ABSTRACT
Even though nanoparticle drug delivery systems (nanoDDSs) have improved antitumor efficacy by delivering more drugs to tumor sites compared to free and unencapsulated satisfactory distribution and penetration of nanoDDSs inside solid tumors, especially in stromal fibrous tumors, remains challenging. As one of the most common stromal cells in solid tumors, tumor-associated fibroblasts (TAFs) not only promote tumor growth and metastasis but also reduce the drug delivery efficiency of nanoparticles through the tumor’s inherent physical and physiological barriers. Thus, TAFs have been emerging as attractive targets, and TAF-targeting nanotherapeutics have been extensively explored to enhance the tumor delivery efficiency and efficacy of various anticancer agents. The purpose of this Review is to opportunely summarize the underlying mechanisms of TAFs on obstructing nanoparticle-mediated drug delivery into tumors and discuss the current advances of a plethora of nanotherapeutic approaches for effectively targeting TAFs.
Keywords: Tumor associated fibroblasts, Nanoparticles, Drug delivery, Tumor therapy
Graphical Abstract
1. INTRODUCTION
Nanoparticles are an effective platform for the delivery of multiple therapeutic and diagnostic agents with high bioavailability, low systemic toxicity, and good patient compliance[1,2]. These nanoparticles can passively target tumor tissues through enhanced permeability and retention (EPR) effects[3,4]. However, the EPR effect may not always produce the maximum effect due to individual variation and tumor heterogeneity leading to inconsistent correlations between in vivo and in vitro and even failed clinical trials of nanotherapeutics[5]. Therefore, the research and development of targeted nanoparticles provides new opportunities for precision treatment of tumors[6]. Although targeted DDSs show great potential for cancer therapy, there are drawbacks including ineffective tumor targeting, permeability, and distribution in solid tumor tissues still limiting their ability to inhibit tumors in vivo.
Solid tumors are complex tissues that contain not only a tumor cell population, but also a variety of non-tumor stromal cells such as endothelial cells, fibroblasts, mesenchymal stem cells, and immune cells, among which TAFs are one of the most important cells[7, 8]. TAFs have the ability to produce cytokines and growth factors as well as remodel the extracellular matrix (ECM), which all together promote the transformational process by stimulating tumor growth, angiogenesis, and inflammation, and contributing to drug resistance[9, 10]. The presence of a larger number of TAFs in tumor stroma has been associated with an increased risk of metastasis and poor clinical prognosis in breast, lung, and pancreatic cancers[11]. In addition, TAFs are mainly located on the lateral side of the tumor tissue and can directly intercept nanoparticles resulting in poor intratumoral uptake of nanoparticles[12]. Meanwhile, TAFs can also secrete ECM, which aggravates the interstitial pressure of the tumor tissue[13], giving rise to increased interstitial fluid pressure (IFP) and decreased blood perfusion[14], further impeding the delivery of nanoparticles to the tumor cells. In the aggregate, the presence of TAFs in tumor tissues has a great effect on the targeted therapy of tumors, making it an attractive target for antitumor nanotherapy[15–17]. In this review, we will review the treatment strategies that target TAFs and related research on TAF-based nanoDDS.
2. TAFS POSE SIGNIFICANT CHALLENGES FOR TUMOR THERAPY
TAFs are one of the important stromal cell types in solid tumor tissue, and are mainly distributed on the outside of tumors or around tumor vascular endothelial cells[18]. The sources of TAFs are diverse, including fibroblasts, vascular smooth muscle cells and pericytes. Additionally, they can stem from transformed tumor cells, mesenchymal stem cells, and inflammatory cells in bone marrow adipose tissue or through the epithelial-mesenchymal transition and endothelial-mesenchymal transition [19, 20]. TAFs are typically fusiform or spindle shaped under physiological conditions and can highly express some specific proteins such as fibroblast-specific protein-1 (FSP-1), also known as S100 calcium binding protein A4(S100A4), fibroblast activation protein (FAP), vimentin and fibroblasts α-smooth muscle actin (α-SMA) [21, 22]. Although TAFs have several highly expressed proteins, they lack a unique protein specific to TAFs. Thus, high expressed FSP-1, FAP, vimentin, and α-SMA are commonly used to identify TAFs.
In addition to expressing hallmark proteins, TAFs also secretes a series of factors that participate in tumor growth, such as TGF-β, HGF, etc.[23]. Studies have shown that ovarian cancer-related fibroblasts can promote the growth of ovarian cancer through a paracrine mechanism dependent on the TGF-signal pathway[24]. Besides that, TAFs can also secrete the chemokine SDF-1, CXC chemokine ligand 1 (CXCL12), which binds to the ligand CXCR4 that is expressed on the surface of tumor cells to directly stimulate tumor cell growth[25]. TAFs can promote not only the growth of tumor cells but also the invasion and metastasis of tumor cells through cell-cell interactions and secretion of various invasion-promoting molecules such as cytokines, chemokines, and proinflammatory factors[26, 27]. Erez et al. found that TAFs can interact with the nuclear factor-κB signaling pathway to promote the expression of inflammatory response signals, which can recruit more macrophages, thereby promoting tumor invasion[28]. Moreover, researchers found that TAFs can promote the migration and invasion of hepatocellular carcinoma (HCC) cells in vitro. This facilitates the HCC metastasis to the bone, brain, and lung tissues as seen in NOD/SCID mice by secreting CCL2, CCL5, CCL7 and CXCL16[29].
More importantly, TAFs can also cause tumor resistance. Recent studies have shown that CD10+GPR77+TAFs can promote tumor formation and chemotherapy resistance by providing protection to cancer stem cells (CSCs). The molecular mechanism is that CD10+GPR77+ TAFs continuously activate NF-kB through p65 phosphorylation and acetylation. This activation is maintained by complement signaling of C5a receptor GPR77[30]. Therefore, the presence of TAFs leads to chemotherapy resistance in solid tumors by altering the physiological signaling pathways of tumor cells[31]. The drug resistance caused by TAFs has provided an additional challenge in the treatment of solid tumors. Solving this issue has become a new direction of tumor research.
3. TAFS AS A FORMIDABLE BARRIER FOR DRUG DELIVERY
TAFs pose an additional challenge by decreasing the overall efficiency of chemotherapeutic agents for the treatment of tumors. Fortunately, the emergence of nanodrug delivery systems has brought new hopes for improving the therapeutic effect of various drugs against tumors. Many targeted nanoparticles have been investigated, developed, and approved for clinical use[32, 33]. However, the interaction between tumors and nanoparticles is complex[34]. Through various mechanisms, tumors can hinder the penetration and distribution of nanoparticles to the tumor. Among these mechanisms, TAFs play a critical role by enhancing tumor fibrosis, increasing interstitial pressure, and secreting various factors and stromal enzymes to restrict the entry of nanoparticles into tumor tissue. All these factors result in the reduced of tumor penetration and decreased antitumor efficacy of DDSs to the tumor[35, 36].
3.1. Physical Barrier
TAFs, one of the abundant stromal cells in tumor microenvironment, are mainly concentrated on the lateral side of the tumor stromal, forming a physical barrier against the delivery of nanoparticles. This physical structure can easily intercept nanoparticles, leading to the off-target effect of nanoparticles[37]. Additionally, TAFs can accelerate the formation of the extracellular matrix (ECM) [38]. ECM is mainly composed of glycoprotein, proteoglycan, collagen, elastin and hyaluronic acid[39, 40] (HA). ECM provides structural support for tumor tissues in addition to regulating the growth of tumor cells. The dense structure of ECM not only provides a good environment for tumor cell growth, but also decrease the penetration of nanoparticles[41]. In addition to directly preventing the delivery of nanoparticles, the high-strength extracellular matrix can also cause an increase in tumor tissue pressure. The elevated pressure can distort and deform the nearby blood vessels around the tumors, damaging the blood vessels and vasculature within tumors. This phenomenon further weakens the blood perfusion, hindering drug and nanoparticle delivery[42]. Furthermore, these structural changes in physical characteristics also lead to the increased interstitial fluid pressure(IFP) in tumor tissues[43]. IFP can enhance the aggregation of nanoparticles through EPR, which only works when the vascular pressure is greater than the intratumoral pressure; otherwise, the penetration and distribution of nanoparticles in tumor tissues will be limited. It has been proven that the penetrating ability of nanoparticles is mainly through penetration from the vasculature to tumors. Therefore, the IFP should be lower than microvascular pressure to achieve effective osmotic pressure. The IFP in normal tissues is about 0–3 mmHg, while the IFP in tumors rise to 5–40 mmHg, and may even reach 75–130 mmHg in some tumor issues[44, 45]. These contrasting pressures greatly impedes the efficiency of nanoparticles in tumor therapy[46]. Additionally, the structure of the fiber, the thickness of collagen, the pore size, and the inhospitable nature of the interstitial space, greatly limit the efficiency of nanoparticle delivery within the tumors.
3.2. Biological Barrier
As constituent cells in tumor tissues, TAFs interact with tumor cells by secreting a variety of cytokines to promote tumor cell proliferation. These factors can further exacerbate the unfavorable changes of the tumor microenvironment, reducing the delivery efficiency of nanoparticles. For example, the excess of vascular endothelial factors produced by TAFs, results in abnormal blood vessel growth in tumor tissues[47]. It is known that vascular disorders are the main reason for the EPR effect. Through this effect, nanoparticles can leak out of the neovasculature and passively accumulate in the tumor. However, this leaky vasculature also increases the penetration of interstitial fluid and blood components, increasing the IFP in tumor tissues, aggravating the diffusion difficulty of nanoparticles into tumors. On the other hand, highly heterogeneous tumor blood vessels can cause abnormal blood flow and poor blood perfusion, leading to hypoxia and an acidic environment in the tumor tissue[48]. This hypoxic environment will induce the production of hypoxia factors, hypoxia-inducible factors aggravate untoward changes in the tumor microenvironment[49], further aggravating the difficulty of drug delivery to tumor. In addition, hypoxia can also intensify the resistance of tumors and lead to clinical treatment failure[50]. In short, to reach the tumor area, the nanoparticles must exude from the blood vessel and penetrate the tumor matrix, which is a long and arduous process. The presence of TAFs keeps the concentration of nanoparticles at a very low level in tumor tissue, impairing the nanoparticle’s therapeutic efficacy. Therefore, targeting TAFs has become a promising research direction for improved cancer therapy.
4. THERAPEUTIC STRATEGIES FOR TARGETING TAFS
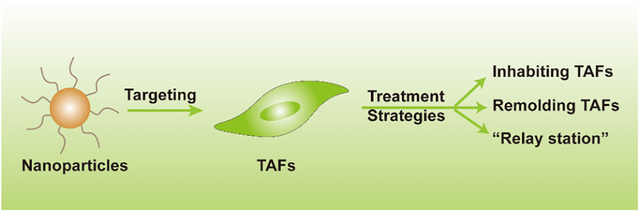
TAFs are resistant to cancer therapy in both physical and physiological ways, suggesting that these cells are promising targets for cancer therapy. Current treatment strategies for TAFs include direct inhibition of the activity of TAFs, remodeling of the activation of TAFs, and using TAFs as intermediate cells to produce therapeutic proteins, also known as the “relay station strategy”.
4.1. Inhibition of the Biological Activity of TAFs
Since TAFs can interact with tumor cells, vascular endothelial cells, and immune cells, inducing TAFs apoptosis is one of the potential strategies for anti-tumor stromal therapy. In a mouse model of lung cancer, removal of FAP positive cells can induce TNF-α and IFN-γ mediated anti-tumor immune responses, leading to rapid tumor necrosis[51]. As mentioned above, TAFs can lead to drug resistance of tumors, so direct killing of TAFs can also increase the effectiveness of chemotherapeutics treating tumors. Studies have indicated that a combination of paclitaxel with a low dose of 5FU, can effectively inhibit tumor growth hormone, and improve the effectiveness of chemotherapy. This is due to the fact that at very low doses, 5-FU can target TAFs and inhibit multi-drug resistance protein (P-gp) [52].
In addition to the direct use of chemo-drugs, nanotechnology-based drugs can also inhibit TAFs and thus improve antitumor efficacy. For example, to promote the penetration of nanomedicines into tumors and block stromal support to cancer cells, novel tumor stromal-targeted nanoparticles (FH-SSL-NAV) were designed to eliminate TAFs. These nanoparticles can effectively downregulate ECM deposition, reduce intratissue fluid pressure and promote blood perfusion, enabling comprehensive tumor microenvironment regulation with more chemotherapeutic nanoDDSs that can penetrate into tumor spheres in vitro and tumor tissues in vivo[53].
Similarly, in order to suppress the activity of TAFs and enhance the efficacy of chemotherapy, an injectable losartan-loaded hydrogel was reported by Hu et al. After injection, the hydrogel could be retained in the tumor for more than nine days, significantly inhibiting 4T1 tumor and collagen synthesis, thus effectively improving the anti-tumor effects of chemotherapy[54]. Additionally, there were experiments revealed in which losartan can enhance the penetration and therapeutic efficacy of nanoparticles (Doxil) both intratumorally and intravenously [55]. In addition, other studies have demonstrated that inhibition of TAFs can effectively boost the effectiveness of cancer treatments. For example, gemcitabine and cisplatin nanoparticles can destroy TAFs in a dose-dependent manner[56]. It has also been reported that the conjugate of PEGylated carboxymethylcellulose and docetaxel (Cellax-DTX particles) can reduce TAFs and destroy tumor stroma, enhancing therapeutic efficacy[57].
Treatment strategies depleting TAFs have showed satisfactory results in avoiding stroma-induced adverse reactions and improving tumor cells’ uptake of nanoparticles. However, there are many limitations for this strategy. First, it was found that inhibition of TAFs by drugs alone sometimes failed to inhibit tumor growth. In one study, the researchers used Pirfenidone (PFD) to target depleted TAFs[58]. The results showed that PFD can inhibit tumor cell viability and suppress collagen induced by TAFs in vitro. PFD alone can inhibit tumor fibrosis and TGF-β signaling in in vivo experiments but could not inhibit tumor growth and lung metastasis. This experiment implies that inhibiting TAFs alone may not necessarily inhibit tumor growth, revealing that TAF-based needs to be combined with other therapeutic modalities to achieve desired anti-cancer effects. We believe that the combination regiment will expand the scope of clinical use of TAFs-based therapy. Second, recent study found that after the elimination of TAFs, the stable state of the matrix may be affected negatively, accelerating the metastasis of tumors and shortening the survival time of tumor-bearing mice[59]. In another report, the researchers found that TAFs damaged by cisplatin could promote the development of survival factors (e.g., Wnt16) that support the proliferation of tumor cells[60]. Therefore, significant efforts remain needed to fully explore the therapeutic potential of targeting TAFs to enhance current cancer therapies.
4.2. Remodeling the Biological Function of TAFs
The phenotype and function of TAFs were found to be reversible. By reversing the phenotype, TAFs with pro-tumor activity can be reversed into a non-pro-tumor fibroblasts, resulting in a reduction in the release of pro-tumor factors[61]. This therapeutic strategy to reshape TAFs function is a promising way to inhibit tumor growth. As a biological phosphonic acid, bisphosphonate is used for the treatment of hypercalcemia and other bone complications caused by tumor bone metastasis [62]. Studies have demonstrated that bisphosphonate drugs can inhibit the expression of matrix metalloproteinases, interfere with the interaction between endothelial cells and extracellular matrix, prevent the formation of the tumor vascular network, and inhibit the expression of VEGF in tumor tissues, thereby suppressing tumor vascular endothelial cell proliferation and neovascularization[63]. Zoledronic acid is one of the most commonly used bisphosphonate drugs in clinical practice and has been widely applied in above aspects[64]. Recent studies have indicated that it can also reverse the phenotype of TAFs and reshape their biological function by inhibiting the RhoA-GTpase activity pathway, which reduces the generation and secretion of pro-oncogenic factors, changes the tumor microenvironment, and inhibits the growth of tumors[65].
In addition to using chemical drugs, regulating the expression of related factors through gene therapy is also a good choice to reconstruct the TAFs phenotype. Research found that in ovarian TAFs, miR-31 and miR-214, are downregulated while miR-155 is upregulated when compared with normal or tumor-adjacent fibroblasts. Mimicking this deregulation, transfection of miRNAs and miRNA inhibitors induce a functional conversion of normal fibroblasts into TAFs, resulting in the reversion of TAFs into normal fibroblasts. The miRNA-reprogrammed normal fibroblasts and patient-derived TAFs shared a large number of upregulated genes highly enriched in chemokines known to be important for TAFs function[66]. These results indicate that TAFs can be reprogrammed to become normal fibroblasts through the therapy of miRNAs.
4.3. Relay Station Strategy
We have known that TAFs have powerful paracrine functions that can secrete a variety of cytokines and proteins into the tumor microenvironment. These secretions play a regulatory role in the growth, invasion, and metastasis of tumor cells. Taking advantage of these characteristics, Huang’s team have put forward a “relay station strategy” to deliver therapeutic genes into TAFs to induce the secretion of therapeutic factors for treating tumors[67]. The results of in vivo studies confirmed that nanoparticles often have off-target effects in solid tumors. The main reason is the retention of nanoparticles in TAFs. They used electrostatic interactions to compress secreted tumor necrosis factor associated apoptosis-inducing ligand (STRAIL) DNA and positively charged protamine to form nanoparticles that were later encapsulated into liposomes. The nanoparticles can be ingested by TAFs through off-target effects. The delivered DNA was transcribed and translated into a large number of proteins within TAFs. With the help of the paracrine function of TAFs, these proteins were secreted into the tumor microenvironment, directly bypassing the stromal barrier, and reaching the tumor cells, which then bound to death receptors on the urface of tumor cells, consequently inducing apoptosis of tumor cells and exerting anti-tumor effects.
5. DESIGN STRATEGIES FOR TAF-TARGETING NANOPARTICLES
As mentioned above, TAFs plays a key role in tumor development, including vascular abnormalities and remodeling the tumor microenvironment[68], such as dense deposition of ECM, nonspecific internalization, high solid stress and increased IFPs. At the same time, their existence also negatively affects the treatment of tumors, including aggravating the multidrug resistance of tumor cells and blocking drug delivery[69]. This accounts for the unsatisfactory clinical antitumor efficacy. Some monoclonal antibodies and small molecule drugs that target crucial regulatory factors during clinical or preclinical evaluation have been reported[70, 71]. But these drugs are highly toxic due to off-target effects. Moreover, traditional drugs treating TAFs are often met with limited success due to the low amount of final drug at the target (TAF), which therefore requires more frequent administration with shorter interval, inevitably contributing to increased adverse effects[72]. Nanotechnology-enabled TAF-targeting strategy has been emerging as a powerful means for the effective treatment of tumors by enhancing the drug delivery efficiency to tumor tissues. NanoDDSs can achieve the passive targeting to TAFs/tumor tissues based on classic enhanced permeability and retention (EPR) effect[73]; or can obtain much higher precision for TAF targeting by anchoring specific ligands that can precisely recognize the receptor proteins on TAFs[74], both of which can reduce the non-specific systemic toxicities. Moreover, nanoDDSs can address the solubility issues plaguing a large portion of the developed therapeutics (eg., small molecule drugs), improving the bioavailability by extending blood circulation because nanocarriers can protect the encapsulated drugs from being recognized and eliminated by opsonization effects[75], increasing the likelihood of reaching the target (eg., TAFs).
In view of the biological functions of TAFs and their role as a formidable barrier for nanoDDSs, the design of nanoparticles targeting TAFs can be multi-faceted. On the one hand, targeted drug delivery can favorably regulate the biological functions of TAFs, alleviating their negative impact on tumor cells. On the other hand, it can reshape the tumor microenvironment and improve the drug delivery efficiency into tumors. To date, various TAFs-targeting nanoparticles have been reported, and these targeted nanoparticles have achieved good anti-tumor effects by eliminating TAFs or remodeling the function of TAFs[76, 77]. These findings proved that TAF-targeting nanoparticles could play a seminal role in tumor therapy. We believe that the discovery of various new therapeutics targeting TAFs and the elucidation of their mechanisms of action will pave the way for further improvement of anti-tumor efficacy and bring more hopes to cancer patients.
5.1. Passive Targeting
In tumor tissues, the rapidly growing neovasculature has poor structural integrity with leaky endothelial conjunction, through which nanoparticles can penetrate and subsequently reach the tumor tissues[78–80]. However, in heathy tissues the endothelial conjunction is intact without leakage, avoiding the untoward distribution of nanoparticles, reducing systemic toxicities [81]. In addition, the lymphatic drainage systems in tumor tissues are impaired, which cannot efficiently pump the nanoparticles back out to the bloodstream, allowing the prolonged retention[82–84]. Taken together, nanoparticles can be passively targeted to tumor tissues based on enhanced permeability and retention (EPR) effects[85–87]. TAFs are mainly located on the lateral side of tumor tissues and are closer to the tumor vasculature. Hence, TAFs possess the natural intercepting capability with improved uptake of nanoparticles, further boosting the passive targeting efficiency.
Recent studies have shown that polyene paclitaxel and polyethylene glycol molecules were covalently coupled to carboxymethyl cellulose by an ester bond, and the polymer can self-assemble into nanoparticles (Cellax-DTX). More than 90% of Cellax-DTX particles passively targeted smooth muscle actin (SMA)-positive cancer-associated fibroblasts. The nanoparticles can eliminate SMA-positive TAFs, reduce matrix density, and increase tumor perfusion by more than 10-fold, thereby facilitating the deep penetration of anti-cancer drugs into tumors, further inhibiting tumor growth and metastasis.
Similarly, passively targeted TAFs nanoparticles can also be used for synergistic co-delivery of therapeutics. In one study, gemcitabine nanoparticles and cisplatin nanoparticles were prepared for the synergistically treating bladder cancer[88]. The results showed that 57% of TAFs were apoptotic after one day of combined treatment. After four days, the proportion of TAFs in cell apoptosis increased to 87% and the tumor stromal level decreased to 85%. Meanwhile, they also found that the combined treatment penetration efficiency of the gemcitabine nanoparticles and cisplatin nanoparticles was 2.75 times higher than that of the free drug.
In addition to conventional nanoparticles, environment-responsive nanoparticles have also been used in the regulation of TAFs. For example, Ji et al. developed a β-cyclodextrin (β-CD) modified matrix metalloproteinase-2 (MMP-2) responsive liposome to deliver pirfenidone and gemcitabine[89]. MMP-2 responsive liposomes passively reach the tumor and decompose into two functional parts under the action of MMP-2. βCD containing pirfenidone was retained in the matrix, downregulating fibrosis and reducing the matrix barrier. On the other hand, liposomes modified with RGD peptides were used to deliver gemcitabine to target pancreatic tumor cells. The targeted nanoparticles significantly increased drug perfusion and provides a potential strategy for improving pancreatic cancer therapy. All these studies have proven that inhibition of TAFs can be achieved by utilizing both the EPR effect arising from neovaculature surrounding tumors, and the off-target effect of TAFs by nanoparticles, both of which can significantly improve the efficiency of drug delivery into tumors.
5.2. Active Targeting
The aforementioned drug-loaded nanoparticles that passively targeted TAFs can effectively improve the deep tumor delivery of nanoparticles and enhance the anti-tumor efficacy of chemotherapy drugs. The nanoparticles in these delivery systems, however, are not specifically ingested by TAFs, and some nanoparticles will be uptaken by tumor cells through EPR effect, reducing the concentration of drug-loaded nanoparticles in TAFs, resulting in the diminished therapeutic activity. In contrast, actively targeting nanoparticles can effectively increase their concentration in the corresponding cells, avoiding the non-specific uptake by surrounding healthy tissues[90]. Active TAFs-targeting nanoparticles can be constructed by covalently coupling or physical adsorption of TAFs-targeting ligands onto the surface of the nanoparticles, which can significantly reinforce their absorption of TAFs[91].
5.2.1. Fibroblast Activation Protein (FAP)-Receptor-Mediated Nanoparticle
FAP is a transmembrane serine protein with expression that is highly specific to TAFs. Its expression is also closely related to the invasion and metastasis of TAFs [92]. In addition, the expression of FAP can also affect tumor immunity by inhibiting IFN-γ, TNF-α and other signaling pathways [93]. Therefore, FAP can be used as a promising target for drug delivery. Loeffler et al. proved that the FAP DNA vaccine can effectively eliminate TAFs and inhibit the growth of primary tumors of colon and breast cancer in non-metastatic mice[94]. Targeting FAP can not only reshape TME and inhibit tumor cell invasion, but can also relieve the immunosuppression in tumors, exerting an immunotherapeutic effect in the body[95]. These changes have significant implications for further improvements in chemotherapy or immunotherapy for tumors treatments. Monoclonal antibodies are widely used in modifying nanocarriers to enhance targeting efficiency. Researchers have used single-chain Fv (scFv) antibody fragments specifically targeting fibroblast activation protein (FAP) to decorate immunoliposomes (ILs), which encapsulated an anti-fibrotic drug, deferoxamine (DFO)[96]. Experimental data proved that purified anti-FAP scFv can bind specifically to these cells without influencing the FAP enzymatic activity. Moreover, DFO-loaded ILs targeted to FAP caused a significant reduction in the collagen deposition, whereas no effect was observed using liposomes without the targeting antibody fragment. These studies suggest that the FAP-specific scFv-conjugated liposomes have considerable potential for cell-specific targeting for reducing excessive collagen deposition during fibrosis. In the same way, Ji et al. used a mouse monoclonal antibody (mAb) molecule that targets the human FAP to modify the surface of nanoparticles via electrostatic binding(Fig.4)[97]. The nanoparticles were inclined to binding with TAFs through interactions between the surface-attached anti-FAP mAb and the FAP-α expressed on the TAFs’ membrane. Compared with free drugs and inactively targeted nanoparticles, the modified nanoparticles increased the penetration of DOX into tumor tissue by depleting TAFs and destroying the stromal barrier.
Figure 4.
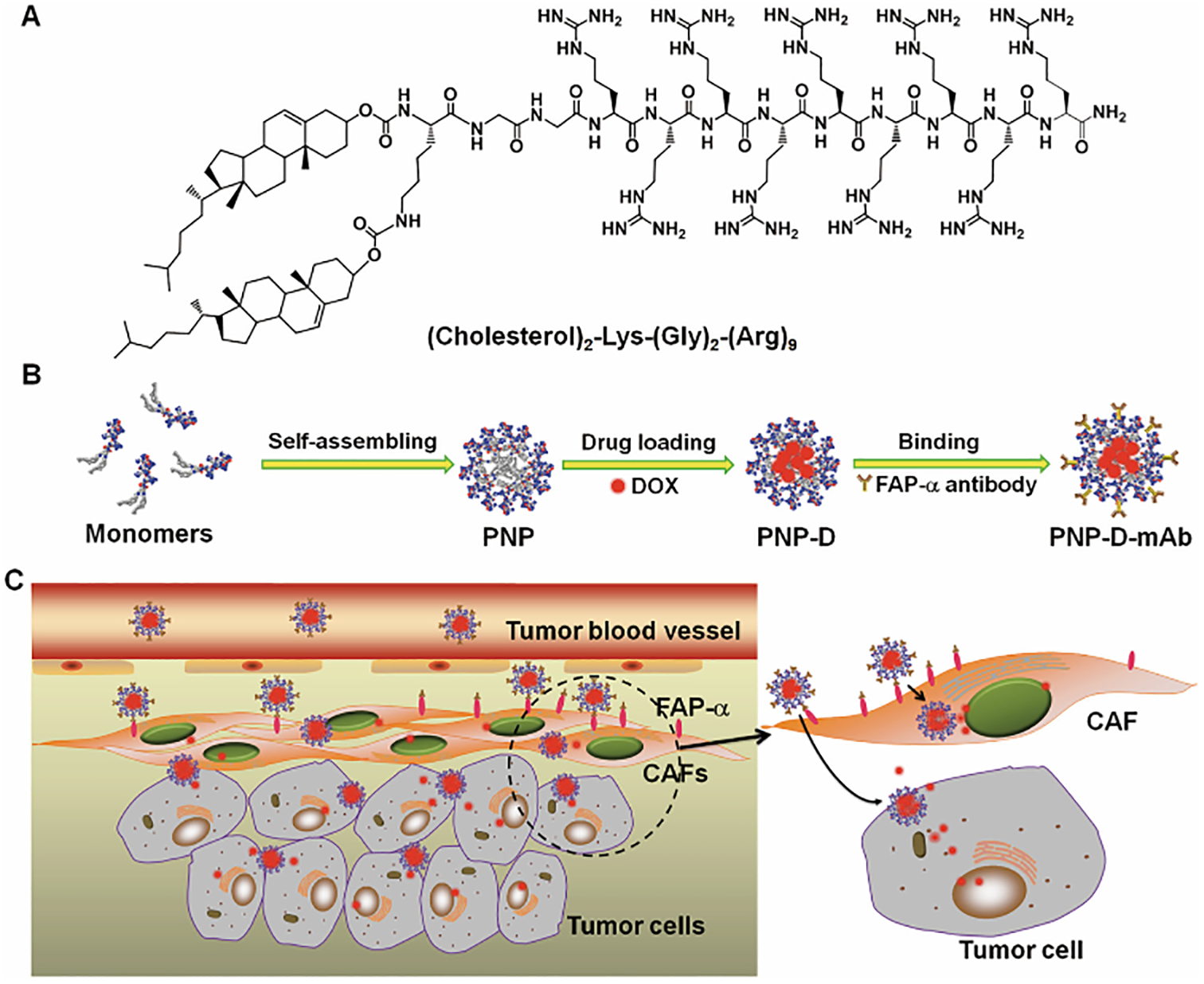
Design and proposed mechanism of PNP-D-mAb. (A) The structure of the cholesterol-modified CPP. (B) Schematic illustration of the nanoparticle formation process including peptide self-assembling, drug loading, and mAb modification. (C) The proposed mechanism of PNP-D-mAb in CAFs targeting and drug penetration. Adapted with permission from ref 97. copyright (2015) Wiley.
In addition to increasing the efficiency of chemotherapy drug delivery, targeting FAP nanoparticles can also increase the effectiveness of immunotherapies. Researchers used an FAP-specific single-chain variable fragment (scFv) to modify a photosensitizer carrier, a ferritin protein cage, to improve the nanoparticle’s TAFs targeting ability[98]. These nanoparticles can selectively act on TAFs in tumor tissues, eliminating TAFs under light conditions. This strategy can destroy the ECM and inhibit the secretion of chemokine 12 (CXCL12), which significantly improved the infiltration and expansion of CD8+T cells. This new type of photoimmunotherapy can selectively remove TAFs and regulate TME, markedly benefiting the anti-cancer immune responses.
In addition to directly anchoring antibodies on the surface of nanoparticles to achieve the active targeting ability, some researchers also used the hydrolytic activity of FAP to design enzyme-responsive response nanoparticles to active targeting to TAFs. FAP protein has both dipeptidase and collagenase activities, and can specifically cleave the peptide bond (Pro-X) formed by proline and other amino acids or small molecules at the second amino acid whose N-terminus is blocked[99, 100]. More importantly, dipeptidyl peptidase IV (DPP-IV), which is widely present in normal tissues in the body, has no dipeptidase activity. Therefore, FAP can be used as a targeted enzyme to design a prodrug or nanoparticles that can be specifically activated within TAFs to release therapeutic drugs directly at the target site and eliminate TAFs. For example, Kim’s team prepared FAP-responsive phospholipid-coated graphene nanoparticles (Dox/PL-rGO) for drug delivery(Fig.5) [101]. The phospholipids of the modified nanoparticles contained the FAP-cleavable promelittin sequence. The in vitro and in vivo results proved that the FAP-specific hemolytic activity of nanoparticles can be activated by the FAP expressed on TAFs in the tumor microenvironment and enhanced the cellular delivery and antitumor efficacy of Dox.
Figure 5.
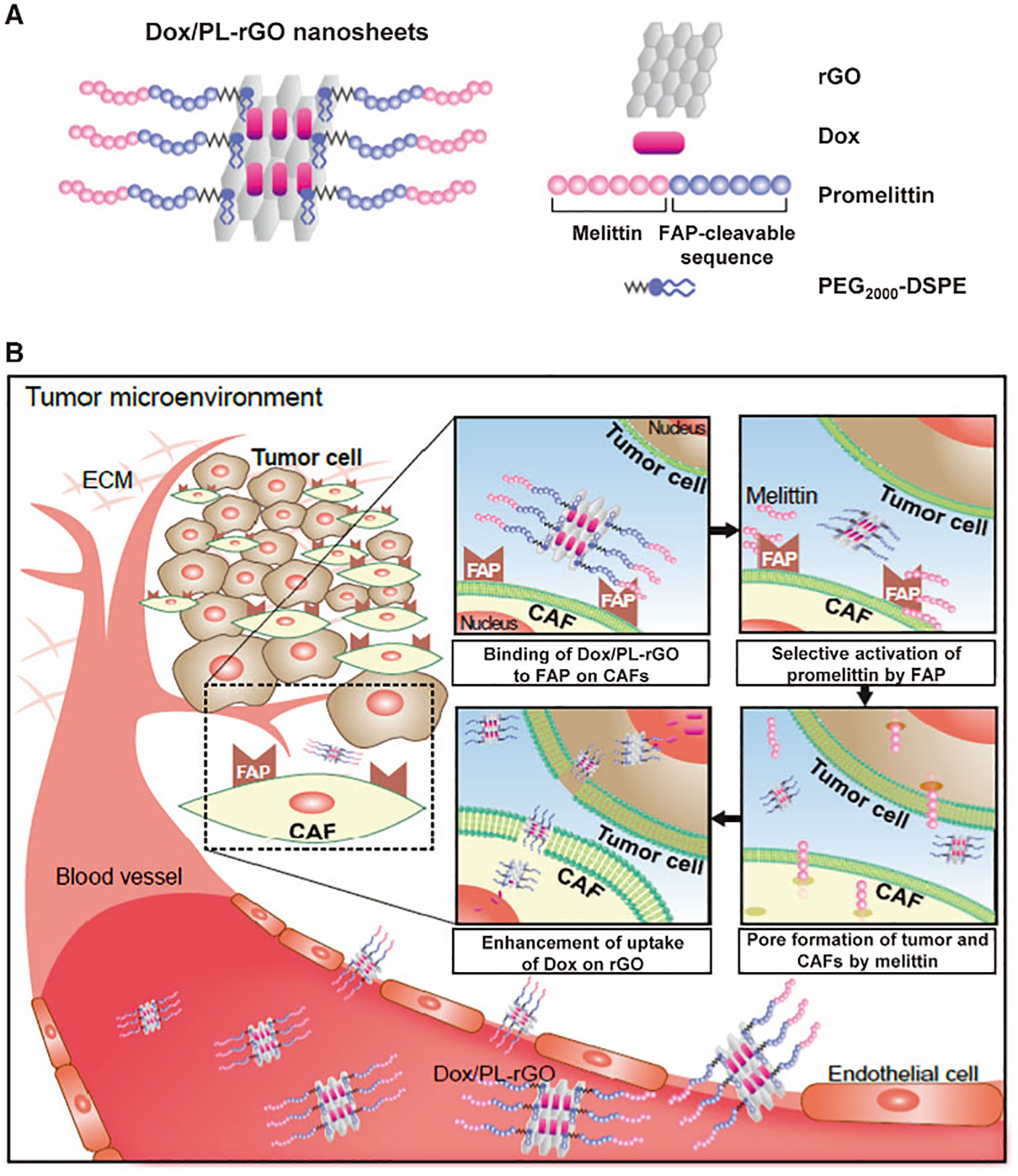
Nanostructure of doxorubicin-loaded promelittin lipid derivative–reduced graphene oxide (Dox/PL-rGO) nanosheets and a schematic illustration of the hypothesized mechanism underlying their action. (A) The promelittin lipid derivative, PL, containing a fibroblast activation protein (FAP)–cleavable sequence, was anchored onto rGO nanosheets. The resulting PL-rGO was further loaded with Dox, yielding Dox/PL-rGO. (B) Schematic of the presumed mechanism of Dox/PL-rGO. Activation of the promelittin moiety of PL-rGO by FAP overexpressed on cancer-associated fibroblasts releases melittin. Diffusion of melittin to surrounding tumor cells and cells in the tumor microenvironment promotes formation of pores in the membrane, increasing the cellular uptake of Dox-loaded rGO nanosheets and enhancing anticancer efficacy. Adapted with permission from ref 101. Copyright (2017) Oxford University Press.
Similarly, another novel cleavable amphiphilic peptide (Ac-Ala-Thr-Ala-Lys (C18) -As-Ala-Thr-Gly-Pro-Ala-Lys(C18)-Thr-Pro-Ala-NH2 (CAP) was designed to produce an enzyme-response (FAP) to fibroblast via the amino acid residue sequence Gly-Pro-Ala-X. The peptide can self-assemble in aqueous solution to form fibrous nanostructures, which were able to encapsulate hydrophobic chemotherapeutics [102]. These nanoparticles were cleaved under the action of FAP, resulting in rapid and effective drug release at the tumor site, effectively improving the tumor delivery efficiency and therapeutic efficacy. Meanwhile, this nanoparticle allowed drugs to disrupt the stromal barrier, enhancing local drug accumulation, further demonstrating the therapeutic potential of FAP-targeted nanoDDSs in anti-tumor therapy. Based on this work, He’s research group constructed a novel nanoparticle which is responsive to the membrane biomarker FAP-α and near-infrared (NIR) laser irradiation[103]. Small paclitaxel albumin nanoparticles (HSA-PTX) with strong tumor penetration were encapsulated into CAP-modified thermosensitive liposomes (CAP-TSL). In addition, CAP-ITSL was obtained by adding IR-780 photothermal agent to CAP-TSL. The HSA-PTX@CAP-ITSL was designed to increase the drug retention of HSA-PTX in solid tumors and to trigger the release of HSA-PTX via FAP-α. IR-780 generated heat to kill tumor cells under near-infrared laser irradiation, and further promoted the release of small volume HSA-PTX in the deep tumor region. Subsequently, HSA-PTX@CAP-ITSL presented improved antitumor efficacy in subcutaneous and orthotopic tumor mouse models. HSA-PTX@CAP-ITSL effectively combined chemotherapy with photothermal therapy, providing a promising drug delivery strategy for pancreatic ductal adenocarcinoma treatment.
5.2.2. Sigma-Receptor-Mediated Nanoparticles
Sigma receptors are well-known membrane-bound proteins that show high affinity for neuroleptics and are overexpressed on many human tumors including melanoma[104, 105]. These receptors have been used to improve the uptake efficiency of nanoDDSs by tumor and stromal cells, especially TAFs. Small molecules such as haloperidol, SA4503, and opipramol have been reported as sigma-receptor ligands[106–108]. These findings suggested that sigma-receptor ligands could also be used for targeted drug delivery. Mukherjee et al. reported that haloperidol-modified liposomes prominently improved the delivery efficiency of DNA compared with the control group[109]. Besides that, another study has shown that anisamide has a high affinity for the sigma receptor. Huang et al. used it as a ligand for a modified tumor-targeting liposome (anisamide-conjugated liposome) which was first reported in 2004[110]. The results showed that the anisamide modified liposome enhanced tumor uptake in vitro and in vivo.
Subsequently, Huang’s team designed a series of DDSs to target TAFs to further reshape the biological function of TME with an aim to improve the antitumor treatment efficacy. They used anisamide to modify the surface of lipid-coated calcium phosphate nanoparticles to augment the TAFs-targeting ability. In this study, the uptake of these anisamide modified nanoparticles by TAFs was about 7-fold higher than that of the other cells[111]. Similarly, they prepared anisamide modified Lipid/Calcium/Phosphate nanoparticles loaded with the quercetin prodrug, quercetin phosphate (Fig.6) [112]. The prodrug was released from the nanoparticles and converted back to the quercetin under proper physiological conditions. Following the systemic administration of nanoparticles, the α-SMA-positive fibroblast and collagen within the tumor decreased significantly. These results demonstrated that the quercetin played a crucial role in remolding tumor microenvironment. Moreover, these nanoparticles significantly increased the penetration distance of the subsequent injection of cisplatin nanoparticles by eliminating the tumor stromal barrier. They also prepared a sigma receptor-targeted lipid-coated protamine DNA complexes (LPD) liposome for delivering gene drugs[67]. All these results indicate that the selection of suitable ligands, with high selective affinity to the sigma receptor for modification of nanoparticles, will contribute to the specific and high-affinity binding of nanoparticles to the TAFs’ surface.
Figure 6.
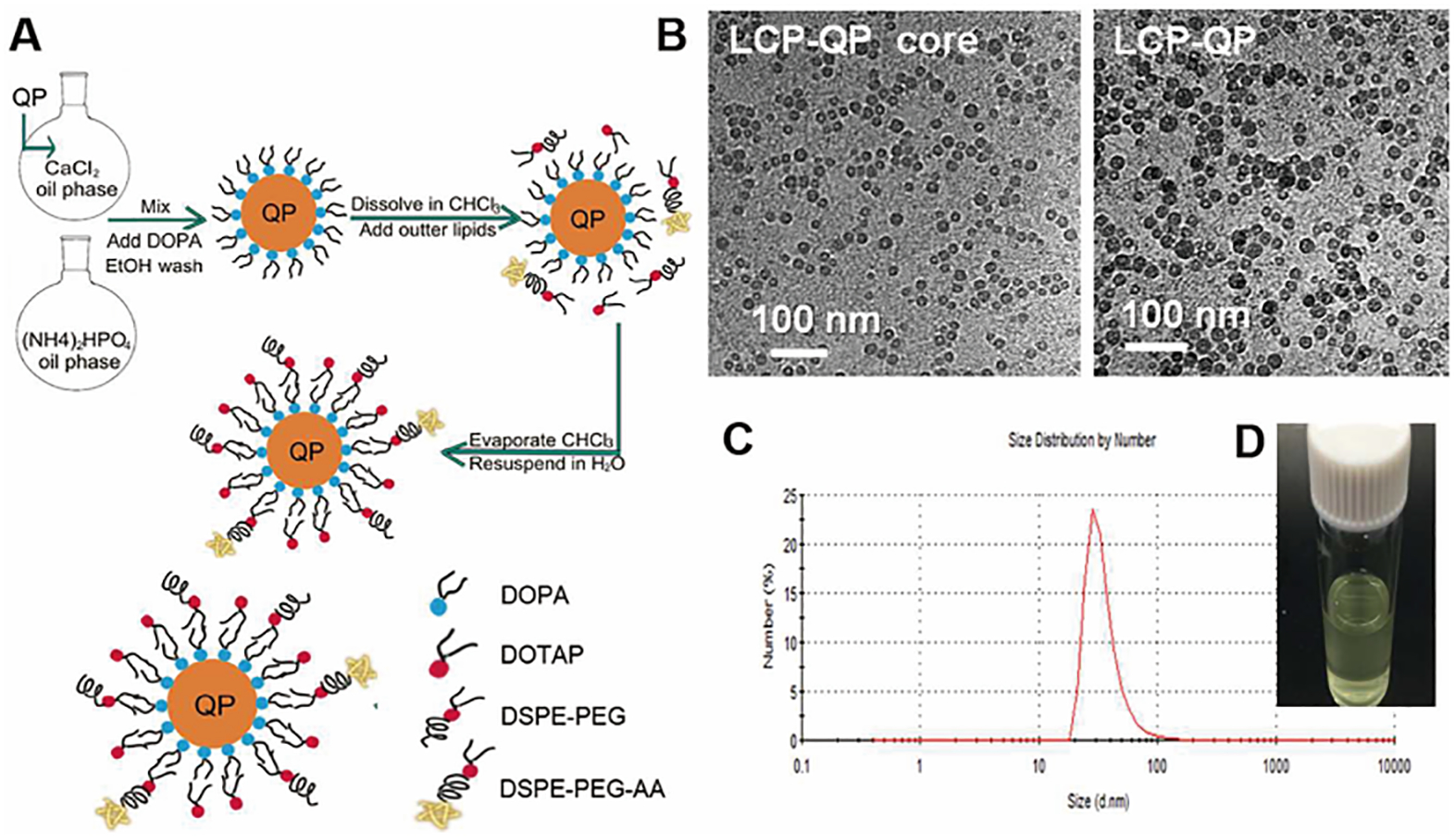
Preparation and characterization of LCP-QP. (A) Preparation procedure for LCP-QP. (B) TEM photograph of LCP-QP cores and final particles. (C) Dynamic light scattering measurements of particle size and distribution of LCP-QP. (D) Photograph of LCP-QP solution. Adapted with permission from ref 112. Copyright (2017) American Chemical Society.
5.2.3. Fibroblast Growth Factor Receptor (FGFR) Mediated Nanoparticles
FGFR, a transmembrane tyrosine kinase receptor located on the surface of TAFs [113], consists of cell surface receptors of four structural subtypes (FGFR1-4) that bind to fibroblast growth factor (FGFs) and play an important role in many biological processes[114]. FGFR expression is highly specific to tumor stromal cells but lacks expression in tumor and other non-tumor cells. Thus, FGFR can be used as a potential target for drug delivery. From 2000, researchers have installed FGF2 to an adenovirus vector to improve the gene transfection efficiency[115]. FGF2 can target an adenovirus vector to TAFs through its high affinity with FGF2 receptor. In order to improve the stability of ligand, another heptamer functional peptide (MQLPLAT) was developed to specifically bind to the FGF2 receptor [116]. Based on this work, another researcher designed MC11 peptide (MQLPLATGGGC) and used it to functionalize polyethyleneimine (PEI), which self-assembled into FGFR targeting nanoparticles (MPC/Ad-SS-PEG) along with cyclodextrin and PEG for gene delivery (Fig.7)[117]. These findings showed that the MPC/Ad-SS-PEG complex can effectively aggregate pDNA into nanoparticles with sizes of 100–200 nm. In vitro gene transfection studies showed that the efficiency of MPC/Ad-SS-PEG mediated transfection was significantly higher than that of the control group. Importantly, MPC/Ad-SS-PEG also realized efficient delivery of tumor-targeting genes in tumor-bearing mouse models after systemic injection in vivo. These discoveries suggested that the MPC/Ad-SS-PEG system can serve as a safe and effective non-viral vector for FGFR-mediated gene therapy by targeting TAFs.
Figure 7.
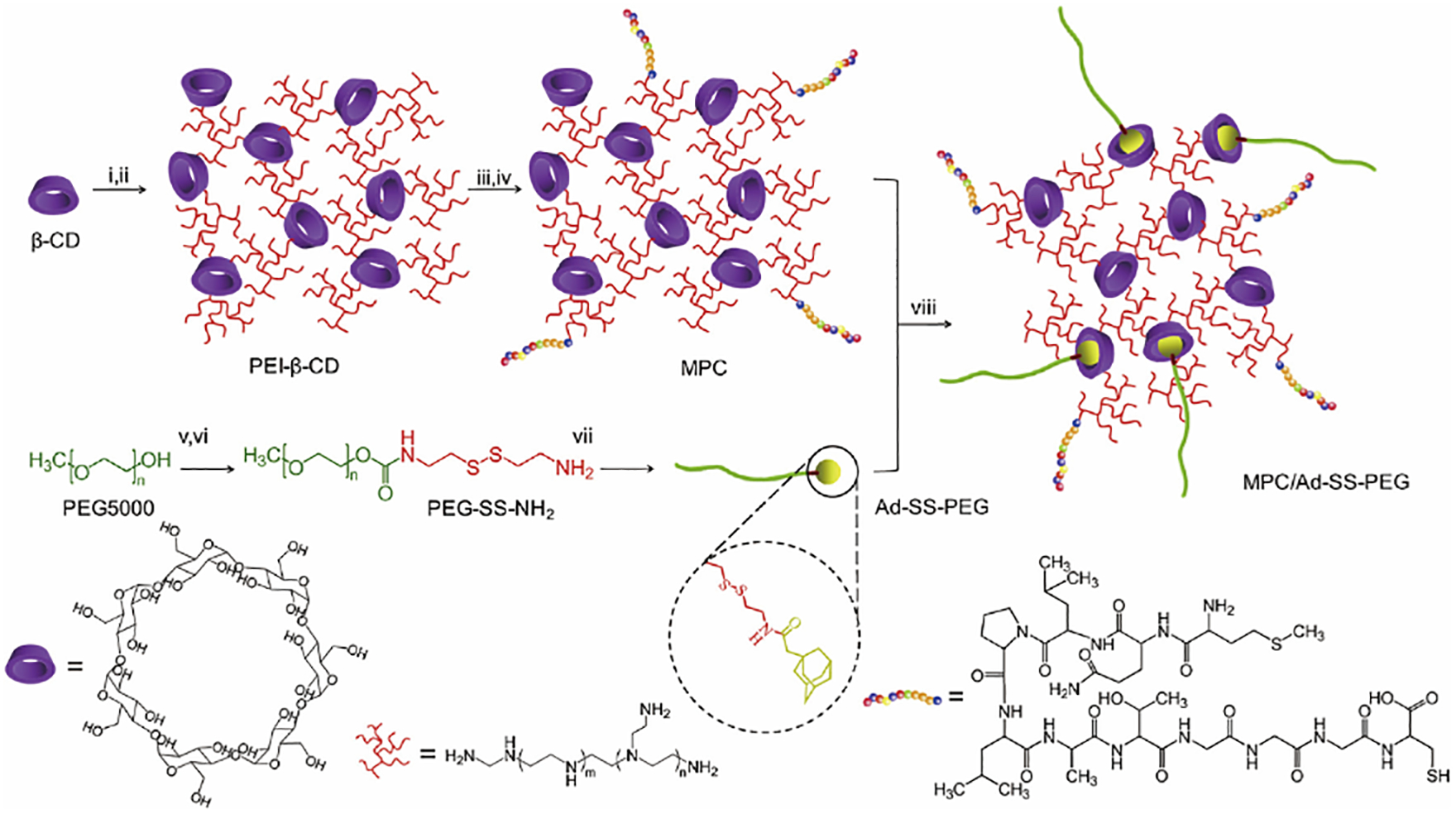
Synthesis route of MPC/Ad-SS-PEG. Conditions: (i) CDI, DMF, 4 h; (ii) PEI (600 Da), DMSO, 12 h; (iii) SPDP, DMSO, 16 h; (iv) MC11, DMSO/PBS, 12 h; (v) CDI, DMF, overnight; (vi) cystamine dihydrochloride, DMSO, 24 h; (vii) 1- adamantaneacetic acid, DCM, 24 h; (viii) r.t., 12 h. Adapted with permission from ref 117. Copyright (2013) Elsevier.
5.2.4. Platelet-Derived Growth Factor (PDGF) Receptor-Mediated Nanoparticles
PDGF receptors include two separate subclasses, PDGFR-α and PDGFR-β, and their structures include five extracellular immunoglobulin (Ig) loops along with intracellular tyrosine kinase domains[118]. PDGFR-α and PDGFR-β exist in the form of homodimer or heterodimer and can specifically bind to the PDGF dimer subunits. The PDGFR-β is one of the well-studied TAFs markers[119–121]. A wide range of ligands are known to bind to PDGFR-β, many of which are[122,123] linear and cyclic peptides[124] or antibody mimetic (affibody) molecules[125]. These ligands have also been used to deliver therapeutics to the tumor stroma[126–128]. One such example is the research work of Alexey Kuzmich and his colleagues. They had fused histone H2A with PDGFR binding peptide (YIPLPPPRRPFFK, YG2) to construct a new type of non-viral fibroblast targeting DNA nanoparticles H2A-YG2[129], which effectively increased the transfection efficiency of foreign genes in PDGFR-positive cells. In addition, other researchers also capitalized on TAF’s PDGF receptors for their DDSs to deliver more drugs to TAFs for improved inhibition of fibrosis or tumor growth (Fig.8)[126]. In order to improve the targeting efficiency and safety of IFN-γ, [(PPB)-polyethylene glycol (PEG)-IFN-γ] nanoparticles were prepared by conjugating PEGylated IFN-γ with PDGFR recognition peptide. In vitro studies showed that PPB-PEG-IFN-γ significantly inhibited the mRNA expression of colla1, colla2 and α-SMA in TGF-β activated NIH3T3 fibroblasts. In vivo, PPB-PEG-IFN-γ specifically aggregates in PDGFR-positive myofibroblasts. PPB-PEG-IFN-γ treatment significantly decreased the expression of type I collagen, fibronectin and α-SMA mRNA and protein. Meanwhile, PPB-PEG-IFN-γ also reduced IFN-γ related side effects. In addition, they also linked IFNγ to PPB-HSA to prepare PPB-HSA-IFN-γ nanoparticles, which further revealed that specific PDGFR-β binding cyclic peptides can be used to deliver IFN-γ to fibroblasts, leading to inhibited tumor growth[130]. Meanwhile, they also conjugated doxorubicin to PPB-HSA to target cells expressing PDGFR-β in C26 tumor mice. Compared with free doxorubicin, targeted nanoparticles appreciably suppressed tumor development[128]. These studies demonstrate that ligand functionalized nanoparticles have the significant potential to improve the drug delivery efficiency to TAFs.
Figure 8.
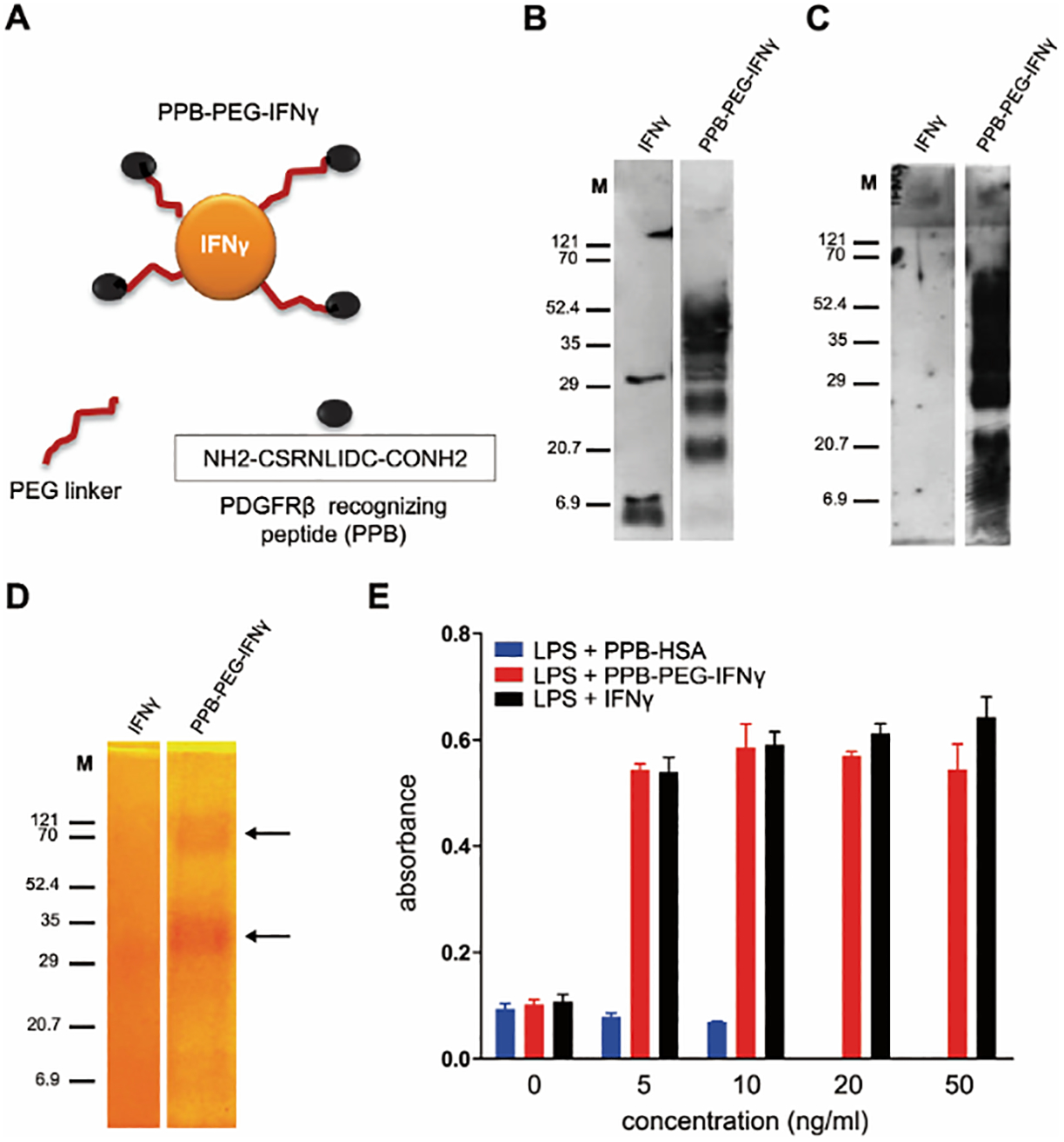
Characterization and biologic activity of PPB-PEG-IFN-γ. (A) Schematic representation of chemically engineered PPB-PEG-IFN-γ conjugate. (B,C) Western blot analysis to characterize PPB-PEG-IFN-γ by using anti–IFN-γ and anti-PPB antibodies, respectively. M: molecular weight. (D) PEG staining showing successful formation of PPB-PEG-IFN-γ. (E) Nitrogen oxide production in mouse RAW macrophages after incubation with PPB-HSA, PPB-PEG-IFN-γ, and unmodified IFN-γ, in the presence of LPS (n = 3). Adapted with permission from ref 126. Copyright (2014) Wiley.
5.2.5. Tenascin-C-Mediated Nanoparticles
Tenascin C (TNC) is mainly secreted by TAFs and is a component of tumor-specific extracellular matrix. It is highly expressed in most solid tumors, but not in normal tissues[131]. It has been reported that the small peptide FH (FHCKKSPALSPVGGG) has high affinity for TNC, revealing active tumor targeting in vivo[132]. Therefore, TNC can be perceived as a specific receptor for developing tumor microenvironment targeting drugs or nanotherapeutics. Based on this principle, Chen et al. designed a novel TAFs-targeted nanoliposome for delivering Navitoclax (FH-SSL-Nav) to specifically eliminate cancer-associated fibroblasts (Fig.9)[133]. The results showed that targeted nanoparticles could significantly kill more TAFs (1.4-fold) compared with unmodified nanoparticles in mouse HCC models. The decrease in the number of TAFs resulted in the inhibition of collagen secreted by TAFs, which effectively downregulated the deposition of ECM, decreased IFP, and promoted blood perfusion. At the same time, they also used the nanoparticles in combination with DOX-loaded targeted nanoparticles (7pep-SSL-DOX) to improve the anti-tumor effect of DOX [132]. The data showed that FH-SSL-Nav destroyed the stromal barrier in tumor microenvironment, reduced the extracellular matrix, enhanced the penetration of 7pep-SSL-DOX in solid tumors, and increased the accumulation in the deep tumor sites, leading to the boosted anti-tumor effects.
Figure 9.
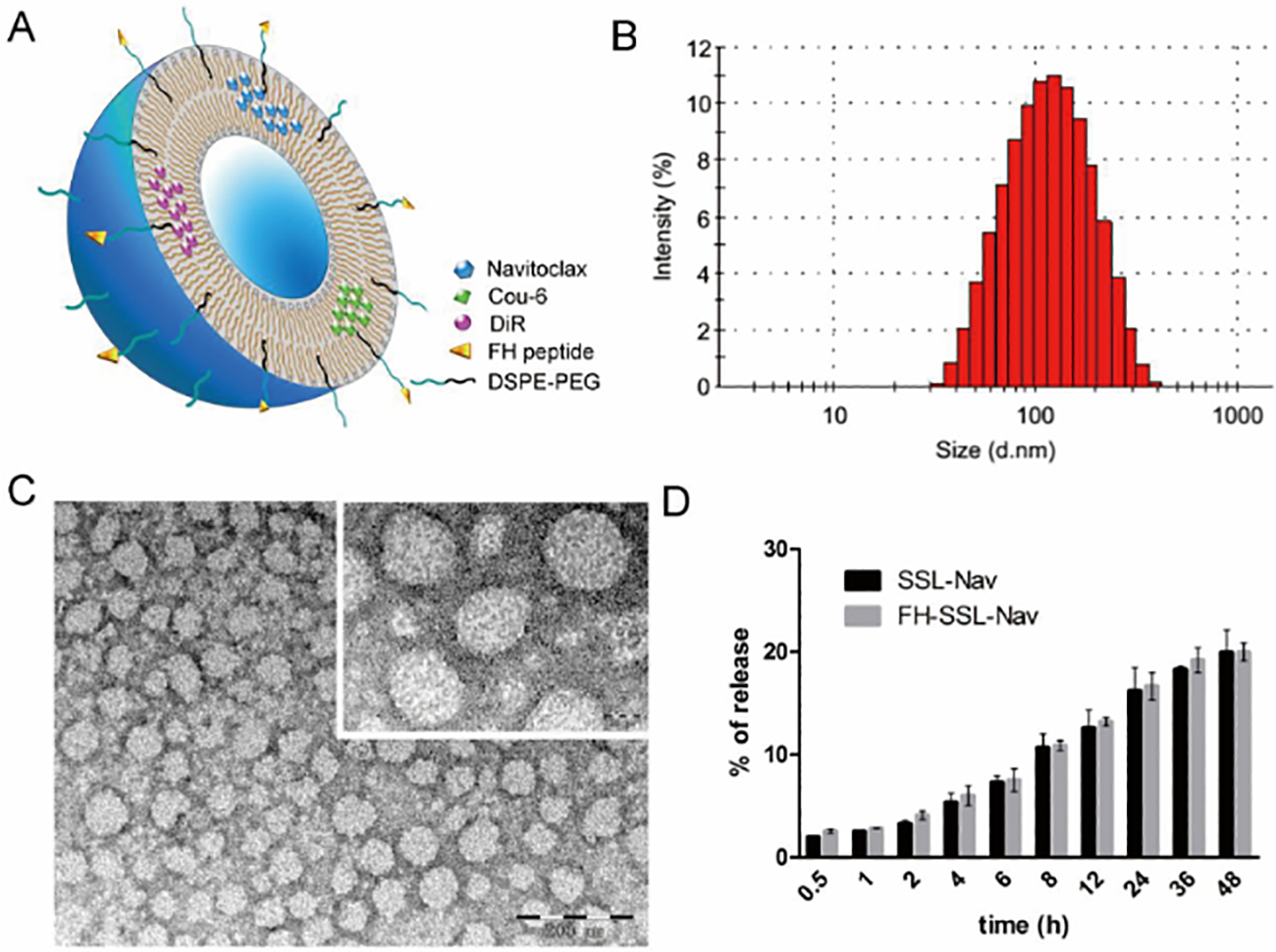
Characterization of Nav-loaded liposomes. (A) Schematic illustration of FH-modified nanoliposomes loaded with various contents. (B) Particle size distribution of FH-SSL-Nav by intensity. (C) Morphology of FH-SSL-Nav by TEM. (D) In vitro release kinetics of Nav from SSL-Nav and FH-SSL-Nav mixed with FBS (1:1, v/v) in PBS containing 0.5% SDS at 37 °C with shaking (100 rpm). Adapted with permission from ref 133. Copyright (2015) Elsevier.
6. SIGNIFICANCE, CHALLENGES, AND FUTURE PERSPECTIVES
Over past decades, the essential roles of TAFs in tumorigenesis, metastasis, and the contribution to inhibit tumor drug delivery are evident. Targeted treatment of TAFs has been emerging as a powerful and attractive therapeutic approach for enhanced cancer therapy[134]. A plethora of therapeutic agents targeting crucial regulatory factors in TAFs have been reported in preclinical or clinical investigation. In TAFs, janus kinase 2 (JAK2) that activates signal transcription and activator of transcription (Stat3) is a potential target of TAFs. JAK2 inhibitors such as SAR302503[135] and Pacritinib[136] inactivate TAFs and deplete the tumor matrix, decreasing tumor collagen. SAR 302503 and Pacritinib are in phase III clinical trials. Similarly, as a biomarker of TAFs, FAP can be specifically recognized by a monoclonal antibody, Sibrotuzumab[137], which is being investigated in patients with colorectal cancer. In addition, the urokinase type plasminogen activator (uPA) secreted by TAFs is an important indicator of tumor metastasis[138] and it has been shown that uPA inhibitor, PAI-2 (currently in preclinical studies)[139], can effectively activate serine protease, producing robust anti-tumor efficacy. These targeted drugs demonstrate the concept that targeting TAFs holds great potential for enhancing cancer treatment. However, the adverse and unwanted off-target effects associated with these TAFs-targeting drugs[140, 141] have markedly dampened the enthusiasm for pursuing this strategy and highlighted the urgent need for further increasing the TAFs targeting specificity. Moreover, traditional chemotherapy drugs in the treatment of TAFs will encounter obstacles such as short half-life and dosing intervals.
Nanotechnology has been extensively employed in the last several decades for targeted drug delivery for improving the therapeutic efficiency for treating various diseases including cancers based on EPR effects for passive targeting and/or specific ligand/receptor-mediated active targeting[142–153]. Despite the fact that a variety of nanoDDSs discussed earlier were able to elicit improved anti-tumor effects by targeted regulation of the TAFs’ function and the favorable remodeling of the tumor microenvironment, their clinical translation is still lacking, which could be attributed to a number of limitations and challenges associated with current TAFs-targeted nanoDDSs.
First, the biocompatibility and drug loading of nanoDDSs need to be improved[154, 155]. Low biocompatibility is not suitable for clinical use and could negatively affect the anti-tumor efficacy[154]. For example, in DOX/PL-rGO nanoparticles, the researchers used rGO as the main carrier material, but the material is not naturally degradable, which could lead to nephrotoxicity in long-term use. In addition, a limited drug loading will not achieve desired therapeutic effect and may even induce drug resistance in the tumor[156]. For instance, the molecular structure of peptides or proteins (e.g. monoclonal antibodies, IFN-γ) is bulky, rendering it challenging to physically encapsulate them into nanoparticles[157, 158]. Therefore, in PPB-PEG-IFN-γ nanoparticles, IFN-γ was covalently bound to the carrier material to improve its drug loading and biological activity. Furthermore, in some cases, when the polarity of the drug may be opposite to that of the carrier material, extremely low drug encapsulation efficiency results. For example, solid lipid nanoparticles[159] or micelles[160] are more suitable for encapsulating hydrophobic drugs. To solve these issues, we can (1) use the naturally occurring materials (e.g., albumin, phospholipids, cholesterol, etc.) or cells (e.g., platelet, red blood cells, neutrophils, etc.) as carrier compositions to significantly improve the biocompatibility of TAFs-targeting nanoDDSs; (2) employ nanoparticles with porous channels to increase the drug loading capacity and efficiency[161]; (3) utilize the prodrug conjugation strategies to modify the polarity of drugs for better drug/nanocarrier compatibility with higher drug loading[162, 163]. For optimal efficacy, we shall also consider the impact of the size[164, 165], charge[166, 167], and shape[168, 169] of nanoparticles targeting TAFs on their pharmacokinetics, distribution and uptake into tumor tissues. It has been demonstrated that nanoparticles with smaller sizes (10–200 nm) have a better tumor penetration efficiency[170–172]; and slightly negative charge can prolong blood circulation (prevent the binding by negative charged blood proteins[173]). Compared to nanospheres, elongated nanoparticles result in a higher number of multivalent occurrences essential for targeting tumor leaky vasculatures because the hemodynamic forces that can detach the nanoparticles away from the endothelium can be effectively offset by geometrically enhanced targeting from elongated nanoparticles[174, 175]. Therefore, optimization of the physicochemical properties of the nanoDDSs targeting TAFs to further improve drug delivery efficiency is indispensable.
Second, the optimal amount of TAF-targeting ligand is required on the surface of the nanoparticles. Too many ligands on the surface may, (1) cause strong immunogenicity leading to rapid clearance of nanoDDS from the blood; (2) give rise to competitive binding within nanoDDSs because a large number of ligands on nanoparticles will compete for a limited number of TAF receptors. Thus, the optimal amount of ligand was inevitably required when designing the TAFs-targeted nanoparticles.
Third, the heterogeneity of TAFs has brought controversy for the treatment strategy aiming at TAFs. The normal state of the matrix may be negatively affected after eliminating TAFs, promoting untoward tumor metastasis[59]. For example, it has been shown that selective elimination of α-SMA+ fibroblasts can inhibit tumor angiogenesis in mouse models[59]. However, removal of α-SMA+ fibroblasts also increased hypoxia and infiltration of immunosuppressive CD3+Foxp3+ Treg cells in tumors, inducing epithelial-mesenchymal transition(EMT) and tumor stem cell generation[59]. To tackle this issue, the application of single-cell sequencing and multi-fluorescence in situ detection technologies to identify the surface marker proteins of TAFs and to distinguish the tumor-promoting or tumor-suppressing subsets of TAFs is of great significance for future precision nanotherapy. Therefore, designing “individualized” nanoDDSs based on the heterogeneity of TAFs to achieve “personalized” treatment is expected to markedly enhance the efficacy and broaden the application of nanoDDSs.
Finally, although above mentioned TAFs-targeting nanoDDSs have achieved certain levels of therapeutic effect, treating TAFs alone only elicited limited efficacy. The interrelationship between tumor cells and TAFs is complex; inhibiting a single cell population does not eliminate tumor cells. Hence, co-treatment of TAFs and tumor cells for synergistic therapeutic effects is desirable. The most commonly used strategy is to produce “all-in-one” nanoDDSs[176], in which two or more drugs are simultaneously encapsulated in a single nanoparticle, and the combination of drugs will orchestrate their distinct mechanism of action for improved cancer therapy. The advantage of this “all-in-one” nanoDDSs is that they can accurately control the ratios between/among drugs spatiotemporally, ensuring the consistency between in vivo and in vitro therapeutic outcome. While nanoparticles are suitable for delivering two or more drugs concurrently in the same cell, they are less ideal for co-delivering drugs targeting different type of cells (TAFs and tumor cells) that are located in different areas of the tumor tissue at the same time. To cope with this issue, utilizing two different nanoDDSs that target the TAFs and tumor cells respectively is another feasible approach via asynchronous or synchronous administration for enhanced cancer therapy. This strategy greatly simplifies the tall complexity of nanoparticles design that would have arisen in developing the co-delivery nanoDDSs that can release drugs in TAFs and in tumor cells separately without comprising the nanoparticle integrity, facilitating the potential clinical translation. Furthermore, TAFs targeting nanoDDSs can also be combined with other therapeutic modalities including radiotherapy, photothermal therapy and immunotherapy, to further boost the therapeutic effects.
7. CONCLUSION
In recent decades, with the development of nanotechnology, nanoparticle-based DDSs have emerged as a powerful platform for improved therapy and diagnosis of tumors. Although the stability, targeting, and safety of nanoparticles have been greatly improved, their tumor delivery efficiency remains not optimal. We have known that during the development of tumor tissue, its surrounding environment will also undergo profound morphological and biological transformations, providing a microenvironment conducive to its growth. TAFs account for a big proportion of the tumor microenvironment. It plays a crucial part in the development, metastasis, and drug resistance of the tumor. Additionally, it also hinders the deep tumor delivery of drugs through various physical and biological effects. Owing to the unique characteristics of TAFs, approaches targeting TAFs to enhance the tumor delivery efficiency have been extensively explored. This article summarizes the current TAFs targeting strategies based on the relevant biological functions of TAFs and elaborates on how to devise the TAFs targeting nanoDDS. These studies have solved the bottleneck of nanoparticle mediated drug delivery into tumors to a certain extent, recognized the potential for deep tumor delivery, and effectively improved the anti-tumor efficacy.
While improved tumor therapy has been accomplished to some degree by nanoparticle-mediated TAF targeting, its full therapeutic potential has yet to be realized. We believe that (1)the new discovery of a variety of targeted drugs for TAFs and the elucidation of their mechanism of action, (2) improving the biocompatibility and drug loading of nanoDDSs, (3) optimizing the physicochemical properties of nanoDDSs, (4) finding the optimal amount of targeting ligand on nanoDDSs, (5) pinpointing and differentiating the tumor-promoting from tumor-suppressing marker proteins on the surface of TAFs, and (6) combining drugs targeting and treating TAFs and tumors will collectively pave the way for further improved antitumor efficacy and shed more light to cancer patients.
Figure 1.
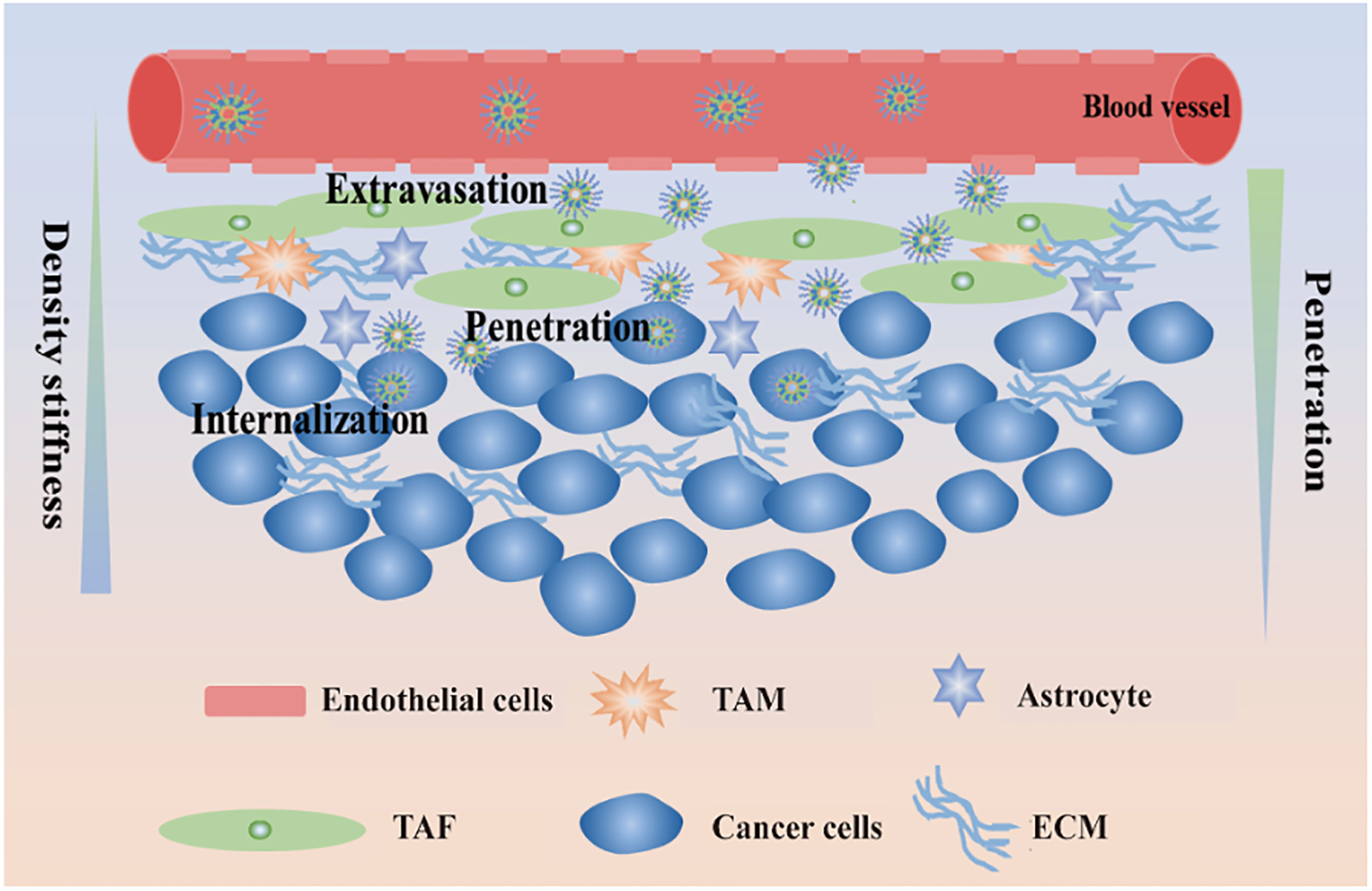
The fate of nanoparticles in tumor tissues. Nanoparticles entered tumor tissues from blood vessel through enhance penetration and retention (EPR) effects, then reach tumor cells through penetration, and finally are endocytosed by cancer cells.
Figure 2.
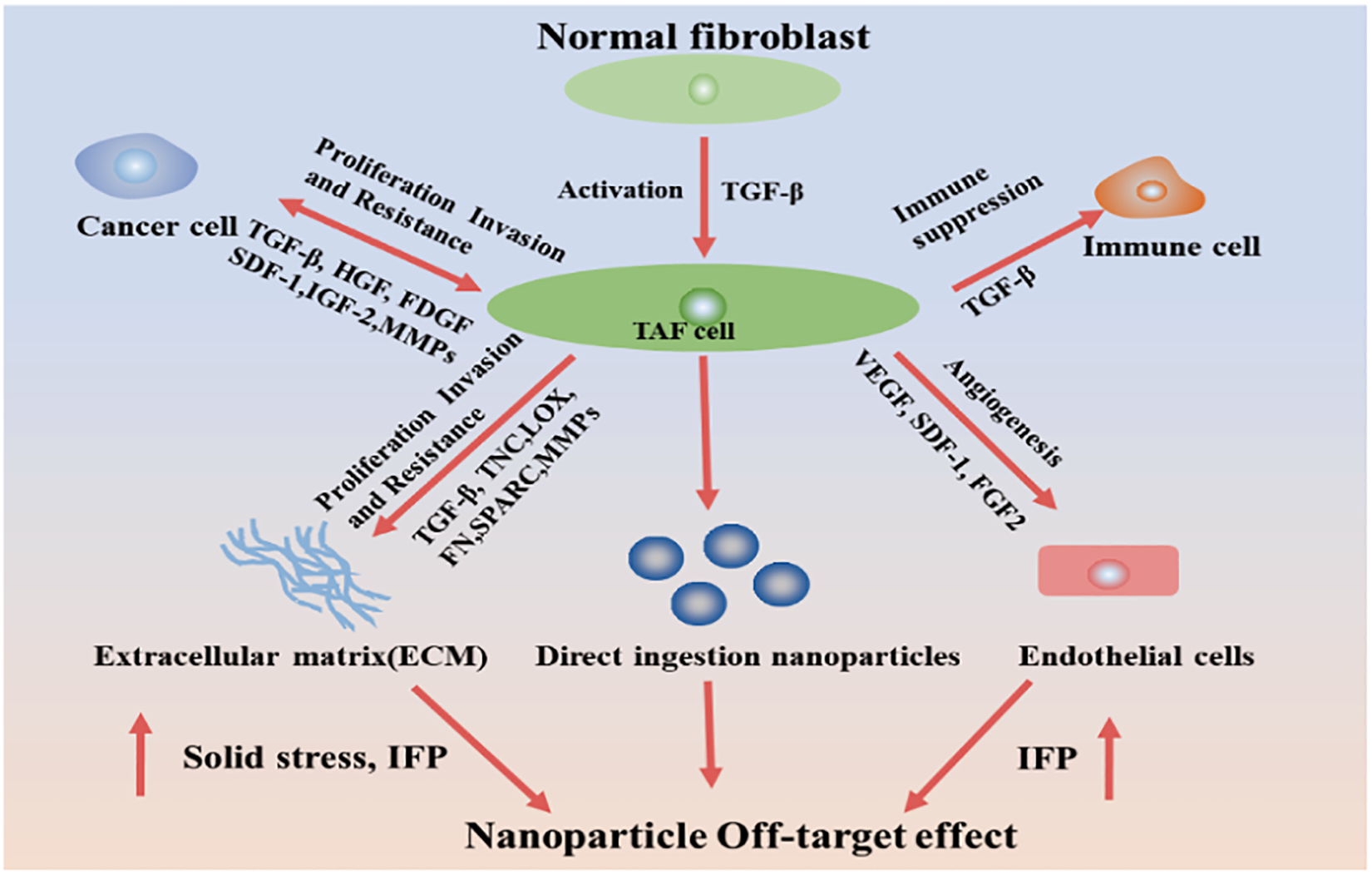
Schematic diagram of the mechanism of TAFs in tumor tissues. TAFs promote tumor proliferation, metastasis, and drug resistance through various mechanisms, reducing the therapeutic effect of chemotherapeutic drugs. In addition, it can also reshape the tumor microenvironment to resist drug delivery.
Figure 3.
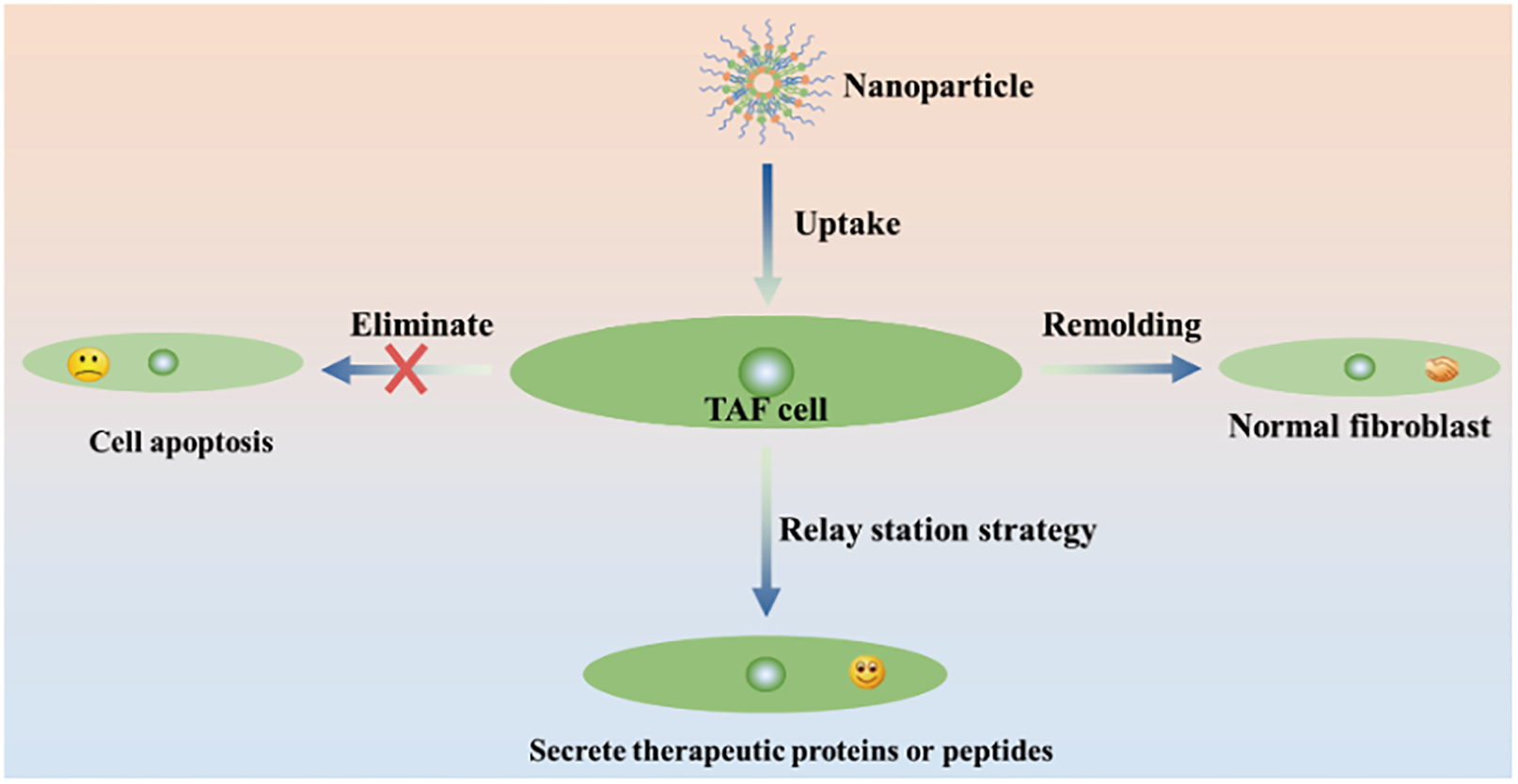
Treatment strategy based on TAFs. Nanoparticles deliver drugs to TAFs to produce biological effects including direct elimination, remodeling of phenotype, and secretion of therapeutic proteins or peptides through TAFs.
Figure 10.
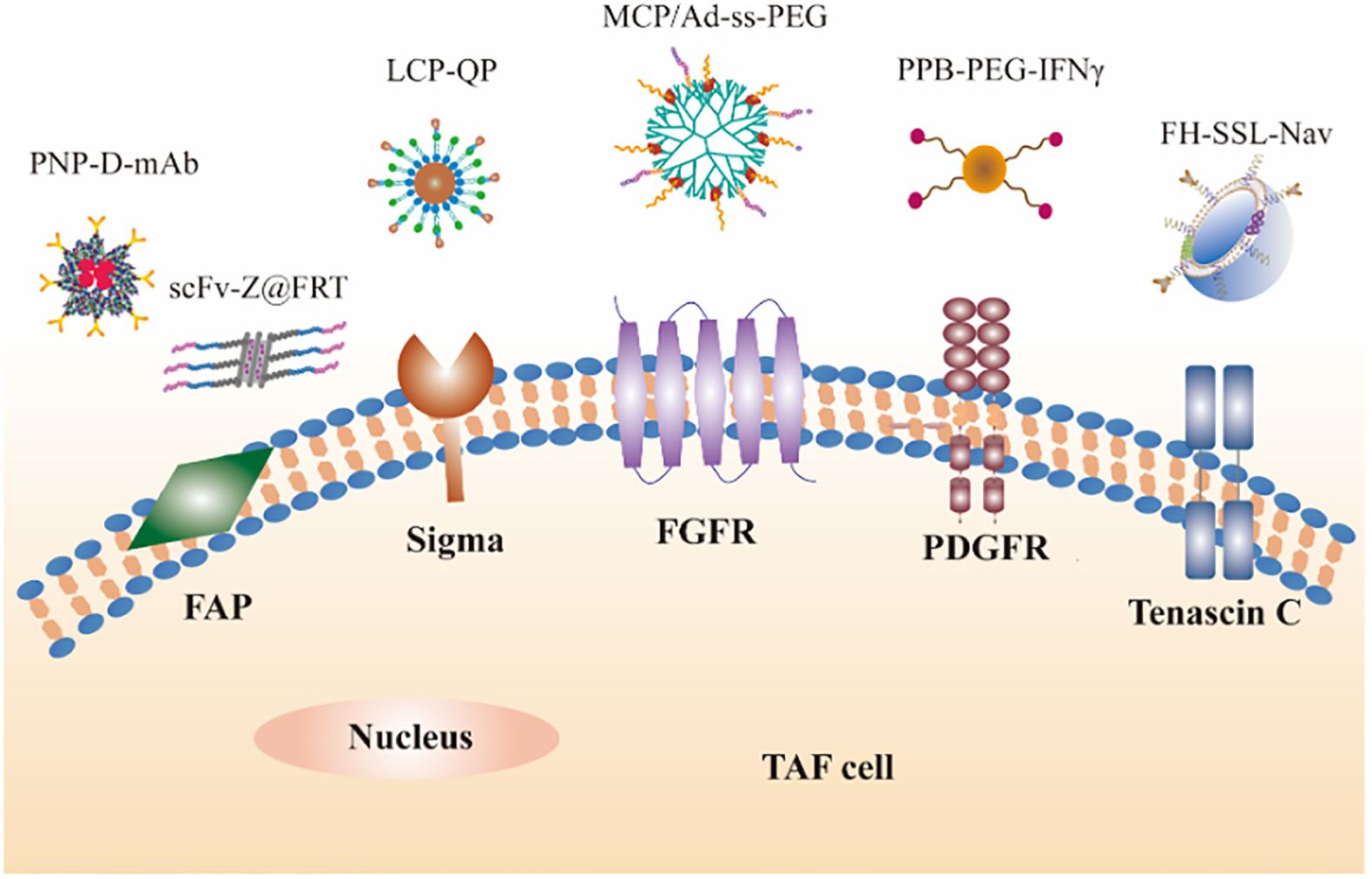
Schematic diagram of receptors or proteins expressed by TAFs and nanoparticles targeting TAFs. (1) Fibroblast activation protein (FAP) mediated nanoparticles (PNP-D-mAb, adapted with permission from ref 97. copyright (2015) Wiley) and FAP-cleavable nanoparticle (Dox/PL-rGO, adapted with permission from ref 101. Copyright (2017) Oxford University Press). (2) Sigma receptors mediated nanoparticles (LCP-QP, adapted with permission from ref 112. Copyright (2017) American Chemical Society). (3) FGFR targeting nanoparticles (MPC/Ad-SS-PEG, adapted with permission from ref 117. Copyright (2013) Elsevier). (4) Platelet-derived growth factor (PDGF) receptors mediated nanoparticles (PPB-PEG-IFNγ, adapted with permission from ref 126. Copyright (2014) Wiley). Tenascin C mediated nanoparticles (FH-SSL-Nav, adapted with permission from ref 133. Copyright (2015) Elsevier).
ACKNOWLEDGEMENTS
This work was supported in part by a Startup Fund from the College of Pharmacy at The University of Arizona (UArizona) and two Seed Grants from the UArizona BIO5 Institute and the State of Arizona’s Technology and Research Initiative Fund, and by NIH NIEHS P30ES006694 grant.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interest.
REFERENCES
- 1.Zahin N, et al. , Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environmental Science and Pollution Research, 2019: p. 1–18. [DOI] [PubMed] [Google Scholar]
- 2.Dadwal A, Baldi A, and Kumar Narang R, Nanoparticles as carriers for drug delivery in cancer. Artificial cells, nanomedicine, and biotechnology, 2018. 46(sup2): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 3.Kalyane D, et al. , Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Materials Science and Engineering: C, 2019. 98: p. 1252–1276. [DOI] [PubMed] [Google Scholar]
- 4.Russell LM, Dawidczyk CM, and Searson PC, Quantitative evaluation of the enhanced permeability and retention (EPR) effect, in Cancer Nanotechnology. 2017, Springer. p. 247–254. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, et al. , Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate chemistry, 2016. 27(10): p. 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, et al. , Therapeutic drugs and drug delivery systems targeting stromal cells for cancer therapy: a review. Journal of drug targeting, 2020. 28(7–8): p. 714–726. [DOI] [PubMed] [Google Scholar]
- 7.Sandha KK, Shukla MK, and Gupta PN, Recent Advances in Strategies for Extracellular Matrix Degradation and Synthesis Inhibition for Improved Therapy of Solid Tumors. Curr Pharm Des, 2020. 26(42): p. 5456–5467. [DOI] [PubMed] [Google Scholar]
- 8.Rigoglio NN, et al. , The Tumor Microenvironment: Focus on Extracellular Matrix. Tumor Microenvironment, 2020: p. 1–38. [DOI] [PubMed] [Google Scholar]
- 9.D’Arcangelo E, et al. , The life cycle of cancer-associated fibroblasts within the tumour stroma and its importance in disease outcome. British journal of cancer, 2020. 122(7): p. 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiori ME, et al. , Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Molecular cancer, 2019. 18(1): p. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwa MQ, et al. , Cancer-associated fibroblasts: how do they contribute to metastasis? Clinical Experimental Metastasis, 2019. 36: p. 71–86. [DOI] [PubMed] [Google Scholar]
- 12.Yunna C, et al. , Emerging strategies against tumor-associated fibroblast for improved the penetration of nanoparticle into desmoplastic tumor. European Journal of Pharmaceutics and Biopharmaceutics, 2021. 165: p. 75–83. [DOI] [PubMed] [Google Scholar]
- 13.Stylianopoulos T, et al. , Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences, 2012. 109(38): p. 15101–15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishihara H, Human pathological basis of blood vessels and stromal tissue for nanotechnology. Advanced drug delivery reviews, 2014. 74: p. 19–27. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Song W, and Huang L, Drug Delivery Systems Targeting Tumor-Associated Fibroblasts for Cancer Immunotherapy. Cancer Letters, 2019. 448: p. 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdani S, Bansal R, and Prakash J, Drug targeting to myofibroblasts: Implications for fibrosis and cancer. Advanced drug delivery reviews, 2017. 121: p. 101–116. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Zeng H, and Chen Y, Emerging nano drug delivery systems targeting cancer-associated fibroblasts for improved antitumor effect and tumor drug penetration. Molecular pharmaceutics, 2020. 17(4): p. 1028–1048. [DOI] [PubMed] [Google Scholar]
- 18.Nissen NI, Karsdal M, and Willumsen N, Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. Journal of Experimental Clinical Cancer Research, 2019. 38(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinz B, et al. , Recent Developments in Myofibroblast Biology: Paradigms for Connective Tissue Remodeling - ScienceDirect. 2012. 180(4): p. 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Z, et al. , Cancer-associated fibroblasts in tumor microenvironment–Accomplices in tumor malignancy. Cellular Immunology, 2019. 343: p. 103729. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, et al. , Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. Journal of hematology & oncology, 2019. 12(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Ahrens D, et al. , The role of stromal cancer-associated fibroblasts in pancreatic cancer. Journal of hematology & oncology, 2017. 10(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdogan B and Webb DJ, Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochemical Society Transactions, 2017. 45(1): p. 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasari S, Fang Y, and Mitra AK, Cancer Associated Fibroblasts: Naughty Neighbors That Drive Ovarian Cancer Progression. Cancers (Basel), 2018. 10(11): p. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng F, et al. , Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. Journal of hematology & oncology, 2016. 9(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oleynikova NA, et al. , Cancer-associated fibroblasts and their significance in tumor progression. Arkhiv patologii, 2020. 82(1): p. 68–77. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y, et al. , Stromal fibroblasts induce metastatic tumor cell clusters via epithelial–mesenchymal plasticity. Life science alliance, 2019. 2(4): p. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erez N, et al. , Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer cell, 2010. 17(2): p. 135–147. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, et al. , Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer letters, 2016. 379(1): p. 49–59. [DOI] [PubMed] [Google Scholar]
- 30.Su S, et al. , CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell, 2018. 172(4): p. 841–856.e16. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R, The biology and function of fibroblasts in cancer. Nature Reviews Cancer, 2016. 16(9): p. 582–598. [DOI] [PubMed] [Google Scholar]
- 32.R Beech J, et al. , Mechanisms for targeted delivery of nanoparticles in cancer. Current pharmaceutical design, 2013. 19(37): p. 6560–6574. [DOI] [PubMed] [Google Scholar]
- 33.Schütz CA, et al. , Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine, 2013. 8(3): p. 449–467. [DOI] [PubMed] [Google Scholar]
- 34.Augustine R, et al. , Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Materials Today Communications, 2020: p. 101692. [Google Scholar]
- 35.Houthuijzen JM and Jonkers J, Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer and Metastasis Reviews, 2018. 37(4): p. 577–597. [DOI] [PubMed] [Google Scholar]
- 36.Yao Y, et al. , Artemisinin derivatives inactivate cancer-associated fibroblasts through suppressing TGF-β signaling in breast cancer. Journal of Experimental & Clinical Cancer Research, 2018. 37(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith NR, et al. , Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clinical Cancer Research, 2013. 19(24): p. 6943–6956. [DOI] [PubMed] [Google Scholar]
- 38.Neri S, et al. , Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. Journal of cancer research and clinical oncology, 2016. 142(2): p. 437–446. [DOI] [PubMed] [Google Scholar]
- 39.He X, et al. , Engineering extracellular matrix to improve drug delivery for cancer therapy. Drug Discovery Today, 2020. 25(9): p. 1727–1734. [DOI] [PubMed] [Google Scholar]
- 40.Jacobetz MA, et al. , Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut, 2013. 62(1): p. 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinger A, et al. , Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS nano, 2019. 13(10): p. 11008–11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khawar IA, Kim JH, and Kuh H-J, Improving drug delivery to solid tumors: priming the tumor microenvironment. Journal of Controlled Release, 2015. 201: p. 78–89. [DOI] [PubMed] [Google Scholar]
- 43.Böckelmann LC and Schumacher U, Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors? Expert opinion on therapeutic targets, 2019. 23(12): p. 1005–1014. [DOI] [PubMed] [Google Scholar]
- 44.Provenzano PP, et al. , Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell, 2012. 21(3): p. 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao H, Shaping tumor microenvironment for improving nanoparticle delivery. Current drug metabolism, 2016. 17(8): p. 731–736. [DOI] [PubMed] [Google Scholar]
- 46.Heldin C-H, et al. , High interstitial fluid pressure—an obstacle in cancer therapy. Nature Reviews Cancer, 2004. 4(10): p. 806–813. [DOI] [PubMed] [Google Scholar]
- 47.Sewell-Loftin MK, et al. , Cancer-associated fibroblasts support vascular growth through mechanical force. Scientific reports, 2017. 7(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin JD, et al. , Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harbor perspectives in medicine, 2016. 6(12): p. a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajizadeh F, et al. , Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life sciences, 2019. 237: p. 116952. [DOI] [PubMed] [Google Scholar]
- 50.Samanta D, et al. , Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proceedings of the National Academy of Sciences, 2014. 111(50): p. E5429–E5438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Kraman M, et al. , Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein–α. Science, 2010. 330(6005): p. 827–830. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y, et al. , Extreme low dose of 5-fluorouracil reverses MDR in cancer by sensitizing cancer associated fibroblasts and down-regulating P-gp. PLOS one, 2017. 12(6): p. e0180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen B, et al. , A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine: Nanotechnology, Biology and Medicine, 2016. 12(1): p. 131–141. [DOI] [PubMed] [Google Scholar]
- 54.Hu C, et al. , Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials, 2017. 144: p. 60–72. [DOI] [PubMed] [Google Scholar]
- 55.Diop-Frimpong B, et al. , Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences, 2011. 108(7): p. 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, et al. , Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. Journal of Controlled Release, 2014. 182(1): p. 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ernsting MJ, et al. , Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. Journal of Controlled Release, 2015. 206: p. 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takai K, et al. , Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget, 2016. 7(50): p. 82889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berna C, et al. , Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell, 2014. 25: p. 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao L, et al. , Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. Journal of controlled release, 2015. 217: p. 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X and Song E, Turning foes to friends: targeting cancer-associated fibroblasts. Nature reviews Drug discovery, 2019. 18(2): p. 99–115. [DOI] [PubMed] [Google Scholar]
- 62.Cole LE, Vargo-Gogola T, and Roeder RK, Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Advanced drug delivery reviews, 2016. 99: p. 12–27. [DOI] [PubMed] [Google Scholar]
- 63.Ouyang Z, et al. , Zoledronic Acid: Pleiotropic Anti-Tumor Mechanism and Therapeutic Outlook for Osteosarcoma. Current Drug Targets, 2015. 18(5): p. 409–421. [DOI] [PubMed] [Google Scholar]
- 64.Zekri J, Mansour M, and Karim SM, The anti-tumour effects of zoledronic acid. Journal of bone oncology, 2014. 3(1): p. 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Comito G, et al. , Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts. Oncotarget, 2017. 8(1): p. 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitra AK, et al. , MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer discovery, 2012. 2(12): p. 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao L, et al. , Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer research, 2017. 77(3): p. 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gascard P and Tlsty TD, Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes & development, 2016. 30(9): p. 1002–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen MFB, Mortensen MB, and Detlefsen S, Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World journal of gastroenterology, 2016. 22(9): p. 2678–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hurwitz HI, et al. , Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. Journal of Clinical Oncology, 2015. 33(34): p. 4039–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mascarenhas J, et al. , Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA oncology, 2018. 4(5): p. 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu M, Song W, and Huang LJCL, Drug Delivery Systems Targeting Tumor-Associated Fibroblasts for Cancer Immunotherapy. Cancer Letters, 2019. 448: p. 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Golombek SK, et al. , Tumor targeting via EPR: Strategies to enhance patient responses. Advanced Drug Delivery Reviews, 2018. 130: p. 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Truffi M, et al. , Nano-Strategies to Target Breast Cancer-Associated Fibroblasts: Rearranging the Tumor Microenvironment to Achieve Antitumor Efficacy. International Journal of Molecular Sciences, 2019. 20(6): p. 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik P, Hoidal JR, and Mukherjee TK, Recent Advances in Curcumin Treated Non-Small Cell Lung Cancers: An Impetus of Pleiotropic Traits and Nanocarrier Aided Delivery. Current Medicinal Chemistry, 2021. 28: p. 3061–3106. [DOI] [PubMed] [Google Scholar]
- 76.Xingli, et al. , Tumor-Associated Fibroblast-Targeted Regulation and Deep Tumor Delivery of Chemotherapeutic Drugs with a Multifunctional Size-Switchable Nanoparticle. ACS applied materials interfaces, 2019. 11(43): p. 39545–39559. [DOI] [PubMed] [Google Scholar]
- 77.Lei, et al. , Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer Research, 2016. 77(3): p. 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao H, et al. , RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm, 2014. 11(3): p. 1042–1052. [DOI] [PubMed] [Google Scholar]
- 79.Anderson SA, et al. , Magnetic resonance contrast enhancement of neovasculature with alpha(v)beta(3)-targeted nanoparticles. Magnetic Resonance in Medicine, 2000. 44(3): p. 433–439. [DOI] [PubMed] [Google Scholar]
- 80.Kang T, et al. , Synergistic targeting tenascin C and neuropilin-1 for specific penetration of nanoparticles for anti-glioblastoma treatment. Biomaterials, 2016: p. 60–75. [DOI] [PubMed] [Google Scholar]
- 81.Khan I, Saeed K, and Khan I, Nanoparticles: Properties, Applications and Toxicities. Arabian Journal of Chemistry, 2017(7): p. 908–931. [Google Scholar]
- 82.Liu J, et al. , Passive Tumor Targeting of Renal-Clearable Luminescent Gold Nanoparticles: Long Tumor Retention and Fast Normal Tissue Clearance. Journal of the American Chemical Society, 2013. 135(13): p. 4978–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X, et al. , Enhanced retention and cellular uptake of nanoparticles in tumors by controlling their aggregation behavior. Acs Nano, 2013. 7(7): p. 6244–6257. [DOI] [PubMed] [Google Scholar]
- 84.George Mattheolabakis BR and Constantinides PP *, Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomedicine, 2012. 7(10): p. 1577–1590. [DOI] [PubMed] [Google Scholar]
- 85.Matsumura Y and Maeda H, A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Research, 1986. 46(12 Pt 1): p. 6387–6392. [PubMed] [Google Scholar]
- 86.Maeda H, The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in Enzyme Regulation, 2001. 41(1): p. 189–207. [DOI] [PubMed] [Google Scholar]
- 87.Greish and Khaled, Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. Journal of Drug Targeting, 2007. 15(7–8): p. 457–464. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, et al. , Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model - ScienceDirect. Journal of Controlled Release, 2014. 182(1): p. 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji T, et al. , An MMP-2 Responsive Liposome Integrating Antifibrosis and Chemotherapeutic Drugs for Enhanced Drug Perfusion and Efficacy in Pancreatic Cancer. ACS applied materials & interfaces, 2016. 8(5): p. 3438–3445. [DOI] [PubMed] [Google Scholar]
- 90.Muro S, Challenges in design and characterization of ligand-targeted drug delivery systems. Journal of Controlled Release, 2012. 164(2): p. 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou L, et al. , Perspective of Targeting Cancer-Associated Fibroblasts in Melanoma. Journal of Cancer, 2015. 6(8): p. 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang J, et al. , A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. International Journal of Cancer, 2016. 138(4): p. 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang X, et al. , FAP Promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 Signaling. Cancer research, 2016. 76(14): p. 4124–4135. [DOI] [PubMed] [Google Scholar]
- 94.Loeffler, et al. , Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. Journal of Clinical Investigation, 2006. 116(7): p. 1955–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanley CJ and Thomas GJ, T-cell tumour exclusion and immunotherapy resistance: a role for CAF targeting. British Journal of Cancer, 2020. 123(9): p. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liane, et al. , Immunoliposomes for Targeted Delivery of an Antifibrotic Drug. Molecular pharmaceutics, 2015. 12(9): p. 3146–3157. [DOI] [PubMed] [Google Scholar]
- 97.Ji T, D.Y., Zhao Y, et al. , Peptide Assembly Integration of Fibroblast‐Targeting and Cell‐Penetration Features for Enhanced Antitumor Drug Delivery. Advanced Materials, 2015. 27(11): p. 1865–1873. [DOI] [PubMed] [Google Scholar]
- 98.Ji T, et al. , Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Letters, 2017. 17(2): p. 862–869. [DOI] [PubMed] [Google Scholar]
- 99.Huber, et al. , Fibroblast Activation Protein: Differential Expression and Serine Protease Activity in Reactive Stromal Fibroblasts of Melanocytic Skin Tumors. Journal of Investigative Dermatology, 2003. 120(2): p. 182–188. [DOI] [PubMed] [Google Scholar]
- 100.Brennen WN, Isaacs JT, and Denmeade SR, Rationale behind targeting fibroblast activation protein–expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Molecular cancer therapeutics, 2012. 11(2): p. 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim M-G, et al. , Selective activation of anticancer chemotherapy by cancer-associated fibroblasts in the tumor microenvironment. JNCI: Journal of the National Cancer Institute, 2017. 109(1): p. djw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ji T, et al. , Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer‐Associated Fibroblast Activation. Wiley-Blackwell Online Open, 2016. 55(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu Q, et al. , Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. Journal of Controlled Release, 2020. 321: p. 564–575. [DOI] [PubMed] [Google Scholar]
- 104.Dasargyri A, et al. , Findings questioning the involvement of Sigma-1 receptor in the uptake of anisamide-decorated particles. Journal of Controlled Release, 2016. 224(1): p. 229–238. [DOI] [PubMed] [Google Scholar]
- 105.Thomas JD, et al. , Sigma1 Targeting to Suppress Aberrant Androgen Receptor Signaling in Prostate Cancer. Cancer Research, 2017. 77(9): p. 2439–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang ONXML and Juliano RL, Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. Journal of the American Chemical Society, 2010. 132(26): p. 8848–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.c, M. T a.b. and S. T-P d, The pharmacology of sigma-1 receptors - ScienceDirect. Pharmacology Therapeutics, 2009. 124(2): p. 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cobos E, et al. , Pharmacology and Therapeutic Potential of Sigma1 Receptor Ligands. Current Neuropharmacology, 2008. 6(4): p. 344–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mukherjee A, et al. , Haloperidol-associated Stealth Liposomes A POTENT CARRIER FOR DELIVERING GENES TO HUMAN BREAST CANCER CELLS. Journal of Biological Chemistry, 2005. 280(16): p. 15619–15627. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee R, et al. , Banerjee R, Tyagi P, Li S and Huang L Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. International Journal of Cancer, 2004. 112(4): p. 693–700. [DOI] [PubMed] [Google Scholar]
- 111.Miao L, et al. , The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS Nano, 2016. 10(10): p. 9243–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu K, et al. , Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS nano, 2017. 11(5): p. 4916–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jafari B, et al. , An alignment-independent 3D-QSAR study of FGFR2 tyrosine kinase inhibitors. Advanced Pharmaceutical Bulletin, 2017. 7(3): p. 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Powers CJ, Mcleskey SW, and Wellstein A, Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer, 2000. 7(3): p. 165–197. [DOI] [PubMed] [Google Scholar]
- 115.Chandler LA, J. D, Gonzalez AM, et al. , FGF2-Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Molecular Therapy, 2000. 2(2): p. 153–162. [DOI] [PubMed] [Google Scholar]
- 116.Maruta F, et al. , Identification of FGF receptor-binding peptides for cancer gene therapy. Cancer Gene Therapy, 2002. 9(6): p. 543–552. [DOI] [PubMed] [Google Scholar]
- 117.Ping Y, et al. , FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials, 2013. 34(27): p. 6482–6494. [DOI] [PubMed] [Google Scholar]
- 118.Bonner JC, Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Reviews, 2004. 15(4): p. 255–273. [DOI] [PubMed] [Google Scholar]
- 119.Gallego-Muñoz P, et al. , Human corneal fibroblast migration and extracellular matrix synthesis during stromal repair: Role played by platelet-derived growth factor-BB, basic fibroblast growth factor, and transforming growth factor-β1. Journal of Tissue Engineering Regenerative Medicine, 2018. 12(2): p. e737–e746. [DOI] [PubMed] [Google Scholar]
- 120.Green J, et al. , Diversity of Interstitial Lung Fibroblasts Is Regulated by Platelet-Derived Growth Factor Receptor α Kinase Activity. American Journal of Respiratory Cell Molecular Biology, 2016. 54(4): p. 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tolmachev V, et al. , Imaging of platelet-derived growth factor receptor β expression in glioblastoma xenografts using affibody molecule 111In-DOTA-Z09591. Journal of Nuclear Medicine, 2014. 55(2): p. 294–300. [DOI] [PubMed] [Google Scholar]
- 122.Marr A, et al. , Peptide arrays for development of PDGFRβ affine molecules. Molecular imaging and biology, 2013. 15(4): p. 391–400. [DOI] [PubMed] [Google Scholar]
- 123.Askoxylakis V, et al. , Peptide-based targeting of the platelet-derived growth factor receptor beta. Molecular imaging and biology, 2013. 15(2): p. 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bansal R, et al. , Targeted recombinant fusion proteins of IFNγ and mimetic IFNγ with PDGFβR bicyclic peptide inhibits liver fibrogenesis in vivo. PloS one, 2014. 9(2): p. e89878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lindborg M, et al. , Engineered high-affinity affibody molecules targeting platelet-derived growth factor receptor β in vivo. Journal of molecular biology, 2011. 407(2): p. 298–315. [DOI] [PubMed] [Google Scholar]
- 126.Poosti F, et al. , Selective delivery of IFN-γ to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. Faseb Journal, 2015. 29(3): p. 1029–1042. [DOI] [PubMed] [Google Scholar]
- 127.Fransien VD, et al. , Targeted Therapies in Liver Fibrosis: Combining the Best Parts of Platelet-Derived Growth Factor BB and Interferon Gamma. Frontiers in Medicine, 2015. 2: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.b, P. J a., et al. , A novel approach to deliver anticancer drugs to key cell types in tumors using a PDGF receptor-binding cyclic peptide containing carrier. Journal of Controlled Release, 2010. 145(2): p. 91–101. [DOI] [PubMed] [Google Scholar]
- 129.Kuzmich A, et al. , Novel Histone-Based DNA Carrier Targeting Cancer-Associated Fibroblasts. Polymers, 2020. 12(8): p. 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bansal R, et al. , Selective Targeting of Interferon γ to Stromal Fibroblasts and Pericytes as a Novel Therapeutic Approach to Inhibit Angiogenesis and Tumor Growth. Molecular Cancer Therapeutics, 2012. 11(11): p. 2419–2428. [DOI] [PubMed] [Google Scholar]
- 131.Kim MY, et al. , Selection and characterization of tenascin C targeting peptide. Molecules Cells, 2012. 33(1): p. 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen B, et al. , Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system. Journal of Controlled Release, 2016. 241: p. 68–80. [DOI] [PubMed] [Google Scholar]
- 133.Chen B, et al. , A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine Nanotechnology Biology Medicine, 2016. 12(1): p. 131–141. [DOI] [PubMed] [Google Scholar]
- 134.Liu H, Shi Y, and Qian F, Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Advanced Drug Delivery Reviews, 2021. 172(1): p. 37–51. [DOI] [PubMed] [Google Scholar]
- 135.Greco R, et al. , Abstract 1796: SAR302503: A Jak2 inhibitor with antitumor activity in solid tumor models. Cancer Research, 2012. 72(8): p. 1796–1796. [Google Scholar]
- 136.Mascarenhas J, et al. , Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis. Jama Oncology, 2018. 4(5): p. 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Est TW, Moosmayer D, and Pfizenmaier K, Construction of a bispecific single chain antibody for recruitment of cytotoxic T cells to the tumour stroma associated antigen fibroblast activation protein. Journal of Biotechnology, 2001. 92(2): p. 159–168. [DOI] [PubMed] [Google Scholar]
- 138.Liu M, et al. , Crosslinked self-assembled nanoparticles for chemo-sonodynamic combination therapy favoring antitumor, antimetastasis management and immune responses. Journal of Controlled Release, 2018. 290: p. 150–164. [DOI] [PubMed] [Google Scholar]
- 139.Qu CF, et al. , Pre-clinical study of 213Bi labeled PAI2 for the control of micrometastatic pancreatic cancer. Clinical Experimental Metastasis, 2005. 22(7): p. 575–586. [DOI] [PubMed] [Google Scholar]
- 140.Camidge DR, et al. , Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncology, 2012. 13(10): p. 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brennan FR, et al. , Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. Mabs, 2010. 2(3): p. 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu J, et al. , Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nature Communications, 2017. 8(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu J, et al. , PEG-derivatized embelin as a nanomicellar carrier for delivery of paclitaxel to breast and prostate cancers. Biomaterials, 2013. 34(5): p. 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu J, et al. , Targeted delivery of Doxorubicin by folic acid-decorated dual functional nanocarrier. Molecular Pharmaceutics, 2014. 11(11): p. 4164–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu J, et al. , Design and Characterization of PEG-Derivatized Vitamin E as a Nanomicellar Formulation for Delivery of Paclitaxel. Molecular Pharmaceutics, 2013. 10(8): p. 2880–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Y, et al. , PEG-Derivatized Embelin as a Dual Functional Carrier for the Delivery of Paclitaxel. Bioconjugate Chemistry, 2012. 23(7): p. 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu J, et al. , An improved D-α-tocopherol-based nanocarrier for targeted delivery of doxorubicin with reversal of multidrug resistance. Journal of Controlled Release, 2014. 196: p. 272–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu J, et al. , The Self-Assembling Camptothecin-Tocopherol Prodrug: An Effective Approach for Formulating Camptothecin. Biomaterials, 2015. 62: p. 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zw A, et al. , Self-assembling prodrug nanotherapeutics for synergistic tumor targeted drug delivery - ScienceDirect. Acta Biomaterialia, 2020. 111: p. 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin, et al. , Hybrid nanoparticles for combination therapy of cancer. Journal of Controlled Release, 2015. 219: p. 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang X, et al. , PEG-Farnesylthiosalicylate Conjugate as a Nanomicellar Carrier for Delivery of Paclitaxel. Bioconjugate chemistry, 2013. 24(3): p. 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang P, et al. , A PEG-Fmoc conjugate as a nanocarrier for paclitaxel. Biomaterials, 2014. 35(25): p. 7146–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao X, et al. , Nanoassembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers. Molecular Pharmaceutics, 2013. 10(1): p. 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Naahidi S, et al. , Biocompatibility of engineered nanoparticles for drug delivery. Journal of Controlled Release, 2013. 166(2): p. 182–194. [DOI] [PubMed] [Google Scholar]
- 155.Pandey NK, et al. , Investigation of influence of shape on drug loading and entrapment efficiency of nanoparticles. Journal of Pharmacy Research, 2017. 1111(20177): p. 850–855. [Google Scholar]
- 156.Hu C and Zhang L, Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochemical pharmacology, 2012. 83(8): p. 1104–1111. [DOI] [PubMed] [Google Scholar]
- 157.He Y, et al. , Engineered High-Loaded Mixed-Monoclonal Antibodies (Adalimumab, Rituximab and Trastuzumab) Polymeric Nanoparticle for Rheumatoid Arthritis Treatment: A Proof of Concept. Journal of Biomedical Nanotechnology, 2020. 16(8): p. 1254–1266. [DOI] [PubMed] [Google Scholar]
- 158.Gdowski A, et al. , Development of Biodegradable Nanocarriers Loaded with a Monoclonal Antibody. International Journal of Molecular Sciences, 2015. 16(2): p. 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li W, et al. , Low density lipoprotein-inspired nanostructured lipid nanoparticles containing pro-doxorubicin to enhance tumor-targeted therapeutic efficiency. Acta Biomaterialia, 2019. 96: p. 456–467. [DOI] [PubMed] [Google Scholar]
- 160.Jia N, et al. , Preparation and evaluation of poly(L-histidine) based pH-sensitive micelles for intracellular delivery of doxorubicin against MCF-7/ADR cells. Asian Journal of Pharmaceutical Sciences, 2017. 12(5): p. 33–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang F, Li L, and Chen D, Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Advanced Materials, 2012. 24(12): p. 1504–1534. [DOI] [PubMed] [Google Scholar]
- 162.Irby D, Du C, and Feng L, Lipid-Drug Conjugate for Enhancing Drug Delivery. Molecular Pharmaceutics, 2017. 14(5): p. 1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang J, et al. , Quantitative and high drug loading of self-assembled prodrug with defined molecular structures for effective cancer therapy. Journal of Controlled Release, 2019. 307: p. 90–97. [DOI] [PubMed] [Google Scholar]
- 164.Feng W, et al. , PH/redox sequentially responsive nanoparticles with size shrinkage properties achieve deep tumor penetration and reversal of multidrug resistance. Biomaterials Science, 2020. 8(32): p. 4767–4778. [DOI] [PubMed] [Google Scholar]
- 165.Ma J-B, et al. , Size-shrinkable and protein kinase Cα-recognizable nanoparticles for deep tumor penetration and cellular internalization. European Journal of Pharmaceutical Sciences, 2021. 159: p. 105693. [DOI] [PubMed] [Google Scholar]
- 166.Chia-Chian, et al. , Erratum: Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy: Erratum. Theranostics, 2017. 7(3): p. 559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X, et al. , Dual pH-responsive “charge-reversal like” gold nanoparticles to enhance tumor retention for chemo-radiotherapy. Nano Research, 2019. 12(11): p. 2815–2826. [Google Scholar]
- 168.Long, et al. , Shape Effect of Nanoparticles on Tumor Penetration in Monolayers Versus Spheroids. Molecular Pharmaceutics, 2019. 16(7): p. 2902–2911. [DOI] [PubMed] [Google Scholar]
- 169.Chaudhuri P, et al. , Shape effect of carbon nanovectors on angiogenesis. Acs Nano, 2010. 4(1): p. 574–582. [DOI] [PubMed] [Google Scholar]
- 170.Tang L, et al. , Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. 2013. 10(3): p. 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oishi M, et al. , Enhanced growth inhibition of hepatic multicellular tumor spheroids by lactosylated poly(ethylene glycol)-siRNA conjugate formulated in PEGylated polyplexes. 2010. 2(9): p. 1290–1297. [DOI] [PubMed] [Google Scholar]
- 172.Yu W, et al. , Size-tunable strategies for a tumor targeted drug delivery system. ACS central science, 2020. 6(2): p. 100–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Z, et al. , Development of zwitterionic polymer-based doxorubicin conjugates: tuning the surface charge to prolong the circulation and reduce toxicity. Langmuir, 2014. 30(13): p. 3764–3774. [DOI] [PubMed] [Google Scholar]
- 174.Decuzzi P and Ferrari M, The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials, 2006. 27(30): p. 5307–5314. [DOI] [PubMed] [Google Scholar]
- 175.Peiris PM, et al. , Imaging Metastasis Using an Integrin-Targeting Chain-Shaped Nanoparticle. ACS Nano, 2012. 6(10): p. 8783–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huynh E and Zheng G, Engineering multifunctional nanoparticles: all-in-one versus one-for-all. Wiley Interdisciplinary Reviews: Nanomedicine Nanobiotechnology, 2013. 5(3): p. 250–265. [DOI] [PubMed] [Google Scholar]


