Abstract
Introduction
Intramuscular corticosteroids (IMC) have gained popularity for the treatment of severe alopecia areata (AA) in recent years; however, evidence on their efficacy and safety is still limited.
Objective
To evaluate the efficacy, relapse rate, and tolerability of IMC in the treatment of AA, as well as factors associated with treatment outcomes.
Methods
Time-to-event analysis was performed on patients with severe, extensive, or rapidly progressive AA receiving IMC. The IMC regimen comprised triamcinolone acetonide 20–40 mg/mL injected every 4–6 weeks. The evaluated outcomes included initial (25% regrowth), significant (75% regrowth), and complete hair regrowth (100% regrowth). Relapse and adverse events were also noted. Factors associated with treatment outcomes and relapse were analyzed using the Cox proportional hazards model.
Results
A total of 101 patients were eligible for analysis. Significant hair regrowth was obtained in 80.2% of the patients (n = 81), in a median time of 3.4 months (95% confidence interval [CI] = 2.9–4.4). Complete hair regrowth was achieved in 48.5% of the subjects (n = 49), and relapse was observed in 47.5% (n = 48). Acneiform eruption was the most common adverse effect. Multivariable analysis revealed that nail involvement was a negative predictor of significant hair regrowth (adjusted hazard ratio [HR] = 0.04, 95% CI = 0.01–0.55; P = 0.015), whereas duration of AA longer than 6 months was associated with disease recurrence (adjusted HR = 4.02, 95% CI = 1.52–4.66; P = 0.005).
Conclusion
This study demonstrated the efficacy and safety of IMC in the treatment of severe or active AA; however, the relapse rate remained relatively high after discontinuation of the therapy. Nail involvement was a negative predictor of significant hair regrowth, while disease duration longer than 6 months predicted AA relapse.
Keywords: acute diffuse and total alopecia, alopecia totalis, alopecia universalis, hair loss, injection, systemic
Introduction
Alopecia areata (AA) is a non-scarring hair loss disorder that affects the scalp and hair-bearing areas.1 Variations on the clinical presentation of AA have been observed, ranging from small, well-defined circular or oval patches of hair loss to diffuse alopecia and complete scalp (alopecia totalis, AT) or total body hair loss (alopecia universalis, AU).2–5 Nail abnormalities, most frequently nail pitting, are occasionally present. Patients with AA often experience major psychological impacts due to the unpredictable clinical course and variable treatment response.6 Various immune-related comorbidities such as autoimmune thyroid diseases (AITD), vitiligo, psoriasis, and atopic diseases have been linked to AA.7–13
The exact etiopathogenesis of AA is unclear, though it is believed to be a cell-mediated autoimmune disease of the hair follicle. Autoreactive T-cell lymphocytes and several cytokines mediate hair follicular destruction and negatively affect the hair growth cycle.14–16 Despite the absence of curative/preventive treatments and FDA-approved regimens for AA, therapeutic approaches have been established through treatment guidelines. Topical and intralesional (IL) corticosteroids, topical minoxidil, and topical anthralin are recommended for AA patients with <50% scalp involvement, whereas in those with ≥50% hair loss, topical contact immunotherapy and systemic corticosteroids are advised.17–23
Corticosteroids, the main treatment option for AA, can be administered in different forms depending on the disease severity.24 Medium- to high-potency topical and IL corticosteroids are indicated as monotherapy or combined therapy in limited and/or slowly progressive AA,25–27 while systemic corticosteroids (eg, oral, intravenous, and intramuscular), are preferred in extensive and/or rapidly progressive disease.17
Among systemic modalities, intramuscular corticosteroids (IMC) may be most beneficial, as its monthly or bimonthly regimen increases patients’ compliance and treatment adherence.28 Moreover, IMC could minimize adverse effects from slowly releasing continuous and small amounts of medication.28 Previous studies of IMC showed a satisfactory outcome in severe and refractory AA, with response rates between 63% and 72.7%.29–31 Although IMC is often the preferred treatment for AA, there are limited data regarding its effectiveness, safety, dosing, and optimal duration. Thus, we conducted a retrospective cohort study based on our 20-year experience to evaluate the efficacy, relapse, and tolerability, as well as factors associated with treatment outcomes of IMC in patients with AA.
Materials and Methods
Study Design and Population
This was a retrospective cohort study conducted at the Dermatology Clinic of Ramathibodi Hospital, Bangkok, Thailand. The study was approved by the Mahidol University Review Board for Ethics in Human Research (MURA2020/1282) and was performed in accordance with the principles of Declaration of Helsinki. The requirement for informed consent was waived, and the data were anonymized before analysis.
We reviewed the medical records of patients clinically and/or histopathologically diagnosed with AA between January 2000 and December 2019. Individuals with AA receiving an IMC treatment regimen were included in the study. Our IMC protocol was triamcinolone acetonide 20–40 mg/mL injected every 4–6 weeks. The treatment was indicated in patients with severe (≥50% scalp involvement), extensive (AT, AU, and ≥50% scalp involvement with body hair loss), or rapidly progressive AA (accelerated hair loss spreading ≥5% of scalp area per week for >2 consecutive weeks with positive hair-pull test results at the periphery of alopecic patch or across the entire scalp), and IMC was performed until patients achieved significant hair regrowth. Patients who did not achieve significant hair regrowth after six IMC injections were switched to other appropriate therapies to minimize adrenocortical alteration.32 We excluded participants treated with systemic immunosuppressive agents or topical contact immunotherapy within 6 months after discontinuation of the IMC protocol; therefore, those who underwent topical treatment (ie, topical corticosteroids and/or topical minoxidil) remained in our cohort. Patients with incomplete medical records, those who received other AA treatments during IMC therapy, those with other hair and scalp disorders, and those with systemic diseases or using medications affecting the hair growth cycle were also excluded.
Data Collection and Clinical Outcomes
Baseline demographics, clinical characteristics, duration of disease, AA subtypes (ie, patch, multiple patches, AT/AU, and ophiasis), body hair involvement, nail abnormalities, and family history of AA were collected. AA severity was evaluated using the Severity of Alopecia Tool (SALT) score. Details regarding IMC regimen were obtained, including dosage, duration, and clinical outcomes. Efficacy and tolerability assessment were performed at baseline and at every follow-up visit. The percentage of scalp area coverage by terminal hair regrowth from baseline was used to evaluate treatment response. Clinical endpoints included initial, significant, and complete hair regrowth, treatment failure, and relapse. Initial hair regrowth (noticeable) was defined as 25% regrowth, while significant hair regrowth (cosmetically acceptable) was defined as 75% regrowth. Patients with 100% regrowth were classified as having complete (full) hair regrowth. Treatment failure was defined as <10% hair regrowth after 6 months of IMC therapy. Relapse was defined as any new appearance of AA during follow-up visits. The cumulative incidence of significant hair regrowth was set as the outcome of interest in the time-to-event analysis. Furthermore, the median time and median cumulative corticosteroid dosage to achieve each clinical endpoint were evaluated. Relevant factors affecting significant hair regrowth and relapse were also analyzed.
Statistical Analysis
Statistical analyses were performed using Stata 14.1 (Stata Corp, College Station, TX, USA). To detect a modestly sized hazard ratio (HR), the sample size was calculated based on data from a previous study regarding the IMC treatment of patients with AA.31 The minimum number of subjects required to achieve a statistical significance level of 5% and a power of 80% was 26. Continuous variables were reported as mean ± standard deviation or median (interquartile range, IQR). Categorical variables were expressed as number or proportion. Survival analysis was performed using the Kaplan–Meier method to estimate outcome rate, median time, and median cumulative corticosteroids to achieve endpoints. The log rank test was used to compare survival distributions between variables. Factors affecting clinical outcomes were assessed using univariable and multivariable stepwise analyses under the Cox proportional hazards regression model and expressed as HRs with 95% confidence intervals (CI). All analyses were two-tailed, and P-values <0.05 were considered statistically significant.
Results
Demographics
A total of 126 patients with AA treated with IMC were initially included. Twenty-five patients were excluded due to coexisting diseases or concomitant use of other medications affecting the hair growth cycle (n = 16), incomplete medical records (n = 5), or because they received systemic treatment after the IMC protocol (n = 4). The majority of the remaining 101 patients were female (n = 70, 69.3%) with a female to male ratio of 2.26:1. The age range of AA onset was 5 to 68 years (mean = 40.4 ± 12.6 years), and the duration of disease varied from 1 week to 11 months (median = 2 [1–4] months). The most common AA subtype was multiple patches (n = 46, 45.5%), followed by patch (n = 20, 19.8%), diffuse (n = 16, 15.8%), AT (n = 9, 8.9%), AU (n = 5, 5.0%), and ophiasis (n = 5, 5.0%). The estimated baseline SALT score was 0–25%, 26–50%, 51–75%, or 76–100% in 11 (10.9%), 16 (15.8%), 60 (59.4%), and 14 patients (13.9%), respectively. Body hair involvement was found in 5.9% (n = 6), and nail abnormalities were observed in 8.9% (n = 9) of the patients. Six patients (5.9%) had coexisting AITD, 2 (1.9%) were atopic, and 2 (1.9%) had a family member with AA. All demographics are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics of Participants with Alopecia Areata
| Variables | N = 101 |
|---|---|
| Sex, n (%) | |
| ● Male | 31 (30.7) |
| ● Female | 70 (69.3) |
| Age at AA onset, year, mean ± SD | 40.4 ± 12.6 |
| Age at AA onset, year, n (%) | |
| ● ≤40 years | 50 (49.5) |
| ● >40 years | 51 (50.5) |
| Duration of disease, month, median (IQR) | 2 (1–4) |
| Duration of disease, n (%) | |
| ● ≤6 months | 77 (76.2) |
| ● >6 months | 24 (23.8) |
| AA subtype, n (%) | |
| ● Patch AA | 20 (19.8) |
| ● Multiple AA | 46 (45.5) |
| ● Diffuse AA | 16 (15.8) |
| ● AT | 9 (8.9) |
| ● AU | 5 (5.0) |
| ● Ophiasis | 5 (5.0) |
| Severity of AA (SALT score), n (%) | |
| ● 0–25% | 11 (10.9) |
| ● 26–50% | 16 (15.8) |
| ● 51–75% | 60 (59.4) |
| ● 76–100% | 14 (13.9) |
| Body hair involvement, n (%) | 6 (5.9) |
| Nail involvement, n (%) | 9 (8.9) |
| Comorbidity, n (%) | |
| ● Atopy | 2 (1.9) |
| ● Autoimmune thyroid diseases | 6 (5.9) |
| Family history of AA, n (%) | 2 (1.9) |
Abbreviations: AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis; IQR, interquartile range; SALT, Severity of Alopecia Tool; SD, standard deviation.
Overall Response
The median overall follow-up time for this cohort was 20 (6–38) months. Patients received 1 to 6 IMC injections (mean = 3.12 ± 0.9) per treatment course. Ninety-eight patients (97.1%) showed improvement, whereas 3 patients (2.9%) showed treatment failure and were further treated by topical contact immunotherapy at approximately 9 months (36 weeks in 2 patients and 37 weeks in another one) after completion of IMC therapy. The mean duration of IMC therapy was 4.2 ± 1.1 months, with a range of 1 to 6 months. Treatment responses are summarized in Table 2.
Table 2.
Treatment Outcomes of Intramuscular Corticosteroid Therapy in Participants with Alopecia Areata
| Treatment Outcomes | N = 101 |
|---|---|
| Treatment failure (<10% regrowth) | |
| ● Incidence, n (%) | 3 (2.9) |
| Initial hair regrowth (25% regrowth) | |
| ● Cumulative incidence, n (%) | 86 (85.1) |
| ● Incidence rate, person-year (CI) | 64.0 (51.9–79.3) |
| ● Median time to achieve endpoint, month (CI) | 0.9 (0.8–1.2) |
| ● Median cumulative dose to achieve endpoint, mg | 100 |
| ● Cumulative dose to achieve endpoint, mg, median (IQR) | 80 (20–160) |
| Significant hair regrowth (75% regrowth) | |
| ● Cumulative incidence, n (%) | 81 (80.2) |
| ● Incidence rate, person-year (CI) | 17.0 (13.6–21.7) |
| ● Median time to achieve endpoint, month (CI) | 3.4 (2.9–4.4) |
| ● Median cumulative dose to achieve endpoint, mg | 200 |
| ● Cumulative dose to achieve endpoint, mg, median (IQR) | 120 (40–200) |
| Complete hair regrowth (100% regrowth) | |
| ● Cumulative incidence, n (%) | 49 (48.5) |
| ● Incidence rate, person-year (CI) | 8.0 (6.2–10.9) |
| ● Median time to achieve endpoint, month (CI) | 6.1 (5.1–8.1) |
| ● Cumulative dose to achieve endpoint, mg, median (IQR) | 160 (60–240) |
| Relapse | |
| ● Cumulative incidence, n (%) | 48 (47.5) |
| ● Incidence rate, person-year (CI) | 5.5 (4.2–7.3) |
| ● Median time to relapse, month (CI) | 11.3 (8.6–15.4) |
Abbreviations: CI, confidence interval; IQR, interquartile range.
Initial Hair Regrowth
According to survival analysis, the cumulative incidence of initial hair regrowth after IMC therapy was 85.1%, with an incidence rate of 64.0 (95% CI = 51.9–79.3) person-years. The median time to achieve initial hair regrowth was 0.9 (95% CI = 0.8–1.2) months, and the average cumulative dose of IMC to achieve initial hair regrowth was 80 (20–160) mg.
Significant Hair Regrowth
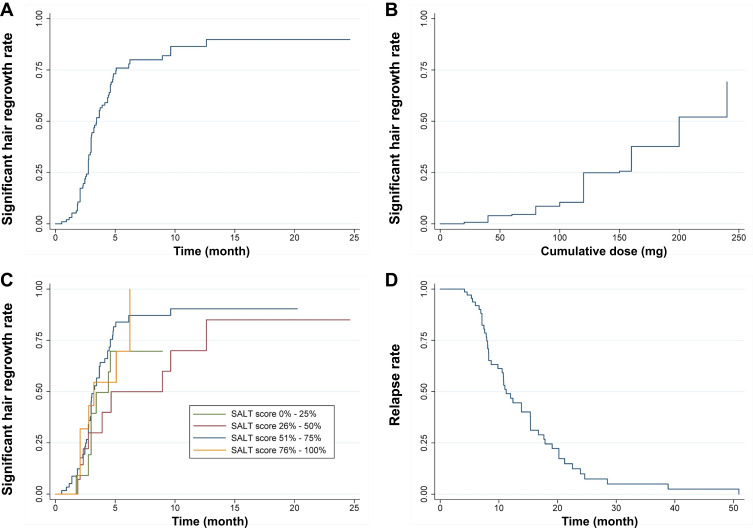
Significant hair regrowth was indicated as the primary outcome and its cumulative incidence in our cohort was 80.2%, with an incidence rate of 17.0 (95% CI = 13.6–21.7) person-years. The median time required to achieve significant hair regrowth was 3.4 (95% CI = 2.9–4.4) months. According to the Kaplan–Meier curve, there was a lag of approximately 1 month from initiation of therapy to the first patients demonstrating significant hair regrowth. Following this initial event, significant hair regrowth was observed in 41.9% of patients at 3 months, 75.9% at 6 months, 81.0% at 9 months, and 86.5% at 12 months. The residual probabilities of significant regrowth as a function of the time after initiation of IMC are also shown in Figure 1A. The average cumulative dose of IMC to achieve significant hair regrowth was 120 (40–200) mg, while a median cumulative dose that 50% of patients attained significant hair regrowth was 200 mg (Figure 1B).
Figure 1.
Kaplan–Meier curve demonstrating (A) the cumulative incidence of patients achieving significant hair regrowth (75% regrowth); (B) the cumulative corticosteroid dose of patients achieving significant hair regrowth; (C) the cumulative incidence of patients achieving significant hair regrowth of four groups according to baseline Severity of Alopecia Tool (SALT) score; and (D) the cumulative incidence of patients with relapse.
The baseline SALT score was identified as a negative factor for IMC treatment response in a previous study.31 To determine the effect of this variable on significant hair regrowth, a stratified Kaplan–Meier analysis was performed. Nine patients (81.8%) with a score of 0–25% obtained significant hair regrowth by a median time of 3.5 months; for the score range 26–50%, 12 (75.0%) patients achieved significant regrowth by 4.8 months; for the score range 51–75%, 48 (80.0%) experienced significant regrowth by 3.1 months; and for the score range 76–100%, 12 (85.7%) ultimately obtained significant regrowth at 3.2 months. However, the log rank test failed to demonstrate statistically significant differences among the four groups (P = 0.287; Figure 1C).
Factors Associated with Significant Hair Regrowth
The Cox proportional hazards model was used to determine the factors associated with significant hair regrowth (Table 3). Univariable analysis revealed that the variables associated with significant hair regrowth were female sex (HR = 2.39, 95% CI = 1.09–4.61; P = 0.035), age at AA onset >40 years (HR = 2.45, 95% CI = 1.49–3.69; P = 0.046), and nail abnormalities (HR = 0.26, 95% CI = 0.08–0.69; P = 0.036). However, when age at AA onset was analyzed as a continuous variable, no association was found (HR = 1.02, 95% CI = 0.89–1.06; P = 0.423). After adjusting for all confounding factors in a multivariable model, nail involvement was the only independent factor negatively affecting the probability of significant hair regrowth (adjusted HR = 0.04, 95% CI = 0.01–0.55; P = 0.015).
Table 3.
Univariate and Multivariable Analyses for the Factor Associated with Significant Hair Regrowth After Intramuscular Corticosteroid Therapy in Patients with Alopecia Areata
| Variables | HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|
| Female | 2.39 (1.09–4.61) | 0.035* | 1.13 (0.81–5.91) | 0.086 |
| Age at AA onset | 1.02 (0.89–1.06) | 0.423 | ||
| Age group at AA onset | ||||
| ● ≤40 years | Reference | |||
| ● >40 years | 2.45 (1.49–3.69) | 0.046* | 1.68 (0.92–3.42) | 0.187 |
| Duration of disease >6 months | 0.63 (0.25–1.49) | 0.141 | ||
| AA subtype | ||||
| ● Patch AA | Reference | |||
| ● Multiple AA | 1.34 (0.25–2.92) | 0.182 | ||
| ● Diffuse AA | 2.63 (0.99–4.61) | 0.062 | ||
| ● AT | 0.82 (0.29–2.85) | 0.845 | ||
| ● AU | 0.16 (0.09–3.15) | 0.753 | ||
| ● Ophiasis | 0.76 (0.09–2.92) | 0.911 | ||
| Severity of AA (SALT score) | ||||
| ● 0–25% | Reference | |||
| ● 26–50% | 0.56 (0.32–2.09) | 0.186 | ||
| ● 51–75% | 0.64 (0.31–2.67) | 0.823 | ||
| ● 76–100% | 1.49 (0.81–3.68) | 0.595 | ||
| Body hair involvement | 0.67 (0.03–4.28) | 0.745 | ||
| Nail involvement | 0.26 (0.08–0.69) | 0.036* | 0.04 (0.01–0.55) | 0.015* |
| Atopy | 0.68 (0.13–5.16) | 0.621 | ||
| Autoimmune thyroid diseases | 1.91 (0.83–3.28) | 0.142 | ||
| Family history of AA | 0.96 (0.41–3.56) | 0.363 |
Note: *Statistically significant.
Abbreviations: AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis; CI, confidence interval; HR, hazard ratio; SALT, Severity of Alopecia Tool.
Complete Hair Regrowth
Regarding complete hair regrowth, the cumulative incidence was 48.5%, with an incidence rate of 8.0 (95% CI = 6.2–10.9) person-years. The median time and average cumulative dose of IMC to achieve complete hair regrowth were 6.1 (95% CI = 5.1–8.1) months and 160 (60–240) mg, respectively.
Relapse and Its Associated Factors
Following 51 months of follow-up, the overall cumulative relapse rate after achievement of clinical hair regrowth with IMC was 47.5% (n = 48; Figure 1D). The incidence rate of AA relapse was 5.5 (95% CI = 4.2–7.3) person-years, and the median time to relapse was 11.3 (95% CI = 8.6–15.4) months. Treatment in patients with recurrence included combination therapy with IL corticosteroids and topical minoxidil (n = 21), topical contact immunotherapy (n = 10), methotrexate (n = 9), IMC (n = 4), and IL corticosteroids (n = 4). All therapies were prescribed at >6 months after discontinuation of the IMC regimen.
The risk of AA relapse was determined using the Cox proportional hazards model. Univariable analysis revealed that coexisting AITD (HR = 2.39, 95% CI = 1.42–5.14; P = 0.041) and disease duration >6 months (HR = 3.12, 95% CI = 1.36–4.94; P = 0.016) were associated with a higher probability of disease recurrence. However, multivariable analysis showed that only AA duration >6 months (adjusted HR = 4.02, 95% CI = 1.52–4.66; P = 0.005) independently increased the probability of AA relapse (Table 4).
Table 4.
Univariate and Multivariable Analyses for the Factor Associated with Relapse After Intramuscular Corticosteroid Therapy in Patients with Alopecia Areata
| Variables | HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
|---|---|---|---|---|
| Female | 1.15 (0.64–1.69) | 0.536 | ||
| Age at AA onset | 1.01 (0.99–1.06) | 0.104 | ||
| Age group at AA onset | ||||
| ● ≤40 years | Reference | |||
| ● >40 years | 1.55 (0.78–2.56) | 0.151 | ||
| Duration of disease >6 months | 3.12 (1.36–4.94) | 0.016* | 4.02 (1.52–4.66) | 0.005* |
| AA subtype | ||||
| ● Patch AA | Reference | |||
| ● Multiple AA | 0.94 (0.66–2.67) | 0.723 | ||
| ● Diffuse AA | 0.85 (0.39–3.62) | 0.264 | ||
| ● AT | 3.12 (0.79–5.64) | 0.459 | ||
| ● AU | 2.92 (0.59–3.95) | 0.644 | ||
| ● Ophiasis | 1.49 (0.08–3.12) | 0.814 | ||
| Severity of AA (SALT score) | ||||
| ● 0–25% | Reference | |||
| ● 26–50% | 0.91 (0.72–2.19) | 0.135 | ||
| ● 51–75% | 1.14 (0.51–2.67) | 0.718 | ||
| ● 76–100% | 0.69 (0.29–1.89) | 0.653 | ||
| Body hair involvement | 1.05 (0.29–3.18) | 0.425 | ||
| Nail involvement | 2.78 (0.98–4.09) | 0.812 | ||
| Atopy | 1.34 (0.63–3.89) | 0.291 | ||
| Autoimmune thyroid diseases | 2.39 (1.42–5.14) | 0.041* | 2.71 (0.82–4.65) | 0.069 |
| Family history of AA | 1.76 (0.87–2.88) | 0.635 |
Note: *Statistically significant.
Abbreviations: AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis; CI, confidence interval; HR, hazard ratio; SALT, Severity of Alopecia Tool.
Adverse Events
Adverse events were experienced by 18.8% of the patients (n = 19) and included acneiform eruption (n = 16, 15.8%), abnormal menstrual cycle (n = 6, 5.9%), weight gain (n = 3, 2.9%), and sleep disturbance (n = 1, 0.9%). Acneiform eruption was managed with topical therapy, while the other side effects resolved spontaneously after discontinuing IMC. The presence of any of these adverse events did not cause dropout from therapy.
Discussion
Although IMC therapy has shown to be effective in the treatment of several dermatological conditions and gained popularity for AA treatment in recent years, information regarding the use of IMC for AA is limited in the literature.32 In this study, we modeled the therapeutic endpoints of the IMC regimen according to multiple variables in patients with AA. To the best of our knowledge, this is the first study to describe the efficacy, cumulative effective dose, and factors associated with treatment outcomes in patients with AA receiving IMC based on a time-to-event analysis. The primary endpoint, defined as significant hair regrowth, had a rate of 80.2%, with a median time of approximately 3–4 months. Nail involvement was demonstrated to be a negative predictor of significant hair regrowth. Relapse was found in 47.5% of the patients, with disease duration >6 months being a risk factor for AA recurrence.
The application of systemic corticosteroids for the treatment of AA was first introduced by Dillaha et al in 1952, with remarkable therapeutic effects.33 Since then, the efficacy of various regimens such as oral, intravenous, and intramuscular administration has been investigated. Oral corticosteroid pulse therapy is generally the most convenient method and has been well studied.34 However, poor medication compliance and adherence may lead to reduced treatment efficacy and substantial worsening of the disease. The therapeutic effect of intravenous pulse corticosteroids has also been confirmed. However, the overall adverse events of intramuscular and oral corticosteroids are relatively high, approaching 41% and 30%, respectively, compared to that of 10% from corticosteroid pulse therapy.29 Hypothalamic–pituitary–adrenal (HPA) axis suppression was reported in 23%, 67%, and 7% of intramuscular, oral, and pulse corticosteroids, respectively.29 Intramuscular injections may offer an advantage over the two methods. IMC maximizes treatment efficacy by providing rapid onset of action comparable to the oral form, and by increasing patients’ compliance and adherence owing to the monthly or bimonthly injection regimen.28 It also minimizes adverse effects due to its pharmacokinetic properties, with a gradual and steady release of corticosteroids over time.28
Our study demonstrated the efficacy of IMC for the treatment of severe, acute, or rapidly progressive AA, as shown by the 80.2% cumulative incidence of significant hair regrowth rate in a median time of 3.4 months. Michalowski and Kuczyfiska reported a 72.7% response rate in 11 cases of extensive AA (AT/AU) receiving 40–80 mg of IMC therapy, initially once a week, then over a gradually lengthened interval up to 6 weeks.31 A retrospective study using IMC 40 mg every 4 weeks for a maximum of 6 months revealed a 63% response rate in refractory AA.30 Administration of IMC 40 mg monthly for 6 months, followed by 40 mg every 1.5 months for 1 year, had significantly superior efficacy, with a 74% response rate, to oral corticosteroid pulse therapy.29 However, our results could not be directly compared to those of earlier studies because of the difference in the severity of AA among the cohorts. The lower percentage of recalcitrant AA cases included in our study compared with previous ones could explain the higher response rate.
Regarding other corticosteroid injection protocols for AA, IL injection of 2.5–10 mg/mL triamcinolone acetonide at four–to-six-week intervals is commonly used in mild forms of AA (ie, patch AA or SALT score <25%) with a response rate between 64% and 97%.23 For severe AA, intravenous injection of 500 mg methylprednisolone for three consecutive days at four-week intervals for three months has a response rate of approximately 80%.23 However, compared to IL injection, the dosage for intramuscular and intravenous injections is higher and may lead to potential adverse effects; therefore, they should be restricted to those with severe AA and administered with caution.
Identifying the factors affecting treatment outcomes of AA is essential for therapeutic success; nevertheless, specific predictors for IMC have been rarely reported. Seo et al indicated that the baseline SALT score was negatively correlated with hair regrowth response to IMC.30 Our stratified analysis using the Kaplan–Meier method did not support this finding, as no association was found between the extent of scalp involvement and treatment response. Earlier age at AA onset, longer disease duration, and nail involvement were identified as indicators of poor prognosis.35–38 A recent meta-analysis demonstrated four negative predictors for treatment response, namely SALT score ≥50, disease duration ≥1 year, atopic diseases, and nail abnormalities.35 Our analysis using a Cox proportional hazards model showed a lack of correlation between significant hair regrowth and SALT score, AA duration, or presence of atopy, but confirmed its negative association with nail abnormalities. This finding could be explained by the characteristics of the enrolled subjects in our cohort, namely the predominance of female patients presenting with rapidly progressive scalp hair loss >50% for <6 months. The clinical characteristics of our patients were compatible to those of patients with acute diffuse and total alopecia, a distinctive AA subtype, which shows a favorable treatment response to systemic corticosteroid therapy.39,40
Emerging evidence suggests that approximately half the patients with AA treated with IMC experience disease recurrence. Seo et al demonstrated a 47.1% relapse rate during a follow-up period of 14.2 months.30 Kurosawa et al reported a comparable relapse rate of 46% after 6 months of treatment.29 Our result was consistent with previous studies, revealing recurrence in approximately half of patients with a median time of 11.3 months. Furthermore, disease duration >6 months was identified as a predictor of AA relapse. Longer AA duration could indicate disease chronicity, and physicians should remain vigilant for AA relapse despite complete IMC therapy. Therefore, clinicians should consider the use of post-IMC treatments such as application of 2% topical minoxidil three times daily, which was reported to limit post-steroid-treatment hair loss.41 For AA patients with chronic relapsing disease, further treatments after initial systemic corticosteroid therapy are challenging owing to a lack of high-quality randomized controlled trials supporting the efficacy and safety of other therapeutic modalities. However, a shared decision-making approach between the physician and patient is crucial as it would ensure that patients have a realistic expectation of treatment outcomes. Topical contact immunotherapy is currently considered the best modality for long-term therapy of patients with recalcitrant AA and is the first line treatment for these cases in our hair clinic.19
HPA axis suppression is considered a major side effect of IMC therapy.32 Previous studies demonstrated adrenocortical alteration in 23% of subjects receiving intramuscular injection.29 Other adverse effects of IMC include abdominal discomfort, dysmenorrhea, and acne.29,30 The incidence of adverse events among our AA patients was low, and acneiform eruption was the most common; no serious side effects were reported. However, HPA axis suppression in our subjects may be underestimated due to a lack of data regarding adrenocortical evaluation in our follow-up protocol.
A major strength of our study is the use of the time-to-event approach to characterize the therapeutic outcomes of patients with AA receiving IMC during the entire follow-up time, including censored observations. This method considers all available treatment and follow-up data, providing more precise and meaningful estimates of overall efficacy and tolerability, and better outcome prediction. The limitations of this study are its retrospective design involving a single tertiary center. These factors might lead to the unavailability of some data and limit the generalizability of the results. Our results may also be limited in their applicability to patients without severe and/or rapidly progressive AA. Additionally, it is difficult to compare our findings to those of previous studies, owing to differences in the definitions of treatment responses and in methodologies. Further large, multicenter randomized controlled trials evaluating the efficacy and safety of IMC therapy are required to overcome these limitations.
Conclusion
Our time-to-event analysis demonstrated the efficacy and safety of intramuscular administration of corticosteroids for the treatment of AA. IMC therapy showed high rates of significant hair regrowth, especially in patients with severe or active AA; however, approximately half of those experienced relapse. Nail involvement was negatively correlated with cosmetically acceptable hair regrowth, while disease duration >6 months was significantly associated with increased risk of relapse. Most importantly, the use of IMC depends on indication and perceived potential benefits and risks; therefore, physicians should be cautious when prescribing this treatment.
Abbreviations
AA, alopecia areata; AITD, autoimmune thyroid disease; AT, alopecia totalis; AU, alopecia universalis; CI, confidence interval; HPA, hypothalamic-pituitary-adrenal; HR, hazard ratio; IL, intralesional; IMC, intramuscular corticosteroids; IQR, interquartile range; SALT, Severity of Alopecia Tool; SD, standard deviation.
Data Sharing Statement
The data produced and analyzed for this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Mahidol University Review Board for Ethics in Human Research (MURA2020/1282) and was performed in accordance with the principles of Declaration of Helsinki. The requirement for informed consent was waived and the data were anonymized before analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1–12. doi: 10.1016/j.jaad.2017.04.1141 [DOI] [PubMed] [Google Scholar]
- 2.Khunkhet S, Vachiramon V, Suchonwanit P. Trichoscopic clues for diagnosis of alopecia areata and trichotillomania in Asians. Int J Dermatol. 2017;56(2):161–165. doi: 10.1111/ijd.13453 [DOI] [PubMed] [Google Scholar]
- 3.Suchonwanit P, Udompanich S, Thadanipon K, Chanprapaph K. Trichoscopic signs in systemic lupus erythematosus: a comparative study with 109 patients and 305 healthy controls. J Eur Acad Dermatol Venereol. 2019;33(4):774–780. doi: 10.1111/jdv.15421 [DOI] [PubMed] [Google Scholar]
- 4.Finner AM. Alopecia areata: clinical presentation, diagnosis, and unusual cases. Dermatol Ther. 2011;24(3):348–354. doi: 10.1111/j.1529-8019.2011.01413.x [DOI] [PubMed] [Google Scholar]
- 5.Leerunyakul K, Suchonwanit P. Asian hair: a review of structures, properties, and distinctive disorders. Clin Cosmet Investig Dermatol. 2020;13:309–318. doi: 10.2147/ccid.S247390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thadanipon K, Suchonwanit P. Measuring patient quality of life following treatment for alopecia. Patient Prefer Adherence. 2021;15:1601–1610. doi: 10.2147/ppa.S282399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG; British Association of D. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149(4):692–699. doi: 10.1046/j.1365-2133.2003.05535.x [DOI] [PubMed] [Google Scholar]
- 9.Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55(6):e338–343. doi: 10.1111/ijd.13061 [DOI] [PubMed] [Google Scholar]
- 10.Roest YBM, van Middendorp HT, Evers AWM, van de Kerkhof PCM, Pasch MC. Nail involvement in alopecia areata: a questionnaire-based survey on clinical signs, impact on quality of life and review of the literature. Acta Derm Venereol. 2018;98(2):212–217. doi: 10.2340/00015555-2810 [DOI] [PubMed] [Google Scholar]
- 11.Chanprapaph K, Udompanich S, Visessiri Y, Ngamjanyaporn P, Suchonwanit P. Nonscarring alopecia in systemic lupus erythematosus: a cross-sectional study with trichoscopic, histopathologic, and immunopathologic analyses. J Am Acad Dermatol. 2019;81(6):1319–1329. doi: 10.1016/j.jaad.2019.05.053 [DOI] [PubMed] [Google Scholar]
- 12.Suchonwanit P, Triamchaisri S, Wittayakornrerk S, Rattanakaemakorn P. Leprosy reaction in Thai population: a 20-year retrospective study. Dermatol Res Pract. 2015;2015:253154. doi: 10.1155/2015/253154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanprapaph K, Mahasaksiri T, Kositkuljorn C, Leerunyakul K, Suchonwanit P. Prevalence and risk factors associated with the occurrence of autoimmune diseases in patients with alopecia areata. J Inflamm Res. 2021;14:4881–4891. doi: 10.2147/jir.S331579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Ovidio R. Alopecia Areata: news on diagnosis, pathogenesis and treatment. G Ital Dermatol Venereol. 2014;149(1):25–45. [PubMed] [Google Scholar]
- 15.Messenger AG, Slater DN, Bleehen SS. Alopecia areata: alterations in the hair growth cycle and correlation with the follicular pathology. Br J Dermatol. 1986;114(3):337–347. doi: 10.1111/j.1365-2133.1986.tb02825.x [DOI] [PubMed] [Google Scholar]
- 16.Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med Sci. 2019;34(9):1857–1864. doi: 10.1007/s10103-019-02783-8 [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Lee WS. Management of alopecia areata: updates and algorithmic approach. J Dermatol. 2017;44(11):1199–1211. doi: 10.1111/1346-8138.13933 [DOI] [PubMed] [Google Scholar]
- 18.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916–926. doi: 10.1111/j.1365-2133.2012.10955.x [DOI] [PubMed] [Google Scholar]
- 19.Mahasaksiri T, Kositkuljorn C, Anuntrangsee T, Suchonwanit P. Application of topical immunotherapy in the treatment of alopecia areata: a review and update. Drug Des Devel Ther. 2021;15:1285–1298. doi: 10.2147/dddt.S297858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suchonwanit P, Iamsumang W, Rojhirunsakool S. Efficacy of Topical Combination of 0.25% Finasteride and 3% Minoxidil Versus 3% Minoxidil Solution in Female Pattern Hair Loss: a Randomized, Double-Blind, Controlled Study. Am J Clin Dermatol. 2019;20(1):147–153. doi: 10.1007/s40257-018-0387-0 [DOI] [PubMed] [Google Scholar]
- 21.Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309(9):729–738. doi: 10.1007/s00403-017-1777-5 [DOI] [PubMed] [Google Scholar]
- 22.Sriphojanart T, Khunkhet S, Suchonwanit P. A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatol Reports. 2017;9(2):7399. doi: 10.4081/dr.2017.7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rattananukrom T, Suchonwanit P. Are drug treatment strategies really effective against alopecia areata? Expert Opin Pharmacother. 2021;22(3):257–260. doi: 10.1080/14656566.2020.1854728 [DOI] [PubMed] [Google Scholar]
- 24.Shreberk-Hassidim R, Ramot Y, Gilula Z, Zlotogorski A. A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol. 2016;74(2):372–374e371-375. doi: 10.1016/j.jaad.2015.09.045 [DOI] [PubMed] [Google Scholar]
- 25.Kumaresan M. Intralesional steroids for alopecia areata. Int J Trichology. 2010;2(1):63–65. doi: 10.4103/0974-7753.66920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62(2):191–202. doi: 10.1016/j.jaad.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 27.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78(1):15–24. doi: 10.1016/j.jaad.2017.04.1142 [DOI] [PubMed] [Google Scholar]
- 28.Thomas LW, Elsensohn A, Bergheim T, Shiu J, Ganesan A, Secrest A. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17(3):323–329. [PubMed] [Google Scholar]
- 29.Kurosawa M, Nakagawa S, Mizuashi M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology. 2006;212(4):361–365. doi: 10.1159/000092287 [DOI] [PubMed] [Google Scholar]
- 30.Seo J, Lee YI, Hwang S, Zheng Z, Kim DY. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44(2):173–179. doi: 10.1111/1346-8138.13533 [DOI] [PubMed] [Google Scholar]
- 31.Michalowski R, Kuczynska L. Long-term intramuscular triamcinolon-acetonide therapy in alopecia areata totalis and universalis. Arch Dermatol Res. 1978;261(1):73–76. doi: 10.1007/BF00455378 [DOI] [PubMed] [Google Scholar]
- 32.Reddy S, Ananthakrishnan S, Garg A. A prospective observational study evaluating hypothalamic-pituitary-adrenal axis alteration and efficacy of intramuscular triamcinolone acetonide for steroid-responsive dermatologic disease. J Am Acad Dermatol. 2013;69(2):226–231. doi: 10.1016/j.jaad.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 33.Dillaha CJ, Rothman S. Therapeutic experiments in alopecia areata with orally administered cortisone. J Am Med Assoc. 1952;150(6):546–550. doi: 10.1001/jama.1952.03680060018006 [DOI] [PubMed] [Google Scholar]
- 34.Lai VWY, Chen G, Gin D, Sinclair R. Systemic treatments for alopecia areata: a systematic review. Australas J Dermatol. 2019;60(1):e1–e13. doi: 10.1111/ajd.12913 [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Kim BJ, Lee YB, Lee WS. Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(10):1145–1151. doi: 10.1001/jamadermatol.2018.2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suchonwanit P, Kositkuljorn C, Mahasaksiri T, Leerunyakul K. A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia areata. J Dermatolog Treat. 2020;1–6. doi: 10.1080/09546634.2020.1773384 [DOI] [PubMed] [Google Scholar]
- 37.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62(2):177–188. doi: 10.1016/j.jaad.2009.10.032 [DOI] [PubMed] [Google Scholar]
- 38.Suchonwanit P, Kositkuljorn C, Pomsoong C. Alopecia Areata: an Autoimmune Disease of Multiple Players. Immunotargets Ther. 2021;10:299–312. doi: 10.2147/ITT.S266409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: a new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol. 2009;60(1):85–93. doi: 10.1016/j.jaad.2008.08.045 [DOI] [PubMed] [Google Scholar]
- 40.Sato-Kawamura M, Aiba S, Tagami H. Acute diffuse and total alopecia of the female scalp. A new subtype of diffuse alopecia areata that has a favorable prognosis. Dermatology. 2002;205(4):367–373. doi: 10.1159/000066435 [DOI] [PubMed] [Google Scholar]
- 41.Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128(11):1467–1473. doi: 10.1001/archderm.1992.01680210045005 [DOI] [PubMed] [Google Scholar]