Abstract
Chronic kidney disease-associated pruritus (CKD-aP), also known as uremic pruritus, has been associated with increased mortality and lower quality of life among patients with chronic kidney disease (CKD). The relentless nature of the condition is mainly due to its diverse and complex etiologies, which are still being studied. Despite the introduction of many agents to treat it, the resolution rates of CKD-aP still remain unsatisfactory. This study sought to review the lesser-known/novel treatments and establish a relationship between their mechanism of action and the proposed etiologies implicated in CKD-aP. We also discuss the role of dialysis modification in managing CKD-aP.
A decent proportion of the reviewed studies have proposed that the agents analyzed in them act through hampering inflammation. Interestingly, the results of two agents alluded to the role of dysbiosis in CKD-aP. The addition of hemoperfusion to the dialysis regimen of patients with CKD-aP improved the severity of their symptoms.
The featured treatments could be tried in patients with intractable symptoms. However, additional research is needed to confirm the findings reported in these studies. A better understanding of the pathologic mechanisms is required to help guide the development of agents that can better treat CKD-aP.
Keywords: dialysis, hemoperfusion, itching, ckd pruritus, ckd, uremic pruritus
Introduction and background
Chronic kidney disease-associated pruritus (CKD-aP), sometimes called uremic pruritus, is a common condition affecting patients with CKD. A meta-analysis of several observational studies has reported a prevalence of at least 50% in patients on dialysis [1]. CKD-aP is more than just an irritating itch; in fact, it has far-reaching effects and has been associated with increased morbidity and mortality and decreased quality of life [2-4]. CKD-aP affects patients on hemodialysis and peritoneal dialysis to a similar degree; however, a study on the topic has found that it affected patients on hemodialysis to a lesser extent [1,5]. Interestingly, it has been observed that CKD-aP may persist even after renal transplantation; a study performed in 2020 to determine the prevalence of chronic pruritus among post-renal transplantees revealed a prevalence of 12% [6]. The presentation of CKD-aP is highly variable; it can be localized or generalized and can occur intermittently or persistently [7,8]. Also, the patients usually do not exhibit skin lesions [7].
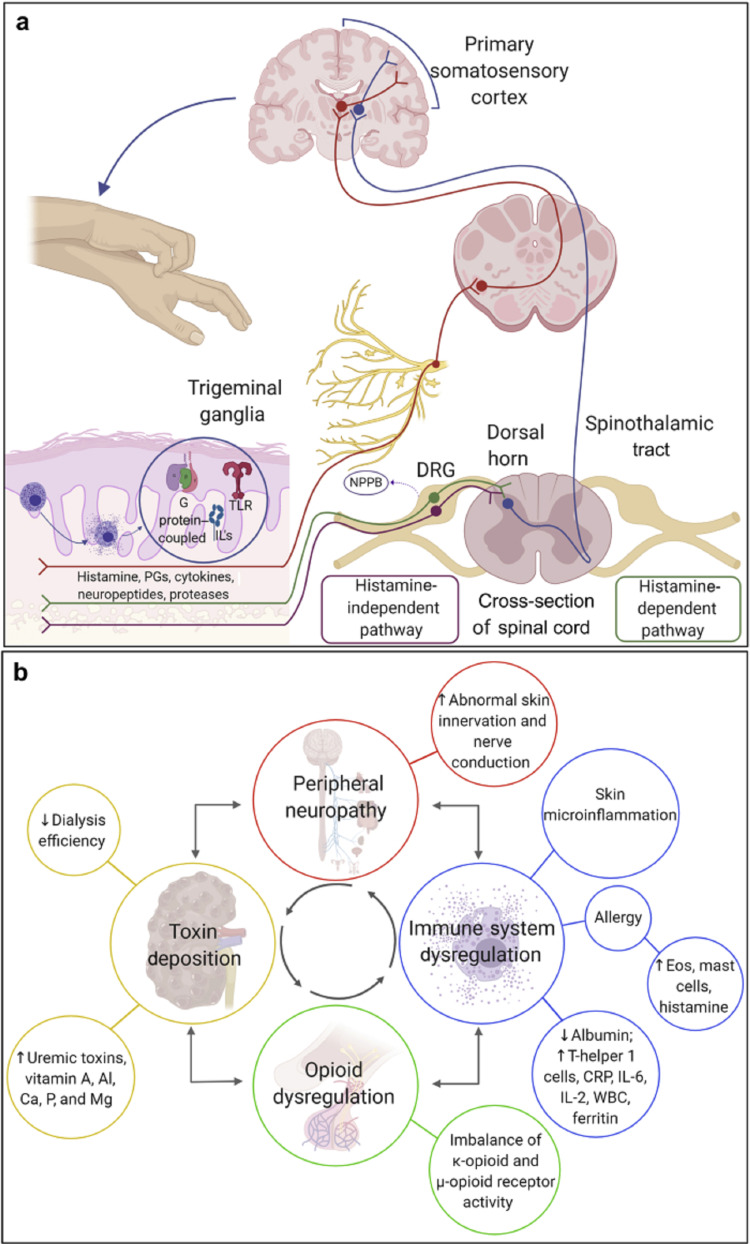
The condition's pathophysiology is complex and is thought to be mediated by the interplay of several mechanisms that are yet to be fully understood [8]. Determining the etiology is further complicated by the potential presence of specific factors associated with a higher chance of developing CKD-aP, such as having hepatitis C and using arteriovenous graft as vascular access for hemodialysis [9-12]. Raised serum calcium, parathyroid hormone (PTH), and phosphorus have also been associated with CKD-aP. The exact mechanism as to how they bring about pruritus is not well elucidated [11,13]. Dry skin has been implicated in the pathogenesis of CKD-aP through a myriad of processes such as a change in skin pH and increased urea excretion in the skin [13].
Figure 1. Pathogenesis of CKD-aP.
CKD-aP: chronic kidney disease-associated pruritus
Image courtesy: Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney International Reports. 2020 Sep 1;5(9):1387-402 [8]
Additionally, the evidence supporting the role of opioid receptors in the pathogenesis of CKD-aP has been strengthened most recently by the KALM-1 trial, which reported pruritus relief in patients treated with difelikefalin [14]. Naltrexone, however, showed conflicting results that suggested the lack of participation of opioids in the condition's pathophysiology [15-17]. Moreover, the contribution of immune dysregulation has been supported by the finding of raised inflammatory markers such as C-reactive protein (CRP) in patients with CKD-aP and their positive response to immunomodulatory drugs [8,13,18]. Furthermore, gabapentin has been shown to be beneficial in treating CKD-aP; this observation suggests a role of neuropathic dysregulation in the pathogenesis of CKD-aP [8,19]. The role of neuropathic dysregulation is further backed by the finding of raised serum levels of neurotrophin-4 and brain-derived nerve growth factors in uremic pruritus patients [20]. Lastly, certain uremic toxins and pruritogenic substances have been implicated in the pathogenesis of CKD-aP. A study conducted by Wu et al. found 22 metabolites that were substantially different between patients with severe uremic pruritus and those with mild uremic pruritus [21]. Interestingly, elevated serum aluminum was associated with pruritus, and its level correlated with the intensity of the itch, but this was not observed in the DOPPS II trial [11,22].
Kt/v, a widely used measure of hemodialysis adequacy, has an inconsistent relationship with pruritus. The DOPPS II study reported no association between Kt/v and pruritus [11]. However, the findings of a cohort study by Ko et al. showed decreased pruritus intensity with a Kt/v target of ≥1.5 [23]. The authors also reported lower pruritus intensity using high-flux dialyzers. The use of polymethylmethacrylate membrane dialyzer, a biocompatible membrane, may relieve pruritus [24].
The treatment of CKD-aP usually comprises various different agents. After obtaining a medical history and examining the patient to exclude similar conditions, it is generally recommended to start the patient on skin moisturizers [25]. However, there are no guidelines concerning the order of treatment agents to be used [26]. Several studies have been performed to address this problem [8,27]. Gabapentin, opioid receptors modulators, antihistamine, topical steroids, and ultraviolet B (UVB) are a few of the agents currently being used [9]. Alternative medicine techniques such as acupuncture have also been used for CKD-aP; a systematic review on the utility of acupuncture and acupressure for uremic pruritus has shown success, but the authors have recommended performing more trials on the topic [28].
A concern regarding the reliability of current medications available for CKD-aP is the inconsistent results in terms of their efficacy and constraints regarding study design [29]. Our incomplete understanding of the complex pathologic processes that bring about CKD-aP is also to blame. Additionally, there are concerns regarding the misuse of gabapentin and the pending adoption of nalfurafine in some countries [25].
This paper aims to review lesser-known treatments for CKD-aP and how their mechanism of action relates to the pathogenesis of CKD-aP. The paper also will look into the contribution of hemodialysis alterations to the treatment of CKD-aP.
Review
Methodology
The evidence for this paper was collected from articles indexed on PubMed and Google Scholar. The search terms used were "CKD associated pruritus," "Uremic pruritus," "ESRD pruritus," and "Treatment of uremic pruritus". Criteria for article selection were as follows: articles in the English language that were published within the last five years.
Pharmacological treatment
Montelukast
Mahmudpour et al. conducted a double-blinded trial on the efficacy of montelukast in treating uremic pruritus by utilizing the visual analog scale (VAS) and detailed pruritus score to assess pruritus intensity. The study reported a significant reduction in the pruritus scores in the montelukast group. Additionally, serum CRP among the montelukast group showed a statistically significant reduction compared to the placebo group [30]. Montelukast acts on cysteinyl leukotriene receptors for leukotrienes D4 and E4, thereby inhibiting the downstream inflammatory effects of these leukotrienes; since mast cells contain the leukotriene receptors, it could explain montelukast's antipruritic effect [31].
Dupilumab
Zhai et al. tested the efficacy of dupilumab on patients with chronic pruritus, including uremic pruritus. The study had five patients with uremic pruritus; they all failed to respond to topical steroids and topical calcineurin inhibitors. They were initiated on a bolus dose of dupilumab (600 mg) and then 300 mg every two weeks. The patients were then followed up for 12 weeks. All patients reported an improvement in the numeric rating scale itch intensity (NRSi). However, it should be noted that only one patient was on hemodialysis [32]. A case report documenting the use of dupilumab in treating a 61-year-old patient with uremic pruritus also reported successful outcomes. Prior to initiating dupilumab, the patient had been on narrow-band UVB phototherapy combined with the use of several agents, including doxepin, pregabalin, and naltrexone at least since the age of 55 years. The patient showed remarkable improvement at the end of six months of treatment [33]. The authors suggest that dupilumab may have relieved the itch by blocking interleukin-4 or interleukin-3 (IL-4 or IL-13) on sensory nerve cells [33]. Oweis et al. have reported IL-13 levels correlating with the severity of uremic pruritus [34]. Given that both studies had patients with refractory pruritus, it is reassuring that dupilumab can be tested in future studies to treat intractable pruritus. However, due to the high cost of dupilumab, it is doubtful that insurance companies will authorize the off-label use of the drug.
Nemolizumab
A randomized, double-blind, placebo-controlled clinical trial was conducted on the itch-reducing effect of nemolizumab. The study subjects were divided into five groups; three groups received nemolizumab at a dose of 0.125 mg/kg, 0.50 mg/kg, and 2 mg/kg, respectively. The fourth group was administered nalfurafine hydrochloride, and the fifth group received a placebo. The study observed no statistically significant improvement in pruritus severity (based on the VAS) between nemolizumab and the placebo group. Also, there was no link between baseline serum IL-31 levels and pruritus score (VAS) in the 48 individuals who received trial therapy. Patients with IL-31 levels of at least 0.86 pg/mL who received nemolizumab demonstrated a decrease in VAS after treatment compared to patients with IL-31 levels <0.86 pg/mL. Neither the placebo nor the nalfurafine groups showed this trend. Surprisingly, the response in the placebo group was higher than that in the nalfurafine group. Nemolizumab is a monoclonal antibody that targets IL-31 [35]. Elevated IL-31 has been found in patients with uremic pruritus [34]. The study findings suggest that nemolizumab is beneficial in a subset of uremic pruritus patients with a high IL-31. Also, the observation that baseline IL-31 did not correlate with baseline VAS score indicates that IL-31 does not correlate with pruritus severity.
Cannabis
A study mentioned in a review by Rein et al. reported an 81% improvement in pruritus among 21 hemodialysis patients after three weeks on a topical agent containing two endocannabinoids [36,37]. The mechanisms by which cannabis is believed to ameliorate the itch are multifold: stimulation of the cannabinoid receptor 1 (CB1) in the central nervous system, action on the transient receptor potential cation channel subfamily V-1 (TRPV-1) present on sensory neurons, modulation of immune cells through cannabinoid receptor 2 (CB2), and reduction in activation of mast cells [38]. Capsaicin acts on TRPV-1, which explains why capsaicin is effective in treating uremic pruritus [9,38]. The study featured in the review employed palmitoylethanolamide (PEA), a ligand for TRPV-1 channels, which has no direct interaction with CB1 and CB2; this suggests that the efficacy and mechanism of action are similar to those of capsaicin. A future study could compare topical PEA with capsaicin to determine any significant difference between the two drugs.
Sodium Thiosulphate IV
A retrospective trial studying the therapeutic effect of sodium thiosulphate in hemodialysis patients complaining of uremic pruritus concluded that patients receiving sodium thiosulphate reported significant relief from pruritus compared to the control group. Given that sodium thiosulphate is used to treat calciphylaxis, one might assume that it ameliorates the pruritus through the removal of calcium; however, no change was observed in calcium and phosphorus levels between the groups [39]. The mechanism of action seems to be non-specific, and further research is needed to identify the mechanism of action.
Butyric Acid Derivative
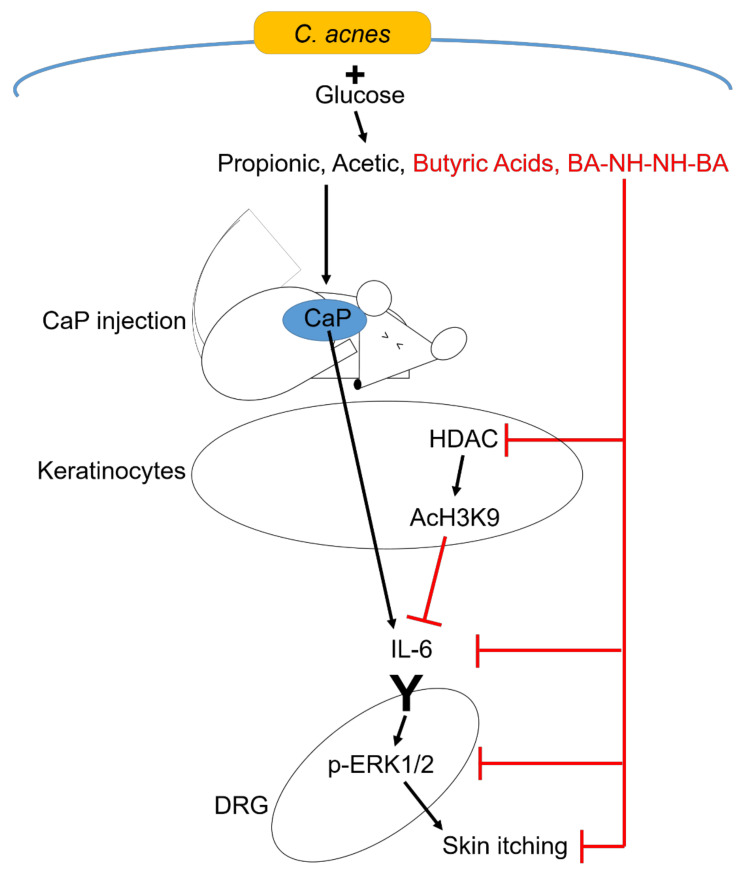
An experimental study conducted on skin Cutibacterium acnes (C. acnes) and a butyric acid derivative about their role in uremic pruritus reported that the number of C. acnes was lower on the skin of CKD patients with pruritus compared to CKD patients with no itch. Secondly, a butyric acid derivative, N-(2-(2-Butyrylamino-ethoxy)-ethyl)-butyramide (BA-NH-NH-BA), topically applied on mice injected with calcium phosphate was found to have the most significant effect on the reduction of pruritus and the reduction in the upregulation of IL-6 [and ultimately extracellular signal-regulated kinases 1/2 (ERK 1/2)] than C. acnes with glucose. The authors observed that the solubilizing ability of butyric acid is less efficient when compared to propionic acid and acetic acid; however, butyric acid was able to diminish IL-6 upregulation by the deposited calcium phosphate in the skin. Interestingly, the authors also observed no change in ERK 1/2 in IL-6 knockout mice, which confirmed the essential role of IL-6 in the upregulation of ERK 1/2.
Calcium phosphate accumulation in the skin leads to the expression of IL-6, which in turn activates ERK 1/2 in the dorsal root ganglia leading to increased sensitivity to certain pruritogens [40]. Butyric acid derivatives obtained by fermentation of glucose by C. acnes dissolve the calcium phosphate and help relieve the itch [40]. This research suggests that calcium phosphate deposition in the skin is an etiological factor in uremic pruritus. Moreover, the role of inflammation through IL-6 upregulation is believed to mediate the itch through the activation of ERK. However, human studies need to be performed to confirm these findings in research and assess safety.
Figure 2. Antipruritic effect of butyric acid and mechanism of calcium phosphate in pruritus.
Image courtesy: Keshari S, Wang Y, Herr DR, et al. Skin cutibacterium acnes mediates fermentation to suppress the calcium phosphate-induced itching: a butyric acid derivative with potential for uremic pruritus. Journal of Clinical Medicine. 2020 Feb;9(2):312 [40]
Non-pharmacological treatment
Omega-3 Fatty Acids
A randomized controlled crossover trial tested the role of omega-3 fatty acids in the treatment of CKD-aP among patients with continuous ambulatory peritoneal dialysis (CAPD); the study assessed the severity of itch intensity using VAS. The study reported significant improvement in the VAS score in both intervention periods [41]. The results of this study are comparable with those of another randomized controlled crossover study [42]. Several anti-inflammatory mechanisms were theorized to have caused the change, such as the reduced activity of the lipoxygenase pathway [43].
Baby Oil
Singh et al. conducted a study on the effectiveness of baby oil in treating uremic pruritus among patients on hemodialysis. The study subjects were divided into an intervention and control group. The pruritus was assessed using a numerical rating system, and the post-intervention assessment was obtained after 10 days. The study reported a statistically significant improvement in pruritus intensity in the intervention group. Besides the emollient properties of baby oil, the product used in the study contained vitamin E, which aids with pruritus [44,45].
Violet Oil
Khorsand et al. ran a randomized control trial on the role of violet oil with or without massage on the severity of pruritus in hemodialysis patients. The study population was divided into two groups; one group used topical violet oil, and the other used topical violet oil and massage. The two groups reported improved dryness scores. However, only the group that used topical violet oil with massage reported statistically significant results regarding pruritus score (VAS). The antipruritic effects of the violet plant can be attributed to sitosterol, a substance similar to analogs of hydrocortisone, which confers anti-inflammatory effects when applied to the skin. Recent studies have shown the presence of tiny nerve endings on the skin that transmit itch sensation, and these fibers are similar to those carrying pain sensation (type C fibers) [46,47]. Massage releases endorphins that might act on these nerve endings, thus alleviating the pruritus [48].
Ostrich Oil
A clinical trial conducted on the therapeutic effect of ostrich oil in reducing the severity of pruritus and improving quality of life in hemodialysis patients reported statistically significant results in those regards. Patients were divided into two groups and were asked to use the intervention for one month. There were no observed changes in the pruritus score during the first and second week; however, during the last two weeks of the trial, there was a reduction in the pruritus score in the group applying the ostrich oil. Oleic acid and linoleic acid are some of the ingredients of ostrich oil, and they relieve pruritus by providing moisture to the skin. Also, linoleic acid is metabolized to an anti-inflammatory prostaglandin analog [49]. The absence of significant changes in pruritus during the first two weeks may discourage physicians from recommending this agent.
Melatonin
A randomized controlled crossover trial conducted by Baharvand et al. studied the antipruritic effect of melatonin on patients with uremic pruritus. The study subjects took either melatonin or placebo for two periods of two weeks, separated by a washout period of one week, and the patients were assessed using the VAS and the 12-item pruritus severity scale questionnaire. The carryover effect was not statistically significant. The study reported better relief from pruritus with melatonin when compared to placebo. It also reported better sleep quality with melatonin [50]. Melatonin production is thought to be decreased in CKD due to the reduced activity of serotonin N-acetyltransferase synthesis [51]. Melatonin is an immunomodulator, and when supplemented, it is thought to relieve the pruritus through an anti-inflammatory mechanism similar to the way it improves the pruritus in atopic dermatitis [50,52].
Charcoal
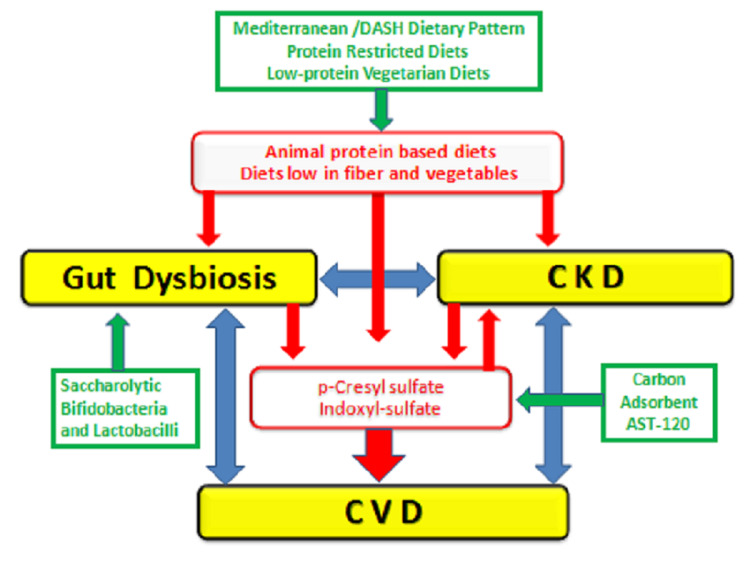
Cupisti et al. reviewed studies on the role of charcoal in treating pruritus in CKD caused by pruritogenic uremic toxins. The authors concluded that charcoal could be suggested to patients with severe refractory pruritus but recommended more research due to conflicting evidence. Also, oral charcoal can affect the absorption of certain drugs given to patients, which has not been studied by many trials [53]. Charcoal seems to relieve pruritus by absorbing small molecules responsible for inducing the itch, such as indoxyl sulfate and p-Cresyl sulfate; these substances have been found to amplify protease-activated receptor-2 expression on the skin, which is linked to non-histaminergic itching [54]. The study also highlights the role of dysbiosis in CKD and its possible contribution to pruritus.
Vegetarian Diet
A cross-sectional study was conducted to find the correlation between a vegetarian diet and uremic pruritus. Pruritus was assessed using the VAS and pruritus scale. Pruritus and inflammatory markers were found to be lower in the vegetarian group (n=15); although the patients were adjusted for dialysis frequency and modality, the low number of participants might have affected the observed finding [55]. Interestingly, six patients in the non-vegetarian group switched to a vegetarian diet for two months, and their CRP and IL-2 decreased significantly. The study results suggest that a vegetarian diet seems to attenuate the inflammatory process mediating the pruritus, which could be due to decreased T cell activity, given that IL-2 is a product of its activation. It would have been interesting if p-Cresyl sulfate and indoxyl-sulfate had been tested to corroborate their relationship with the diet [53].
Figure 3. Relationship between chronic kidney disease (CKD), cardiovascular disease (CVD), and microbiota-derived uremic toxins.
Image courtesy: Cupisti A, Piccoli GB, Gallieni M. Charcoal for the management of pruritus and uremic toxins in patients with chronic kidney disease. Current Opinion in Nephrology and Hypertension. 2020 Jan 1;29(1):71-9 [53]
Dialysis alteration
Increasing Blood Flow
A study by Aliasgharpour et al. tested the role of raising the blood flow in patients with uremic pruritus on hemodialysis. The study population was divided into a control group and an experimental group. Two sessions before the intervention, the mean pump velocity was examined and was raised by 25 rounds per minute (rpm) in the first two weeks and 50 rpm in the final two weeks. The frequency of pruritus in the experimental group showed a significant difference after four weeks, while the severity of pruritus between two hemodialysis sessions showed earlier improvement at two weeks [56]. The study participants were matched on age and gender, but the differences in hemoglobin, calcium, CRP, and other biochemical markers were not mentioned. Increasing the blood flow has been associated with better adequacy of dialysis and higher Kt/v [57]. Hence, the results observed by the authors can be attributed to improved dialysis adequacy.
Altering Membrane Flux
Lim et al. studied the quality of life outcomes (including pruritus) among hemodialysis patients on medium cut-off flux dialyzer vs. high-flux dialyzer. After 12 weeks, the medium cut-off dialyzer group's morning pruritus dispersion was found to be lower. In addition, the medium cut-off dialyzer group had fewer sleep disruptions due to pruritus-related itching. Contrarily, the VAS score in the medium cut-off dialyzer group was higher but not statistically significant compared to the other group (Table 1) [58].
Table 1. Comparision in values between baseline and after 12 weeks of intervention in the study by Lim et al.*.
*[58]
MCO: medium cut-off; SD: standard deviation; VAS: visual analog scale
| Variables | Baseline | 12 weeks | ||||
| MCO (n=24), mean ± SD | High-flux (n=25) | P-value | MCO (n=24) | High-flux (n=25) | P-value | |
| Severity | ||||||
| Morning | 1.92 ± 1.06 | 1.40 ± 0.50 | 0.033 | 1.54 ± 0.72 | 1.64 ± 0.86 | 0.667 |
| Afternoon | 2.00 ± 1.14 | 1.72 ± 0.84 | 0.332 | 1.88 ± 0.95 | 1.84 ± 1.07 | 0.904 |
| Distribution | ||||||
| Morning | 1.42 ± 0.58 | 1.48 ± 0.71 | 0.736 | 1.29 ± 0.46 | 1.64 ± 0.64 | 0.034 |
| Afternoon | 1.46 ± 0.59 | 1.56 ± 0.96 | 0.659 | 1.38 ± 0.65 | 1.56 ± 0.71 | 0.347 |
| Sleep disturbance | ||||||
| Frequency of waking from sleep | 0.83 ± 1.05 | 0.68 ± 1.28 | 0.650 | 0.75 ± 0.85 | 1.32 ± 1.60 | 0.126 |
| Frequency of scratching during sleep | 0.38 ± 0.92 | 0.24 ± 0.72 | 0.571 | 0.25 ± 0.53 | 1.00 ± 1.47 | 0.023 |
| Total score by measuring system | 8.58 ± 7.74 | 7.20 ± 7.58 | 0.530 | 6.92 ± 5.98 | 9.92 ± 8.23 | 0.152 |
| VAS scoring system | ||||||
| Morning | 2.58 ± 2.24 | 2.14 ± 2.28 | 0.496 | 2.50 ± 1.93 | 3.34 ± 2.82 | 0.232 |
| Afternoon | 3.04 ± 2.57 | 2.74 ± 2.53 | 0.680 | 3.46 ± 2.32 | 4.24 ± 3.18 | 0.333 |
| Average | 2.81 ± 2.19 | 2.44 ± 2.31 | 0.565 | 2.98 ± 1.98 | 3.79 ± 2.91 | 0.262 |
Hemoperfusion
Gu et al. conducted a two-year study on patients on hemodialysis to assess if hemoperfusion combined with hemodialysis improves sleep and mortality rates. The study subjects were divided into groups. One group included 80 patients on hemodialysis only, whereas the other group included 78 patients on hemodialysis combined with hemoperfusion. Hemoperfusion was performed one to two times biweekly for two hours. Besides meeting the primary outcome, patients in the combined hemodialysis and hemoperfusion group demonstrated better amelioration of pruritus and better sleep quality. Additionally, biochemical parameters such as calcium and phosphorus showed improvement [59]. The study findings are reassuring and provide a means of symptom control for those who dislike taking medications or are concerned about polypharmacy.
A trial by Zhang et al. studied patients on combined hemodialysis and hemoperfusion vs. combined hemodiafiltration and hemoperfusion. The authors conducted the trial among a study population of 40 patients. Both groups reported significant improvement in pruritus score and biochemical parameters such as calcium, phosphate, PTH, and β2-microglobulin; however, the combined hemodiafiltration and hemoperfusion group showed much more remarkable improvement [60].
Limitations of the study
This study has some limitations. Most of the selected studies did not have large sample sizes. Additionally, the type of vascular access was not delineated in most studies. Also, only a few studies involved patients on peritoneal dialysis. Lastly, not all agents were included in the study due to time constraints.
Conclusions
CKD-aP is a condition with diverse etiologies and treatment options. Immune dysregulation and systemic inflammation seem to be the central pathology by which a sizable proportion of the featured agents work. Also, dysbiosis seems to contribute to CKD-aP through an inflammatory mechanism. A study looking into the use of probiotics in CKD-aP would be interesting.
Additional research is needed to validate the observed effects of the treatments, preferably with a more extensive study population. Future research should also involve patients on peritoneal dialysis. Due to the numerous factors that need to be adjusted for, a single-center study will not be sufficient. Hence, sample collection from multiple centers is recommended. In the meantime, we recommend some of the featured agents as a complementary treatment to patients not responding adequately to their current management. The addition of hemoperfusion or hemodiafiltration is worthwhile and is recommended in CKD-aP patients.
Acknowledgments
I appreciate Dr. Hamid’s support and guidance.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: a meta-analysis of cross-sectional studies. Hu X, Sang Y, Yang M, Chen X, Tang W. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uremic pruritus and long-term morbidities in the dialysis population. Ting SW, Fan PC, Lin YS, Lin MS, Lee CC, Kuo G, Chang CH. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0241088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortality of haemodialysis patients with and without chronic itch: a follow-up study of the German Epidemiological Hemodialysis Itch Study (GEHIS) Grochulska K, Ofenloch RF, Mettang T, Weisshaar E. Acta Derm Venereol. 2019;99:423–428. doi: 10.2340/00015555-3125. [DOI] [PubMed] [Google Scholar]
- 4.A longitudinal study of uremic pruritus in hemodialysis patients. Mathur VS, Lindberg J, Germain M, et al. Clin J Am Soc Nephrol. 2010;5:1410–1419. doi: 10.2215/CJN.00100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Min JW, Kim SH, Kim YO, et al. Kidney Res Clin Pract. 2016;35:107–113. doi: 10.1016/j.krcp.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevalence of pruritus in a single cohort of long-term kidney transplant recipients. Schricker S, Weisshaar E, Kupfer J, Mettang T. Acta Derm Venereol. 2020;100:0. doi: 10.2340/00015555-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recent advances in the treatment of uremic pruritus. Trachtenberg AJ, Collister D, Rigatto C. Curr Opin Nephrol Hypertens. 2020;29:465–470. doi: 10.1097/MNH.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 8.CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Verduzco HA, Shirazian S. Kidney Int Rep. 2020;5:1387–1402. doi: 10.1016/j.ekir.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pruritus associated with chronic kidney disease: a comprehensive literature review. Swarna SS, Aziz K, Zubair T, Qadir N, Khan M. Cureus. 2019;11:0. doi: 10.7759/cureus.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insulin resistance and hepatitis C virus-associated subclinical inflammation are hidden causes of pruritus in Egyptian hemodialysis patients: a multicenter prospective observational study. Abdelsalam M, Tawfik M, Reda EM, Eldeeb AA, Abdelwahab A, Zaki ME, Abdelkader Sobh M. Nephron. 2019;143:120–127. doi: 10.1159/000501409. [DOI] [PubMed] [Google Scholar]
- 11.Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Pisoni RL, Wikström B, Elder SJ, et al. Nephrol Dial Transplant. 2006;21:3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 12.Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS) Kimata N, Fuller DS, Saito A, et al. Hemodial Int. 2014;18:657–667. doi: 10.1111/hdi.12158. [DOI] [PubMed] [Google Scholar]
- 13.Chronic kidney disease-associated pruritus. Agarwal P, Garg V, Karagaiah P, Szepietowski JC, Grabbe S, Goldust M. Toxins (Basel) 2021;13:527. doi: 10.3390/toxins13080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. N Engl J Med. 2020;382:222–232. doi: 10.1056/NEJMoa1912770. [DOI] [PubMed] [Google Scholar]
- 15.Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. Ekelem C, Juhasz M, Khera P, Mesinkovska NA. JAMA Dermatol. 2019;155:229–236. doi: 10.1001/jamadermatol.2018.4093. [DOI] [PubMed] [Google Scholar]
- 16.A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Legroux-Crespel E, Clèdes J, Misery L. Dermatology. 2004;208:326–330. doi: 10.1159/000077841. [DOI] [PubMed] [Google Scholar]
- 17.Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. Pauli-Magnus C, Mikus G, Alscher DM, et al. J Am Soc Nephrol. 2000;11:514–519. doi: 10.1681/ASN.V113514. [DOI] [PubMed] [Google Scholar]
- 18.Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Malekmakan L, Malekmakan A, Sayadi M, Pakfetrat M, Sepaskhah M, Roozbeh J. Saudi J Kidney Dis Transpl. 2015;26:890–895. doi: 10.4103/1319-2442.164565. [DOI] [PubMed] [Google Scholar]
- 19.Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Eusebio-Alpapara KM, Castillo RL, Dofitas BL. Int J Dermatol. 2020;59:412–422. doi: 10.1111/ijd.14708. [DOI] [PubMed] [Google Scholar]
- 20.Evaluation of serum levels of neurotrophin 4 and brain-derived nerve growth factor in uremic pruritus patients. Sorour NE, Elesawy FM, Tabl HA, Ibrahim ME, Akl EM. Clin Cosmet Investig Dermatol. 2019;12:109–114. doi: 10.2147/CCID.S190917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UPLC-QTOF MS-based serum metabolomic profiling analysis reveals the molecular perturbations underlying uremic pruritus. Wu Q, Zhang H, Ding JR, et al. Biomed Res Int. 2018;2018:4351674. doi: 10.1155/2018/4351674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Association between serum aluminum level and uremic pruritus in hemodialysis patients. Hsu CW, Weng CH, Chan MJ, Lin-Tan DT, Yen TH, Huang WH. Sci Rep. 2018;8:17251. doi: 10.1038/s41598-018-35217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. Ko MJ, Wu HY, Chen HY, et al. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0071404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uraemic itching: do polymethylmethacrylate dialysis membranes play a role? Aucella F, Vigilante M, Gesuete A, Maruccio G, Specchio A, Gesualdo L. Nephrol Dial Transplant. 2007;22:0–12. doi: 10.1093/ndt/gfm293. [DOI] [PubMed] [Google Scholar]
- 25.Combs S: CKD-associated pruritus. [ Oct; 2021 ];25] Combs S. https://kdigo.org/wp-content/uploads/2019/09/1.-Combs-CKD_Itch_KDIGO2019.pdf 2019
- 26.Development and validation of a uremic pruritus treatment algorithm and patient information toolkit in patients with chronic kidney disease and end stage kidney disease. Ragazzo J, Cesta A, Jassal SV, Chiang N, Battistella M. J Pain Symptom Manage. 2020;59:279–292. doi: 10.1016/j.jpainsymman.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Have we just scratched the surface? A narrative review of uremic pruritus in 2020. Martin CE, Clotet-Freixas S, Farragher JF, Hundemer GL. Can J Kidney Health Dis. 2020;7:2054358120954024. doi: 10.1177/2054358120954024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A systematic review and meta-analysis of using acupuncture and acupressure for uremic pruritus. Badiee Aval S, Ravanshad Y, Azarfar A, Mehrad-Majd H, Torabi S, Ravanshad S. http://www.ijkd.org/index.php/ijkd/article/view/3273/990. Iran J Kidney Dis. 2018;12:78–83. [PubMed] [Google Scholar]
- 29.Treatment of uremic pruritus: a systematic review. Simonsen E, Komenda P, Lerner B, et al. Am J Kidney Dis. 2017;70:638–655. doi: 10.1053/j.ajkd.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Therapeutic effect of montelukast for treatment of uremic pruritus in hemodialysis patients. Mahmudpour M, Roozbeh J, Raiss Jalali GA, Pakfetrat M, Ezzat Zadegan S, Sagheb MM. http://www.ijkd.org/index.php/ijkd/article/view/2791/898. Iran J Kidney Dis. 2017;11:50–55. [PubMed] [Google Scholar]
- 31.Wermuth HR, Badri T, Takov V. Treasure Island, FL: StatPearls; 2021. Montelukast. [Google Scholar]
- 32.Chronic pruritus responding to dupilumab—a case series. Zhai LL, Savage KT, Qiu CC, Jin A, Valdes-Rodriguez R, Mollanazar NK. Medicines (Basel) 2019;6:72. doi: 10.3390/medicines6030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A successful case of dupilumab treatment for severe uremic pruritus. Silverberg JI, Brieva J. JAAD Case Rep. 2019;5:339–341. doi: 10.1016/j.jdcr.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Oweis AO, Al-Qarqaz F, Bodoor K, et al. Cytokine. 2021;138:155369. doi: 10.1016/j.cyto.2020.155369. [DOI] [PubMed] [Google Scholar]
- 35.Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: a phase II, randomized, double-blind, placebo-controlled clinical study. Kinugasa E, Igawa K, Shimada H, et al. Clin Exp Nephrol. 2021;25:875–884. doi: 10.1007/s10157-021-02047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijuana and cannabinoids in ESRD and earlier stages of CKD. Rein JL, Wyatt CM. Am J Kidney Dis. 2018;71:267–274. doi: 10.1053/j.ajkd.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Emollients with endocannabinoids in the treatment of uremic pruritus: discussion of the therapeutic options. Szepietowski JC, Reich A, Szepietowski T. Ther Apher Dial. 2005;9:277–279. doi: 10.1111/j.1774-9987.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Cannabinoids for the treatment of chronic pruritus: a review. Avila C, Massick S, Kaffenberger BH, Kwatra SG, Bechtel M. J Am Acad Dermatol. 2020;82:1205–1212. doi: 10.1016/j.jaad.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Therapeutic effect of intravenous sodium thiosulfate for uremic pruritus in hemodialysis patients. Song YH, Wang SY, Lang JH, Xiao YF, Cai GY, Chen XM. Ren Fail. 2020;42:987–993. doi: 10.1080/0886022X.2020.1822867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skin cutibacterium acnes mediates fermentation to suppress the calcium phosphate-induced itching: a butyric acid derivative with potential for uremic pruritus. Keshari S, Wang Y, Herr DR, et al. J Clin Med. 2020;9:312. doi: 10.3390/jcm9020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omega-3 supplementation improves pruritus in continuous ambulatory peritoneal dialysis patients: a crossover randomized pilot clinical trial. Lahiji AP, Mortazavi M, Tirani SA, et al. J Res Pharm Pract. 2018;7:195–199. doi: 10.4103/jrpp.JRPP_18_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The effects of omega-3 on the sleep quality of patients with uremic pruritus undergoing hemodialysis: a randomized crossover study. Heydarbaki M, Amerian M, Abbasi A, Amanpour F, Mohammadpourhodki R, Ebrahimi H. J Complement Integr Med. 2020;18:217–222. doi: 10.1515/jcim-2019-0081. [DOI] [PubMed] [Google Scholar]
- 43.Therapeutic effects of omega-3 fatty acids on chronic kidney disease-associated pruritus: a literature review. Panahi Y, Dashti-Khavidaki S, Farnood F, Noshad H, Lotfi M, Gharekhani A. Adv Pharm Bull. 2016;6:509–514. doi: 10.15171/apb.2016.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Effectiveness of baby oil therapy for uremic pruritus in hemodialysis patients. Singh VS, Vinayadev V. Saudi J Kidney Dis Transpl. 2021;32:163–169. doi: 10.4103/1319-2442.318518. [DOI] [PubMed] [Google Scholar]
- 45.10 benefits of vitamin E oil. [ Oct; 2021 ];https://www.medicalnewstoday.com/articles/318168 2020
- 46.The effect of foot reflexology massage on pruritus in hemodialysis patients. Shahriari A, Sarani H, Sheikh S, Arbabisarjou A. J Educ Health Promot. 2021;10:81. doi: 10.4103/jehp.jehp_494_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall JE, Hall ME. Health Sciences Division. Philadelphia, PA: Elsevier; 2020. Guyton and Hall Textbook of Medical Physiology. [Google Scholar]
- 48.Massage therapy research review. Field T. Complement Ther Clin Pract. 2014;20:224–229. doi: 10.1016/j.ctcp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The effect of ostrich oil as a complementary medicine on the severity of pruritus and quality of life in hemodialysis patients. Sadeghnejad Z, Karampourian A, Borzou SR, Gholyaf M, Mohammadi Y, Hadadi R. Complement Med Res. 2021;28:40–45. doi: 10.1159/000508288. [DOI] [PubMed] [Google Scholar]
- 50.Evaluation of antipruritic effect of melatonin on hemodialysis patients with uremic pruritus, a double-blind, randomized, crossover trial. Baharvand P, Abbasi MR, Ziaee Ardestani S, Esmaeili A, Namazi S. http://www.ijkd.org/index.php/ijkd/article/view/5319/1242. Iran J Kidney Dis. 2021;1:38–47. [PubMed] [Google Scholar]
- 51.Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study) Koch BC, van der Putten K, Van Someren EJ, Wielders JP, Ter Wee PM, Nagtegaal JE, Gaillard CA. Nephrol Dial Transplant. 2010;25:513–519. doi: 10.1093/ndt/gfp493. [DOI] [PubMed] [Google Scholar]
- 52.A review of the multiple actions of melatonin on the immune system. Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 53.Charcoal for the management of pruritus and uremic toxins in patients with chronic kidney disease. Cupisti A, Piccoli GB, Gallieni M. Curr Opin Nephrol Hypertens. 2020;29:71–79. doi: 10.1097/MNH.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 54.Uremic solutes of indoxyl sulfate and p-cresol enhance protease-activated receptor-2 expression in vitro and in vivo in keratinocytes. Kim SJ, Zhang X, Cho SB, Kim CH, Park HC, Moon SJ. Hum Exp Toxicol. 2021;40:113–123. doi: 10.1177/0960327120945758. [DOI] [PubMed] [Google Scholar]
- 55.Vegetarian diet may ameliorate uremic pruritus in hemodialysis patients. Tseng CY, Wu TT, Lai CW, Lin HJ, Chou CY, Chang CT, Chen HC. Ren Fail. 2018;40:514–519. doi: 10.1080/0886022X.2018.1512871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The effect of increasing blood flow rate on severity of uremic pruritus in hemodialysis patients: a single clinical trial. Aliasgharpour M, Zabolypour S, Asadinoghabi A, Haghani H, Lesanpezeshki M. J Natl Med Assoc. 2018;110:270–275. doi: 10.1016/j.jnma.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 57.The effect of increasing blood flow rate on dialysis adequacy in hemodialysis patients. Borzou SR, Gholyaf M, Zandiha M, Amini R, Goodarzi MT, Torkaman B. https://www.sjkdt.org/article.asp?issn=1319-2442;year=2009;volume=20;issue=4;spage=639;epage=642;aulast=Borzou. Saudi J Kidney Dis Transpl. 2009;20:639–642. [PubMed] [Google Scholar]
- 58.Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Lim JH, Park Y, Yook JM, et al. Sci Rep. 2020;10:7780. doi: 10.1038/s41598-020-64622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Additional hemoperfusion is associated with improved overall survival and self-reported sleep disturbance in patients on hemodialysis. Gu YH, Yang XH, Pan LH, Zhan XL, Guo LL, Jin HM. Int J Artif Organs. 2019;42:347–353. doi: 10.1177/0391398819837546. [DOI] [PubMed] [Google Scholar]
- 60.Randomised, open-label, multicentre trial comparing haemodialysis plus haemoperfusion versus haemodialysis alone in adult patients with end-stage renal disease (HD/HP vs HD): study protocol. Lu W, Jiang GR. BMJ Open. 2018;8:0. doi: 10.1136/bmjopen-2018-022169. [DOI] [PMC free article] [PubMed] [Google Scholar]