both of which contained mercury in our patients. This may be important since mercury in corticomotoneurons could affect the hyperdirect pathway of motor control [57]. Mercury was seen in many medium-sized neurons in the striatum, which are implicated in the pathophysiology of PD [58]. The subthalamic nucleus and globus pallidus, regions affected by changes to striatal neurons in the classical model, did not have mercury-containing neurons. This distribution suggests mercury could contribute to bradykinesia and rigidity in idiopathic PD by disrupting basal ganglia/thalamic/motor cortex communications.
Fig 6. Mercury in neurons that could contribute to bradykinesia, rigidity and tremor.
Regions of the brain implicated in PD motor disorders whose neurons contain mercury are shown in red. The classical pathway of PD motor pathophysiology is included in the upper half of the diagram. Neurons in the substantia nigra compacta (SNc), striatum, thalamus and motor cortex contain mercury, but not those in the subthalamic nucleus (STN), or the globus pallidus interna (GPi) and externa (GPe). Mercury in the locus ceruleus could decrease noradrenaline output to the substantia nigra, leaving it more susceptible to toxicants, and to blood vessels, making the blood-brain-barrier more permeable to circulating toxicants. Mercury in neurons of the cerebellar cortex, dentate nucleus (DN) and inferior olivary nucleus could underlie types of tremor in PD (lower left in diagram). Also shown (bottom left) is a stimulatory pathway from the subthalamic nucleus via the anterior pontine nuclei to the cerebellar cortex and dentate nucleus (both containing mercury), that could cause a resting tremor. BV: blood vessel, DIRECT: direct pathway, INDIRECT: indirect pathway, DOP: dopamine, GABA: gamma-aminobutyric acid, NOR: noradrenaline.
Tremor. A resting tremor is characteristic of PD, but about a quarter of people with PD do not have a tremor, and of those who do, most have a mixed resting and action tremor, while a few have an action tremor only [59]. The pathogenesis of tremor in PD is unclear, and is not readily explained by the classical pathophysiology model, with possible sources being the basal ganglia, thalamus, or cerebellum. Recent findings that a subthalamic nucleus/anterior pons/cerebellar cortex pathway can generate a resting tremor suggests a key role for the cerebellum in the genesis of PD tremors [60] (Fig 6). It has long been known that the most frequent sign of occupational mercury exposure is an action tremor, probably from mercury in the basal ganglia or cerebellum [61]. The cerebellar cortex has also been implicated in action tremors in post mortem studies while others have suggested the inferior olivary nucleus could be involved [62]. In our PD cases, mercury was present variably in cerebellar Purkinje and granule neurons, and in neurons of the cerebellar dentate and inferior olivary nuclei (Fig 6). This variability in toxicant distribution could explain the differences in tremor type between PD patients.
Non-motor CNS symptoms. Several non-motor PD CNS symptoms could be triggered by a toxicant such as mercury. (1) The incidence of dementia in people with PD is fourfold higher than in the general population, with the average time to dementia after PD diagnosis being about 10 years [63, 64]. One possible contributor to PD dementia could be widespread damage to oligodendrocytes from contained mercury, as seen in our PD cases. Oligodendrocytes contain iron [38], and a large amount of iron was present in hippocampal white matter where oligodendrocytes contained mercury, so a synergistic effect of these two metals could reduce oligodendrocyte function in this region that is vital for memory. Oligodendrocytes have previously been implicated in both Alzheimer’s disease [65] and in a-synucleinopathies [66], and so may be involved in the combination of a-synuclein and Alzheimer pathology often present in PD dementia [63]. Non-myelinating oligodendrocytes in PD that contain a-synuclein inclusions are damaged late in the disease [67], when dementia usually occurs. Widespread damage to oligodendrocytes could also be the basis for the white matter changes seen on MRI in PD [68], and it has been suggested that white matter impairment precedes neuronal loss in PD [69]. (2) Visual hallucinations are frequent in PD, and can be a harbinger of future dementia [64]. Damage to the lateral geniculate nucleus, with disturbances of the visual pathway, may be triggered by mercury in geniculate neurons [32]. Both of our PD cases had marked uptake of mercury into lateral geniculate neurons. (3) Depression and anxiety are common in PD [1]. Mercury in serotonergic brain stem raphe neurons could contribute to depression, and mercury in amygdala neurons may play a part in anxiety. (4) It is unclear why pain should accompany PD [1], but mercury was present in thalamic neurons in our PD cases and is also taken up by human posterior root ganglia neurons [52], so either central or peripheral regions of the pain pathway could be disturbed after mercury exposure. (5) Both retinal thinning on optical coherence tomography [70–72] and retinal pathology [73] have been noted in people with PD. This may relate to mercury being found commonly in the human retinal pigment epithelium and choriocapillaris [74], as well as mercury being present in retinal ganglion cells, pigment epithelium and endothelial cells of mercury-exposed mice [75] and primates [76].
Non-CNS associated symptoms. In our PD cases, mercury was present in cells of the kidney, thyroid, adrenal medulla and anterior pituitary. This could explain the presence of some extra-CNS disorders associated with PD. Chronic kidney disease [23] and hypertension [77, 78] appear to be more common in PD, and mercury in both the renal cortex and medulla can be found in older adults [79], including our PD patients. Kidney mercury could be responsible for the low levels of uric acid seen in PD [80], since chronic exposure to heavy metals can increase urate secretion [81]. Mercury can be taken up by thyroid follicular cells and could be responsible for the thyroid disorders that are found in about 10% of PD patients [21, 82]. Mercury in the adrenal medulla may contribute to hypertension by raising noradrenaline levels [83] and therefore raise blood pressure in PD patients [77, 78]. Pancreatic samples were not available for our PD cases, but mercury can be taken up by human pancreatic insulin-producing beta cells [16], so mercury exposure could contribute to the increased incidence of type 2 diabetes in PD [22, 84].
This study has several limitations. (1) No detailed clinical information was available from these forensic cases, so we were not able to match neurological signs and symptoms with the distribution of mercury in the brain. (2) No extensive data on environmental exposure to mercury, such as occupations, fish consumption, and numbers of dental amalgam fillings [85], were available. (3) We limited the study to people with evidence of previous mercury exposure (ie, with mercury in the locus ceruleus) to compare the multifocal distribution of mercury in the brains of people with or without PD. This reduced the number of people with PD available for study. Future multimodal elemental studies of larger numbers of PD post mortem brains, both with and without histochemical evidence of mercury exposure, are likely to give further insights into the role of toxic metals in PD. (4) In many people with PD a long latent period between the last toxicant exposure and the time of death is likely, and clearing of toxicants from cells over time, for example by the glymphatic pathway [32], is to be expected. It is therefore unlikely that in all PD patients, even those in whom toxicants played a role, brain toxicants will readily be identified using current techniques based on post mortem samples. Future in vivo multi-elemental imaging studies of younger people with PD, or those with rapidly progressive disease, could give a more comprehensive picture of toxicant distribution in PD brains. (5) The numbers of surviving substantia nigra neurons in our PD patients were too small to confirm the presence of mercury, or to look for other neuronal toxic elements, with LA-ICP-MS. However, others have previously shown the presence of mercury and other metals in neuromelanin from the substantia nigra [86]. (6) It is rare to obtain human post mortem brain samples that have been subjected to modern elemental analysis from people with a known exposure to mercury, and our two mercury-exposure cases were younger than the PD cases, and of different gender. However, we think these cases are worth including since they do suggest that people without PD handle mercury differently, with a restricted brain uptake. Furthermore, PD has a long prodromal period, so it is likely that the initial toxicant exposure occurs years before the clinical manifestations of PD appear, when the patient is younger. (7) The mean age of our control brain samples (82 years) was higher than that of the PD cases (74 years). However, since mercury accumulates with age in human cells [16], the relative lack of mercury in this older control group is a further indication of the restricted distribution of mercury in most non-PD cases.
In conclusion, mercury can be found in neurons and oligodendrocytes in regions of the brain that are affected by PD, and mercury often co-localises with the aggregated a-synuclein found in Lewy bodies and neurites. Although post mortem tissue studies cannot provide a direct link between toxicants and PD pathogenesis, the varied toxic mechanisms of mercury make it a candidate for an environmental toxicant that could play a role in the disease, probably in conjunction with genetic susceptibilities to metal uptake, elimination, or toxicity, and synergies with other potentially toxic elements. The development of future in vivo bio-elemental imaging techniques will be needed to further study the role of toxic elements in the prodromal and early symptomatic phases of the disease. In the longer term, it will of interest to see if current attempts to decrease the burning of fossil fuels, a major contributor to rising atmospheric mercury levels and related mercury pollution of fish, will result in a future decrease in the incidence of idiopathic PD.
Supporting information
The labels indicate the outlined regions. Phosphorus images (top row) indicate the nuclear density of the tissues. (A) Particulate metals detected in the autometallography-positive locus ceruleus are mercury, iron, aluminium, and nickel. Iron in prominent in the pontine white matter. Chromium is widespread in the posterior pons. (B) Speckled mercury is present in the lateral geniculate nucleus, where neurons were autometallography-positive. Iron is seen in the adjacent white matter. (C) Mercury is present in the region of facial motor neurons. (D) Particulate mercury is present in the frontal white matter, which contains more iron than the cortex. (E) Particulate mercury is seen in the hippocampal white matter, which contains a large amount of iron. Chromium is widespread in the hippocampus. (F) No mercury is seen in the cerebellar cortex or white matter, which were both autometallography-negative in this patient. Iron is present in the cerebellar subcortical white matter. No significant amounts of cadmium, lead, bismuth, silver or gold are detected in any sections. Scale = counts per second (proportional to abundance).
(TIF)
Acknowledgments
RP is supported by the Aimee Stacy Memorial and Ignacy Burnett bequests.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397: 2284–2303. doi: 10.1016/S0140-6736(21)00218-X [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Sherer T, Okun MS, Bloem BR. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis. 2018;8: S3–S8. doi: 10.3233/JPD-181474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54: 141–164. doi: 10.1146/annurev-pharmtox-011613-135937 [DOI] [PubMed] [Google Scholar]
- 4.Caudle WM, Guillot TS, Lazo C, Miller GW. Parkinson’s disease and the environment: beyond pesticides. Neurotoxicology. 2012;33: 585. doi: 10.1016/j.neuro.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Ball N, Teo WP, Chandra S, Chapman J. Parkinson’s Disease and the Environment. Front Neurol. 2019;10: 218. doi: 10.3389/fneur.2019.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raj K, Kaur P, Gupta GD, Singh S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett. 2021;753: 135873. doi: 10.1016/j.neulet.2021.135873 [DOI] [PubMed] [Google Scholar]
- 7.Pamphlett R, Bishop DP, Kum Jew S, Doble PA. Age-related accumulation of toxic metals in the human locus ceruleus. PLoS One. 2018;13: e0203627. doi: 10.1371/journal.pone.0203627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streets DG, Devane MK, Lu Z, Bond TC, Sunderland EM, Jacob DJ. All-time releases of mercury to the atmosphere from human activities. Environ Sci Technol. 2011;45: 10485–10491. doi: 10.1021/es202765m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schartup AT, Thackray CP, Qureshi A, Dassuncao C, Gillespie K, Hanke A, et al. Climate change and overfishing increase neurotoxicant in marine predators. Nature. 2019;572: 648–650. doi: 10.1038/s41586-019-1468-9 [DOI] [PubMed] [Google Scholar]
- 10.Obrist D, Kirk JL, Zhang L, Sunderland EM, Jiskra M, Selin NE. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio. 2018;47: 116–140. doi: 10.1007/s13280-017-1004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustin MS, Bank MS, Bishop K, Bowman K, Branfireun B, Chetelat J, et al. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci Total Environ. 2020;737: 139619. doi: 10.1016/j.scitotenv.2020.139619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaborators GBDPsD. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17: 939–953. doi: 10.1016/S1474-4422(18)30295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36: 609–662. doi: 10.1080/10408440600845619 [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Finley EJ, Aschner M. Recent Advances in Mercury Research. Curr Environ Health Rep. 2014;1: 163–171. doi: 10.1007/s40572-014-0014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM. Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol. 1995;134: 273–284. doi: 10.1006/taap.1995.1193 [DOI] [PubMed] [Google Scholar]
- 16.Pamphlett R. The prevalence of inorganic mercury in human cells increases during aging but decreases in the very old. Sci Rep. 2021;11: 16714. doi: 10.1038/s41598-021-96359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18: 321–336. doi: 10.1016/0891-5849(94)00159-h [DOI] [PubMed] [Google Scholar]
- 18.Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH. Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta Gen Subj. 2019;1863: 129299. doi: 10.1016/j.bbagen.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang LW, Hartmann HA. Electron microscopic histochemical study on the localization and distribution of mercury in the nervous system after mercury intoxication. Exp Neurol. 1972;35: 122–137. doi: 10.1016/0014-4886(72)90064-7 [DOI] [PubMed] [Google Scholar]
- 20.Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem. 2003;278: 27630–27635. doi: 10.1074/jbc.M303302200 [DOI] [PubMed] [Google Scholar]
- 21.Munhoz RP, Teive HA, Troiano AR, Hauck PR, Herdoiza Leiva MH, Graff H, et al. Parkinson’s disease and thyroid dysfunction. Parkinsonism Relat Disord. 2004;10: 381–383. doi: 10.1016/j.parkreldis.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Chohan H, Senkevich K, Patel RK, Bestwick JP, Jacobs BM, Bandres Ciga S, et al. Type 2 Diabetes as a Determinant of Parkinson’s Disease Risk and Progression. Mov Disord. 2021;36: 1420–1429. doi: 10.1002/mds.28551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melendez-Flores JD, Estrada-Bellmann I. Linking chronic kidney disease and Parkinson’s disease: a literature review. Metab Brain Dis. 2021;36: 1–12. doi: 10.1007/s11011-020-00623-1 [DOI] [PubMed] [Google Scholar]
- 24.Pamphlett R, Waley P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. 1996;92: 525–527. doi: 10.1007/s004010050556 [DOI] [PubMed] [Google Scholar]
- 25.Ahn JS, Kang KW, Kang WY, Lim HM, Cho S, Moon JD, et al. Mercury poisoning in a fisherman working on a pelagic fishing vessel due to excessive tuna consumption. J Occup Health. 2018;60: 89–93. doi: 10.1539/joh.16-0274-CS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danscher G, Moller-Madsen B. Silver amplification of mercury sulfide and selenide: a histochemical method for light and electron microscopic localization of mercury in tissue. J Histochem Cytochem. 1985;33: 219–228. doi: 10.1177/33.3.2579122 [DOI] [PubMed] [Google Scholar]
- 27.Danscher G, Rungby J. Differentiation of histochemically visualized mercury and silver. Histochem J. 1986;18: 109–114. doi: 10.1007/BF01675364 [DOI] [PubMed] [Google Scholar]
- 28.Pamphlett R, Png FY. Shrinkage of motor axons following systemic exposure to inorganic mercury. J Neuropathol Exp Neurol. 1998;57: 360–366. doi: 10.1097/00005072-199804000-00009 [DOI] [PubMed] [Google Scholar]
- 29.Danscher G, Stoltenberg M, Juhl S. How to detect gold, silver and mercury in human brain and other tissues by autometallographic silver amplification. Neuropathol Appl Neurobiol. 1994;20: 454–467. doi: 10.1111/j.1365-2990.1994.tb00996.x [DOI] [PubMed] [Google Scholar]
- 30.Danscher G, Stoltenberg M, Kemp K, Pamphlett R. Bismuth autometallography: protocol, specificity, and differentiation. J Histochem Cytochem. 2000;48: 1503–1510. doi: 10.1177/002215540004801107 [DOI] [PubMed] [Google Scholar]
- 31.Lockwood TE, Westerhausen MT, Doble PA. Pew(2): Open-Source Imaging Software for Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Anal Chem. 2021;93: 10418–10423. doi: 10.1021/acs.analchem.1c02138 [DOI] [PubMed] [Google Scholar]
- 32.Pamphlett R, Kum Jew S, Doble PA, Bishop DP. Elemental imaging shows mercury in cells of the human lateral and medial geniculate nuclei. PLoS One. 2020;15: e0231870. doi: 10.1371/journal.pone.0231870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobbina SJ, Chen Y, Zhou Z, Wu X, Feng W, Wang W, et al. Low concentration toxic metal mixture interactions: Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere. 2015;132: 79–86. doi: 10.1016/j.chemosphere.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 34.Andrade VM, Aschner M, Marreilha Dos Santos AP (2017) Neurotoxicity of Metal Mixtures. In: Aschner M, Costa LG, editors. Neurotoxicity of Metals. 2017/09/11 ed. Cham, Switzerland: Springer Nature. pp. 227–265. [Google Scholar]
- 35.Uversky VN, Li J, Bower K, Fink AL. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology. 2002;23: 527–536. doi: 10.1016/s0161-813x(02)00067-0 [DOI] [PubMed] [Google Scholar]
- 36.Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, et al. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J Neural Transm (Vienna). 2006;113: 757–767. doi: 10.1007/s00702-006-0453-2 [DOI] [PubMed] [Google Scholar]
- 37.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17: 83–93. doi: [DOI] [PubMed] [Google Scholar]
- 38.Reinert A, Morawski M, Seeger J, Arendt T, Reinert T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019;20: 25. doi: 10.1186/s12868-019-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol. 2017;155: 96–119. doi: 10.1016/j.pneurobio.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, et al. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38: 4–24. doi: 10.1111/j.1365-2990.2011.01234.x [DOI] [PubMed] [Google Scholar]
- 41.Pamphlett R, Stoltenberg M, Rungby J, Danscher G. Uptake of bismuth in motor neurons of mice after single oral doses of bismuth compounds. Neurotoxicol Teratol. 2000;22: 559–563. doi: 10.1016/s0892-0362(00)00083-0 [DOI] [PubMed] [Google Scholar]
- 42.Ross JF, Switzer RC, Poston MR, Lawhorn GT. Distribution of bismuth in the brain after intraperitoneal dosing of bismuth subnitrate in mice: implications for routes of entry of xenobiotic metals into the brain. Brain Res. 1996;725: 137–154. doi: 10.1016/0006-8993(96)00146-1 [DOI] [PubMed] [Google Scholar]
- 43.Andreoli V, Sprovieri F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int J Environ Res Public Health. 2017;14: E93. doi: 10.3390/ijerph14010093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci Biobehav Rev. 2000;24: 655–668. doi: 10.1016/s0149-7634(00)00028-2 [DOI] [PubMed] [Google Scholar]
- 45.Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C. The Noradrenergic System in Parkinson’s Disease. Front Pharmacol. 2020;11: 435. doi: 10.3389/fphar.2020.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harik SI, McGunigal T Jr. The protective influence of the locus ceruleus on the blood-brain barrier. Ann Neurol. 1984;15: 568–574. doi: 10.1002/ana.410150609 [DOI] [PubMed] [Google Scholar]
- 47.Hassani OK, Rymar VV, Nguyen KQ, Huo L, Cloutier JF, Miller FD, et al. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: implications for development and Parkinson’s disease. Neurobiol Aging. 2020;85: 22–37. doi: 10.1016/j.neurobiolaging.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 48.Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329: 294–299. doi: 10.1016/0006-8993(85)90537-2 [DOI] [PubMed] [Google Scholar]
- 49.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292: R18–36. doi: 10.1152/ajpregu.00327.2006 [DOI] [PubMed] [Google Scholar]
- 50.Cai Z, Zhang J, Li H. Selenium, aging and aging-related diseases. Aging Clin Exp Res. 2019;31: 1035–1047. doi: 10.1007/s40520-018-1086-7 [DOI] [PubMed] [Google Scholar]
- 51.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68: 816–822. doi: 10.1097/NEN.0b013e3181ac10a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hargreaves RJ, Evans JG, Janota I, Magos L, Cavanagh JB. Persistent mercury in nerve cells 16 years after metallic mercury poisoning. Neuropathol Appl Neurobiol. 1988;14: 443–452. doi: 10.1111/j.1365-2990.1988.tb01336.x [DOI] [PubMed] [Google Scholar]
- 53.Kalia LV, Lang AE. Parkinson’s disease. The Lancet. 2015;386: 896–912. doi: 10.1016/s0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 54.McGregor MM, Nelson AB. Circuit Mechanisms of Parkinson’s Disease. Neuron. 2019;101: 1042–1056. doi: 10.1016/j.neuron.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 55.Dum RP, Levinthal DJ, Strick PL. The mind-body problem: Circuits that link the cerebral cortex to the adrenal medulla. Proc Natl Acad Sci U S A. 2019. doi: 10.1073/pnas.1902297116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levinthal DJ, Strick PL. The motor cortex communicates with the kidney. J Neurosci. 2012;32: 6726–6731. doi: 10.1523/JNEUROSCI.0406-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foffani G, Obeso JA. A Cortical Pathogenic Theory of Parkinson’s Disease. Neuron. 2018;99: 1116–1128. doi: 10.1016/j.neuron.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 58.Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64: 545–547. doi: 10.1212/01.WNL.0000150591.33787.A4 [DOI] [PubMed] [Google Scholar]
- 59.Gironell A, Pascual-Sedano B, Aracil I, Marin-Lahoz J, Pagonabarraga J, Kulisevsky J. Tremor Types in Parkinson Disease: A Descriptive Study Using a New Classification. Parkinsons Dis. 2018;2018: 4327597. doi: 10.1155/2018/4327597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19: 338–350. doi: 10.1038/s41583-018-0002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fawer RF, de Ribaupierre Y, Guillemin MP, Berode M, Lob M. Measurement of hand tremor induced by industrial exposure to metallic mercury. Br J Ind Med. 1983;40: 204–208. doi: 10.1136/oem.40.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louis ED, Faust PL. Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol. 2020;16: 69–83. doi: 10.1038/s41582-019-0302-1 [DOI] [PubMed] [Google Scholar]
- 63.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci. 2013;14: 626–636. doi: 10.1038/nrn3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13: 217–231. doi: 10.1038/nrneurol.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6: 22. doi: 10.1186/s40478-018-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fellner L, Stefanova N. The role of glia in alpha-synucleinopathies. Mol Neurobiol. 2013;47: 575–586. doi: 10.1007/s12035-012-8340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26: 6–17. doi: 10.1002/mds.23455 [DOI] [PubMed] [Google Scholar]
- 68.Wei X, Luo C, Li Q, Hu N, Xiao Y, Liu N, et al. White Matter Abnormalities in Patients With Parkinson’s Disease: A Meta-Analysis of Diffusion Tensor Imaging Using Tract-Based Spatial Statistics. Front Aging Neurosci. 2020;12: 610962. doi: 10.3389/fnagi.2020.610962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rektor I, Svatkova A, Vojtisek L, Zikmundova I, Vanicek J, Kiraly A, et al. White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS One. 2018;13: e0187939. doi: 10.1371/journal.pone.0187939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127: 737–741. doi: 10.1001/archophthalmol.2009.106 [DOI] [PubMed] [Google Scholar]
- 71.Eraslan M, Cerman E, Yildiz Balci S, Celiker H, Sahin O, Temel A, et al. The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: enhanced depth imaging optical coherence tomography study. Acta Ophthalmol. 2016;94: e68–75. doi: 10.1111/aos.12809 [DOI] [PubMed] [Google Scholar]
- 72.Satue M, Obis J, Alarcia R, Orduna E, Rodrigo MJ, Vilades E, et al. Retinal and Choroidal Changes in Patients with Parkinson’s Disease Detected by Swept-Source Optical Coherence Tomography. Curr Eye Res. 2018;43: 109–115. doi: 10.1080/02713683.2017.1370116 [DOI] [PubMed] [Google Scholar]
- 73.Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, et al. Characterization of Retinal Microvascular and Choroidal Structural Changes in Parkinson Disease. JAMA Ophthalmol. 2021;139: 182–188. doi: 10.1001/jamaophthalmol.2020.5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pamphlett R, Cherepanoff S, Too LK, Kum Jew S, Doble PA, Bishop DP. The distribution of toxic metals in the human retina and optic nerve head: Implications for age-related macular degeneration. PLoS One. 2020;15: e0241054. doi: 10.1371/journal.pone.0241054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pamphlett R, Kum Jew S, Cherepanoff S. Mercury in the retina and optic nerve following prenatal exposure to mercury vapor. PLoS One. 2019;14: e0220859. doi: 10.1371/journal.pone.0220859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warfvinge K, Bruun A. Mercury accumulation in the squirrel monkey eye after mercury vapour exposure. Toxicology. 1996;107: 189–200. doi: 10.1016/0300-483x(95)03257-g [DOI] [PubMed] [Google Scholar]
- 77.Hou L, Li Q, Jiang L, Qiu H, Geng C, Hong JS, et al. Hypertension and Diagnosis of Parkinson’s Disease: A Meta-Analysis of Cohort Studies. Front Neurol. 2018;9: 162. doi: 10.3389/fneur.2018.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Zhang C, Wu Y, Zhang D. Association between Hypertension and the Risk of Parkinson’s Disease: A Meta-Analysis of Analytical Studies. Neuroepidemiology. 2019;52: 181–192. doi: 10.1159/000496977 [DOI] [PubMed] [Google Scholar]
- 79.Pamphlett R, Doble PA, Bishop DP. The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension. Toxics. 2021;9. doi: 10.3390/toxics9010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen M, Zhou B, Chen YH, Ma ZL, Gou Y, Zhang CL, et al. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS One. 2017;12: e0173731. doi: 10.1371/journal.pone.0173731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lentini P, Zanoli L, Granata A, Signorelli SS, Castellino P, Dell’Aquila R. Kidney and heavy metals—The role of environmental exposure (Review). Mol Med Rep. 2017;15: 3413–3419. doi: 10.3892/mmr.2017.6389 [DOI] [PubMed] [Google Scholar]
- 82.Bonuccelli U, D’Avino C, Caraccio N, Del Guerra P, Casolaro A, Pavese N, et al. Thyroid function and autoimmunity in Parkinson’s disease: a study of 101 patients. Parkinsonism Relat Disord. 1999;5: 49–53. doi: 10.1016/s1353-8020(99)00010-3 [DOI] [PubMed] [Google Scholar]
- 83.Pamphlett R, Kum Jew S, Doble PA, Bishop DP. Mercury in the human adrenal medulla could contribute to increased plasma noradrenaline in aging. Sci Rep. 2021;11: 2961. doi: 10.1038/s41598-021-82483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Gomez A, Diaz Y, Duarte-Salles T, Compta Y, Marti MJ. Prediabetes, type 2 diabetes mellitus and risk of Parkinson’s disease: A population-based cohort study. Parkinsonism Relat Disord. 2021;89: 22–27. doi: 10.1016/j.parkreldis.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 85.Parkin Kullmann JA, Pamphlett R. A Comparison of Mercury Exposure from Seafood Consumption and Dental Amalgam Fillings in People with and without Amyotrophic Lateral Sclerosis (ALS): An International Online Case-Control Study. Int J Environ Res Public Health. 2018;15. doi: 10.3390/ijerph15122874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zecca L, Tampellini D, Gatti A, Crippa R, Eisner M, Sulzer D, et al. The neuromelanin of human substantia nigra and its interaction with metals. J Neural Transm (Vienna). 2002;109: 663–672. doi: 10.1007/s007020200055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The labels indicate the outlined regions. Phosphorus images (top row) indicate the nuclear density of the tissues. (A) Particulate metals detected in the autometallography-positive locus ceruleus are mercury, iron, aluminium, and nickel. Iron in prominent in the pontine white matter. Chromium is widespread in the posterior pons. (B) Speckled mercury is present in the lateral geniculate nucleus, where neurons were autometallography-positive. Iron is seen in the adjacent white matter. (C) Mercury is present in the region of facial motor neurons. (D) Particulate mercury is present in the frontal white matter, which contains more iron than the cortex. (E) Particulate mercury is seen in the hippocampal white matter, which contains a large amount of iron. Chromium is widespread in the hippocampus. (F) No mercury is seen in the cerebellar cortex or white matter, which were both autometallography-negative in this patient. Iron is present in the cerebellar subcortical white matter. No significant amounts of cadmium, lead, bismuth, silver or gold are detected in any sections. Scale = counts per second (proportional to abundance).
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.