ABSTRACT
Bacteriophage-encoded lysins are increasingly reported as alternatives to combat Acinetobacter baumannii infections, for which limited therapeutic options are available. Some lysins, such as LysMK34, have a C-terminal amphipathic helix allowing them to penetrate the otherwise-impermeable outer membrane barrier. Another approach to kill Gram-negative pathogens with lysins relies on fusion of a peptide with outer membrane-permeabilizing properties to the lysin. In this work, we aimed to leverage the intrinsic antibacterial activity of LysMK34 by fusing the peptide cecropin A to its N terminus via a linker of three Ala-Gly repeats, resulting in engineered LysMK34 (eLysMK34). The engineered lysin has an improved antibacterial activity compared to that of the parental lysin, LysMK34, in terms of MICs (0.45 to 1.2 μM), killing rate, and killing extent. eLysMK34 has a ≥2-fold-increased activity against stationary-phase cells, and the bactericidal effect becomes less dependent on the intracellular osmotic pressure. In particular, colistin-resistant strains become highly susceptible to eLysMK34, and enhanced antibacterial activity is observed in complement-deactivated human serum. These observations demonstrate that fusion of a lysin with intrinsic antibacterial activity with a selected outer membrane-permeabilizing peptide is a useful strategy to further improve the in vitro antibacterial properties of such lysins.
IMPORTANCE Phage lysins are a new class of enzyme-based antibiotics that increasingly gain interest. Lysins kill cells through rapid degradation of the peptidoglycan layer, resulting in sudden osmotic lysis. Whereas Gram-positive bacteria are readily susceptible to the actions of lysins, Gram-negative bacteria are naturally resistant, as the outer membrane protects their peptidoglycan layer. This work reveals that fusing an outer membrane-permeabilizing peptide to a lysin with intrinsic antibacterial activity results in a superior lysin that shows improved robustness in its antibacterial activity, including against the most worrisome colistin-resistant A. baumannii strains.
KEYWORDS: Acinetobacter baumannii, lysin, antibacterial, lysis, protein engineering
INTRODUCTION
Antibiotic resistance is a global threat that may pose an unprecedented burden on both health care and the economy. Consequently, the World Health Organization (WHO) has called for an urgent development of novel antibacterial agents to fill the large void caused by a high resistance development rate and an insufficiently filled antibiotic development pipeline (1). Carbapenem-resistant Acinetobacter baumannii is chiefly present at the top of the WHO list of critical pathogens. A. baumannii is associated with a wide range of community- and hospital-acquired infections, including ventilator-associated pneumonia, bacteremia, wound infections, and meningitis (2, 3). Its high ability to acquire resistance genes in addition to its natural resistance to a wide range of antibiotics makes treatment of A. baumannii infections challenging (4). Colistin is frequently used as a last-resort antibiotic for controlling A. baumannii infections; however, a growing number of reports on colistin-resistant strains announce a possible postantibiotic era (5, 6).
Lysins are a bacteriophage-derived class of antimicrobial agents. They breach the bacterial cell wall by enzymatic degradation at the beginning and end of the lytic cycle for genome ejection and progeny release, respectively (7). They have been developed relatively fast throughout the (pre)clinical pipeline up to a currently ongoing first phase III clinical trial (ClinicalTrials.gov, NCT04160468) (8). These agents were initially thought to be ineffective against Gram-negative bacteria because of the protective outer membrane (OM) that hinders large molecules, such as lysins, from accessing the peptidoglycan layer (9). Nevertheless, different lysins are able to penetrate the OM barrier by means of a C-terminal amphipathic helix or a cationic N terminus and exert intrinsic antibacterial activity (9–15). In particular, A. baumannii appears to show an enhanced susceptibility to this group of lysins. Furthermore, the addition of organic outer membrane permeabilizers (for example, EDTA and citric acid) improve the overall activities of lysins due to OM destabilization and enhanced penetration of the lysin (16). Many engineering efforts have released enhanced versions of lysins effective against Gram-negative bacteria. These engineered lysins pass through the outer membrane with the help of fused OM-permeabilizing peptides (i.e., Artilysins) (17), pyocin domains (i.e., lysocins) (18), or receptor-binding proteins of phages (i.e., Innolysins) (19). Artilysins have been shown to have a high and rapid bactericidal activity against challenging bacterial phenotypes, including resistant and persistent bacteria (17, 20). Lysin 1D10, which is engineered with an OM-permeabilizing cecropin A (CecA) peptide from Aedes aegypti at its N terminus, shows antibacterial activity against A. baumannii under human serum conditions and in an ex vivo piglet skin wound model (21). Cecropins are a class of amphipathic peptides that have both hydrophobic and cationic properties and are secreted by insects. The amphipathic nature of these peptides facilitates outer membrane permeabilization of Gram-negative bacteria through interaction and disruption of both hydrophobic (mediated by the lipid A moiety) and ionic (mediated by divalent cations interacting with phosphate groups) stabilizing forces of the bacterial outer membrane (22). In a previous study, we evaluated the antibacterial activity of the peptidoglycan-degrading lysin of A. baumannii phage MK34 (here referred to as LysMK34) (15). LysMK34 shows a turgor pressure-dependent, intrinsic antibacterial activity with limited activity in high-tonicity buffers containing physiological concentrations of NaCl (150 mM) (0.6-log-unit reduction in cell number after 120 min) but high activity in low-tonicity buffer (>5 log units). No LysMK34 MIC was detected up to 1 mg/mL in Mueller-Hinton (MH) broth. However, LysMK34 displays synergy with colistin, which is most pronounced for colistin-resistant strains (up to 16- and 32-fold reductions of the MIC of colistin in MH broth and 50% human serum, respectively). The synergy is not present when LysMK34 is combined with polymyxin B nonapeptide, a colistin derivative without its fatty acid chain, highlighting the role of the fatty acid chain of colistin and its interaction with the cytoplasmic membrane in the overall antibacterial activity of LysMK34/colistin combinations (15).
In this work, CecA was selected based on our previous study that included 38 outer membrane-permeabilizing peptides with different physiochemical properties when we screened for hits against A. baumannii. The best lysin (1D10) from this screening has CecA at its N terminus (21). Based on these observations, we constructed a chimeric protein fusing a lysin with intrinsic activity (LysMK34) to an OM-permeabilizing peptide (cecropin A from Aedes aegypti), and we show that the antibacterial activity of engineered LysMK34 (eLysMK34) is enhanced compared to that of LysMK34, particularly against colistin-resistant strains and under deactivated human serum conditions.
RESULTS
eLysMK34 specifically inhibits the growth of A. baumannii in Mueller-Hinton broth.
eLysMK34 comprises a fusion of CecA from Aedes aegypti to a short flexible linker (Gly-Ala)3 at the N terminus of LysMK34. Both LysMK34 and eLysMK34 were purified using metal affinity chromatography and a C-terminal 6×His tag, with total yields of 36 and 20 mg/L of expression culture, respectively. SDS-PAGE analysis of the purified fractions showed major bands with electrophoretic homogeneity (>95%) and estimated molecular masses close to the theoretical masses of both the wild-type (22-kDa) and engineered (26.4-kDa) lysins (see Fig. S1 in the supplemental material).
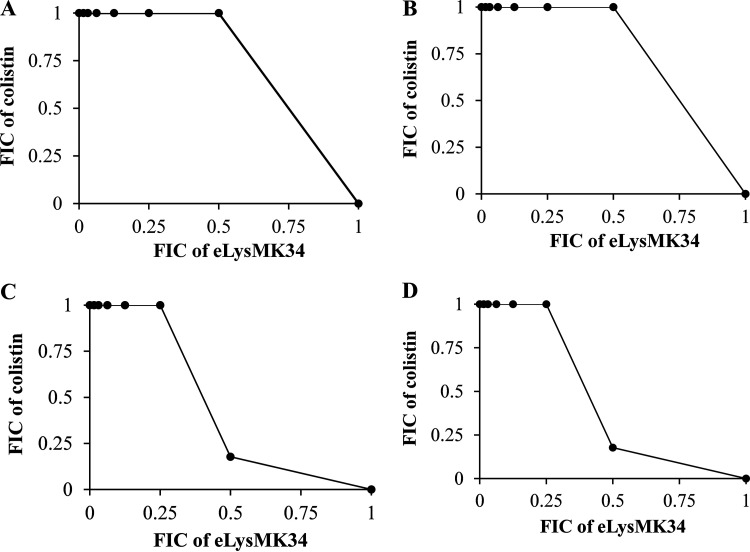
We investigated the MICs of LysMK34 and eLysMK34 in MH broth. In contrast to LysMK34 (MIC > 45.5 μM), eLysMK34 shows MIC values for all A. baumannii strains tested (MK34, NCTC13423, RUH134, Greek46, and Greek47) ranging from 0.45 to 1.2 μM (Table 1). Notably, the highest MIC (1.2 μM) was observed for Greek47, a colistin-resistant strain for which the MIC of colistin was 27.8 μM. Neither wild-type LysMK34 nor eLysMK34 inhibits Pseudomonas aeruginosa (PA14, PAO1, and Br667) or Escherichia coli (BL21 and ATCC 25922) within the range tested (Table 1). In addition, we evaluated the combinatorial growth inhibition by eLysMK34 and colistin against representative colistin-sensitive (MK34 and RUH134) and colistin-resistant (Greek46 and Greek47) A. baumannii strains using a checkerboard assay (Fig. 1). Fractional inhibitory concentrations (FICs) for each combination were calculated as the MIC of eLysMK34 in the presence of colistin divided by the MIC of (e)LysMK34 alone. Then, the FIC index (FICI) was calculated as the sum of FIC values for both compounds. The interaction is interpreted as synergistic, additive, or antagonistic if FICI ≤ 0.5, 0.5 < FICI ≤ 4, or FICI > 4, respectively. Unlike with the previously observed synergy between LysMK34 and colistin (15), eLysMK34/colistin combinations show an additive effect against the same strains, with FICs between 0.5 and 4. The loss of synergy observed for LysMK34 and colistin suggests that either a common molecular site is targeted by CecA and colistin or more generally that eLysMK34 can permeate the OM more efficiently than LysMK34 and thus does not rely on increased OM permeability induced by colistin.
TABLE 1.
MICs of eLysMK34 against different A. baumannii, P. aeruginosa, and E. coli strainsa
| Strain | Origin/source (reference) | Colistin resistance | Resistance (reference) | MIC (μM) | MIC (μg/mL) |
|---|---|---|---|---|---|
| A. baumannii MK34 | Sputum isolate from a hospitalized patient in Beni-Suef Hospital, Egypt (15) | Sensitive | XDR, resistant to 11 tested antibiotics (15) | 0.76 | 20 |
| A. baumannii RUH134 | Burn wound isolate from a Belgian burn wound center (43) | Sensitive | MDR, resistant to β-lactams, aminoglycosides, and quinolones but sensitive to carbapenem (43) | 0.76 | 20 |
| A. baumannii Greek46 | Collection of the Laboratory of Medical Microbiology (University of Antwerp, Belgium) | Resistant | 0.76 | 20 | |
| A. baumannii Greek47 | Collection of the Laboratory of Medical Microbiology (University of Antwerp, Belgium) | Resistant | 1.2 | 32 | |
| A. baumannii NCTC13423 | Iraqibacter strain, a problematic strain responsible for wound infections in American soldiers in the Iraqi war, 2003 (43) | Sensitive | MDR, resistant to β-lactams, aminoglycosides, and carbapenem (43) | 0.45 | 12 |
| P. aeruginosa PA14 | Highly virulent laboratory strain (44) | Sensitive | Highly virulent laboratory strain (44) | >1.5 | >40 |
| P. aeruginosa Br667 | Burn wound isolate (44) | Sensitive | MDR, resistant to 10 out of 11 tested antibiotics (44) | >1.5 | >40 |
| E. coli ATCC 25922 | Reference strain (https://www.atcc.org/products/25922#detailed-product-information) | Not tested | Reference strain for susceptibility testing (CLSI, M2-A9 [48]) | >1.5 | >40 |
| E. coli BL21 (DE3) RIL | Reference strain | Not tested | >1.5 | >40 |
The experiment was carried out according to the conventional microdilution assay in MH broth with an inoculum size of 5 × 105 CFU/well. The MIC of eLysMK34 was tested for all strains within a range of 0 to 1.5 μM. The mode of three independent replicates is shown. XDR, extremely drug resistant; MDR, multiple-drug resistant.
FIG 1.
Isobolograms of eLysMK34 and colistin combinations against A. baumannii MK34 (A), A. baumannii RUH134 (B), A. baumannii Greek46 (C), and A. baumannii Greek47 (D). The FIC values represent the means from three independent replicates. The FIC index (FICI) was calculated as the sum of FIC values for both drugs. The interaction is interpreted as synergistic, additive, or antagonistic if FICI ≤ 0.5, 0.5 < FICI ≤ 4, or FICI > 4, respectively.
eLysMK34 has a faster bactericidal activity than the parental lysin.
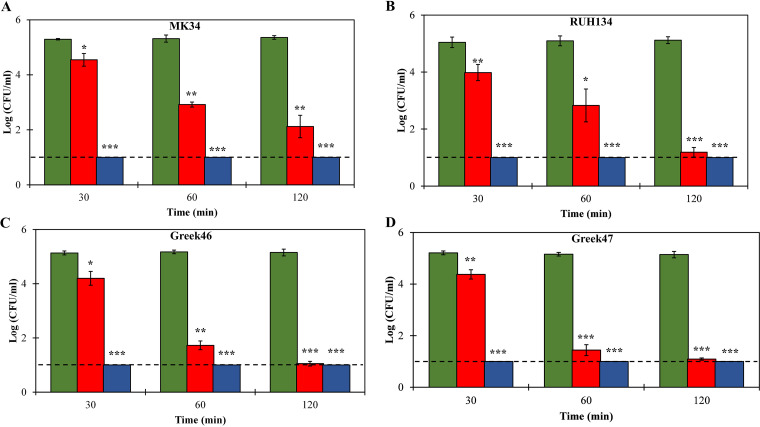
We analyzed the bactericidal activities of both 1 μM LysMK34 and 1 μM eLysMK34 against the same colistin-sensitive and colistin-resistant strains and compared their killing kinetics. Cells were resuspended in a low-tonicity buffer (20 mM HEPES-NaOH, pH 7.4), yielding cells with a high intracellular osmotic pressure. Such osmotic pressure enhances killing, as has been observed before for LysMK34 (15). The bactericidal activity was quantified at three different time points (30, 60, and 120 min). eLysMK34 shows a significantly higher bactericidal activity (P < 0.01), causing a complete reduction of the bacterial cell count of all tested strains below the detection limits after a 30-min exposure (Fig. 2). An equimolar concentration of LysMK34 shows time-dependent killing, with an almost 1-log-unit reduction after 30 min. Only after 120 min, comparable levels of killing were observed for LysMK34 and eLysMK34 (P > 0.05). The colistin-resistant strains Greek46 and Greek47 are slightly more responsive to LysMK34, with higher reductions in cell counts observed at 60 min than for the sensitive strains. Fusion of the CecA peptide thus results in an engineered lysin with a higher killing rate.
FIG 2.
Time-kill assay of different A. baumannii strains, MK34 (A), RUH134 (B), Greek46 (C), and Greek47 (D), resuspended in low-tonicity buffer (20 mM HEPES-NaOH, pH 7.4; ∼2 × 105 CFU/ml) upon exposure to 1 μM LysMK34 (red bars) or 1 μM eLysMK34 (blue bars). Green bars show the results for untreated cells. The numbers of surviving bacteria are expressed as log numbers of CFU per milliliter after 30, 60, and 120 min of exposure. Each value represents the mean ± standard deviation from three independent replicates. Asterisks represent statistical differences from values for untreated cells (Student's t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001). The dashed line represents the limit of detection (10 CFU/ml).
eLysMK34 kills colistin-resistant A. baumannii strains with low and high intracellular osmotic pressure.
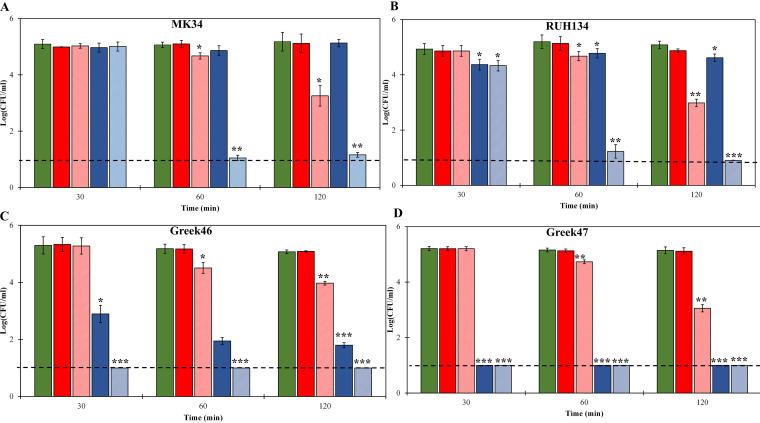
Our previous characterization of LysMK34 revealed a limited antibacterial activity against different A. baumannii strains (MK34, RUH134, Greek46, and Greek47) when these bacterial cells have a low intracellular osmotic pressure due to suspension in a high-tonicity buffer (20 mM HEPES-NaOH, 150 mM NaCl, pH 7.4, reflecting physiological conditions), even when we used a (practically too) high concentration of 22.7 μM. We hypothesize that under these conditions, extensive peptidoglycan degradation is needed to trigger full osmotic lysis. However, addition of 0.5 mM EDTA, a well-known OM destabilizer that works by chelating the stabilizing divalent cations, could partially compensate for the lack of a high intracellular osmotic pressure, resulting in a more effective killing of cells with low osmotic pressure (15). Here, we analyzed whether a fusion with CecA could eliminate the need of 0.5 mM EDTA to effectively kill cells with low intracellular osmotic pressure. Therefore, we repeated the experiment with cells suspended in high-tonicity buffer (20 mM HEPES-NaOH, 150 mM NaCl, pH 7.4), again using equimolar concentrations (1 μM) and analyzing at three different time points (Fig. 3). In the case of colistin-sensitive A. baumannii strains (MK34 and RUH134), both lysins showed no or limited (≤0.6 log) activities against both strains for up to 120 min. Addition of 0.5 mM EDTA strongly enhanced the bactericidal activities for both lysins, most noticeably for eLysMK34, resulting in ≥2.1-log reductions and complete eradication (≥4.1 log) for the parental and engineered lysins, respectively. Killing thus remains slower than for cells with a high intracellular osmotic pressure. Nevertheless, a 10-fold increase of the eLysMK34 concentration up to 10 μM resulted in a complete eradication of both colistin-sensitive strains after 120 min (≥4.1 log) (data no shown).
FIG 3.
Time-kill assay of different A. baumannii strains, MK34 (A), RUH134 (B), Greek46 (C), and Greek47 (D), resuspended in high-tonicity buffer (20 mM HEPES-NaOH, 150 mM NaCl, pH 7.4; ∼2 × 105 CFU/ml), resulting in a low intracellular osmotic pressure, upon exposure to 1 μM LysMK34 (red bars) or 1 μM eLysMK34 (blue bars). Green bars show the untreated cells. In the cases of the light-red and light-blue bars, 0.5 mM EDTA was included along with LysMK34 and eLysMK34, respectively. The numbers of surviving bacteria are expressed as log numbers of CFU per milliliter after 30, 60, and 120 min of exposure. Each value represents the mean ± standard deviation from three independent replicates. Asterisks represent statistical differences from values for untreated cells (Student's t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001). The dashed line represents the limit of detection (10 CFU/ml).
The observations were quite different for colistin-resistant strains (Greek46 and Greek47). Although LysMK34 showed almost no activity against both colistin-resistant strains after 120 min of incubation, the antibacterial effect of eLysMK34 was noticeable. For instance, eLysMK34 showed a 2.41 ± 0.19-log-unit reduction of the A. baumannii strain Greek46 (MIC colistin, 7 μM) after the first 30 min of incubation. This figure further increased to 3.26 ± 0.24 after 120 min. When tested against the more colistin-resistant strain (Greek47; MIC colistin, 27.8 μM), eLysMK34 completely reduced the cell number below the detection limit, with a 4.14-log-unit reduction from 30 min on. Also, for colistin-resistant strains, the addition of 0.5 mM EDTA strengthened the effect for both the parental and engineered lysins.
Taken together, these observations underline the interplay of osmotic pressure, the presence of CecA, the presence of EDTA, the lysin concentration, and the degree of colistin resistance, all of which affect the eventual antibacterial activity. The N-terminal fusion of CecA generally improves the antibacterial activity of LysMK34, a lysin with intrinsic antibacterial activity, against A. baumannii strains with different resistance profiles. Fusion of the peptide thus renders the activity less dependent on intracellular osmotic pressure and EDTA.
eLysMK34 has an improved activity against cells in the stationary growth phase.
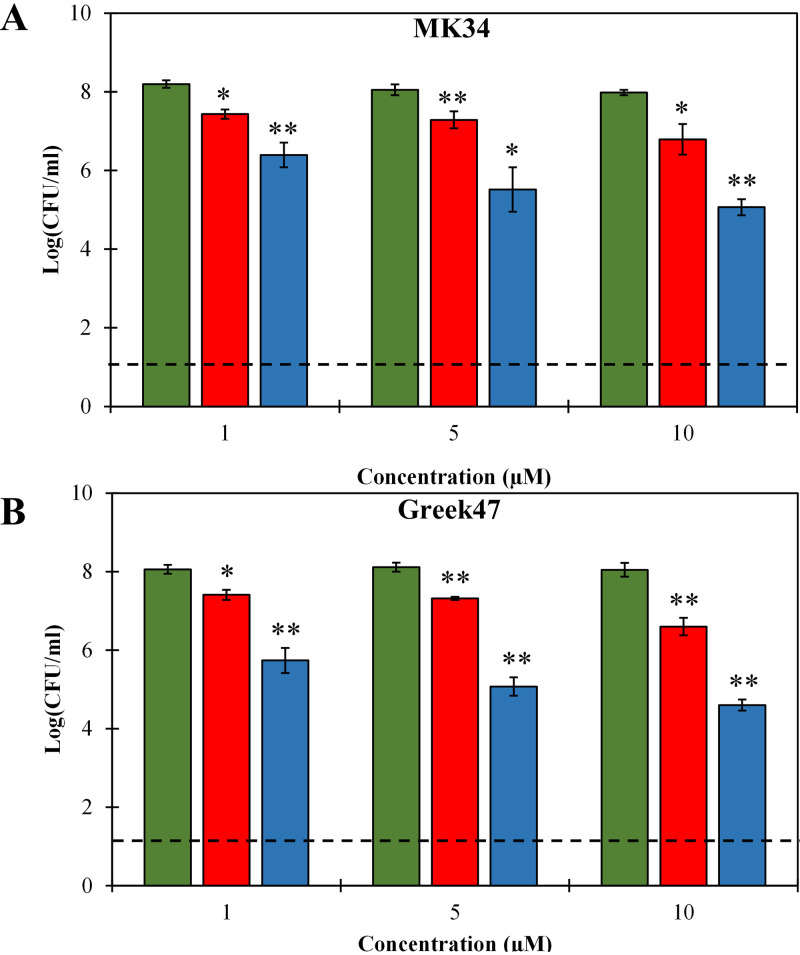
Bacterial cells in the stationary growth phase are known to be less susceptible to antibacterials due to a lowered metabolic activity and outer membrane permeability (23). Therefore, we investigated the antibacterial activities of both lysins against stationary-phase-grown A. baumannii MK34 and Greek47 cells, as representatives of colistin-sensitive and -resistant strains, respectively, with high intracellular osmotic pressure (20 mM HEPES-NaOH, pH 7.4). We used three escalating doses (1, 5, and 10 μM) of both lysins for 2 h. LysMK34 showed moderate antibacterial activity against stationary-phase cells, with maximum activities of 1.19 ± 0.32- and 1.44 ± 0.16-log reductions for MK34 and Greek47, respectively, when 10 μM was used (Fig. 4). Under all conditions, the activity of eLysMK34 was significantly improved 2- to 3-fold. In sum, we conclude that the engineering of LysMK34 further improves its intrinsic activity against stationary-phase cells.
FIG 4.
Dose-dependent antibacterial activity of LysMK34 (red bars) and eLysMK34 (blue bars) against A. baumannii MK34 (A) and A. baumannii Greek47 (B) cells in the stationary growth phase with high intracellular osmotic pressure (suspended in 20 mM HEPES-NaOH, pH 7.4; ∼108 CFU/ml). The cells were exposed to either 1, 5, or 10 μM protein. Green bars show the untreated cells. The numbers of surviving bacteria are expressed as log numbers of CFU per milliliter after 120 min of exposure. Each value represents the mean ± standard deviation from three independent replicates. Asterisks represent statistical differences from values for buffer-treated cells (Student's t test; *, P < 0.05, **; P < 0.01; ***, P < 0.001). The dashed line represents the limit of detection (10 CFU/ml).
eLysMK34 sublethally injures bacterial cells in complement-deactivated human serum.
Human serum has been proven to be a challenging environment that may inhibit the antibacterial activities of lysins (10, 15). In a preliminary experiment, we evaluated the antibacterial activity of eLysMK34 against all four A. baumannii strains in 100% human serum, but the observed log reductions were limited to 0.07 and 0.39 in a strain-dependent way (Fig. S2). Possible explanations for this lack of antibacterial activity are the lowered osmotic pressure inside the bacterial cells in the presence of serum, a direct inhibitory effect of a serum component on the lysin (e.g., a reduction of the kinetic parameters) or physiological changes of the outer membrane of the cells in serum. To analyze a possible effect of the osmotic pressure, cells were washed twice and resuspended in 100% human serum, followed by exposure to eLysMK34. After incubation, we followed two different plating procedures to differentiate between lethally and sublethally injured cells. In the first plating procedure, lysin-exposed cells were diluted in the same human serum; thus, isotonicity was maintained and osmotic shock during dilution prior to plating was avoided. In the alternative procedure, the cells were diluted in a low-tonicity buffer (20 mM HEPES-NaOH, pH 7.4) prior to being plated to induce an osmotic shock that would cause the lysis of cells, which had a partially disintegrated cell wall due to the action eLysMK34. Sublethally injured cells are thus lysed by the osmotic shock. We used 100% complement-deactivated human serum to exclude any interference, since we observed before that intact serum substantially retards the growth of A. baumannii.
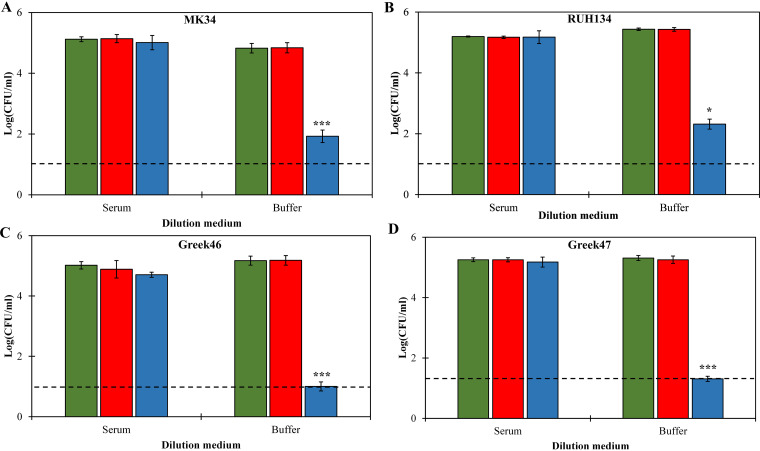
Application of 20 μM LysMK34 or eLysMK34 did not reduce the bacterial count for any of the tested strains when cells were diluted with complement-deactivated human serum, again demonstrating the inhibitory effect of human serum for these lysins (Fig. 5). However, when eLysMK34-treated cells were subjected to an osmotic shock by exposing them to the low-tonicity buffer, a strain-dependent antibacterial activity was observed. In line with the observations described above, the colistin-resistant strains were more sensitive to the latter condition; antibacterial activity was up to 4.2 log units (detection limit), compared to that for colistin-sensitive strains (2.9 ± 0.1 and 3.0 ± 0.02 log units for MK34 and RUH134, respectively). These observations suggest that the engineered lysin partially digested the peptidoglycan layer in complement-deactivated human serum, in contrast to the parental lysin, but fusion with the CecA peptide is not sufficient to induce full lysis under these conditions.
FIG 5.
Antibacterial activity of LysMK34 (red bars) and eLysMK34 (blue bars) against A. baumannii strains MK34 (A), RUH134 (B), Greek46 (C), and Greek47 (D) resuspended in 100% complement-deactivated human serum. The bacterial cells were treated with equimolar concentrations for 2 h. The treated cells were then washed twice and diluted prior to being plated in either complement-deactivated human serum (left) or 20 mM HEPES-NaOH, pH 7.4 (right). Green bars show the untreated cells. Each value represents the mean ± standard deviation from three independent replicates. Asterisks represent statistical differences from values for buffer-treated cells (Student's t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001). The dashed line represents the limit of detection (10 CFU/ml).
eLysMK34 is not cytotoxic against the HaCaT human epithelial cell line.
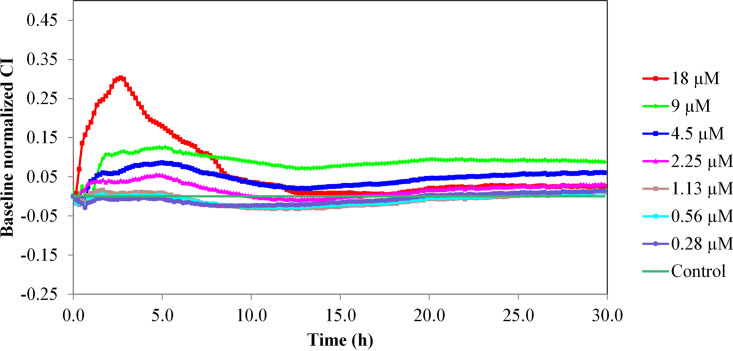
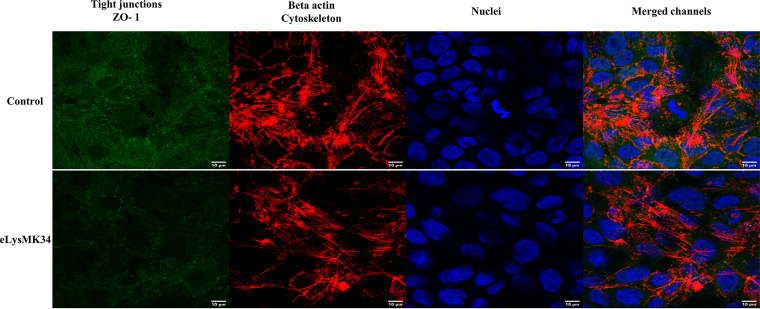
Since one of the main future applications of eLysMK34 may be topical application for removal of A. baumannii from wound and burn wound infections, we tested its cytotoxicity on a monolayer of human keratinocyte cells. The potential cytotoxicity of eLysMK34 was evaluated using the HaCaT human epithelial cell line, which was treated with concentrations of eLysMK34 ranging from 0.28 to 18 μM. Cell behavior was monitored in terms of the normalized cell index (CI); values obtained at all tested concentrations were higher than the baseline (nontreated cells) (Fig. 6), indicating the lack of cytotoxicity of eLysMK34. Notably, a transient increase in the normalized CI was observed at concentrations between 2.25 and 18 μM, suggesting a temporal modification in cellular receptors, such as, among others, G protein-coupled receptors (24). The lack of cytotoxic effects was further confirmed by confocal laser scanning microscopy (CLSM) images obtained after HaCaT cell treatment with the protein for 6 h (Fig. 7), which showed no evident morphological changes from the control. We therefore conclude that eLysMK34 is not cytotoxic toward this human epithelial cell line, even at the highest concentration tested, which corresponds to 15× and 23× the MIC, depending on the strain tested.
FIG 6.
Variation in the normalized cell index (CI) of HaCaT monolayers treated with different concentrations of eLysMK34 (0.28 to 18 μM). eLysMK34 was added to the RTCA wells after the HaCaT epithelial cells had formed a monolayer (reached after 20 h of incubation and indicated by a plateau in the cell index). The time point of eLysMK34 addition corresponds to time point zero. Subsequently, the cell index was monitored for 30 h upon addition of eLysMK34. Normalization of data was performed at 10 min after protein addition, with respect to the CI observed in the control sample (value 0 in the graph). Values represent means ± standard deviations from three replicates.
FIG 7.
Fluorescence images obtained by CLSM of HaCaT cells after 6 h of incubation with eLysMK34 (18 μM). As a control, cells were incubated without protein. The tight junctions, specifically, zonula occludes (ZO-1), were labeled with anti-ZO-1-Alexa Fluor 488 (green). The actin of the cytoskeleton was detected by labeling with Alexa Fluor 568 phalloidin probe (red), and the nuclei were labeled with DAPI (blue). The scale bars measure 10 μm.
DISCUSSION
An increasing number of lysins with intrinsic antibacterial activity has been reported in recent years. In particular, A. baumannii appears to be susceptible to this group of lysins (10–12, 15, 25–31). A C-terminal amphipathic helix was identified as a common feature of these lysins (11, 12, 25, 26). This peptide has been suggested to act in its natural habitat as a spanin in spanin-less phages, disrupting the OM from within during phage-induced cell lysis (32), and has been shown to retain its OM-disrupting activity when acting from without. We previously identified LysMK34, a globular lysin encoded by A. baumannii phage (PMK34) and found similar intrinsic antibacterial characteristics and a C-terminal amphipathic helix. However, upon elaboration, we found that LysMK34’s in vitro antibacterial activity is dependent on experimental conditions, such as the medium used, the tonicity of buffers, the intracellular osmotic pressure, and plating procedures, rendering its antibacterial activity condition dependent, less robust, and difficult to compare to the activities of other reported lysins for which these parameters have not been studied, which has led to researchers unknowingly making skewed interpretations (15). We reasoned that fusion of LysMK34 to a peptide that in its biological role disrupts the OM from without would yield a more robust lysin. The same approach was successfully followed for the construction of Artilysins (17, 21, 33, 34), but here we combine the strengths of a lysin with a particularly high intrinsic activity due to a C-terminal amphipathic helix, with so-called artilysation (35–37), i.e., engineering with an additional peptide to cross the outer membrane.
We found that eLysMK34 exceeds LysMK34 in its antibacterial properties. For instance, LysMK34 has a MIC value of >45.5 μM by the conventional broth microdilution assay, whereas eLysMK34 completely inhibits the growth of all A. baumannii test strains within a concentration range of 0.45 to 1.2 μM. In addition, eLysMK34 kills faster, is less dependent on a high intracellular osmotic pressure, and is more active against stationary-phase cells. As such, the interplay between several factors that contribute to antibacterial activity is uncovered in this work. The mode of action of lysins relies on the combined actions of peptidoglycan degradation and pressure-induced osmotic lysis, the latter being the eventual killing event, as bacterial spheroplasts (and even protoplasts) remain viable and can multiply (38, 39). The additional CecA peptide may enhance the bactericidal activity by transferring more lysin moieties through the OM, resulting in a more weakened peptidoglycan layer across the cell wall and requiring a lower intracellular osmotic pressure to complete cell lysis. Additionally, the possibly remaining antibacterial activity of CecA (membrane depolarization) may contribute to the antibacterial effect but is unknown for this fusion protein.
Our observations can be compared with those of a previous study evaluating the changes in antibacterial activity after fusion of the modular endolysin (OBPgp279) with a truncated CecA peptide at its N terminus. Alone, OBPgp279 shows a modest antibacterial activity of a 1.38-log-unit reduction against A. baumannii in low-tonicity buffer (20 mM Tris-HCl, pH 7.4). An equimolar concentration (100 μg/ml) of the engineered lysin (PlyA) completely eradicates A. baumannii strains, with over 5-log reductions. Nevertheless, no activity was observed against A. baumannii grown in complex media, including lysogeny broth (LB) and MH media (35). Another recent study was conducted to assess the effect of fusing truncated CecA (KWKLFKKI) to both the N and C termini of LysAB2, a lysin with intrinsic antibacterial activity. Interestingly, LysAB2-KWK (C-terminally modified LysAB2) showed a superior activity (a 6-log-unit reduction) compared to that of the N-terminally modified counterpart (a 3-log-unit reduction) using equimolar concentrations (8 μM). Furthermore, LysAB2-KWK showed up to a 105-fold increase in antibacterial activity against A. baumannii in addition to improved activity against A. baumannii in both stationary-phase and biofilm forms compared to that of the native lysin. Moreover, LysAB2-KWK showed serum concentration-dependent activity in serum, with retained activity in up to 4% serum (40). However, these studies did not study in detail the influence of external conditions on LysAB2-KWK’s antibacterial activity or their interplay, as elaborated in this work.
OM permeation is still a limitative factor and appears to be enhanced in colistin-resistant strains. Indeed, in high-tonicity buffer inducing a low intracellular osmotic pressure, 0.5 mM EDTA is required to observe significant killing of colistin-sensitive strains, but not for colistin-resistant strains tested in this work (Fig. 3). A similar observation was previously described for Art-175, which shows an improved activity in terms of its log reduction of colistin-resistant E. coli strains with the mcr-1 mechanism compared to that for colistin-sensitive strains (41). Although the colistin resistance mechanism of Greek46 and Greek47 is not known, the widespread mcr-1 mechanism is based on replacement of the negatively charged phosphate groups of lipid A with phosphoethanolamine, resulting in a less overall negative charge (42). This modification results in the loss of the target site of colistin but may apparently also results in a less effective barrier or overall increased outer membrane permeability for eLysMK34. Other colistin resistance mechanisms (e.g., different routes for lipopolysaccharide [LPS] modification, overexpression of efflux pumps, and overproduction of capsule polysaccharide) have been reported as well (43). Further investigation of the mode of action of LysMK34 and eLysMK34 against a larger set of strains with well-characterized resistance mechanisms may yield refined insights into the mode of action of OM permeation. Notably, the observed synergy between colistin and LysMK34, which is most pronounced for colistin-resistant strains (15), is lost in the case of eLysMK34, suggesting that artilysation has taken over the role of colistin in colistin/LysMK34 mixtures. This is a particularly welcomed feature since colistin is a last-resort antibiotic but may cause nephrotoxicity as an undesired side effect.
Lysins often show limited activity in complex environments, such as serum (10, 28, 35), and also here, we observed that both intact and complement-deactivated human serum is inhibitive of the actions of LysMK34 and eLysMK34. However, the observed differences in killing after application of an osmotic shock suggest more extensive peptidoglycan degradation, and thus better OM penetration, by eLysMK34 than by LysMK34. Under these conditions, sufficient osmotic pressure is still needed to induce osmotic lysis by eLysMK34. We should note that we used complement-deactivated serum in order not to obscure the individual effects of either LysMK34 or eLysMK34. It cannot be excluded that other factors of the immune system (lysozyme, lactoferrin, innate immunity peptides) may affect the susceptibility of bacteria to such lysins in vivo.
Recently, our group created approximately 10,000 modular lysin variants that were screened to find the optimal lysin with antibacterial activity against A. baumannii in human serum. The MIC values of the final lead lysin (1D10) were slightly lower (0.13 to 0.82 μM) than those of eLysMK34 (0.45 to 1.2 μM) (21). These differences may be explained by the presence of an additional peptidoglycan binding domain in lysin 1D10 or the mere fact that the CecA and lysin moieties in lysin 1D10 have a more optimal orientation or positioning. The latter is supported by the fact that many other CecA-lysin fusions from that study show inferior activity compared to that of lysin 1D10. Notably, also the MIC of Art-175, which is based on a modular endolysin fused to the SMAP-29 antibacterial peptide, for A. baumannii ranges from 4 to 20 μg/mL, and Art-175 reduces the cell number of stationary-phase A. baumannii cells by 8 to 9 log units. In addition, Art-175 is active against both A. baumannii and P. aeruginosa strains (17, 20). These differences in activity and specificity highlight again the modularity principle of engineered lysins and the unique possibility of tuning their antibacterial properties. This study demonstrates that at least in the case of LysMK34, peptide engineering is a useful approach to further improve the in vitro antibacterial properties of a lysin with high intrinsic antibacterial activity without introducing cytotoxicity.
Conclusions.
A. baumannii infections are challenging due to a limited number of effective treatments. Therefore, the development of new effective approaches is urgent. Lysins are a clinically advanced route of investigation toward new antibacterial alternatives with a novel mode of action. Whereas Gram-negative bacteria were initially considered out of the scope of this new class of antibacterials, diverse strategies to tackle the outer membrane barrier have been demonstrated in recent years, including artilysation and the use of lysins with intrinsic antibacterial activity. In this study, we have shown that artilysation improves the intrinsic antibacterial activity of LysMK34 and makes it less dependent on experimental conditions and growth phase, with pronounced activity against colistin-resistant strains, showing that both approaches are complementary, at least in the case of LysMK34. Furthermore, eLysMK34 shows improved antibacterial activity in complement-deactivated serum but requires further osmotic perturbation to result in full cell lysis and killing. Steadily improving our insights into how lysins can successfully interfere with and permeate the outer membrane will strengthen the portfolio of lysin-based molecules that kill Gram-negative bacteria.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Five previously characterized A. baumannii strains with different resistance patterns were included in this study (Table 1): the multidrug-resistant epidemiological RUH134 strain (EU clone II) (44), the extensively drug-resistant strain MK34 (15), colistin-resistant strains Greek46 and Greek47 (15), and strain NCTC13423 (44) (Iraqibacter strain, a problematic strain responsible for wound infections among American soldiers in the Iraqi war of 2003). Furthermore, highly virulent P. aeruginosa PA14 (45), multidrug-resistant P. aeruginosa Br667 (45), and E. coli ATCC 25922 were included as well for the determination of the MIC of the engineered lysin. E. coli TOP10 and E. coli BL21(DE3)-RIL (Agilent Technologies, Belgium) were used for plasmid storage and protein expression, respectively.
All strains were subcultured from their respective glycerol stocks in lysogeny broth (LB; 1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37°C while shaking (200 rpm) or on LB agar plates (LB supplemented with 2% agar). For selection of E. coli strains transformed with the cloned plasmids, LB agar plates were supplemented with 5% sucrose in addition to 100 μg/mL ampicillin, or with kanamycin (50 μg/mL) and chloramphenicol (25 μg/mL) for E. coli TOP10 and E. coli BL21(DE3)-RIL, respectively.
Creation of building modules and the chimeric lysin eLysMK34.
In this study, we assembled the full coding sequence for the chimeric protein (referred to as eLysMK34) using the VersaTile DNA assembly technique as described previously (21). The engineered lysin is composed of the following three building modules (tiles) from the N to the C terminus: an amphipathic outer membrane-permeabilizing peptide, CecA, from Aedes aegypti (CecA; NCBI accession no. AF117886.1), a flexible linker of three Ala-Gly repeats (21), and LysMK34 (NCBI accession no. QGF20176.1) derived from A. baumannii phage vB_AbaP_PMK34 (15). The respective coding sequences were converted to tiles by flanking the amplicons (CecA/LysMK34) or primer cassette (linker) with tail sequences, including a position tag, a BsaI recognition site (for assembly), and a SapI restriction and recognition site (for cloning in the entry vector) in the outward orientation. The position tag determines the position of the respective tile in the final assembled sequence: CecA in position 1, the flexible linker in position 2, and LysMK34 in position 3. Tiles for CecA and the flexible linker integrated in the entry vector pVTE were utilized from our previously prepared repository (21). The tile for LysMK34 at position 3 was prepared in this work using the primers 5′-TATGCTCTTCTAGAGGTCTCGCAGGCATTCTGACTAAAGACGGGTTTAG-3′ (forward) and 5′-TATGCTCTTCACTTGGTCTCATACTTTAAACTCCGTAGAGCGCG-3′ (reverse), with a position tag (italic), a BsaI recognition site (underlined), and a SapI recognition site (bold). The amplicon was made using Phusion high-fidelity DNA polymerase (Thermo Fischer Scientific, Belgium) with the A. baumannii vB_AbaP_PMK34 phage genome (NCBI accession no. MN433707.1) as the template. Then, the amplicon was integrated into the entry vector pVTE using repetitive restriction/ligation cycles with SapI (ThermoFischer Scientific, Belgium) and T4 DNA ligase (ThermoFisher Scientific, Belgium) by following conditions described before (21). Afterwards, chemocompetent E. coli TOP10 cells were transformed with pVTE/LysMK34. A sequence-verified plasmid was used for an assembly reaction as described before (21). Briefly, 1 μL of each tile (DNA concentration, 50 ng/μL), 1 μL of 100 ng/μL pVTD3, 1 μL BsaI (10 U/μL), 3 μL T4 DNA ligase (1 U/μL), and 2 μL T4 DNA ligation buffer were mixed. The reaction volume was supplemented to 20 μL using ultrapure water. Finally, the assembly mixture was subjected to 50 cycles of 37°C for 2 min and 16°C for 3 min, followed by 5 min at 50°C and finally 5 min at 80°C. E. coli BL21(DE3)-RIL was transformed with 5 μL of this reaction mixture, and a correct clone was verified using Sanger sequencing (LGC Genomics, Germany).
Protein expression and purification of LysMK34 and eLysMK34.
Overexpression of LysMK34 and eLysMK34 was carried out as described earlier (15). Briefly, 2 to 3 colonies were picked from an overnight culture plated over LB agar containing 50 μg/mL kanamycin and 25 μg/mL chloramphenicol, transferred to LB broth supplemented with 50 μg/mL kanamycin and 25 μg/mL chloramphenicol, and incubated for 18 h at 37°C. This culture was then transferred to 1 L LB broth supplemented with 50 μg/mL kanamycin and incubated until we obtained an optical density (OD) of 0.5 to 0.6 at 37°C with shaking (200 rpm). Then, IPTG (isopropyl-β-d-thiogalactopyranoside; final concentration of 0.5 mM) was added, followed by incubation at 16°C (for eLysMK34) or 30°C (for LysMK34) for 18 h with 200-rpm shaking. Next, the pelleted cells were suspended in 20 mL lysis buffer (20 mM NaH2PO4/Na2HPO4, 0.5 M NaCl, 50 mM imidazole, pH 7.4) and disrupted by three freeze-thawing steps and sonication (Q125; Qsonica, USA). The soluble fraction was collected by centrifugation (16,000 × g for 30 min at 4°C) and filtered through a 0.22-μm-pore-size filter (polyethersulfone; Novolab, Belgium). Purification of overexpressed C-terminally 6×His-tagged protein was achieved by immobilized metal affinity chromatography (IMAC) with His GraviTrap columns (GE Healthcare, Belgium) according to the manufacturer’s instructions. Dialysis was performed using 3,500-molecular-weight-cutoff (MWCO) Slide-A-Lyzer MINI dialysis devices (ThermoFisher Scientific, Belgium) against 20 mM HEPES-NaOH, 500 mM NaCl, pH 7.4, or 20 mM HEPES-NaOH, 150 mM NaCl, pH 7.4, for eLysMK34 or LysMK34, respectively. Finally, protein purity was examined by SDS-PAGE. Protein concentration was estimated using a DS-11 spectrophotometer (DeNovix Inc., USA) using an extinction coefficient of 33,350 M−1 cm−1 and molecular masses of 22.1 and 26.4 kDa for LysMK34 and eLysMK34, respectively.
MIC assay and synergy analysis.
The MIC was determined for eLysMK34 according to the broth microdilution assay in MH broth (44). eLysMK34 was prepared in MH broth with a final concentration range between 0 and 40 μg/mL, with 4-μg/mL increments, and dispensed in a 96-well microtiter plate. Afterwards, test strains in the exponential growth phase (106 CFU/mL) were added, followed by incubation at 37°C for 18 h without shaking. Different A. baumannii (NCTC13423, RUH134, MK34, Greek46, and Greek47), P. aeruginosa (PA14 and Br667), and E. coli (ATCC 25922 and BL21) strains were included. The MIC values were also used to evaluate a potential synergy between eLysMK34 and polymyxin E (colistin; ThermoFisher Scientific) against A. baumannii (RUH134, MK34, Greek46, and Greek47) using the checkerboard approach (46), with a concentration range from 1/64 to 2× the MIC.
Time-kill assay.
A. baumannii strains (MK34, RUH134, Greek46, and Greek47) were grown in LB broth to the mid-log growth phase (OD at 600 nm [OD600] = 0.6). Subsequently, they were washed twice and diluted 1:100 in 20 mM HEPES-NaOH, pH 7.4, buffer. Then, 100 μL of this culture was mixed with an equal volume of equimolar concentrations of either LysMK34 or eLysMK34 (both after a buffer change to 20 mM HEPES-NaOH, pH 7.4) to obtain a final concentration of 1 μM. As a control, the same cell suspension was mixed with 100 μL of 20 mM HEPES-NaOH, pH 7.4, buffer instead of the tested lysin. The mixtures were then incubated at room temperature for 30, 60, and 120 min. At each time point, a sample of 50 μL was diluted in 20 mM HEPES-NaOH, pH 7.4, buffer, plated on LB agar plates, and incubated at 37°C for 18 h. The antibacterial activity was quantified according to the formula log10[N0/Ni], with N0 as the initial number of buffer-treated cells and Ni as the number of cells counted after lysin treatment.
In vitro antibacterial assay of LysMK34 and eLysMK34 under different conditions.
To evaluate the antibacterial activities of both lysins in high-tonicity buffer, cells grown in LB to the exponential growth phase were washed twice and then resuspended using 20 mM HEPES, 150 mM NaCl, pH 7.4. The prepared cells were then 100-fold diluted in the same buffer and mixed with each lysin (eLysMK34 was dialyzed against the same buffer) to obtain final concentrations of 1 μM. Controls were conducted using 100 μL 20 mM HEPES, 150 mM NaCl, pH 7.4, in some cases supplemented with EDTA (final concentration of 0.5 mM) instead of the tested lysin. For colistin-sensitive strains (MK34 and RUH134), we further tested the antibacterial activity with increased concentrations of both lysins up to 10 μM. The mixture was then incubated at room temperature for 30, 60, and 120 min, diluted in the same buffer, plated, and incubated for 18 h at 37°C for bacterial counts.
Similarly, we evaluated the antibacterial activities of both lysins against A. baumannii MK34 and Greek47 cells that had been grown for18 h (to stationary phase, using a final cell density of 108 CFU/mL) as representatives for colistin-sensitive and -resistant strains, respectively. The buffer used was 20 mM HEPES-NaOH, pH 7.4, and lysin concentrations were adjusted to 1, 5, and 10 μM. Parallel controls were performed by adding 20 mM HEPES-NaOH, pH 7.4, to the cells to rule out a possible effect of the buffer on bacterial cell survival. Finally, we assessed the antibacterial activities of both lysins in human serum. For this, we started with a preliminary experiment to test the antibacterial activity of eLysMK34 in 100% human serum (MP Biomedicals, Belgium) as described before (21). Exponentially growing A. baumannii strains (MK34, RUH134, Greek46, and Greek47) in LB were pelleted, washed twice, and then 100-fold diluted in 100% human serum. Next, 100 μl cells was mixed with 100 μl of eLysMK34 (diluted in 20 mM HEPES-NaOH, 150 mM NaCl, pH 7.4) to obtain a final concentration of 20× the MIC (15 to 24 μM) in 50% serum. The mixture was incubated for 120 min at 37°C. The treated cells were then diluted in either 20 mM HEPES-NaOH, pH 7.4, to induce a hypotonic shock or intact human serum prior to being plated. The plates were incubated for 18 h at 37°C before the cell counting. To exclude any possible inference of serum antibacterial proteins with the overall result of the assay, we repeated the experiment using the complement-deactivated human serum (56°C, 15 min) and evaluated the antibacterial activities of 20 μM concentrations of both LysMK34 and eLysMK34.
HaCaT epithelial cell line and culture conditions.
HaCaT cells were cultured in DMEM containing heat-inactivated fetal bovine serum (FBS) (10%, vol/vol), 4.5 g/liter glucose, 2 mM l-glutamine, and a mixture of antibiotics (50 μg/mL of penicillin, streptomycin, and gentamicin) and a fungicide (1.25 μg/mL amphotericin B) at 37°C in a 5% CO2 atmosphere in a CO2-Series Shel-Lab incubator (Sheldon Manufacturing Inc., OR, USA). All media and reagents were purchased from Sigma-Aldrich. Maintenance of the cell line was performed two times per week. When cells reached approximately 90% confluence, they were harvested with TrypLE Express solution and were used to carry out the further assays.
Cytotoxicity analysis.
The potential cytotoxicity of eLysMK34 against epithelial cells (a monolayer of human skin keratinocytes, HaCaT) was analyzed using a real-time cell analyzer (RTCA) on the basis of impedance measurements, revealing major cytotoxic events, such as cell lysis, detachment, rounding up, variations in cell morphology, and receptor activity (47). The procedure followed was identical to that of the cytotoxicity analysis of LysMK34 reported before (15). Briefly, RTCA xCELLigence equipment (ACEA Bioscience Inc., San Diego, CA; currently belonging to Agilent) was placed into a Heracell-240 incubator (Thermo Electron LDD GmbH, Langenselbold, Germany) at 37°C and a 5% CO2 atmosphere. A HaCaT cell suspension up to 105 cells/mL was grown in DMEM (ThermoFisher Scientific, Madrid, Spain), and 100 μL was added to each well of 16-well E plates. The plates were then connected to the equipment and incubated at 37°C with 5% CO2 to record the cell index (CI; impedance measurement) every 10 min. When the monolayer of HaCaT cells was formed, indicated by a plateau of the cell index (after 20 h of incubation), different concentrations (2-fold dilutions) of eLysMK34 ranging from 0.28 to 18 μM were added. Every condition was tested in triplicate. As a control, 100 μL of DMEM was used. The recording of the CI was carried out for an extra 30 h in order to determine HaCaT changes in short- and long-term exposure. Data normalization was performed with the RTCA software as previously described (45). First, normalization of the CI was obtained by dividing the CI of a measurement by the CI at a specific time (in this case, 10 min after the addition of the protein). Then, all data were referred to the baseline CI, which is the result of subtracting the normalized CI of each well to the normalized CI of the control. With these settings, the baseline of the control is 0. The cytotoxicity of a protein is described as the decrease below the control in CI after normalization.
Additionally, to confirm the lack of toxicity observed in the previous experiment, HaCaT monolayers were subjected to treatment with the maximum concentration of eLysMK34 (18 μM) and were analyzed by confocal laser scanning microscopy (CLSM) after 6 h of exposure, as described before with some modifications (47). To achieve this, 300 μL of HaCaT cell suspension (105 cells/mL) in DMEM was added to the wells of an 8-well μ-Slide chamber (IbiTreat; Ibidi GmbH, Gräfelfing, Germany). Plates were then incubated at 37°C with 5% CO2 for 20 h. After this, medium was removed, and the same volume of DMEM with the diluted protein was poured into the well. Experiments were performed in duplicate. As a control, 300 μL DMEM was added. After 6 h, the liquid was removed, cell monolayers were fixed with 4% paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 10 min, and then washed with PBS, permeabilized for 5 min with 0.3% Triton X-100 (Sigma-Aldrich) in PBS, and blocked for unspecific binding with 2% bovine serum albumin (BSA; Fisher BioReagents) in PBS for 30 min. For staining, cells were incubated with anti-ZO-1-Alexa Fluor 488 (Invitrogen, ThermoFisher Scientific) and phalloidin-Alexa Fluor 568 (Invitrogen) in 0.5% BSA buffer for 1 h at room temperature. After the cells were washed, a secondary antibody was used to amplify the signal from the ZO-1 primary antibody using goat anti-mouse-fluorescein isothiocyanate (FITC; Invitrogen) at a 1:400 dilution factor in 0.5% BSA buffer for an additional 1 h at room temperature. DAPI (4′,6-diamidino-2-phenylindole; Sigma-Aldrich) was used for nucleic acid staining at 1 μM for 5 min. Finally, images were acquired using a Leica DMi4 confocal microscope using a 63×/1.4-numerical aperture (NA) oil objective and Leica LAS X image software version 3.0.11, with the excitation lasers and emission filters specified for the fluorescent components in the suppliers’ instructions. ImageJ version 1.52 was used for image analysis.
Statistical analysis.
SPSS Statistics for Windows v. 22.0 (IBM Corp.) was used for all calculations. Student's t test was used to compare the differences between the treated and untreated bacterial cultures at a level of significance of <0.05 (unless otherwise stated).
ACKNOWLEDGMENTS
K.A. was funded by the Missions Sector, Ministry of Higher Education, Egypt. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. D.G. is supported by the Research Foundation-Flanders (FWO) under grant G066919N. Y.B. is a coinventor of patents related to the Artilysin field.
We thank S. Malhotra-Kumar (Laboratory of Medical Microbiology, Vaccine & Infectious Disease, University of Antwerp, Antwerp, Belgium) for providing the colistin-resistant A. baumannii strains (Greek46 and Greek47) and J. P. Pirnay (Laboratory for Molecular and Cellular Technology, Queen Astrid Hospital, Neder-over-Heembeek, Belgium) for providing A. baumannii RUH134 and P. aeruginosa PA14 and Br667.
Footnotes
Supplemental material is available online only.
Contributor Information
Yves Briers, Email: yves.briers@ugent.be.
Charles M. Dozois, INRS–Institut Armand-Frappier
REFERENCES
- 1.World Health Organization. 2019. Antibacterial agents in preclinical development: an open access database. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub M, Hammoudi H. 2020. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9:119. 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirel L, Bonnin RA, Nordmann P. 2011. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life 63:1061–1067. 10.1002/iub.532. [DOI] [PubMed] [Google Scholar]
- 5.Nowak J, Zander E, Stefanik D, Higgins PG, Roca I, Vila J, McConnell MJ, Cisneros JM, Seifert H, MagicBullet Working Group WP4 . 2017. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother 72:3277–3282. 10.1093/jac/dkx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohd Sazlly Lim S, Zainal Abidin A, Liew SM, Roberts JA, Sime FB. 2019. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: a systematic review and meta-analysis. J Infect 79:593–600. 10.1016/j.jinf.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Abdelkader K, Gerstmans H, Saafan A, Dishisha T, Briers Y. 2019. The preclinical and clinical progress of bacteriophages and their lytic enzymes: the parts are easier than the whole. Viruses 11:96. 10.3390/v11020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dams D, Briers Y. 2019. Enzybiotics: enzyme-based antibacterials as therapeutics, p 233–253. In Labrou N (ed), Therapeutic enzymes: function and clinical implications. Springer, Singapore, Singapore. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez D, Briers Y. 2021. Lysins breaking down the walls of Gram-negative bacteria, no longer a no-go. Curr Opin Biotechnol 68:15–22. 10.1016/j.copbio.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Larpin Y, Oechslin F, Moreillon P, Resch G, Entenza JM, Mancini S. 2018. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria. PLoS One 13:e0192507. 10.1371/journal.pone.0192507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Schuch R, Fischetti VA. 2015. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991. 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonova N, Vasina D, Lendel A, Usachev E, Makarov V, Gintsburg A, Tkachuk A, Gushchin V. 2019. Broad bactericidal activity of the Myoviridae bacteriophage lysins LysAm24, LysECD7, and LysSi3 against Gram-negative ESKAPE pathogens. Viruses 11:284. 10.3390/v11030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thummeepak R, Kitti T, Kunthalert D, Sitthisak S. 2016. Enhanced antibacterial activity of Acinetobacter baumannii bacteriophage ØABP-01 endolysin (LysABP-01) in combination with colistin. Front Microbiol 7:1402. 10.3389/fmicb.2016.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotka M, Kapusta M, Dorawa S, Kaczorowska A-K, Kaczorowski T. 2019. Ts2631 endolysin from the extremophilic Thermus scotoductus bacteriophage vB_Tsc2631 as an antimicrobial agent against Gram-negative multidrug-resistant bacteria. Viruses 11:657. 10.3390/v11070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelkader K, Gutiérrez D, Grimon D, Ruas-Madiedo P, Lood C, Lavigne R, Safaan A, Khairalla AS, Gaber Y, Dishisha T, Briers Y. 2020. Lysin LysMK34 of Acinetobacter baumannii bacteriophage PMK34 has a turgor pressure-dependent intrinsic antibacterial activity and reverts colistin resistance. Appl Environ Microbiol 86:e01311-20. 10.1128/AEM.01311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerstmans H, Criel B, Briers Y. 2018. Synthetic biology of modular endolysins. Biotechnol Adv 36:624–640. 10.1016/j.biotechadv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Defraine V, Schuermans J, Grymonprez B, Govers SK, Aertsen A, Fauvart M, Michiels J, Lavigne R, Briers Y. 2016. Efficacy of Artilysin Art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob Agents Chemother 60:3480–3488. 10.1128/AAC.00285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heselpoth RD, Euler CW, Schuch R, Fischetti VA. 2019. Lysocins: bioengineered antimicrobials that deliver lysins across the outer membrane of Gram-negative bacteria. Antimicrob Agents Chemother 63:e00342-19. 10.1128/AAC.00342-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zampara A, Sørensen MCH, Grimon D, Antenucci F, Vitt AR, Bortolaia V, Briers Y, Brøndsted L. 2020. Exploiting phage receptor binding proteins to enable endolysins to kill Gram-negative bacteria. Sci Rep 10:12087. 10.1038/s41598-020-68983-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briers Y, Walmagh M, Grymonprez B, Biebl M, Pirnay J-P, Defraine V, Michiels J, Cenens W, Aertsen A, Miller S, Lavigne R. 2014. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:3774–3784. 10.1128/AAC.02668-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstmans H, Grimon D, Gutiérrez D, Lood C, Rodríguez A, van Noort V, Lammertyn J, Lavigne R, Briers Y. 2020. A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci Adv 6:eaaz1136. 10.1126/sciadv.aaz1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock REW, Chapple DS. 1999. Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323. 10.1128/AAC.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell AM, Wang W, Silhavy TJ. 2017. Novel RpoS-dependent mechanisms strengthen the envelope permeability barrier during stationary phase. J Bacteriol 199:e00708-16. 10.1128/JB.00708-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stallaert W, Dorn JF, van der Westhuizen E, Audet M, Bouvier M. 2012. Impedance responses reveal β2-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PLoS One 7:e29420. 10.1371/journal.pone.0029420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira H, Vilas BD, Mesnage S, Kluskens LD, Lavigne R, Sillankorva S, Secundo F, Azeredo J. 2016. Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-Gram-negative bacterial activity. Front Microbiol 7:208. 10.3389/fmicb.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai M-J, Lin N-T, Hu A, Soo P-C, Chen L-K, Chen L-H, Chang K-C. 2011. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both Gram-positive and Gram-negative bacteria. Appl Microbiol Biotechnol 90:529–539. 10.1007/s00253-011-3104-y. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Lavigne R, Neves-Petersen MT, Kluskens LD, Azeredo J. 2014. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against Gram-negative pathogens in presence of weak acids. PLoS One 9:e108376. 10.1371/journal.pone.0108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz A, Serrano A, Hernandez A, Euler CW, Fischetti VA. 2019. Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection. Antimicrob Agents Chemother 63:e00024-19. 10.1128/AAC.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M, Hu K, Xie Y, Liu Y, Mu D, Guo H, Zhang Z, Zhang Y, Chang D, Shi Y. 2018. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front Microbiol 9:3302. 10.3389/fmicb.2018.03302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Lee D-W, Jin J-S, Kim J. 2020. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J Glob Antimicrob Resist 22:32–39. 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Yuan Y, Li X, Wang L, Li G, Cong C, Li R, Cui H, Murtaza B, Xu Y. 2020. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb Biotechnol 14:403–418. 10.1111/1751-7915.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kongari R, Rajaure M, Cahill J, Rasche E, Mijalis E, Berry J, Young R. 2018. Phage spanins: diversity, topological dynamics and gene convergence. BMC Bioinformatics 19:326. 10.1186/s12859-018-2342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerstmans H, Rodríguez-Rubio L, Lavigne R, Briers Y. 2016. From endolysins to Artilysin®s: novel enzyme-based approaches to kill drug-resistant bacteria. Biochem Soc Trans 44:123–128. 10.1042/BST20150192. [DOI] [PubMed] [Google Scholar]
- 34.Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, Oliveira H, Azeredo J, Verween G, Pirnay J-P, Miller S, Volckaert G, Lavigne R. 2014. Engineered endolysin-based “Artilysins” to combat multidrug-resistant Gram-negative pathogens. mBio 5:e01379-14. 10.1128/mBio.01379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Wang M, Yu J, Wei H. 2015. Antibacterial activity of a novel peptide-modified lysin against Acinetobacter baumannii and Pseudomonas aeruginosa. Front Microbiol 6:1471. 10.3389/fmicb.2015.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez-Rubio L, Chang W-L, Gutiérrez D, Lavigne R, Martínez B, Rodríguez A, Govers SK, Aertsen A, Hirl C, Biebl M, Briers Y, García P. 2016. ‘Artilysation’ of endolysin λSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci Rep 6:35382. 10.1038/srep35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonova NP, Vasina DV, Rubalsky EO, Fursov MV, Savinova AS, Grigoriev IV, Usachev EV, Shevlyagina NV, Zhukhovitsky VG, Balabanyan VU, Potapov VD, Aleshkin AV, Makarov VV, Yudin SM, Gintsburg AL, Tkachuk AP, Gushchin VA. 2020. Modulation of endolysin LysECD7 bactericidal activity by different peptide tag fusion. Biomolecules 10:440. 10.3390/biom10030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross T, Ransegnola B, Shin J-H, Weaver A, Fauntleroy K, VanNieuwenhze MS, Westblade LF, Dörr T. 2019. Spheroplast-mediated carbapenem tolerance in Gram-negative pathogens. Antimicrob Agents Chemother 63:e00756-19. 10.1128/AAC.00756-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott T, Lambert P. 2001. Cell-wall-deficient bacteria. Lancet 357:1885. 10.1016/S0140-6736(00)04991-6. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Liu M, Zhang P, Leung SSY, Xia J. 2021. Membrane-permeable antibacterial enzyme against multidrug-resistant Acinetobacter baumannii. ACS Infect Dis 7:2192–2204. 10.1021/acsinfecdis.1c00222. [DOI] [PubMed] [Google Scholar]
- 41.Schirmeier E, Zimmermann P, Hofmann V, Biebl M, Gerstmans H, Maervoet VET, Briers Y. 2018. Inhibitory and bactericidal effect of Artilysin Art-175 against colistin-resistant mcr-1-positive Escherichia coli isolates. Int J Antimicrob Agents 51:528–529. 10.1016/j.ijantimicag.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aghapour Z, Gholizadeh P, Ganbarov K, Bialvaei AZ, Mahmood SS, Tanomand A, Yousefi M, Asgharzadeh M, Yousefi B, Samadi Kafil H. 2019. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect Drug Resist 12:965–975. 10.2147/IDR.S199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Vos D, Pirnay J-P, Bilocq F, Jennes S, Verbeken G, Rose T, Keersebilck E, Bosmans P, Pieters T, Hing M, Heuninckx W, De Pauw F, Soentjens P, Merabishvili M, Deschaght P, Vaneechoutte M, Bogaerts P, Glupczynski Y, Pot B, van der Reijden TJ, Dijkshoorn L. 2016. Molecular epidemiology and clinical impact of Acinetobacter calcoaceticus-baumannii complex in a Belgian burn wound center. PLoS One 11:e0156237. 10.1371/journal.pone.0156237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirnay J-P, De Vos D, Cochez C, Bilocq F, Pirson J, Struelens M, Duinslaeger L, Cornelis P, Zizi M, Vanderkelen A. 2003. Molecular epidemiology of Pseudomonas aeruginosa colonization in a burn unit: persistence of a multidrug-resistant clone and a silver sulfadiazine-resistant clone. J Clin Microbiol 41:1192–1202. 10.1128/JCM.41.3.1192-1202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat J-M, Pancholi P. 2010. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:4678–4683. 10.1128/AAC.00497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valdés-Varela L, Alonso-Guervos M, García-Suárez O, Gueimonde M, Ruas-Madiedo P. 2016. Screening of Bifidobacteria and Lactobacilli able to antagonize the cytotoxic effect of Clostridium difficile upon intestinal epithelial HT29 monolayer. Front Microbiol 7:577. 10.3389/fmicb.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial disk susceptibility tests; approved standard—9th edition. CLSI M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2. Download AEM.01515-21-s0001.pdf, PDF file, 0.1 MB (109.3KB, pdf)