Abstract
Background
It is well documented that malnutrition is a common complication of paediatric malignancy and its treatment. Malnutrition can often be a consequence of cancer itself or a result of chemotherapy. Nutritional support aims to reverse malnutrition seen at diagnosis, prevent malnutrition associated with treatment and promote weight gain and growth. The most effective and safe forms of nutritional support in children and young people with cancer are not known.
Objectives
To determine the effects of any form of parenteral (PN) or enteral (EN) nutritional support, excluding vitamin supplementation and micronutrient supplementation, in children and young people with cancer undergoing chemotherapy and to determine the effect of the nutritional content of PN and EN. This is an update of a previous Cochrane review.
Search methods
We searched the following databases for the initial review: CENTRAL (The Cochrane Library, Issue 2, 2009), MEDLINE (1950 to 2006), EMBASE (1974 to 2006), CINAHL (1982 to 2006), the National Research Register (2007) and Dissertations & Theses (2007). Experts in the field were also contacted for information on relevant trials. For this update, we searched the same electronic databases from 2006 to September 2013. We also scrutinised the reference lists of included articles to identify additional trials.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing any form of nutritional support with another, or control, in children or young people with cancer undergoing chemotherapy.
Data collection and analysis
Two authors independently selected trials. At least two authors independently assessed quality and extracted data. We contacted trialists for missing information.
Main results
The current review included the eight trials from the initial review and six new trials which randomised 595 participants (< 21 years of age) with leukaemias or solid tumours undergoing chemotherapy. The trials were all of low quality with the exception of two of the trials looking at glutamine supplementation. One small trial found that compared to EN, PN significantly increased weight (mean difference (MD) 4.12, 95% CI 1.91 to 6.33), serum albumin levels (MD 0.70, 95% CI 0.14 to 1.26), calorie intake (MD 22.00, 95% CI 5.12 to 38.88) and protein intake (MD 0.80, 95% CI 0.45 to 1.15). One trial comparing peripheral PN and EN with central PN found that mean daily weight gain (MD ‐27.00, 95% CI ‐43.32 to ‐10.68) and energy intake (MD ‐15.00, 95% CI ‐26.81 to ‐3.19) were significantly less for the peripheral PN and EN group, whereas mean change in serum albumin was significantly greater for that group (MD 0.47, 95% CI 0.13 to 0.81, P = 0.008). Another trial with few participants found an increase in mean energy intake (% recommended daily amount) in children fed an energy dense feed compared to a standard calorie feed (MD +28%, 95% CI 17% to 39%). Three studies looked at glutamine supplementation. The evidence suggesting that glutamine reduces severity of mucositis was not statistically significant in two studies (RR 0.64, 95% CI 0.19 to 2.2 and RR 0.85, 95% CI 0.66 to 1.1) and differences in reduction of infection rates were also not significant in two studies (RR 1.0, 95% CI 0.72 to 1.4 and RR 0.98, 95% CI 0.63 to 1.51). Only one study compared olive oil based PN to standard lipid containing PN. Despite similar calorie contents in both feeds, the standard lipid formula lead to greater weight gain (MD ‐0.34 z‐scores, 95% CI ‐0.68 to 0.00). A single study compared standard EN with fructooligosaccharide containing EN. There was no difference in weight gain between groups (mean difference ‐0.12, 95% CI ‐0.57 to 0.33), with adverse effects (nausea) occurring equally between the groups (RR 0.92, 95% CI 0.48 to 1.74).
Authors' conclusions
There is limited evidence from individual trials to suggest that PN is more effective than EN in well‐nourished children and young people with cancer undergoing chemotherapy. The evidence for other methods of nutritional support remains unclear. Limited evidence suggests an energy dense feed increases mean daily energy intake and has a positive effect on weight gain. Evidence suggesting glutamine supplementation reduces incidence and severity of mucositis, infection rates and length of hospital stay is not statistically significant. Further research, incorporating larger sample sizes and rigorous methodology utilising valid and reliable outcome measures, is essential.
Plain language summary
Nutritional support in children and young people with cancer undergoing chemotherapy
The provision of safe, appropriate and effective nutritional support for children and young people undergoing treatment for cancer is now well recognised as an important part of their care. It may help to reverse malnutrition seen at diagnosis, prevent malnutrition associated with the cancer, promote weight gain and growth and improve quality of life. Nutritional support may be provided by one of two methods: intravenous nutritional liquids delivered through a central or peripheral vein which bypass the gut (parenteral nutrition); or nutritional liquids or solids that pass through any part of the gut, regardless of method of delivery (e.g. orally or via a tube; enteral nutrition).
We found evidence from one small trial to suggest that parenteral nutrition may result in an increase in weight, serum albumin levels and calorie and protein intake when compared to enteral nutrition (usual food intake). However, the effect of other methods of delivery of nutritional support remains unclear. Results from another small study suggested that the use of energy dense enteral feeds resulted in greater average daily energy intake and subsequently improved weight gain. Three studies looked at glutamine supplementation and did not show a benefit from its use. One study looked at the effect of using olive oil based parenteral feeds rather than those containing standard fats and found that it lead to less weight gain. One study considered the effect of adding fructooligosaccharide to enteral feeds and found that it did not effect the amount of weight gained or how often participants felt nauseated. No studies were identified that compared the nutritional content in either the PN or EN groups of studies. The trials were all of low quality and very different in terms of outcome measures used. In the future, much larger, rigorously conducted trials are needed in order to address this important question.
Background
Description of the condition
Childhood cancers differ from those seen in adults in both type and outcome (Task Force on Cancer 2002; Boklan 2006). Children have tumours that respond better to chemotherapy, and children tolerate chemotherapy better than adults do (Marsoni 1985; Balis 1997). Children also differ metabolically from adults and continued growth and development are desired throughout therapy that often spans several years.
Progressive nutritional depletion is frequently seen in cancer patients, manifested by anorexia, weight loss, fatigue, muscle wasting, fat mass loss and impaired immune function. The initial nutritional problems resulting from the tumour are soon compounded by iatrogenic nutritional abnormalities, the consequence of the treatment and its complications. Anorexia, mucositis, vomiting, diarrhoea and alterations in taste are important contributory factors to weight loss seen in children and young people undergoing treatment for cancer (Barr 2002); metabolic and psychological factors also have a role (Mauer 1990; Van Cutsem 2005; von Meyenfeldt 2005; Antoun 2006). The consequences of malnutrition are serious, with children who are underweight at diagnosis having a poorer outcome compared to those who are adequately nourished at diagnosis (Donaldson 1981; Argiles 2005; Lange 2005). Malnutrition contributes to a reduced tolerance to therapy, and protein calorie intake may affect sensitivity to chemotherapy agents (Andressy 1998; Charland 1994; Sala 2004; Ladas 2005). Malnutrition may also contribute to problems of drug toxicity due to altered pharmacokinetics secondary to changes in body composition, and the relationship between body surface area and lean body mass (Ladas 2005; Tambori 2005). In addition, the relationship between malnutrition and increased infection rates is well documented in the child with cancer (Taj 1993; Sala 2004). However, the evidence regarding the effect of malnutrition at diagnosis or during treatment on overall survival is unclear and may depend on the disease and its extent (Weir 1998; Yaris 2002; Sala 2004).
Description of the intervention
Nutritional support involves the administration of nutrients in place of, or in addition to, that provided by normal eating and incorporates interventions relating to the methods of delivery (e.g. via parenteral or enteral route) and/or nutritional content (e.g. glutamine supplementation, high energy density). Enteral nutrition (EN) is defined as any method of supplying nutrients via the gastrointestinal tract. It includes the intake of oral food and fluids, but usually indicates the use of nasogastric, nasojejunal, gastrostomy or jejunostomy feeding, which are generally referred to as enteral tube feeding. Parenteral nutrition (PN) is the administration of intravenous nutrition that bypasses the gastrointestinal tract. PN can be administered via a peripheral or central line. Central lines allow infusion of more concentrated solutions and therefore can maximise nutritional intake in fluid restricted patients or those with higher nutrient requirements, and are suitable for long term parenteral nutritional support. The nutritional constituents of the parenteral or enteral feed usually include as a minimum amino acids, glucose, fat, electrolytes, vitamins and trace elements.
How the intervention might work
The main aims of nutritional support are to reverse any malnutrition seen at diagnosis, prevent any future malnutrition associated with treatment and to promote normal weight gain and growth. Nutritional support should improve immune competence, tolerance to treatment and quality of life (Ladas 2005). Successful nutritional support must take the form of the most safe, appropriate and effective method for children and young people with cancer.
Children and young people undergoing treatment for cancer are at risk of depleted nutrient stores due to a decreased oral intake or increased losses due to vomiting, diarrhoea or renal losses. They are often unable to meet their nutritional requirements by oral food intake alone due to problems such as mucositis, taste changes, nausea or a poor appetite, and enteral or parenteral nutritional support is frequently instigated. In general, EN tends to be the method of choice in children and young people undergoing treatment for cancer as it is practical and has advantages over PN including a lower risk of infection and other catheter related complications. It helps to preserve the integrity of the intestinal mucosa, reduces the risk of bacterial translocation and is more economical (Han‐Markey 2000; Deswarte‐Wallace 2001). PN tends to be reserved for when the gastrointestinal tract is not functioning or cannot be accessed or for patients whose enteral feed regimen cannot provide enough nutrients. PN is commonly indicated for children and young people treated for cancer who develop severe mucositis and enteritis. Other indications include typhlitis, neutropenic enterocolitis, ileus, chylous ascites post surgery or severe graft verus host of the gut following bone marrow transplantation (BMT). Metabolic and infection related complications of PN are well documented (Beghetto 2005; Koletzko 2005; Yilmaz 2007). It has been proposed that careful consideration is recommended before commencement of PN due to its limited nutritional benefit if given for less than one week; fluid replacement therapy may be given instead (Schmid 2006).
In the last decade, novel substrates such as glutamine are increasingly being added to feeds to enhance nutritional support. It is thought that glutamine support may improve nutritional and immunological parameters in children and reduce the risk of mucositis whilst receiving chemotherapy (Aquino 2005; Okur 2006; Ward 2009). Choosing the most effective and safe form of nutritional support is essential, yet current knowledge relating to evidence for nutrition support is poor (NICE 2006).
Why it is important to do this review
Prior to the last version of this review (Jones 2010), two non‐Cochrane reviews were conducted, which were in need of updating (McGeer 1990; Klein 1994). One indicated that PN, when compared with control (not defined), had a detrimental effect: decreased survival, poorer tumour response and a significant increase in infectious complications. This review included studies of children, but the literature search was restricted to MEDLINE (McGeer 1990). The other narrative review of randomised controlled trials aimed to evaluate separately the clinical efficacy of PN and EN in children and adults with cancer undergoing a variety of treatments such as surgery, chemotherapy, radiotherapy and BMT (Klein 1994). The review concluded that PN made no difference to survival or haematological or gastrointestinal toxicity, worsened tumour response rates and increased infection rates. No obvious therapeutic benefit was seen with respect to survival, tumour response or chemotherapy toxicity in the EN studies. This review was assessed by the Centre for Reviews and Dissemination (CRD), York. The CRD concluded that they were unable to determine the completeness of the review in terms of the resources searched and the methods used for quality assessment. The review was limited to inclusion of English language studies.
The aim of the current review was to update the previous review (Jones 2010), examine any new evidence in terms of the effectiveness of any form of nutritional support (PN or EN) and/or nutritional content of enteral/parenteral nutrition, focusing on children and young people with cancer undergoing chemotherapy.
Objectives
To determine the effects of nutritional support (excluding vitamin and micronutrient supplementation) in children and young people with cancer undergoing chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials or quasi‐randomised trials (e.g. alternate patient admissions).
Types of participants
Children and young people (age ≤ 21 years) with any form of malignant disease (leukaemias, lymphomas, solid tumours) which required chemotherapy. We considered studies which included radiotherapy treatment in combination with chemotherapy.
Types of interventions
One form of nutritional support compared with another or with no nutritional support (i.e. usual food intake, fluid therapy).
Nutritional support is defined here as the administration of nutrients in place of or in addition to normal eating and incorporates interventions relating to method of delivery (e.g. via parenteral or enteral route) and/or nutritional content (e.g. glutamine supplementation, high energy density feeds, high fibre feeds). It excludes specific vitamin or micronutrient supplementation, as these strategies differ from those of general nutritional support in their prescription and their aims, for example bone health and vitamin D.
No nutritional support comprises:
Usual food intake ‐ defined here as oral feeding from a selection of foods that are eaten by the patient as part of their normal diet.
Fluid therapy (FT) ‐ the administration of a solution of glucose, saline and electrolytes.
PN is the intravenous administration of nutrients containing, as a minimum, glucose and amino acids. It is administered through the central or peripheral venous system and therefore bypasses the gastrointestinal tract. PN normally consists of a standardised PN solution containing proteins, carbohydrates, lipids and electrolytes that may have additions of vitamins, trace elements or glutamine.
EN is the delivery of any substance of nutritional value in solid or liquid form that passes any part of the digestive tract, regardless of the method of delivery (orally or via a tube, e.g. nasogastric, nasojejunal, gastrostomy, jejunostomy). Since EN also includes usual food intake, for the purposes of this review it can be considered to be a form of no nutritional support or control, e.g. selection of favourite foods and/or beverages, as well as a form of nutritional support, e.g. oral calorie supplements added to usual food intake, calorie dense or hydrolysed or elemental formulas delivered via a tube.
Examples of interventions that could have been compared include:
PN versus EN, e.g. PN versus usual food intake, PN versus enteral tube feeding.
EN versus EN, e.g. nasogastric versus usual food intake, nasogastric versus gastrostomy, usual food intake and oral calorie supplements versus usual food intake alone.
PN versus PN, e.g. peripheral PN versus central PN, PN and glutamine versus PN alone.
PN and EN versus PN alone.
PN and EN versus EN alone.
PN versus FT.
Types of outcome measures
Primary outcomes
-
Change in nutritional indices.
Weight.
Height.
Body mass index.
Fat‐free body mass.
Total body water.
Arm anthropometry (triceps/biceps skinfold thickness, mid‐upper arm circumference, arm circumference, arm muscle area).
Serum albumin.
Pre‐albumin.
-
Adverse events.
Infection rate (line infection, positive blood culture, catheter tip infection).
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting, or severity or duration of mucositis.
Abnormal biochemical profiles (serum glutamine or serum ammonia levels or dyslipidaemia)
-
Calorie and nutritional intake.
Total energy intake.
Total protein intake.
Secondary outcomes
Number of deaths at end of study.
Length of hospital stay.
Patient tolerance of or adherence to nutritional intervention.
Participant perceived health status, where possible using validated tools for measuring performance and/or quality of life.
Search methods for identification of studies
Electronic searches
The authors of the original review searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 2, 2009), MEDLINE via DataStar (from 1950 to 31 March 2006), EMBASE via DataStar (from 1974 to 31 March 2006) and CINAHL via DataStar (from 1982 to 30 April 2006). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text word terms) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4). No language restrictions were applied to the searches. The reviewers also searched Dissertations & Theses (via ProQuest) to identify additional published or unpublished data on 31 January 2007, and the National Research Register (NRR; electronic register of ongoing research) on 31 January 2007.
For this update, we searched MEDLINE and EMBASE via Ovid, the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 9, 2014), and CINAHL (via Ebsco) on 7 October 2014. The search strategies are in the appendices (Appendix 5; Appendix 6; Appendix 7; Appendix 8).
Searching other resources
We reviewed the bibliographies of the randomised trials and review articles identified, and contacted the study authors and known experts in the field.
Data collection and analysis
Selection of studies
In the original review, one author (LJ) scanned the titles and abstracts of every record retrieved by the searching process for eligibility. Hard copies of all studies which appeared to meet the inclusion criteria were then retrieved in full. Two authors (LJ, RW) then independently assessed the eligibility of each study using an inclusion/exclusion form designed specifically for this review, without blinding. There were no discrepancies between authors in trial selection.
For the update, two authors (EW, LH) independently reviewed the titles and abstracts of every record retrieved by the searching process for eligibility. All 'include' studies and any discordant studies were discussed with a third author (RSP or SW) and consensus was reached. We retrieved a full text copy of any study which could not be confirmed to meet the exclusion criteria. Two authors (EW, LH) then independently assessed the eligibility of each study using an inclusion/exclusion form designed specifically for this review. No disagreements occurred at this stage of the process.
Data extraction and management
In the original review three authors (LJ, RW, SW) independently extracted data (using a customised data extraction form designed by the Cochrane Cystic Fibrosis and Genetic Disorders Group). Where information was lacking, those reviewers contacted primary authors for clarification.
They analysed the different nutritional support interventions identified within the following comparative groups: PN versus EN (usual food intake); EN (nasogastric) versus EN (usual food intake); PN and EN versus PN; and PN versus FT. Statistical analysis of data from nine out of a total of 16 outcomes was possible. Varying numbers of trials were included in each analysis. They reported the remaining outcomes narratively because the clinical endpoints and measurements differed widely across trials.
To avoid duplicate reporting of outcome data from 14 of the patients enrolled in a single‐centre trial (Ghavimi 1982) that were also enrolled into a multi‐institutional trial (Donaldson 1982) on PN versus EN, where both trials reported on the same outcome measure, the authors of the original review only entered the data from one of the trials into the statistical analysis. They took the following approach when looking at the effects of interventions.
When both studies provided outcome data in a format amenable to analysis, they reported both sets of results narratively and only incorporated the data from the multi‐institutional study conducted by Donaldson 1982 into an analysis (diarrhoea, nausea, vomiting, number of deaths).
When both studies provided outcome data, but only one study provided it in a format amenable to analysis (e.g. mean, standard deviation), they reported both sets of results narratively and only incorporated the data from the study which provided the data in an appropriate format into an analysis (arm anthropometry).
For some outcomes both studies presented data either only narratively or in a format not amenable to analysis; in these instances they reported both sets of outcome data narratively (arm muscle circumference, serum albumin).
For all other outcomes, only one of the studies reported on a particular outcome and so duplicate reporting of data was not an issue (arm circumference, infection rate).
For the review update, two authors (EW, LH) independently extracted the data and checked the data from each included study using a customised data extraction form based on that used for the original review.
The new studies included in the updated review addressed different questions regarding the benefit of nutritional support compared to the original review. The additional areas were: the benefit of energy dense or fibre supplemented nasogastric feeds, the value of glutamine supplementation and the comparative value of different lipid formulations for PN.
Varying numbers of trials were included in each analysis, either statistical or narrative. There were no issues with duplicate reporting in the trials added to this review.
Assessment of risk of bias in included studies
For the original review, three authors (LJ, RW, SW) independently assessed the methodological quality of the selected trials. The evaluation of methodological quality was based on the method described by Jüni as outlined below (Jüni 2001).
For this update, two review authors (EW, LH) assessed the risk of bias of each study independently. The following aspects that may cause bias were examined: selection bias (including sequence generation and allocation concealment), performance bias (including blinding of participants and personnel), detection bias (including blinding of outcome assessors), attrition bias (including incomplete outcome data), and reporting bias (including selective outcome reporting) as described in the latest module of the Childhood Cancer Group (CCG 2012), which is based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Where a mixture of subjective and objective outcomes were present, and outcome assessment was undertaken without blinding, we chose to take the 'worst case' approach and describe all outcomes as having high risk of bias. We assessed each trial to see if the study was free of suggestion of selective outcome reporting by comparing the outcomes described in the methods section to the reported in the results section as a proxy, as original trial protocols were not available. If other biases particular to the study type or intervention were thought possible, these were recorded as 'Other bias' (for example, the use of an unverified and unreported symptom questionnaire may have introduced bias). Each item was scored as having a low, high, or unclear risk of bias. In case of disagreement between the two review authors, a third review author (RSP) assessed the risk of bias. The assessments in the original review were also updated.
Measures of treatment effect
For both dichotomous and continuous outcomes, we performed available case analyses using data only on those whose results were known (Higgins 2011b). We analysed dichotomous data using risk ratios (RR) and reported them with 95% confidence intervals (CI). For continuous outcomes, depending on how data were reported, we recorded the mean change or mean post‐intervention values with their corresponding standard deviations (SD) and reported them with 95% CIs.
Dealing with missing data
Where information was lacking, authors of the original review contacted primary authors for clarification. Responses were received with additional information from two (Van Eys 1980; Schmid 2006). One author supplied additional information on methods of randomisation and allocation concealment (Van Eys 1980); the other author supplied additional information on methods of randomisation and allocation concealment as well as individual patient data for change in nutritional indices outcomes (Schmid 2006). Authors of the updated review did not seek information from original study authors.
Assessment of heterogeneity
Where data were sufficient to allow meaningful meta‐analysis, we investigated statistical heterogeneity between trials using the I2 statistic (Higgins 2003). This measure describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). The values of I2 lie between 0% and 100% and we used a simplified categorisation of heterogeneity: low I2 (between 0% and 25%), moderate I2 (between 25% and 50%), high I2 (between 50% and 75%) and very high I2 (over 75%) (Higgins 2003).
Data synthesis
Where meta‐analyses were performed, we reported average summary estimates of measures of relevant outcomes with 95% confidence intervals using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
As with the original review, we had planned to explore statistical heterogeneity by performing subgroup analyses by:
age group;
type of malignancy;
type of chemotherapy;
intensity of chemotherapy;
However, an insufficient number of trials was identified to allow this. Where reports had moderate or greater statistical heterogeneity, we planned to calculate a prediction interval (Higgins 2009), which estimates the likely result that would be found in further studies.
Sensitivity analysis
We had planned to conduct sensitivity analyses to assess the robustness of the review results by repeating the analyses including only results from reviews that had low risk of bias. No single meta‐analysis included more than three trials, however ‐ an insufficient number to allow this.
Results
Description of studies
Results of the search
The original search of electronic databases identified a total of 8209 citations. No ongoing trials were found from the National Research Register. After initial screening for eligibility by one author (LJ), 37 potential citations were identified from the database searches and one further potential citation was identified by scanning reference lists. Two authors (LJ, RW) independently assessed these 38 potential citations using an inclusion/exclusion form. We excluded 17 studies and included eight individual trials (21 citations) which examined the use of a nutritional intervention in children and young people with cancer (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1985; Rickard 1989; Smith 1992; Schmid 2006).
The reviewers conducted an updated search of the CENTRAL database (The Cochrane Library 2009, issue 2) after the review was finished, and identified three trials (den Broeder 2000; Zheng 2006; Ward 2009) for inclusion in the eventual update.
For the 2014 update, our searches revealed a total of 2808 potentially relevant new citations. Full texts of 12 trials were retrieved for further analysis. Of these, two trials were included (Hartman 2009; Uderzo 2011) along with the three articles previously identified (den Broeder 2000; Zheng 2006); Ward 2009 and another which was previously excluded (Aquino 2005). The original review authors had classified this study as "Not chemotherapy," however it concerns treatment after high dose conditioning chemotherapy for stem cell transplantation. Six newly identified studies were excluded. No further studies were found for inclusion from reference lists or content experts.
Included studies
A total of 14 individual trials (27 citations) are now included in this updated review which examined the use of a nutritional intervention in children and young people with cancer. These comprise eight trials from the initial review (21 citations) (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1985; Rickard 1989; Smith 1992; Schmid 2006) and a further six trials (den Broeder 2000; Aquino 2005; Zheng 2006; Hartman 2009; Ward 2009; Uderzo 2011).
Trial characteristics
All included studies were randomised controlled trials except for Ward 2009 which was quasi‐randomised. This updated review contains information from more than twice the original number of participants, with a total of 562 patients randomised (previously 159 participants).
Participants
The participants in the trials were all children and young people (under 21 years of age) with leukaemias or solid tumours undergoing chemotherapy (including hematopoietic stem cell transplants), radiotherapy or both.
Interventions
The studies from the initial review largely focused on the benefit of active, interventional approaches to nutritional supplementation compared to usual care. Four trials compared PN to EN (usual food intake) in well‐nourished patients (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983); one trial compared EN (nasogastric) to EN (usual food intake) in malnourished patients (Smith 1992); two trials compared peripheral PN and EN (usual food intake) to central PN alone in malnourished patients (Rickard 1985; Rickard 1989); and one trial compared PN to FT in patients with mucositis (Schmid 2006).
The trials added by the update consider questions relating to the benefits of modifications of supplemental nutrition, either in EN (nasogastric) feeds ‐ where one trial compared standard versus calorie‐enriched feeds (den Broeder 2000), and one examined fibre‐enriched vs. standard feeds (Zheng 2006) ‐ or in PN (central), where one trial compared the short term effects of different lipid formulations (Hartman 2009). The final three studies evaluated the effect of the addition of glutamine to EN (Aquino 2005; Ward 2009) or PN (Uderzo 2011).
Outcomes measured
The relevant outcomes reported in each trial are presented in the Characteristics of included studies table. Outcomes relevant to this review comprise:
Primary outcomes
1. Change in nutritional indices.
a. Weight.
Weight was reported as an outcome in ten trials, but methods of reporting varied as follows: mean percentage weight change for each group (Ghavimi 1982); mean change in weight (kg) for each group (Hays 1983; Rickard 1985; Uderzo 2011); mean daily weight gain (g/day) and the mean percentage weight change for each group (Rickard 1989); median change in weight for each group (Schmid 2006); mean percentage change in weight z‐score (Hartman 2009), proportion of group with weight‐for‐height z‐score ≥ 0 (den Broeder 2000), and final values of group differences in weight for height (Zheng 2006; Ward 2009) and weight‐for‐age (Zheng 2006).
b. Height.
This outcome was reported in only two studies; as the individual heights of each participant in each group at the start of the trial (Rickard 1985) or as mean heights during the trial (Zheng 2006). Height was also used in calculating weight‐for‐height z‐score ≥ 0 (den Broeder 2000), but the height itself was not reported.
c. Body mass index (BMI).
This was not reported in any of the trials.
d. Fat‐free body mass.
This was reported in one trial as the median change in fat‐free mass for each group at the end of the study (Schmid 2006).
e. Total body water.
This was reported in one trial as the median change in total body water for each group at the end of the study (Schmid 2006).
f. Arm anthropometry.
Arm anthropometry was reported in eight trials as follows: median percentage change from baseline for triceps skinfold, arm circumference and arm muscle circumference (Donaldson 1982); mean skinfold thickness and mean arm muscle circumference (cm) at the end of the study for each group (Ghavimi 1982); initial and final percentiles for triceps skinfold and arm muscle area for each group (Hays 1983); mean mid‐upper arm circumference (cm) for each group at three different time points (Smith 1992); mean change in triceps skinfold and subscapular skinfold (mm) for each group (Rickard 1985); mean percentage change in triceps skinfold and subscapular skinfold for each group (Rickard 1989); mean percentage different from reference populations for triceps skinfold, biceps skinfold and mid‐upper arm circumference, mean change in overall arm muscle area (den Broeder 2000); and mean mid‐upper arm circumference (cm) for each group at the end of the study (Ward 2009).
g. Serum albumin.
This was reported in ten trials as follows: narratively (Donaldson 1982; Ghavimi 1982; Zheng 2006); mean change in serum albumin (units not reported) for each group (Hays 1983); mean change in serum albumin (g/dl) for each group (Rickard 1985; Rickard 1989); median change in serum albumin (g/dl) for each group (Schmid 2006); mean pre (Uderzo 2011) and post intervention values of serum albumin (g/dl) for each group (den Broeder 2000; Hartman 2009; Uderzo 2011).
h. Pre‐albumin.
Pre‐albumin was reported in six trials as follows: narratively (Hays 1983; Zheng 2006); mean change in pre‐albumin (mg/dl) for each group (Rickard 1989); median change in pre‐albumin (mg/dl) for each group (Schmid 2006); and mean pre and post intervention values of serum pre‐albumin (g/dl) for each group (den Broeder 2000; Uderzo 2011).
2. Adverse events.
a. Infection rate.
Infection rate was reported in seven trials as follows: rates of sepsis and all infections requiring antibiotic therapy (Van Eys 1980); rates of positive blood culture (Ghavimi 1982; Rickard 1989; Uderzo 2011); rates of positive culture from blood, urine or stool (Hays 1983; Schmid 2006), episodes of bacteraemia (Aquino 2005).
b. Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting, or severity or duration of mucositis.
Diarrhoea, nausea and/or vomiting was reported in seven studies and mucositis in three studies as follows: the number of patients in each group attaining a maximum score for symptoms of severity for nausea or vomiting and bowel movement (Ghavimi 1982); the number of patients experiencing nausea or vomiting and diarrhoea in each group (Donaldson 1982; Zheng 2006; Ward 2009); the number of vomiting events (den Broeder 2000); the number of patients experiencing recurrent vomiting in each group (Smith 1992); the number of patients developing diarrhoea in each group (Rickard 1989; Zheng 2006; Ward 2009); and stool culture positive for enterobacteria, bifidobacteria, Lactobacillus or Clostridium (Zheng 2006). Mucositis was reported by number of patients affected (Ward 2009; Uderzo 2011) and by severity of mucositis (Aquino 2005; Uderzo 2011).
c. Abnormal biochemical profiles (serum glutamine or serum ammonia levels or dyslipidaemia).
The reporting methods of abnormal biochemical profiles were specifically driven by the interventions being studied. In the study of glutamine supplementation by the enteral route, it was examined using the highest (Aquino 2005; Ward 2009) and mean (Ward 2009) serum ammonia values. Serum lipid profiles were examined by Hartman 2009, who studied the use of different PN lipids.
3. Calorie and nutritional intake.
a. Total energy intake.
Descriptions of total energy intake were reported in six of the trials as follows: mean calorie intake (cal/kg/day) in each group (Hays 1983; Hartman 2009); percentage recommended daily allowance (RDA) of energy for each group (Smith 1992); mean change in energy intake (% healthy children) for each group (Rickard 1985); and mean energy intakes (% healthy children) in each group (Rickard 1989den Broeder 2000).
b. Total protein intake.
This was reported in five trials as follows: mean protein intake (g/kg/day) in each group (Hays 1983; den Broeder 2000); mean change in protein intake (g/kg/day) in each group (Rickard 1985); and mean protein intake (g protein/kg) in each group (Rickard 1989; Hartman 2009).
Secondary outcomes
1. Number of deaths at end of study.
The number of deaths in each group at the end of the study was reported in eight trials (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1989; Smith 1992; Aquino 2005; Uderzo 2011).
2. Length of hospital stay.
Length of hospital stay was reported in three trials, as the median hospital length of stay (days) in each group (Schmid 2006) and the mean hospital length of stay (days) per group (Aquino 2005; Uderzo 2011).
3. Patient tolerance of, or adherence to, nutritional intervention.
Patient tolerance of the nutritional intervention was reported as the acceptability of the mode of feeding in the nasogastric group (Smith 1992). The average volume of feed was also reported in den Broeder 2000, and an unspecified measure of feed tolerance was reported in Zheng 2006.
4. Participants’ perceived health status
Participants' perceived health status was reported in two trials as follows: performance status, defined as the level of activity compared to baseline activity for each group (Donaldson 1982), and performance status, defined as the level of activity during play, and reported median play scores for each group (Smith 1992).
Excluded studies
We excluded 22 studies because they did not fulfil all of the inclusion criteria (see 'Characteristics of excluded studies' table).
Risk of bias in included studies
Details of quality assessment for each study are given in the Characteristics of included studies and visualised in Figure 1 and Figure 2.
1.
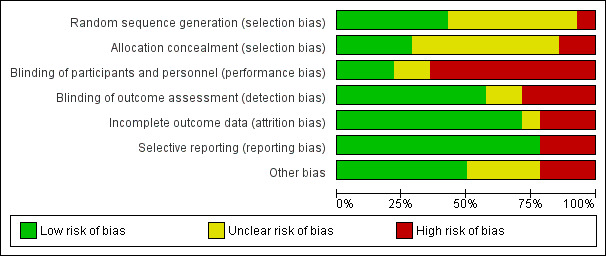
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
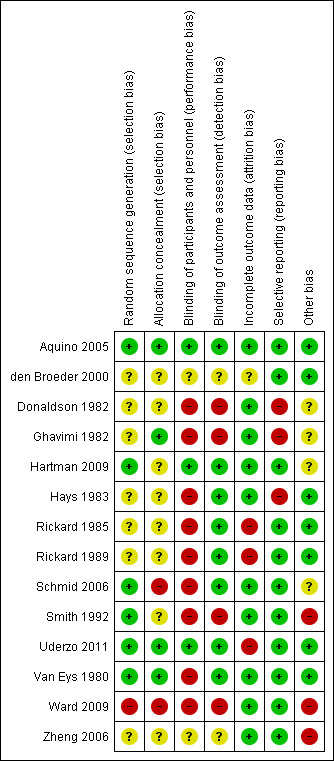
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation information was initially provided by four trials. These were graded as having a 'low risk of bias' (Smith 1992; Aquino 2005; Hartman 2009; Uderzo 2011). Two trials initially graded as 'unclear' were changed to 'low risk of bias' following correspondence with authors (Van Eys 1980; Schmid 2006). The one quasi‐randomised trial was graded as having a 'high risk of bias' (Ward 2009). The remaining trials were graded as 'unclear' as the authors failed to provide sufficient information (Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1985; Rickard 1989; den Broeder 2000; Zheng 2006).
Three trial reports provided information on allocation concealment and were graded having a 'low risk of bias' (Ghavimi 1982; Aquino 2005; Uderzo 2011). Two trials were initially graded as 'unclear', but after correspondence with the author, one was changed to 'high risk of bias' (Schmid 2006), and one was changed to 'low risk of bias' (Van Eys 1980). Eight of the trials provided no information on allocation concealment and were graded as 'unclear' (Donaldson 1982; Hays 1983; Rickard 1985; Rickard 1989; Smith 1992; den Broeder 2000; Zheng 2006; Hartman 2009). The one quasi‐randomised trial was graded as having a 'high risk of bias' (Ward 2009).
Blinding
Clinicians or persons delivering treatment or participants
Three trials undertook masking of the clinicians to the treatment delivered and were considered to be at 'low risk of bias' (Aquino 2005; Hartman 2009; Uderzo 2011). In seven trials blinding was considered impossible (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1985; Rickard 1989; Smith 1992). In two other trials, correspondence with the author confirmed that no blinding was performed, and in these it was considered that there was a 'high risk of bias' (Schmid 2006; Ward 2009). It remained unclear in the final two studies, as we were uncertain whether clinicians were blinded (den Broeder 2000; Zheng 2006).
Outcome assessor
Three trials undertook blinding of the outcome assessors to the treatments delivered and were considered to be at 'low risk of bias' (Aquino 2005; Hartman 2009; Uderzo 2011). In seven trials no information was provided on the blinding of outcome assessors (Donaldson 1982; Ghavimi 1982; Hays 1983; Rickard 1985; Rickard 1989; Smith 1992; Van Eys 1980). In two other trials, correspondence with the author confirmed that no blinding of outcome assessment was performed (Schmid 2006; Ward 2009). The objective nature of the outcomes in five of these studies means that these were considered to be at 'low risk of bias' (Van Eys 1980; Hays 1983; Rickard 1985; Rickard 1989; Schmid 2006), while the subjective nature of some outcomes in the other four led to assessments of 'high risk of bias' (Donaldson 1982; Ghavimi 1982; Smith 1992; Ward 2009). In the final two studies, blinding of outcome assessor was uncertain (den Broeder 2000; Zheng 2006).
Incomplete outcome data
Only Aquino 2005 clearly undertook a full intention to treat analysis, and was considered to be at 'low risk of bias'.
In seven trials withdrawals from treatment were described, although the amount of missing data varied between outcomes (Van Eys 1980; Ghavimi 1982; Rickard 1985; Rickard 1989; den Broeder 2000; Ward 2009; Uderzo 2011). The den Broeder 2000 study did not give adequate information on the amount of missing data, and so was considered to be at 'unclear risk of bias'. The studies by Ghavimi 1982 and Van Eys 1980 provided sufficient information for the incomplete outcomes to be accounted for, and so were considered to be at 'low risk of bias'. Ward 2009 specifically reported safety data in those who had not completed the interventions, and was also considered at 'low risk of bias'. The other studies (Rickard 1985; Rickard 1989; Uderzo 2011) were considered at 'high risk of bias' related to attrition.
In the remaining trials there were very few withdrawals (Donaldson 1982; Hays 1983; Smith 1992; Schmid 2006; Zheng 2006; Hartman 2009) and these were therefore considered to be at 'low risk of bias'.
Selective reporting
For this review we looked at two aspects of outcome reporting bias.
1. Outcomes relevant to this review were described in the trial as being measured but the results were not reported. 2. Time points where measurements were taken were described in the trial but the results for those corresponding time points were not reported.
Outcomes listed but not reported
In two trials, outcomes were described as being measured, but the trials did not report the results for these particular outcomes or only reported them narratively (Ghavimi 1982; Hays 1983). These outcomes related to nutritional status and quality of life. For some studies, outcomes irrelevant to this systematic review (for example, daily blood tests in patients following stem‐cell transplant) were measured by not reported (Van Eys 1980; Schmid 2006; Zheng 2006; Uderzo 2011); these were not felt to impact any risk of bias.
Time points listed but not reported
In three trials, outcomes were reported as being measured at particular time points, but the results for these corresponding time points were not presented (Donaldson 1982; Ghavimi 1982; Hartman 2009). In two of these studies, the reason for the choice of time points reported was not clear, and so these studies were judged to be at 'high risk of bias' (Donaldson 1982; Ghavimi 1982). For the Hartman 2009 study, day zero and day 14 time points were reported and only daily weights not detailed, and this was considered to carry a 'low risk of bias' related to selective outcome reporting.
In the other studies, no inconsistencies leading to concerns over selective outcome reporting were noted (Rickard 1985; Rickard 1989; Smith 1992; den Broeder 2000; Aquino 2005; Ward 2009). In no case was an original trial protocol available with which to compare the stated and reported outcomes.
Other potential sources of bias
In three trials, additional sources of potential bias were noted. In Ward 2009 the investigator determined the use of enteral and parenteral nutrition, and may have affected these parameters in the two groups. In the Zheng 2006 and Smith 1992 studies no details of an assessment questionnaire were provided, which makes interpretation of the subjective reports of feed tolerance difficult. In seven studies it was felt that information provided was sufficient to allow a conclusion that there were no other risks of bias (Van Eys 1980; Hays 1983; Rickard 1985; Rickard 1989; den Broeder 2000; Aquino 2005; Uderzo 2011). In four further studies issues were noted which were of uncertain significance: the nature of nausea assessment (Donaldson 1982; Ghavimi 1982), the interpretation of safety profiles after limited duration of exposure to intravenous lipid (Hartman 2009), and multiple randomisations of the same patient, where the uncertainty in the magnitude of how responses varied between individuals receiving each intervention compared with the magnitude of variation in response between individuals were unknown, and so we were unsure if this would introduce significant bias or not (Schmid 2006).
Effects of interventions
Parenteral nutrition versus enteral nutrition (usual food intake)
Four trials reported on this comparison (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983).
Primary outcomes
Change in nutritional indices
Weight
This outcome was reported in two trials (Ghavimi 1982; Hays 1983).
Hays 1983 (N = 10) found that there was a significant difference in mean change in weight (kg) between trial groups in favour of the PN group at the end of the study (mean difference (MD) 4.12, 95% CI 1.91 to 6.33, P = 0.0003; see Analysis 1.1).
1.1. Analysis.
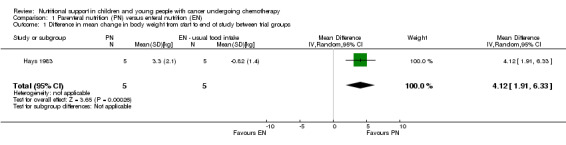
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 1 Difference in mean change in body weight from start to end of study between trial groups.
Ghavimi 1982 (N = 25) found that the PN group had an average weight gain of 12.9% compared to the usual food intake group, who had an average weight loss of 1.40% at the end of radiotherapy (P = 0.006). Standard deviations were not reported and so it was not possible to produce a forest plot for these data. At three months follow up there was no significant difference in weight change between the two groups. When the study was complete and all patients were receiving ad libitum oral intake, the former PN group experienced marked weight loss, whereas weight in the usual food intake group remained fairly stable. However, the actual results for weight change at the two time points were not reported (Ghavimi 1982).
Arm anthropometry
Skinfold thickness or triceps skinfold
This outcome was reported in three trials (Donaldson 1982; Ghavimi 1982; Hays 1983).
Ghavimi 1982 (N = 25) found that there was no significant difference in mean skinfold thickness between trial groups at the end of the study, although units of skinfold thickness were not reported (MD ‐0.74, 95% CI ‐3.15 to 1.67, P = 0.55; see Analysis 1.2).
1.2. Analysis.
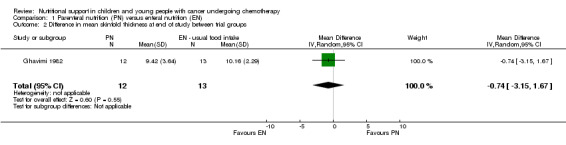
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 2 Difference in mean skinfold thickness at end of study between trial groups.
Donaldson 1982 (N = 23) found that the median percentage change from baseline to the end of radiotherapy for triceps skinfold was an increase of 13.9% for the PN group compared to no change for the usual food intake group(P = 0.05). At three months follow up the median percentage change from baseline was an increase of 16.7% for the PN group compared to an increase of 0.9% for the usual food intake group (P = 0.08). Since median values were reported by Donaldson 1982, and to avoid the possibility of duplicate reporting with the Ghavimi 1982 data (14 of the patients in the Ghavimi 1982 single‐centre trial were also enrolled into the multi‐institutional paediatric nutrition trial by Donaldson 1982), we did not analyse the data from Donaldson 1982 in a forest plot.
In the Hays 1983 trial (N = 10), initial and final percentiles for triceps skinfold were presented for each patient and it was reported that skinfold measurements were similar between groups. However, the actual skinfold measurements were not reported.
Arm circumference/mid upper arm circumference
This outcome was reported in one trial (Donaldson 1982). This trial (N = 23) found that the median percentage change from baseline to the end of radiotherapy for arm circumference was an increase of 2.65% for the PN group compared to no change for the usual food intake group (P = 0.03). At the three‐month follow up the median percentage change from baseline was an increase of 2.4% for the PN group compared to a decrease of 0.7% for the usual food intake group (P = 0.89). Since median values were reported, we did not analyse these data in a forest plot.
Arm muscle circumference
This outcome was reported in two trials (Donaldson 1982; Ghavimi 1982).
Donaldson 1982 (N = 23) found that the median percentage change from baseline to the end of radiotherapy for arm muscle circumference was a decrease of 0.2% for the PN group compared to an increase of 0.4% for the usual food intake group (P = 0.42). At the three‐month follow up, the median percentage change from baseline was a decrease of 0.8% for the PN group compared to a decrease of 1.9% for the usual food intake group (P = 0.86). Since median values were reported, and to avoid the possibility of duplicate reporting with the Ghavimi 1982 data (14 of the patients in the Ghavimi 1982 single‐centre trial were also enrolled into the multi‐institutional paediatric nutrition trial by Donaldson 1982), we did not include the data from Donaldson 1982 in a forest plot.
Ghavimi 1982 (N = 25) found that arm muscle circumference was significantly different between the two groups at the end of the study (PN 18.1 cm and control 22.4 cm, P = 0.018). However, standard deviations were not reported and so it was not possible to produce a forest plot for these data.
Arm muscle area
This outcome was only reported in Hays 1983 (N = 10), where initial and final percentiles were presented for each patient. It was reported that arm muscle area increased in three of five patients and remained the same in two patients in the PN group and increased in three of five patients and remained the same in two patients in the usual food intake group. However, the actual arm muscle area measurements were not reported.
Serum albumin
This outcome was reported in three trials (Donaldson 1982; Ghavimi 1982; Hays 1983).
Results from two trials for this outcome were only reported narratively (Donaldson 1982; Ghavimi 1982). Fourteen of the patients in the Ghavimi 1982 single‐centre trial were also enrolled into the Donaldson 1982 multi‐institutional paediatric nutrition trial. Both sets of data are reported as, since it is not possible to perform an analysis, there is no issue of duplicate reporting. In Donaldson 1982 (N = 23), the following was reported: "Values for serum albumin did not differ significantly between the groups at completion of radiotherapy (median increase = 0.1 in each group) or at the later three‐month follow‐up (median increase = 0.05 for controls, 0.0 for PN)."
In Ghavimi 1982 (N = 25), the following was reported: "No significant differences were noted between the two groups in relation to albumin either at the initiation or end of the study."
Hays 1983 (N = 10) found that there was a significant difference in mean change in serum albumin (units not reported) between trial groups in favour of the PN group at the end of the study (MD 0.70, 95% CI 0.14 to 1.26, P = 0.01; see Analysis 1.3).
1.3. Analysis.
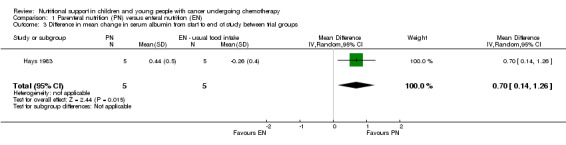
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 3 Difference in mean change in serum albumin from start to end of study between trial groups.
Pre‐albumin
This outcome was reported in Hays 1983 (N = 10). Results for this outcome were only reported narratively: "Prealbumin increased in all patients in the experimental group and in a majority of patients in the control group, these are not significant differences."
Adverse events
Infection rate (line infection, positive blood culture, catheter tip infection)
This outcome was reported in three trials (Van Eys 1980; Ghavimi 1982; Hays 1983), although the different definitions of 'infection' make this outcome challenging to interpret clearly.
In Van Eys 1980, rates of sepsis and all infections requiring antibiotic therapy (e.g. sepsis, UTI, pneumonia) were reported. In Ghavimi 1982, rates of positive blood culture were reported. Hays 1983 reported the rates of a positive culture from blood, urine or stool. The three studies (N = 54) found that there was no significant difference in the number of infections in the PN group compared to the usual food intake group (relative risk (RR) 2.47, 95% CI 0.86 to 7.11, P = 0.09; see Analysis 1.4). The I2 value of 13% suggests low statistical heterogeneity but this assessment is based on very few trials.
1.4. Analysis.
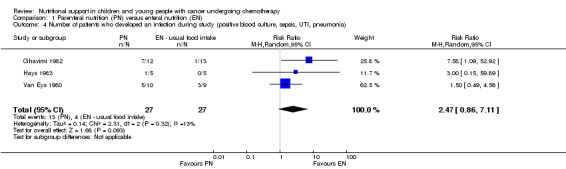
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 4 Number of patients who developed an infection during study (positive blood culture, sepsis, UTI, pneumonia).
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
Two trials reported on the number of patients who experienced nausea and vomiting and the number of patients who developed diarrhoea during the study periods (Donaldson 1982; Ghavimi 1982). Fourteen of the patients in the Ghavimi 1982 single‐centre trial were also enrolled into the multi‐institutional paediatric nutrition trial by Donaldson 1982. In order to avoid duplicate reporting of outcome data, only the data from Donaldson 1982 has been entered into the analysis.
In Ghavimi 1982 (N = 25), there was no significant difference between groups in numbers of people experiencing the most severe nausea and vomiting with a maximum severity score of 10 (severity measured using a scoring system of 0 to 10, with 10 being most severe symptoms; RR 2.17, 95% CI 0.22 to 20.94, P = 0.50). No definitions of nausea and vomiting were reported. Donaldson 1982 (N = 23) found that there was no significant difference in the number of people who experienced nausea and vomiting during the study (RR 1.09, 95% CI 0.71 to 1.68, P = 0.69; see Analysis 1.5).
1.5. Analysis.
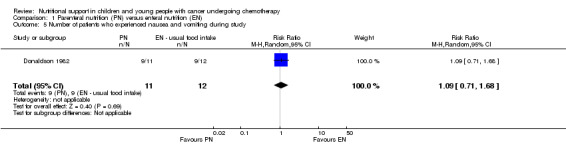
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 5 Number of patients who experienced nausea and vomiting during study.
However, Ghavimi 1982 (N = 25) found that there was a significant difference in the number of people experiencing the most severe diarrhoea with a maximum severity score of five (RR 1.81, 95% CI 0.95 to 3.42, P = 0.07). Donaldson 1982 (N = 23) did not find a significant difference in the number of people who developed diarrhoea during the study (RR 1.64, 95% CI 0.87 to 3.07, P = 0.13; see Analysis 1.6). No definition of diarrhoea was reported.
1.6. Analysis.
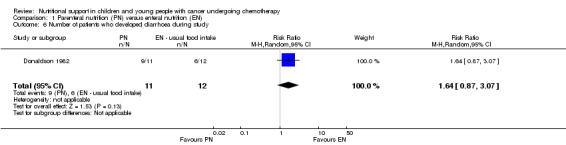
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 6 Number of patients who developed diarrhoea during study.
Calorie and nutritional intake
Total energy intake
This was only reported in Hays 1983 (N = 10), which found that mean calorie intake (cal/kg/day) was significantly greater in the PN group compared to the usual food intake group at the end of the study (MD 22.00, 95% CI 5.12 to 38.88, P = 0.01; see Analysis 1.7).
1.7. Analysis.
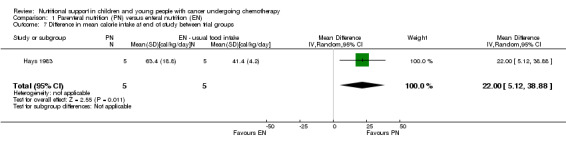
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 7 Difference in mean calorie intake at end of study between trial groups.
Total protein intake
This was only reported in Hays 1983 (N = 10), which found that mean protein intake (g/kg/day) was significantly greater in the PN group compared to the usual food intake group at the end of the study (MD 0.80, 95% CI 0.45 to 1.15, P < 0.00001; see Analysis 1.8).
1.8. Analysis.
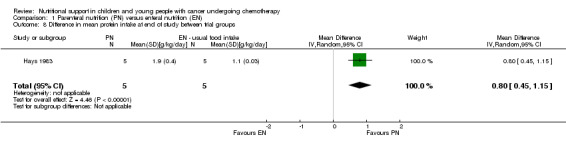
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 8 Difference in mean protein intake at end of study between trial groups.
Secondary outcomes
Number of deaths at end of study
Four trials reported the number of deaths in each trial group at the end of the study period (Van Eys 1980; Donaldson 1982; Ghavimi 1982; Hays 1983). However, 14 of the patients in the Ghavimi 1982 single‐centre trial were also enrolled into the Donaldson 1982 multi‐institutional paediatric nutrition trial. In order to avoid duplicate reporting of outcome data, we did not enter the data from Ghavimi 1982 into the analysis.
The three trials in the analysis (N = 52) found that there was no difference between the number of deaths at the end of the study period in the PN group compared to the usual food intake group (RR 1.19, 95% CI 0.32 to 4.39, P = 0.80; see Analysis 1.9). In Ghavimi 1982 (N = 25), there was no difference between the number of deaths at the end of the study period in the PN group compared to the usual food intake group (RR 0.81, 95% CI 0.23 to 2.91, P = 0.75).
1.9. Analysis.
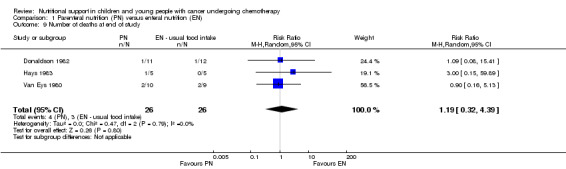
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 9 Number of deaths at end of study.
The I2 value from the analysis suggests low statistical heterogeneity but this measurement is of low power with few studies.
Participants' perceived health status, where possible using validated tools for measuring performance and/or quality of life
Performance
Only Donaldson 1982 (N = 23) reported on the performance status of patients, which was defined as the level of activity compared to baseline activity at the end of the radiotherapy and at three‐month follow up. Performance status was scored as either activity similar to peers, activity less than peers but not bedridden, or bedridden. The tool used to evaluate performance status was not reported. The majority of patients in both groups either maintained or improved their level of performance at both time points. The trial found that there was no difference between groups with respect to the number of patients who improved or maintained their level of performance at the end of follow up (RR 1.09, 95% CI 0.71 to 1.68, P = 0.69; see Analysis 1.10).
1.10. Analysis.
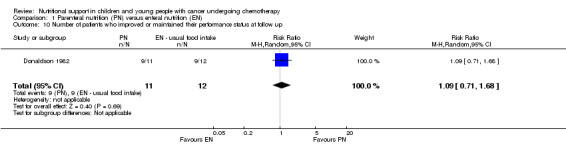
Comparison 1 Parenteral nutrition (PN) versus enteral nutrition (EN), Outcome 10 Number of patients who improved or maintained their performance status at follow up.
Enteral nutrition (nasogastric) versus enteral nutrition (usual food intake)
Only one trial (N = 10) reported on this comparison (Smith 1992).
Primary outcomes
Change in nutritional indices
Arm anthropometry
Arm circumference/mid‐upper arm circumference
Mid‐upper arm circumference (MAC) in centimetres was recorded at the start of the study and at two, six and 12 weeks follow up for the nasogastric group and usual food intake group. We used data presented in the paper to calculate the mean and SD values for each group at the different time points. At two and 12 weeks there was no significant difference between groups in mean MAC in cm (MD 0.60, 95% CI ‐0.32 to 1.52, P = 0.20 and MD 0.06, 95% CI ‐1.22 to 1.34, P = 0.93, respectively). At six weeks from diagnosis there was a significant difference in favour of the nasogastric group in mean MAC in cm (MD 1.76, 95% CI 0.34 to 3.18, P = 0.02; see Analysis 2.1).
2.1. Analysis.
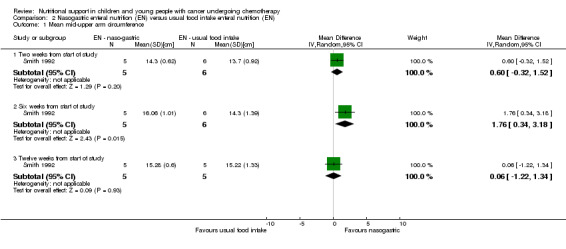
Comparison 2 Nasogastric enteral nutrition (EN) versus usual food intake enteral nutrition (EN), Outcome 1 Mean mid‐upper arm circumference.
Adverse events
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
Smith 1992 reported on the number of patients who experienced recurrent vomiting during the study. The trial found that there was no significant difference between the nasogastric group and the usual food intake group in terms of the incidence of recurrent vomiting (RR 3.50, 95% CI 0.17 to 70.94, P = 0.41; see Analysis 2.2).
2.2. Analysis.
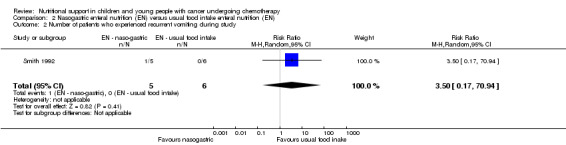
Comparison 2 Nasogastric enteral nutrition (EN) versus usual food intake enteral nutrition (EN), Outcome 2 Number of patients who experienced recurrent vomiting during study.
Calorie and nutritional intake
Total energy intake
Energy intake at diagnosis was given as a percentage of recommended daily allowance (RDA) of energy and the range and median values given for each group. At diagnosis all participants in the nasogastric group had energy intakes less than 50% of their RDA (median 15%) and intakes of the usual food intake group ranged from 10% to 108% of RDA (median 51%). Three weeks after diagnosis all of the nasogastrically‐fed participants, compared to only two of five controls, had intakes greater than 90% of RDA, and this was still the case six weeks after diagnosis. Since median values were reported, we have not analysed these data in a forest plot.
Secondary outcomes
Number of deaths at end of study
There was no difference between the number of deaths at the end of the study period in the nasogastric group compared to the usual food intake group (RR 0.33, 95% CI 0.02 to 6.65, P = 0.47; see Analysis 2.3).
2.3. Analysis.
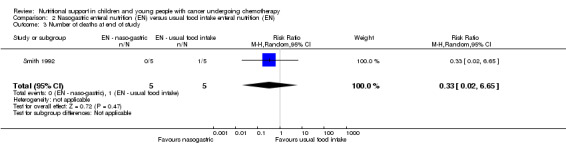
Comparison 2 Nasogastric enteral nutrition (EN) versus usual food intake enteral nutrition (EN), Outcome 3 Number of deaths at end of study.
Patient tolerance of/adherence with nutritional intervention
Acceptability of the mode of feeding was reported. Parents of five participants randomised to receive nasogastric feeding, and two usual food intake controls who ultimately received nasogastric feeding, completed nasogastric questionnaires to ascertain their views on the advantages and disadvantages of feeding. All parents were very positive about the value of nasogastric feeding. All considered that the passing of the tube had been a distressing experience for their child, but none considered that the tube was distressing once placed. All seven attributed weight gain and improved wellbeing to nasogastric feeding. No parent considered that night‐time disturbance was a problem and only two felt that daytime activities had been restricted as a direct consequence of feeding. All seven stated that they would readily consent to their child undergoing nasogastric feeding again if necessary.
Participants' perceived health status
Activity was measured using the Lansky play performance scale ‐ a parent‐completed questionnaire which requires parents to choose one of ten statements which best describe the 'average' of their child's activities over the previous week. This tool appears to have been validated (Lansky 1987). Each statement carries a numerical score facilitating group comparisons. At diagnosis, the median play score was 20 for the nasogastric group (range 10 to 40) and 50 for the usual food intake group (range 0 to 60). At six weeks from diagnosis the median play score was 80 in the nasogastric group (range 70 to 100) and 80 for the usual food intake group (range 50 to 100). The median time from diagnosis for improvement by 20% on the play scale was two weeks for the nasogastric group and three weeks for the usual food intake group. Since median values were reported, we have not analysed these data in a forest plot.
Peripheral parenteral nutrition and enteral nutrition (usual food intake) versus central parenteral nutrition
Two trials reported on this comparison (Rickard 1985; Rickard 1989).
Primary outcomes
Change in nutritional indices
In Rickard 1985 (N = 16), individual patient data presented in tables allowed us to calculate the mean change in weight, triceps skinfold, subscapular skinfold, serum albumin, pre‐albumin, energy intake and protein intake for each group from the start (week zero) to the end of the nutritional support period (week four), with the corresponding SDs.
Weight
Both trials reported on this outcome. Rickard 1985 (N = 16) found that there was no significant difference in mean change in body weight (kg) between trial groups at the end of the nutritional support period (MD ‐0.05, 95% CI ‐0.64 to 0.54, P = 0.87; (see Analysis 3.1). Rickard 1989 (N = 13) found that mean daily weight gain (g/day) was significantly greater for the central parenteral nutrition (CPN) group compared to the peripheral parenteral nutrition (PPN) and EN group during the study (MD ‐27.00, 95% CI ‐43.32 to ‐10.68, P = 0.001; see Analysis 3.2). Mean weekly changes in weight were also illustrated in figures (Rickard 1985). The mean group percentage change in weight (data read from figures) increased by 6% for the PPN group compared to 17% for the CPN group (P < 0.01) for the four weeks of intense nutritional support. No values for SD were provided and so percentage change in weight data has not been analysed in a forest plot.
3.1. Analysis.
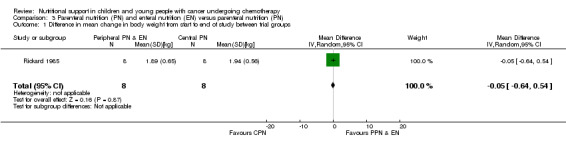
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 1 Difference in mean change in body weight from start to end of study between trial groups.
3.2. Analysis.
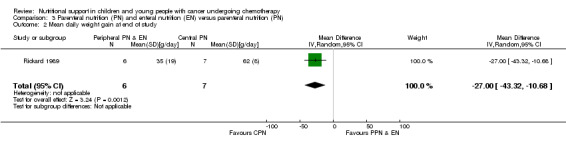
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 2 Mean daily weight gain at end of study.
Height
Only one trial reported on this outcome (Rickard 1985). However, individual patient data were only presented for patients in each group at the start of the trial and not at the end of the nutritional support (week four) and so the data have not been analysed.
Arm anthropometry
Two trials reported on triceps skinfold and subscapular skinfold (Rickard 1985; Rickard 1989).
Triceps skinfold
Rickard 1985 (N = 16) found that there was no significant difference between groups in mean change in triceps skinfold (mm) from the start to the end of nutritional support (MD 0.09, 95% CI ‐1.20 to 1.38, P = 0.89; see Analysis 3.3). In Rickard 1989 (N = 13), mean weekly group percentage changes were illustrated in figures. The mean group percentage change in triceps skinfold (data read from figures) increased by 28% for the PPN group compared to 26% for the CPN group for the four weeks of intense nutritional support. No values for SD were provided and so percentage change in triceps skinfold has not been analysed in a forest plot.
3.3. Analysis.
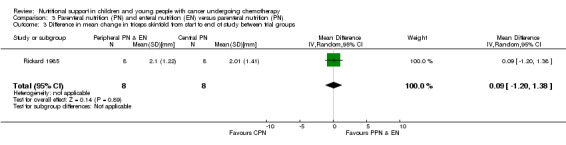
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 3 Difference in mean change in triceps skinfold from start to end of study between trial groups.
Subscapular skinfold
Rickard 1985 (N = 16) found that there was no significant difference between groups in mean change in subscapular skinfold (mm) from the start to the end of nutritional support (MD ‐0.70, 95% CI ‐1.59 to 0.19, P = 0.12; see Analysis 3.4). In Rickard 1989, mean weekly group percentage changes were illustrated in figures. The mean group percentage change in subscapular skinfold (data read from figures) increased by 44% for the PPN group compared to 40% for the CPN group for the four weeks of intense nutritional support. No values for SD were provided and so percentage change in subscapular skinfold has not been analysed in a forest plot.
3.4. Analysis.
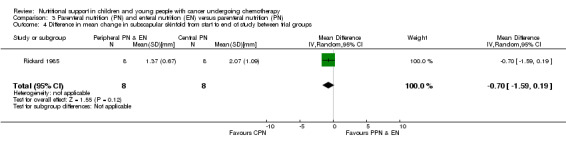
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 4 Difference in mean change in subscapular skinfold from start to end of study between trial groups.
Serum albumin
Both trials reported on this outcome. Rickard 1985 found that there was a significant difference in favour of the PPN group in mean change in serum albumin from the start to the end of nutritional support (MD 0.47, 95% CI 0.13 to 0.81, P = 0.008; see Analysis 3.5). In Rickard 1989, mean weekly concentrations of serum albumin were illustrated in figures. The mean change in serum albumin from the start (week zero) to the end of the nutritional support period (week four) was an increase of 0.09 g/dl for the PPN group compared to an increase of 0.4 g/dl for the CPN group (P < 0.05; data read from figures). No values for SD were provided and so data have not been analysed in a forest plot.
3.5. Analysis.
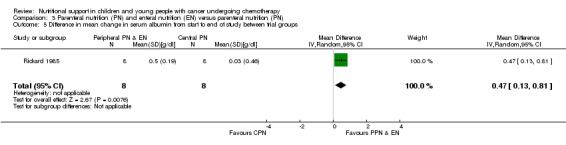
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 5 Difference in mean change in serum albumin from start to end of study between trial groups.
Pre‐albumin
Only Rickard 1989 reported this outcome and mean weekly concentrations of pre‐albumin were illustrated in figures. The mean change in pre‐albumin from the start (week zero) to the end of the nutritional support period (week four) was an increase of 4 mg/dl for the PPN group compared to an increase of 12 mg/dl for the CPN group (P < 0.01; data read from figures). No values for SD were provided and so data have not been analysed in a forest plot.
Adverse events
Infection rate (line infection, positive blood culture, catheter tip infection)
This outcome was only reported by Rickard 1989 (N = 13). Blood cultures were positive for Staphylococcus epidermis and Staphylococcus aureus in the CPN group and Staphylococcus aureus in the PPN group. The study found no significant difference in the number of infections between trial groups (RR 0.58, 95% CI 0.07 to 4.95, P = 0.62; see Analysis 3.6).
3.6. Analysis.
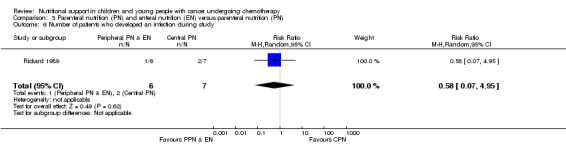
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 6 Number of patients who developed an infection during study.
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
Again, only Rickard 1989 (N = 13) reported this outcome and found that patients in the PPN and EN groups were no more likely than the CPN group to develop diarrhoea during the course of the study (RR 0.93, 95% CI 0.45 to 1.95, P = 0.85; see Analysis 3.7). No definition of diarrhoea was reported.
3.7. Analysis.
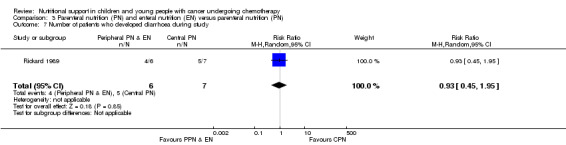
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 7 Number of patients who developed diarrhoea during study.
Calorie and nutritional intake
Total energy intake
Both trials reported on this outcome. Rickard 1985 (N = 16) found that there was no significant difference between groups in mean change in energy intake (% healthy children) from the start to the end of nutritional support (MD ‐1.71, 95% CI ‐26.31 to 22.89, P = 0.89; see Analysis 3.8). Rickard 1989 (N = 13) found that mean energy intakes (% healthy children) were significantly greater for the CPN group compared to the PPN and EN group during the course of the study (MD ‐15.00, 95% CI ‐26.81 to ‐3.19, P = 0.01; see Analysis 3.9).
3.8. Analysis.
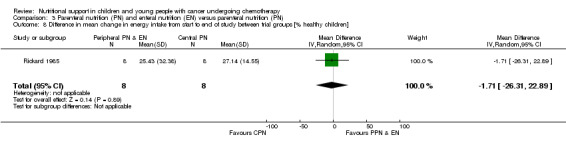
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 8 Difference in mean change in energy intake from start to end of study between trial groups [% healthy children].
3.9. Analysis.
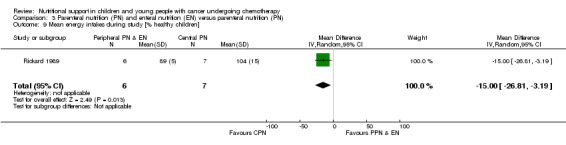
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 9 Mean energy intakes during study [% healthy children].
Total protein intake
Both trials reported on this outcome. Rickard 1985 (N = 16) found that there was no significant difference between groups in mean change in protein intake (g/kg/day) from the start to the end of nutritional support (MD ‐0.03, 95% CI ‐0.93 to 0.87, P = 0.95; see Analysis 3.10). Rickard 1989 (N = 13) found that there was no difference between groups in average protein intake (g protein/kg) during the course of the study (MD ‐0.10, 95% CI ‐0.27 to 0.07, P = 0.24; see Analysis 3.11).
3.10. Analysis.
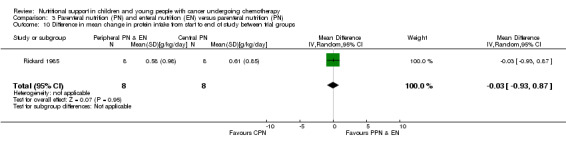
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 10 Difference in mean change in protein intake from start to end of study between trial groups.
3.11. Analysis.
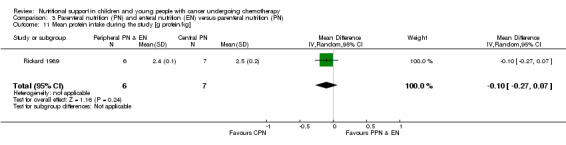
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 11 Mean protein intake during the study [g protein/kg].
Secondary outcomes
Number of deaths at end of study
Only Rickard 1989 reported on this outcome. There was no significant difference between the number of deaths in each group at the end of the study (RR 0.38, 95% CI 0.02 to 7.93, P = 0.53; see Analysis 3.12).
3.12. Analysis.
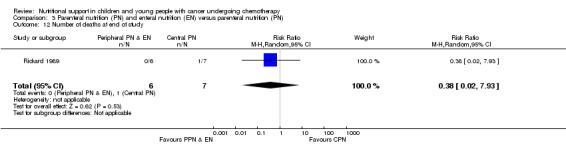
Comparison 3 Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN), Outcome 12 Number of deaths at end of study.
Parenteral nutrition versus fluid therapy
One trial reported on this comparison (Schmid 2006).
Primary outcomes
Change in nutritional indices
Data were presented graphically as median and 25th and 75th percentiles for weight, fat‐free mass and total body water; values could not be distinguished from the graphs. Since median values were reported it was not possible to analyse data in forest plots.
Weight
Data obtained from the author allowed us to calculate the median change in weight and interquartile range (IQR) for each group at the end of the study, which were found to be 1.4 kg (IQR ‐0.3 to 2.3) for the PN group and ‐0.1 kg (IQR ‐0.5 to 0.5) for the FT group.
Fat‐free mass
Data obtained from the author allowed us to calculate the median change in fat‐free mass and IQR for each group at the end of the study, which were found to be 0.95 kg (IQR 0.03 to 1.56) for the PN group and ‐0.09 kg (IQR ‐0.82 to 2.48) for the FT group.
Total body water
Data obtained from the author allowed us to calculate the median change in total body water and IQR for each group at the end of the study, which were found to be 0.3 kg (IQR 0.1 to 0.9) for the PN group and 0.15 kg (IQR ‐0.5 to 0.9) for the FT group.
Serum albumin
The results were reported narratively: "levels of albumin remained unchanged compared to baseline." However, data obtained from the author allowed us to calculate the median change in albumin g/dl and IQR for each group at the end of the study, which were found to be ‐0.1 g/dl (IQR ‐0.7 to 0.3) for the PN group and ‐0.3 g/dl (IQR ‐0.5 to 0.4) for the FT group.
Pre‐albumin
The results were reported narratively: "levels of prealbumin remained unchanged compared to baseline." However, data obtained from the author allowed us to calculate the median change in pre‐albumin mg/dl and IQR for each group at the end of the study, which were found to be ‐0.85 mg/dl (IQR ‐5.4 to 3.7) for the PN group and ‐3.5 mg/dl (IQR ‐12.9 to 1.4) for the FT group.
Adverse events
Infection rate (line infection, positive blood culture, catheter tip infection)
An infection was diagnosed as soon as blood, stool or urine culture turned out to be positive for bacteria, virus or fungus. This trial (N = 30) found that there was no significant difference in the number of culture positive infections between groups (RR 2.00; 95% CI 0.76 to 5.24, P = 0.16; see Analysis 4.1).
4.1. Analysis.
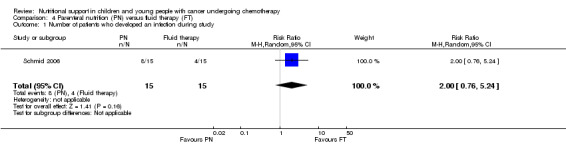
Comparison 4 Parenteral nutrition (PN) versus fluid therapy (FT), Outcome 1 Number of patients who developed an infection during study.
Secondary outcomes
Length of hospital stay
In this study the PN group had a median hospitalisation time of 14 days compared to 13 days for children in the FT group (P = 0.817).
Energy‐dense versus standard calorie content enteral (nasogastric) nutrition
One trial reported on this comparison (den Broeder 2000).
Primary outcomes
Change in nutritional indices
Weight
The trial reported that calorie dense feeds allowed a greater proportion of patients to achieve a non‐negative weight z‐score (weight for height ≥ 0) which means that their weight was at, or greater than, expected for an average child of their height after 10 weeks therapy (Analysis 5.1). In the energy‐dense group, 73% of patients increased their z‐score from below to above zero, compared to 17% of patients in the standard formula group (RR 4.40, 95% CI 1.20 ‐ 16.17, P = 0.03).
5.1. Analysis.
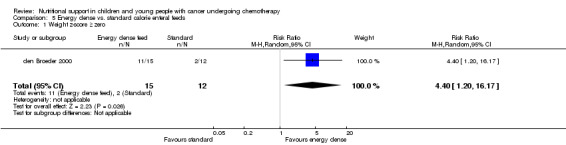
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 1 Weight z‐score ≥ zero.
Arm anthropometry
Anthropometric measurements were given as relative to the 'normal' reference population. Mid upper arm circumference and biceps and triceps skinfold measurements reportedly did not vary significantly between the two groups with energy dense nutrition, though both groups showed improvement in these indices (see Analysis 5.2; Analysis 5.3; Analysis 5.4). Overall arm muscle area increased by 14.9% ± 10.1 in the standard group compared to 20.7% ± 8.4 in the energy dense group (MD 5.80%, 95% CI ‐1.32 to 12.92, P = 0.11).
5.2. Analysis.
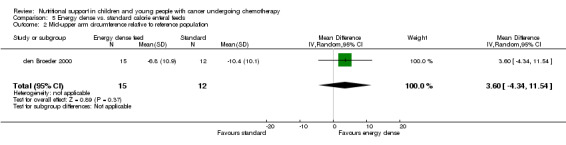
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 2 Mid‐upper arm circumference relative to reference population.
5.3. Analysis.
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 3 Triceps skinfold thickness relative to reference population.
5.4. Analysis.
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 4 Biceps skinfold thickness relative to reference population.
Serum albumin
Narratively, the difference in serum albumin was reported to not be significant between the groups, but both groups showed improvements.
Serum pre‐albumin
Narratively, the difference in serum pre‐albumin was reported to not be significant between the groups, but both groups showed improvements.
Adverse events
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
All patients experienced therapy‐related vomiting at least once. The mean number of days on which such vomiting was experienced did not differ between the groups (Analysis 5.8). Therapy‐related vomiting occurred on 2.2 ± 1.1 days per week in the standard group and 2.0 ± 0.6 days per week in the energy‐dense group (MD ‐0.20 days, 95% CI ‐0.89 to 0.49, P > 0.05).
5.8. Analysis.
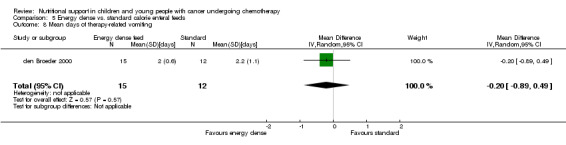
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 8 Mean days of therapy‐related vomiting.
Calorie and nutritional intake
Total energy intake
A significantly higher energy intake was achieved in the group receiving energy‐dense feeds. A mean daily energy intake of 84 ± 14% of requirements was achieved in the standard group, compared to 112 ± 15% in the energy dense group (MD 28.00, 95% CI 17.03 to 38.97%, P < 0.001).
Total protein intake
The average daily protein intake was greater in the group receiving energy dense feeds (1.54g/kg compared with 1.27 g/kg) although no statistical test comparing these values could be undertaken as the authors did not provide variance data.
Secondary Outcomes
Patient tolerance of nutritional intervention
The consumption of energy‐dense feeds led to a smaller average volume of feeds being ingested (75% required vs. 84% required; MD ‐9%, 95% CI ‐18.40 to 0.40, P = 0.06).
Fructooligosaccharide (fibre) supplementation of enteral feeds versus enteral feeds
One trial reported on this comparison (Zheng 2006).
Primary outcomes
Change in nutritional indices
Weight‐for‐height
Data from the study allowed us to calculate the mean increase in mean weight‐for‐height z‐scores in the two feed groups. There was no clear difference in mean z‐score gain between the groups: fructooligosaccharides (FOS) +0.33, standard feeds +0.45 (MD ‐0.12, 95% CI ‐0.57 to 0.33, P = 0.60; see Analysis 6.1).
6.1. Analysis.
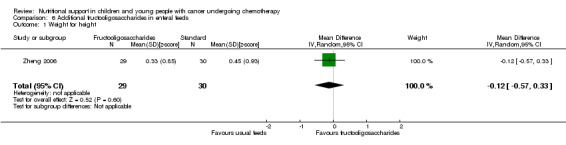
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 1 Weight for height.
Height
Mean increase in height‐for‐age z‐scores were not significantly different between feed groups (standardised mean difference (SMD) ‐0.06, 95% CI ‐0.57 to 0.44, P = 0.80; see Analysis 6.2).
6.2. Analysis.
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 2 Mean increase in height‐for‐age z‐score.
Weight
Mean increase in weight‐for‐age z‐scores in the two feed groups was not significantly different (SMD 0.48, 95% CI ‐0.03 to 1.00, P = 0.07; see Analysis 6.3).
6.3. Analysis.
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 3 Mean increase in weight‐for‐age z‐score.
Serum albumin
The results were reported narratively to show "from day 0‐30 no significant difference between the treatment groups".
Pre‐albumin
The results were reported narratively to show "from day 0‐30 no significant difference between the treatment groups".
Adverse events
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
Nausea was reported "on at least one occasion" by equal numbers in the two groups (RR 0.92, 95% CI 0.48 to 1.74, P = 0.79; see Analysis 6.5).
6.5. Analysis.
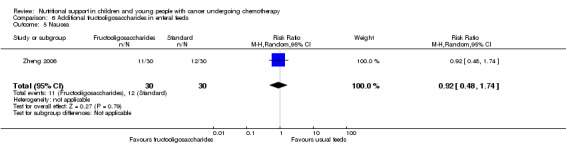
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 5 Nausea.
Diarrhoea was reported by 1/30 patients receiving FOS compared with 0/30 in the standard group (RR 3.0, 95% CI 0.13 to 71, P = 0.50; see Analysis 6.4).
6.4. Analysis.
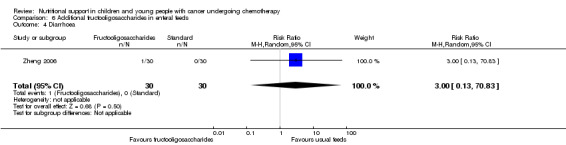
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 4 Diarrhoea.
Bacterial concentrations in stool
There were no significant differences between bacterial concentrations in stool between the two groups (P > 0.05).
Secondary outcomes
Patient tolerance of or adherence to nutritional intervention
Tolerance was assessed by evaluation of abdominal pain, diarrhoea (as above) and stool consistency in the two groups. Stool consistency was not reported in detail but described as not significantly different between the two groups. Abdominal pain was infrequent; 2/30 patients receiving FOS compared with 0/30 in the standard group (RR 5.0, 95% CI 0.25 to 100, P = 0.29; see Analysis 6.6).
6.6. Analysis.
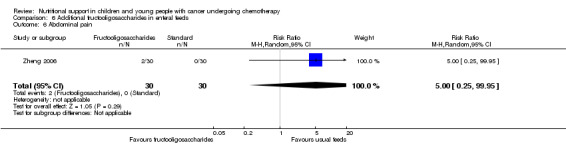
Comparison 6 Additional fructooligosaccharides in enteral feeds, Outcome 6 Abdominal pain.
Glutamine supplementation of enteral or parenteral nutrition
Three trials reported on this comparison: one using glutamine for EN in standard chemotherapy (Ward 2009), or after hematopoietic stem cell transplantation (HSCT; Aquino 2005), and one using glutamine parenterally after HSCT (Uderzo 2011).
Primary outcomes
Change in nutritional indices
Weight
Weight data were reported differently in two studies. Ward 2009 reported the mean percentage weight‐for‐height at the end of the study comparing glutamine enriched supplementation to no supplementation (95.5% vs. 96.9%, P = 0.275).Uderzo 2011 provided data to allow us to calculate the mean change in absolute weight, revealing a mean weight loss among patients receiving glutamine‐supplemented PN of 0.8 kg compared to 1.0 kg in those receiving unsupplementated PN (P = 0.703).
Arm anthropometry
Mid upper arm circumference was only reported by Ward 2009. No difference was demonstrated between the two groups (20.2 cm supplemented vs. 20.3 cm unsupplemented (MD ‐0.10 cm, 95% CI ‐2.0 to 1.8 cm, P = 0.92; see Analysis 7.1).
7.1. Analysis.
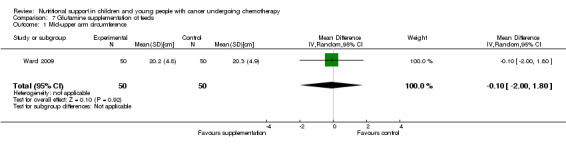
Comparison 7 Glutamine supplementation of feeds, Outcome 1 Mid‐upper arm circumference.
Serum albumin
The mean differences in albumin levels were reported in the Uderzo 2011 study. These did not demonstrate a significant difference (16.8 g/L supplemented versus 14.8 g/L unsupplemented, P = 0.552).
Pre‐albumin
The mean differences in prealbumin levels were reported in the Uderzo 2011 study. These did not demonstrate a significant difference (46.6 g/L supplemented versus ‐49.6 g/L unsupplemented, P = 0.513).
Adverse events
Infection rate (line infection, positive blood culture, catheter tip infection)
An infection was diagnosed in close to half of patients in the post‐transplant studies (Uderzo 2011: 55% of each group, RR 1.0, 95% CI 0.72 to 1.4, P = 0.98; Aquino 2005: 40% glutamine versus 41% glycine, RR 0.98, 95% CI 0.63 to 1.51, P = 0.92), and not reported in Ward 2009 (see Analysis 7.2).
7.2. Analysis.
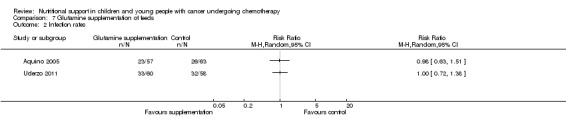
Comparison 7 Glutamine supplementation of feeds, Outcome 2 Infection rates.
Frequency, duration or severity (author defined) of diarrhoea, nausea or vomiting
Nausea and/or vomiting was reported only in the Ward 2009 study. A similar proportion of patients experienced severe nausea and/or vomiting (24/50 versus 27/50, RR 0.89, 95% CI 0.60 to 1.3, P = 0.55; see Analysis 7.3), and with a similar mean duration (e.g. mean duration of vomiting 0.31 days versus 0.42 days, mean difference ‐0.11 days, 95% CI ‐0.55 to 0.33, P = 0.62; see Analysis 7.4). Severe diarrhoea was also reported to be similar both in frequency (RR 1.1, 95% CI 0.68 to 1.8, P = 0.68; see Analysis 7.5) and duration (MD ‐0.18 days, 95% CI ‐0.81 to 0.45, P = 0.57; see Analysis 7.6).
7.3. Analysis.
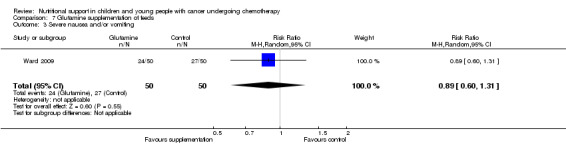
Comparison 7 Glutamine supplementation of feeds, Outcome 3 Severe nausea and/or vomiting.
7.4. Analysis.
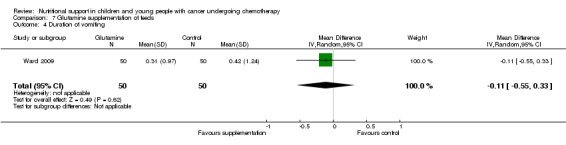
Comparison 7 Glutamine supplementation of feeds, Outcome 4 Duration of vomiting.
7.5. Analysis.
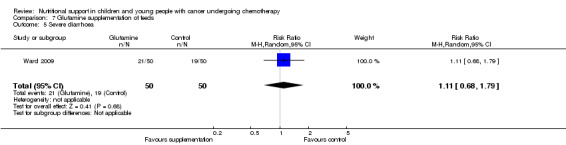
Comparison 7 Glutamine supplementation of feeds, Outcome 5 Severe diarrhoea.
7.6. Analysis.
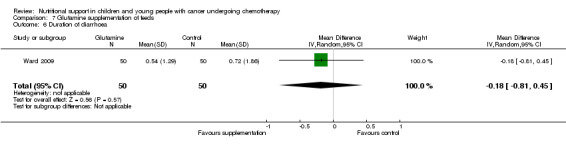
Comparison 7 Glutamine supplementation of feeds, Outcome 6 Duration of diarrhoea.
Severe mucositis, a related outcome, was described by all three studies. The results showed a similar lack of efficacy of glutamine supplementation when defined as grade 3 or greater toxicity (Uderzo 2011: RR 0.64, 95% CI 0.19 to 2.2, P = 0.48; Ward 2009: RR 0.85, 95% CI 0.66 to 1.1, P = 0.19; see Analysis 7.7). A similar lack of difference was seen in Aquino 2005 when this outcome was assessed by a mean graded severity score (3.0 +/‐ 0.3 for the glutamine group versus 3.9 +/‐ 0.4 for the glycine group, P = 0.07) and highest graded severity score (7.2 +/‐ 0.6 for the glutamine group versus 7.5 +/‐ 0.6 for the glycine group, P = 0.70).
7.7. Analysis.
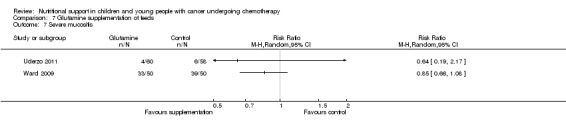
Comparison 7 Glutamine supplementation of feeds, Outcome 7 Severe mucositis.
Abnormal biochemical profiles
Ammonia levels were reported by Ward 2009 and Aquino 2005. Ward 2009 reported that one patient on glutamine supplementation had an abnormally high level of plasma ammonia (> 100 micromol/L) and withdrew from the study, and the mean peak serum ammonia levels were greater in the supplemented group in this trial (mean difference 21.30 micromol/L, 95% CI 13.97 to 28.63, P < 0.001: see Analysis 7.9). Aquino 2005 found no difference in average maximum serum ammonia levels (mean difference 1.60 micromol/L, 95% CI ‐0.56 to 3.76, P = 0.15; see Analysis 7.9).
7.9. Analysis.
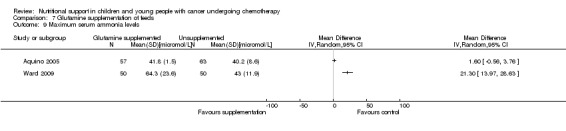
Comparison 7 Glutamine supplementation of feeds, Outcome 9 Maximum serum ammonia levels.
Secondary outcomes
Number of deaths at end of study
The mortality rates were not statistically significantly different between groups in these studies. Uderzo 2011 reported treatment‐related mortality rates of 11.7% (7/60) supplemented patients compared with 8.6% (5/58) in the unsupplemented group (RR 1.35, 95% CI 0.46 to 4.0, P = 0.59). A similar result was seen in Aquino 2005 (6/57 glutamine versus 6/63 glycine; RR 1.11, 95% CI 0.38 to 3.23, P = 0.85; See Analysis 7.8.)
7.8. Analysis.
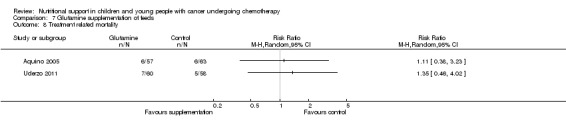
Comparison 7 Glutamine supplementation of feeds, Outcome 8 Treatment related mortality.
Length of hospital stay
Uderzo 2011 and Aquino 2005 both reported that the length of hospital stay did not differ between the two groups (Uderzo 2011: mean 45 days versus 44 days, P = 0.20; Aquino 2005: 36.1 days vs 34.1 days, P = 0.42).
Value of different lipid formulations for parenteral nutrition
One trial reported on this comparison (Hartman 2009), comparing an olive oil‐derived lipid to standard lipid formulations over a 14 day period.
Primary outcomes
Change in nutritional indices
Weight
Weight data were reported as before‐after and mean change weight‐for‐height z‐scores, allowing us to calculate the average z‐score change which revealed a greater increase in weight in the standard lipid group (MD ‐0.34 z‐scores for standard lipid, 95% CI ‐0.68 to ‐0.00, P = 0.05; see Analysis 8.1).
8.1. Analysis.
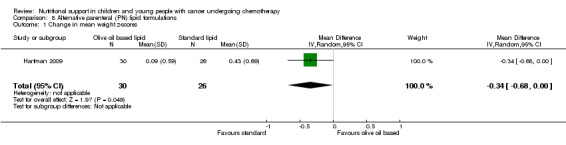
Comparison 8 Alternative parenteral (PN) lipid formulations, Outcome 1 Change in mean weight z‐scores.
Serum albumin
The mean albumin levels did not change or differ between groups in the 14 day treatment period (mean values 3.8 g/dL at start and end of treatment in both groups).
Adverse events
Abnormal biochemical profiles
A variety of lipid levels were reported in the study (including palmitic, steric, oelitic, linoeleic, arachadonic, EPA, DHA, total cholesterol and triglycerides). These have unclear clinical significance at 14 days of treatment.
Calorie and nutritional intake
Total energy intake
Total PN energy intake was documented, and did not show a difference (36.9 kcal/kg/day for olive oil‐based lipid compared with 38.8 kcal/kg/day for standard lipid, MD ‐1.9 kcal/kg/day, 95% CI ‐5.2 to 1.4, P = 0.26; see Analysis 8.2).
8.2. Analysis.
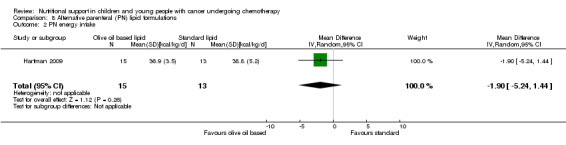
Comparison 8 Alternative parenteral (PN) lipid formulations, Outcome 2 PN energy intake.
Total protein intake
Total PN protein intake was also similar; 1.4 g/kg/day vs 1.4 g/kg/day (MD 0.0 g/kg/day, 95% CI ‐0.09 to 0.09, P = 1.0; see Analysis 8.3).
8.3. Analysis.
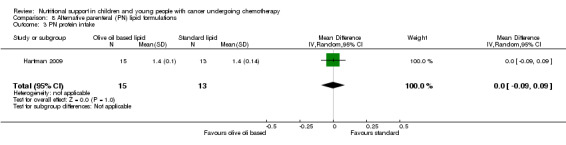
Comparison 8 Alternative parenteral (PN) lipid formulations, Outcome 3 PN protein intake.
Discussion
Eight trials were eligible for inclusion in the previous review, which encompassed four comparisons: four trials of PN compared to EN (usual food intake) in well‐nourished patients (n = 80); one trial of EN (nasogastric) compared to EN (usual food intake) in malnourished patients (n = 12); two trials of PN (peripheral) and EN (usual food intake) compared to central PN alone in malnourished patients (n = 37); and one trial of PN compared to FT in patients with mucositis (n = 30). The majority of trials were published more than 17 years ago.
In the studies comparing PN to EN there was limited evidence from one small trial that suggested PN was beneficial in terms of increasing body weight, serum albumin levels, and calorie and protein intake when compared to EN. The evidence for other methods of delivery of nutritional support was also limited to single studies and remains unclear. One trial comparing peripheral PN and EN (usual food intake) with central PN found that mean daily weight gain and energy intakes were significantly lower for the peripheral PN and EN group (Rickard 1989), whereas another trial found that mean change in serum albumin was significantly greater for the peripheral PN and EN group (Rickard 1985). In the one study comparing EN (nasogastric) versus EN (usual food intake), the only significant finding was that mid‐upper arm circumference was significantly greater in the nasogastric group at six weeks follow up (Smith 1992). In the one most recent study comparing PN to FT, all the data for change in nutritional indices were reported as medians and so it was not possible to analyse the data in forest plots (Schmid 2006). The study does report narratively that weight gain significantly increased in the PN group compared to the FT group where weight remained stable, whereas fat‐free mass and total body water decreased in the PN group compared to the FT group (Schmid 2006). However, P values were not reported and so it is not possible to ascertain whether these results are statistically significant. It is worth noting that there was no significant difference between the PN and FT group in terms of number of culture‐positive infections.
A further six trials were eligible for inclusion in the current review, which encompassed a further four comparisons: one trial of energy‐dense EN compared to standard calorie content EN (n = 27); one trial of FOS supplemented EN compared to a non‐FOS EN (n= 67); three trials comparing glutamine‐supplemented EN (oral or nasogastric) or PN compared to standard non‐glutamine‐supplemented EN (oral or nasogastric) or PN (n = 288); and one trial of an olive oil‐derived lipid PN compared to standard lipid PN (n = 28). All six trials were published between 2000 and 2011.
The current study therefore did not highlight any new significant evidence in terms of method of nutritional support as the newly included trials investigated nutrient content (energy density, FOS, glutamine and oliveoil‐derived PN lipid) rather than method of delivery. Therefore the questions addressed by the initial review regarding the most effective method of nutritional support in children and young people with cancer undergoing chemotherapy remains unanswered, with the initial review reporting limited evidence from one small trial that compared to EN, PN significantly increased weight gain, serum albumin levels, calorie intake and protein intake (Hays 1983). There have been no subsequent studies that fulfilled the inclusion criteria which focused on the method of nutrient delivery by comparing EN with PN or oral food intake/oral nutritional supplements with EN or PN.
One study in the current review comparing an energy dense enteral feed (1.5 Kcal/ml) to a standard calorie feed (1.0 Kcal/ml) reported, as expected, a significantly greater mean energy intake in the energy dense feed group which resulted in a significantly higher increase in z‐score of weight for height in the energy dense enteral feed group compared to the standard formula group after 10 weeks of feeding (den Broeder 2000). However the evidence is limited due to the small sample size and unclear risk of bias. The study comparing an FOS supplemented feed compared to a non fibre containing feed showed no difference in terms of nutritional indices (weight, albumin and pre albumin). Although less abdominal pain and diarrhoea were reported in the FOS supplemented feed group, this was not significant and the risk of bias in the study was unclear (Zheng 2006).
In the three studies comparing glutamine supplementation, two as EN in standard chemotherapy (Ward 2009) and after HSCT (Aquino 2005) and the third added to PN after HSCT (Uderzo 2011), no significance was found in change in nutritional indices. Infection rates were reported in both Aquino 2005 and Uderzo 2011 but no significant difference was reported in either study. Nausea and vomiting was reported in Ward 2009 with no significance in terms of severity or duration between courses of chemotherapy with or without glutamine supplementation. The glutamine supplementation showed no significant improvement in severity of mucositis in any of the three studies. Slightly higher treatment related mortality rates were reported in the glutamine supplemented groups in Aquino 2005 and Uderzo 2011 compared to the non glutamine controls (11% versus 9%, non‐significant). Ward 2009 did not report length of stay but both Aquino 2005 and Uderzo 2011 found no difference in length of hospital stay between the two groups. Previously there have been several studies looking at the role of glutamine supplementation in adult oncology patients (Murray 2009), however there are very few published studies looking at glutamine in children with cancer. The current review highlights the fact that evidence for the use of glutamine supplementation either enterally or parenterally in children treated for cancer is weak, particularly following HSCT. Ward 2009 had a high risk of bias, but both Aquino 2005 and Uderzo 2011 had a low risk of bias yet found no significant benefit to glutamine supplementation.
The final study in the current review looked at an olive oil based parenteral lipid compared to a standard parenteral lipid in a small number of BMT patients (Hartman 2009). After 14 days the plasma lipid profile of those tested in the study remained favourable. Despite no difference in calorie intake between the two lipid groups, a greater increase in weight gain average z‐score in the standard lipid group was observed. The study period was only 14 days so it remains unclear if there are any potential adverse effects or abnormal lipid profiles if an olive oil based lipid was to be given for a longer period of time.
The question addressed by this review was broad in scope, and consequently a number of comparisons of nutritional interventions were included. The advantages of the broad scope are that the review provides a comprehensive summary of the evidence to date. The studies in the previous review focused mainly on the method of delivery of the nutritional intervention. As more studies have become available, this review has been able to address the nutrient content within the parenteral or enteral feed (e.g. high energy dense EN, FOS containing feeds, glutamine supplementation and olive oil based PN). The included studies consisted of children and young people with cancer who were described as being either malnourished or well‐nourished at the beginning of the nutritional therapy. In the trials that examined the usage of PN versus EN (usual food intake), the interventions were clinically heterogenous. In some trials, both the PN and EN groups (Donaldson 1982), or the EN group alone (Van Eys 1980; Ghavimi 1982), received additional dietary advice or counselling, and in one trial both groups were allowed oral nutrition as tolerated (Hays 1983).
A diverse range of clinical endpoints for each of our specified primary and secondary outcomes were reported in the trials. Due to this, and the inconsistencies in expression of results and statistical reporting across trials (for example, measures of change in weight were reported in a variety of ways), it was not possible to include many of the outcome measures in the statistical analyses. Meta‐analysis was therefore only possible for two outcome measures: infection rate and mortality. Furthermore, most of the outcome measures included in the statistical analysis consisted of data from only one or two trials. The use of a diverse range of clinical outcome measures is a common theme amongst Cochrane reviews of nutritional interventions (Poustie 1999; Cairns 2000; Ferreira 2001; Huang 2002; Al‐Omran 2003; Collins 2003; Ferreira 2005; Simmer 2005; Andersen 2006; Lai 2006; Soghier 2006; Wasiak 2006; Dewey 2007; Mahlungulu 2007; Wasiak 2007; Joffe 2009; Murray 2009). Recent work highlights the fact that more methodological research is needed in defining pertinent outcome measures in paediatric trials (Clarke 2007; Sinha 2008). Future work in this important area could lead to a more clearly defined set of core outcomes for measuring the effectiveness and safety of nutritional interventions in paediatric oncology trials. An insufficient number of trials within each of the comparisons, and lack of consistent outcome data presented within the included trials, meant we were unable to use sensitivity and subgroup analyses to examine effects of methodological quality of trials, the condition of individuals (i.e. severity of disease, nutritional status in terms of malnourished or well‐nourished), type of malignancy or type/intensity of chemotherapy. Future studies should be multi‐centre trials with standard outcome measurements.
The methodological quality of the included trials was generally poor with only four trials reporting 'adequate' methods of allocation concealment and six trials reporting 'adequate' methods for sequence generation. Three of the studies attempted blinding of clinicians and participants, and two of the studies attempted blinding of outcome assessors. Selective outcome reporting bias was also a concern in three of the included trials. All of the included trials were small with the exception of Aquino 2005 and Uderzo 2011, which each had over 100 participants.
In summary, the previous review identified four main comparisons of nutritional interventions: PN versus EN; EN versus EN; PN and EN versus PN; and PN versus FT. The evidence suggests that: PN may be more effective than EN in terms of greater weight gain, serum albumin levels and calorie and protein intake; CPN is more effective than PPN and EN in terms of greater weight gain and energy intakes; and PPN and EN is more effective than CPN in terms of greater serum albumin levels. However, much of the evidence was limited to single studies. The evidence for other comparisons remains unclear as data were limited to one or two small trials with a diverse range of clinical outcome measures.
Evidence in the current review additionally suggested that a higher calorie intake and subsequently better weight gain is achievable by using an energy dense enteral feed; an FOS containing feed was well tolerated but demonstrated no significant difference in terms of nutritional indices, abdominal pain or diarrhoea compared to a standard enteral feed; and similarly, an olive oil based parenteral lipid was tolerated for a limited period of time in terms of plasma lipid profile but there was no significantly clear clinical benefit to its use. The evidence was limited to small single studies. There was evidence from three studies relating to the use of enteral or parenteral glutamine, two of which had a low risk of bias. The evidence for all three studies was weak with regard to the benefits of glutamine supplementation, showing no reduction in the incidence or severity of mucositis, infection rates or length of hospital stay.
Authors' conclusions
Implications for practice.
There is limited evidence from individual trials to suggest that PN is more effective than EN in well‐nourished children and young people with cancer undergoing chemotherapy. There is limited evidence to suggest that glutamine supplementation given either enterally or parenterally has no significant effect on mucositis, infection rates or length of hospital stay. The evidence for other comparisons and in malnourished patients remains unclear. Until results of adequately‐powered, preferably multi‐centre trials with clear outcome measures are available, nutritional support needs to be selected on an individual basis following a discussion involving the patient, the carers (if appropriate) and the multidisciplinary treatment team, which should include a dietitian experienced in managing oncology patients. The possible benefits and harms of any nutritional support must be discussed with the patient.
Implications for research.
There is a need for more well designed, adequately powered trials assessing the effectiveness and safety of nutritional support in children and young people with cancer. Consideration should be given to the adoption of standard multi‐centre clinical outcome measures that are important to people with cancer and their carers. Trialists should report results fully in a standardised and consistent manner that should be appropriate to the data.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2014 | New search has been performed | Searches updated to October 2014. |
| 6 November 2014 | New citation required and conclusions have changed | Evidence in the current review now includes information on calorie density of feeds, fructooligosaccharide feeds, glutamine supplementation and an olive oil based parenteral lipid. The evidence is limited to small single studies. |
Acknowledgements
The original review team offered the following acknowledgements:
"We would like to thank Nikki Jahnke and Tracey Remmington, Cochrane Cystic Fibrosis & Genetic Disorders Group, for their assistance during all stages in the preparation of this review, Ashley Jones and Kerry Dwan for their statistical advice, and Dr Jean Craig, for her ongoing support and encouragement throughout. We would like to thank the editorial base of the Cochrane Childhood Cancer Group, which is funded by Kinderen Kankervrij (KIKA). Finally, we would like to thank all trialists who provided us with additional information in response to our queries: Irene Schmid; Jan van Eys."
We commend their thoughts and add our own thanks to the editorial base of the Cochrane Childhood Cancer Group Trials Search Coordinator, Edith Leclercq, as well as Candlelighters, the Yorkshire Childrens' Cancer Charity, who financially supported the review, and the children and families who have raised the money which the charity distributes.
Appendices
Appendix 1. Original search strategy for CENTRAL
| For the original version of the review we used the following search strategy: Search #1 to #26 relates to terms describing the intervention; search #27 to #43 relates to terms describing the disease; search #44 to #48 relates to terms describing the population. #1 tpn in All Fields in all products #2 parenteral nutrition in All Fields in all products #3 parenteral feed* in All Fields in all products #4 enteral nutrition in All Fields in all products #5 enteral feed* in All Fields in all products #6 central line* in All Fields in all products #7 hydrolysate* in All Fields in all products #8 polymeric in All Fields in all products #9 elemental in All Fields in all products #10 nasogastr* in All Fields in all products #11 naso‐gastr* in All Fields in all products #12 gastrostom* in All Fields in all products #13 jejunostom* in All Fields in all products #14 tube feed* in All Fields in all products #15 intravenous therapy in All Fields in all products #16 intubation in All Fields in all products #17 MeSH descriptor Parenteral Nutrition explode all trees in MeSH products #18 MeSH descriptor Enteral Nutrition explode all trees in MeSH products #19 MeSH descriptor Nutritional Support, this term only in MeSH products #20 MeSH descriptor Intubation, Gastrointestinal explode all trees in MeSH products #21 MeSH descriptor Gastrostomy explode all trees in MeSH products #22 MeSH descriptor Jejunostomy explode all trees in MeSH products #23 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #24 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #25 (#18 OR #19 OR #20 OR #21 OR #22) #26 (#23 OR #24 OR #25) #27 chemotherap* in All Fields in all products #28 anticancer treatment in All Fields in all products #29 anti cancer treatment in All Fields in all products #30 leukemia OR leukemias in All Fields in all products #31 leukaemia OR leukaemias in All Fields in all products #32 lymphoma OR lymphomas in All Fields in all products #33 tumour OR tumours in All Fields in all products #34 tumor OR tumors in All Fields in all products #35 cancer OR cancers in All Fields in all products #36 neoplasm OR neoplasms in All Fields in all products #37 oncology in All Fields in all products #38 MeSH descriptor Chemotherapy, Adjuvant explode all trees in MeSH products #39 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees in MeSH products #40 MeSH descriptor Chemotherapy, Cancer, Regional Perfusion explode all trees in MeSH products #41 (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34) #42 (#35 OR #36 OR #37 OR #38 OR #39 OR #40) #43 (#41 OR #42) #44 MeSH descriptor Adolescent explode all trees in MeSH products #45 MeSH descriptor Child explode all trees in MeSH products #46 MeSH descriptor Child, Preschool explode all trees in MeSH products #47 child OR children OR childhood in All Fields in all products #48 (#44 OR #45 OR #46 OR #47) #49 (#26 AND #43 AND #48) |
Appendix 2. Original search strategy for MEDLINE
| For the original version of the review we used the following search strategy: Dialog DataStar via the National Library for Health (http://www.library.nhs.uk) Search 1 to 26 relates to terms describing the intervention; search 27 to 43 relates to terms describing the disease; search 44 to 46 relates to terms describing the population. 1 TPN OR PN OR EN 2 PARENTERAL ADJ NUTRITION 3 PARENTERAL ADJ FEED$ 4 ENTERAL ADJ NUTRITION 5 ENTERAL ADJ FEED$ 6 CENTRAL ADJ LINE$ 7 HYDROLYSATE$ 8 POLYMERIC 9 ELEMENTAL 10 NASOGASTR$ 11 NASO‐GASTR$ 12 GASTROSTOM$ 13 JEJUNOSTOM$ 14 TUBE ADJ FEED$ 15 INTRAVEN$ 16 INTUBATION 17 PARENTERAL‐NUTRITION#.DE. 18 ENTERAL‐NUTRITION#.DE. 19 INTUBATION‐GASTROINTESTINAL#.DE. 20 GASTROSTOMY#.W..DE. 21 JEJUNOSTOMY#.W..DE. 22 FOOD‐FORMULATED#.DE. 23 PROTEIN‐HYDROLYSATES#.DE. 24 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 25 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 26 24 OR 25 27 CHEMOTHERAP$ 28 ANTICANCER ADJ TREATMENT 29 ANTI ADJ CANCER ADJ TREATMENT 30 LEUKEMIA OR LEUKEMIAS 31 LEUKAEMIA OR LEUKAEMIAS 32 LYMPHOMA OR LYMPHOMAS 33 TUMOUR OR TUMOURS 34 TUMOR OR TUMORS 35 CANCER OR CANCERS 36 NEOPLASM OR NEOPLASMS 37 ONCOLOGY 38 CHEMOTHERAPY‐ADJUVANT#.DE. 39 NEOPLASMS#.W..DE. 40 27 OR 28 OR 29 OR 30 OR 31 OR 32 41 33 OR 34 OR 35 OR 36 OR 37 42 38 OR 39 43 40 OR 41OR 42 44 CHILD# OR ADOLESCENT.DE. OR INFANT# 45 CHILD OR CHILDREN OR CHILDHOOD 46 44 OR 45 47 26 AND 43 AND 46 The following search strategy was adapted from the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE and combined with the search strategy above to identify randomised controlled trials: 1 PT=RANDOMIZED‐CONTROLLED‐TRIAL 2 PT=CONTROLLED‐CLINICAL‐TRIAL 3 RANDOMIZED‐CONTROLLED‐TRIALS#.DE. 4 RANDOM‐ALLOCATION#.DE. 5 DOUBLE‐BLIND‐METHOD#.DE. 6 SINGLE‐BLIND‐METHOD#.DE. 7 1 OR 2 OR 3 OR 4 OR 5 OR 6 8 ANIMAL=YES 9 HUMAN=YES 10 8 NOT (8 AND 9) 11 7 NOT 10 12 PT=CLINICAL‐TRIAL 13 CLINICAL‐TRIALS#.DE. 14 (CLINICAL ADJ TRIAL$).TI. 15 (CLINICAL ADJ TRIAL$).AB. 16 (SINGLE OR DOUBLE OR TREBLE OR TRIPLE) ADJ (BLIND OR MASK) 17 16.TI. 18 16.AB. 19 PLACEBOS#.DE. 20 PLACEBO$.TI. 21 PLACEBO$.AB. 22 RANDOM$.TI. 23 RANDOM$.AB. 24 RESEARCH‐DESIGN#.DE. 25 12 OR 13 OR 14 OR 15 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 26 25 NOT 10 27 26 NOT 11 28 COMPARATIVE‐STUDY#.DE. 29 EVALUATION‐STUDIES#.DE. 30 FOLLOW‐UP‐STUDIES#.DE. 31 PROSPECTIVE‐STUDIES#.DE. 32 (CONTROL$ OR PROSPECTIV$ OR VOLUNTEER$).TI. 33 (CONTROL$ OR PROSPECTIV$ OR VOLUNTEER$).AB. 34 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 35 34 NOT 10 36 35 NOT (11 OR 27) 37 11 OR 27 OR 36 |
Appendix 3. Original search strategy for EMBASE
| Dialog DataStar via the National Library for Health (http://www.library.nhs.uk) Search 1 to 28 relates to terms describing the intervention; search 29 to 44 relates to terms describing the disease; search 45 to 47 relates to terms describing the population; search 48 to 53 relates to terms describing the study design. 1 TPN 2 PARENTERAL ADJ NUTRITION 3 PARENTERAL ADJ FEED$ 4 ENTERAL ADJ NUTRITION 5 ENTERAL ADJ FEED$ 6 CENTRAL ADJ LINE 7 CENTRAL ADJ LINES 8 INTRAVEN$ 9 INTUBATION 10 HYDROLYSATE$ 11 POLYMERIC 12 ELEMENTAL 13 NASOGASTR$ 14 NASO‐GASTR$ 15 GASTROSTOM$ 16 JEJUNOSTOM$ 17 TUBE ADJ FEED$ 18 PARENTERAL‐NUTRITION#.DE. 19 ENTERIC‐FEEDING#.DE. 20 DIGESTIVE‐TRACT‐INTUBATION#.DE. 21 GASTROSTOMY.W..DE. 22 JEJUNOSTOMY.W..DE. 23 ELEMENTAL‐DIET.DE. 24 PROTEIN‐HYDROLYSATE.DE. OR PROTEIN‐HYDROLYSATE.DE. 25 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 26 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 27 20 OR 21 OR 22 OR 23 OR 24 28 25 OR 26 OR 27 29 CHEMOTHERAP$ 30 ANTICANCER ADJ TREATMENT 31 ANTI ADJ CANCER ADJ TREATMENT 32 LEUKEMIA OR LEUKEMIAS 33 LEUKAEMIA OR LEUKAEMIAS 34 LYMPHOMA OR LYMPHOMAS 35 TUMOUR OR TUMOURS 36 TUMOR OR TUMORS 37 CANCER OR CANCERS 38 NEOPLASM OR NEOPLASMS 39 ONCOLOGY 40 CANCER‐CHEMOTHERAPY#.DE. 41 29 OR 30 OR 31 OR 32 OR 33 42 34 OR 35 OR 36 43 37 OR 38 OR 39 OR 40 44 41 OR 42 OR 43 45 CHILD# OR ADOLESCENT.DE. 46 CHILD OR CHILDREN OR CHILDHOOD 47 45 OR 46 48 RANDOMIZED‐CONTROLLED‐TRIAL.DE. 49 CLINICAL‐TRIAL# 50 COMPARATIVE‐STUDY#.DE. 51 CONTROLLED‐STUDY#.DE. 52 CONTROL‐GROUP.DE. OR DOUBLE‐BLIND‐PROCEDURE.DE. OR TRIPLE‐BLIND‐PROCEDURE.DE. 53 48 OR 49 OR 50 OR 51 OR 52 54 28 AND 44 AND 47 55 53 AND 54 |
Appendix 4. Original search strategy for CINAHL
| Dialog DataStar via the National Library for Health (http://www.library.nhs.uk) Search 1 to 27 relates to terms describing the intervention; search 28 to 43 relates to terms describing the disease; search 44 to 46 relates to terms describing the population. 1 TPN 2 PARENTERAL ADJ NUTRITION 3 PARENTERAL ADJ FEED$ 4 ENTERAL ADJ NUTRITION 5 ENTERAL ADJ FEED$ 6 CENTRAL ADJ LINE OR CENTRAL ADJ LINES 7 HYDROLYSATE$ 8 POLYMERIC 9 ELEMENTAL 10 NASOGASTR$ 11 NASO‐GASTR$ 12 GASTROSTOM$ 13 JEJUNOSTOM$ 14 TUBE ADJ FEED$ 15 INTUBATION 16 INTRAVENOUS ADJ THERAPY 17 PARENTERAL‐NUTRITION#.DE. 18 ENTERAL‐NUTRITION#.DE. 19 FEEDING‐TUBES#.DE. 20 GASTROSTOMY#.W..DE. 21 JEJUNOSTOMY#.W..DE. 22 NUTRITIONAL‐SUPPORT#.DE. 23 FOOD‐FORMULATED#.DE. 24 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 25 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 26 19 OR 20 OR 21 OR 22 OR 23 27 24 OR 25 OR 26 28 CHEMOTHERAP$ 29 ANTICANCER ADJ TREATMENT 30 ANTI ADJ CANCER ADJ TREATMENT 31 LEUKEMIA OR LEUKEMIAS 32 LEUKAEMIA OR LEUKAEMIAS 33 LYMPHOMA OR LYMPHOMAS 34 TUMOUR OR TUMOURS 35 TUMOR OR TUMORS 36 CANCER OR CANCERS 37 NEOPLASM OR NEOPLASMS 38 ONCOLOGY 39 CHEMOTHERAPY‐CANCER#.DE. 40 NEOPLASMS#.W..DE. 41 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 42 37 OR 38 OR 39 OR 40 OR 41 43 41 OR 42 44 INFANT.W..DE. OR CHILD‐PRESCHOOL OR CHILD.W..DE. OR ADOLESCENCE.DE. 45 CHILD OR CHILDREN OR CHILDHOOD 46 44 OR 45 47 27 AND 43 AND 46 |
Appendix 5. Updated seach strategy for CENTRAL
Search strategy used for the update (October 2014):
1. For Nutrition the following kewords were used:
tpn OR parenteral nutrition OR parenteral feed* OR enteral nutrition OR enteral feed* OR central line* OR hydrolysate* OR polymeric OR elemental OR nasogastr* OR naso‐gastr* OR gastrostom* OR jejunostom* OR tube feed* OR intravenous therapy OR intubation OR Nutritional Support OR Gastrointestinal Intubation OR Gastrostomy OR Jejunostomy
2. For Cancer the following keywords were used:
(cancer OR cancers OR cancer* OR oncology OR oncolog* OR neoplasm OR neoplasms OR neoplasm* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR tumors OR tumours OR malignan* OR malignant OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo*)
3. For Children the following keywords were used
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy
Final search 1 AND 2 AND 3
[*] = zero or more characters
The search was performed in title, abstract or keywords
Appendix 6. Updated search strategy for MEDLINE
Search strategy used for the update (October 2014): via Ovid
1. For Nutrition the following MeSH terms and keywords were used:
1. exp Parenteral Nutrition/ or exp Parenteral Nutrition, Total/ or tpn.mp. 2. (parenteral adj nutrition).mp. 3. (parenteral adj feed$).mp. 4. enteral nutrition.mp. or exp Enteral Nutrition/ 5. (((enteral adj nutrition) or enteral) adj feed$).mp. 6. (central adj line$).mp. 7. exp Protein Hydrolysates/ or hydrolysate.mp. 8. hydrolysate$.mp. 9. polymeric.mp. 10. exp Food, Formulated/ or elemental.mp. 11. (nasogastr$ or naso‐gastr$).mp. 12. gastrostomy.mp. or exp Gastrostomy/ 13. gastrostom$.mp. 14. jejunostomy.mp. or exp Jejunostomy/ 15. (tube adj feed$).mp. 16. intraven$.mp. 17. exp Intubation, Gastrointestinal/ 18. or/1‐17
2. For Cancer the following MeSH terms and keywords were used:
1. (cancer or cancers or cancer$).mp. 2. (oncology or oncolog$).mp. 3. (neoplasm or neoplasms or neoplasm$).mp. or exp neoplasms/ 4. (carcinoma or carcinom$).mp. or exp carcinoma/ 5. (tumor or tumour or tumor$ or tumour$ or tumors or tumours).mp. 6. (malignan$ or malignant).mp. 7. (hematooncological or hemato oncological or hemato‐oncological or hematologic neoplasms or hematolo$).mp. or exp hematologic neoplasms/ 8. or/1‐7
3. For Children the following MeSH terms and keywords were used:
1. Infant, Newborn/ or Infant/ or Child, Preschool/ or Child/ or Adolescent/ or Minors/ or Puberty/ or pediatrics/ or Schools.mp. or Schools, Nursery/ 2. infant.ti,ab. or infant$.mp. or infanc$.mp. 3. (newborn or newborn$ or (new adj born$)).mp. 4. (baby or baby$ or babies).mp. 5. (neonat$ or perinat$ or postnat$).mp. 6. (child or child$).mp. 7. (schoolchild or schoolchild$ or (school adj child) or (school adj child$)).mp. 8. (kid or kids).mp. 9. (toddler or toddler$).mp. 10. adolesc$.mp. 11. (teen or teens or teenager or teenager$).mp. 12. (boy or boy$).mp. 13. (girl or girl$).mp. 14. (minors or minors$).mp. 15. (underag$ or (under adj ag$)).mp. 16. juvenil$.mp. 17. youth$.mp. 18. kindergar$.mp. 19. (puberty or puber$ or pubescen$).mp. 20. (prepuberscen$ or prepuberty$).mp. 21. (pediatrics or pediatric$ or paediatric$ or peadiatric$).mp. 22. (schools or preschool$ or (pre adj school$)).mp. 23. ((primary adj school$) or (secondary adj school$)).mp. 24. ((elementary adj school) or (elementary adj school$)).mp. 25. ((high adj school$) or highschool$).mp. 26. (nursery adj school$).mp. 27. (schoolage or (school adj age$) or schoolage$).mp. 28. or/1‐27
4. For RCTs and CCTs the following MeSH terms and keywords were used:
1. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ti,ab. or placebo.ti,ab. or drug therapy.fs. or randomly.ti,ab. or trial.ti,ab. or groups.ti,ab.) and humans/
Final search 1 AND 2 AND 3 AND 4
[mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier; $= zero or more charaxcters; / = MeSH term; pt= publication type; ti,ab=title or abstract; fs‐floating subheading]
Appendix 7. Updated search strategy for EMBASE
Search strategy used for the update (October 2014): Via Ovid
1. For Nutrition the following Emtree terms and keywords were used:
1. tpn.mp. 2. (parenteral adj nutrition).mp. 3. exp parenteral nutrition/ 4. (parenteral adj feed$).mp. 5. (enteral adj nutrition).mp. 6. (enteral adj feed$).mp. 7. exp enteric feeding/ 8. ((central adj line) or (central adj lines)).mp. 9. intraven$.mp. 10. exp DIGESTIVE TRACT INTUBATION/ or intubation.mp. 11. exp PROTEIN HYDROLYSATE/ or hydrolysate$.mp. 12. polymeric.mp. 13. elemental.mp. or exp elemental diet/ 14. (nasogastr$ or naso‐gastr$).mp. 15. gastrostom$.mp. or exp gastrostomy/ 16. jejunostom$.mp. or exp jejunostomy/ 17. exp tube feeding/ 18. (tube adj feed$).mp. 19. or/1‐18
2. For Cancer the following Emtree terms and keywords were used:
1. (cancer or cancers or cancer$).mp. 2. (oncology or oncolog$).mp. or exp oncology/ 3. (neoplasm or neoplasms or neoplasm$).mp. or exp neoplasm/ 4. (carcinoma or carcinom$).mp. or exp carcinoma/ 5. (tumor or tumour or tumor$ or tumour$ or tumors or tumours).mp. or exp tumor/ 6. (malignan$ or malignant).mp. 7. (hematooncological or hemato oncological or hemato‐oncological or hematologic neoplasms or hematolo$).mp. or exp hematologic malignancy/ 8. or/1‐7
3. For Children the following Emtree terms and keywords were used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors or minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
4. For RCTs and CCTs the following Emtree terms and keywords were used:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. Human/ 11. 9 and 10
Final search 1 AND 2 AND 3 AND 4
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer; $=zero or more characters; /=Emtree term; ti,ab=title or abstract; sh=subheading]
Appendix 8. Updated search strategy for CINAHL
Search strategy used for the update (October 2014): Via Ebsco:
1. ForNutrition the following Subject Headings and keywords were used:
S1 (MH "Total Parenteral Nutrition") OR (MH "Enteral Nutrition") OR (MH "Parenteral Nutrition Solutions") OR "TPN" S2 (MH "Gastrostomy") OR "gastrostomy" S3 "parenteral nutrition" S4 "parenteral feed*" S5 "enteral nutrition" S6 "enteral feed*" S7 "hydrolysate*" S8 "polymeric" S9 "elemental" S10 "gastrostom*" OR (MH "Gastrostomy") S11 (MH "Jejunostomy") OR "jejunostom*" S12 (MH "Feeding Tubes+") OR "tube feed*" S13 (MH "Intubation+") OR "intubation" S14 (MH "Intravenous Therapy+") OR "intravenous therapy" S15 (MH "Nutritional Support+") OR "nutritional support" S16 (MH "Food, Formulated+") OR "formulated food" S17 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16)
2. For Cancer the following Subject Headings and keywords were used:
S1 "cancer" S2 cancer or cancers or cancer* S3 (MH "Oncology+") OR "oncology" S4 "oncolog*" S5 "neoplasm" OR (MH "Childhood Neoplasms") S6 (MH "Neoplasms+") OR "neoplasms" S7 "neoplasm*" S8 (MH "Carcinoma+") OR "carcinoma" S9 "carcinom*" S10 tumor OR tumour OR tumor* OR tumour* OR tumors OR tumours S11 "malignan* OR malignant" S12 hematooncological OR hemato oncological OR hemato‐oncological S13 (MH "Hematologic Neoplasms+") OR "hematologic neoplasms" S14 hematolog* S15 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14)
3. For Children the following Subject Headings and keywords were used:
S1 (MH "Infant+") OR "Infant" OR (MH "Infant, Newborn+") S2 (MH "Child+") OR "Child" S3 (MH "Child, Preschool") S4 (MH "Adolescence+") OR "Adolescent" S5 "Minors" OR (MH "Minors (Legal)") S6 (MH "Puberty+") OR "Puberty" S7 (MH "Pediatrics+") OR "pediatrics" S8 (MH "Schools, Nursery") OR (MH "Schools+") OR "schools" S9 "infancy or infanc*" S10 "newborn" S11 baby or baby* or babies S12 neonat* S13 perinat* S14 postnat* S15 child* or children* S16 schoolchild S17 kid OR kids S18 toddler OR toddler* S19 adolesc* S20 teen OR teens OR teenager OR teenager* S21 boy OR boy* S22 girl OR girl* S23 minors* S24 underag* S25 juvenil* S26 youth* S27 kindergarten S28 puber* S29 pubescen* S30 prepuberty* S31 pediatric* S32 paediatric* S33 peadiatric* S34 preschool* S35 primary school S36 (MH "Schools, Secondary") OR "secondary school" S37 (MH "Schools, Elementary") OR "elementary school" S38 high school OR highschool S39 nursery school S40 school age OR schoolage S41 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40)
4. For RCTs and CCTs the following Subject Headings and keywords were used:
S1 (MH "Randomized Controlled Trials") OR "randomized controlled trial" S2 (MH "Clinical Trials+") OR "Clinical Trials" S3 "controlled clinical trial" S4 TI randomized S5 AB randomized S6 TI placebo S7 AB placebo S8 (MH "Drug Therapy+") OR "drug therapy" S9 TI randomly S10 AB randomly S11 TI trial S12 AB trial S13 TI groups S14 AB groups S15 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14)
Final search 1 AND 2 AND 3 AND 4
[MH=Exact Subject Heading; *=zero or more character; +=explosion; TI‐title; AB=abstract]
Data and analyses
Comparison 1. Parenteral nutrition (PN) versus enteral nutrition (EN).
Comparison 2. Nasogastric enteral nutrition (EN) versus usual food intake enteral nutrition (EN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean mid‐upper arm circumference | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Two weeks from start of study | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.32, 1.52] |
| 1.2 Six weeks from start of study | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 1.76 [0.34, 3.18] |
| 1.3 Twelve weeks from start of study | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐1.22, 1.34] |
| 2 Number of patients who experienced recurrent vomiting during study | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [0.17, 70.94] |
| 3 Number of deaths at end of study | 1 | 10 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 6.65] |
Comparison 3. Parenteral nutrition (PN) and enteral nutrition (EN) versus parenteral nutrition (PN).
Comparison 4. Parenteral nutrition (PN) versus fluid therapy (FT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients who developed an infection during study | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.76, 5.24] |
Comparison 5. Energy dense vs. standard calorie enteral feeds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight z‐score ≥ zero | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 4.4 [1.20, 16.17] |
| 2 Mid‐upper arm circumference relative to reference population | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 3.60 [‐4.34, 11.54] |
| 3 Triceps skinfold thickness relative to reference population | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐146.39, 148.39] |
| 4 Biceps skinfold thickness relative to reference population | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐4.5 [‐28.51, 19.51] |
| 5 Mean change in overall muscle arm area | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 5.80 [‐1.32, 12.92] |
| 6 Mean energy intake | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 28.0 [17.03, 38.97] |
| 7 Proportion of daily estimated feed requirement consumed | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐18.40, 0.40] |
| 8 Mean days of therapy‐related vomiting | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.89, 0.49] |
5.5. Analysis.
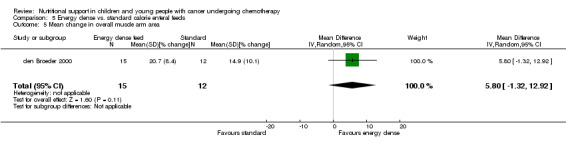
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 5 Mean change in overall muscle arm area.
5.6. Analysis.
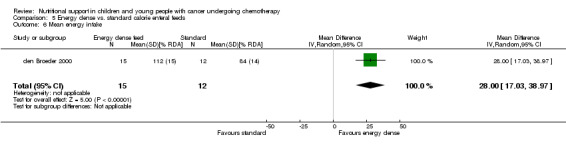
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 6 Mean energy intake.
5.7. Analysis.
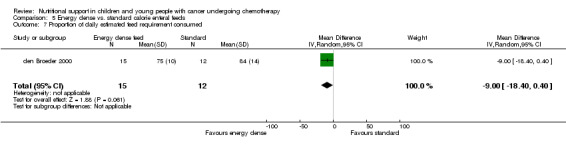
Comparison 5 Energy dense vs. standard calorie enteral feeds, Outcome 7 Proportion of daily estimated feed requirement consumed.
Comparison 6. Additional fructooligosaccharides in enteral feeds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight for height | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.57, 0.33] |
| 2 Mean increase in height‐for‐age z‐score | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.57, 0.44] |
| 3 Mean increase in weight‐for‐age z‐score | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.03, 1.00] |
| 4 Diarrhoea | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 5 Nausea | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.48, 1.74] |
| 6 Abdominal pain | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 99.95] |
Comparison 7. Glutamine supplementation of feeds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mid‐upper arm circumference | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.00, 1.80] |
| 2 Infection rates | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Severe nausea and/or vomiting | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.60, 1.31] |
| 4 Duration of vomiting | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.55, 0.33] |
| 5 Severe diarrhoea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.68, 1.79] |
| 6 Duration of diarrhoea | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.81, 0.45] |
| 7 Severe mucositis | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Treatment related mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Maximum serum ammonia levels | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Alternative parenteral (PN) lipid formulations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in mean weight z‐scores | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.68, ‐0.00] |
| 2 PN energy intake | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐5.24, 1.44] |
| 3 PN protein intake | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aquino 2005.
| Methods | RCT Parallell design Multi‐centred (participating centres of the Pediatric Blood and Marrow Transplant Consortium ) United States of America |
|
| Participants | Patients were eligible for enrolment if they were undergoing allogeneic or autologous stem cell transplant from any stem cell source (including bone marrow, umbilical cord blood, or peripheral blood stem cells) at participating institutions, were younger than 21 years of age and their planned conditioning regimen was associated with at least a documented 50% risk of grade III or IV mucositis by National Cancer Institute criteria. Subjects were ineligible if they were to receive vancomycin paste or nonabsorbable antibiotics, were to receive glutamine supplemented TPN, had a history of veno‐occlusive disease, or were undergoing a second HSCT. 130 enrolled, 120 followed up. AGE Glutamine 8.9 ± 1.0 years Glycine 10.5 ± 0.6 years SEX Glutamine – male 37 (65%), female 20 (35%) Glycine – male 36 (57%), female 27 (43%) DISEASE STATUS Glutamine (n = 57) Leukemia 30 (53%) Lymphoma 3 (5%) Neuroblastoma 12 (21%) Other solid tumours 7(12%) Nonmalignancy 5 (9%) Glycine (n = 63) Leukemia 32 (51%) Lymphoma 5 (8%) Neuroblastoma 10 (16%) Other solid tumours 11 (17%) Nonmalignancy 5 (8%) HSV positive status ‐ glutamine 12 (21%), glycine ‐ 24 (38%) CHEMOTHERAPY/RADIOTHERAPY Total body irradiation ‐ glutamine 24 (42%), glycine 26 (41%) Methotrexate as graft‐versus‐host disease prophylaxis ‐ glutamine 16 (28%), glycine 19 (30%) Autologous (total) ‐ glutamine 25 (44%), glycine 29 (46%) Allogeneic (total) ‐ glutamine 32 (56%), glycine 34 (54%) |
|
| Interventions | 120 patients reported to be randomised Both glutamine and glycine were administered at a dose of 2 g/m²/dose (maximum dose 4 g) in a solution of 500 mg/ml twice daily. The drug was dissolved in water or other liquid at the local institution. The solution had the consistency of a thickened liquid. |
|
| Outcomes | Reported for throughout the 28 days post transplant, or until discharge. 130 enrolled, 2 unable to take part, 8 refused to take any drug. Primary outcomes Adverse events ‐ Average mucositis score. ‐ Highest mucositis score. ‐ Episodes of bacteraemia. ‐ Abnormal biochemical profiles. Secondary outcomes ‐ Number of deaths. ‐ Duration of hospitalisation. |
|
| Notes | The drug was started on the first day of admission for HSCT, and given until 28 days post transplant or until the day of discharge, whichever occurred first. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer generated |
| Allocation concealment (selection bias) | Low risk | Comment: random permutation table at a central pharmacy (Children’s Medical Center Dallas). The drug was then shipped to the transplanting centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded. Both glutamine and glycine (placebo) were administered in a solution of 500 mg/ml twice daily. The drug was dissolved in water or other liquid at the local institution. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded. Outcomes reported include objective and subjective measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 130 patients enrolled, but it appears clear that 10 patients who were not included in the analysis were excluded for reason which would not affect randomisation (failure to undergo BMT, refusal to take any medicine, inability to transport), therefore complete follow‐up and analysis was probably done. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes that were said to be collected were reported. |
| Other bias | Low risk | Comment: none noted |
den Broeder 2000.
| Methods | RCT Parallel design Single‐centre University Hospital Nijmegen, the Netherlands | |
| Participants | 27 patients aged 1 to 18 years, newly diagnosed with cancer AGE EN nasogastric (energy enriched) ‐ 6.5 ± 4.5 years (mean ± SD) EN nasogastric (standard) ‐ 5.7 ± 3.8 years (mean ± SD) SEX EN nasogastric (energy enriched) ‐ 7 male, 8 female EN nasogastric (standard) ‐ 5 male, 7 female DISEASE STATUS Nephroblastoma Rhabdomyosarcoma Ewing sarcoma Neuroblastoma Osteosarcoma Germ cell tumour Hepatoblastoma Brain tumour Malnourished at diagnosis CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy and/or radiotherapy |
|
| Interventions | 27 patients randomly assigned:
EN nasogastric (energy enriched) ‐ (n = 15), EN nasogastric (standard) ‐ (n = 12) EN tube feeding was administered at home and during hospital admission via a small bore silicone duodenal feeding tube with a weighted tip that was inserted into the stomach. During hospital admissions, the tube feeding was administered by continuous infusion over a 24‐hour period. At home the feeding routine was flexible and tailored to individual needs. The total volume of tube feeding to be administered was set to provide each child with 100% of the total daily energy requirement for the standard formula and 150% of the total energy requirement for the energy enriched formula. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Weight for height measurements weekly
‐ Mid‐upper arm circumference weekly
‐ Triceps skinfold weekly
‐ Biceps skinfold weekly ‐ Calculated muscle (arm area) ‐ Serum albumin at diagnosis, at initiation of tube feeding and weekly thereafter ‐ Pre‐albumin at diagnosis, at initiation of tube feeding and weekly thereafter Adverse events ‐ Occurrence of vomiting (mean number of days per week on which vomiting occurred) Calorie and nutritional intake ‐ Mean daily energy intake per week ‐ Mean daily total protein intake Secondary outcomes ‐ Patient tolerance/adherence with feeding |
|
| Notes | The study period started at the initiation of tube feeding (week 0) and ended after 10 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: random generation unclear, but undertaken in pairs (block randomisation) before study |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated how undertaken |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not clear if formulas were unlabelled or concealed in any way so as researchers or participants were blinded. However it does state in the tube feed regimen description in the methods that it was unknown whether the child received the standard or energy dense formula, as volume of feed was calculated on the assumption that all patients received the standard feed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not clear if formulas were unlabelled or concealed in any way so as researchers or participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: many outcomes were measured from patient diaries ‐ unclear how much missing data was present. Also, 27/29 patients were analysed; missing patients were presumed to have dropped out of the control group (15 patients analysed in intervention group). As missing data was not imputed, this is not intention to treat. |
| Selective reporting (reporting bias) | Low risk | Comment: all measurements stated were reported |
| Other bias | Low risk | Comment: no other risks of bias noted |
Donaldson 1982.
| Methods | RCT Parallel design Multi‐centre ‐ 3 paediatric oncology centres USA | |
| Participants |
AGE
PN ‐ median = 5.8 years, EN ‐ median = 13.4 years SEX Not reported DISEASE STATUS Wilms' tumour Neuroblastoma Soft tissue sarcoma including rhabdomyosarcoma Bone sarcoma including pelvic, Ewing's sarcoma and osteogenic sarcoma Abdominal non‐Hodgkin's lymphoma Ovarian cancer Only well‐nourished patients randomised CHEMOTHERAPY/RADIOTHERAPY All patients received radiotherapy and all but 2 patients received additional chemotherapy |
|
| Interventions | 25 patients randomly assigned:
PN ‐ n = 12, EN (usual food intake) ‐ n = 13 PN ‐ consisted of crystalline amino acid solution, dextrose, minerals and vitamins. PN patients were fed by central line throughout the period of the study. PN patients were allowed only non‐calorie oral intake during the period of PN. EN (usual food intake) ‐ consisted of no restriction in terms of dietary intake Both groups profited from regular dietary counselling throughout the period of the study |
|
| Outcomes | Outcomes measured at the following time points: ‐ End of radiotherapy/chemotherapy ‐ 3‐month follow up Primary outcomes Change in nutritional indices ‐ Median percentage change in triceps skinfold ‐ Median percentage change in arm circumference ‐ Median percentage change in arm muscle circumference ‐ Median change in serum albumin Adverse events ‐ Number of patients with nausea and vomiting ‐ Number of patients with diarrhoea Secondary outcomes ‐ Number of deaths ‐ Performance status (activity compared to baseline) |
|
| Notes | PN was begun simultaneously with irradiation and continued throughout radiotherapy and for a 3‐ to 5‐day post‐therapy period as a PN "weaning" period. Patients received PN or EN treatment throughout the course of the radiotherapy treatment which lasted for approximately 6 weeks. All but 2 randomised patients received chemotherapy in addition to radiotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients randomized to receive or not to receive PN", "patients were stratified on the basis of cancer therapy, i.e. radiation dose and volume, with/or without chemotherapy" Comment: actual method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not possible (PN infusion obvious) Participants: not possible (PN infusion obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not discussed. Some outcomes were subjective (e.g. nausea) so lack of blinding may have influenced outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Patients randomised: PN n = 12 EN n = 13 Withdrawals described PN n = 1 One randomised PN patient complained of hunger and withdrew from the study after only 4 days of PN EN n = 4 Four of the children randomised to the EN arm of the study received PN at some point during their oncologic treatment. One of these 4 requested PN; the other 3 became malnourished and were crossed‐over to PN per protocol. Patients analysed: PN n = 11 EN n = 12 In the evaluation of the effect of PN on nutritional status during oncologic treatment, the EN patient who requested PN and the PN patient who withdrew from the study were excluded from the analysis, making 11 PN and 12 EN patients. Intention‐to‐treat analysis was therefore not conducted. |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes described in trial as being measured but results not reported: Dietary history Skin tests for delayed hypersensitivity Detailed laboratory studies ‐ haematologic studies Time points: States children were evaluated at 3‐weekly intervals following radiotherapy ‐ but only end of radiotherapy and 3‐month follow up endpoints reported |
| Other bias | Unclear risk | Nausea scale ‐ appears unvalidated, so unclear as to how well it performs. |
Ghavimi 1982.
| Methods | RCT Single‐centre study, although 14 of the patients in this study were also enrolled into a multi‐institutional paediatric nutrition study (Donaldson 1982) sponsored by the National Cancer Institute USA | |
| Participants |
AGE
PN ‐ median = 13 years, range 2.5 to 17 years
EN (usual food intake) ‐ median = 15 years, range 5.5 to 21 years SEX Not reported DISEASE STATUS Embryonal rhabdomyosarcoma, non‐Hodgkin's lymphoma, Ewing's sarcoma, endodermal sinus tumour, Grade III teratoma, dysgerminoma, Wilms' tumour Only well‐nourished patients randomised CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy and radiotherapy. |
|
| Interventions | 25 patients randomly assigned:
PN ‐ n = 12, EN (usual food intake) ‐ n = 13 PN ‐ consisted of daily amounts of calories and amino acids, given as Freamine II, 8.5% and hypertonic glucose, designed to meet the recommended dietary allowances for the particular age group and adjusted if necessary to meet any special nutritional needs. Fat was given as Intralipid 10%, at a minimum of 3% of caloric intake, at least once a week to supply essential fatty acids. Minerals, vitamins and trace elements were given at levels as large or larger than the recommended dietary allowances. PN patients were not fed orally during this period of PN except for oral medications and limited amounts of water. EN (usual food intake) ‐ consisted of the established regimen of care, including oral diet ad libitum with the advice and assistance of dieticians. Dextrose (5%), saline, and other electrolytes, fluids and blood products were administered intravenously as required. These patients were not hospitalised except at the initiation of therapy. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Percentage weight gain
‐ Mean skinfold thickness
‐ Mean arm muscle circumference
‐ Serum albumin Adverse events ‐ Number of positive blood cultures ‐ Number of patients with nausea and vomiting ‐ Number of patients with diarrhoea Secondary outcomes ‐ Number of deaths |
|
| Notes | PN ‐ mean duration = 44 days
EN (usual food intake) ‐ mean duration = 42 days PN ‐ was started 2 to 4 days prior to initiation of radiotherapy. The PN solutions were delivered through tubing with an in‐line filter and continuously over 24 hours. These patients were hospitalised for the duration of PN, which was the time involved in the first cycle of chemotherapy and radiation therapy. Radiotherapy was combined with simultaneous or sequential systemic chemotherapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients randomized to the control or PN group" Comment: actual method of randomisation not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "Two groups were randomly selected by the Statistical Laboratory of Memorial Sloan‐Kettering Cancer Center and by the Diet, Nutrition and Cancer program at the National Cancer Institute." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not possible (PN infusion obvious) Participants: not possible (PN infusion obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not discussed. Some outcomes were subjective (e.g. nausea) so lack of blinding may have influenced outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients randomised:
PN n = 12
EN n = 13 Withdrawals described PN n = 1 One patient randomised to PN refused it and the authors state that this patient "was treated as a non‐randomised EN/control. The data on this patient are included with those in the EN group." EN n = 4 Four of the EN patients lost weight during the study and were crossed over to PN. Their results were recorded under the EN group. Patients analysed: PN n = 11 EN n = 14 Intention‐to‐treat analysis: not used, since 1 of the PN patients was treated as and analysed as a non‐randomised EN/control. We have taken account of this in our analysis and not included the non‐randomised control patient in the denominator. |
| Selective reporting (reporting bias) | High risk | Outcomes described in trial as being measured but results not reported: Dietary history Skin tests for delayed hypersensitivity Detailed laboratory studies ‐ haematologic studies Time points: States children were evaluated every 3 weeks following radiotherapy ‐ but only end of radiotherapy and 3‐month follow up endpoints reported |
| Other bias | Unclear risk | Nausea scale ‐ appears unvalidated, so unclear as to how well it performs |
Hartman 2009.
| Methods | RCT Single centre study Israel |
|
| Participants |
AGE Study group 6.6 +/‐ 1.2 years Control group 7.1 +/‐ 1.2 years SEX Study group 8 male, 7 female Control group 8 male, 5 female DISEASE STATUS Study group 3 autologous BMT 12 allogenic BMT 5 thalaesemia 2 neuroblastoma 1 acute lymphocytic leukemia 2 acute myeloid leukemia 1 lymphoma 1 Ewings sarcoma 1 aplastic anaemia 0 Hurler syndrome Control group 5 autologous BMT 8 allogenic BMT 4 thalaesemia 4 neuroblastoma 3 acute lymphocytic leukemia 0 acute myeloid leukemia 1 lymphoma 1 Ewings sarcoma 0 aplastic anaemia 1 Hurler syndrome CHEMOTHERAPY/RADIOTHERAPY Allogeneic transplant patients received total body irradiation, autologous transplant patients were conditioned with busulphan +/‐ melphalan. Exact details not given. |
|
| Interventions | 28 patients randomly assigned; 15 in treatment group, 13 in control group Treatment group received olive oil based Cinoleic 20% (Baxter SAS, Maurepas, France) Control group received standard Lipofundin 20% (B. Braun, Melsungen, Germany) These were adjusted to give 30% of child’s energy intake as lipid; 1.1+/‐ 0.1 g/kg/day |
|
| Outcomes | Primary outcomes were recorded at day 0 and day 14 (weight was recorded daily) Change in nutritional indices ‐ Change in weight z‐score ‐ Serum albumin Adverse events ‐ Serum lipid values Calorie and nutritional intake ‐ Total and fractional PN energy supplied ‐ Total daily protein intake |
|
| Notes | Children aged 1‐18 years who underwent BMT and needed PN for at least 2 weeks. Children who received PN for less than 8 days, had abnormal liver function tests before initiation of PN (total bilirubin > 2.5 mg/dl, SGOT/SGPT 2.5 x upper limit of normal) and enteral intake of more than 50% of total calories were not included in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer generated random numbers used |
| Allocation concealment (selection bias) | Unclear risk | Comment: appears to be allocation in a site away from the treating hospital, but not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators, attending and primary physicians,
patients, the nursing staff and the study pharmacist who received coded PN solutions were blinded to randomization and treatment scheme." Comment: participants not mentioned, but unlikely to have been informed given the blinding of all healthcare personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigators, attending and primary physicians,
patients, the nursing staff and the study pharmacist who received coded PN solutions were blinded to randomization and treatment scheme" Comment: the outcome measurements are largely laboratory values which are objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Only daily weights not reported in paper (weights on day 0 and day 14 given) |
| Other bias | Unclear risk | Small dose of lipid given and short duration of treatment; absence of any adverse effects is not evidence of safety. |
Hays 1983.
| Methods | RCT Parallel design Single‐centre California, USA | |
| Participants |
AGE
Age range 2.5 to 12 years SEX Not reported DISEASE STATUS All had a diagnosis of acute non‐lymphocytic leukaemia Only well‐nourished patients randomised CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy |
|
| Interventions | 10 patients randomly assigned:
PN ‐ n = 5, EN (usual food intake) ‐ n = 5 PN ‐ consisted of a standardised PN regimen given to patients throughout the period of study except when interrupted by sepsis, mechanical problems or competing therapy EN ‐ consisted of a sterilised oral diet without PN Both groups were allowed oral nutrition as tolerated. Also states that the control group received intravenous fluids (not PN) only when clinically indicated. PN was administered for the duration of the study at a rate of 1800 ml/metre2, lipid emulsion (10%) was infused 3 times per week at a dose of 10 to 20 ml/kg. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Mean change in weight
‐ Arm muscle area percentiles
‐ Triceps skinfold percentiles
‐ Mean change in serum albumin
‐ Pre‐albumin Adverse events ‐ Number of positive cultures from blood, urine or stool Calorie and nutritional intake ‐ Mean calorie intake/day ‐ Mean protein intake/day Secondary outcomes ‐ Number of deaths |
|
| Notes | PN (mean duration = 55.8 days, range 30 to 104 days)
EN (mean duration = 75.2 days, range 51 to 111 days) PN was administered for the duration of the study, confined to induction phase of chemotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: actual method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not possible (PN infusion obvious) Participants: not possible (PN infusion obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not discussed. All outcomes objective, so lack of blinding unlikely to influence outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Patients randomised: PN n = 5 EN n = 5 Withdrawals: no withdrawals Patients analysed: PN n = 5 EN n = 5 All patients entering into trial appear to have been analysed according to the intervention to which they were allocated Intention‐to‐treat analysis appears to have been used |
| Selective reporting (reporting bias) | High risk | Comment: outcomes described in trial as being measured but results only reported narratively: Retinol‐binding protein Pre‐albumin Transferrin levels |
| Other bias | Low risk | Comment: None noted |
Rickard 1985.
| Methods | RCT Parallel design Single‐centre ‐ Riley Hospital for Children ‐ patients enrolled between December 1978 and March 1983 USA | |
| Participants |
AGE
Age range 6 months to 10 years
CPN ‐ range 1 year to 10 years 6.5 months
PPN and EN ‐ range 1 year 3 months to 5 years 6 months SEX CPN ‐ 6 male, 4 female PPN and EN ‐ 4 male, 4 female DISEASE STATUS Stage 3 or 4 neuroblastoma Details of primary site for each patient:CPN ‐ 7 abdominal, 3 other PPN and EN ‐ 7 abdominal, 1 other Only malnourished patients randomised CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy and 7 of 10 CPN patients and 3 of 8 PPN and EN patients received additional radiotherapy |
|
| Interventions | 18 patients randomly assigned: CPN ‐ n = 10, PPN and EN ‐ n = 8 EN ‐ consisted of age‐appropriate counselling, nutritious favourite foods and oral supplements. Supplements were offered during chemotherapy‐free periods to reduce the possibility of conditioned aversion. Neither vitamin nor iron supplements were used. CPN ‐ consisted of synthetic nutrient mixture of 25 g/dl glucose, 2.55 g/dl crystalline amino acids and multivitamin infusion. Some patients received FreAmine II and some received FreAmine III when FreAmine II became unavailable. Trace elements were provided according to the American Medical Association guidelines. Nutrients were administered through a silastic central venous catheter placed into the superior vena cava. One milligram of AquaMEPHYTON was given weekly by intramuscular injection and intravenous fat emulsion was administered 3 times a week. Concentrations of glucose and rate of administration were increased over a 5 to 7 day period to provide an arbitrary goal of 100% of the 50th percentile energy intake of healthy children of a similar age. PPN ‐ consisted of a 12.5 g/dl glucose and 2.55 g/dl crystalline amino acid solution. Concentrations of vitamins and minerals were similar to that provided by CPN solution. Nutrients were administered through an intravenous catheter into peripheral veins. Intralipid was administered via a Y‐connector at a concentration of 10 g/dl and initiated at a rate of 2 g lipid/kg/day. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Mean change in weight
‐ Height
‐ Mean change in triceps skinfold
‐ Mean change in subscapular skinfold
‐ Mean change in serum albumin Calorie and nutritional intake ‐ Mean change in energy intake ‐ Mean change in protein intake |
|
| Notes | PN was begun at the onset of therapy and was provided for an average of 28 days through the first 2 cycles of chemotherapy Median length of follow up reported ‐ 10 weeks 14 of 18 malnourished children entered into the study had PN for approximately 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "children were randomized" Comment: actual method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "children were randomized" Comment: actual method of randomisation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not discussed (not possible as nasogastric tube obvious) Participants: not discussed (not possible as nasogastric tube obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not discussed. All outcomes objective, so lack of blinding unlikely to influence outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Patients randomised: CPN n = 10 PPN & EN n = 8 Withdrawals described CPN n = 2 due to gross oedema and pleural effusion PPN & EN n = 0 Patients analysed: CPN n = 8 PPN & EN n = 8 No imputation of missing data, therefore intention‐to‐treat analysis not undertaken. As drop outs are not accounted for and may be related to the intervention, there is a high risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes described in trial as being measured are reported |
| Other bias | Low risk | Comment: none noted |
Rickard 1989.
| Methods | RCT Parallel design Single‐centre ‐ Riley Hospital for Children ‐ enrolled between July 1979 and May 1985 USA | |
| Participants |
AGE
CPN ‐ median 2 years 6 months
PPN and EN ‐ median 6 years 6 months SEX CPN ‐ 7 male, 3 female PPN and EN ‐ 7 male, 2 female DISEASE STATUS All malnourished with newly diagnosed Wilms' tumour stage 1 to 5 Only malnourished patients considered HNR were randomised HNR = Stage 2 to 5 disease who: 1) were treated with NWTS protocol 3, i.e. operation, intense chemotherapy and abdominal radiation; or 2) were severely malnourished with > 15% weight loss CHEMOTHERAPY All patients received chemotherapy and all but one CPN patient received additional radiotherapy |
|
| Interventions | 19 patients randomly assigned:
CPN ‐ n = 10, PPN and EN ‐ n = 9 EN ‐ consisted of age appropriate counselling, nutritious favourite foods and oral supplements. Favourite foods and supplements were offered during chemotherapy‐free period to reduce the possibility of conditioned aversion. Vitamin and iron supplements were not used. A concerted effort was made to provide adequate EN for patients who received PPN. CPN patients who had catheter interruptions received PPN plus EN support during this period. CPN ‐ provided a synthetic nutrient mixture of 25 g/dl glucose, 2.55 g/dl crystalline amino acids and a multivitamin infusion. An intravenous fat emulsion was administered (2 g lipid/kg/day) 3 times per week. Nutrients were administered through a silastic central intravenous catheter placed into the superior vena cava just above the right atrium. PPN ‐ consisted of 12.5 g/dl glucose and 2.55 g/dl crystalline amino acid solution. Concentrations of vitamins and minerals were similar to those provided in the CPN solution. Nutrients were administered through an intravenous catheter into peripheral veins. Intralipid was administered through a Y‐connector at a concentration of 10 g/dl and initiated at a rate of 2 g/kg/day. CHEMOTHERAPY/RADIOTHERAPY All patients received radiotherapy and all but 2 patients received additional chemotherapy. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Mean daily weight changes
‐ Mean percentage change in weight
‐ Mean percentage change in triceps skinfold
‐ Mean percentage change in subscapular skinfold
‐ Mean change in serum albumin
‐ Mean change in pre‐albumin Adverse events ‐ Number of positive blood cultures ‐ Number of patients with diarrhoea Calorie and nutritional intake ‐ Mean percentage change in energy intake ‐ Mean protein intake Secondary outcomes ‐ Number of deaths |
|
| Notes | In HNR children were randomised to receive either CPN or PPN for approximately 4 weeks of initial intense treatment followed thereafter by EN up to 26 weeks. CPN for 4 weeks, then EN up to 26 weeks PPN and EN for 4 weeks, then EN up to 26 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized" Comment: actual method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not possible (CPN/PPN/EN obvious) Participants: not possible (CPN/PPN/EN obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Outcome assessor: not discussed All outcomes objective, so unlikely to influence outcome measurements |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Patients randomised: CPN n = 10 PPN & EN n = 9 Withdrawals described CPN n = 3 PPN & EN n = 3 6 patients excluded from short‐term analysis at 4 weeks ‐ 5 excluded because of inability to adhere to nutritional protocol and 1 excluded because of fluid limitations Patients analysed: CPN n = 7 PPN and EN n = 6 As not all patients were analysed and no imputation undertaken, an intention‐to‐treat analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes described in trial as being measured but results not reported: None ‐ all outcomes measured are reported upon either in graphical form or in the text. Although weekly measurements taken, some are reported as % changes and some actual changes |
| Other bias | Low risk | Comment: None noted |
Schmid 2006.
| Methods | RCT Parallel design Single‐centre Germany | |
| Participants |
AGE
Age range 1.0 to 18 years
PN ‐ mean = 8.2 years, range 2.0 to 15.2 years)
FT ‐ mean = 8.1 years, range 2.1 to 17.2 years) SEX PN ‐ 7 male, 8 female FT ‐ 11 male, 4 female All had oral mucositis. All had WHO grade IV tumours. CHEMOTHERAPY/RADIOTHERAPY All patients received first‐line intensive standard‐dose chemotherapy for leukaemias and solid tumours |
|
| Interventions | 30 patients randomly assigned:
PN ‐ n = 15, FT ‐ n = 15 PN ‐ consisted of the same amount of intravenous fluid and electrolytes as the children on fluid replacement therapy with standard formulations of carbohydrates, lipids, amino acids, vitamins and trace elements given at doses recommended by the German Nutrition Society FT ‐ fluid replacement therapy ‐ consisted of intravenous fluids and electrolytes according to the baseline recommendations for the German Nutrition Society |
|
| Outcomes |
Primary outcomes Change in nutritional indices ‐ Median change in weight ‐ Median change in fat‐free mass ‐ Median change in total body water ‐ Median change in serum albumin ‐ Median change in pre‐albumin Adverse events ‐ Number of infections diagnosed from positive blood, stool or urine culture of bacteria, virus or fungus Secondary outcomes ‐ Median hospitalisation time in days |
|
| Notes | PN ‐ median duration = 14 days, range 6 to 27 days
FT ‐ median duration = 13 days, range 7 to 25 days Starting on the day that oral mucositis WHO grade IV developed and IV opioid analgesics were initiated, patients were randomised to PN or FT for a duration of 10 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (response from author): "There was a numbered list which allocated every consecutive patient to PN or FT by throwing dice for PN or for FT." |
| Allocation concealment (selection bias) | High risk | Quote: (response from author): "The sequence was not concealed but known to the physicians on ward for starting the programme." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment; Clinician/person delivering treatment: not discussed (possible as PN and fluid infusions could look similar) Participants: not discussed (possible as PN and fluid infusions could look similar) However, given response from author regarding allocation concealment ‐ "The sequence was not concealed but known to the physicians on ward for starting the programme" ‐ it is likely that the clinicians, and perhaps the participants, were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not discussed. Given response from author regarding allocation concealment ‐ "The sequence was not concealed but known to the physicians on ward for starting the programme" ‐ it is likely that the clinicians, and perhaps the participants, were not blinded. However, as all outcomes were objective, lack of blinding unlikely to influence outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Patients randomised: PN n = 15 FT n = 15 Description of withdrawals: no formal description, but from table 2 it appears that there were no withdrawals. From table 2 of patient infections during course of treatment, it appears that all patients were analysed in the groups to which they were randomised. Patients analysed: PN n = 15 FT n = 15 Intention‐to‐treat analysis: numbers and tables imply that this has been undertaken. |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes described in trial as being measured but results only reported narratively: Blood urea nitrogen Creatinine Serum albumin (results obtained from author) Pre‐albumin (results obtained from author) |
| Other bias | Unclear risk | Quote: "five children recruited twice, two were randomized in both arms, one twice in the PN group and two twice in the FT group." Comment: the role of individual variation is unclear in this intervention; if analysis as if variations were independent may have biased results. |
Smith 1992.
| Methods | RCT Parallel design Single‐centre UK | |
| Participants |
AGE
Median age of 12 patients = 3.0 years
EN (nasogastric) ‐ age range 2.0 to 5.5 years
EN (usual food intake) ‐ age range 1.9 to 9.3 years SEX EN (nasogastric) ‐ 4 males, 2 females EN (usual food intake) ‐ 4 males, 2 females DISEASE STATUS Juvenile chronic myeloid leukaemia (1) Wilms' tumour (4) Neuroblastoma (4) Rhabdomyosarcoma (1) Liver sarcoma (1) Megakaryocytic leukaemia (1) Patients were malnourished CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy |
|
| Interventions | 12 patients randomly assigned:
EN (nasogastric) ‐ n = 6, EN (usual food intake) ‐ n = 6 EN (nasogastric) ‐ consisted of high energy density feed (1.5 kcal/ml) Fortisip Energy Plus (Cow and Gate). Seravit (SHS) was added to provide standard requirements of vitamins and minerals. The aim of the supplementation was to provide at least 100% of recommended daily allowance of energy or 100% of previous daily intake, whichever was higher. Feeds were administered via a Kangaroo 330 pump. Oral feeding was permitted ad libitum but did not influence the quantity of supplementary feed administered. EN (usual food intake) ‐ consisted of regular dietary counselling, the emphasis of which was to promote as high an energy intake as possible. |
|
| Outcomes |
Primary outcomes
Change in nutritional indices
‐ Mean change in mid‐upper arm circumference Adverse events ‐ Number of patients with recurrent vomiting Calorie and nutritional intake ‐ Median percentage of RDA of energy intake Secondary outcomes ‐ Number of deaths ‐ Acceptability of nasogastric feeding ‐ parental questionnaire ‐ Activity ‐ median play scores |
|
| Notes | EN (nasogastric) ‐ median duration = 4 weeks, range 3 to 6 weeks Subjects commenced supplementary nasogastric feeding as soon after diagnosis as possible. The feeding routine for nasogastric was flexible, with the expectation that most children would eventually receive nasogastric feeding overnight at home. Feeding was usually commenced at a continuous infusion of 10 ml/h and was increased by 10 ml/h/day, if tolerated. If children were in bed throughout most of each 24‐hour period, nasogastric feeding was continued throughout the 24 hours. In children were more active by day, feeding was restricted to 10 to 14 hours overnight. Once MAC was documented to be increasing, no attempt was made to increase total daily intake any further. Nasogastric feeding was continued until MAC was above the tenth percentile for at least 2 weeks. Follow up was for a period of 3 months from diagnosis. This permitted study during the most intensive phase of disease specific treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to study or control groups using a computer generated random sequence" |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not discussed (not possible as nasogastric tube obvious) Participants: not discussed (not possible as nasogastric tube obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not discussed, Some outcomes were subjective (e.g. nausea) so lack of blinding may have influenced outcome measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Patients randomised: EN (nasogastric) n = 6 EN (usual food intake) n = 6 Withdrawals described: EN (nasogastric) n = 1 One nasogastric patient withdrew from the study before nasogastric feeding established ‐ remaining 5 patients completed the study EN (usual food intake) n = 3 Two of the 6 EN (usual food intake) patients received nasogastric feeding; one 4 months from diagnosis by which time the study was completed; one 2 weeks from diagnosis, but data kept within EN (usual food intake) group up to 2 weeks. One EN (usual food intake) patient died 2 months following diagnosis. Patients analysed: EN (nasogastric) n = 5 EN (usual food intake) n = 6 Intention‐to‐treat analysis was not undertaken as described in the Cochrane handbook, as an eligible patient dropped out and was not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes described in the trial as being measured were reported.on |
| Other bias | High risk | The reproducibility of data from the parental report sheets is unclear. |
Uderzo 2011.
| Methods | RCT Parallel design 4 Italian paediatric HSCT centres (Associazione Italiana di Ematologia ed Oncologia Pediatrica) | |
| Participants |
AGE S‐TPN ‐ median age 8.4 years (range 0.4‐18.6 years) GE‐TPN ‐ median age 8.0 years (range 0.9‐18.6 years SEX S‐TPN ‐ n=58 39 males (67.2%), 19 females GE‐TPN ‐ n=60 42 males (70%), 18 females DISEASE (S‐TPN, GE‐TPN respectively) Acute lymphoblastic leukaemia (31, 30) Acute myeloid leukaemia (13, 15) Chronic myeloid leukaemia (1, 8) B‐cell non‐Hodgkin's lymphoma (4, 2) Hodgins lymphoma (2, 1) Myelodysplatic syndrome (3, 3) Malignant lymphohistiocytosis (1, 0) Secondary acute myeloid leukaemia (0, 1) Rhabdomyosarcoma (1, 0) Juvenile chronic myeloid leukaemia (2, 0) CHEMOTHERAPY/RADIOTHERAPY Chemotherapy + TBI conditioning S‐TPN 34 (58.6) GE‐TPN 31 (51.7) |
|
| Interventions | S‐TPN ‐ standard TPN, modified as required GE‐TPN ‐ 0.4 g/kg/day L‐alanine glutamine dipeptide (equal to 0.25 g free glutamine) The first use was on the day of HSCT after the randomisation and continued until the end of TPN when the patients could orally cover more than 50% of their daily energy requirements for at least 3 days. |
|
| Outcomes |
Primary outcomes
Anthropometric parameters ‐ Weight Biochemical parameters ‐ Albumin and pre‐albumin Adverse events ‐ Number of infections requiring antibiotics and infections due to sepsis ‐ Mucositis Secondary outcomes ‐ Number of deaths ‐ Length of hospital stay Changes in nutritional status were measured before HSCT, 10 days after, and at the end of PN. Data concerning the immunological recovery was obtained at 1, 3, and 6 months after HSCT. |
|
| Notes | The children eligible for this double‐blind study had malignant hematological diseases and underwent allogeneic HSCT from June 2005 to June 2008 after high dose chemotherapy and total body irradiation. Type of transplant Related S‐TPN 18 (31), GE‐TPN 17 (28.3) Unrelated S‐TPN 35 (60.3), GE‐TPN 30 (50) Cord blood S‐TPN 4 (6.9), GE‐TPN 7 (11.7) Haploidentical S‐TPN 1 (1.7), GE‐TPN 6 (10.0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer generated table |
| Allocation concealment (selection bias) | Low risk | Comment: each centre investigator requested the random assignment from the central location 2 days before starting the supportive treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the patients and physician received the corresponding parenteral formula from the pharmacy without knowing the type. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the physicians and nurses who assessed outcomes received the corresponding parenteral formula from the pharmacy without knowing the type. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: varied with outcome. Pre‐albumin & albumin had poorer reporting. |
| Selective reporting (reporting bias) | Low risk | Comment: although many routine tests were not reported, they are not relevant to this review |
| Other bias | Low risk | Comment: none identified. |
Van Eys 1980.
| Methods | RCT Parallel design Single‐centre USA | |
| Participants |
AGE
Age range not reported ‐ patients were 21 years of age or younger SEX Not reported DISEASE STATUS Patients with metastatic disease to or from bone PN: Neuroblastoma (3) Osteosarcoma (4) Giant cell tumour (1) Wilms' tumour (1) Undifferentiated ectodermal neoplasm (1) EN (usual food intake): Neuroblastoma (2) Ewing's sarcoma (2) Osteosarcoma (3) Lymphoid malignancy (2) Unknown primary (1) Only well‐nourished patients randomised CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy |
|
| Interventions | 20 patients randomly assigned:
PN ‐ n = 10, EN (usual food intake) ‐ n = 10 PN ‐ consisted of a standard solution, which was adjusted for sodium or potassium content as needed. All PN was administered through a subclavian vein, except during periods when infections or other complications required temporary peripheral PN administration. Patients were allowed to continue on oral intake. EN (usual food intake) ‐ consisted of dietary advice and monitoring |
|
| Outcomes |
Primary outcomes
Adverse events
‐ Number of infections requiring antibiotics and infections due to sepsis Secondary outcomes ‐ Number of deaths |
|
| Notes | PN ‐ mean duration = 106.7 days, SD ± 70.93 days
EN ‐ mean duration = 145.1 days, SD ± 116.98 days During an episode of chemotherapy, PN was administered for a minimum of 10 days, starting 3 days before chemotherapy was administered. Each chemotherapy episode could have had nutritional sub‐episodes in which the patient crossed over from PN to control, or vice versa, as nutritional status mandated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (correspondence from author): "randomization was assigned by computer generated random numbers by the Department of Biostatistics at the University of Texas MD Anderson Cancer Center" |
| Allocation concealment (selection bias) | Low risk | Quote (correspondence from author): "When a new eligible patient was admitted, the assignment was obtained from the Statistician by telephone. The physicians had no advanced knowledge what the assignment was going to be." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Clinician/person delivering treatment: not discussed (not possible as PN obvious) Participants: not discussed (not possible as PN obvious) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not discussed. All outcomes objective, so lack of blinding unlikely to influence outcome measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Patients randomised: PN n = 10 EN n = 10 Withdrawals described: EN n = 3 One randomised EN patient was lost to follow up. Two EN patients deteriorated and were put onto PN. Patients analysed: PN n = 10 EN n = 9 From tables, all PN patients are included in analyses (n = 10) and 9 EN patients are included in analyses (n = 9). Intention‐to‐treat analysis was therefore not undertaken as drop‐outs we not imputed. |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes described in trial as being measured but results not reported: Biochemical values, but this is justified ‐ the study was not designed to, nor could it be expected to answer questions regarding the effect of PN on white cell count, lymphocyte count, drug toxicity, and biochemical parameters, and these are routine investigations in clinical care. |
| Other bias | Low risk | Comment: None noted |
Ward 2009.
| Methods | Quasi‐RCT Cross‐over design Single‐centre St James's University Hospital, Leeds, UK | |
| Participants | 76 patients 2 to 21 years undergoing treatment for paediatric malignancy and at risk of developing mucositis AGE Mean (± SD) = 8.76 ± 5.78 years, range 2 to 21 SEX 28 male (56%), 22 female (44%) DISEASE STATUS Acute myeloid leukaemia ‐ 10 (20%) B‐cell non‐Hodgkin's lymphoma ‐ 17 (34%) Ewing's sarcoma/primitive neuroectodermal tumour ‐ 9 (18%) Rhabdomyosarcoma ‐ 5 (10%) Osteosarcoma ‐ 3 (6%) Other ‐ 6 (12%) Nutritional status not reported CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy |
|
| Interventions | 50 patients randomly assigned ‐ used as their own controls: One course of chemotherapy with glutamine One course of chemotherapy without glutamine Glutamine ‐ dose was 0.65 g/kg‐1administered orally or via an enteral feeding tube. Oral dose was administered as a once‐daily dose starting on day 1 of chemotherapy and given every subsequent day for a total of 7 days. The oral dose was mixed with water at a maximum concentration of 10 g/100 ml water and a total volume of not more than 300 ml. This was flavoured with fruit cordial. Whether taken orally or via a feeding tube the dose was given within 30 minutes. |
|
| Outcomes |
Primary outcomes Change in nutritional indices ‐ Percentage weight/height ‐ Mid‐upper arm circumference Adverse events ‐ Mucositis ‐ Number of patients with nausea ‐ Number of patients with vomiting ‐ Number of patients with diarrhoea ‐ Abnormal biochemical profiles |
|
| Notes | The oral dose of glutamine was administered as a once‐daily dose starting on day 1 of chemotherapy and given every subsequent day for a total of 7 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: quasi RCT ‐ alternate patients |
| Allocation concealment (selection bias) | High risk | Comment: quasi RCT ‐ alternate patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: some outcomes measured by an unblinded investigator (Mid‐upper arm circumference, days of PN, days of TPN) or unblinded participant (nausea, vomiting) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 18 patients did not complete study, but were reported in safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported (some included in appendix) |
| Other bias | High risk | Comment: investigator involved in decision‐making about type and duration of enteral feed, investigator involved in decision‐making about TPN |
Zheng 2006.
| Methods | RCT Parallel design Single‐centre Children's Hospital, Shanghai Medical University, China | |
| Participants | 67 patients 1 to 12 years of age diagnosed with cancer in stages 1 to 3 AGE EN and FOS ‐ 7.5 ± 2.9 years EN alone ‐ 5.0 ± 3.1 years SEX EN and FOS ‐ 34% females EN alone ‐ 43% females DISEASE STATUS Neuroblastoma Wilms' tumour Malignant teratoma Hepatoblastoma Rhabdomyosarcoma Nutritional status not reported CHEMOTHERAPY/RADIOTHERAPY All patients received chemotherapy |
|
| Interventions | 66 patients randomly assigned: EN and FOS ‐ n = 32, EN alone ‐ n = 34 EN ‐ consisted of 400 mL (1674 kJ) of a commercial enteral feed (Nutren Junior, Nestle, Konolfingen, Switzerland). No restriction in terms of dietary intake. FOS ‐ 2 g/L of FOS, Nestle, containing 70:30 Raftilose/Raftiline from Orafti (Brussels, Belgium). All products were delivered orally or with a nasogastric or gastrostomy tube on gravity administration. Both products contained proteins, carbohydrates, and fats with vitamins and minerals in amounts intended for full nutritional support of paediatric patients. |
|
| Outcomes |
Primary outcomes Change in nutritional indices ‐ Change in weight‐for‐age (day 0 to 13; day 13 to 30; day 0 to 30) ‐ Change in height‐for‐age (day 0 to 13; day 13 to 30; day 0 to 30) ‐ Change in weight‐for‐height (day 0 to 13; day 13 to 30; day 0 to 30) ‐ Serum albumin ‐ Pre‐albumin Adverse events ‐ Stool culture positive for enterobacteria, bifidobacteria, lactobacillus, Clostridium (days 0, 3, 13, 30 stool samples analysed) ‐ Number of patients with nausea ‐ Number of patients with diarrhoea (stool frequency and consistency) Secondary outcomes "Feed tolerance" |
|
| Notes | Patients received at least 400 mL of an assigned formula for 13 to 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear how random assignment generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear how allocation assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: although states double blind, no further clarification |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: although states double blind, no further clarification |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data from 66/67 participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: blood pressure, heart rate, body temperature, respiratory rate recorded but not reported, but not related to outcomes in this review |
| Other bias | High risk | Comment: no copy of standardised questionnaire to record 24 hour dietary intake and feed tolerance measures included or cited in paper to assess quality/reproducibility |
BMT: bone marrow transplant CPN: central parenteral nutrition EN: enteral nutrition FOS: fructooligosaccharides FT: fluid replacement therapy GE‐TPN: glutamine enritched TPN HNR: high nutritional risk HSCT: hematopoietic stem cell transplant MAC: mid‐upper arm circumference NWTS: Mational Wilms' Tumor Study PN: parenteral nutrition PPN: peripheral parenteral nutrition RCT: randomised controlled trial RDA: recommended daily allowance S‐TPN: standard TPN TBI: total body irradiation TPN: total parenteral nutrition
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blijlevens 2005 | Study in adults |
| Cohen 2010 | Not a randomised or quasi‐randomised controlled study |
| Coquin 1981 | Study in adults |
| Dacou 1983 | Not a nutritional intervention |
| Fang 2007 | Study in adults |
| Filler 1976 | Not a randomised or quasi‐randomised controlled study |
| Forchielli 1997 | Not a randomised or quasi‐randomised controlled study |
| Gassas 2006 | Not a randomised or quasi‐randomised controlled study |
| Okur 2006 | Not a randomised or quasi‐randomised controlled study |
| Piccirillo 2003 | Study in adults |
| Rickard 1979 | Not a randomised or quasi‐randomised controlled study |
| Rickard 1980 | Not a randomised or quasi‐randomised controlled study |
| Scheid 2004 | Study in adults |
| Scheltinga 1991 | Study in adults |
| Shamberger 1983 | Not exclusively children ‐ data for children not reported separately |
| Shamberger 1984 | Duplicate publication of Shamberger 1983 |
| Sornsuvit 2008 | Not exclusively children ‐ data for children not reported separately |
| Sykorova 2005 | Study in adults, not chemotherapy |
| Van Eys 1982a | Not a randomised or quasi‐randomised controlled study |
| Wada 2010 | Inappropriate intervention (probiotic) |
| Ward 2003 | Not a randomised or quasi‐randomised controlled study |
| Yildirim 2013 | Not a randomised or quasi‐randomised controlled study |
Differences between protocol and review
Differences between the original protocol and original review
In the original protocol we stated that we would consider studies where participants were children aged 18 years or less. Once the search for studies had been conducted, a post hoc change to the review was made. We changed the inclusion criteria to participants up to the age of 21 years. This change was made because several paediatric oncology units in the UK and elsewhere now accept patients of 18 years and older. There has also been a recent trend to extend the upper age limit for inclusion in paediatric oncology trials to 21 years.
In the original protocol we specified that we would consider studies that included the following outcomes:
Primary outcomes:
Nutritional status as measured by weight change, biochemical indices.
Diarrhoea, nausea, vomiting or any other outcomes examined which may be associated with the feeding or chemotherapy regimen.
Infection rate (catheter‐related sepsis or any other infection).
Secondary outcomes:
Death.
Remission of cancer (tumour response, other measures of disease progression).
Measures of quality of life.
Length of hospital stay/frequency of admissions.
Cost.
Once data had been extracted on outcomes and the review completed it was decided, based on peer review comments, that the original outcome measures were too broad and these were defined more precisely as outlined in the Methods section under 'Types of outcome measures'.
Differences between the 2014 update review and the original review
A largely new team took over the update of this review.
The risk of bias assessment has been undertaken according to the most recent guidelines from the Childhood Cancer Group (CCG 2012).
Explicit exclusion of vitamins and micronutrient supplementation has been introduced into this review. This change is driven by the different often pharmacological nature of these supplements, and the different outcomes which they are intended to promote (for example, bone health in Vitamin D supplements).
Inclusion of mucositis and abnormal biochemical profiles as outcomes. This was motivated by the inclusion of glutamine supplementation which was hypothesised to reduce mucositis and thereby improve nutritional absorption, but concerns were raised of hyperammonaemia as a side effect. Different lipid formulations for PN were hypothesised to cause dyslipidaemia, and this was included as an outcome.
The defunct NRR and Dissertation and Thesis databases were not searched for the update, as we did not have access to the Dissertations register and the NRR is no longer functional (the final issue of the NRR was published in October 2007).
Contributions of authors
The original review was conceived by the Department of Nutrition and Dietetics, Alder Hey Children's NHS Foundation Trust and the Evidence‐Based Child Health Unit, University of Liverpool and designed by Leanne Jones and Ruth Watling. Searches for relevant studies were conducted by Leanne Jones. Leanne Jones, Ruth Watling and Simone Wilkins screened, appraised and abstracted data for the review. Additional information from authors was sought by Leanne Jones where necessary. Data entry was performed by Leanne Jones. Barry Pizer commented on drafts of the protocol and review.
The update review questions were developed by all the update review authors. Simon Wilkins, Amanda Friend, Bob Phillips, Evelyn Ward and Lisa Henry undertook screening; Evelyn Ward and Lisa Henry undertook the initial data extraction and appraisal, with support from Bob Phillips. Bob Phillips and Amanda Friend undertook data entry and analysis. All authors contributed to the drafting of the review.
Sources of support
Internal sources
Evidence‐Based Child Health Unit, Institute of Child Health, University of Liverpool and Alder Hey Children's NHS Foundation Trust, UK.
External sources
-
Candlelighters, the Yorkshire Childrens' Cancer Charity, UK.
Funding for interlibrary loans and specialist dietician input.
Declarations of interest
One of the authors of this review (Evelyn Ward) was also the principal investigator of an included study (Ward 2009). The data was extracted and checked independently by the other review authors.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aquino 2005 {published data only}
- Aquino VM, Harvey AR, Garvin JH, Godder KT, Nieder ML, Adams RH, et al. A double‐blind randomized placebo‐controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplantation 2005;36(7):611‐6. [DOI] [PubMed] [Google Scholar]
den Broeder 2000 {published data only}
- Broeder E, Lippens RJ, 't Hof MA, Tolboom JJ, Sengers RC, Berg AM, et al. Nasogastric tube feeding in children with cancer: the effect of two different formulas on weight, body composition, and serum protein concentrations. Journal of Parenteral and Enteral Nutrition 2000;24:351‐60. [DOI] [PubMed] [Google Scholar]
Donaldson 1982 {published data only}
- Donaldson SS, Wesley MN, Ghavimi F, Shils ME, Suskind RM, DeWys WD. A prospective randomized clinical trial of total parenteral nutrition in children with cancer. Medical & Pediatric Oncology 1982;10(2):129‐399. [DOI] [PubMed] [Google Scholar]
- Eys J, Wesley MN, Cangir A, Copeland EM, Donaldson SS, Ghavimi F. Safety of intravenous hyperalimentation in children with malignancies: a cooperative group trial. Journal of Parenteral and Enteral Nutrition 1982;6(4):291‐4. [DOI] [PubMed] [Google Scholar]
Ghavimi 1982 {published data only}
- Ghavimi F, Shils ME, Scott BF. Comparison of morbidity in children requiring abdominal radiation and chemotherapy, with and without total parenteral nutrition. Journal of Pediatrics 1982;101(4):530‐7. [DOI] [PubMed] [Google Scholar]
- Shils ME, Ghavimi F, Scott BF, Brown M. Total parenteral nutrition (TPN) as an adjunct to cancer therapy in children [abstract]. Proceedings of American Society of Clinical Oncology 1977;19:346. [Google Scholar]
Hartman 2009 {published data only}
- Hartman C, Ben AE, Berkowitz D, Elhasid R, Lajterer N, Postovski S, et al. Olive oil‐based intravenous lipid emulsion in pediatric patients undergoing bone marrow transplantation: a short‐term prospective controlled trial. Clinical Nutrition 2009;28:631‐5. [DOI] [PubMed] [Google Scholar]
Hays 1983 {published data only}
- Hays DM, Merritt RJ, Ashley J. Effect of total parenteral nutrition on marrow recovery during therapy for acute non‐lymphocytic leukemia in childhood [abstract]. Journal of Parenteral and Enteral Nutrition 1982;6:584. [Google Scholar]
- Hays DM, Merritt RJ, White L, Ashley J, Siegel SE. Effect of total parenteral nutrition on marrow recovery during induction therapy for acute nonlymphocytic leukemia in childhood. Medical & Pediatric Oncology 1983;11(2):134‐400. [DOI] [PubMed] [Google Scholar]
Rickard 1985 {published data only}
- Corcoran M, Rickard K, Loghmani E, Grosfeld J, Godshall B, Coates T, et al. Effectiveness of two methods of parenteral nutrition in improving muscle mass of children treated for neuroblastoma of Wilms' tumor: a randomized study. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists 1987;1030:262. [Google Scholar]
- Rickard KA, Becker MC, Loghmani E, Grosfeld JL, Godshall BJ, Weetman RM, et al. Effectiveness of two methods of parenteral nutrition support in improving muscle mass of children with neuroblastoma or Wilms' tumor. A randomized study. Cancer 1989;64(1):116‐255. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Detamore CM, Coates TD, Grosfeld JL, Weetman RM, White NM, et al. Effect of nutrition staging on treatment delays and outcome in Stage IV neuroblastoma. Cancer 1983;52(4):587‐98. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Foland BB, Detamore CM, Coates TD, Grosfeld JL, White NM, et al. Effectiveness of central parenteral nutrition versus peripheral parenteral nutrition plus enteral nutrition in reversing protein‐energy malnutrition in children with advanced neuroblastoma and Wilms' tumor: a prospective randomized study. American Journal of Clinical Nutrition 1983;38(3):445‐566. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Loghmani ES, Grosfeld JL, Detamore CM, White NM, Foland BB, et al. Effectiveness of enteral and parenteral nutrition in preventing and/or reversing PEM in children with advance neuroblastoma: a prospective randomised study. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists. 1984.
- Rickard KA, Loghmani ES, Grosfeld JL, Lingard CD, White NM, Foland BB, et al. Short‐ and long‐term effectiveness of enteral and parenteral nutrition in reversing or preventing protein‐energy malnutrition in advanced neuroblastoma. A prospective randomized study. Cancer 1985;56(12):2881‐977. [DOI] [PubMed] [Google Scholar]
Rickard 1989 {published data only}
- Corcoran M, Rickard K, Loghmani E, Grosfeld J, Godshall B, Coates T, et al. Effectiveness of two methods of parenteral nutrition in improving muscle mass of children treated for neuroblastoma of Wilms' tumor: a randomized study. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists 1987;1030:262. [Google Scholar]
- Godshall BJ, Rickard KA, Loghmani ES, Coates TD, Grosfeld JL, Weetman RM, et al. Integration of nutritional support into oncologic treatment protocols for high and low nutritional risk children with Wilm's tumor: a prospective randomized study. Proceedings of the Annual Meeting of the American Society of Clinical Oncologists. 1987.
- Rickard KA, Becker MC, Loghmani E, Grosfeld JL, Godshall BJ, Weetman RM, et al. Effectiveness of two methods of parenteral nutrition support in improving muscle mass of children with neuroblastoma or Wilms' tumor. A randomized study. Cancer 1989;64(1):116‐255. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Foland BB, Detamore CM, Coates TD, Grosfeld JL, White NM, et al. Effectiveness of central parenteral nutrition versus peripheral parenteral nutrition plus enteral nutrition in reversing protein‐energy malnutrition in children with advanced neuroblastoma and Wilms' tumor: a prospective randomized study. American Journal of Clinical Nutrition 1983;38(3):445‐566. [DOI] [PubMed] [Google Scholar]
- Rickard KA, Godshall BJ, Loghmani ES, Coates TD, Grosfeld JL, Weetman RM, et al. Integration of nutrition support into oncologic treatment protocols for high and low nutritional risk children with Wilms' tumor. A prospective randomized study. Cancer 1989;64(2):491‐509. [DOI] [PubMed] [Google Scholar]
Schmid 2006 {published data only}
- Schmid I, Schmitt M, Streiter M, Meilbeck R, Albert MH, Reinhardt D, et al. Parenteral nutrition is not superior to replacement fluid therapy for the supportive treatment of chemotherapy induced oral mucositis in children. European Journal of Cancer 2006;42(2):205‐11. [DOI] [PubMed] [Google Scholar]
Smith 1992 {published data only}
- Smith DE, Handy DJ, Holden CE, Stevens MCG, Booth IW. An investigation of supplementary naso‐gastric feeding in malnourished children undergoing treatment for malignancy: results of a pilot study. Journal of Human Nutrition & Dietetics 1992;5(2):85‐91. [Google Scholar]
Uderzo 2011 {published data only}
- Uderzo C, Rebora P, Marrocco E, Varotto S, Cichello F, Bonetti M, et al. Glutamine‐enriched nutrition does not reduce mucosal morbidity or complications after stem‐cell transplantation for childhood malignancies: a prospective randomized study. Transplantation 2011;91(12):1321‐5. [DOI] [PubMed] [Google Scholar]
Van Eys 1980 {published data only}
- Eys J, Copeland EM, Cangir A. A clinical trial of hyperalimentation in children with metastatic malignancies. Medical & Pediatric Oncology 1980;8(1):63‐73. [DOI] [PubMed] [Google Scholar]
- Eys J, Wesley MN, Cangir A, Copeland EM, Donaldson SS, Ghavimi F. Safety of intravenous hyperalimentation in children with malignancies: a cooperative group trial. Journal of Parenteral and Enteral Nutrition 1982;6(4):291‐4. [DOI] [PubMed] [Google Scholar]
Ward 2009 {published data only}
- Ward E, Smith M, Henderson M, Reid U, Lewis I, Kinsey S, et al. The effect of high‐dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. European Journal of Clinical Nutrition 2009;63:134‐40. [DOI] [PubMed] [Google Scholar]
Zheng 2006 {published data only}
- Zheng S, Steenhout P, Kuiran D, Qihong W, Weiping W, Hager C, et al. Nutritional support of pediatric patients with cancer consuming an enteral formula with fructooligosaccharides. Nutrition Research 2006;26:154‐62. [Google Scholar]
References to studies excluded from this review
Blijlevens 2005 {published data only}
- Blijlevens NM, Donnelly JP, Naber AH, Schattenberg AV, DePauw BE. A randomised, double‐blinded, placebo‐controlled, pilot study of parenteral glutamine for allogeneic stem cell transplant patients. Supportive Care in Cancer 2005;13(10):790‐6. [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen J, Maurice L. Adequacy of nutritional support in pediatric blood and marrow transplantation. Journal of Pediatric Oncology Nursing 2010;27(1):40‐7. [DOI] [PubMed] [Google Scholar]
Coquin 1981 {published data only}
- Coquin JY, Maraninchi JA, Gastaut JA, Carcassonne Y. Influence of parenteral nutrition (PN) on chemotherapy and survival of acute leukaemias (AL): preliminary results of a randomized trial. Journal of Parenteral and Enteral Nutrition. 1981; Vol. 5.
Dacou 1983 {published data only}
- Dacou VC, Palis J, Haidas S. Abnormal glucose tolerance in children with acute leukemia. Effect of induction chemotherapy including L‐asparaginase. American Journal of Pediatric Hematology‐Oncology 1983;5(2):139‐46. [PubMed] [Google Scholar]
Fang 2007 {published data only}
- Fang BJ, Song YP, Zhang L, Wei XD, Lin QD. Total parenteral alimentation with glutamine supplementation for the prevention of common complications in patients undergoing hematopoietic stem cell transplantation. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11:8483‐6. [Google Scholar]
Filler 1976 {published data only}
- Filler R, Jaffe N. Support with total parenteral nutrition (TPN) in childhood malignancy. Proceedings of the American Association of Cancer Research 1976;17:140. [Google Scholar]
Forchielli 1997 {published data only}
- Forchielli ML, Lo CW, Richardson D, Gura K, Walker WA, Tonelli E. Central venous line related bacteremia during total parenteral nutrition and/or chemotherapy infusions in children. Annali di Igiene 1997;9(1):35‐40. [PubMed] [Google Scholar]
Gassas 2006 {published data only}
- Gassas A, Kennedy J, Green G, Connolly B, Cohen J, Dag E, et al. Risk of ventriculoperitoneal shunt infections due to gastrostomy feeding tube insertion in pediatric patients with brain tumors. Pediatric Neurosurgery 2006;42(2):95‐9. [DOI] [PubMed] [Google Scholar]
Okur 2006 {published data only}
- Okur A, Ezgü FS, Tümer L, Cinasal G, Oguz A, Hasanoglu A, et al. Effects of oral glutamine supplementation on children with solid tumors receiving chemotherapy. Pediatric Hematology and Oncology 2006;23:277‐85. [DOI] [PubMed] [Google Scholar]
Piccirillo 2003 {published data only}
- Piccirillo N, De M, Laurenti L, Chiusolo P, Sora F, Pittiruti M, et al. Glutamine‐enriched parenteral nutrition after autologous peripheral blood stem cell transplantation: effects on immune reconstitution and mucositis. Haematologica 2003;88(2):192‐200. [PubMed] [Google Scholar]
Rickard 1979 {published data only}
- Rickard KA, Grosfeld JL, Kirksey A, Ballantine TV, Baehner RL. Reversal of protein‐energy malnutrition in children during treatment of advanced neoplastic disease. Annals of Surgery 1979;190(6):771‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rickard 1980 {published data only}
- Rickard KA, Kirksey A, Baehner RL, Grosfeld JL, Provisor A, Weetman RM, et al. Effectiveness of enteral and parenteral nutrition in the nutritional management of children with Wilms' tumors. American Journal of Clinical Nutrition 1980;33(12):2622‐9. [DOI] [PubMed] [Google Scholar]
Scheid 2004 {published data only}
- Scheid C, Hermann K, Kremer G, Holsing A, Heck G, Fuchs M, et al. Randomized, double‐blind, controlled study of glycyl‐glutamine‐dipeptide in the parenteral nutrition of patients with acute leukemia undergoing intensive chemotherapy. Nutrition 2004;20(3):249‐54. [DOI] [PubMed] [Google Scholar]
Scheltinga 1991 {published data only}
- Scheltinga MR, Young LS, Benfell K, Bye RL, Ziegler TR, Santos AA, et al. Glutamine‐enriched intravenous feedings attenuate extracellular fluid expansion after a standard stress. Annals of Surgery 1991;214(4):385‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shamberger 1983 {published data only}
- Shamberger RC, Pizzo PA, Goodgame JT Jr, Lowry SF, Maher MM, Wesley RA, et al. The effect of total parenteral nutrition on chemotherapy‐induced myelosuppression. A randomized study. American Journal of Medicine 1983;74(1):40‐8. [DOI] [PubMed] [Google Scholar]
Shamberger 1984 {published data only}
- Shamberger RC, Brennan MF, Goodgame JT Jr, Lowry SF, Maher MM, Wesley RA, et al. A prospective, randomized study of adjuvant parenteral nutrition in the treatment of sarcomas: results of metabolic and survival studies. Surgery 1984;96(1):1‐13. [PubMed] [Google Scholar]
Sornsuvit 2008 {published data only}
- Sornsuvit C, Komindr S, Chuncharunee S, Wanikiat P, Archararit N, Santanirand P. Pilot Study: effects of parenteral glutamine dipeptide supplementation on neutrophil functions and prevention of chemotherapy‐induced side‐effects in acute myeloid leukaemia patients. Journal of International Medical Research 2008;36(6):1383‐91. [DOI] [PubMed] [Google Scholar]
Sykorova 2005 {published data only}
- Sykorova A, Horacek J, Zak P, Kmonicek M, Bukac J, Maly J. A randomized, double blind comparative study of prophylactic parenteral nutritional support with or without glutamine in autologous stem cell transplantation for hematological malignancies ‐ three years' follow‐up. Neoplasma 2005;52(6):476‐82. [PubMed] [Google Scholar]
Van Eys 1982a {published data only}
- Eys J, Cangir A, Carter P, Coody D. Effect of nutritional supportive therapy on children with advanced cancer. Cancer Research 1982;Suppl 2:713s‐14s. [PubMed] [Google Scholar]
Wada 2010 {published data only}
- Wada M, Nagata S, Saito M, Shimizu T, Yamashiro Y, Matsuki T, et al. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Supportive Care in Cancer 2010;18(6):751‐9. [DOI] [PubMed] [Google Scholar]
Ward 2003 {published data only}
- Ward E, Picton S, Reid U, Thomas D, Gardener C, Smith M, et al. Oral glutamine in paediatric oncology patients: a dose finding study. European Journal of Clinical Nutrition 2003;57(1):31‐6. [DOI] [PubMed] [Google Scholar]
Yildirim 2013 {published data only}
- Yildirim ZK, Bidev D, Buyukavci M. Parenteral glutamine supplementation has no effect on chemotherapy‐induced toxicity in children with non‐Hodgkin lymphoma. Journal of Pediatric Hematology/Oncology 2013;35:371‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Omran 2003
- Al‐Omran M, Groof A, Wilke D. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002837] [DOI] [PubMed] [Google Scholar]
Andersen 2006
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004080.pub2] [DOI] [PubMed] [Google Scholar]
Andressy 1998
- Andressy RJ, Chwals WJ. Nutritional support of the paediatric oncology patient. Nutrition 1998;14:124‐9. [DOI] [PubMed] [Google Scholar]
Antoun 2006
- Antoun S, Merad M, Raynard B, Ruffie P. Malnutrition in cancer patients. La Revue du Praticien 2006;56(18):2025‐9. [PubMed] [Google Scholar]
Argiles 2005
- Argiles JM. Cancer‐associated malnutrition. European Journal of Oncology Nursing 2005;9(Suppl 2):S39‐50. [DOI] [PubMed] [Google Scholar]
Balis 1997
- Balis FM. The challenge of developing new therapies for childhood cancers. Oncologist 1997;2(1):I‐II. [PubMed] [Google Scholar]
Barr 2002
- Barr RD. Nutrition and Cancer. Nutrition 2002;18(5):1302‐3. [DOI] [PubMed] [Google Scholar]
Beghetto 2005
- Beghetto MG, Victorino J, Teixeira L, Azevedo MJ. Parenteral nutrition as a risk factor for central venous catheter‐related infection. Journal of Parenteral and Enteral Nutrition 2005;29(5):367‐3. [DOI] [PubMed] [Google Scholar]
Boklan 2006
- Boklan J. Little patients, losing patience: pediatric cancer drug development. Molecular Cancer Therapeutics 2006;5(8):1905‐8. [DOI] [PubMed] [Google Scholar]
Cairns 2000
- Cairns PA, Stalker DJ. Carnitine supplementation of parenterally fed neonates. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000950] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charland 1994
- Charland SL, Bartlett D, Torosian MH. Effect of protein‐calorie malnutrition on methotrexate pharmacokinetics. Journal of Parenteral and Enteral Nutrition 1994;18(1):45‐9. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2003
- Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003743] [DOI] [PubMed] [Google Scholar]
Deswarte‐Wallace 2001
- Deswarte‐Wallace J, Firouzbakhsh S, Finklestein JZ. Using research to change practice: enteral feeding for pediatric oncology patients. Journal of Pediatric Oncology Nursing 2001;18(5):81‐5. [DOI] [PubMed] [Google Scholar]
Dewey 2007
- Dewey A, Baughan C, Dean TP, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega‐3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004597.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 1981
- Donaldson SS, Wesley MN, DeWys WD, Suskind RM, Jaffe N, vanEys J. A study of nutritional status in pediatric cancer patients. American Journal of Disease in Children 1981;135(12):1107‐12. [DOI] [PubMed] [Google Scholar]
Ferreira 2001
Ferreira 2005
- Ferreira I, Brooks D, Lacasse Y, Goldstein R, White J. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000998.pub2] [DOI] [PubMed] [Google Scholar]
Han‐Markey 2000
- Han‐Markey T. Nutritional considerations in pediatric oncology. Seminars in Oncology Nursing 2000;16(2):146‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huang 2002
- Huang RC, Forbes D, Davies MW. Feed thickener for newborn infants with gastro‐oesophageal reflux. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003211] [DOI] [PubMed] [Google Scholar]
Joffe 2009
- Joffe A, Anton N, Lequier L, Vandermeer B, Tjosvold L, Larsen B, et al. Nutritional support for critically ill children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005144.pub2] [DOI] [PubMed] [Google Scholar]
Klein 1994
- Klein S, Koretz RL. Nutrition support in patients with cancer: what do the data really show?. Nutrition in Clinical Practice 1994;9:91‐100. [DOI] [PubMed] [Google Scholar]
Koletzko 2005
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). Journal of Pediatric Gastroenterology and Nutrition 2005;41(Supp 2):S1‐87. [DOI] [PubMed] [Google Scholar]
Ladas 2005
- Ladas EJ, Sacks N, Meacham L, Henry D, Enriquez L, Lowry G, et al. A multidisciplinary review of nutrition considerations in the pediatric oncology population: a perspective from children's oncology group. Nutrition in Clinical Practice 2005;20(4):377‐93. [DOI] [PubMed] [Google Scholar]
Lai 2006
- Lai NM, Rajadurai SV, Tan K. Increased energy intake for preterm infants with (or developing) bronchopulmonary dysplasia/chronic lung disease. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005093.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lange 2005
- Lange BJ, Gerbing RD, Feusner J, Skolnik J Sacks N, Smith FO, et al . Mortality in overweight and underweight children with acute myeloid leukaemia.. Journal of the American Medical Association 2005;293(2):303‐11. [DOI] [PubMed] [Google Scholar]
Lansky 1987
- Lansky SB, List MA, Lansky LL, Ritter‐Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer 1987;60(7):1651‐6. [DOI] [PubMed] [Google Scholar]
Mahlungulu 2007
- Mahlungulu SN, Grobler L, Visser ME, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004536.pub2] [DOI] [PubMed] [Google Scholar]
Marsoni 1985
- Marsoni S, Ungerleider RS, Hurson SB, Simon RM, Hammershaimb LD. Tolerance to antineoplastic agents in children and adults. Cancer Treatment Reports 1985;69(11):1263‐9. [PubMed] [Google Scholar]
Mauer 1990
- Mauer AM, Burgess JB, Donaldson SS. Special nutritional needs of children with malignancies. Journal of Parenteral and Enteral Nutrition 1990;14(3):315‐23. [DOI] [PubMed] [Google Scholar]
McGeer 1990
- McGeer AJ, Detsky AS, O'Rourke K. Parenteral nutrition in cancer patients undergoing chemotherapy: a meta‐analysis. Nutrition 1990;6(3):233‐40. [PubMed] [Google Scholar]
Murray 2009
- Murray SM, Pindoria S. Nutrition support for bone marrow transplant patients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002920.pub3] [DOI] [PubMed] [Google Scholar]
NICE 2006
- CG32 Nutrition support in adults. NICE guideline 20 February 2006.
Poustie 1999
- Poustie VJ, Smyth RL, Watling RM. Oral protein calorie supplementation for children with chronic disease. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001914] [DOI] [PubMed] [Google Scholar]
Sala 2004
- Sala A, Pencharz P, Barr RD. Children, cancer and nutrition – a dynamic triangle in review. Cancer 2004;109(4):677‐87. [DOI] [PubMed] [Google Scholar]
Simmer 2005
- Simmer K, Rao SC. Early introduction of lipids to parenterally‐fed preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005256] [DOI] [PubMed] [Google Scholar]
Sinha 2008
- Sinha I, Jones L, Smyth RL, Williamson PR. A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLoS Medicine 2008; Vol. 5, issue 4:e96. [DOI] [PMC free article] [PubMed]
Soghier 2006
- Soghier LM, Brion LP. Cysteine, cystine or N‐acetylcysteine supplementation in parenterally fed neonates. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004869.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taj 1993
- Taj MM, Pearson AD, Mumford DB, Price L. Effect of nutritional status on incidence of infection in childhood cancer. Pediatric Hematology/Oncology 1993;10(3):283‐7. [DOI] [PubMed] [Google Scholar]
Tambori 2005
- Tambori U, Sung L, Hukin J, Laperriere N, Crooks B, Carret A‐S, et al . Medulloblastoma in the second decade of life: a specific group with respect to toxicity and management. Cancer 2005;103(9):1874‐80. [DOI] [PubMed] [Google Scholar]
Task Force on Cancer 2002
- Task Force on Cancer Prevention. Task Force on Cancer Prevention, Early Detection and Treatment in New Jersey. New Jersey Comprehensive Cancer Control Plan. Report to the Governor, July 2002. Trenton, NJ, New Jersey Department of Health and Senior Services 2002:59.
Van Cutsem 2005
- Cutsem E, Arends J. The causes and consequences of cancer‐associated malnutrition. European Journal of Oncology Nursing 2005;9(Suppl 2):S51‐63. [DOI] [PubMed] [Google Scholar]
von Meyenfeldt 2005
- Meyenfeldt M. Cancer‐associated malnutrition: an introduction. European Journal of Oncology Nursing 2005;9(Suppl 2):S35‐8. [DOI] [PubMed] [Google Scholar]
Wasiak 2006
- Wasiak J, Cleland H, Jeffery R. Early versus delayed enteral nutrition support for burn injuries. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005489.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasiak 2007
Weir 1998
- Weir J, Reilly JJ, McColl JH, Gibson BE. No evidence for an effect of nutritional status at diagnosis on prognosis in children with acute lymphoblastic leukaemia. Pediatric Hematology/Oncology 1998;20(6):534‐38. [DOI] [PubMed] [Google Scholar]
Yaris 2002
- Yaris N, Akyuz C, Coskun T, Kutluk T, Buyukpamukcu M. Nutritional status of children with cancer and its effect on survival. Turkish Journal of Pediatrics 2002;44(1):35‐9. [PubMed] [Google Scholar]
Yilmaz 2007
- Yilmaz G, Koksal I, Aydin K, Caylan R, Sucu N, Aksoy F. Risk factors of catheter‐related bloodstream infections in parenteral nutrition catheterization. Journal of Parenteral and Enteral Nutrition 2007;31(4):284‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2010
- Jones L, Watling RM, Wilkins S, Pizer B. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD003298.pub2] [DOI] [PubMed] [Google Scholar]


