Abstract
Introduction:
WEE1 is a serine kinase central to the G2 checkpoint. Inhibition of WEE1 can lead to cell death by permitting cell-cycle progression despite unrepaired DNA damage. AZD1775 is a WEE1 inhibitor that is in clinical development for children and adults with cancer.
Methods:
AZD1775 was tested using a dose of 120 mg/kg administered orally for days 1 to 5. Irinotecan was administered intraperitoneally at a dose of 2.5 mg/kg for days 1 to 5 (one hour after AZD1775 when used in combination). AZD1775 and irinotecan were studied alone and in combination in neuroblastoma (n = 3), osteosarcoma (n = 4), and Wilms tumor (n = 3) xenografts.
Results:
AZD1775 as a single agent showed little activity. Irinotecan induced objective responses in two neuroblastoma lines (PRs), and two Wilms tumor models (CR and PR). The combination of AZD1775 + irinotecan-induced objective responses in two neuroblastoma lines (PR and CR) and all three Wilms tumor lines (CR and 2 PRs). The objective response measure improved compared with single-agent treatment for one neuroblastoma (PR to CR), two osteosarcoma (PD1 to PD2), and one Wilms tumor (PD2 to PR) xenograft lines. Of note, the combination yielded CR (n = 1) and PR (n = 2) in all the Wilms tumor lines. The event-free survival was significantly longer for the combination compared with single-agent irinotecan in all models tested. The magnitude of the increase was greatest in osteosarcoma and Wilms tumor xenografts.
Conclusions:
AZD1775 potentiates the effects of irinotecan across most of the xenograft lines tested, with effect size appearing to vary across tumor panels.
Keywords: AZD1775, irinotecan, neuroblastoma, osteosarcoma, preclinical testing, Wilms tumor
1 |. INTRODUCTION
Reproducible and precise progression through the cell cycle is dependent on the activation of the cyclin-dependent kinases (CDKs). There are nine CDKs involved in cell-cycle regulation. Inhibition of CDK function may result in cell-cycle arrest and apoptosis. CDK1 is critical for progression from G2 to the M phase of the cell cycle. Through phosphorylation of CDK1 at tyrosine 15, the WEE1 kinase halts cell-cycle progression in response to DNA damage.1 WEE1 is also involved in stabilizing DNA replication forks and homologous recombination repair.2 The ataxia-telangiectasia-related (ATR) kinase is a key mediator for the repair of DNA single-strand breaks through phosphorylation of the checkpoint kinase CHK1.3 CHK1 in turn phosphorylates and activates WEE1. Phosphorylated WEE1 inhibits CDK1/cyclin B function, thus inducing cell-cycle arrest at G2 and permitting DNA-damage repair. Conversely, inhibition of WEE1 will permit premature entry into the M phase leading to mitotic catastrophe and apoptosis.1 WEE1 kinase also plays a central role in the G2-M cell-cycle checkpoint and is also required during S phase. WEE1 phosphorylation of CDK2 during S phase allows the maintenance of stalled replication forks.
AZD1775 is a potent and selective ATP-competitive inhibitor of WEE1, with promising activity potentiating the genotoxic activity of anticancer agents. Preclinical studies demonstrate AZD1775 potentiation of topoisomerase-I inhibitors, antimetabolites, DNA cross-linking agents and radiation. In osteosarcoma xenografts, a 50% reduction in tumor growth is reported with single-agent AZD1775 and a 70% reduction in combination with gemcitabine. In these models, AZD1775 predictably induced G2-M escape and apoptosis. AZD1775 enhanced the effect of gamma radiation in orthotopic glioma xenograft tumors.5 The combination of AZD1775 with irinotecan and vincristine improved response for orthotopic xenograft models of high-risk rhabdomyosarcoma relative to irinotecan and vincristine alone.6 In acute myeloid leukemia, AZD1775 enhanced the antitumor effect of the histone deacetylase inhibitor panobinostat,7 the CDK inhibitor roscovitine,8 and cytarabine.9 It is possible that G1 checkpoint control is deficient in tumors with TP53 mutations. Tumors harboring such mutations may therefore be more dependent on the S and G2 phase checkpoints, suggesting that S and G2 checkpoint abrogation by WEE1 inhibition may selectively sensitize TP53-deficient tumors.10 However, others have demonstrated that AZD1775 effect is independent of TP53.11,12 It is likely that the relationship between AZD1775 activity and TP53 status is context dependent and may not be the critical determinant of activity.
Two pediatric phase 1 trials that are assessing the safety and feasibility of combining of AZD1775 either with local radiation therapy in treating newly diagnosed DIPG (NCT01922076) or with irinotecan hydrochloride in advanced solid tumors (NCT02095132). These data will inform the pediatric development of AZD1775 in combination with conventional therapies for pediatric malignancies. The PPTC sought to supplement these data by evaluating the ability of AZD1775 to potentiate the in vivo activity of irinotecan across a range of pediatric solid tumors, given the broad use of irinotecan in the relapse setting for pediatric patients with solid tumors.
2 |. METHODS
2.1 |. In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY) were used to propagate subcutaneously implanted tumors as previously described.13 All mice were maintained under barrier conditions, and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Female mice were used irrespective of the gender from which the tumor was derived. Ten mice were used per group.
2.2 |. Statistical analysis
A tumor event is defined as a quadrupling of tumor volume from the day of treatment initiation, where tumor volume is estimated from caliper measurements as (4/3) × π × [(length + width)/4].3 The exact time to event is estimated by interpolating between the measurements directly preceding and following the event, assuming log-linear growth. Differences in event-free survival (EFS) between experimental groups are tested using the Gehan-Wilcoxon test. Relative tumor volume (RTV) is defined for each mouse as the ratio of its current tumor volume divided by baseline tumor volume. Comparisons between treatments groups of the minimum attained RTV are performed using the Wilcoxon rank sum test.
The objective response categories are progressive disease (PD, which is subdivided among treated mice into PD without and with growth delay, PD1 and PD2, respectively), stable disease (SD), partial response (PR), complete response (CR), and maintained complete response (MCR), defined below.
| PD | < 50% tumor regression throughout study and > 25% tumor growth at the end of the study. |
| PD1 | PD and the mouse’s time to event ≤ 200% the Kaplan-Meier (KM) median time to event in the control group. |
| PD2 | PD and the mouse’s time to event > 200% of the KM median time to event in the control group. |
| SD | < 50% tumor regression throughout study and ≤25% tumor growth at the end of the study. |
| PR | ≥50% tumor regression at any point during study, but measurable tumor throughout the study period. |
| CR | Disappearance of measurable tumor mass during the study period. |
| MCR | No measurable tumor mass for at least three consecutive weekly readings at any time after treatment has been completed. |
Overall group response is determined by the median response among evaluable mice. Each individual mouse is assigned a score from 0 to 10 based on its response: PD1 = 0, PD2 = 2, SD = 4, PR = 6, CR = 8, and MCR = 10, with the median for each group determining overall response. If the median score is half-way between response categories, the objective response is assigned to the lower response category (e.g., a median response score of 9 is scored CR).
Mice experiencing a possibly treatment-related death (i.e., drug toxicity), mice with failed engraftment, and mice which unexpectedly die for reasons unrelated to treatment are excluded from statistical analyses of time to event, minimum tumor volume, and objective response.
For combination testing projects, one objective was to demonstrate that the combination is significantly more effective than either agent utilized at their optimal/standard single-agent dose/schedule. This condition is termed therapeutic enhancement, which represents a therapeutic effect for which a tolerated regimen of a combination treatment exceeds the optimal effect achieved at any tolerated dose of monotherapy associated with the same drugs used in the combination.14,15 This definition was operationalized as follows: therapeutic enhancement was considered present when the tumor growth delay (T-C) for a combination was greater than the tumor growth delay for each of the single agents tested at their maximum tolerated dose (MTD) and when the EFS distribution for the combination treatment was significantly better than the EFS distributions for both of the single agents tested at their MTD. In order to control experiment-wise Type I error at 5%, statistical tests were evaluated at the Bonferroni-corrected significance level α = 0.01, due to the five comparisons being made (combination vs agent 1 alone, combination vs agent 2 alone, agent 1 vs control, agent 2 vs control, and combination vs control). Testing was considered not evaluable for therapeutic enhancement if either single agent used alone produced median EFS beyond the observation period.
Combination testing results were also analyzed for evidence of supra-additivity for the combination, in comparison with the expected treatment effect assuming additivity. As described previously,15 a linear regression model for time to event (T) was employed, with testing to determine whether the treatment interaction of the two-drug combination is significantly different from zero. The interaction model was as follows: T = a0 +a1 × I1 + a2 × I2 + a3 × I1 × I2, in which I1 and I2 are indicator functions for drugs 1 and 2, respectively. The coefficients are for the no-treatment control (a0), the two-drug effects relative to control (a1 and a2), and the treatment interaction effect of the two drugs relative to control (a3). Whether the value of a3 is significantly greater or less than zero (P < 0.01) indicates supra-additivity or sub-additivity of the drug combination, respectively; otherwise, the drug combination is considered additive. In addition, the estimated values for these coefficients are the estimated time to event (or additional time to event) associated with the corresponding treatment effects. To allow comparison of the a3 values across xenografts and across doses of the standard agents, values were normalized by computing the ratio of a3 to the expected effect of the combination under the assumption of additivity (i.e., a1 + a2). If any animals were censored in any treatment group, the combination was not considered evaluable unless (1) the censoring occurred exclusively in the combination group, and the combination effect was supra-additive, or (2) the censoring occurred exclusively in one or both single-agent groups, and the combination effect was sub-additive.
2.3 |. Drugs and formulation
AZD1775 was provided to the PPTC by AstraZeneca. It was administered by oral gavage as a suspension in a vehicle of 0.5% w/vmethyl cellulose in deionized water on days 1 to 5 at a dose of 120 mg/kg. Irinotecan was purchased from the University Hospital Pharmacy (camptosar) and was administered intraperitoneally at a dose of 2.5 mg/kg on days 1 to 5. The combination of AZD1775 and irinotecan used the same doses and schedules of agents as applied for single-agent testing.
3 |. RESULTS
AZD1775 was tested alone and in combination with irinotecan. Detailed testing results are provided in Supporting Information Table S1. As a single agent, the average maximum weight loss across all lines tested was 1.4% ± 0.6% for AZD1775, whereas irinotecan produced an average weight loss of 0.9% ± 0.4%. In combination, the average maximum weight loss was 6.7% ± 1.8%, which was greater than the weight loss observed with either AZD1775 or irinotecan when administered as a single agent. However, no mortality occurred following the administration of AZD1775 alone or in combination with irinotecan.
3.1 |. Neuroblastoma
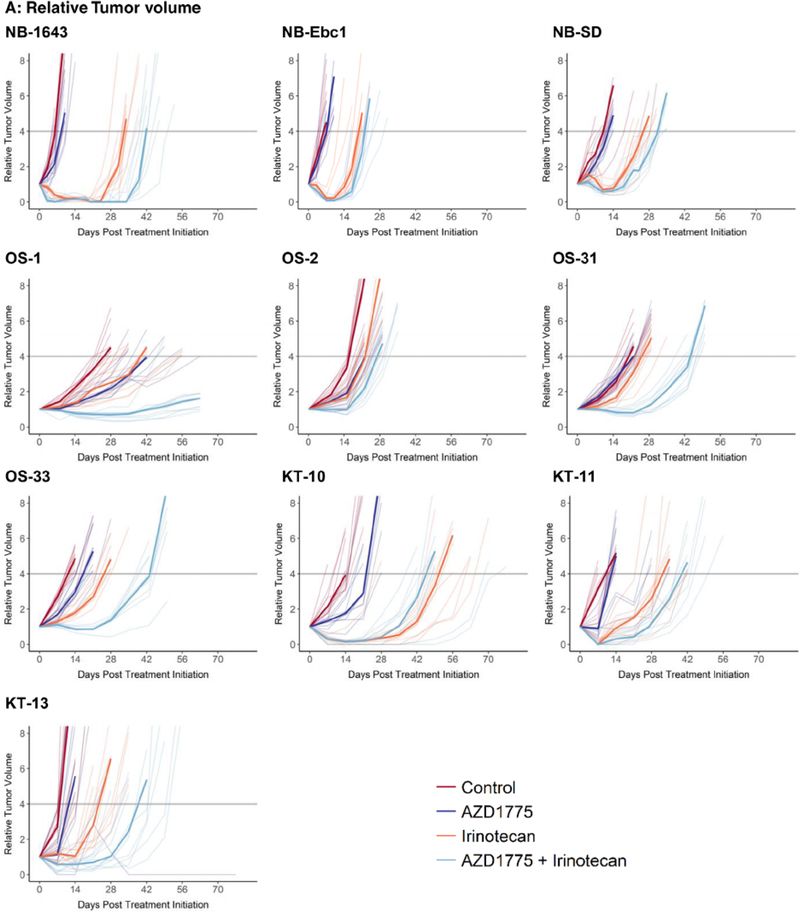
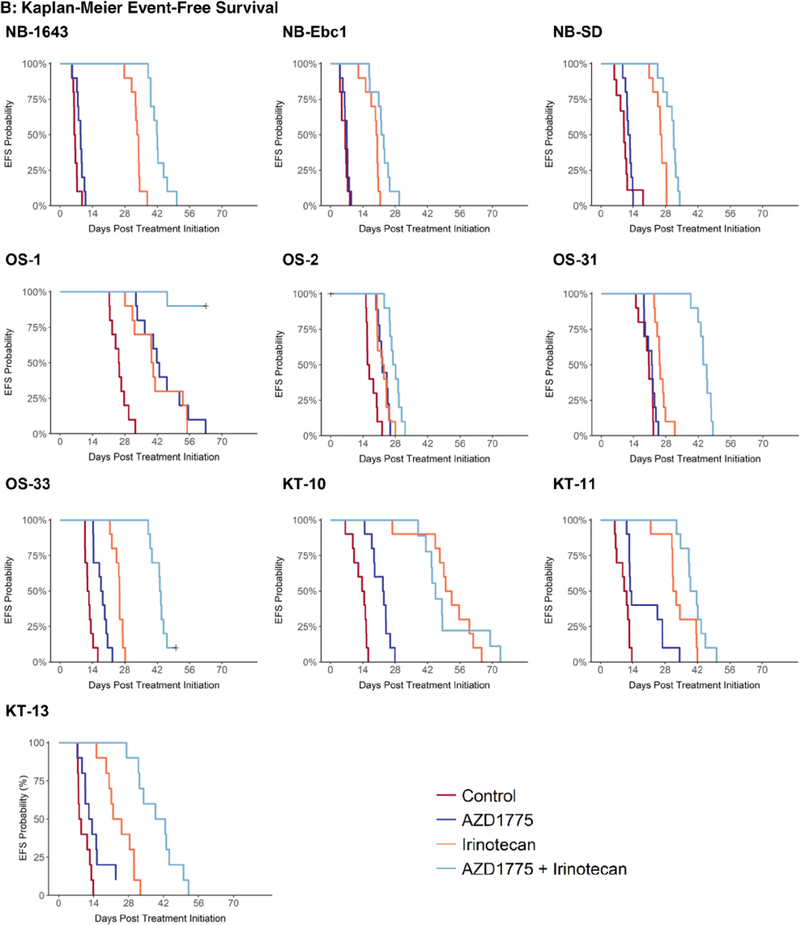
Three neuroblastoma lines (NB-1643, NB-SD, and NB-EBc-1) were evaluated (Table 1 and Figure 1). Single-agent AZD1775 showed minimal activity, with only one of three lines showing a significant prolongation in time to event for the treated versus control groups. All three lines showed PD1 responses with EFS T/C values ranging from 1.12 to 1.42. Irinotecan as a single agent caused significant differences in EFS distribution for all three lines evaluated. Two of three lines showed objective responses (both PRs) and EFS T/C values ranged from 2.58 to 5.37. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with control in all lines tested and produced objective responses in two lines (CR and PR) and a PD2 response in the remaining line. EFS T/C values ranged from 3.13 to 6.7. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with single-agent irinotecan for all three neuroblastoma lines, although the prolongation in time to event was only two to eight days. The combination also produced for each of the neuroblastoma models minimum tumor volumes that were significantly smaller than those produced by single-agent irinotecan.
TABLE 1.
Summary of the in vivo activity of AZD1775 and irinotecan as single agents and in combination
| Line | Histology | Treatment | EFS (d) | P | EFS T-C | EFS T/C | MinRTV | Response |
|---|---|---|---|---|---|---|---|---|
| NB-1643 | Neuroblastoma | AZD1775 | 8.9 | P = 0.009 | 2.6 | 1.42 | 1.455 | PD1 |
| NB-1643 | Neuroblastoma | IR | 33.6 | P < 0.001 | 27.3 | 5.37 | 0.032 | PR |
| NB-1643 | Neuroblastoma | AZD1775 + IR | 41.9 | P < 0.001 | 35.6 | 6.70 | 0.000 | CR |
| NB-SD | Neuroblastoma | AZD1775 | 12.2 | P = 0.021 | 2.1 | 1.21 | 1.544 | PD1 |
| NB-SD | Neuroblastoma | IR | 26 | P < 0.01 | 15.9 | 2.58 | 0.730 | PD2 |
| NB-SD | Neuroblastoma | AZD1775 + IR | 31.6 | P < 0.01 | 21.5 | 3.13 | 0.532 | PD2 |
| NB-Ebc1 | Neuroblastoma | AZD1775 | 7.1 | P = 0.257 | 0.8 | 1.12 | 1.997 | PD1 |
| NB-Ebc1 | Neuroblastoma | IR | 20.1 | P < 0.001 | 13.7 | 3.18 | 0.232 | PR |
| NB-Ebc1 | Neuroblastoma | AZD1775 + IR | 22.5 | P < 0.001 | 16.2 | 3.56 | 0.091 | PR |
| OS1 | Osteosarcoma | AZD1775 | 12.7 | P < 0.001 | 16.9 | 1.66 | 1.063 | PD1 |
| OS1 | Osteosarcoma | IR | 23.5 | P < 0.001 | 14.4 | 1.57 | 1.127 | PD1 |
| OS1 | Osteosarcoma | AZD1775 + IR | 40.6 | P < 0.001 | >37.6 | >2.48 | 0.579 | PD2 |
| OS2 | Osteosarcoma | AZD1775 | 42.4 | P < 0.001 | 6.0 | 1.37 | 1.403 | PD1 |
| OS2 | Osteosarcoma | IR | 39.9 | P < 0.001 | 6.2 | 1.38 | 1.385 | PD1 |
| OS2 | Osteosarcoma | AZD1775 + IR | >63 | P < 0.001 | 11.2 | 1.69 | 0.957 | PD1 |
| OS31 | Osteosarcoma | AZD1775 | 21.9 | P = 0.327 | 1.3 | 1.06 | 1.765 | PD1 |
| OS31 | Osteosarcoma | IR | 25.3 | P < 0.001 | 4.7 | 1.23 | 1.349 | PD1 |
| OS31 | Osteosarcoma | AZD1775 + IR | 44.9 | P < 0.001 | 24.3 | 2.18 | 0.789 | PD2 |
| OS33 | Osteosarcoma | AZD1775 | 18.1 | P < 0.001 | 5.9 | 1.49 | 1.714 | PD1 |
| OS33 | Osteosarcoma | IR | 25.7 | P < 0.001 | 13.6 | 2.12 | 1.365 | PD2 |
| OS33 | Osteosarcoma | AZD1775 + IR | 43.4 | P < 0.001 | 31.3 | 3.58 | 0.837 | PD2 |
| KT-10 | Wilms | AZD1775 | 22.8 | P < 0.001 | 8.8 | 1.62 | 1.278 | PD1 |
| KT-10 | Wilms | IR | 51.1 | P < 0.001 | 37.1 | 3.64 | 0.149 | PR |
| KT-10 | Wilms | AZD1775 + IR | 45.4 | P < 0.001 | 31.4 | 3.23 | 0.090 | PR |
| KT-11 | Wilms | AZD1775 | 13.1 | P = 0.003 | 2.3 | 1.21 | 0.832 | PD1 |
| KT-11 | Wilms | IR | 32.2 | P < 0.001 | 21.3 | 2.97 | 0.107 | CR |
| KT-11 | Wilms | AZD1775 + IR | 40.3 | P < 0.001 | 29.4 | 3.72 | 0.050 | CR |
| KT-13 | Wilms | AZD1775 | 12.7 | P = 0.055 | 4.1 | 1.48 | 1.356 | PD1 |
| KT-13 | Wilms | IR | 23.5 | P < 0.001 | 14.9 | 2.71 | 0.973 | PD2 |
| KT-13 | Wilms | AZD1775 + IR | 40.6 | P < 0.001 | 21.0 | 4.71 | 0.311 | PR |
FIGURE 1.
Four treatment groups were employed: A, control; B, single-agent AZD1775; C, single-agent irinotecan; D, combination of AZD1775 and irinotecan. Doses and schedules are provided in the Methods section. Results are shown for neuroblastoma, osteosarcoma, and Wilms tumor xenograft lines
3.2 |. Osteosarcoma
Four osteosarcoma lines (OS-1, OS-2, OS-31, and OS-33) were evaluated (Table 1 and Figure 1). Single-agent AZD1775 showed limited activity, with three of the four lines showing a significant prolongation in time to event for the treated versus control groups. However, all four lines showed only PD1 responses with EFS T/C values ranging from 1.06 to 1.66. Irinotecan as a single agent caused significant differences in EFS distribution for all four lines evaluated. Three of four lines showed PD1 responses and a PD2 response in the remaining line. The EFS T/C values for single-agent irinotecan ranged from 1.23 to 2.12. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with control in all lines tested and produced a PD2 response in three of the lines. EFS T/C values ranged from 1.69 to 3.48. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with single-agent irinotecan for all osteosarcoma lines, with prolongation in time to event of 17 to 23 days. The combination also produced for each of the osteosarcoma models minimum tumor volumes that were significantly smaller than those produced by single-agent irinotecan.
3.3 |. Wilms tumor
Three tumor lines (KT10, KT-11, and KT-13) were evaluated (Table 1 and Figure 1). Single-agent AZD1775 showed limited activity. All three lines showing a significant prolongation in time to event for the treated versus control groups, but each showed only PD1 responses with EFS T/C values ranging from 1.21 to 1.62. Irinotecan as a single agent caused significant differences in EFS distribution for all three lines evaluated. Irinotecan showed objective responses in two lines (CR and PR) and a PD2 response in the remaining line. The EFS T/C values ranged from 2.72 to 3.64. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with control in each of the three tumor lines tested and produced objective responses in all the lines (CR and two PRs). EFS T/C values ranged from 3.23 to 4.71. The combination of AZD1775 and irinotecan significantly prolonged time to event compared with single-agent irinotecan for two tumor lines with prolongation in time to event of 8.1 and 17 days. The combination significantly reduced minimum tumor volume in only one tumor model when compared with irinotecan alone.
3.4 |. Evaluation for therapeutic enhancement and for supra-additivity for the AZD1775 and irinotecan combination
Therapeutic enhancement requires that the activity of the combination under evaluation be superior to either of the single agents given at tolerable preclinical doses. Therapeutic enhancement was observed for the AZD1775 plus irinotecan combination for each of the neuroblastoma and osteosarcoma models tested, and it was observed for two of three Wilms tumor models evaluated (Table 2).
TABLE 2.
Evaluation for therapeutic enhancement
| Disease | Xenograft | P (combination vs AZD1775) | P (combination vsirinotecan) | Therapeutic enhancement |
|---|---|---|---|---|
| Neuroblastoma | NB-1643 | <0.001 | <0.001 | Yes |
| Neuroblastoma | NB-Ebc1 | <0.001 | 0.009 | Yes |
| Neuroblastoma | NB-SD | <0.001 | 0.002 | Yes |
| Osteosarcoma | OS1 | <0.001 | <0.001 | Yes |
| Osteosarcoma | OS2 | <0.001 | <0.001 | Yes |
| Osteosarcoma | OS31 | <0.001 | <0.001 | Yes |
| Osteosarcoma | OS33 | <0.001 | <0.001 | Yes |
| Wilms tumor | KT-10 | <0.001 | 0.281 | No |
| Wilms tumor | KT-11 | <0.001 | 0.010 | Yes |
| Wilms tumor | KT-13 | 0.001 | <0.001 | Yes |
P values reflect differences in EFS between experimental groups and are tested using the Gehan-Wilcoxon test.
A model-based analysis for supra-additivity was applied using analysis methods previously applied to rapamycin plus chemotherapy combinations.15 Supra-additivity was identified for one of three neuroblastoma lines (NB-1643), two of four osteosarcoma lines (OS-31 and OS-33), and zero of three Wilms tumor lines (Table 3). The combination effect for the remaining lines was consistent with additivity. The “normalized interaction term” shown in Table 3 can be conceptualized as representing the percentage gain or loss of the expected treatment effect observed for the combination under additivity. The normalized interaction term was greatest for the OS-31 followed by OS-33 and NB-1643. The greater magnitude for the combination effect for OS-31 compared with OS-33 and NB-1643 can be visually appreciated in Figure 1.
TABLE 3.
Evaluation for supra-additivity
| Disease | Xenograft | Normalized interaction term | Total effect expected | Interaction parameter estimate | P | R 2 | Interpretation |
|---|---|---|---|---|---|---|---|
| Neuroblastoma | NB-1643 | 0.26 | 28.56 | 7.32 | <0.001 | 0.98 | Supra-additive |
| Neuroblastoma | NB-Ebc1 | 0.22 | 13.54 | 2.97 | 0.085 | 0.89 | Additive |
| Neuroblastoma | NB-SD | 0.18 | 17.22 | 3.11 | 0.083 | 0.92 | Additive |
| Osteosarcoma | OS1 | 0.03 | 34.53 | 1.16 | 0.812 | 0.75 | Additive |
| Osteosarcoma | OS2 | 0.24 | 8.16 | 1.98 | 0.481 | 0.44 | Additive |
| Osteosarcoma | OS31 | 2.34 | 7.39 | 17.30 | <0.001 | 0.94 | Supra-additive |
| Osteosarcoma | OS33 | 0.69 | 18.17 | 12.47 | <0.001 | 0.95 | Supra-additive |
| Wilms tumor | KT-10 | −0.19 | 47.35 | −8.80 | 0.117 | 0.82 | Additive |
| Wilms tumor | KT-11 | −0.06 | 32.31 | −1.87 | 0.626 | 0.82 | Additive |
| Wilms tumor | KT-13 | 0.17 | 25.36 | 4.35 | 0.615 | 0.41 | Additive |
4 |. DISCUSSION
Evaluation of the WEE1 kinase inhibitor AZD1775, alone and in combination against xenograft models of neuroblastoma (n = 3), osteosarcoma (n = 4), and Wilms tumor (n = 3), demonstrated that all treatment regimens were relatively well tolerated with no toxic mortality observed. Murine dosing of irinotecan is well described.16,17 The dose of irinotecan used (2.5 mg/kg/day for five days) was intended to provide an SN-38 concentration to approximate the 50mg/m2/day for five days dosing in children. The maximally tolerated dose for irinotecan in the mouse is upward of 50 mg/kg for five days (approximately 20 times the dose used in the current experiment). Toxicity mortality was not observed, nor would it be expected at the administered dose or irinotecan. Extrapolation of murine to human dosing for AZD1775 is not as well established. For the experiments described herein, AZD1775 dosing was 120 mg/kg administered orally for days 1 to 5. At a dose of 50 mg/kg in athymic mice, Pokorny et al. reported peak whole blood AZD1755 concentration of 7.78 μM (±2.15 μM) 1 to 2 hours after dosing.18 In a phase 1 pharmacokinetic analysis, Do et al. reported peak AZD1775 serum concentrations of 1.65 μM after five doses at 225 mg twice daily.19 Stewart et al. performed a detailed pharmacokinetic analysis of AZD1755 in mice and found that a dose of 60 mg/kg twice daily best approximated the achievable human clinical exposure based on a comparison of murine plasma AUCinf and Cavg with reported human plasma pharmacokinetic values.6 Hence, it is likely that the murine dose of AZD1775 that we used produces peak concentrations beyond those achievable in humans, but the dosing used in these experiments is within the range of other preclinical evaluations of AZD1775. The fact that weight loss for the combination was more than additive (1.4% and 0.9% in single-agent groups vs 6.7% in the combination) suggests that there may be increased toxicity with the addition of AZD1775 to irinotecan.
As a single-agent AZD1775 shows little activity with all groups demonstrating progressive disease with some tumor growth delay. Irinotecan as a single agent yielded objective responses in the neuroblastoma and Wilms tumor lines. In all lines tested except one (KT-10), the AZD1775 with irinotecan combination demonstrated significantly improved EFS and induced smaller minimum relative tumor volumes when compared with irinotecan alone. The impact on survival was modest, likely because the predominant response was a delay in tumor regrowth following single cycle of therapy. These results suggest that this rationally designed combination of a cell-cycle checkpoint inhibitor with a DNA-damaging agent may have clinical utility in pediatric cancers.
Although AZD1775 potentiated the effects of irinotecan across most of the xenograft lines tested, the magnitude of potentiation of irinotecan by AZD1775 appeared greater for the osteosarcoma and Wilms tumor xenograft lines compared with the neuroblastoma lines. Others have previously reported similarly favorable combination strategies with AZD1775, both in osteosarcoma cell lines and in vitro and in vivo for neuroblastoma and in vivo for rhabdomyosarcoma.4,6,20,21 This is the first report of combination activity in Wilms tumor. Though the in vivo combination effect in the current study is significant, we did not observe complete, sustained responses. Russell et al. report improved activity with combination CHK1 and WEE1 inhibition that may provide more potent means for cells to escape G2-M with subsequent apoptosis.16 Additionally, other DNA-damaging agents (e.g., cisplatin) or antimetabolites (e.g., gemcitabine) may enhance cell dependence on cell-cycle checkpoint control for survival. Nonetheless, together these data demonstrate the role of WEE1 in limiting anticancer activity following irinotecan-induced DNA damage in osteosarcoma, neuroblastoma, and Wilms tumor.
The relationship between TP53 status and AZD1775-mediated chemopotentiation remains poorly understood. There is evidence in some tumor types that AZD1775-mediated chemopotentiation is greatest when TP53 is defective.10,22,23 Others have found no association with TP53 status and AZD1775 effect.12,24,25 In the current study, the osteosarcoma lines lack TP53 demonstrated through absent expression. KT-13 and NB-SD have p.C176Y and p.C176FTP53 mutations, respectively. The degree of chemopotentiation among the xenograft lines tested in this study was greatest for lines with defective TP53, although there was variability in the magnitude of potentiation, suggesting that there are additional unknown mediators adding to the role of WEE1 in cell-cycle regulation in these malignant tumor lines.
When considered with recently reported AZD1775 in vivo combination testing results for rhabdomyosarcoma,6 these results support pediatric clinical evaluations of AZD1775 in combination with irinotecan, as is ongoing in NCT02095132. The European Proof-of-Concept Therapeutic Stratification Trial of Molecular Anomalies in Relapse or Refractory Tumors (ESMART, NCT2813135) includes an arm combining AZD1775 with carboplatin. Future studies should focus on combination strategies to enhance G2-M escape as well as additional genotoxic therapies.
Supplementary Material
Acknowledgments
Funding information
U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute; Grant# CA199221.
Abbreviations:
- CDKs
cyclin-dependent kinases
- CR
complete response
- MCR
maintained complete response
- PD
progressive disease
- PR
partial response
- SD
stable disease
Footnotes
CONFLICTS OF INTEREST
The authors consider that there are no actual or perceived conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1.Do K, Doroshow JH, Kummar S. Wee1 kinase as a target for cancer therapy. Cell Cycle. 2013;12(19):3159–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krajewska M, Heijink AM, Bisselink YJ, et al. Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene 2013;32(24):3001–3008. [DOI] [PubMed] [Google Scholar]
- 3.Puigvert JC, Sanjiv K, Helleday T. Targeting DNA repair, DNA metabolism and replication stress as anti-cancer strategies. FEBS J 2016;283(2):232–245. [DOI] [PubMed] [Google Scholar]
- 4.Kreahling JM, Foroutan P, Reed D, et al. Wee1 inhibition by MK-1775 leads to tumor inhibition and enhances efficacy of gemcitabine in human sarcomas. PLoS One 2013;8(3):e57523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller K, Scheithauer H, Pietschmann S, et al. Reirradiation as part of a salvage treatment approach for progressive non-pontine pediatric high-grade gliomas: preliminary experiences from the German HIT-HGGstudy group. Radiat Oncol 2014;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart E, Federico SM, Chen X, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 2017;549(7670):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi W, Zhang W, Edwards H, et al. Synergistic anti-leukemic interactions between panobinostat and MK-1775 in acute myeloid leukemia ex vivo. Cancer Biol Ther 2015;16(12):1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri L, Vincelette ND, Koh BD, et al. CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo. Haematologica 2014;99(4): 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tibes R, Bogenberger JM, Chaudhuri L, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood 2012;119(12):2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai H, Iwasawa Y, Okada M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther 2009;8(11):2992–3000. [DOI] [PubMed] [Google Scholar]
- 11.Ford JB, Baturin D, Burleson TM, Van Linden AA, Kim YM, Porter CC. AZD1775 sensitizes T cell acute lymphoblastic leukemia cells to cytarabine by promoting apoptosis over DNA repair. Oncotarget 2015;6(29):28001–28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller S, Hashizume R, Yang X, et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol 2014;16(3):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer 2007;49(7):928–940. [DOI] [PubMed] [Google Scholar]
- 14.Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clin Cancer Res 2004;10(21):7413–7417. [DOI] [PubMed] [Google Scholar]
- 15.Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther 2010;9(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson J, Zamboni WC, Cheshire PJ, et al. Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res 1997;3(3):423–431. [PubMed] [Google Scholar]
- 17.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol 1999;17(6):1815–1824. [DOI] [PubMed] [Google Scholar]
- 18.Pokorny JL, Calligaris D, Gupta SK, et al. The efficacy of the Wee1 inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood-brain barrier in glioblastoma. Clin Cancer Res 2015;21(8):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do K, Wilsker D, Ji J, et al. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol 2015;33(30):3409–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu D, Kahen E, Cubitt CL, et al. Identification of synergistic, clinically achievable, combination therapies for osteosarcoma. Sci Rep 2015;5:16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell MR, Levin K, Rader J, et al. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res 2013;73(2):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridges KA, Hirai H, Buser CA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res 2011;17(17):5638–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajeshkumar NV, De Oliveira E, Ottenhof N, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res 2011;17(9):2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Linden AA, Baturin D, Ford JB, et al. Inhibition of Wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality. Mol Cancer Ther 2013;12(12):2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guertin AD, Li J, Liu Y, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther 2013;12(8):1442–1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.