Abstract
Background:
Whether perioperative glycemic control is associated with neurocognitive decline (NCD) after cardiac surgery was examined.
Methods:
Thirty patients undergoing cardiac surgery utilizing cardiopulmonary bypass (CPB) were screened for NCD preoperatively and on post-operative day four (POD4). Indices of glucose control were examined. Serum cytokine levels were measured and human transcriptome analysis was performed on blood samples. Neurocognitive data are presented as a change from baseline to POD4 in a score standardized with respect to age and gender.
Results:
A decline in neurocognitive function was identified in 73% (22/30) of patients on POD4. There was no difference in neurocognitive function between patients with elevated HbA1c levels preoperatively (p=0.973) or elevated fasting blood glucose levels the morning of surgery (>126mg/dL, p=0.910), or a higher maximum blood glucose levels during CPB (>180mg/dL, p=0.252), or higher average glucose levels during CPB (>160mg/dL, p=0.639). Patients with postoperative leukocytosis (WBC ≥ 10.5) had more NCD when compared to their baseline function (p=0.03). Patients with elevated IL-8 levels at 6 hours postoperatively had a significant decline in NCD at POD4 (p=0.04). Human transcriptome analysis demonstrated unique and differential patterns of gene expression in patients depending on the presence of DM and NCD.
Conclusions:
Perioperative glycemic control does not have an effect on NCD soon after cardiac surgery.The profile of gene expression was altered in patients with NCD with or without diabetes.
Introduction
Although there have been many advances in technology to help with intraoperative neurocognitive protection, the adverse effects from cardiopulmonary bypass (CPB) during cardiac surgery remain a major threat to patient outcomes. Advances in risk stratification, CPB, anesthesia methods, along with pre and postoperative care have changed cardiac surgical outcomes significantly over the past few decades, however optimizing protection of the central nervous system remains an area of ongoing effort. Furthermore, short-term neurocognitive deficits appear to be predictive of both long-term outcomes and quality of life.1
Given that the majority of cardiac surgery is still performed using CPB, it is important to better understand which patients will experience neurocognitive decline, and to what degree, in order to assist in both preoperative and perioperative counseling along with treatment decisions. Previous studies from our group suggest that neurocognitive outcomes in certain patients are related to transcriptional upregulation of specific genes that may play a role in inflammation and immunomodulation.2 Furthermore, activation of the inflammatory response may lead to penetration of the blood-brain barrier by cytokines either directly or via vagal nerve stimulation.3 Additionally, cognitive impairment has been shown to be a result of cytokine-mediated interactions between neurons and glial cells within the CNS.3 It remains unclear whether these inflammatory interactions occur in genetically predisposed patients or if there are risk factors that contribute to this inflammatory response, i.e., hyperglycemia during and after cardiac surgery. Since diabetes is an inflammatory disorder, it would follow that perioperative hyperglycemia or presence of diabetes would be a risk factor for NCD after cardiac surgery. The aim of the present study is to elucidate if perioperative glucose control or presence of diabetes is a risk factors of early NCD in patients undergoing cardiac surgery with CPB.
Methods
Patient Enrollment
We enrolled thirty patients on a rolling basis with a planned elective or urgent valvular procedure (aortic or mitral), coronary artery bypass grafting, or both; requiring CPB. We included minimally invasive aortic valvular replacements (n=1). Our exclusion criteria included: planned aortic arch or root procedures, patients with a recent stroke (within the last year), heavily calcified aortas, high-grade carotid stenosis, chronic renal failure (Cr>2.0mg/dL), hepatic cirrhosis, severe neurological deficits, severe or total vision impairment, or an inability to complete baseline neurocognitive testing. Patients were not randomized as they were compared to their own pre-operative neurocognitive assessment. Furthermore, we only included patients who were native English speakers.
Patients were divided into two groups based on pre-existing diabetes either via documentation in the chart or a pre-operative HbA1c >7.0% or not having diabetes. Both male and female patients were included (female=9; 30%). The post-operative timepoint for the RBANS analysis was chosen as POD 4 as it was the anticipated discharge date for patients without complications. All patients provided informed consent and all procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University.
Surgical Technique
Standard cardiac surgical technique was utilized including induction of general anesthesia, invasive monitoring, midline sternotomy or anterior thoracotomy for minimally invasive cases, and systemic heparinization. Cerebral oxygenation was measured in all patients per usual protocol. CPB was initiated via cannulation of the right atrium and ascending aorta with a non-pulsatile system, membrane oxygenator, and an arterial filter. All patients underwent hypothermic CPB with intermittent cold blood cardioplegia. Intraoperative serum glucose levels were monitored and maintained at a goal of <200mg/dL by intravenous insulin administration. All patients had intra-operative transesophageal echocardiography and ejection fraction was determined at the beginning of the case by a cardiac anesthetist. Post-operatively, if any patients were unable to be extubated within a reasonable time and complete the fast-tracking to discharge and able to perform the neurocognitive assessment on POD4, they were excluded from analysis.
Neurocognitive Assessment
Within one week prior to their operation and on postoperative day four, all patients were administered a Repeatable Battery Assessment of Neuropsychological Status (RBANS) neuropsychological assessment, which has been shown to detect even mild impairment in cognitive function.4 This assessment comes in two forms, with the questions administered before the operation being different than those administered postoperatively but testing the same areas of cognitive function. These include immediate and delayed memory, language, attention, global cognition, and visuospatial function. Each of these cognitive domains were scored to produce both a raw and scaled score that were collectively added for a total score. The mean raw score is 100 with a standard deviation of 15.6 and is the summation of each cognitive domain. The scaled score is produced in reference to a normative sample of healthy individuals and considers age and gender to produce a score with a mean of 10 and a standard deviation of 3. The forms were administered by individuals who were native English speakers and had been trained by a neuropsychologist. The pre and postoperative tests were administered by the same individual for each patient.
Genetic Analysis
Blood samples were drawn directly into PAXgene tubes (Qiagen Inc, Valencia, Calif) and immediately frozen and stored at −80 degrees Celsius for mRNA stabilization and extraction, per the manufacturer’s recommendation.
Human transcriptome profiling assay were done with Applied Biosystems™ Clariom™ D Pico Assay, human (ThermoFisher Scientific) according to the manufacturer’s protocol in Brown University Genomics Core Facility for use on the GeneChip™ 3000 instrument system. Transcriptome Analysis Console (TAC) software was used to analyze and visualize global expression patterns of genes, exons, pathways, and alternative splicing events.
Cytokine Analysis
Blood samples were collected at three time points: immediately preoperatively, six hours postoperatively, and on postoperative day four. Cytokine concentrations were measured from patients’ serum (preoperatively, 6 hours postoperatively, and POD4) using a Luminex bead-based multiplex assay (Luminex Corporation, Austin, TX).
Statistics
The RBANS scores were compared pre and postoperatively and analyzed using a univariate analysis against characteristics including laboratory data and interventions intra-operatively and during their hospital stay. For laboratory data such as white blood cell counts and creatinine, measurements were compared on the morning of postoperative day four or the closest lab measurement within 24 hours of this timepoint. These RBANS scores were also compared to the cytokines analyzed at the three time points: pre-op, six hours post-op, and POD4, as well as the genetics pre- and post-operatively. Patient randomization was not performed because the patients were compared to themselves pre- and post-operatively.
Neurocognitive data are presented as a change from baseline to POD4 in standardized score for age and gender on RBANS. Data were analyzed by an unpaired Mann-Whitney U test.
Results
Neurocognitive function was observed in 22/30 patients (73%) at postoperative day four when defined by a decrease from baseline RBANS score. Patients with acute kidney injury (Cr ≥ 1.2) on POD4 trended towards impaired neurocognitive function (4/30 patients, 13%, p=0.06). Postoperative leukocytosis (WBC ≥ 10.5×103mm) on POD4 was associated with NCD when compared to their baseline function (p=0.03) [Figure 1B]. Further demographic information including age, race, education and comorbidities can be found in a previous publication, along with time on bypass and hospital length of stay.5
Figure 1:
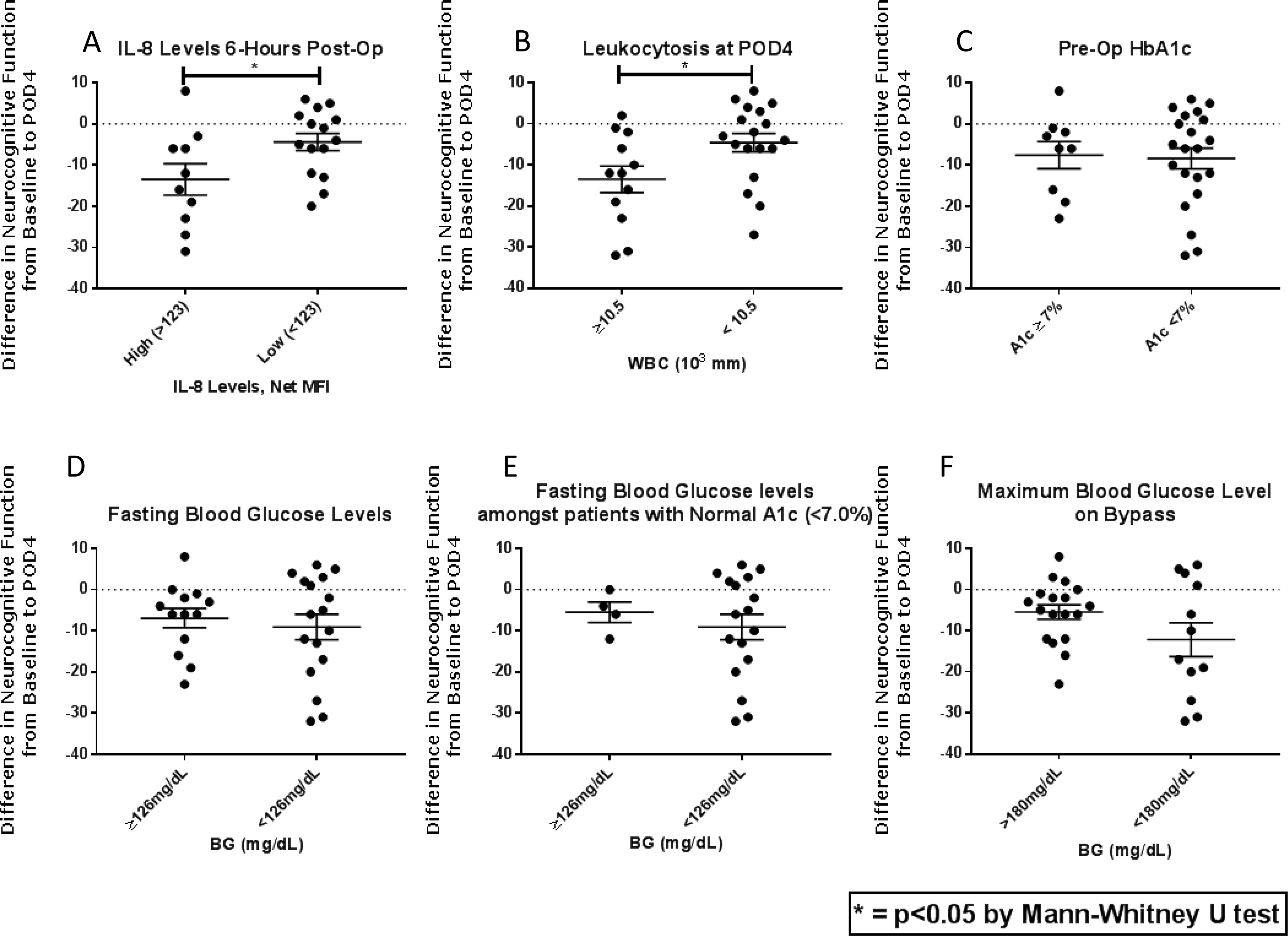
1A: Elevated levels of IL-8 at 6 hours post-op are correlated with neurocognitive decline at postoperative day 4 (POD4, p=0.04). 1B: Postoperative leukocytosis (WBC ≥ 10.5×103mm) was associated with NCD at POD4 when compared to their baseline function (p=0.03). 1C: HbA1c levels are not correlated with NCD at POD4 (p=0.973). 1D: Elevated fasting glucose levels (>126mg/dL) are not associated with NCD at POD4 (p=0.910). 1E: Among patients with normal HbA1c levels, elevated fasting glucose levels are also not associated with NCD. 1F: Glucose levels during CPD are not associated with postoperative NCD (p=0.639). NCD=neurocognitive decline, POD4=postoperative day 4, CPD=cardiopulmonary bypass
Influence of diabetes or glycemic control
No difference was observed in neurocognitive function postoperatively (POD4) between patients with pre-operative elevated HbA1c levels (p=0.973), [Figure 1C]. There was also no difference observed between patients who had elevated fasting glucose levels (>126mg/dL) on the morning of their operation (p=0.910). Intraoperative average and maximum glucose levels were compared and while all were largely well-controlled, there was no difference in postoperative neurocognitive function in patients who had either higher maximum blood glucose levels during CPB (>180mg/dL, p=0.252), or average glucose levels during CPB (>160mg/dL, p=0.639), [Figures 1D–F].
Cytokines, Chemokines, Growth Factors
We analyzed 42 cytokines, chemokines, and growth factors in patient serum and compared pre-operative levels to those at six-hours post-op and on POD4, then correlated them to RBANS scores to determine any association with neurocognitive decline. There were many cytokines and growth factors that were significantly elevated or diminished at six-hours post-operatively when compared to baseline and levels on POD4. Specifically, there was significant upregulation of IL-6, IL-8, IL-10, IP-10, Eotaxin, IL-1RA, SCF, HGF, MIP1β, MCP1, VEGF-A and VEGF-D and downregulation of IL-1β, IL-5, TNF-α, and IL-23 at 6 hours postoperatively (p≤0.05 for all). With the exception of IL-8, these changes in expression were not associated with NCD on POD4 (p=0.04), [Figure 1A]. There was no difference detected in levels of other inflammatory cytokines when correlated to NCD at any time point (p>0.3 for all).
Cardiac Function
Lower ejection fraction at the commencement of the case or preoperatively (<55%, n=11) was significantly correlated with neurocognitive decline postoperatively (p=0.01).
Transcriptional analysis
Patients who experienced neurocognitive decline had an upregulation of 445 genes and a downregulation of 40 genes at POD 4, compared to patients without NCD. In patients with diabetes, there was an upregulation of 12 genes and downregulation of 7 genes when compared to patients without diabetes. In patients with diabetes that experienced neurocognitive decline there was an upregulation of 277 genes and downregulation of 220 genes when compared to those patients with diabetes that had no neurocognitive decline. Within the subset of patients with NCD, those with diabetes upregulated 121 genes and downregulated 159 genes when compared to patients who did not have diabetes. [Figures 2–5]. A list of the top upregulated and downregulated transcripts for the patients with diabetes, post-cardiopulmonary bypass, with and without NCD can be found in tables 1–4. Annexin A1 was found to be upregulated 3.14-fold (p= 0.02) above baseline in the same group of patients.
Figure 2:
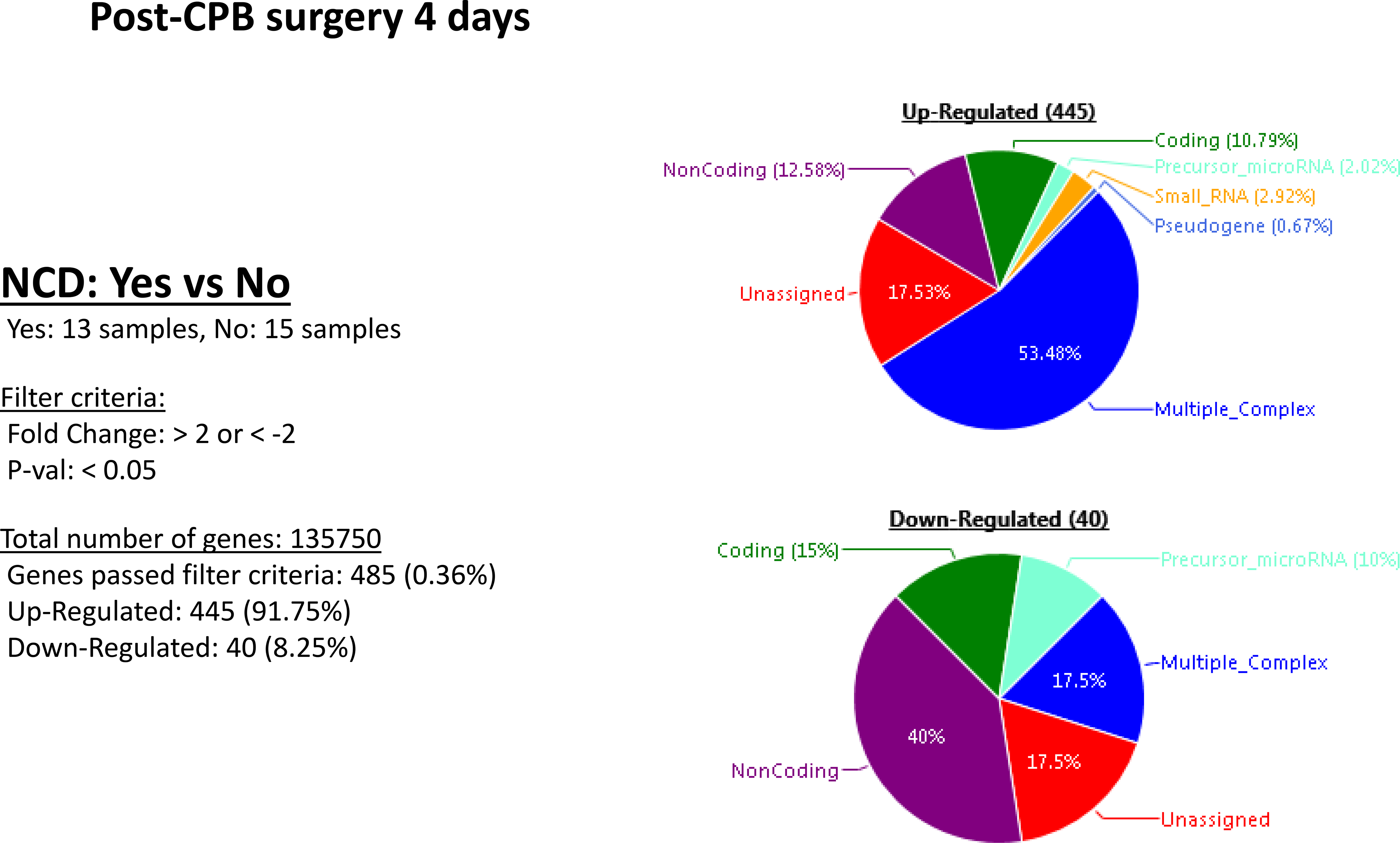
Postoperative genetic regulation in patients with or without neurocognitive decline. Patients who experienced neurocognitive decline had an upregulation of 445 genes and a downregulation of 40 genes on POD4.
Figure 5:
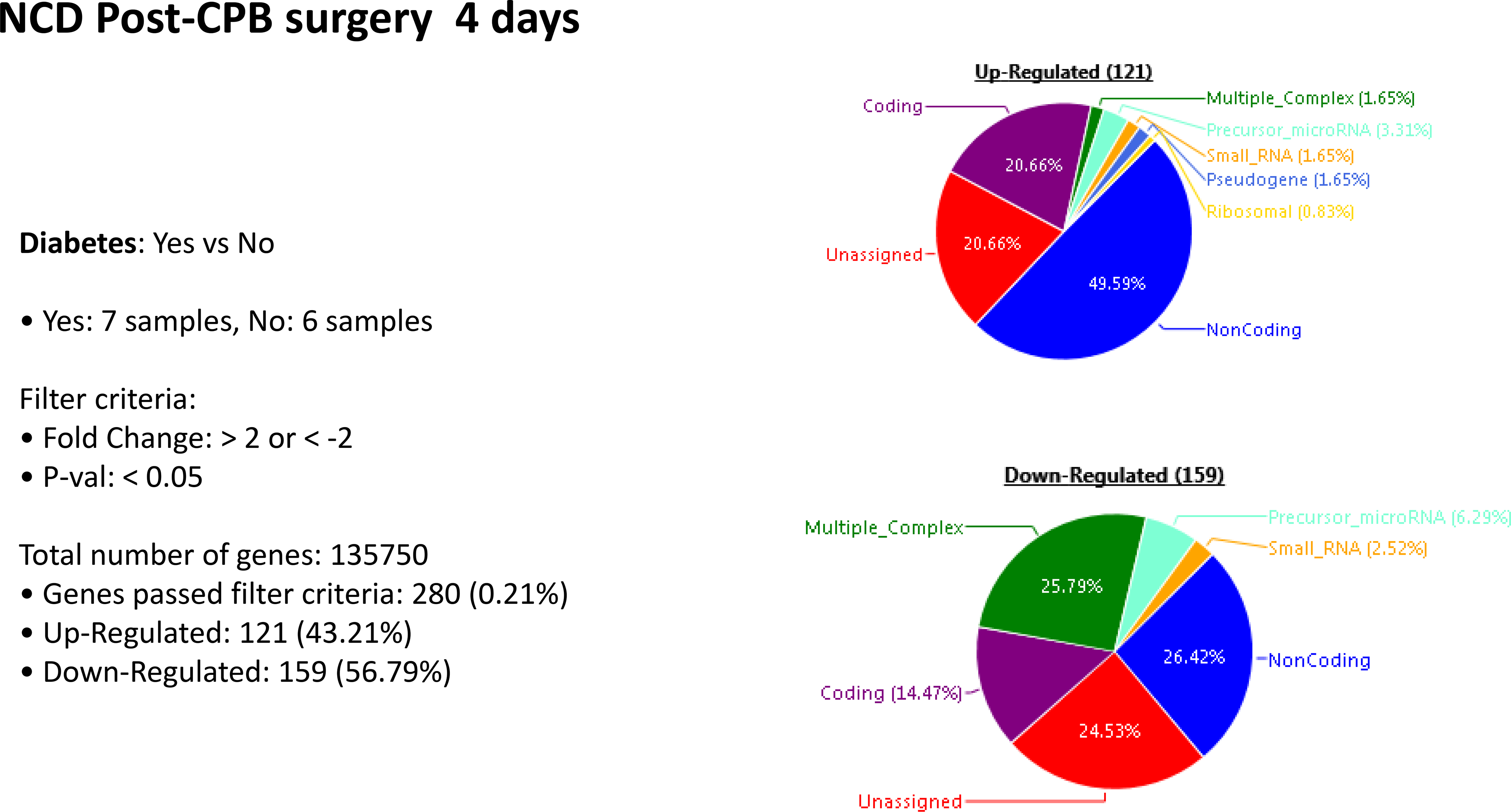
Postoperative genetic regulation in patients with neurocognitive decline with or without diabetes. Within the subset of patients with NCD, those with diabetes upregulated 121 genes and downregulated 159 genes compared to patients who also had NCD but not diabetes.
Table 1:
Top upregulated transcripts from the post-CPB NCD + DM group
| Fold Change | P-Value | Gene Symbol |
|---|---|---|
| 3.65 | 0.0005 | plufubu |
| 3.40 | 0.0014 | blamorbo |
| 3.33 | 0.027 | spoyzabu |
| 3.25 | 0.0193 | narvee |
| 3.20 | 0.0219 | pawkloy |
| 3.00 | 0.02 | nanoru |
| 2.88 | 0.0055 | kukluby |
| 2.82 | 0.009 | swoyjubo |
| 2.68 | 0.0052 | CLEC4F |
| 2.60 | 0.0065 | sleepaw |
| 2.58 | 0.0466 | AC242988.1 |
| 2.56 | 0.0433 | klybloyby |
| 2.53 | 9.82E-05 | pusmeyby |
| 2.51 | 0.0111 | nawgy |
| 2.41 | 0.0117 | sleeplubu |
| 2.40 | 0.0213 | mawzu |
| 2.40 | 0.0265 | neebubu |
| 2.33 | 0.0103 | OR2A9P; OR2A20P |
| 2.31 | 0.0172 | klorfawby |
Table 4:
Top down-regulated genes from the post-CPB non-NCD + DM group
| Fold Change | P-val | Gene Symbol |
|---|---|---|
| −3.48 | 0.0237 | puflor |
| −3.26 | 0.0027 | FCRL5 |
| −3.00 | 0.0121 | voychy |
| −2.71 | 0.0211 | CIITA |
| −2.64 | 0.0033 | bytor |
| −2.59 | 0.0431 | jeereybo |
| −2.50 | 0.0423 | RASGRP3 |
| −2.48 | 0.0243 | gerfubo |
| −2.48 | 0.0385 | rerfly |
| −2.45 | 0.0387 | chujybu |
| −2.43 | 0.0419 | vasmuby |
| −2.39 | 0.0004 | meefoby |
| −2.33 | 0.0024 | klugoy |
| −2.32 | 0.0362 | shersoby |
| −2.30 | 0.0154 | smoyswer |
| −2.28 | 0.0115 | plerdarby |
| −2.27 | 0.0002 | marflor |
| −2.27 | 0.0051 | jarflobu |
| −2.26 | 0.0129 | borgawby |
| −2.25 | 0.0009 | weyber |
Discussion
In this study, we aimed to examine the role of glycemic control on the development of NCD after cardiac surgery. Further we determined to identify risk factors such as inflammatory markers or gene expression that may contribute the neurocognitive dysfunction following cardiac surgery in patients with diabetes. We found a large portion of patients are affected by decreased neurocognitive function in the early postoperative period (73%). While this is higher than often reported in other studies, our RBANS test was designed to pick up even minor neurocognitive deficits and reflect any decrease from baseline. Previous studies have shown that the majority of these patients (40 out of 41) do return to normal neurocognitive function at 30 days.6 Surprisingly, we found no effect of blood glucose or HbA1c on the rate of NCD after surgery. Furthermore, we only included patients who underwent cardiac surgery utilizing CPB; therefore, future studies will be necessary to compare the effects on NCD in patients with and without CPB and the contributions of genetics.
Acute kidney injury is known to cause inflammatory mediators to reach the brain and have downstream effects including diminished neurologic function.7 Furthermore, it has been recently shown that patients who experience AKI during hospitalization are significantly more likely to develop dementia even after controlling for other various comorbidities.8 It is therefore not surprising that we observed a correlation between patients who had an AKI during their hospitalization and suffered NCD (as defined as Cr≥1.2mg/dL). CKD is not associated with dementia given the influx of neurotoxic uremic substances and the permeability of the blood-brain barrier9, therefore we did omit patients from this study who had an elevated baseline creatinine. However, even mild inflammation from acute kidney injury appears to be correlated to neurocognitive decline at POD4, suggesting that fluid status and renal perfusion are of utmost importance in both short- and long-term outcomes of cardiac surgery.
Regarding inflammatory markers, postoperative leukocytosis (WBC ≥ 10.5×103mm) was correlated with a decreased scoring in neurocognitive testing at POD4. One marker for systemic inflammatory response syndrome (SIRS) is leukocytosis and it has been shown that SIRS is known to negatively affect cognitive function, particularly in the elderly, and can last for years following the event.10
The increased expression of cytokines, chemokines, and growth factors such as IL-6 six-hours postoperatively have been previously shown; and we demonstrated this again, along with other cytokines including IL-10, SCF, HGF, MCP1, VEGF-A and VEGF-D.11 Increased IL-6 levels have been shown to result in neurocognitive decline in animal models as it disrupts the blood-brain barrier and activates microglia. However, our findings do not support this effect in our study population.12 Surprisingly, we saw a decrease in other inflammatory cytokines including TNF-α, IL-1β and IL-23.
IL-8 is a chemokine which is involved in chemotaxis of neutrophils as well as angiogenesis. Release of IL-8 from astrocytes and microglia results in activation of neutrophils and their subsequent adhesion to endothelial cells causing, leakiness in the blood-brain barrier.13 Therefore, increased IL-8 levels in patients at six-hours postoperatively, may contribute to the penetration of inflammatory cytokines across the blood-brain barrier, leading to neurocognitive decline in the post-operative period. Furthermore, inhibition of IL-8 could serve as a valuable therapeutic target for prevention of neurocognitive decline in cardiac surgery. It is important to note the while increased expression of inflammatory cytokines is associated with NCD, sepsis and other pathologic conditions, most of the studies no not clearly demonstrate a causal relationship.14 This is the case in this study.
Patients with diabetes, particularly those with poorly controlled diabetes and elevated HbA1c levels, have been shown to have higher rates of baseline cognitive dysfunction.15,16 Additionally, patients with diabetes have been shown to have higher rates of complications following CPB.17,18 Thus, it was hypothesized that patients with diabetes, and especially those with poorly control diabetes, would have greater neurocognitive decline following cardiac surgery. It was further hypothesized that this may be related to microvascular changes and altered regulation of perfusion that occurs in the brain and other organs during and after cardiac surgery.19–22 Marked alterations in vasomotor regulation have been documented after cardiac surgery utilizing cardiopulmonary bypass both in vivo23 and in vitro.19–22,24,25 These changes in vasomotor regulation and other cellular signaling are exacerbated or different in patients with poorly controlled diabetes compared to patients without diabetes or well controlled diabetes.24–27
While hyperglycemia is often seen as an inflammatory state and therefore theorized to be associated with inflammatory markers penetrating the blood-brain barrier and causing decreased neurocognitive function, we did not observe that in this study. Neither did pre-existing diabetes, as determined by an elevated pre-operative HbA1c level, nor elevations of glucose levels the morning of or intra-operatively have any effect on early neurocognitive function. It is possible that the tight intra-operative glucose control regularly achieved may have minimized this risk to an undetectable finding in our small sized study but would perhaps be more evident in a much larger cohort; or perhaps, the insidious nature of the complications of diabetes are such that an operation is not a sufficiently large enough event to trigger earlier development and detection of such complications like neurocognitive dysfunction. Interestingly, a subset of the ACCORD trial showed that while total brain volume was smaller in patients with less-tightly controlled diabetes, more subtle neurocognitive outcomes were not different between groups at 20 and 40 months, suggesting that these more subtle differences may be well-compensated in the early years of progression of poorly controlled diabetes.28
Anemia and heart failure are potential risk factors for NCD. Low output heart failure, in particular, after cardiac surgery may be contributory to postoperative neurological decline. Unfortunately, while anemia has been shown to be a greater risk, patients who receive intraoperative RBC transfusions are at higher risk of low output heart failure.29 In our study, we found that lower ejection fractions (<55%) were associated with neurocognitive decline on POD4. This is not a surprising finding given the presumed etiology of lower blood flow to the brain having negative consequences. However, increasing hematocrit intraoperatively does not appear to alleviate the low output heart failure, and therefore is unlikely to alleviate neurocognitive decline. Furthermore, our center has shown that while pre-operative anemia is associated with neurocognitive decline, transfusion does not improve this outcome.5
Genetic regulation has been previously shown to be associated with neurocognitive decline via pathways of inflammation, cell-death, and neurological dysfunction in blood samples of patients before and after cardiopulmonary bypass.2,6 Our study similarly demonstrates differences in genetic expression amongst groups, but further subdivides between those with and without diabetes. Neurocognitive decline appears to be associated with upregulation of a variety of genes (over 400) at POD4, which may relate to a higher inflammatory state than those who do not experience neurocognitive decline postoperatively. The presence of pre-existing diabetes did not seem to have a large difference in genetic upregulation or downregulation when compared to patients without diabetes. This once again may reflect the tight glucose control in both the intra- and postoperative period; therefore, minimizing any potential difference due to the lack of significant time spent with hyperglycemia. However, the variability in genetic upregulation and downregulation among patients with and without pre-existing diabetes who experienced NCD, suggests a closer look at the role diabetes plays in neurocognitive outcomes in cardiac surgery is warranted. Compared to patients with diabetes who experienced NCD following cardiac surgery, patients with diabetes without NCD were found to upregulate a number of genes involved in immune system functioning. Although it is difficult to evaluate the role that single genes play in the greater context of an inflammatory response; patients with diabetes and no NCD were found to upregulate several genes with anti-inflammatory effects. Among these genes was Annexin A1 which is the main downstream effector of the anti-inflammatory effects of glucocorticoids.30 Among its anti-inflammatory functions, annexin A1 has been found to inhibit phospholipase A2 and therefore the production of eicosanoids, thus reducing neutrophil extravasation, promoting apoptosis, and inducing the conversion of macrophages to anti-inflammatory phenotypes that promote the resolution of the inflammatory response.30,31 Additionally, TGF-β was upregulated in patients with diabetes but without NCD following cardiac surgery. TGF-β has well known anti-inflammatory properties which include the inhibition of NF-κB activity via NF-κB/REI inhibitor protein, increased expression of IL-10, and promoting the differentiation of anti-inflammatory M2 macrophages.32,33 Neurocognitive decline after cardiac surgery has been partly attributed to the inflammatory milieu that may lead to increased vascular permeability in the brain.34 Therefore, it is plausible that in patients with diabetes that did not experience NCD, the actions of TGF-β and annexin A1 collectively help limit the inflammatory response; although more research is needed to elucidate the complex regulatory mechanisms that modulate the inflammatory response and its role in NCD.
Limitations
The major limitation of this study was the small sample size and short time course, further compounded by the self-selecting group of patients who completed all study time points and did not withdraw. Additionally, this sample was composed primarily of white males, which impacts the generalizability of all of the results. We aimed to enroll an equal and diverse population, but given the exclusion criteria, diversity both in race and sex as well as medical comorbidities was not well-reflected in this small sample size. Finally, while correlations were established between expression of IL-8 and various genes, no causal relationship was established.
Conclusion
While hyperglycemia and most inflammatory cytokines do not appear to correlate with increased risk of postoperative neurocognitive dysfunction following cardiac surgery, certain genetic expression trends are apparent. Future directions will need to focus on improving the understanding of factors contributing to genetic expression patterns before and after cardiac surgery, as well as potential ways to modulate that expression and its impact on peri-operative patient care.
Figure 3:
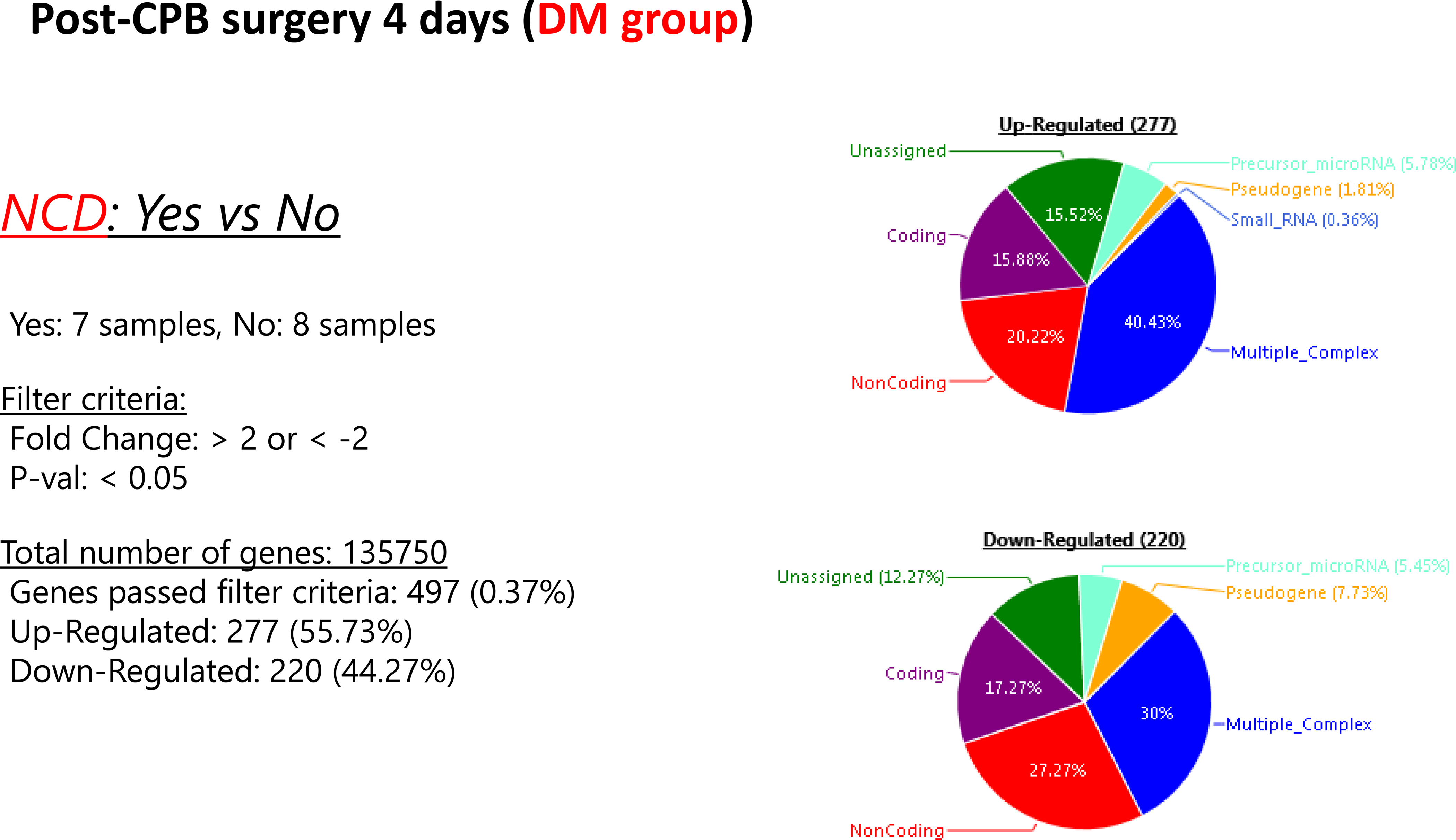
Post-operative genetic regulation in patients with diabetes with or without neurocognitive decline. Patients with diabetes who experienced NCD at POD demonstrated upregulation of 277 genes and downregulation of 220 genes compared to those patients with diabetes but no neurocognitive decline.
Figure 4:
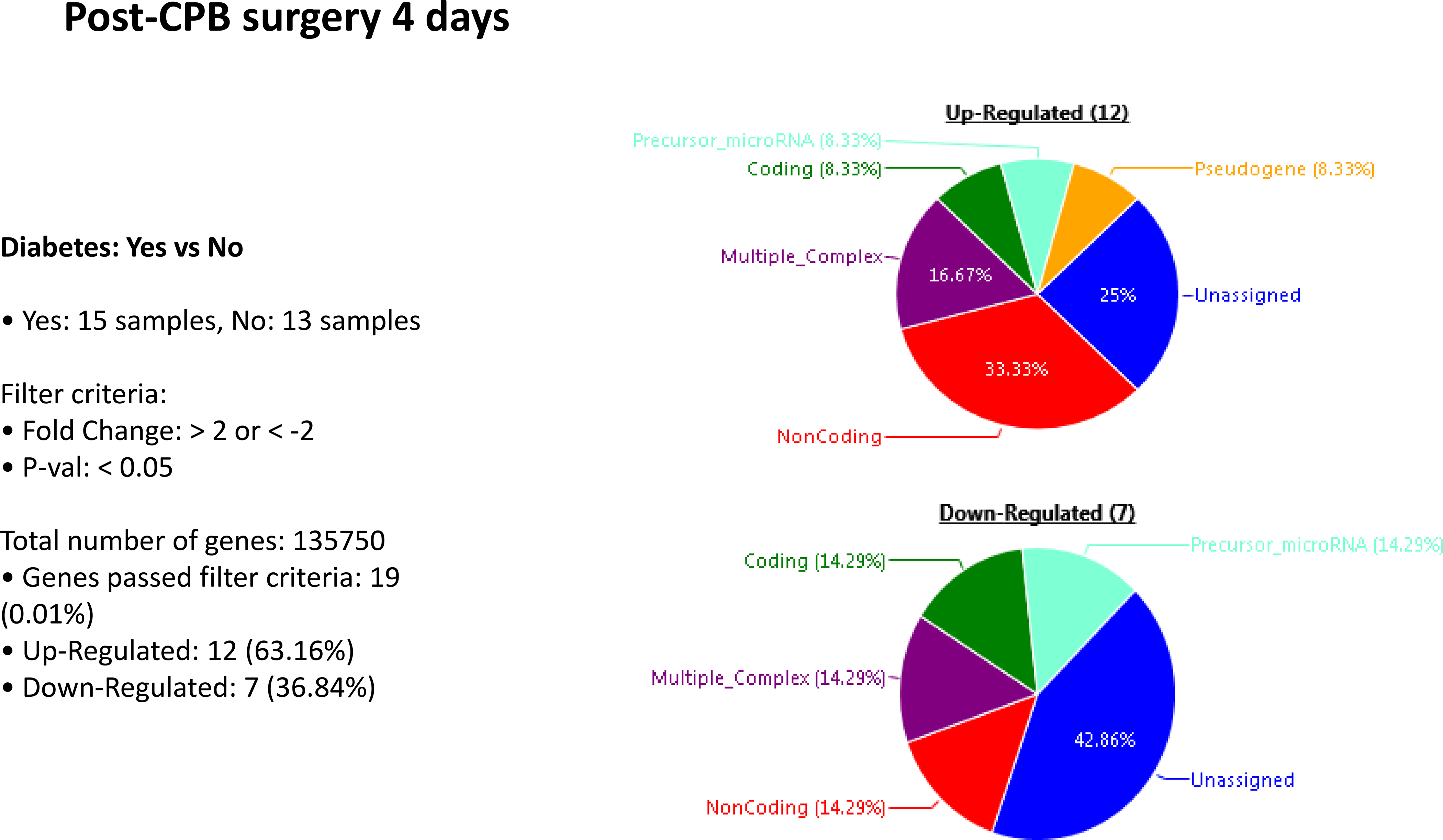
Postoperative genetic regulation between patients with and without diabetes after cardiopulmonary bypass demonstrates minimal differences.
Table 2:
Top down-regulated transcripts from the post-CPB + DM group
| Fold Change | P-Value | Gene Symbol |
|---|---|---|
| −5.21 | 0.0011 | PVALB |
| −4.65 | 0.004 | NFXL1 |
| −4.03 | 0.0147 | HBG2; HBG1 |
| −3.81 | 0.0318 | RDXP2 |
| −3.64 | 0.015 | smorflor |
| −3.60 | 0.0124 | perpee |
| −3.25 | 0.0265 | GCOM1; MYZAP; POLR2M |
| −3.18 | 0.0417 | NSFP1 |
| −2.95 | 0.0027 | WDR73 |
| −2.92 | 0.0374 | MYL4 |
| −2.91 | 0.0356 | MIR548AU |
| −2.87 | 0.0092 | ANTXRLP1 |
| −2.82 | 0.0388 | lusto |
| −2.80 | 0.0093 | smablee |
| −2.76 | 0.0406 | merlybu |
| −2.66 | 0.0034 | ATP1B1 |
| −2.66 | 0.0272 | ATP1B1 |
| −2.63 | 0.0296 | sperwy |
| −2.58 | 0.0104 | MIER3 |
Table 3:
Top upregulated transcripts from the post-CPB non-NCD + DM group
| Fold Change | P-val | Gene Symbol |
|---|---|---|
| 5.62 | 0.0189 | lermaw |
| 4.37 | 0.0064 | chawdabu |
| 4.25 | 0.0025 | BCAS2 |
| 4.06 | 0.0066 | HLA-U |
| 3.65 | 0.015 | RP11-490N5.3 |
| 3.63 | 0.0468 | FLNA |
| 3.43 | 0.0349 | F11R |
| 3.39 | 0.0473 | CORO1C |
| 3.27 | 0.0353 | OVOS2 |
| 3.24 | 0.0095 | RDXP2 |
| 3.18 | 0.033 | PDCD4; MIR4680 |
| 3.14 | 0.0179 | ANXA1 |
| 3.11 | 0.0076 | LGALS1 |
| 2.94 | 0.0317 | tutoru |
| 2.93 | 0.0141 | HLA-H |
| 2.89 | 0.0357 | swawshyby |
| 2.88 | 0.0405 | ACSM3 |
| 2.85 | 0.0014 | CD48 |
| 2.82 | 0.0451 | PPT1 |
Acknowledgements
Funding
Funding for this research was provided by the National Heart, Lung, and Blood Institute (NHLBI). [R01HL46716 (F.W.S.), RO1HL128831-01A1 (F.W.S.]; and 2T32 GM065085-13 (L.A.S.). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interests/Disclosures:
The authors have no conflicts of interests associated with this work.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Roach GW, Kanchuger M, Mangano CM, et al. Adverse Cerebral Outcomes after Coronary Bypass Surgery. N Engl J Med. 1996;335(25):1857–1864. doi: 10.1056/nejm199612193352501 [DOI] [PubMed] [Google Scholar]
- 2.Sabe AA, Dalal RS, Chu LM, et al. Preoperative gene expression may be associated with neurocognitive decline after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2015;149(2):613–623. doi: 10.1016/j.jtcvs.2014.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition - The case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x [DOI] [PubMed] [Google Scholar]
- 4.Merz ZC, Hurless N, Wright JD. Examination of the Construct Validity of the Repeatable Battery for the Assessment of Neuropsychological Status Language Index in a Mixed Neurological Sample. Arch Clin Neuropsychol. 2018;33:889–894. doi: 10.1093/arclin/acx115 [DOI] [PubMed] [Google Scholar]
- 5.Gorvitovskaia AY, Scrimgeour LA, Potz BA, et al. Lower preoperative hematocrit, longer hospital stay, and neurocognitive decline after cardiac surgery. Surg (United States). 2020. doi: 10.1016/j.surg.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramlawi B, Otu H, Rudolph JL, et al. Genomic expression pathways associated with brain injury after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;134(4). doi: 10.1016/j.jtcvs.2007.01.096 [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai HH, Yen RF, Lin CL, Kao CH. Increased risk of dementia in patients hospitalized with acute kidney injury: A nationwide population-based cohort study. PLoS One. 2017;12(2). doi: 10.1371/journal.pone.0171671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol. 2013;24(3):353–363. doi: 10.1681/ASN.2012050536 [DOI] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA - J Am Med Assoc. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramlawi B, Rudolph JL, Mieno S, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006;244(4):593–600. doi: 10.1097/01.sla.0000239087.00826.b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120(3):537–545. doi: 10.1016/j.bja.2017.11.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich LC, Hu S, Sheng WS, et al. Cytokine Regulation of Human Microglial Cell IL-8 Production. J Immunol. 1998;160(4):1944–1948. [PubMed] [Google Scholar]
- 14.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/JAMA.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett-Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Heal Aging. 2006;10(4):292–295. https://pubmed.ncbi.nlm.nih.gov/16886099/. Accessed December 5, 2020. [PubMed] [Google Scholar]
- 16.Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29(8):1794–1799. doi: 10.2337/dc06-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maze M, Feng X. Comorbidities and Postoperative Neurocognitive Disorder. In: The Perioperative Neurocognitive Disorders. Cambridge University Press; 2019:115–122. doi: 10.1017/9781316402504.011 [DOI] [Google Scholar]
- 18.Feng J, Sellke F. Microvascular dysfunction in patientswith diabetes after cardioplegic arrest and cardiopulmonary bypass. Curr Opin Cardiol. 2016;31(6):618–624. doi: 10.1097/HCO.0000000000000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellke FW, Shafique T, Schoen FJ, Weintraub RM. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. J Thorac Cardiovasc Surg. 1993;105(1):52–58. doi: 10.1016/s0022-5223(19)33847-4 [DOI] [PubMed] [Google Scholar]
- 20.Sellke FW, Wang SY, Stamler A, Johnson RG, Cohn WE, Weintraub RM. Changes in autonomic response of the cerebral circulation after normothermic extracorporeal circulation. J Thorac Cardiovasc Surg. 1996;112(2):450–461. doi: 10.1016/s0022-5223(96)70273-8 [DOI] [PubMed] [Google Scholar]
- 21.Stamler A, Wang SY, Li J, Thurer RL, Schoen FJ, Sellke FW. Moderate hypothermia reduces cardiopulmonary bypass-induced impairment of cerebrovascular responses to platelet products. Ann Thorac Surg. 1996;62(1):191–198. doi: 10.1016/0003-4975(96)00240-8 [DOI] [PubMed] [Google Scholar]
- 22.Mirman B, Ikeda I, Zhang Z, et al. Effects of neuropeptide Y on the microvasculature of human skeletal muscle. In: Surgery (United States). Vol 168. Mosby Inc.; 2020:155–159. doi: 10.1016/j.surg.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiratzka LF, Eastham CL, Carter JG, et al. The Effects of Cardiopulmonary Bypass and Cold Cardioplegia on Coronary Flow Velocity and the Reactive Hyperemic Response in Patients and Dogs. Ann Thorac Surg. 1988;45(5):474–481. doi: 10.1016/S0003-4975(10)64518-3 [DOI] [PubMed] [Google Scholar]
- 24.Sellke N, Kuczmarski A, Lawandy I, et al. Enhanced coronary arteriolar contraction to vasopressin in patients with diabetes after cardiac surgery. J Thorac Cardiovasc Surg. 2018;156(6):2098–2107. doi: 10.1016/j.jtcvs.2018.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J, Liu Y, Singh AK, et al. Effects of diabetes and cardiopulmonary bypass on expression of adherens junction proteins in human peripheral tissue. In: Surgery (United States). Vol 161. Mosby Inc.; 2017:823–829. doi: 10.1016/j.surg.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J, Liu Y, Dobrilovic N, et al. Altered apoptosis-related signaling after cardioplegic arrest in patients with uncontrolled type 2 diabetes mellitus. Circulation. 2013;128(SUPPL.1):S144–S151. doi: 10.1161/CIRCULATIONAHA.112.000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng J, Liu Y, Chu LM, et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126(11 SUPPL.1):S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): A randomised open-label substudy. Lancet Neurol. 2011;10(11):969–977. doi: 10.1016/S1474-4422(11)70188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surgenor SD, DeFoe GR, Fillinger MP, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114(SUPPL. 1). doi: 10.1161/CIRCULATIONAHA.105.001271 [DOI] [PubMed] [Google Scholar]
- 30.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. doi: 10.1038/nri2470 [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res. 2016;2016. doi: 10.1155/2016/8239258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arsura M, Wu M, Sonenshein GE. TGFβ1 inhibits NF-κB/Rel activity inducing apoptosis of B cells: Transcriptional activation of IκB. Immunity. 1996;5(1):31–40. doi: 10.1016/S1074-7613(00)80307-6 [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAILmediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–52306. doi: 10.18632/oncotarget.10561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel N, Minhas JS, Chung EML. Risk Factors Associated with Cognitive Decline after Cardiac Surgery: A Systematic Review. Cardiovasc Psychiatry Neurol. 2015;2015:1–12. doi: 10.1155/2015/370612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


