Abstract
Tau immunotherapies have advanced from proof-of-concept studies to over a dozen clinical trials for Alzheimer’s disease (AD) and other tauopathies. Mechanistic studies in animal and culture models have provided valuable insight into how these therapies may work but multiple pathways are likely involved. Different groups have emphasized the importance of intracellular vs. extracellular antibody-mediated clearance of the tau protein and there is no consensus on which pool of tau should ideally be targeted. Likewise, various normal and disease-selective epitopes are being targeted, and the antibody isotypes either favor phagocytosis of the tau-antibody complex or are neutral in that aspect. Most of the clinical trials are in early stages, thus their efficacy is not yet known but all have been without any major adverse effects and some have reported target engagement. A few have been discontinued. One in Phase 1, presumably because of a poor pharmacokinetic profile, and three in Phase 2 for lack of efficacy although this trial stage is not well powered for efficacy measures. In these Phase 2 studies, trials with two antibodies in patients with progressive supranuclear palsy or other primary tauopathies were halted but are continuing in AD patients, and one antibody trial was stopped in early-stage AD but is continuing in moderate AD. These three antibodies have been reported to only work extracellularly and tau is not increased in the cerebrospinal fluid of primary tauopathies, which may explain the failures of two of them. In the discontinued AD trial, there are some concerns about how much of extracellular tau contains the N-terminal epitope that is being targeted. In addition, extracellular tau is only a small part of total tau, compared to intracellular tau. Targeting only the former may not be sufficient for functional benefits. Given these outcomes, decision makers within the pharmaceutical companies who green light these trials should attempt to target tau not only extracellularly but also intracellularly to increase their chances of success. Hopefully, some of the ongoing trials will provide some functional benefits to the large number of patients with tauopathies.
1. Introduction
Alzheimer’s disease (AD) is a leading cause of dementia. Accumulation of extracellular amyloid-β (Aβ) deposits and intracellular hyperphosphorylated tau in neurofibrillary tangles are pathological hallmarks of the disease [1, 2]. Given that the degree of tau pathology is more closely correlated to the decline of cognition in AD patients than Aβ burden, and because of the relative failures of anti-Aβ immunotherapies, attention has shifted from Aβ to pathological tau as a viable target for disease intervention [2-4]. Although the exact mechanisms of tau pathogenesis are still unknown, neutralizing and clearing pathological tau by immunotherapy has shown promising efficacy, including functional improvements in various preclinical models [1, 2, 5-8]. With much progress being made and lessons being learned in preclinical and clinical studies, two tau vaccines and ten tau antibodies are currently in clinical trials for AD and primary tauopathies, mostly for the most common one, progressive supranuclear palsy (PSP). In recent reviews, we have covered in detail the mechanisms of tau immunotherapies [1, 9-11]. The purpose of this review is to provide an up-to-date overview of clinical trials of tau immunotherapies with a brief summary of the mechanisms behind this promising therapeutic approach. Because most of these clinical trials are still ongoing and their detailed outcome has yet to be published, related conference abstracts and press releases have been reviewed and when necessary are cited, with these sources clearly marked in the text.
2. Mechanisms of tau immunotherapies
The tau protein is physiologically abundant in neurons. The dynamic interaction between tau and microtubules plays important roles to support normal neuronal function, such as axonal transport and synaptic signaling [12, 13]. There are six isoforms of the human tau protein in the central nervous system, resulting from its alternative splicing. These isoforms vary in their apparent sizes of about 45-65 kDa [14, 15]. The tau protein undergoes multiple post-translational modifications, such as phosphorylation, acetylation, ubiquitination, glycosylation, amidation, nitration, sumoylation, oxidation, and proteolysis [16, 17]. All these factors contribute to a diverse pool of tau proteome and a complicated tau interactome.
Intrinsically, tau is an unfolded protein [13], but under pathological conditions, excessive post-translational modifications lead to its misfolding and aggregation [18]. Hyperphosphorylation is the most common form, which decreases the affinity of tau to microtubules [18]. In patients with AD and other tauopathies, hyperphosphorylated tau is found in tau monomers, oligomers, and higher order soluble and insoluble tau aggregates, many of which are neurotoxic [19]. Neutralization or removal of these toxic tau species is likely neuroprotective.
Most of the tau protein resides in the somatodendritic compartment and axon of neurons. It is also found in the nucleus [13]. During disease, tau redistributes from an axon enriched to a more somatodendritic localization [12, 20-23]. Pathological forms of tau as oligomers and aggregates that can form neurofibrillary tangles are mostly intracellular [13], but low levels of tau are also found in brain interstitial fluid (ISF) and cerebrospinal fluid (CSF) [24-28]. In a recent study on a group of patients with AD or mild cognitive impairment (MCI), it was estimated that CSF tau is roughly 0.001-0.0001% of total brain soluble tau [29]. In another study in transgenic tauopathy mice, CSF tau was measured to be approximately 10% of tau levels in ISF [26]. If this ratio is similar in humans, it can be predicted that ISF tau is about 0.01-0.001% of intracellular tau. It should be noted though that the ratio of intra- vs. extracellular tau varies between tauopathies. Importantly, compared to control subjects, CSF tau is only increased in AD patients and not in any of the primary tauopathies [30-39]. With this in mind, antibodies that target both the intra- and extracellular pool of tau hold greater therapeutic promise to treat all tauopathies [40], than antibodies that only work extracellularly, as we have pointed out over the years.
Extracellular tau can exist as monomers, oligomers and larger aggregates, which are either actively released from neurons or come from remnants of degenerated neurons [24-28, 41-44]. These tau species can be taken up, and serve as seeds to template aggregation in neighboring neurons [45-47]. Transneuronal propagation of tau aggregation may contribute to the spreading of pathologic tau during disease progression [46, 48, 49], and different molecular conformers of tau aggregates/seeds exist across tauopathies [50-54]. Therefore, the seeding competencies of extracellular tau varies. This phenomenon may have some relevance to the spread of AD tau pathology within a brain region but spread between brain regions is more likely to be governed by neuronal network and cell autonomous factors. It is important to note as well in this context that seeding and toxicity may not necessarily go hand in hand [40].
Oligomeric and soluble tau aggregates can mediate acute neurotoxicity [40, 55-57], and these species are primarily found within neurons, although they can be released into extracellular space and serve as seeds to spread tau pathology [48, 49]. In the short term, seeding is likely a way to sequester soluble toxic tau species, although these higher order aggregates will eventually kill the neuron but on a longer time scale. Therefore, to focus on an antibody’s ability to prevent tau seeding without considering its ability to block tau toxicity is shortsighted. Our studies have shown that some tau antibodies are good at both preventing tau-mediated neurotoxicity and tau seeding, while others only block seeding and not toxicity [41, 43]. It is also important to note that tau seeds are different across different tauopathies [45], and toxicity of pathological tau enriched from human tauopathy brains can vary greatly (personal observations). These issues need to be taken into account when examining the efficacy of tau antibodies in different tauopathies.
Most of the preclinical studies on the therapeutic potential of tau antibodies have not focused on whether these antibodies are working extra- and/or intracellularly but we have shown that many can work in both compartments [40, 56]. We and others have detected the antibodies within neurons ([40, 56, 58-67], whereas some other antibodies do not appear to be taken up into neurons [24, 40, 68-70]. Within neurons, the antibodies are typically seen associated with tau within the endosomal/lysosomal pathway, in which they may facilitate tau clearance by promoting disassembly of tau aggregates and thereby allow for better access of lysosomal enzymes for their degradation [60, 61]. In addition, these antibodies have also been detected outside these vesicles in the cytosol where they can bind to the cytosolic Fc receptor TRIM21, which as ubiquitin E3 ligase will promote tau ubiquitination and its proteosomal clearance [71].
Extracellularly, antibody binding to tau may promote their microglial clearance or in some undefined way neutralize extracellular tau [56, 60, 72-74]. In this context, it is interesting to note that most of the tau antibodies in clinical trials that have been predicted to only work extracellularly are described not to have effector function, and would therefore not promote microglial phagocytosis of the tau-antibody complex. This is presumably done for safety reasons to limit inflammation but considering the low levels of extracellular tau, such a side effect is not very likely, and this may limit clearance efficacy. Therefore, the odds of success are stacked against these antibodies.
3. Considerations for vaccine/antibody design
Characteristics of an anti-tau antibody can greatly affect its efficacy, safety and mechanisms of action. These features include epitope, isotype, affinity, charge and size.
3.1. Epitope
Various epitopes of tau have been targeted and shown promising results in both pre-clinical models and clinical studies (see Figure 1). These epitopes included non-phosphorylated, phosphorylated, oligomeric, conformational and truncated tau ([67-70, 73-88]; abstracts [89, 90]). The epitope in the original studies, phospho-serine 396,404 (pSer396,404), has been shown to be an excellent target by us and many other groups in various cell-culture and transgenic mice models [5-7, 56, 58-61, 66, 76, 81, 82, 85, 91-95]. This epitope is the focus in two clinical trials. It is found within the active vaccine ACI-35, and was used to generate the tau antibody Lu AF87908. However, more tau antibodies in clinical trials target the N-terminus, and most of the mouse antibodies that these were derived from have been reported to only work extracellularly to block tau seeding and reduce tau pathology in preclinical models [79, 96].
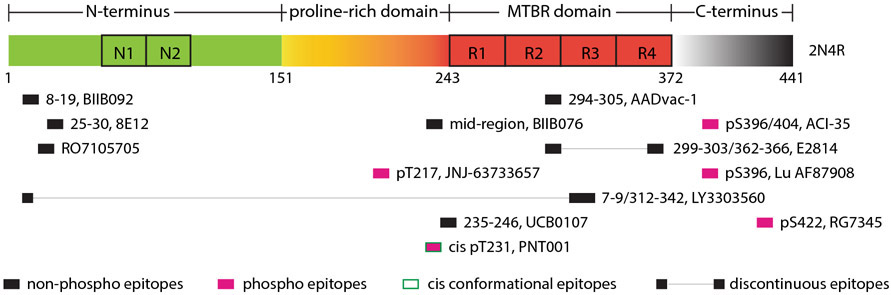
Figure 1.
Epitopes of tau vaccines/antibodies that are currently in clinical trials. MTBR: microtubule binding domain.
Concerns have been raised about these N-terminal antibodies because of the low levels of extracellular tau compared to intracellular tau [11, 35]. In addition, mass spectroscopy studies show that most of CSF tau lacks N and C-termini, which then further diminishes the target pool of these antibodies [30, 43]. If the antibody is predicted to only work extracellularly, targeting the mid-region of tau may be more efficacious in preventing tau seeding and propagation [97, 98]. Several such antibodies are currently in clinical trials but the exact epitopes of all have not been revealed and some may be outside the most prominent extracellular tau region (approximately tau150-250). The first tau vaccine that entered clinical trials targets the 294-305 epitope [99]. Some of these antibodies/peptide vaccines are likely to be selective for certain tau conformations and two are defined as conformational antibodies, one against a discontinuous epitope that incorporates tau 7-9 and tau 312-342, and another one against a cis-conformation of phospho-threonine 231 (pT231) (Alzforum [100-102]).
3.2. Isotype
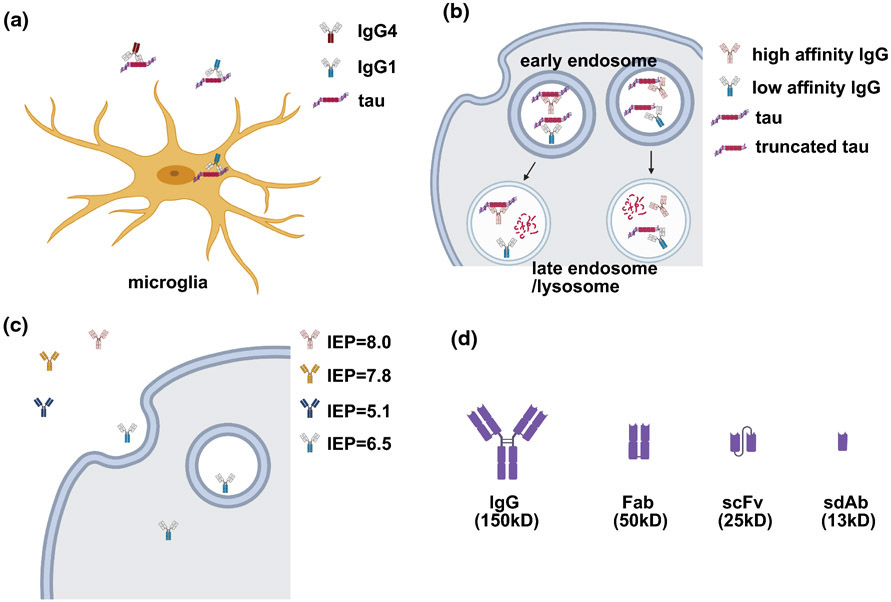
There are four different isotypes of human immunoglobulin G (IgG), which vary in binding affinities to Fc gamma receptors (FcγRs) and effector functions. Interaction between the Fc region of IgG and the FcγRs on the cell surface can mediate the entry of antibody into cells. In the central nervous system, microglia are the major effector cells to take up extracellular tau/antibody complex for degradation [56, 60, 72-74]. Neurons also express FcγRs, which mediate antibody uptake for intraneuronal tau clearance [103-106]. Human IgG1 is the most effective isotype to promote microglia phagocytosis, whereas IgG4 is the least effective one (see Figure 2a). Both IgG1 and IgG4 tau antibodies are currently being tested in the clinical trials.
Figure 2.
Considerations on antibody isotype (a), affinity (b), charge (c) and size (d) when designing and/or modifying a tau antibody. (a) IgG1 has better effector functions compared to IgG4; therefore, it can mediate microglia-dependent clearance of antibody-tau complex. (b) A high affinity antibody sometimes can block the dissociation of tau oligomers/aggregates and thereby prevent their intracellular degradation; whereas, a relatively lower affinity antibody may promote the disassembly of tau oligomers/aggregates and subsequent degradation. However, this is likely epitope dependent as reflected in greater efficacy of a high affinity antibody for tau truncated at Asp421 compared to a low affinity antibody generated against the same immunogen. (c) A slightly acidic charge appears to be optimal for antibody uptake into cells. IEP, isoelectric point. (d) Size of different antibody fragments. Fab, antigen-binding fragment; scFv, single-chain variable fragment; sdAb, single domain antibody fragment.
Whether an effective antibody should have effector function is debatable. One study found that the whole antibody is more effective than its antigen-binding fragment (Fab) to promote the clearance of pathological tau in microglia, indicating an Fc-dependent clearance pathway [74]. Another study showed that antibody mediated modulation of tau pathology in transgenic mice varied depending on the isotype but the binding sites of the antibodies were not identical, although they were against the same region [92]. Conversely, another report suggested that antibody effector function and microglia engagement were not necessary for efficacy [107]. It is a bit puzzling in this context that several of the antibodies that are reported to only target extracellular tau are relatively effectorless (IgG4 or mutated IgG1), and can therefore not take advantage of the enhanced clearance associated with microglial phagocytosis of the antibody-tau complex.
It should be noted that most of the mouse tau antibodies that have been examined in preclinical studies are of the mouse IgG1 isotype, which is more similar to human IgG2b that is not present in any of the ongoing clinical trials. Clearly, more work is needed to clarify the importance of antibody isotypes, as this feature can not only affect the efficacy, but also the safety of a tau antibody.
3.3. Affinity
Antibody affinity does not equal efficacy (see Figure 2b). Studies from our laboratory have repeatedly shown that a low affinity antibody against pSer396,404 is effective in various culture and in vivo models, whereas a high affinity antibody against the same epitope is ineffective [5, 40, 58, 61, 94, 108]. Both antibodies are mouse IgG1κ. Similarly, a low affinity antibody against a conformational epitope (MC1; aa7-9 and 312-342) is more effective in tauopathy mice than a high affinity antibody recognizing tau150-190 [109, 110]. However, this phenomenon is likely epitope dependent. For example, our studies on antibodies targeting truncated tau at aspartate 421 show that a high affinity antibody against this truncated epitope is much more effective than a low affinity antibody (abstract [90]). For certain epitopes, the tight binding of an antibody to tau may render these aggregates more resistant to instead of promoting their degradation.
3.4. Charge
Antibody charge is an important feature to consider, as it can influence the binding characteristics, and in particular cellular uptake and therefore efficacy (see Figure 2c). Modulating antibody charge to influence tissue penetration has been extensively studied in cancer antibody therapeutics and is regularly taken into account in their development. With this in mind, it is interesting that this feature had not been well explored for tau antibodies. To address this issue, we first examined how charge (isoelectric point (IEP)) of mouse tau antibodies against different tau epitopes influenced their neuronal uptake and efficacy [40]. The antibodies were taken up into primary cortical neurons from tauopathy mice to a varying degree. Antibody 4E6 with IEP = 6.5 had the most neuronal uptake and greatest intracellular efficacy compared to three other antibodies (1B9, IEP = 8.0; 2C11, IEP = 7.8; Tau-5, IEP = 5.1). Interestingly, a partial humanization of 4E6 shifted its IEP from 6.5 to 9.6, and thereby prevented its uptake and intracellular efficacy in blocking tau toxicity and promoting tau clearance. However, this humanized 4E6 retained in large part its extracellularly efficacy. These data indicate that a slightly acidic IEP is favorable for neuronal uptake of antibodies, whereas more acidic or basic charge interferes with antibody uptake and thereby reduces its overall intracellular availability. These findings highlight the need to take charge into consideration when the mouse monoclonal antibodies targeting tau are humanized for clinical trials.
3.5. Size
Only whole antibodies are being examined in the ongoing clinical trials. Antibody fragments such as Fabs (50 kDa), single-chain variable fragments (scFv, 25 kDa) and single domain antibody fragments (sdAbs, 13 kDa) have better tissue penetration compared to whole antibodies (150 kDa) (see Figure 2d). For example, we have detected increased uptake of Fab in brain slices and of scFv into brain in vivo compared to whole antibodies [60, 62]. However, their half-lives are much shorter than for intact antibodies (minutes to a day vs. several weeks). Therefore, the smaller entities have potential as diagnostic imaging agents [62, 111], but as treatments would only be suitable as gene therapies.
Given the small size of antibody fragments, they can be directly expressed in neurons for better and sustained efficacy. For example, we have shown that neuronal expression of an scFv targeting tau decreases tau pathology and rescues behavioral deficits in a tauopathy fly model [112]. Others have reported therapeutic benefits of scFvs targeting tau that were administered in mouse models via ultrasound or by using a carrier protein or vectored expression [113-116]. Directing an anti-tau scFv into the proteasome has also been reported to be more effective than directing it to lysosomal clearance of the scFv-tau complex [117]. A recent study also showed that targeting intracellular tau by AAV-mediated expression of a modified scFv is more effective than targeting extracellular tau with secreted scFv in transgenic tauopathy JNPL3 mice [118].
Antibodies that can enter or be expressed in neurons followed by degradation of the tau-antibody complex are likely to have improved therapeutic potential over antibodies that only work extracellularly as we have pointed out over the years, because an overwhelming majority of pathological tau resides within neurons. Importantly, in our first publication on this topic, we showed antibody entry into the brain and into neurons, where they bound to pathological tau [58]. The therapeutic potential of the smallest antibody fragments, sdAbs, is beginning to be explored [119, 120] (abstracts [121-123]). These are typically derived from llamas and as a single unit may be the most suitable of antibody fragments as a gene therapy or a diagnostic imaging agent.
4. Clinical Trials on Tau Immunotherapies
4.1. AADvac-1
AADvac1 is an active vaccine that is being developed by Axon Neuroscience SE. The epitope was selected based on features of a monoclonal antibody, DC8E8, and inspired by research on N-terminally truncated tau fragments [99, 124]. AADvac-1 consists of a synthetic peptide tau 294-305, coupled to keyhole limpet hemocyanin with aluminum hydroxide as an adjuvant.
In 2013, a first-in-man Phase 1 trial began in patients with mild to moderate AD to evaluate the safety, tolerability and immunogenicity of this vaccine [124]. Assessment of cognition was also explored in this trial. Patients received three subcutaneous monthly injections of a single dose of AADvac-1. After this, patients entered the open-label phase, and another three doses were administered at monthly intervals. The treatment group received six doses in total, while the placebo group got three. Twenty-nine out of thirty patients developed an IgG response with no cases of encephalitis or vasogenic edema. The primary endpoint of the safety evaluation was treatment-emergent adverse events. A separate analysis for injection site reactions was also conducted. The most common adverse event was limited to inflammation at the injection site, which occurred in 53% of patients. No deleterious immunological responses were elicited. Five patients in the treatment group had serious adverse events during the trial. Three of those were deemed to be unrelated to treatment but the remaining two (viral infection and epileptic seizure) might have been related to treatment, although this was considered to be unlikely by the independent data and safety monitoring board. Two patients withdrew from the trial due to adverse events. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) scores remained stable in all patients. Overall, AADvac-1 had excellent immunogenicity and a favorable safety profile.
After this trial, a follow-up study to monitor these patients lasted for a further 18 months [125]. Twenty out of twenty-six patients had completed this study when reported. Six months after the last injection, the antibody titer declined but booster doses restored the titer. No additional treatment related serious adverse events were observed. A trend for lower hippocampal atrophy rate and better performance on some cognitive tests were observed in patients with higher IgG titers.
In March 2016, a Phase 2 trial (NCT02579252) was conducted in a larger group of patients with mild to moderate AD as supported with a magnetic resonance imaging (MRI) scan. The enrolled patients were not pre-screened for amyloid or tau pathology, but had medial temporal lobe atrophy. In this trial, patients in the treatment group received six doses of AADvac1 over 6 months, with five quarterly boosters. The primary outcome was safety. The secondary outcomes included immunogenicity, cognitive and clinical batteries, the Clinical Dementia Rating (CDR) Sum of Boxes (CDR-SB) scale, and the Alzheimer’s Disease Cooperative Study – Activities of Daily Living questionnaire (ADCS-ADL). Exploratory outcomes included fluorodeoxyglucose positron emission tomography (FDG PET), MRI volumetry, and CSF biochemistry. Initial results of this trial were announced in a company’s press conference (press release [126]), and more details were presented at the 2020 virtual AAT-AD/PD Focus Meeting (Alzforum news [127]). No differences in the types of adverse events between treatment and placebo groups were observed. More than 80 percent of participants developed high affinity tau antibodies that recognize aggregated tau in brain tissue from AD, PSP, corticobasal degeneration and frontotemporal dementia patients. These antibodies can also block neuronal uptake of pathological tau in a cell model. Furthermore, patients who received AADvac-1 had a smaller increase of neurofilament light chain (NfL) protein in blood, a marker for neurodegeneration, compared to those in the placebo group (p=0.0039). Twenty treated patients and seven with placebo provided CSF. A trend for reduction of CSF tau phosphorylated at Thr181 (pT181) and tau phosphorylated at Thr217 (pT217) in treated patients was observed. There were no significant cognitive benefits. However, a trend toward slower decline on CDR-SB, Mini-Mental State Examination (MMSE) and ADCS-ADL in younger participants with treatment compared to placebo was found in a preplanned age subgroup analysis. This subgroup also had greater reductions in plasma NfL and significant slowing of cortical atrophy compared to the group as a whole.
A separate open-label Phase 1 pilot trial (NCT03174886) is also underway in patients with non-fluent/agrammatic variant progressive aphasia. In this trial, patients receive two doses of AADvac-1 over 6 weeks, followed by five booster shots every 13 weeks. Safety and immunogenicity are evaluated as the primary outcomes. Secondary outcomes include changes in CSF and serum biomarkers, such as neurogranin, phosphorylated neurofilament heavy chain protein, tau, pT181, N-terminal tau, amyloid-β, NfL, MRI, and immunological responses. Assessment of cognition will also be explored in this trial. The clinical measures include Frontotemporal Lobar Degeneration Clinical Dementia Rating Sum of Boxes (FTLD-CDR-SB), Clinician’s Global Impression – Improvement (CGI-I), Instrumental Activities of Daily Living (ADL), a Custom Cognitive Battery, Addenbrooke’s Cognitive Examination, Unified Parkinson’s Disease Rating Scale (UPDRS) part III, and Frontal Systems Behavior Scale (FrSBe).
4.2. ACI-35
ACI-35 is a liposome-based vaccine that was initially developed by AC Immune and then licensed to Janssen Pharmaceutics. ACI-35 targets phosphorylated tau encompassing residues serine-396 and serine-404 (pSer396,404), which is greatly elevated in pathological forms of tau. Therefore, the elicited immune response will not target much of the abundant physiological forms of this ubiquitous protein. Sixteen copies of a synthetic tau fragment were encapsulated in liposomes and were initially delivered with the adjuvant Monophosphoryl Lipid A (MPLA) [128].
Efficacy of ACI-35 was tested in a pre-clinical study using P301L tauopathy mice, which develop progressive motor impairment [95]. These mice received two doses of subcutaneously injected ACI-35 over a 3-month regimen. Serum samples from the immunized mice contained antibodies that selectively bound to pSer396,404 over its non-phosphorylated equivalent tau peptide. The resulting antibodies bound neurofibrillary tangles and reduced soluble and insoluble tau in the mouse brains. The vaccinated mice showed delayed onset of the motor deficits, retention of body weight and an extended lifespan. There was no gliosis, T cell activation or other inflammatory responses.
Safety and immunogenicity of the ACI-35 vaccine were evaluated in a Phase 1b study (ISRCTN13033912) initiated in December 2013. Twenty-four patients with mild to moderate AD received two to five injections of ACI-35 at low, medium, or high doses over a 6-month period, followed by a subsequent booster shot at 6 or 16 months after this initial dosing period. Safety, tolerability and immunogenicity were evaluated as primary outcomes. Analyses included adverse events, antibody titers in blood, and unspecified biochemistry measures in CSF, MRI scans and electrocardiograms (ECG). Secondary outcomes were biomarkers and cognitive assessments, including ADAS-Cog, MMSE, trail-making and fluency tests, Clinical Global Impression of Change Disability Assessment in Dementia, and Neuropsychiatric Inventory Scale. The results of this trial were presented at the 2020 virtual AAT-AD/PD Focus Meeting (Alzforum news [127]). While ACI-35 raised no safety concerns, antibody titers were low, even after booster shots. A second-generation vaccine ACI-35.030 includes a second adjuvant and an epitope to activate T helper cells. The redesigned vaccine induces a stronger immune response in rhesus monkeys and booster shots increase antibody titers. The antibodies specifically bind to phosphorylated tau, and recognize paired helical filaments in AD brains.
In July 2019, AC Immune and Janssen started a Phase 1b/2a trial (NCT04445831) to test the ACI-35.030 in patients with early AD. Twenty-four patients were enrolled. Three different doses vs. placebo are administered multiple times over 48 weeks. Primary outcomes are adverse events, other safety measures and antibody titers in blood up to 74 weeks. Cognition and behavior changes will also be evaluated. In July 2020, AC Immune announced that positive safety and immunogenicity data had been collected on the lowest dosing group, and subsequent initiation of the second highest dosing group (press release [129]). The interim results showed a potent antigen specific antibody response was observed in all older patients with early AD after the first injection, with no clinically relevant adverse events (press release [130]). Recruitment for the highest dose is currently ongoing (Alzforum [131]). These results support plans to further this vaccine into Phase 2/3.
In addition to ACI-35.030, AC Immune and Janssen Pharmaceuticals, Inc. recently added an arm to the current Phase 1b/2a trial (NCT04445831) to evaluate an alternative phospho-tau vaccine candidate, JACI-35.054. It is not clear if this is a different tau epitope, a different formulation or both. Participants were increased to 32. Promising interim safety, tolerability and immunogenicity results were collected in the low-dose group. Enrollment for higher dose group has started (press release [132]).
4.3. BIIB076
BIIB076 is a human monoclonal anti-tau IgG1 antibody. It was originally developed by Neurimmune and later acquired by Biogen. BIIB076 recognizes the mid-domain of tau, and blocks tau aggregation in vitro and its propagation between neurons [81]. In preclinical studies, BIIB076 binds to human and cynomolgus monkey recombinant tau with subnanomolar affinity. It recognizes monomeric and fibrillary tau as well as tau isolated from healthy human and AD brains. In young monkeys, its single dose (100 mg/kg) had a half-life of 8 to 11 days in blood. CSF concentration reached its maximum in 24-48 h after administration but was 1,000 times lower than in plasma. The antibody led to an increase in total tau protein in plasma but did not affect total tau in CSF. Unbound tau in CSF dropped 75 percent 24 h after antibody administration and returned to baseline after 3-weeks, indicating a target engagement (Alzforum [133]).
A toxicity study was carried out to evaluate three doses of up to 16 times the highest predicted efficacious dose in young cynomolgus monkeys. BIIB076 was injected intravenously or subcutaneously over the course of a month. Its levels in serum increased as the dose increased. Total and free tau in CSF were reduced at the highest doses used. No toxicity or pathology related to BIIB076 were reported in this study [134].
In February 2017, a Phase 1 trial (NCT03056729) began in healthy volunteers and in subjects with mild or probable AD. The participants were screened by CSF levels of Aβ42, total tau, and phosphorylated tau and given a single intravenous infusion. Healthy volunteers were grouped into five successive dosing cohorts while AD patients were grouped into two. Primary outcomes include adverse events, vital signs, neurological exams, ECG, and MRI; secondary outcomes include pharmacokinetic parameters of exposure and clearance, and immunogenicity. In June 2019, Biogen modified the trial protocol by eliminating the more advanced AD cohort and adopting adverse events as the sole primary outcome. This trial was completed in March, 2020, but results have not yet been made public (Alzforum [133]).
4.4. BIIB092
BIIB092, also named gosuranemab, is a humanized IgG4 monoclonal antibody that was developed against extracellular N-terminal tau fragments (eTau) isolated from human neurons differentiated from familial AD patient-derived pluripotent stem cells ([24], Alzforum [135]). This antibody was initially developed by iPerian, which was acquired by Bristol-Myers Squibb, and this antibody was subsequently acquired by Biogen.
In preclinical studies, iPerian showed that exogenously added eTau increased Aβ production and neuronal hyperactivity in primary human cortical neurons [24]. Neutralizing the eTau by an antibody reduced Aβ production. This result was also observed in two different human tau transgenic mouse models. Epitope mapping studies conducted by Biogen showed that BIIB092 binds to human tau residues 15-22 [136]. It recognizes tau monomer, fibrils and insoluble tau from different tauopathies with high affinity, and pathological human brain homogenates or seed-competent AD-tau pretreated with BIIB092 has significantly reduced ability to seed tau aggregation in cellular models.
Two phase 1 trials were conducted to assess the safety of this antibody. The first trial (NCT02294851) performed by Bristol-Myers Squibb from December 2014 to April 2016 was a single-center, single ascending-dose study in 65 healthy volunteers. A follow-up study lasted for 8 months after administration. No severe adverse events were observed. A dose-dependent increase of BIIB092 in blood and CSF caused 67 to 97 percent decrease of CSF unbound eTau 4 weeks after administration. Doses of 210 mg and higher resulted in sustained eTau reduction for up to 12 weeks [137]. The second trial (NCT02460094) run by Biogen from September 2015 was a multi-center, multiple ascending-dose Phase 1b trial in 48 PSP patients. Patients received three doses every 4 weeks. The highest dose was 2100 mg. Safety, pharmacology and immunogenicity of BIIB092 were assessed. According to the published results [138], BIIB092 is safe and well-tolerated in PSP patients. No severe adverse events were reported. Most adverse events were mild to moderate. The half-life of BIIB092 in CSF is about 28 days, and there is a dose-dependent accumulation of BIIB092 in blood and CSF. All BIIB092 doses decreased the unbound eTau in CSF by more than 90 percent in treated PSP patients, whereas no change was observed in the placebo group. The reduction was sustained for 85 days after administration. No change in MRI scans was detected. Likewise, CSF biomarkers including total tau, pT181, Aβ42, or neurofilament light chain were not altered in the treatment group compared to controls. An 18-month open-label extension study (NCT02658916) on the participants in the phase 1b study was then terminated due to the failure to meet the primary endpoint.
In April 2017, Biogen started a subsequent Phase 2 study named PASSPORT (NCT03068468) with a longer treatment period and a larger group of PSP patients. It was a 52-week multiple-site study. Primary outcomes were safety and the PSP rating scale, which measures movement problems. Unfortunately, this trial was terminated in December 2019 (press release [139], Alzforum news [140]). BIIB092 showed no significant improvement of the PSP rating scale compared to the placebo control. This was not particularly surprising, as discussed previously [31, 35, 141]. Briefly, this antibody was originally described not to enter neurons and only work extracellularly [24]. As mentioned above, CSF tau levels are not increased in PSP compared to controls [30-32, 35, 39], indicating that extracellular tau has not a major role in its pathogenesis, and targeting it solely there, would therefore be futile. In addition, its epitope may not be prominent extracellularly because it does not affect total tau levels.
Another Phase 2 study, named TANGO (NCT03352557), is ongoing in patients with mild cognitive impairment (MCI) or mild AD, who have a positive Aβ PET scan. Patients enrolled were not scanned with PET for tau lesions. Three different doses of BIIB092 and placebo were infused monthly for 18 months, followed by 3 years of extension. The placebo-controlled phase is still ongoing, and the long-term extension is expected to end in 2024. The primary objective of this trial is to evaluate the long-term safety and tolerability of the antibody. The secondary objectives are immunogenicity and to evaluate the efficacy of BIIB092 in modifying cognitive impairment in patients with MCI or mild AD. In MCI and mild AD, CSF tau is known to be increased relative to healthy controls. Therefore, BIIB092 has a greater chance to work here than in PSP patients [35, 141].
Lastly, a Phase 1b placebo-controlled “basket” trial (NCT03658135) began in September 2018 but ended in April 2020. BIIB092 was tested in four different primary tauopathies: amyloid-β PET negative corticobasal syndrome, non-fluent variant primary progressive aphasia, frontotemporal lobar degeneration with MAPT mutation, and traumatic encephalopathy syndromes. Participants in this trial were randomized to the drug and placebo, and infused with up to 2000 mg of antibody or placebo monthly for six doses. Primary outcome was safety; secondary outcomes were pharmacokinetics, pharmacodynamics of CSF tau, exploratory MRI scan and CSF biomarkers, and cognitive and functional measures. Results of this trial were presented at the CTAD conference in November 2020 (press release [139], Alzforum [135]). There were no adverse events associated with the treatment. Although the treatment led to a 100 percent decrease of unbound eTau in CSF, there were no effects on exploratory measures of disease severity. This is likely due to differences in disease pathology between primary tauopathies and secondary tauopathies [39]. As for PSP, these other primary tauopathies have not been shown to have increased tau levels in CSF [30, 37-39]. Therefore, similar to the PSP trial, targeting extracellular tau is not sufficient to modify disease progression in these particular tauopathies.
4.5. CN2-8E12 (ABBV-8E12)
Humanized IgG4 antibody 8E12, currently named tilavonemab, was developed by C2N Diagnostics and advanced to clinical trials by AbbVie to treat tauopathies (Alzforum [142]). This antibody has been described to recognize amino acids 25-30 at the N-terminal sequence of tau in aggregated and extracellular form, and not be taken up into neurons [73, 87, 143]. Therefore, this antibody has been said to only work extracellularly. In preclinical studies, the mouse version of this antibody blocks tau seeding caused by exogenous tau aggregates, uptake of AD-derived tau aggregates, and prevents transneuronal propagation of tau pathology in cell-based assays [73, 143]. Infusion of this antibody into transgenic tauopathy mice reduced brain neurofibrillary pathology, insoluble tau, microglial activation, seeding activity of the lysate of treated brains and deficits in the conditioned fear response [68, 87].
Between July 2015 and August 2016, a Phase 1 trial (NCT02494024) of 8E12 was conducted in a single-ascending-dose study in PSP patients. This trial compared four doses from 2.5 to 50 mg/kg of 8E12 to placebo. A follow-up study was run in successive three-to-one randomization groups 84 days after administration. Primary outcomes were safety, tolerability, immunogenicity and pharmacokinetics. Results show that 8E12 is safe at all doses, but the maximum tolerated dose was yet to be determined. The serum half-life of 8E12 was about 27-37 days in a dose-dependent manner. The CSF-to-blood ratio was 0.18-0.35 [144]. In 2018, an open-label extension study (NCT03413319) on long-term safety and tolerability was conducted to determine the eligibility of participants for the subsequent Phase 2 trial.
A Phase 2 trial (NCT02985879) on PSP patients was initiated in 2016 by Abbvie. In this trial, patients aged ≥40 years with symptoms for less than 5 years were enrolled. The primary outcomes were adverse events and Total Score on the PSP Rating Scale (PSPRS). Secondary outcomes included pharmacokinetic parameters, MRI scan and global and Parkinson’s measures. A 4-year randomized extension to this trial (NCT03391765) began on participants who had completed the placebo-controlled treatment phase in 2018. Primary outcome was PSPRS Total Score, and secondary outcomes were Parkinson’s and global clinical measures. Unfortunately, this trial was halted by Abbvie in July 2019 because the antibody provided no benefit over placebo (Alzforum [145], [146]). The company also announced the cancellation of the extension studies of 8E12 in PSP patients and the expanded access (NCT03744546) of this antibody to Corticobasal Degeneration (CBD) patients. Like BIIB092, 8E12 is reported to only work extracellularly. Therefore, it is unlikely to show efficacy in PSP or CBD patients, given that CSF tau levels, which notably are only a small fraction of total tau, are not increased in these patients [30, 38, 39].
However, the Phase 2 trial (NCT02880956) in early stage Alzheimer’s patients is still ongoing. Abbvie started this trial in October 2016 at multiple sites. A long-term extension study (NCT03712787) on long-term safety and tolerability is offered to participants who complete the study for up to 5.5 years of dosing. More than 400 individuals with positive amyloid PET scan were enrolled. Their early disease stage was determined by a CDR rating of 0.5, an MMSE of 22 or higher, and Repeatable Battery for Assessment of Neuropsychological Status (RBANS) of 85 or lower. Three doses of 8E12 over placebo are being compared over 96 weeks and during a 16-week follow-up. The primary outcomes are adverse events and decline on the CDR Sum of Boxes. Secondary outcomes are clinical and functional measures, such as Alzheimer's Disease Assessment Scale (14-Item) Cognition Portion (ADAS-Cog14), RBANS, Functional Activities Questionnaire (FAQ), and 24-Item Alzheimer's Disease Cooperative Study/Activities of Daily Living Scale Adapted for Patients with Mild Cognitive Impairment (ADCS-MCI-ADL-24).
4.6. E2814
E2814 is a humanized IgG1 antibody that recognizes HVPGG motifs in the second (aa299–303) and fourth (aa362–366) repeat of the microtubule binding domain in 2N4R-tau, which as mentioned above contributes to seeding and transcellular propagation of tau pathology [147]. It recognizes both the 4R and 3R tau isoforms, and is described to bind to extracellular tau [147]. In vitro, this antibody prevents tau aggregation and seeding [147]. In a transgenic mouse model, antibody treatment attenuated deposition of tau aggregates in mice injected with tau fibrils [147]. In non-human primates, it showed dose-dependent binding to mid-domain tau fragments, and reduced the levels of free tau containing the mid-domain (press release [148]).
In December 2019, Eisai started a Phase 1 trial (NCT04231513) to test the safety and tolerability of this antibody in healthy participants. All subjects received a single intravenous infusion and were followed for up to 4 months. Three different doses were compared to placebo treatment. Primary outcomes were treatment related adverse events and serious adverse events. Secondary outcomes were pharmacokinetic measures in serum and CSF, and immunogenicity. An exploratory outcome is to assess target engagement in CSF. This trial was completed in August 2020. Results show no severe drug related adverse events. Serum and CSF pharmacokinetic measures were proportional to the levels of antibody injected. Two participants developed anti-E2814 antibodies. Liquid chromatography–mass spectrometry (LC/MS) analysis revealed a dose-dependent increase of antibody-tau association in CSF ([149], abstract [150]). Using this method, mid domain containing tau was quantified in human CSF, and was shown to be significantly increased in CSF of AD patients, compared to PSP patients and healthy adults ([149], press release [148], abstract [150]). There was a dose-related increase of antibody-tau association, which persisted for at least a month and indicated target engagement (press release [148]). It is not clear if the antibody affected CSF tau levels.
In March 2021, E2814 was chosen to be evaluated in the Dominantly Inherited Alzheimer’s Network Trials Unit (DIAN-TU) prevention trial, which enrolls people carrying pathogenic APP and presenilin mutations (Alzforum [151]).
4.7. Lu AF87908
Lu AF 87908 is a humanized monoclonal IgG1 antibody targeting phospho-serine 396 region of tau. The original mouse version of this antibody has shown efficacy in reducing tau seeding in cellular and mouse models of tauopathy [94]. It can neutralize seed-competent pathological tau, and mediate its uptake and lysosomal degradation in microglia [72].
Currently, a Phase 1 study (NCT04149860) is ongoing to test the safety, tolerability and pharmacokinetics in healthy individuals and patients with AD. This study is expected to complete in May 2021.
4.8. LY3303560
LY3303560, named zagotenemab, is a humanized anti-tau antibody targeting a conformational epitope of tau, MC1, which is an early pathological conformation of tau [76, 110]. In transgenic mice, treatment with MC1 reduces phosphorylated tau levels and neurofibrillary pathology, which is mediated by microglia-dependent or neuronal-dependent tau/antibody clearance [74, 116]. In preclinical studies, LY3303560 selectively binds tau aggregates over monomers with high affinity as reported by Eli Lilly at the 2017 AAIC meeting (abstract [152]). Intravenous injection of LY3303560 into monkeys has a half-life of 13 days in serum and clearance rate of 0.15 ml/h/kg. Subcutaneous administration shows 79 percent bioavailability. In a rat model, CSF concentration was 0.1 percent of plasma at 24 h after intravenous injection.
Eli Lilly initiated a Phase 1 trial (NCT02754830) in 2016. Both healthy individuals and patients with MCI or mild to moderate AD were enrolled. A single ascending dose of an intravenous infusion or a subcutaneous injection was evaluated in this study. Adverse effects were measured up to 85 days after administration. Maximum drug concentration in both serum and CSF was also evaluated.
A second Phase 1 study of a multiple-ascending dose (NCT03019536) was initiated in identical cohort of patients in 2017. LY3303560 was delivered intravenously. That trial lasted for 6 months with 4 additional months of follow-up. Primary outcomes were adverse effects and pharmacokinetic measures. Results from these two trials have not been published.
In April 2018, a Phase 2 trial (NCT03518073) was started in patients with early symptomatic AD, who have had a gradual and progressive decline in memory for more than 6 months. LY3303560 was delivered intravenously. Two doses were administrated. Primary outcomes include a change on Lilly's integrated Alzheimer's Disease Rating Scale (iADRS). Secondary outcomes were the Alzheimer's Disease Assessment Scale Cognitive subscale 13-item version (ADAS-Cog13), the Alzheimer’s Disease Cooperative Study – instrumental Activities of Daily Living (ADCS-iADL), CDR-SB, MMSE, the CogState Brief Battery (CBB), as well as tau PET, volumetric MRI, the Columbia Suicide Severity Rating Scale (C-SSRS), and immunogenicity of LY3303560. This trial finished enrolling in August 2019 and will run until August 2021.
4.9. JNJ-63733657
JNJ-63733657 is a monoclonal antibody that has been described to target the mid-domain of phosphorylated tau. This antibody has high affinity for tau phosphorylated at Thr217 (pT217). Although it is not clear which exact region of tau it targets, the general mid-domain is the most abundant extracellular region of tau and likely to mediate cell to cell transmission of pathological tau. In preclinical studies, this antibody binds to tau “seeds” and prevents spread of tau pathology in a transgenic mice model (Alzforum [153]).
A two-part Phase 1 trial (NCT03375697) was initiated by Janssen in 2017. The first part is a single-ascending-dose study in healthy subjects, and the second part is a multiple-ascending-dose study in patients with prodromal or mild AD, who have a CDR Scale global rating of 0.5 or 1.0 at the time of screening and CSF consistent with AD pathology. JNJ-63733657 is being delivered intravenously to participants. Primary outcomes are safety and tolerability. Secondary outcomes are pharmacokinetic parameters and immunogenicity of JNJ-63733657 in serum and CSF. Results of this trial were presented in the 2019 AAIC conference (abstract [154]) and the 2020 CTAD conference (Alzforum [153]). Single dose administration of JNJ-63733657 was safe and well tolerated. A dose-dependent increase of serum exposure was observed. CSF exposure was about 0.2 percent of serum levels. Multiple doses were also tolerable, with similar pharmacokinetics in healthy and AD participants. Both antibody administration paradigms resulted in dose-dependent decrease of pT217 in CSF.
In 2018, a separate Phase 1 trial (NCT03689153) was initiated in healthy Japanese participants. This trial is to assess safety, tolerability, pharmacokinetics, and pharmacodynamics of JNJ-63733657 following a single ascending intravenous dose.
In November 2020, a Phase 2 study (NCT04619420) has been registered to evaluate the safety, tolerability and efficacy of this antibody in patients with early AD with elevated brain tau. This trial includes a 13-week screening study, up to 4.5 years’ treatment, and a 13-week follow-up period. Participants will receive a single dose of low or high dose antibody or placebo every 4 weeks by intravenous infusion. Primary outcome is change of ADAS-Cog13 Total Score. Secondary outcomes are RBANS, CDR-SB, ADCS-ADL-MCI, iADRS, Neuropsychiatric Inventory (NPI), Clinical Dementia Rating- Global Score (CDR-GS), brain tau burden as measured by tau PET, CSF concentrations of total, free, and bound pTau217 fragments, serum and CSF concentration of JNJ-63733657, anti-drug antibody to JNJ-63733657, adverse events, ECG, as well as clinical and functional measures. This trial will run until 2025.
4.10. UCB0107
UCB0107, named bepranemab, is a monoclonal IgG4 antibody that targets amino acids 235-246 of tau. This epitope is near the microtubule-binding domain. Similar to JNJ-63733657, targeting this mid-domain is likely to prevent propagation of pathogenic tau; therefore, it might be more efficacious to block the spread of tau pathology than antibodies that target the N-terminus. Indeed, this antibody showed greater efficacy than other antibodies in preventing pathological tau seeding and aggregation in a cell-based assay [98]. In transgenic mice, the mouse version of UCB0107 prevented the induction of tau pathology by injection of tau seeds derived from AD brain extracts [97]. In addition, it blocked the propagation of tau pathology to distal brain regions. Since the isotype of this antibody is human IgG4, it is not likely to recruit microglial phagocytosis for antibody bound tau clearance.
In February 2018, a single-ascending-dose Phase 1 study (NCT03464227) of UCB0107 was initiated in healthy subjects. Up to seven doses were administrated during a 20-week course. Primary outcome was adverse effects. Secondary outcomes were antibody exposure in blood and CSF, pharmacokinetic parameters, and immunogenicity. Results of this trial showed no treatment-related adverse events or anti-drug antibodies (press release [155]). There was a dose-dependent increase of UCB0107 in serum and CSF, and the CSF/serum ratio was constant among different doses. No anti-drug antibody was detected (abstract [156]).
A second Phase 1 trial (NCT03605082) was completed in March 2019 in 24 healthy Japanese men. This is a single dose study to examine safety, tolerability, and serum pharmacokinetics in healthy participants.
In December 2019, a placebo-controlled Phase 1 trial (NCT04185415) in 24 PSP patients began at multiple sites. The primary outcome is treatment related adverse events up to 68 weeks after administration. This study is expected to conclude in April 2022. An open-label extension study (NCT04658199) was registered in December 2020 to evaluate the long-term safety and tolerability of UCB0107 in PSP patients. Primary outcome is adverse events up to 60 months of treatment. Because this antibody is proposed to act extracellularly, the odds are against it to be efficacious in PSP because CSF tau levels are not increased in this tauopathy compared to controls, as mentioned above [30-32, 35, 39].
In July 2020, a licensing agreement was reached between UCB and Roche/Genentech to develop this antibody for Alzheimer’s disease. A Phase 2 trial (NCT04867616) is registered and expected to start in June 2021. This trial will test the efficacy, safety and tolerability of UCB0107 in patients with MCI or mild AD.
4.11. PNT001
PNT001 is a monoclonal antibody against cis-pT231. This form of pathological tau is considered to be highly neurotoxic [80, 157]. Preclinical studies have identified cis-pT231 in brain tissue from people with AD and after traumatic brain injury (TBI) [80, 158]. Cis pT231 is resistant to dephosphorylation and degradation, and it promotes tau aggregation and drives neurodegeneration [80, 158]. In an animal model of TBI, mice treated with an IgG2b mouse antibody against cis-pT231 prevented the development and spreading of tauopathy. Furthermore, it restored TBI-related structural and functional sequelae.
A Phase 1 study (NCT04096287) in healthy individuals was initiated in 2019. Six single ascending doses are being tested in this study, which assesses safety, tolerability and pharmacokinetic parameters of PNT001. Primary outcomes are adverse events and clinical and laboratory measures after 16 weeks of treatment. According to the results presented at the 2021 AD/PD conference, antibody administration led to a dose-dependent increase in blood and CSF exposure, which stayed at a consistent level for 28 days. This antibody was well tolerated (Alzforum [159]).
The company also plans to move into one or more Phase 1 trials in patients with tau pathology, including TBI, PSP or AD. Currently, a Phase 1 trial (NCT04677829) has been registered, and plans to examine the safety and tolerability of PNT001 in patients with acute TBI. Multiple ascending doses will be tested in this trial. Patients will be randomized to receive three doses of either PNT001 or placebo by intravenous infusion. Safety, tolerability, pharmacokinetic, biomarker, imaging and cognitive data will be collected over 12 weeks after the first dosing, followed by subsequent visits for dosing and safety, pharmacokinetic, biomarker and clinical assessments.
4.12. RG7345
RG7345 is a humanized monoclonal antibody targeting tau phosphorylated at serine 422 (pS422). This form of pathological tau has been linked to the mislocalization of tau to somatodendritic compartments of neurons. In preclinical studies, targeting the tau pS422 epitope with active vaccination in Thy-tau22 transgenic mice decreased levels of insoluble tau and improved performance in the Y maze [84]. Passive immunization of TauPS2APP triple transgenic mice with anti-pS422 antibodies reduced tau pathology [67]. In addition, the antibody enters neurons, and localizes in lysosomes.
In January 2015, a single-ascending-dose phase 1 trial (NCT02281786) was initiated by Roche in 48 healthy young male patients. This trial was to assess safety, tolerability, and pharmacokinetics following an intravenous infusion. However, this trial has been discontinued and the results have not been made available to the public (Alzforum [160]). Presumably, this antibody had an unfavorable pharmacokinetic profile, because no safety or efficacy concerns have been raised [1].
4.13. RO7105705
RO7105705, named semorinemab, is an IgG4 antibody developed by AC Immune and Genentech. This antibody targets extracellular tau, and has reduced effector function like all IgG4 antibodies [107]. At the 2017 AD/PD conference, Genentech reported that RO7105705 binds to the N-terminus of all six forms of human tau, in both monomeric and oligomeric forms, and independent of phosphorylation. In a preclinical study, administration of this antibody for 13 weeks reduced brain tau pathology and increased plasma tau levels in P301L tau transgenic mice. This antibody was safe in mice and cynomolgus monkeys (Alzforum [161]).
From June 2016 to June 2017, a Phase I trial (NCT02820896) of this antibody was conducted in healthy individuals and patients with mild to moderate AD. This trial compared single and multiple doses, given intravenously and subcutaneously. Primary outcomes were adverse effects, dose limiting adverse events, and suicidal ideation and behavior using the C-SSRS score. Secondary outcomes were global function based on the CDR global score, cognitive function using the MMSE, serum concentrations, and percentage of participants with anti-therapeutic antibodies. No severe adverse effects were observed in this trial, even in the highest single dose in healthy volunteers at 16,800 mg. In addition, 70 percent of subcutaneous injection was bioavailable, with the plasma and CSF concentration increased with dose. The serum half-life of RO7105705 was 32 days (abstract [162]).
Two separate Phase 2 trials have already been initiated. The first one TAURIEL (NCT03289143) enrolls prodromal or probably AD patients with a positive Aβ PET or high CSF Aβ42 levels, and mild symptoms. Placebo or one of three doses are given to participants over 18 months. A 96-week open label extension study is being conducted on participants who completed the blinded portion of the trial. Primary outcomes are safety and CDR score. Secondary outcomes are RBANS, ADAS, the Amsterdam Instrumental Activity of Daily Living questionnaire, the ADCS-ADL Inventory, as well as serum drug concentration and immunogenicity. This trial also assesses tau pathology as detected by the tau PET tracer Genentech Tau Probe 1 (GTP1) at week 73. In September 2020, the results of the placebo-controlled phase were reported. This antibody was safe. However, it failed to rescue the decline on the CDR sum of boxes or either of two secondary endpoints, the ADAS-Cog13 and ADCS-ADL (press release [163]). No change was observed in any other secondary clinical endpoints, nor did the antibody administration slow tangle accumulation. The antibody pharmacokinetics were dose-proportional (Alzforum [159]).
The second phase 2 trial LAURIET (NCT03828747) enrolls patients with moderate AD as confirmed by positive Aβ PET or high CSF Aβ42 levels, and moderate dementia. This study is still ongoing despite the results of the TAURIEL trial. It consists of a double-blind treatment, an optional open-label extension period, and a safety follow-up period. Participants will be divided into three cohorts and receive treatment for 48, 60, or 72 weeks. Primary outcome is a change in both ADAS-Cog11 and ADCS-ADL. Secondary outcomes include changes in CDR-SB and MMSE, adverse events, serum concentration and immunogenicity.
5. Conclusion and perspectives
While we wait for the results of several clinical trials, it is reasonable to be optimistic that some of these will provide functional benefits (see Table 1 for a summary of ongoing clinical trials). Overall, there are no major safety and tolerability issues with tau immunotherapies as many Phase 1 trials have been successfully completed. Although a few trials targeting primary tauopathies and one AD trial have been halted due to lack of efficacy, lessons have been learned from these failed trials.
Table 1.
Current Clinical Trials of Tau Immunotherapies
| Name | Synonyms | Therapy Type |
Tau Epitope | Isotype | Subjects (Current Status) | Primary Completion Date of Active Trials |
Company |
|---|---|---|---|---|---|---|---|
| AADvac-1 | Axon peptide 108 conjugated to KLH | Active | 294-305 | Mild to moderate Alzheimer's Disease (Phase 2 - completed), Progressive Nonfluent Aphasia (Phase 1 - active) | Nov 2020 | Axon Neuroscience SE | |
| ACI-35 | Active | p-Ser396,404 | Early Alzheimer's Disease (Phase 1 - active) | Oct 2023 | AC Immune SA, Janssen | ||
| BIIB076 | NI-105, 6C5 huIgG1/l | Passive | mid-region | IgG1 | Healthy, mild Alzheimer's Disease (Phase 1 - active) | Mar 2020 | Biogen, Neurimmune, Eisai Co., Ltd. |
| BIIB092 | Gosuranemab, BMS986168, IPN007 | Passive | 8-19 | IgG4 | Mild Alzheimer's Disease (Phase 2 - active) Progressive Supranuclear Palsy (Phase 2 - discontinued) Corticobasal Syndrome, Progressive Nonfluent Aphasia, Frontotemporal Lobar Degeneration due to tau mutations, and Traumatic Encephalopathy (Phase 1 - discontinued), | Mar 2024 | Biogen, Bristol-Meyers Squibb |
| CN2-8E12 | Tilavonemab, ABBV-8E12, HJ9.3 | Passive | 25-30 | IgG4 | Early Alzheimer's Disease (Phase 2 - active), Progressive Supranuclear Palsy (Phase 2 - discontinued), | Apr 2021, July 2026 (extension study) | AbbVie, C2N Diagnostics, LLC |
| E2814 | Passive | HVPGG motif in the microtubule binding domain, (299-303, 362-366) | IgG1 | Healthy participants (Phase 1 - active) | Oct 2020 | Eisai Co., Ltd. | |
| Lu AF87908 | Passive | p-Ser396 | IgG1 | Healthy, Alzheimer's Disease (Phase 1 - active) | May 2021 | H. Lundbeck A/S | |
| LY3303560 | Zagotenemab | Passive | conformational (7-9, 312-342) | Early Alzheimer's Disease (Phase 2 - active) | Aug 2021 | Eli Lilly & Co. | |
| JNJ-63733657 | Passive | mid-region, pT217 | Early Alzheimer's Disease (Phase 2 - active) | Mar 2025 | Janssen | ||
| UCB0107 | Bepranemab, Antibody D | Passive | 235-246 | IgG4 | Healthy participants (Phase 1 - completed), Progressive Supranuclear Palsy (Phase 1 - active) | Nov 2021, Oct 2026 (extension study) | UCB Biopharma, Genentech |
| PNT001 | Passive | cis-pT231 | Healthy participants (Phase 1 - active), Patients with acute traumatic brain injury (Phase 1 - active) | Jan 2021, July 2022 | Pinteon Therapeutics | ||
| RG7345 | RO6926496 | Passive | p-Ser422 | Healthy participants (Phase 1 - discontinued) | F. Hoffman La Roche AG | ||
| RO7105705 | Semorinemab, MTAU9937A, RG6100 | Passive | N-terminus of all six isoforms | IgG4 | Moderate Alzheimer's Disease (Phase 2 - active) | May 2021 | AC Immune SA, Genentech |
More research is needed to clarify the fundamental difference of tauopathies. AD tauopathy differs from primary tauopathies, such as PSP, Pick’s disease and corticobasal degeneration. As discussed above, CSF tau is increased in AD but not in any of the primary tauopathies, compared to controls. Thereby, targeting extracellular tau may have some benefits in AD but is unlikely to be of much use in any of the primary tauopathies. This may explain the two failed Phase 2 trials in PSP patients, and the discontinuation of the trial in other primary tauopathies, since those two antibodies reportedly only target extracellular tau. Likewise, the failure of the N-terminal antibody in early-stage AD may be explained by relatively low CSF levels of that epitope in AD, and perhaps because that antibody as well only targets extracellular tau. Considering that levels of intracellular tau are at least several thousand-fold higher than extracellular tau, the most efficacious antibodies should be able to enter neurons. Although recent cryo-EM studies have revealed distinct structures of tau fibrils among AD and other tauopathies [51, 52], this is unlikely to influence the efficacy of most of the antibodies since they target epitopes that are common to all tauopathies.
Considering that humanized antibodies may have a very different charge than mouse antibodies [40], their neuronal uptake and thereby efficacy may differ substantially compared to their mouse counterparts. Additionally, humanization of antibody parts outside the binding regions (complementary determining regions [CDRs]) can change their affinity, which also can influence efficacy [40]. Notably, higher affinity does not necessarily translate into greater efficacy [40, 56, 66]. Since it is difficult if not impossible to thoroughly evaluate efficacy of humanized tau antibodies in animal models prior to clinical trials, it is, therefore, entirely unclear if the efficacy of the humanized antibodies will relate to their mouse counterparts. In conclusion, luck may determine which of the tau antibodies in clinical trials may be successful. Active immunization in general has a greater chance of success because of the polyclonal response that leads to the generation of antibodies of different charges and different affinities, of which at least some will be efficacious. However, the likelihood of side effects is greater with that approach, which in part explains why most pharmaceutical companies have focused on the antibody approach. The other reason is a better return on investment because of a higher cost of antibodies compared to vaccines. With these trials gradually advancing into Phase 3, we will hopefully, in the coming decade, have at least a few efficacious immunotherapies targeting the tau protein in AD and other tauopathies.
Key Points.
Tau immunotherapies have advanced from proof-of-concept studies to numerous clinical trials.
How these therapies work likely involves multiple pathways.
This approach appears to be very safe in humans but its clinical efficacy remains to be determined and is likely to vary between the different trials.
A few trials have failed and this negative outcome may have been predicted based on current knowledge within the field.
Acknowledgments
EMS is supported by NIH grants R01 AG032611, R01 NS077239, R21 AG069475 and RF1 NS120488. We thank Ashley Vincenty-Acosta for help with preparing the references.
Footnotes
Conflicts of interest/Competing interests (include appropriate disclosures):
EMS is an inventor on several patents that are assigned to New York University. Some of the patents on tau immunotherapies and related diagnostics are licensed to H. Lundbeck A/S. In the last 36 months, EMS received a speaking honorarium, consulting fee and travel support to give a seminar at Biogen, Cambridge, MA.
CJ has no potential conflict of interest.
Reference
- 1.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018. Jun 12; 14:399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015. Jun;21(6):394–402. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdsson EM. Tau Immunotherapy. Neurodegener Dis. 2016;16(1-2):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arriagada PV, Growdon JH, Hedley-Whyte ET, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992. Mar;42(3 Pt 1):631–9. [DOI] [PubMed] [Google Scholar]
- 5.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010. Dec 8;30(49): 16559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutajangout A, Sigurdsson EM, Krishnamurthy PK. Tau as a therapeutic target for Alzheimer's disease. Curr Alzheimer Res. 2011. Sep;8(6):666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamohamedsait H, Rasool S, Rajamohamedsait W, et al. Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice. Sci Rep. 2017. Dec 6;7(1):17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Bai Y, Li W, et al. Increased neuronal activity in motor cortex reveals prominent calcium dyshomeostasis in tauopathy mice. Neurobiol Dis. 2021. Jan; 147:105165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandusky-Beltran LA, Sigurdsson EM. Tau immunotherapies: Lessons learned, current status and future considerations. Neuropharmacology. 2020. Sep 15; 175:108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies. J Alzheimers Dis. 2008. Oct; 15(2):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurdsson EM. Alzheimer's therapy development: A few points to consider. Prog Mol Biol Transl Sci. 2019;168:205–17. [DOI] [PubMed] [Google Scholar]
- 12.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010. Aug 6;142(3):387–97. [DOI] [PubMed] [Google Scholar]
- 13.Tapia-Rojas C, Cabezas-Opazo F, Deaton CA, et al. It's all about tau. Prog Neurobiol. 2019. Apr; 175:54–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013. Jun;12(6):609–22. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Gong C-X. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008. 2008/July/10;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong CX, Liu F, Grundke-Iqbal I, et al. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm (Vienna). 2005. Jun;112(6):813–38. [DOI] [PubMed] [Google Scholar]
- 17.Martin L, Latypova X, Terro F. Post-translational modifications of tau protein: implications for Alzheimer's disease. Neurochem Int. 2011. Mar;58(4):458–71. [DOI] [PubMed] [Google Scholar]
- 18.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986. Jul;83(13):4913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol. 2016. Jan;12(1):15–27. [DOI] [PubMed] [Google Scholar]
- 20.Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010. Dec 22;68(6):1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zempel H, Thies E, Mandelkow E, et al. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010. Sep 8;30(36):11938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia D, Li C, Gotz J. Pseudophosphorylation of Tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein Tau to dendritic spines. Biochimica et biophysica acta. 2015. May; 1852(5):913–24. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Gotz J. Somatodendritic accumulation of Tau in Alzheimer's disease is promoted by Fyn-mediated local protein translation. Embo J. 2017. Nov 2;36(21):3120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bright J, Hussain S, Dang V, et al. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015. Feb;36(2):693–709. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Holth JK, Liao F, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014. Mar 10;211(3):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K, Cirrito JR, Stewart FR, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011. Sep 14;31(37):13110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holth JK, Fritschi SK, Wang C, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019. Jan 24;363:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai X, Dage JL, Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis. 2012. Dec;48(3):356–66. [DOI] [PubMed] [Google Scholar]
- 29.Han P, Serrano G, Beach TG, et al. A Quantitative Analysis of Brain Soluble Tau and the Tau Secretion Factor. J Neuropathol Exp Neurol. 2017. Jan 1;76(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthelemy NR, Gabelle A, Hirtz C, et al. Differential Mass Spectrometry Profiles of Tau Protein in the Cerebrospinal Fluid of Patients with Alzheimer's Disease, Progressive Supranuclear Palsy, and Dementia with Lewy Bodies. J Alzheimers Dis. 2016;51(4):1033–43. [DOI] [PubMed] [Google Scholar]
- 31.Colin M, Dujardin S, Schraen-Maschke S, et al. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020. Jan;139(1):3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlin D, Irwin DJ. Emerging Diagnostic and Therapeutic Strategies for Tauopathies. Curr Neurol Neurosci Rep. 2017. Sep;17(9):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hales CM, Hu WT. From frontotemporal lobar degeneration pathology to frontotemporal lobar degeneration biomarkers. Int Rev Psychiatry. 2013. Apr;25(2):210–20. [DOI] [PubMed] [Google Scholar]
- 34.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016. Jun;15(7):673–84. [DOI] [PubMed] [Google Scholar]
- 35.Sigurdsson EM. Tau Immunotherapies for Alzheimer's Disease and Related Tauopathies: Progress and Potential Pitfalls. J Alzheimers Dis. 2018;64(s1):S555–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigurdsson EM, Congdon EE, Asuni AA, et al. Comment on “Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement”. Aug 27, 2016. Available from: https://www.alzforum.org/papers/antibody-mediated-targeting-tau-vivo-does-not-require-effector-function-and-microglial. Accessed May 19, 2021. [Google Scholar]
- 37.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008. May 6;70(19 Pt 2):1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol. 2005. May;57(5):721–9. [DOI] [PubMed] [Google Scholar]
- 39.Wagshal D, Sankaranarayanan S, Guss V, et al. Divergent CSF τ alterations in two common tauopathies: Alzheimer's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015. Mar;86(3):244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Congdon EE, Chukwu JE, Shamir DB, et al. Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy. EBioMedicine. 2019. Apr;42:157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicognola C, Brinkmalm G, Wahlgren J, et al. Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease. Acta Neuropathol. 2019. Feb;137(2):279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meredith JE Jr., Sankaranarayanan S, Guss V, et al. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer's disease. PLoS One. 2013;8(10):e76523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato C, Barthelemy NR, Mawuenyega KG, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018. Mar 21;97(6):1284–98 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta U, Portelius E, Hansson O, et al. Tau oligomers in cerebrospinal fluid in Alzheimer's disease. Ann Clin Transl Neurol. 2017. Apr;4(4):226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada K. Extracellular Tau and Its Potential Role in the Propagation of Tau Pathology. Front Neurosci. 2017;11:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerson JE, Kayed R. Formation and propagation of tau oligomeric seeds. Front Neurol. 2013;4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerson JE, Sengupta U, Lasagna-Reeves CA, et al. Characterization of tau oligomeric seeds in progressive supranuclear palsy. Acta Neuropathol Commun. 2014. Jun 14;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009. Jul;11(7):909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goedert M, Spillantini MG. Propagation of Tau aggregates. Mol Brain. 2017. May 30;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature. 2017. Jul 13;547(7662):185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falcon B, Zhang W, Murzin AG, et al. Structures of filaments from Pick's disease reveal a novel tau protein fold. Nature. 2018. Sep;561(7721):137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falcon B, Zivanov J, Zhang W, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019. Apr;568(7752):420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arakhamia T, Lee CE, Carlomagno Y, et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell. 2020. Feb 20;180(4):633–44.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kametani F, Yoshida M, Matsubara T, et al. Comparison of Common and Disease-Specific Post-translational Modifications of Pathological Tau Associated With a Wide Range of Tauopathies. Front Neurosci. 2020;14:581936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fa M, Puzzo D, Piacentini R, et al. Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci Rep. 2016. Jan 20;6:19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Congdon EE, Lin Y, Rajamohamedsait HB, et al. Affinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy. Mol Neurodegener. 2016. Aug 30;11(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sebastian-Serrano A, de Diego-Garcia L, Diaz-Hernandez M. The Neurotoxic Role of Extracellular Tau Protein. Int J Mol Sci. 2018. Mar 27;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asuni AA, Boutajangout A, Quartermain D, et al. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007. Aug 22;27(34):9115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Congdon EE, Gu J, Sait HB, et al. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcgamma receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013. Dec 6;288(49):35452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu J, Congdon EE, Sigurdsson EM. Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J Biol Chem. 2013. Nov 15;288(46):33081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnamurthy PK, Deng Y, Sigurdsson EM. Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model. Front Psychiatry. 2011;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnaswamy S, Lin Y, Rajamohamedsait WJ, et al. Antibody-derived in vivo imaging of tau pathology. J Neurosci. 2014. Dec 10;34(50):16835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shamir DB, Deng Y, Sigurdsson EM. Live Imaging of Pathological Tau Protein and Tau Antibodies in a Neuron-Like Cellular Model. Methods Mol Biol. 2018;1779:371–9. [DOI] [PubMed] [Google Scholar]
- 64.Shamir DB, Deng Y, Wu Q, et al. Dynamics of Internalization and Intracellular Interaction of Tau Antibodies and Human Pathological Tau Protein in a Human Neuron-Like Model. Front Neurol. 2020;11:602292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shamir DB, Rosenqvist N, Rasool S, et al. Internalization of tau antibody and pathological tau protein detected with a flow cytometry multiplexing approach. Alzheimers Dement. 2016. Oct;12(10):1098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q, Lin Y, Gu J, et al. Dynamic assessment of tau immunotherapies in the brains of live animals by two-photon imaging. EBioMedicine. 2018. Sep;35:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collin L, Bohrmann B, Gopfert U, et al. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain. 2014. Oct;137(Pt 10):2834–46. [DOI] [PubMed] [Google Scholar]
- 68.Yanamandra K, Jiang H, Mahan TE, et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015. Mar;2(3):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, et al. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014. Mar 19;34(12):4260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.d'Abramo C, Acker CM, Jimenez H, et al. Passive Immunization in JNPL3 Transgenic Mice Using an Array of Phospho-Tau Specific Antibodies. PLoS One. 2015;10(8):e0135774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McEwan WA, Falcon B, Vaysburd M, et al. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A. 2017. Jan 17; 114(3):574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andersson CR, Falsig J, Stavenhagen JB, et al. Antibody-mediated clearance of tau in primary mouse microglial cultures requires Fcγ-receptor binding and functional lysosomes. Sci Rep. 2019. Mar 15;9(1):4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funk KE, Mirbaha H, Jiang H, et al. Distinct Therapeutic Mechanisms of Tau Antibodies: Promoting Microglial Clearance Versus Blocking Neuronal Uptake. J Biol Chem. 2015. Aug 28;290(35):21652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo W, Liu W, Hu X, et al. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci Rep. 2015. Jun 9;5:11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castillo-Carranza DL, Gerson JE, Sengupta U, et al. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J Alzheimers Dis. 2014;40 Suppl 1:S97–S111. [DOI] [PubMed] [Google Scholar]
- 76.Chai X, Wu S, Murray TK, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011. Sep 30;286(39):34457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai CL, Chen X, Kazim SF, et al. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J Neural Transm (Vienna). 2015. Apr;122(4):607–17. [DOI] [PubMed] [Google Scholar]
- 78.Dai CL, Tung YC, Liu F, et al. Tau passive immunization inhibits not only tau but also Abeta pathology. Alzheimers Res Ther. 2017. Jan 10;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davtyan H, Chen WW, Zagorski K, et al. MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice. Vaccine. 2017. Apr 11;35(16):2015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kondo A, Shahpasand K, Mannix R, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015. Jul 23;523(7561):431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nobuhara CK, DeVos SL, Commins C, et al. Tau Antibody Targeting Pathological Species Blocks Neuronal Uptake and Interneuron Propagation of Tau in Vitro. Am J Pathol. 2017. Jun;187(6):1399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sankaranarayanan S, Barten DM, Vana L, et al. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One. 2015;10(5):e0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subramanian S, Savanur G, Madhavadas S. Passive immunization targeting the N-terminal region of phosphorylated tau (residues 68-71) improves spatial memory in okadaic acid induced tauopathy model rats. Biochem Biophys Res Commun. 2017. Jan 29;483(1):585–9. [DOI] [PubMed] [Google Scholar]
- 84.Troquier L, Caillierez R, Burnouf S, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012. May;9(4):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Umeda T, Eguchi H, Kunori Y, et al. Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann Clin Transl Neurol. 2015. Mar;2(3):241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walls KC, Ager RR, Vasilevko V, et al. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice. Neurosci Lett. 2014. Jul 11;575:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013. Oct 16;80(2):402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yanamandra K, Patel TK, Jiang H, et al. Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci Transl Med. 2017. Apr 19;9(386). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Modak SR, Sigurdsson EM. Antibodies targeting truncated Asp421 tau protein clear human Alzheimer’s tau and prevent its toxicity in primary neuronal and mixed cortical cultures. Society for Neuroscience Abstract. 2017;478.19. [Google Scholar]
- 90.Modak SR, Solesio M, Krishnaswamy S, et al. Antibodies targeting truncated tau protein reduce tau pathology in primary and mixed cortical cultures. Society for Neuroscience Abstract. 2015; 579.14. [Google Scholar]
- 91.Bi M, Ittner A, Ke YD, et al. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6(12):e26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ittner A, Bertz J, Suh LS, et al. Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice. J Neurochem. 2015. Jan;132(1):135–45. [DOI] [PubMed] [Google Scholar]
- 93.Liu W, Zhao L, Blackman B, et al. Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice. J Neurosci. 2016. Dec 7;36(49):12425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenqvist N, Asuni AA, Andersson CR, et al. Highly specific and selective anti-pS396-tau antibody C10.2 targets seeding-competent tau. Alzheimers Dement (N Y). 2018;4:521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agadjanyan MG, Zagorski K, Petrushina I, et al. Humanized monoclonal antibody armanezumab specific to N-terminus of pathological tau: characterization and therapeutic potency. Mol Neurodegener. 2017. May 5;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Albert M, Mairet-Coello G, Danis C, et al. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain. 2019. Jun 1;142(6):1736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Courade JP, Angers R, Mairet-Coello G, et al. Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer Tau. Acta Neuropathol. 2018. Nov;136(5):729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kontsekova E, Zilka N, Kovacech B, et al. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther. 2014;6(4):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alzforum.org. Therapeutics; AADvac1 Apr 17, 2020. Available from: https://www.alzforum.org/therapeutics/aadvac1 Accessed May 19, 2021.
- 101.Alzforum.org. Therapeutics; PNT001. Apr 23, 2021. Available from: https://www.alzforum.org/therapeutics/pnt001 Accessed May 19, 2021.
- 102.Alzforum.org. Therapeutics; Zagotenemab. Sep 29, 2020. Available from: https://www.alzforum.org/therapeutics/zagotenemab Accessed May 19, 2021.
- 103.Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004. Jan;18(1):182–4. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez-Vizarra P, Lopez-Franco O, Mallavia B, et al. Immunoglobulin G Fc receptor deficiency prevents Alzheimer-like pathology and cognitive impairment in mice. Brain. 2012. Sep;135(Pt 9):2826–37. [DOI] [PubMed] [Google Scholar]
- 105.Suemitsu S, Watanabe M, Yokobayashi E, et al. Fcgamma receptors contribute to pyramidal cell death in the mouse hippocampus following local kainic acid injection. Neuroscience. 2010. Mar 31;166(3):819–31. [DOI] [PubMed] [Google Scholar]
- 106.van der Kleij H, Charles N, Karimi K, et al. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J Allergy Clin Immunol. 2010. Mar;125(3):757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SH, Le Pichon CE, Adolfsson O, et al. Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell Rep. 2016. Aug 9;16(6):1690–700. [DOI] [PubMed] [Google Scholar]
- 108.Boutajangout A, Ingadottir J, Davies P, et al. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011. Aug;118(4):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.d'Abramo C, Acker CM, Jimenez HT, et al. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One. 2013;8(4):e62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jicha GA, Bowser R, Kazam IG, et al. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997. Apr 15;48(2):128–32. [DOI] [PubMed] [Google Scholar]
- 111.Krishnaswamy S, Wu Q, Lin Y, et al. In Vivo Imaging of Tauopathy in Mice. Methods Mol Biol. 2018;1779:513–26. [DOI] [PubMed] [Google Scholar]
- 112.Krishnaswamy S, Huang HW, Marchal IS, et al. Neuronally expressed anti-tau scFv prevents tauopathy-induced phenotypes in Drosophila models. Neurobiol Dis. 2020. Apr; 137:104770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ising C, Gallardo G, Leyns CEG, et al. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J Exp Med. 2017. May 1;214(5):1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nisbet RM, Van der Jeugd A, Leinenga G, et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain. 2017. May 1;140(5):1220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spencer B, Bruschweiler S, Sealey-Cardona M, et al. Selective targeting of 3 repeat Tau with brain penetrating single chain antibodies for the treatment of neurodegenerative disorders. Acta Neuropathol. 2018. Jul;136(1):69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vitale F, Giliberto L, Ruiz S, et al. Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice. Acta Neuropathol Commun. 2018. Aug 22;6(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gallardo G, Wong CH, Ricardez SM, et al. Targeting tauopathy with engineered tau-degrading intrabodies. Mol Neurodegener. 2019. Oct 22;14(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goodwin MS, Sinyavskaya O, Burg F, et al. Anti-tau scFvs Targeted to the Cytoplasm or Secretory Pathway Variably Modify Pathology and Neurodegenerative Phenotypes. Mol Ther. 2021. Feb 3;29(2):859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dupre E, Danis C, Arrial A, et al. Single domain antibody fragments as new tools for the detection of neuronal tau protein in cells and in mice studies. ACS Chem Neurosci. 2019. Sep 18;10(9):3997–4006. [DOI] [PubMed] [Google Scholar]
- 120.Li T, Vandesquille M, Koukouli F, et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J Control Release. 2016. Dec 10;243:1–10. [DOI] [PubMed] [Google Scholar]
- 121.Congdon EE, Lin Y, Sigurdsson EM. Prevention of intra- and extracellular alpha-synuclein toxicity and seeding by single domain antibodies. Society for Neuroscience Abstract. 2019;537.07. [Google Scholar]
- 122.Marchal IS, Huang HW, Krishnaswamy S, et al. Neuronally expressed anti-tau scFvs and sdAbs prevent tauopathy-induced phenotypes in Drosophila models. Society for Neuroscience Abstract. 2019;446.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sandusky-Beltran LA, Congdon EE, Modak SR, et al. Examining the impact of single domain anti-tau immunotherapies in an animal model of tauopathy. Alzheimer’s disease & Parkinson’s disease (ADPD) Conference Abstract. 2019;Symposium 14: Treatment of Tauopathies. [Google Scholar]
- 124.Novak P, Schmidt R, Kontsekova E, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16(2):123–34. [DOI] [PubMed] [Google Scholar]
- 125.Novak P, Schmidt R, Kontsekova E, et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease. Alzheimers Res Ther. 2018. Oct 24;10(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Axon announces positive results from Phase II ADAMANT trial for AADvac1 in Alzheimer’s Disease. Sep 9, 2019. [press release]. Available from: https://www.axon-neuroscience.eu/docs/press_release_Axon_announces_positive_result_9-9-2019.pdf Accessed May 19, 2021.
- 127.Alzforum.org. Active tau vaccine: hints of slowing neurodegeneration. AAT-AD/PD™ 2020 Conference: Advances in Alzheimer's and Parkinson's Therapies. Apr 15, 2020. [conference coverage series]. Available from: https://www.alzforum.org/news/conference-coverage/active-tau-vaccine-hints-slowing-neurodegeneration Accessed May 19, 2021. [Google Scholar]
- 128.Hickman DT, Lopez-Deber MP, Ndao DM, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011. Apr 22;286(16):13966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.AC Immune advances phospho-Tau Alzheimer’s vaccine in Phase 1b/2a Study. July 16, 2020. [press release]. Available from: https://ir.acimmune.com/news-releases/news-release-details/ac-immune-advances-phospho-tau-alzheimers-vaccine-phase-1b2a Accessed May 19, 2021.
- 130.AC Immune’s Alzheimer’s vaccine generates potent anti-pTau antibody response in a Phase 1b/2a Study. Feb 11, 2021. [press release]. Available from: https://ir.acimmune.com/news-releases/news-release-details/ac-immunes-alzheimers-vaccine-generates-potent-anti-ptau Accessed May 19, 2021.
- 131.Alzforum.org. Therapeutics; ACI-35. Feb 25, 2021. Available from: https://www.alzforum.org/therapeutics/aci-35 Accessed May 19, 2021.
- 132.AC Immune Announces Expansion of Phase 1b/2a phospho-Tau Alzheimer’s Vaccine Trial and Provides a Program Update. May 17, 2021. [press release]. Available from: https://www.globenewswire.com/news-release/2021/05/17/2230554/0/en/AC-Immune-Announces-Expansion-of-Phase-1b-2a-phospho-Tau-Alzheimer-s-Vaccine-Trial-and-Provides-a-Program-Update.html Accessed May 19, 2021.
- 133.Alzforum.org. Therapeutics; BIIB076. Sep 29, 2020. Available from: https://www.alzforum.org/therapeutics/biib076 Accessed May 19, 2021.
- 134.Czerkowicz J, Chen W, Wang Q, et al. Pan-tau antibody BIIB076 exhibits promising safety and biomarker profile in cynomolgus monkey toxicity study. Alzheimers Dement. 2017;13(7):P1271. [Google Scholar]
- 135.Alzforum.org. Therapeutics; Gosuranemab. Nov 13, 2020. Available from: https://www.alzforum.org/therapeutics/gosuranemab Accessed May 19, 2021.
- 136.Sopko R, Golonzhka O, Arndt J, et al. Characterization of tau binding by gosuranemab. Neurobiol Dis. 2020. Dec;146:105120. [DOI] [PubMed] [Google Scholar]
- 137.Qureshi IA, Tirucherai G, Ahlijanian MK, et al. A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimers Dement (N Y). 2018;4:746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boxer AL, Qureshi I, Ahlijanian M, et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 2019;18(6):549–58. [DOI] [PubMed] [Google Scholar]
- 139.Biogen reports top-line results from Phase 2 study in progressive supranuclear palsy. Dec 13, 2019. [press release]. Available from: https://investors.biogen.com/news-releases/news-release-details/biogen-reports-top-line-results-phase-2-study-progressive Accessed May 19, 2021.
- 140.Alzforum.org. Gosuranemab, Biogen’s anti-tau immunotherapy, does not fly for PSP. Dec 13, 2019. Available from: https://www.alzforum.org/news/research-news/gosuranemab-biogens-anti-tau-immunotherapy-does-not-fly-psp Accessed May 19, 2021.
- 141.Sigurdsson EM. Comment on “Nice Catch? Antibodies Stabilize Tau in the Blood; Mark Levels in Brain”. Apr 20, 2017. Available from: https://www.alzforum.org/news/research-news/nice-catch-antibodies-stabilize-tau-blood-mark-levels-brain#comment-23756 Accessed May 19, 2021. [Google Scholar]
- 142.Alzforum.org. Therapeutics; Tilavonemab. Apr 23, 2021. Available from: https://www.alzforum.org/therapeutics/tilavonemab Accessed May 19, 2021.
- 143.Kfoury N, Holmes BB, Jiang H, et al. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012. Jun 01;287(23):19440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.West T, Hu Y, Verghese PB, et al. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer's Disease and Other Tauopathies. J Prev Alzheimers Dis. 2017;4(4):236–41. [DOI] [PubMed] [Google Scholar]
- 145.Alzforum.org. AbbVie’s tau antibody flops in progressive supranuclear palsy. Jul 26, 2019. Available from: https://www.alzforum.org/news/research-news/abbvies-tau-antibody-flops-progressive-supranuclear-palsy Accessed May 19, 2021.
- 146.Höglinger GU, Litvan I, Mendonca N, et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial. Lancet Neurol. 2021. Mar;20(3):182–92. [DOI] [PubMed] [Google Scholar]
- 147.Roberts M, Sevastou I, Imaizumi Y, et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease. Acta Neuropathol Commun. 2020. Feb 4;8(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eisai presents data showing quantification of tau microtubule binding region in cerebrospinal fluid and the identification of a target engagement biomarker for the new anti-tau antibody e2814 at alzheimer’s association international conference (AAIC) 2019. July 19, 2019. [press release]. Available from: https://www.eisai.com/news/2019/news201955.html Accessed May 19, 2021.
- 149.Horie K, Barthélemy NR, Sato C, et al. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021. Mar 3;144(2):515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Horie K, Takahashi E, Aoyama M, et al. Quantification of the Tau Microtubule Binding Region (Mtbr) in Cerebrospinal Fluid and Subsequent Validation of Target Engagement Assay for E2814, a Novel Anti - Tau Therapeutic Antibody. Alzheimer's Association International Conference, July 2019; 2019. p. P4–696. [Google Scholar]
- 151.Alzforum.org. Aiming at the tangle’s heart? DIAN-TU trial to torpedo tau’s core. Mar 18, 2021. Available from: https://www.alzforum.org/news/research-news/aiming-tangles-heart-dian-tu-trial-torpedo-taus-core Accessed May 19, 2021.
- 152.Alam R, Driver D, Wu S, et al. Preclinical Characterization of an Antibody [Ly3303560] Targeting Aggregated Tau. Alzheimers Dement 2017;13(7):P592–P3. [Google Scholar]
- 153.Alzforum.org. Therapeutics; JNJ-63733657. Jan 11, 2021. Available from: https://www.alzforum.org/therapeutics/jnj-63733657 Accessed May 19, 2021.
- 154.Galpern WR, Mercken M, Kolen KV, et al. P1 - 052: A single ascending dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the anti - phospho - tau antibody JNJ - 63733657 in healthy subjects. Alzheimers Dement. 2019;15(7S):252–3. [Google Scholar]
- 155.UCB presents UCB0107 anti-Tau immunotherapy Phase I study results at World Movement Disorders Conference. Sep 25, 2019. [press release]. Available from: https://www.ucb.com/stories-media/Press-Releases/article/UCB-presents-UCB0107-anti-Tau-immunotherapy-Phase-I-study-results-at-World-Movement-Disorders-Conference Accessed May 19, 2021.
- 156.Buchanan T, De Bruyn S, Fadini T, et al. A randomised, placebo-controlled, first-inhuman study with a central Tau epitope antibody – UCB0107. International Congress of Parkinson’s Disease and Movement Disorders, 2019 Late-Breaking Abstracts. 2019;LBA3. [Google Scholar]
- 157.Naserkhaki R, Zamanzadeh S, Baharvand H, et al. cis pT231-Tau Drives Neurodegeneration in Bipolar Disorder. ACS Chem Neurosci. 2019. Mar 20;10(3):1214–21. [DOI] [PubMed] [Google Scholar]
- 158.Albayram O, Kondo A, Mannix R, et al. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat Commun. 2017. Oct 17;8(1): 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alzforum.org. N-terminal tau antibodies fade, mid-domain ones push to the fore. International Conference on Alzheimer's and Parkinson's Diseases 2021 (Virtual). Mar 27, 2021. [conference coverage]. Available from: https://www.alzforum.org/news/conference-coverage/n-terminal-tau-antibodies-fade-mid-domain-ones-push-fore Accessed May 19, 2021. [Google Scholar]
- 160.Alzforum.org. Therapeutics; RG7345. Nov 20, 2015. Available from: https://www.alzforum.org/therapeutics/rg7345 Accessed May 19, 2021.
- 161.Alzforum.org. Treating tau: finally, clinical candidates are stepping into the ring. AD/PD 2017 Draws Record Number of Scientists To Vienna. Apr 27, 2017. Available from: https://www.alzforum.org/news/conference-coverage/treating-tau-finally-clinical-candidates-are-stepping-ring Accessed May 19, 2021. [Google Scholar]
- 162.Kerchner GA, Ayalon G, Brunstein F, et al. A Phase I study to evaluate the safety and tolerability of RO7105705 in healthy volunteers and patients with mild-to-moderate AD. Alzheimers Dement 2017;13(7): P601–P601. [Google Scholar]
- 163.AC Immune reports top line results from TAURIEL Phase 2 trial evaluating Semorinemab in early Alzheimer’s Disease. Sep 23, 2020. [press release]. Available from: https://ir.acimmune.com/static-files/7296e650-85ea-4151-aca5-6f63ec71653c Accessed May 19, 2021.