Abstract
Genomic species diversity among 147 Acinetobacter clinical isolates not belonging to the A. calcoaceticus- A. baumannii (ACB) complex was investigated by phenotypic and genotypic identification methods. The isolates were obtained between 1991 and 1999 from numerous diagnostic laboratories in the Czech Republic and were studied by numerical probabilistic identification using two biochemical frequency matrices and amplified rDNA restriction analysis (ARDRA). Their final identification was derived from the combined phenotypic and ARDRA results. In total, 102 isolates were unambiguously (n = 89) or presumptively (n = 13) identified as A. lwoffii (n = 63), genomic species 13BJ/14TU (n = 9), A. johnsonii (n = 7), A. haemolyticus (n = 6), A. junii (n = 5), and other genomic species (n < 5 isolates each). Forty-five isolates could not be identified as belonging to any described species. Among the unidentified isolates two large groups of non-glucose-acidifying, nonhemolytic, and non-gelatinase-producing isolates were distinguished. These groups, designated phenon 1 (n = 17) and phenon 2 (n = 15), had distinctive phenotypic features and novel ARDRA profiles, which suggests that they represent hitherto undescribed Acinetobacter species. Phenon 2 included mainly clinically insignificant isolates from outpatients, while phenon 1 comprised clinically relevant isolates mostly from the blood of hospitalized patients, and its precise taxonomic definition may therefore be of medical importance. Overall, the development of practical methods for identification required for the elucidation of the biological significance of the (genomic) species within the genus Acinetobacter remains a challenging task.
The genus Acinetobacter comprises at least 18 genomic species seven of which have a species rank (5, 7, 26). The vast majority of Acinetobacter strains of clinical origin belong to the genetically closely related genomic species 2 (A. baumannii), 3, and 13 sensu Tjernberg and Ursing (TU) (6, 17, 24). These (genomic) species are phenotypically highly similar and have been lumped together with A. calcoaceticus in the so-called A. calcoaceticus-A. baumannii (ACB) complex (14). Little is known about the clinical relevance of other genomic species in the genus. Some of these may occur on the skin and mucous membranes of healthy people or have occasionally been found in clinical specimens (1, 2, 6, 8, 24, 25). However, many studies reporting on Acinetobacter infections have either used names which are no longer valid or applied methods which are inappropriate for species identification. Therefore, it is not always clear which of the genomic species were involved in such cases.
The widely adopted biochemical scheme of Bouvet and Grimont (6) showed promising results (6, 24) for identification of Acinetobacter species, but further studies have shown that this scheme does not cover the phenotypic variability of all genomic species (14). Commercial phenotypic identification systems have also been shown to have a limited capacity for the differentiation of all genomic species (3, 4). However, the efficiency of these phenotypic identification methods would be considerably higher if strains belonging to A. baumannii and genomic species 1, 3, and 13TU are identified as members of the ACB complex rather than as (genomic) species (4, 14).
Apart from phenotypic methods, a variety of genotypic methods have been explored for species identification (12, 13, 18, 27). One of these, amplified rDNA restriction analysis (ARDRA), has been tested on a large set of reference strains and has shown good correlation with DNA-DNA hybridization results (10, 19). However, in more recent studies a number of strains could not be identified by this method (1, 8, 25). Either these findings may indicate the existence of additional genomic species or they follow from yet unrecognized intraspecies diversity (25).
In an exploratory study, the prevalence of Acinetobacter genomic species in 700 prospective human isolates from numerous institutions in the Czech Republic was investigated using phenotypic (6) and genotypic methods (10, 13). A total of 553 isolates were identified as belonging to the ACB complex. In the present study, the species diversity of the remaining 147 isolates was further investigated. Given the limitations of identification by a single method, ARDRA was used in combination with biochemical identification according to the method of Bouvet and Grimont (6). Two novel phenons were delineated among the strains not belonging to the ACB complex and are described in detail.
MATERIALS AND METHODS
Isolates.
In the period from 1991 to 1999, a total of 700 isolates of the genus Acinetobacter were received by the National Institute of Public Health (NIPH) from different diagnostic laboratories in the Czech Republic. The isolates were recovered from a variety of specimens from patients in hospitals or general practice and represented different levels of clinical significance, ranging from mere colonization to life-threatening infections. The majority of these isolates were sent to the NIPH for reasons of genus or species identification, epidemiological typing, or antibiotic susceptibility determination. All isolates had the properties of the genus Acinetobacter (21); i.e., they were gram-negative, strictly aerobic, oxidase-negative, nonmotile coccobacilli and were positive in the transformation assay of Juni (20). In total, 549 out of 700 isolates were allocated to the ACB complex by phenotypic methods (6) combined with computer-assisted identification (14). Four additional isolates were allocated to the ACB complex by ARDRA (10) and ribotyping (13). The remaining 147 isolates could not be identified as belonging to the ACB complex and were further investigated in the present study. Ninety-seven of these isolates were from outpatients or were obtained in general practice, 38 were from hospitalized patients, and 12 were from persons with unknown outpatient or inpatient status. These non-ACB complex isolates were recovered from nasal or throat swabs (n = 33), vaginal or cervical swabs (n = 21), urine (n = 20), blood (n = 16), wound swabs (n = 14), ear swabs (n = 13), eye swabs (n = 12), feces (n = 4), pus (n = 3), intravenous catheters (n = 2), and other specimens (n = 9).
Phenotypic characterization.
The isolates were characterized using the tests of Bouvet and Grimont (6) and Gerner-Smidt et al. (14), with some minor modifications. In short, all tests were done at 30°C and recorded after 2 days unless indicated otherwise. Aerobic acid production from glucose was tested in Hugh and Leifson's agar medium (Difco) supplemented with 1% (wt/vol) d-glucose. The gelatinase test was performed on Trypticasein agar containing 4% (wt/vol) gelatin; Frazier reagent (15% [wt/vol] HgCl2 in 20% [vol/vol] HCl) was used for the detection of gelatin hydrolysis. Hemolysis was tested on 5% sheep blood agar plates. Utilization of dl-lactate, dl-4-aminobutyrate, trans-aconitate, glutarate, l-aspartate, azelate, β-alanine, l-histidine, d-malate, malonate, histamine, l-phenylalanine, and phenylacetate was tested in tubes containing the defined fluid medium of Cruze et al. (9) supplemented with 0.1% (wt/vol) carbon (C) source. Each tube was inoculated with a drop of cell suspension of standardized turbidity (5 · 107 to 1 · 108 CFU/ml) prepared in saline from an overnight culture. Utilization of citrate was tested on Simmons citrate agar (Difco) and recorded after 6 days of incubation, while growth on the other C sources was evaluated after 2 and 6 days. Growth tests at 37, 41, and 44°C were performed in brain heart infusion broth (Difco) inoculated as described for the C source utilization tests.
Numerical probabilistic identification.
The principles of probabilistic identification have been explained in detail by Priest and Williams (23). Briefly, by this method the likelihood that an unknown strain belongs to a given taxon is calculated. This likelihood is defined as the probability of obtaining the observed test results with a strain of this taxon. There are several options of using likelihoods for identification decisions. In the present study, computer-assisted probabilistic identification was performed as done by Gerner-Smidt et al. (14) who used the identification score (22)—often referred to as Willcox probability—and modal likelihood fraction (11) as identification parameters. Phenotypic data of each isolate were tested against two probability matrices represented by the computer-stored data tables in which the responses of each taxon to each test was recorded as a probability figure for a positive result. The first matrix (called the matrix of Bouvet and Grimont in this study) was derived from the data of Bouvet and Grimont (5) for genomic species 1 to 12 and from the data of Bouvet and Jeanjean (7) for genomic species 13BJ to 17BJ. This compiled matrix was also published by Grimont and Bouvet (15). The second matrix was adapted from that in the paper of Gerner-Smidt et al. (14) and included data for genomic species 1 to 12 and 13TU to 15TU described by Tjernberg and Ursing (26). Both matrices comprised all phenotypic tests listed above (except for hemolysis and phenylacetate, which were absent in the matrix of Bouvet and Grimont and the matrix of Gerner-Smidt et al., respectively), while the lower and upper limits of the reaction probabilities were set at 0.01 and 0.99, respectively. This was justified by the assumption of an error rate of 1% for any test result. The identification coefficients were determined as follows. First, the likelihood that a tested isolate belonged to each of the genomic species of a given matrix was calculated in turn by multiplication of the probabilities for the individual test results. The identification score (IS) was then determined by dividing this likelihood for a given genomic species by the sum of likelihoods for all genomic species of the matrix, while the modal likelihood fraction was calculated as the likelihood for a given genomic species divided by the maximum likelihood possible for that genomic species with the given tests. All the calculations were performed by using the software package BACTID, which was developed by J. Schindler, Jr., and J. Schindler (NIPH) and is available on request. An isolate was accepted as identified by a given probability matrix if the IS was ≥0.95 and modal likelihood fraction was ≥0.0001 (14).
ARDRA.
The method was carried out as described (10, 25) with minor modifications. Briefly, isolates were grown overnight on Mueller-Hilton agar (Oxoid) at 30°C. A 1-μl loopful of colony growth was suspended in 20 μl of 0.05 M NaOH–0.25% (wt/vol) sodium dodecyl sulfate solution and heated at 98°C for 15 min. The suspension was diluted to a 200-μl final volume, agitated thoroughly, and centrifuged briefly. Aliquots of 1 μl of supernatant were used for amplification by PCR. The reaction mixture contained a 0.2 mM concentration of each nucleoside triphosphate a 0.2 μM concentration of each primer, 1.5 mM MgCl2, 10 mM Tris-HCl, 50 mM KCl, and 0.5 U of Taq polymerase (Takara Shuzo Co., Shiga, Japan). The sequences of the primers were 5′TGGCTCAGATTGAACGCTGGCGGC3′ (5′ end of the 16S rRNA gene) and 5′TACCTTGTTACGACTTCACCCCA3′ (3′ end of the 16S rRNA gene). Amplification was performed under the following conditions: initial denaturation for 6 min at 94°C; 35 cycles at 94°C for 45 s, at 60°C for 45 s, and at 72°C for 1 min; followed by 7 min at 72°C. Fractions of the amplimers were digested with the respective restriction enzymes Hin6I (a CfoI isoschizomer), AluI, MboI, RsaI, MspI (Fermentas, Vilnius, Lithuania), BfaI, or BsmAI (New England Biolabs, Beverly, Mass.). Restriction fragments were resolved by horizontal electrophoresis in 3% Metaphor agarose (FMC BioProducts, Rockland, Maine). ARDRA profiles, defined as a combination of the restriction patterns obtained with the respective enzymes, were interpreted according to the scheme of Dijkshoorn et al. (10), which is a revised and extended version of the scheme of Vannechoutte et al. (27). Additional CfoI and AluI patterns described for A. johnsonii by Seifert et al. (25) were also taken into account. As a rule, the isolates were first investigated with restriction enzymes Hin6I (a CfoI isoschizomer), AluI, and MboI. Analysis by other enzymes was carried out only if the combination of patterns obtained by these enzymes was not unique for a particular genomic species or if there was disagreement between the ARDRA results and phenotypic identification.
RESULTS
Consensus identification.
The results of the combined identification of the non-ACB complex isolates are shown in Table 1. An isolate was considered unambiguously identified as belonging to a particular genomic species if it was concordantly identified by ARDRA and at least one biochemical scheme. Thus, 89 of the isolates were unambiguously identified. Of these, 77 isolates were identified concordantly by both biochemical schemes, while 9 isolates were not identified using the matrix of Bouvet and Grimont and 3 isolates remained unidentified using the matrix of Gerner-Smidt et al. In addition, the correlation of the ARDRA and biochemical results allowed for presumptive identification of 13 other isolates deviating either in one biochemical reaction or in the ARDRA pattern obtained with one enzyme. Altogether 102 isolates were unambiguously or presumptively identified as belonging to A. lwoffii (n = 63), genomic species 13BJ/14TU (n = 9), A. johnsonii (n = 7), A. haemolyticus (n = 6), A. junii (n = 5), A. radioresistens (n = 4), genomic species 10 (n = 3), genomic species 11 (n = 2), genomic species 6 (n = 1), genomic species 15TU (n = 1), and genomic species 16 (n = 1). A total of 45 (31%) isolates could either not be identified by any method or their identifications by ARDRA and by phenotypic methods were discordant.
TABLE 1.
Genotypic and phenotypic identification of 147 isolates not belonging to the ACB complex
| Genomic speciesa (species) as determined by:
|
No. of isolates | |||
|---|---|---|---|---|
| ARDRA | Phenotypic identificationb using probability matrices of:
|
Consensus identificationc | ||
| Gerner-Smidt et al. (14) | Bouvet and Grimont (15) | |||
| 4 or 13BJ/14TUd | 4 | 4 | 4 (A. haemolyticus) | 4 |
| 4 or 13BJ/14TUd | 4 | Unidentified | 4 (A. haemolyticus) | 2 |
| 5 | 5 | 5 | 5 (A. junii) | 5 |
| 7 | 7 | 7 | 7 (A. johnsonii) | 4 |
| 8/9 | 8 | 8/9 | 8/9 (A. lwoffii) | 29 |
| 8/9 | 8 or 15TUe | 8/9 | 8/9 (A. lwoffii) | 25 |
| 8/9 | Unidentified | 8/9 | 8/9 (A. lwoffii) | 1 |
| 8/9 | 8 | Unidentified | 8/9 (A. lwoffii) | 1 |
| 10 | 10 | 10 | 10 | 3 |
| 11 | 11 | 11 | 11 | 1 |
| 11 | 11 | Unidentified | 11 | 1 |
| 12 | 12 | Unidentified | 12 (A. radioresistens) | 4 |
| 13BJ/14TU | 14TU | 13BJ | 13BJ/14TU | 6 |
| 4 or 13BJ/14TUd | Unidentifiedf | 13BJ | 13BJ/14TU | 1 |
| 15TU | 15TU | Unidentified | 15TU | 1 |
| 16 | Unidentified | 16 | 16 | 1 |
| 6 | Unidentifiedg | Unidentifiedg | 6 | 1 |
| 4 or 7 or 13BJ/14TUh | 7 | 7 | 7 (A. johnsonii) | 2 |
| 4 or 7 or 13BJ/14TUh | Unidentified | 7 | 7 (A. johnsonii) | 1 |
| 8/9i | 8 | 8/9 | 8/9 (A. lwoffii) | 4 |
| 8/9i | 8 or 15TUe | 8/9 | 8/9 (A. lwoffii) | 2 |
| 8/9i | 8 or 15TUe | Unidentified | 8/9 (A. lwoffii) | 1 |
| 4 or 13BJ/14TUd | Unidentifiedf | Unidentified | 13BJ/14TU | 1 |
| 4 or 13BJ/14TUd | 6 | 13BJ | 13BJ/14TU | 1 |
| 5 | 4 | 4 | Unidentified | 1 |
| 13BJ/14TU | 4 | 4 | Unidentified | 1 |
| 13BJ/14TU | 4 | Unidentified | Unidentified | 1 |
| Unidentified | 8 or 15TUe | 8/9 | Unidentified | 1 |
| Unidentified | 8 or 15TUe | Unidentified | Unidentified | 3 |
| Unidentified | 15TU | Unidentified | Unidentified | 2 |
| Unidentified | Unidentified | Unidentified | Unidentified | 36 |
Genomic species are numbered according to Bouvet and Grimont (5) (no suffix), Bouvet and Jeanjean (7) (suffix BJ), or Tjernberg and Ursing (25) (suffix TU).
For an explanation of phenotypic identification and numerical probabilistic identification, see Materials and Methods.
Criteria for consensus identification are given in Results. According to these criteria, 89 isolates were unambiguously identified, 13 isolates were presumptively identified, and 45 isolates remained unidentified. These three groups appear, respectively, in the three major blocks in the table [i.e., results of consensus identification 4 (A. haemolyticus) to 16, 6 to 13BJ/14TU, and unidentified].
Genomic species 4 and 13BJ/14TU cannot be differentiated; CfoI, AluI, MboI, RsaI, MspI, and BfaI patterns are common to both these genomic species (10).
Genomic species 8 and 15TU cannot be differentiated (14); the sum of their ISs is ≥0.95.
Identified as genomic species 14TU (0.95 > IS ≥0.90).
Biochemical properties intermediate between those of A. haemolyticus and genomic species 6.
CfoI, AluI, MboI, RsaI, and MspI patterns as in genomic species 4, 7, and 13BJ/14TU, but BfaI pattern is aberrant (10).
CfoI, AluI, MboI, RsaI, and MspI patterns specific for A. lwoffii, except for one additional fragment in AluI pattern 3.
Delineation of phenon 1 and phenon 2.
The majority of the 45 unidentified isolates could be allocated to two groups called phenon 1 (n = 17) and phenon 2 (n = 15). Each of these groups contained isolates that were strikingly similar in both biochemical properties and ARDRA profiles.
Phenotypic characteristics of both phenons are summarized in Table 2. Phenon 1 isolates were biochemically almost homogeneous, the exceptions being two isolates (NIPH 706 and NIPH 709) which were glutarate negative and NIPH 376, which was l-aspartate negative. Phenon 2 isolates diversely utilized citrate and azelate and with two exceptions were homogeneous in the other tests. NIPH 293 did not grow on d-malate, while NIPH 907 did not grow on glutarate (this isolate was incorrectly identified as A. lwoffii by both biochemical schemes). The isolates of phenon 1 and phenon 2 could be separated by the tests of growth at 41°C and on l-aspartate (except NIPH 376).
TABLE 2.
Phenotypic characteristics of phenon 1 and phenon 2a
| Test | Phenon 1 (n = 17) | Phenon 2 (n = 15) |
|---|---|---|
| Growth in BHI broth at: | ||
| 44°C | − | − |
| 41°C | − | +b |
| 37°C | +b | + |
| Acid from d-glucose | − | − |
| Gelatinase | − | − |
| Hemolysis of sheep blood | − | − |
| Utilization of: | ||
| dl-Lactate | + | + |
| dl-4-Aminobutyrate | − | − |
| trans-aconitate | − | − |
| Citrate (Simmons) | + | 60 |
| Glutarate | 88c | 93 |
| l-Aspartate | 94b | − |
| Azelate | + | 60 |
| β-Alanine | − | − |
| l-Histidine | − | − |
| d-Malate | +b | 93b |
| Malonate | − | − |
| Histamine | − | − |
| l-Phenylalanine | − | − |
| Phenylacetate | − | − |
+, all strains positive; −, all strains negative. The numbers are percentages of positive strains.
Weak reactions in some strains.
Two gluratate-negative isolates were epidemidemiologically related and represented one strain as confirmed by RAPD typing (see Results).
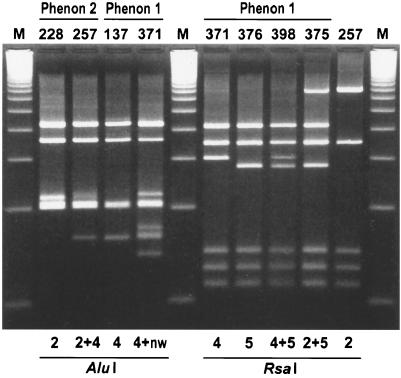
ARDRA profiles are shown in Table 3. The profiles of the phenon 1 isolates were characterized by a new RsaI pattern designated pattern 5, as an extension of the existing classification (10). This RsaI pattern 5 showed only a minor difference with the previously described pattern 4 in migration of a fragment of approximately 300 bp (Fig. 1) and was found either alone or in combination with patterns 2 or 4 in all but two isolates. In addition, five isolates yielded a new AluI pattern containing all the fragments of pattern 4 and three additional fragments (Fig. 1). In spite of their diversity, both RsaI and AluI patterns (except for two isolates with RsaI pattern 4) were characterized by one predominating pattern, i.e., RsaI pattern 5 and AluI pattern 4, which occurred either alone or in combination with an additional pattern, e.g., RsaI pattern 4.
TABLE 3.
ARDRA patterns of the phenon 1 and phenon 2 isolates
| Phenon and isolate no.a | Restriction patternb with enzyme
|
|||||
|---|---|---|---|---|---|---|
| CfoIc | AluI | MboI | RsaI | MspI | BfaI | |
| 1 (n = 17) | ||||||
| 137, 280, 376, 439, 930, 950, 993, 1118 | 1 | 4 | 3 | 5d | 3 | NDe |
| 1025 | 1 | 4 | 3 | 2+5 | 3 | ND |
| 398 | 1 | 4 | 3 | 4+5 | 3 | ND |
| 1048 | 1 | 4 | 1f+3 | 4+5 | 3 | ND |
| 706, 709, 1120 | 1 | 4+nwg | 3 | 4+5 | 3 | ND |
| 177, 371 | 1 | 4+nw | 3 | 4 | 3 | ND |
| 375 | 1 | 2+4 | 1f+3 | 2+5 | 3 | ND |
| 2 (n = 15) | ||||||
| 257, 286, 291, 293, 296, 383, 883, 904, 907, 933, 1034 | 1+5 | 2+4h | 1 | 2 | 2 | 10 |
| 285, 369, 900 | 1+5 | 2 | 1 | 2 | 2 | 10 |
| 228 | 1+5f | 2 | 1 | 2 | 2 | 10 |
Isolates are identified by NIPH number.
Pattern designation according to Dijkshoorn et al. (10).
Hin6I, an isoschizomer of CfoI, was used in this study.
5 is a new RsaI pattern (see Fig. 1).
ND, not determined.
A weakly expressed restriction pattern.
4+nw is a new combined AluI pattern (see Fig. 1).
Presumptive pattern 2+4 (see Fig. 1).
FIG. 1.
AluI and RsaI restriction patterns found in phenon 1 and phenon 2 isolates (designated by the NIPH numbers). AluI patterns 2 and 4 and RsaI patterns 2 and 4 were previously described (10, 27); RsaI patterns 5, 4+5, and 2+5 are new patterns or pattern combinations. The AluI pattern of NIPH 371 was tentatively interpreted as the mixture of pattern 4 and a new pattern (4+nw). The AluI pattern of NIPH 257 was considered to be pattern 2+4, with the diffuse band (210 bp) specific for pattern 2. Lanes M, 100-bp ladder.
The phenon 2 isolates were characterized by the uniformity of ARDRA profiles, the only exception being the patterns obtained by AluI (Table 3). Four isolates yielded AluI pattern 2 while 11 isolates had combination patterns with all fragments of patterns 2 and 4. In the latter case, the band specific for pattern 2 was diffuse (Fig. 1) even when the amplified DNA was purified (High Pure PCR Product Purification kit; Boehringer Mannheim) and digested with a fourfold-increased amount of AluI enzyme (not shown).
Origin of the phenon 1 and phenon 2 isolates.
Eleven out of the 17 isolates of phenon 1 were obtained from seriously ill patients hospitalized in five geographically distant hospitals: seven of these isolates were from blood cultures, two were from intravenous catheters, and two were from pus (drainage). Retrospective analysis of clinical and diagnostic data revealed that three blood isolates (NIPH 371, NIPH 706, and NIPH 1048) were recovered from patients with diagnosed bacteremia (three positive consecutive blood cultures). One of these patients (NIPH 371) suffered bronchopneumonia with a fatal outcome. One blood isolate (NIPH 137) originated from a patient with endocarditis.
There was no apparent epidemiological link between phenon 1 isolates from the same hospital, except for two cases. In the first case, two isolates (NIPH 706 and NIPH 709, from a blood culture and an intravenous catheter, respectively) were recovered from two patients who were at the same time in a surgical intensive care unit. These isolates shared an ARDRA profile and unique phenotype characterized by the inability to grow on glutarate and were indistinguishable by random amplified polymorphic DNA analysis (RAPD) using primer DAF4 (16) (not shown). In the second case, two isolates (NIPH 375 and NIPH 376) were obtained during 1-day from two patients hospitalized after surgery in an orthopedic ward. NIPH 375 was associated with a suppurative fracture of a femur, while NIPH 376 was recovered from the focus of a suppurative coxarthritis. In spite of their apparent epidemiological relationship, these two isolates differed both in ARDRA patterns (Table 3; Fig. 1) and in DAF4-RAPD profiles (not shown).
In contrast to phenon 1 isolates, all phenon 2 isolates were obtained in general practice, and their presence in patient specimens (vaginal, cervical, throat, nasal, ear, or conjunctival swabs or urine) was mostly regarded as clinically nonsignificant.
DISCUSSION
In recent years, considerable progress has been made in resolving the taxonomy of the genus Acinetobacter. Although many methods for identification of genomic species have been advocated (6, 12, 13, 18, 27), differentiation between some Acinetobacter species can be difficult (10, 12, 14). Moreover, the published identification schemes have not always been validated with a sufficient number of strains to assess the actual intra- and interspecies variability. The initial schemes may consequently either require revision (10, 14), or are inadequate for identification of some (genomic) species (19).
In the present study the species diversity of 147 clinical isolates not belonging to the ACB complex was investigated. A combination of two methods was used which compensate to a certain extent for each other's limitations. This combination included biochemical identification associated with numerical probabilistic approach and genotypic identification by ARDRA. Thus, almost one-third of the non-ACB complex isolates remained unidentified. Apart from three isolates that were allocated to different (genomic) species using the ARDRA and biochemical identification, the unidentified isolates had ARDRA profiles distinct from those of all the genomic species described to date (10, 25). In addition, the large majority of these isolates were not identified biochemically either, which strongly suggests that at least some of these isolates do not belong to any of the known genomic species.
The most important result of this study was the delineation of two phenetically distinct groups (phenons) that included 71% of all unidentified isolates. Despite certain intraphenon variability in the ARDRA profiles (phenon 1) or phenotypic features (phenon 2), the overall similarity and exclusivity of the features investigated suggest that these phenons may represent two hitherto undescribed Acinetobacter species. Comparison with published results indicates that strains similar to those of phenon 1 and phenon 2 are not restricted to the Czech Republic, since strains with similar ARDRA patterns and not identified by other methods have been described in previous studies (2, 10). The clinical specimens from which the isolates of both phenons were detected are noteworthy. While phenon 2 included mainly clinically insignificant isolates originating exclusively from outpatients, phenon 1 comprised clinically relevant isolates recovered mostly from blood cultures of hospitalized patients. Similarly, all strains that have been described in other studies with ARDRA profiles consistent with those of phenon 1 were isolated from the blood of septicemic patients (2, 17).
In summary, despite the use of two well-established complementary identification methods, a considerable proportion of the Acinetobacter isolates remained unidentified. These isolates were clearly distinct from the (genomic) species included in the ACB complex, and most of them could be grouped into two novel phenons. Of these, phenon 1 grouped isolates associated with infections in hospitalized patients, and its precise taxonomic definition may therefore be of essential medical importance. Overall, the development of practical methods for identification required for the elucidation of the biological significance of the (genomic) species within the genus Acinetobacter remains a challenging task.
ACKNOWLEDGMENTS
This work was supported by research grant 310/98/1602 of the Grant Agency of the Czech Republic.
We are grateful to K. Gavurová and R. Fryštacká for their excellent technical assistance.
REFERENCES
- 1.Berlau J, Aucken H, Malnick H, Pit T. Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis. 1999;18:179–183. doi: 10.1007/s100960050254. [DOI] [PubMed] [Google Scholar]
- 2.Bernards A T, de Beaufort A J, Dijkshoorn L, van Boven C P A. Outbreak of Acinetobacter junii septicemia in a neonatal intensive care unit possibly caused by contaminated parenteral nutritional fluid. J Hosp Infect. 1997;35:129–140. doi: 10.1016/s0195-6701(97)90101-8. [DOI] [PubMed] [Google Scholar]
- 3.Bernards A T, Dijkshoorn L, van der Toorn J, Bochner B R, van Boven C P A. Phenotypic characterization of Acinetobacter strains of 13 DNA-DNA hybridization groups by means of the Biolog system. J Med Microbiol. 1995;42:113–119. doi: 10.1099/00222615-42-2-113. [DOI] [PubMed] [Google Scholar]
- 4.Bernards A T, van der Toorn J, van Boven C P A, Dijkshoorn L. Evaluation of the ability of the API 20NE system to identify Acinetobacter genomic species. Eur J Clin Microbiol Infect Dis. 1996;15:303–308. doi: 10.1007/BF01695662. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet P J M, Grimont P A D. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov., and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–240. [Google Scholar]
- 6.Bouvet P J M, Grimont P A D. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987;138:569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- 7.Bouvet P J M, Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989;140:291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 8.Chu Y W, Leung C M, Houang E T S, NG K C, Leung C B, Leung H Y, Cheng A F B. Skin carriage of acinetobacters in Hong Kong. J Clin Microbiol. 1999;37:2962–2967. doi: 10.1128/jcm.37.9.2962-2967.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruze J A, Singer J T, Finnerty W R. Conditions for quantitative transformation in Acinetobacter calcoaceticus. Curr Microbiol. 1979;3:129–132. [Google Scholar]
- 10.Dijkshoorn L, van Harsselaar B, Tjernberg I, Bouvet P J M, Vaneechoutte M. Evaluation of amplified ribosomal DNA restriction analysis for identification of Acinetobacter genomic species. Syst Appl Microbiol. 1998;21:33–39. doi: 10.1016/S0723-2020(98)80006-4. [DOI] [PubMed] [Google Scholar]
- 11.Dybowski W, Franklin D A. Conditional probability and the identification of bacteria: a pilot study. J Gen Microbiol. 1968;54:215–229. doi: 10.1099/00221287-54-2-215. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenstein B, Bernards A T, Dijkshoorn L, Gerner-Smidt P, Towner K J, Bouvet P J M, Daschner F D, Grundman H. Acinetobacter species identification using tRNA fingerprinting. J Clin Microbiol. 1996;34:2414–2420. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992;30:2680–2685. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991;29:277–282. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimont P A D, Bouvet P J M. Taxonomy of Acinetobacter. In: Towner K J, Bergogne-Bérézin E, Fewson C A, editors. The biology of Acinetobacter. New York, N.Y: Plenum Press; 1991. pp. 25–36. [Google Scholar]
- 16.Grundmann H J, Towner K J, Dijkshoorn L, Gerner-Smidt P, Maher M, Seifert H, Vaneechoutte M. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J Clin Microbiol. 1997;35:3071–3077. doi: 10.1128/jcm.35.12.3071-3077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horrevorts A, Bergman K, Kollée L, Breuker I, Tjernberg I, Dijkshoorn L. Clinical and epidemiological investigations of Acinetobacter genomospecies 3 in a neonatal intensive care unit. J Clin Microbiol. 1995;33:1567–1572. doi: 10.1128/jcm.33.6.1567-1572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim A, Gerner-Smidt P, Sjostedt A. Amplification and restriction endonuclease digestion of a large fragment of genes coding for rRNA as a rapid method for discrimination of closely related pathogenic bacteria. J Clin Microbiol. 1996;34:2894–2896. doi: 10.1128/jcm.34.12.2894-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jawad A, Snelling A M, Heritage J, Hawkey P M. Comparison of ARDRA and recA-RFLP analysis for genomic species identification of Acinetobacter spp. FEMS Microbiol Lett. 1998;165:357–362. doi: 10.1111/j.1574-6968.1998.tb13170.x. [DOI] [PubMed] [Google Scholar]
- 20.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juni E. Genus III. Acinetobacter Brisou and Prévot 1954, 727AL. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. 8th ed. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 303–307. [Google Scholar]
- 22.Lapage S P, Bascomb S, Willcox W R, Curtis M A. Identification of bacteria by computer: general aspects and perspectives. J Gen Microbiol. 1973;77:273–290. doi: 10.1099/00221287-77-2-273. [DOI] [PubMed] [Google Scholar]
- 23.Priest F G, Williams S T. Computer-assisted identification. In: Goodfellow M, O'Donnell A G, editors. Handbook of new bacterial systematics. London, United Kingdom: Academic Press; 1993. pp. 361–381. [Google Scholar]
- 24.Seifert H, Baginski R, Schulze A, Pulverer G. The distribution of Acinetobacter species in clinical culture materials. Zentbl Bakteriol. 1993;279:544–552. doi: 10.1016/s0934-8840(11)80427-5. [DOI] [PubMed] [Google Scholar]
- 25.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic methods. J Clin Microbiol. 1997;35:2819–2825. doi: 10.1128/jcm.35.11.2819-2825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjernberg I, Ursing J. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. APMIS. 1989;97:595–605. doi: 10.1111/j.1699-0463.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 27.Vaneechoutte M, Dijkshoorn L, Tjernberg I, Elaichouni A, de Vos P, Claeys G, Verschraegen G. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1995;33:11–15. doi: 10.1128/jcm.33.1.11-15.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]