TO THE EDITOR:
The role of T cells in the pathogenesis of acute coronavirus disease-19 (COVID-19) and the subsequent establishment of short-term and long-term immune response to the natural infection and to vaccination is under intense investigation.1-3 It is generally agreed that a coordinated humoral and cellular immune response plays a key role in protection from disease.4-7 In this context, we examined the study by Dhakal et al,8 which describes the immune response to COVID-19 vaccinations in patients who have received an allogeneic hematopoietic cell transplantation or chimeric antigen receptor T-cell therapy (CART). Their results are concordant with those of the studies by Ram et al9 and Parvathaneni et al.10 They found that the humoral response to vaccination was obtunded in CART patients with ongoing B-cell aplasia, although Dhakal et al did not have information on B-cell counts or nor did they measure T-cell responses, whereas Ram et al and Parvathaneni et al found that some CART patients demonstrated a good in vitro T-cell response in the absence of a response to serum antibodies. We report a case that further highlights the crucial role of the T-cell response in controlling severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with potential implications for how immune responses are best monitored in the CART patient group.
A 16-year-old male with Down syndrome–related CD19+ acute lymphoblastic leukemia (ALL) received tisagenlecleucel CART therapy on the CASSIOPEIA trial (this trial was registered at www.clinicaltrials.gov as #NCT03876769 [CCTL019G2201J]) for persistent flow cytometric minimal residual disease of 0.2% after induction and consolidation therapy according to UKALL 2019 guidelines. He had several preexisting conditions: epilepsy, high body mass index, obstructive sleep apnea, recurrent folliculitis, and parathyroid adenoma with mild hypercalcemia. He had several complications associated with ALL therapy, including non–insulin-dependent diabetes requiring metformin and neuromyopathy related to treatment with dexamethasone and vincristine, which had resolved or were improving before CART therapy. His immediate course of treatment after CART therapy was uncomplicated apart from prolonged neutropenia and thrombocytopenia, and at 12 months, he was in flow cytometric minimal residual disease–negative remission with ongoing B-cell aplasia. He had started monthly intravenous immunoglobulin (Ig) infusions at 3 months after CART therapy and was maintaining pre-infusion IgG levels of >4 g/L.
Seven months after receiving CART therapy, he presented to his local hospital with fever, cough, tachypnea, and a requirement for oxygen (Table 1). He was found to be positive for SARS-CoV-2 by polymerase chain reaction (PCR), and computed tomography of his chest showed changes consistent with COVID-19 pneumonitis. He required escalation of therapy to receive continuous positive airway pressure support in the intensive care unit. After discussion among multidisciplinary caregivers, he received 5 days of intravenous (IV) remdesivir and 10 days of IV dexamethasone. He had 2 more episodes over the next 3 months that also required brief admissions to the intensive care unit, with superadded bacterial and Aspergillus infections associated with the third visit. For the second admission, he received the same treatment as during the first, but given his immunocompromised state and the recurrence of symptoms, tocilizumab (a single dose of 8 mg/kg) was added to control the inflammatory component present at the second visit along with 500 mg of nitazoxanide twice per day. For the third admission, he received an additional 5 days of IV remdesivir, and additional antibiotics and antifungals to treat the superadded infections. Forty days from the third episode, he had no respiratory symptoms and was negative for SARS-CoV-2 via PCR testing on 3 consecutive once-per-week throat and stool samples.
Table 1.
Timeline of events and laboratory investigations after CART therapy
| Days after COVID infection | B-cell or lymphocyte count | Length of hospital stay with COVID pneumonitis (d) | Treatment received for COVID pneumonitis | SARS-CoV-2 IgG status via PCR assay | T-cell response assay |
|---|---|---|---|---|---|
| –200 | Complete B-cell aplasia, measured at days –200, –46, +17, +52, and +108 from first admission with COVID pneumonitis. Lymphocyte count <1 × 109/L at all of these time points | ||||
| 0 | 10 | Remdesivir (5 d) plus dexamethasone (10 d) | PCR-positive | ||
| 17 | 19 | Remdesivir (5 d) plus dexamethasone (10 d) plus tocilizumab (1 dose) plus nitazoxanide | PCR-positive | ||
| 70 | 16 | Remdesivir (5 d) plus meropenem (Klebsiella in bronchoalveolar lavage) plus amphotericin B/voriconazole (increased galactomannan) | PCR-positive | ||
| 110 | PCR-negative in stool and nasopharyngeal aspirate | ||||
| 128 | Persistent B-cell aplasia, lymphocyte count 1.38 × 109/L | SARS-CoV-2 IgG negative in serum | Good proliferative response to spike, nucleocapsid, and membrane proteins |
PCR, polymerase chain reaction.
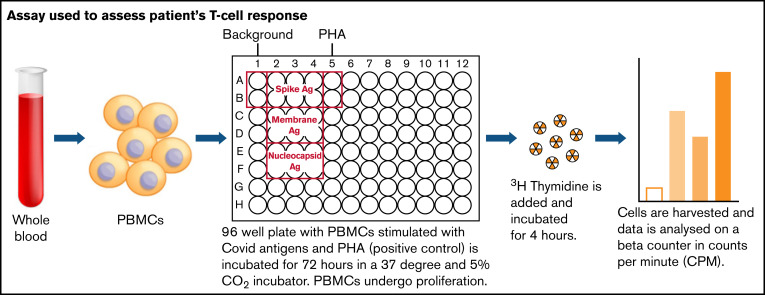
Because there was no measurable SARS-CoV-2–specific anti-spike antibody in his serum, we investigated whether he had mounted a T-cell response to the virus. Separated peripheral blood mononuclear cells from the patient were isolated from lithium heparin blood and then assayed as shown in Figure 1. The cells were cultured for 72 hours with media alone, with the addition of phytohemagglutinin as a positive control, or in the presence of peptides to SARS-CoV-2 spike, nucleocapsid, and membrane proteins. Cells were then pulsed with tritiated thymidine, and incorporation of tritium into proliferating cells was measured by scintillation counting. The results are the means of duplicate tests; proliferation to spike antigen was 7857 counts per minute (cpm), to membrane antigen was 5876 cpm, and to nucleocapsid antigen was 7119 cpm. The mean proliferative response in 4 health care workers, after symptomatic SARS-CoV-2 PCR-positive infection were 4906 cpm to spike antigen, 7260 cpm to membrane antigen, and 5551 cpm to nucleocapsid antigen. We showed that he had proliferative responses to spike, nucleocapsid, and membrane proteins in the assay that are comparable to those of healthy controls with no known immunologic dysfunction. The batch of Ig infusion he was receiving for replacement therapy tested negative for SARS-CoV-2 spike and nucleocapsid-specific antibody.
Figure 1.
Assay used to assess patient’s T-cell response. Ag, antigen; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin.
To our knowledge, this is the first report of a CART patient who cleared SARS-CoV-2 despite ongoing severe B-cell aplasia and lack of a humoral response. We did not have samples to test T-cell responses at earlier time points, so it is possible that viral clearance may have been in part achieved by the antiviral drugs he received for the last episode of pneumonitis.11 However, the presence of strong T-cell responses to all 3 viral proteins in the in vitro assay coinciding with in vivo viral clearance provides strong circumstantial evidence that cell-mediated immunity, along with innate immunity, was the primary mechanism for viral control. Antibody testing does not provide a complete measure of immunity to SARS-CoV-2 infection (and, by extrapolation, vaccination) in patients incapable of mounting a humoral response. Studies in this context must include T-cell responses to determine the presence or absence of immunity. Development of well-validated clinical assays for assessing in vitro T-cell immunity to SARS-CoV-2 will be of use in assessing immunity to SARS-CoV-2 in immunocompromised patients.
Recent literature9,10 has shown that patients with complete B-cell aplasia can mount T-cell responses to messenger RNA (mRNA) vaccines, suggesting that T-cell–driven immunity is possible in this patient group. Our case study supports the fact that T-cell responses to SARS-CoV-2 infection and vaccination are central in patients with B-cell aplasia and also suggests that T-cell responses can lead to viral clearance. Whether T-cell responses triggered by mRNA vaccines are functionally equivalent to those resulting from natural infection and in vitro correlates of protection or viral clearance warrants further investigation.
Contribution: A.H. and A.V. conceived of the study and wrote the first draft; K.G., A.A., and F.T. performed the T-cell assays; and K.G., A.A., F.T., L.W., J.C., A.B., C.R., C.D., and O.M. reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ajay Vora, Great Ormond Street Hospital, Great Ormond St, London WC1N 3JH, United Kingdom; e-mail: ajay.vora@gosh.nhs.uk.
References
- 1.Shrotri M, van Schalkwyk MCI, Post N, et al. T cell response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2021;16(1):e0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457-462. [DOI] [PubMed] [Google Scholar]
- 3.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoletti A, Le Bert N, Qui M, Tan AT. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol Immunol. 2021;18(10):2307-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng Y, Mentzer AJ, Liu G, et al. ; ISARIC4C Investigators . Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group . Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158-168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy – a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvathaneni K, Torres-Rodriguez K, Meng W, et al. SARS-CoV-2 spike-specific T-cell responses in patients with B-cell depletion who received chimeric antigen receptor T-cell treatments [published online ahead of print 18 November 2021]. JAMA Oncol. doi: 10.1001/jamaoncol.2021.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckland MS, Galloway JB, Fhogartaigh CN, et al. ; MRC-Toxicology Unit COVID-19 Consortium . Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11(1):6385. [DOI] [PMC free article] [PubMed] [Google Scholar]