Abstract
Aims/hypothesis
Methods to identify individuals at highest risk for type 1 diabetes are essential for the successful implementation of disease-modifying interventions. Simple metabolic measures are needed to help stratify autoantibody-positive (Aab+) individuals who are at risk of developing type 1 diabetes. HOMA2-B is a validated mathematical tool commonly used to estimate beta cell function in type 2 diabetes using fasting glucose and insulin. The utility of HOMA2-B in association with type 1 diabetes progression has not been tested.
Methods
Baseline HOMA2-B values from single-Aab+ (n=2652; mean age, 21.1±14.0 years) and multiple-Aab+ (n=3794; mean age, 14.5±11.2 years) individuals enrolled in the TrialNet Pathway to Prevention study were compared. Cox proportional hazard models were used to determine associations between HOMA2-B tertiles and time to progression to type 1 diabetes, with adjustments for age, sex, HLA status and BMI z score. Receiver operating characteristic (ROC) analysis was used to test the association of HOMA2-B with type 1 diabetes development in 1, 2, 5 and 10 years.
Results
At study entry, HOMA2-B values were higher in single- compared with multiple-Aab+ Pathway to Prevention participants (91.1±44.4 vs 83.9±38.9; p<0.001). Single- and multiple-Aab+ individuals in the lowest HOMA2-B tertile had a higher risk and faster rate of progression to type 1 diabetes. For progression to type 1 diabetes within 1 year, area under the ROC curve (AUC-ROC) was 0.685, 0.666 and 0.680 for all Aab+, single-Aab+ and multiple-Aab+ individuals, respectively. When correlation between HOMA2-B and type 1 diabetes risk was assessed in combination with additional factors known to influence type 1 diabetes progression (insulin sensitivity, age and HLA status), AUC-ROC was highest for the single-Aab+ group’s risk of progression at 2 years (AUC-ROC 0.723 [95% CI 0.652, 0.794]).
Conclusions/interpretation
These data suggest that HOMA2-B may have utility as a single-time-point measurement to stratify risk of type 1 diabetes development in Aab+ individuals.
Keywords: Autoantibody, Biomarker, HOMA2-B, Risk prediction, TrialNet Pathway to Prevention, Type 1 diabetes
Graphical Abstract
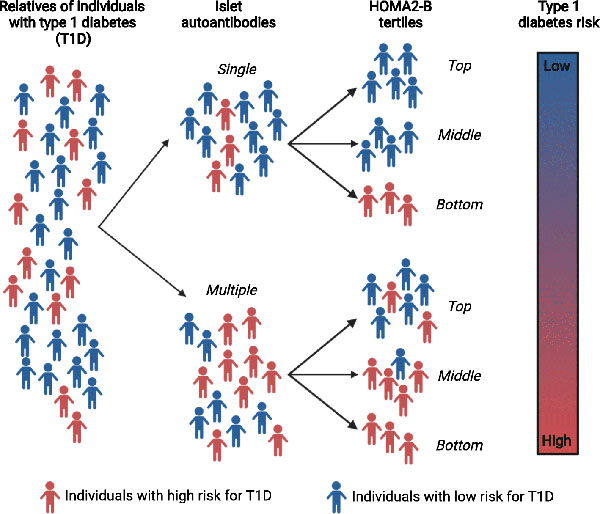
Introduction
The clinical diagnosis of type 1 diabetes occurs after a threshold loss of beta cell mass and function leads to the development of symptomatic hyperglycaemia. The preclinical phase of type 1 diabetes is characterised by the presence of circulating islet autoantibodies, and type 1 diabetes risk increases with the number of islet autoantibodies detected [1]. Data from birth cohort studies suggest that nearly 70% of individuals with multiple autoantibodies will develop type 1 diabetes over 10 years of follow-up [1, 2]. The power of autoantibodies to predict disease led to the adoption of a new staging system for type 1 diabetes diagnosis during presymptomatic periods: stage 1 is diagnosed in individuals with multiple autoantibodies and normal glucose tolerance; stage 2 is diagnosed in individuals with multiple autoantibodies and abnormal glucose tolerance; and stage 3 is diagnosed in individuals with multiple autoantibodies and overt hyperglycaemia [3]. However, not all individuals with autoantibodies progress to clinical disease, and the time to develop type 1 diabetes is highly variable, even among individuals with similar autoantibody profiles. Recently, teplizumab, an Fc receptor-non-binding anti-CD3ε monoclonal antibody, was found to delay the onset of clinical type 1 diabetes in individuals with stage 2 disease, providing the first evidence that immune interventions have the ability to alter the course of type 1 diabetes progression [4]. In autoantibody-positive (Aab+) individuals followed in research studies, progression to type 1 diabetes is typically assessed using stimulated, dynamic measures of beta cell function, including IVGTT, OGTT and hyperglycaemic clamp analysis, with results from these tests often used to determine trial eligibility [4–6]. In addition to being costly and time consuming, these assays require significant expertise and resources, limiting their application in large cohorts or in population-based screening and intervention efforts. Thus, a necessary precursor to the successful and widespread implementation of disease-modifying interventions is the development of simple, yet reliable methods to identify individuals with the highest risk of progression to type 1 diabetes.
The HOMA model estimates insulin resistance (HOMA-IR) and beta cell function (HOMA-B) as percentages of a normal reference population from a pair of fasting glucose and insulin or C-peptide values. The original HOMA model derived mathematical equations from physiological dose–responses of glucose uptake and insulin production to quantify beta cell function and insulin sensitivity [7]. It was updated as HOMA2-B in 1998 to account for hepatic and peripheral glucose resistance variations, increases in insulin secretion for higher plasma glucose concentrations and effects of circulating proinsulin [8]. HOMA2-B has been used extensively to predict progression to type 2 diabetes due, in large part, to its ease of measurement and cost-effectiveness compared with more invasive ‘gold standard’ measures of insulin resistance and beta cell function. In this regard, insulin resistance and beta cell function derived from HOMA models strongly correlate with estimates from euglycaemic and hyperglycaemic clamps, respectively [7, 9]. Further, when compared with the more complex IVGTT, HOMA2-B is better able to discriminate between individuals with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes [10].
The goal of this study was to determine how beta cell function as estimated by HOMA2-B correlates with the progression to type 1 diabetes in at-risk individuals. The TrialNet Pathway to Prevention cohort consists of first-, second- and third-degree relatives of individuals with type 1 diabetes who were screened for the presence of islet autoantibodies and followed longitudinally. We used TrialNet data to determine whether HOMA2-B is associated with type 1 diabetes progression in Aab+ individuals.
Methods
Participants
Data from the TrialNet Pathway to Prevention study (TN01; ClinicalTrials.gov registration no. NCT00097292) as of 31 May 2019 were analysed. Details of the study design have been previously described [11]. Non-diabetic first-, second- or third-degree relatives of individuals with type 1 diabetes were screened for the presence of islet autoantibodies using methods described in the Diabetes Antibody Standardization Program [12].
Laboratory analysis
Initial testing for GAD65 autoantibody, micro-insulin autoantibody (mIAA), or islet antigen-2 (IA-2) autoantibody was followed by measurement of islet cell autoantibody (ICA) or zinc transporter 8 (ZnT8) autoantibody if any one of the initial autoantibody tests was positive [13]. ZnT8 autoantibody was measured in a limited group of study participants beginning in 2004 and for the entire Pathway to Prevention cohort beginning in 2012 [14].
Between 2004 and 2019, a total of 218,865 individuals were screened for the presence of islet autoantibodies and offered enrolment into the Pathway to Prevention cohort. Until 2019, single- and multiple-Aab+ participants enrolled in longitudinal monitoring underwent semi-annual or annual visits where a standard 2 h OGTT was performed [15]. Glucose during the OGTT was measured using the glucose oxidase method [16]. Insulin was assayed by RIA until 2009–2010 [5] and afterwards by the TOSOH (San Francisco, CA, USA) Automated Immunoassay Analyzer platform [17]. Individuals who had baseline fasting glucose and insulin measured using the TOSOH assay were included in the current analysis. Confirmatory testing was performed for single-Aab+ participants. Single-Aab+ participants who developed additional autoantibodies were kept in the single-Aab+ group. Diabetes was diagnosed according to the ADA criteria, which included a confirmed fasting plasma glucose ≥7.0 mmol/l, random glucose ≥11.1 mmol/l or HbA1c ≥48 mmol/mol (6.5%) [18]. Fasting C-peptide measured at the start of the OGTT was used to calculate C-peptide AUC using Simpson’s rule. Informed consent was obtained from all participants prior to data collection. The study was approved by the ethical boards of all participating institutions and was conducted according to standards established by the Declaration of Helsinki.
Statistical methods
Based on OGTTs obtained at the initial visit, HOMA2-B (beta cell function) and HOMA2-S (insulin sensitivity) were calculated from fasting glucose and insulin measures using the publicly available HOMA2 Calculator (www.dtu.ox.ac.uk/homacalculator/; accessed19 May 2021). Output values for beta cell function and insulin sensitivity were reported as percentages of a normal reference population and log transformed for comparisons.
Baseline Pathway to Prevention participant characteristics were compared by autoantibody status (single-Aab+ vs multiple-Aab+) using the Wilcoxon rank-sum test, two-sample t test, or χ2 test based on variable type. These characteristics were also compared within autoantibody status by HOMA2-B tertile using the χ2 test, the Kruskal–Wallis test or ANOVA, again, based on variable type. Kaplan–Meier analysis was used to investigate diabetes-free survival rate, and differences between groups were determined by the logrank test. Cox proportional hazards regression was used to estimate HRs and 95% CIs for type 1 diabetes development for HOMA2-B as a continuous variable and by HOMA2-B tertile, with adjustments for age, sex, HLA status and BMI z score. The area under the receiver operating characteristic (ROC) curve (AUC-ROC) was used to determine the association of HOMA2-B with the development of type 1 diabetes at 1, 2, 5 and 10 years for all participants, and also separately for single- and multiple-Aab+ participants and those with stage 1 and stage 2 type 1 diabetes. ROC multivariate modelling [19] was used to assess the association of HOMA2-B in combination with HOMA2-S, HLA status and age. The covariate values used to develop these survival curves were based on an ‘average’ participant profile for the single-Aab+ and the multiple-Aab+ groups separately. For the single-Aab+ group, this represented a participant profile of someone who was aged 21.0 years, male sex, either DR3- or DR4-positive, and who had a BMI z score of 0.55. For the multiple-Aab+ group, this represented a participant profile of someone who was aged 14.3 years, male sex, either DR3- or DR4-positive, and who had a BMI z score of 0.44. Youden’s index [20] was calculated to assess optimal HOMA2-B cut-off values.
Data were analysed using the Statistical Analysis System software (version 9.4; SAS Institute, Cary, NC, USA). Two-tailed p values <0.05 were considered to be statistically significant. No adjustment in type 1 error was made for multiple comparisons.
Results
Baseline demographic and metabolic characteristics of TrialNet Pathway to Prevention participants by autoantibody status
A total of 6446 individuals were included in the final analysis cohort. Of these, 2652 were single-Aab+ and 3794 were multiple-Aab+ (Fig. 1). Baseline characteristics of study participants are shown in Table 1. Single-Aab+ individuals were older at study entry (mean±SD age 21.1±14.0 years) compared with multiple-Aab+ individuals (14.5±11.2 years). Length of follow-up for the entire cohort ranged from less than 6 months to greater than 3 years (electronic supplementary material [ESM] Fig. 1). Mean length of follow-up was slightly longer for the multiple-Aab+ group (1.9 years) compared with the single-Aab+ group (1.7 years; p<0.001). The single-Aab+ group had a higher proportion of female participants (57.5%; p<0.001), whereas the multiple-Aab+ group had a slight predominance of male participants (52.4%). Both groups were predominantly non-Hispanic white and siblings of individuals with type 1 diabetes. Multiple-Aab+ participants were more likely to have at least one high-risk HLA (DR3 or DR4) (84.0%; p<0.001) compared with single-Aab+ participants (74.6%). A higher percentage of multiple-Aab+ participants were positive for both DR3 and DR4 (24.6%), compared with single-Aab+ participants (12.5%). Study groups were not obese, based on BMI z-scores.
Fig. 1.
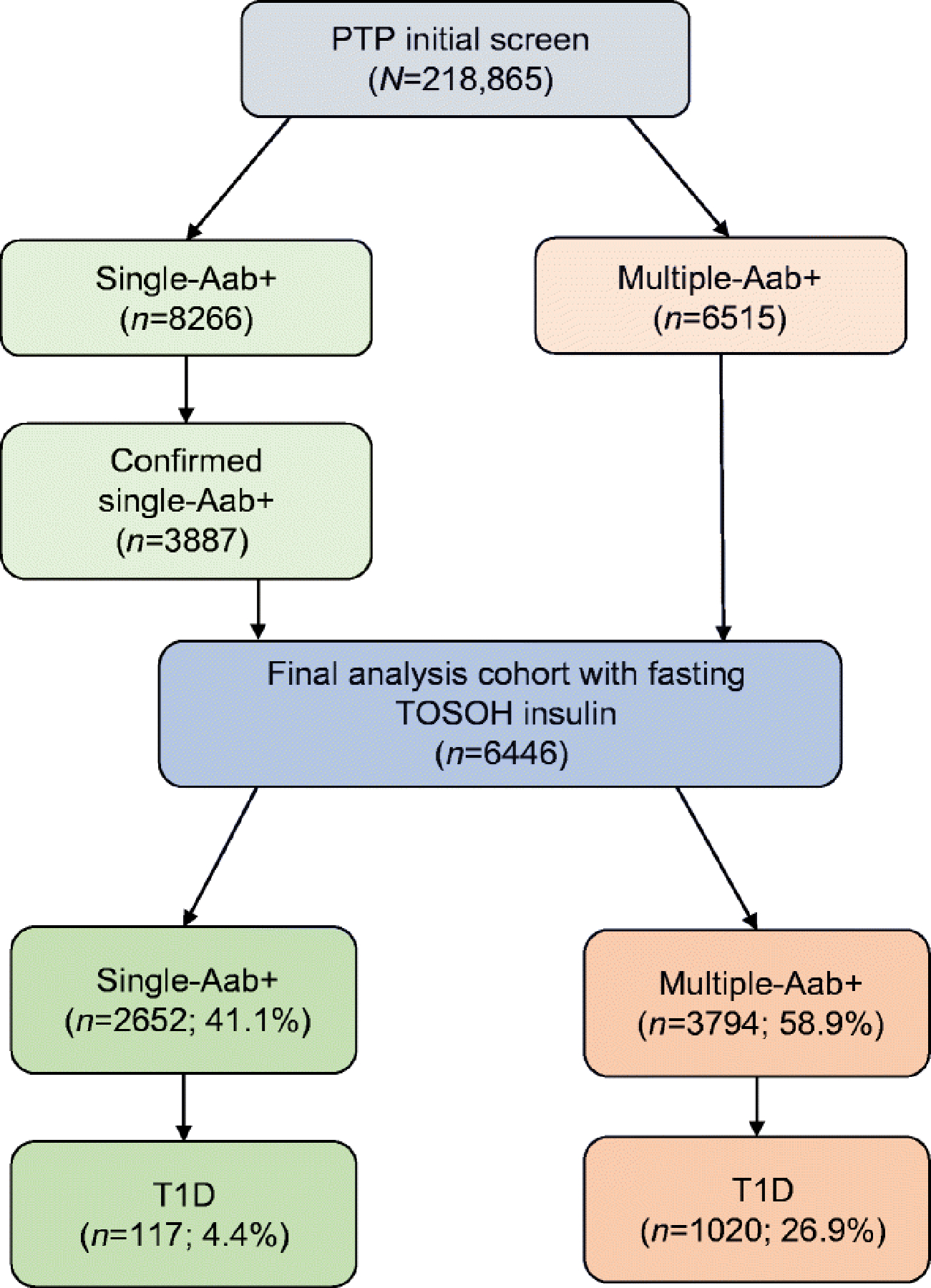
CONsolidated Standards of Reporting Trials (CONSORT) diagram for Pathway to Prevention screening and confirmatory testing. Numbers of participants identified as of 31 May 2019 are indicated. First-, second- and third-degree relatives of individuals with type 1 diabetes were screened for the presence of islet autoantibodies. A total of 218,865 eligible individuals were screened. Aab+ participants were invited to participate in longitudinal monitoring. Multiple-Aab+ participants who had at least one OGTT and fasting TOSOH insulin (n=3794) were included in the final analysis cohort. Single-Aab+ participants received confirmatory testing, and those with confirmed Aab+ status and who had at least one OGTT and fasting TOSOH insulin (n=2652) were also included in the final analysis cohort. Type 1 diabetes developed in 4.4% and 26.9% of the single-Aab+ and multiple-Aab+ groups, respectively. Within the single-Aab+ group, 483 participants (18.2%) developed additional autoantibodies and remained classified as single-Aab+ participants. T1D, type 1 diabetes
Table 1.
Baseline Pathway to Prevention participant characteristics by autoantibody status
| Characteristic | Single-Aab+ (n=2652) | Multiple-Aab+ (n=3794) | p value |
|---|---|---|---|
| Age at initial screening, years | |||
| Mean±SD | 21.1±14.0 | 14.5±11.2 | <0.001a |
| Median (Q1, Q3) | 15.0 (9.4, 35.8) | 10.9 (7.0, 16.3) | |
| Length of followup, years | |||
| Mean±SD | 1.7±2.7 | 1.9±2.5 | <0.001a |
| Median (Q1, Q3) | 0.0 (0.0, 2.5) | 0.9 (0.0, 2.8) | |
| Sex, n (%) | <0.001b | ||
| Female | 1525 (57.6) | 1799 (47.6) | |
| Male | 1125 (42.5) | 1983 (52.4) | |
| Missing | 2 (0.1) | 12 (0.3) | |
| Race, n (%) | <0.001b | ||
| Hispanic | 307 (11.6) | 316 (8.3) | |
| White/NH | 2018 (76.1) | 3067 (80.8) | |
| African American | 75 (2.8) | 129 (3.4) | |
| Other | 127 (4.8) | 127 (3.4) | |
| Unknown or not reported | 125 (4.7) | 155 (4.1) | |
| Relationship to proband, n (%) | <0.001b | ||
| Offspring | 487 (18.4) | 715 (18.9) | |
| Parent | 731 (27.6) | 439 (11.6) | |
| Sibling | 1131 (42.7) | 2258 (59.5) | |
| Other | 256 (9.7) | 248 (6.5) | |
| Unknown or not reported | 47 (1.8) | 134 (3.5) | |
| High-risk HLA (either DR3 or DR4), n (%) | <0.001b | ||
| Neither | 649 (25.4) | 582 (16.0) | |
| Either or both | 1910 (74.6) | 3048 (84.0) | |
| High-risk HLA (either DR3 or DR4), n (%) | <0.001b | ||
| Neither | 649 (25.4) | 582 (16.0) | |
| One | 1590 (62.1) | 2157 (59.4) | |
| Both | 320 (12.5) | 891 (24.6) | |
| Autoantibody type, n (%) | <0.001b | ||
| GAD65 | 1890 (71.3) | 3365 (88.7) | |
| mIAA | 659 (24.9) | 1928 (50.8) | |
| IA-2 | 93 (3.5) | 2016 (53.2) | |
| ICA | 9 (0.4) | 2518 (68.3) | |
| ZnT8 | 1 (0.1) | 1654 (57.4) | |
| BMI-for-age z score, mean±SD | 0.6±1.2 | 0.4±1.1 | <0.001c |
| Mean C-peptide AUC, mean±SD | 6.6±3.0 | 5.3±2.6 | <0.001c |
| Peak C-peptide (ng/ml), mean±SD | 9.0±4.2 | 7.2±3.5 | <0.001c |
| Fasting glucose (mmol/l), mean±SD | 5.1±0.6 | 5.1±0.9 | 0.419c |
| Fasting insulin (pmol/l), mean±SD | 56.9±47.2 | 49.3±38.2 | <0.001c |
| HOMA2-S | |||
| Mean±SD | 173.9±253.4 | 193.5±274.3 | <0.001a |
| Median (Q1, Q3) | 120.3 (75.8, 191.3) | 136.2 (86.8, 212.7) | |
| HOMA2-B | |||
| Mean±SD | 91.1±44.5 | 83.9±38.9 | <0.001a |
| Median (Q1, Q3) | 81.3 (61.8, 110.1) | 76.2 (58.5, 100.7) |
p values reflect comparisons between groups using Wilcoxon rank-sum test
p values reflect comparisons between groups using χ2 test
p values reflect comparisons between groups using two-sample t test NH, non-Hispanic; Q1, quartile 1; Q3, quartile 3
Baseline demographic characteristics of TrialNet Pathway to Prevention participants by HOMA2-B tertile
Single-Aab+ participants had higher baseline HOMA2-B values at study entry compared with multiple-Aab+ participants (91.1 vs 83.9; p<0.001; Table 1). Single- and multiple-Aab+ groups were divided into tertiles based on their HOMA2-B values at study entry, with the bottom tertiles corresponding to those individuals with the lowest beta cell function (Table 2). As expected, mean HOMA2-B for all tertiles was significantly lower in multiple-Aab+ participants compared with single-Aab+ participants (bottom 48.9±12.2 vs 52.4±12.3; middle 76.9±7.7 vs 82.5±8.5; top 125.4±36.4 vs 138.7±43.4; p<0.001 for all).
Table 2.
Baseline Pathway to Prevention participant characteristics by HOMA2-B tertile
| Characteristic | Single-Aab+ | Multiple-Aab+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bottom tertile (n=885) | Middle tertile (n=886) | Top tertile (n=881) | p value | Bottom tertile (n=1265) | Middle tertile (n=1266) | Top tertile (n=1263) | p value | ||
| HOMA2-B, mean±SD | 52.4±12.3 | 82.5±8.5 | 138.7±43.4 | <0.001a | 48.9±12.2 | 76.9±7.7 | 125.4±36.4 | <0.001a | |
| Autoantibody type, n (%) | |||||||||
| GAD65 | 632 (71.4) | 640 (72.3) | 618 (70.2) | 0.62b | 1078 (85.2) | 1140 (90.1) | 1146 (90.8) | <0.001b | |
| mIAA | 217 (24.5) | 214 (24.2) | 228 (25.9) | 0.68b | 703 (55.6) | 657 (51.9) | 568 (45.0) | <0.001b | |
| IA-2 | 31 (3.5) | 29 (3.3) | 33 (3.8) | 0.86b | 713 (56.4) | 650 (51.3) | 653 (51.7) | 0.02b | |
| ICA | 5 (0.6) | 3 (0.3) | 1 (0.1) | 0.26b | 842 (68.7) | 845 (68.8) | 831 (67.2) | 0.64b | |
| ZnT8 | 0 | 0 | 1 | 0.35b | 583 (59.2) | 525 (54.7) | 546 (58.3) | 0.11b | |
| No. of antibodies, mean±SD | 1±0.0 | 1±0.0 | 1±0.0 | 3.1±1.0 | 3.0±1.0 | 3.0±1.0 | 0.01b | ||
| Age at initial screening, years | |||||||||
| Mean±SD | 19.8±15.5 | 21.5±13.8 | 21.9±12.5 | <0.001a | 12.6±11.8 | 14.2±11.1 | 16.6±10.2 | <0.001a | |
| Median (Q1, Q3) | 11.6 (6.6, 36.6) | 15.7 (9.7, 35.9) | 15.9 (12.3, 34.8) | 7.9 (5.2, 13.0) | 10.5 (7.0, 16.1) | 13.4 (10.3, 17.7) | |||
| Length of follow-up, years | |||||||||
| Mean±SD | 1.8±2.7 | 1.7±2.7 | 1.6±2.6 | 0.19b | 1.7±2.4 | 2.0±2.6 | 2.0±2.5 | <0.001b | |
| Median (Q1, Q3) | 0.5 (0.0, 2.5) | 0.0 (0.0, 2.6) | 0.0 (0.0, 2.5) | 0.6 (0.0, 2.5) | 0.9 (0.0, 3.0) | 1.0 (0.0, 3.0) | |||
| Sex, n (%) | <0.001b | <0.001b | |||||||
| Female | 420 (47.5) | 521 (58.8) | 584 (66.3) | 501 (39.7) | 595 (47.2) | 703 (55.9) | |||
| Male | 464 (52.4) | 365 (41.2) | 296 (33.6) | 762 (60.3) | 667 (52.9) | 554 (44.1) | |||
| High-risk HLA (either DR3 or DR4), n (%) | 0.25b | 0.16b | |||||||
| Neither | 200 (23.4) | 225 (26.1) | 224 (26.6) | 181 (15.0) | 188 (15.4) | 213 (17.7) | |||
| Either or both | 656 (76.6) | 637 (73.9) | 617 (73.4) | 1025 (85.0) | 1030 (84.6) | 993 (82.3) | |||
| High-risk HLA (either DR3 or DR4), n (%) | 0.26b | 0.08b | |||||||
| Neither | 200 (23.4) | 225 (26.1) | 224 (26.6) | 181 (15.0) | 188 (15.4) | 213 (17.7) | |||
| One | 537 (62.7) | 528 (61.3) | 525 (62.4) | 700 (58.0) | 742 (60.9) | 715 (59.3) | |||
| Both | 119 (13.9) | 109 (12.7) | 92 (10.9) | 325 (27.0) | 288 (23.7) | 278 (23.1) | |||
| BMI-for-age z score, mean±SD | 0.2±1.1 | 0.4±1.1 | 1.1±1.1 | <0.001 | 0.1±1.1 | 0.3±1.0 | 0.9±1.1 | <0.001 | |
p values reflect comparisons between groups using Kruskal–Wallis test
p values reflect comparisons between groups using χ2 test
p values reflect comparisons between groups using ANOVA Q1, quartile 1; Q3, quartile 3
Because different autoantibody subtypes are associated with different type 1 diabetes risk profiles, we wondered whether autoantibody subtypes were also associated with metabolic risk variables. For the single-Aab+ group, GAD65 was the most prevalent autoantibody, followed by mIAA, IA-2 autoantibody, ICA and then ZnT8 autoantibody. Notably, the frequencies of each autoantibody were distributed evenly among bottom, middle and top tertiles in the single-Aab+ group. For the multiple-Aab+ group, GAD65 was also the most frequent autoantibody detected; however, its frequency was significantly higher in the top HOMA2-B tertile (90.8%), compared with the middle (90.1%) and bottom tertiles (85.2%; p<0.001). In contrast, mIAA and IA-2 were more commonly observed in the bottom HOMA2-B tertile of multiple-Aab+ individuals. The mean number of autoantibodies among tertiles in the multiple-Aab+ group was similar for all tertiles (3.1, 3.0 and 3.0 for the bottom, middle and top tertile, respectively). For both single- and multiple-Aab+ groups, participants in the bottom tertile were younger and male. Interestingly, the likelihood of carrying at least one high-risk HLA (DR3 and/or DR4) was not different among tertiles for either the single- or multiple-Aab+ groups.
Progression to type 1 diabetes stratified to HOMA2-B tertile
Consistent with a previous report [1], type 1 diabetes development was more common in multiple-Aab+ participants (26.9%) compared with the single-Aab+ participants (4.4%) (Fig. 1). Of the single-Aab+ participants who developed diabetes, 58 (49.6%) became multiple-Aab+ prior to clinical diabetes diagnosis. To determine whether HOMA2-B at study entry was associated with progression to type 1 diabetes, survival analysis by HOMA2-B tertile was performed for both Aab+ groups (Fig. 2). In Kaplan–Meier analysis, significant differences were identified for single-Aab+ (p=0.0142, logrank) and multiple-Aab+ (p<0.001, logrank) groups, though these differences were more pronounced in the multiple-Aab+ group. At 5 years, the percentage of diabetes-free participants in the single-Aab+ group was 86.5% (95% CI 81.5, 90.2), 88.7% (95% CI 83.5, 92.4) and 91.2% (95% CI 86.2, 94.5) for the bottom, middle and top tertile, respectively, compared with 42.4% (95% CI 38.1, 46.6), 55.6% (95% CI 50.9, 59.9) and 62.3% (95% CI 58.5, 67.4) for the bottom, middle, and top tertile of the multiple-Aab+ group, respectively.
Fig. 2.
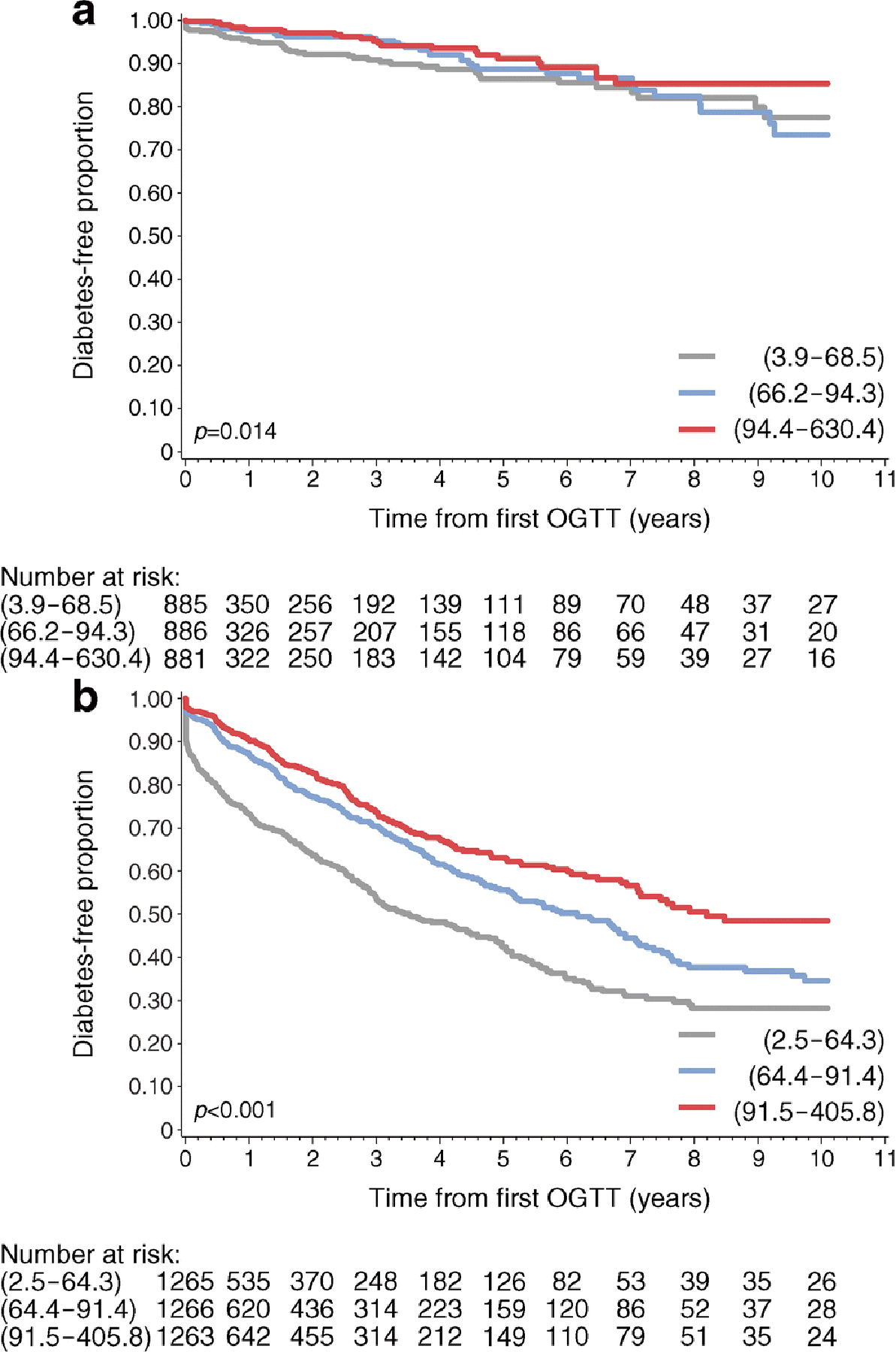
Diabetes-free survival by HOMA2-B tertile. Kaplan–Meier curves of diabetes-free survival by HOMA2-B tertile for single-Aab+ (a) and multiple-Aab+ (b) cohorts. Grey lines, bottom tertile; blue lines, middle tertile; red lines, top tertile. HOMA2-B ranges for the corresponding tertiles are indicated in parentheses. p values were determined by logrank test
Cox regression analysis
Cox proportional hazards analysis was performed for single- and multiple-Aab+ participants using HOMA2-B as a continuous variable and by tertiles of HOMA2-B, using the respective top HOMA2-B tertile as the reference group, with adjustment for age, sex, HLA status and BMI z score. In the first analysis, when single- and multiple-Aab+ participants were considered together, the HR from a univariate model for the prediction of type 1 diabetes for HOMA2-B was 0.989 (95% CI 0.988, 0.991, p<0.001), whilst for the single-Aab+ group, the HR was 0.993 (95% CI 0.988, 0.999, p=0.013) and for the multiple-Aab+ population the HR was 0.990 (95% CI 0.987, 0.992, p <0.001). When tertiles of HOMA2-B were considered, type 1 diabetes HRs were significant for the bottom HOMA2-B tertile in single-Aab+ participants (HR 1.91 [95% CI 1.12, 3.24], p=0.017), compared with the reference group, but not for the middle tertile (Table 3). For the multiple-Aab+ group, progression to type 1 diabetes was higher for the bottom compared with the middle tertile, and type 1 diabetes HRs were significantly increased for both of these groups when compared with the top tertile reference population (HR 2.43 [95% CI 2.03, 2.91], p<0.001 and HR 1.36 [95% CI 1.14, 1.64], p=0.001, respectively) (Table 3). Multivariate analysis demonstrated that for both single- and multiple-Aab+ groups, progression to type 1 diabetes was highest for the bottom tertiles and lowest for the top tertiles when adjusted for age, sex, HLA status and BMI z score (Fig. 3). Overall, these results suggest that when HOMA2-B levels are considered as tertiles, a high-risk population within the single-Aab+ group can be identified and improved risk stratification for multiple-Aab+ participants is achieved.
Table 3.
Multivariate Cox proportional hazards analysis of type 1 diabetes development by autoantibody status
| HOMA2-B tertile | Single-Aab+ | Multiple-Aab+ | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Top | Reference | Reference | |||
| Middle | 1.29 (0.75, 2.23) | 0.362 | 1.36 (1.14, 1.64) | 0.001 | |
| Bottom | 1.91 (1.12, 3.24) | 0.017 | 2.43 (2.03, 2.91) | <0.001 | |
Data are adjusted for age, sex, HLA status and BMI z score
Fig. 3.
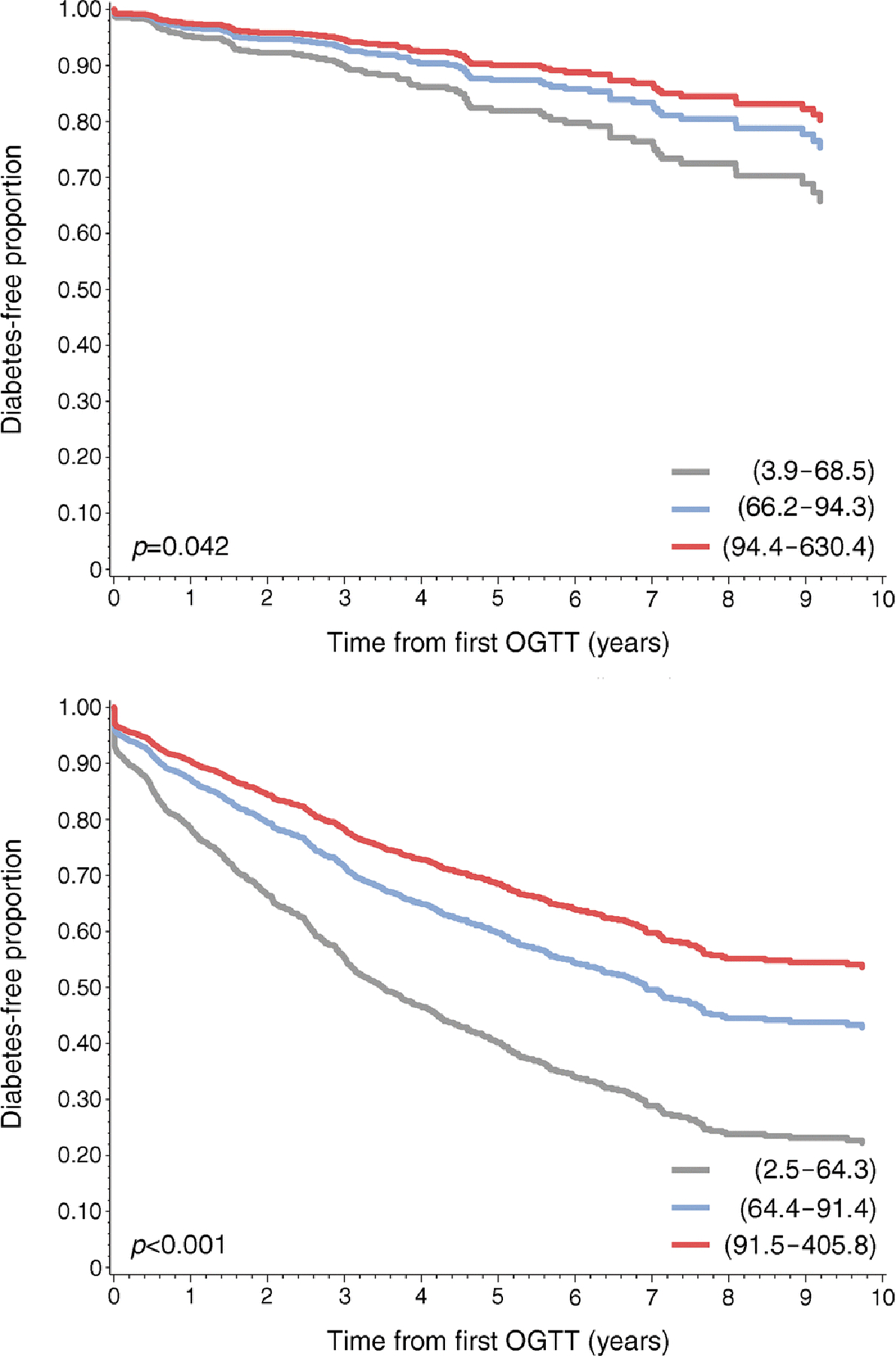
Lower HOMA2-B tertiles correlate with increased risk for type 1 diabetes. Cox proportional hazards regression analysis by autoantibody status for single-Aab+ (a) and multiple-Aab+ (b) cohorts with adjustment for age, sex, HLA status and BMI z score. The lowest HOMA2-B tertile corresponds with the highest type 1 diabetes development risk for single- and multiple-Aab+ cohorts. Grey lines, bottom tertile; blue lines, middle tertile; red lines, top tertile. HOMA2-B ranges for the corresponding tertiles are indicated in parentheses. p values were determined by logrank test
Logistic regression analysis
To further evaluate how HOMA2-B is associated with progression to type 1 diabetes, sensitivity, specificity and ROC curves were calculated for HOMA2-B in all participants combined, and for single-Aab+ participants and multiple-Aab+ participants. Type 1 diabetes development at 1, 2, 5 and 10 years from study entry was assessed (Fig. 4). For progression to type 1 diabetes within 1 year, AUC-ROC was 0.685, 0.666 and 0.680 for all Aab+, single-Aab+ and multiple-Aab+ individuals, respectively. For single-Aab+ participants, AUC-ROC was highest at 2 years (AUC-ROC 0.668 [95% CI 0.596, 0.741]). For multiple-Aab+ participants, AUC-ROC was highest at 1 year (AUC-ROC 0.680 [95% CI 0.652, 0.707]). The strongest correlation between HOMA2-B and type 1 diabetes risk was identified in the combined single- and multiple-Aab+ group at 1 year (0.685 [95% CI 0.660, 0.711]). Youden’s index was calculated to determine HOMA2-B cut-off values with highest specificity and sensitivity for each group within 1, 2, 5 and 10 years (Table 4). Youden’s index was highest for single-Aab+ participants with progression to type 1 diabetes within 1 and 2 years, though all values were <0.5.
Fig. 4.
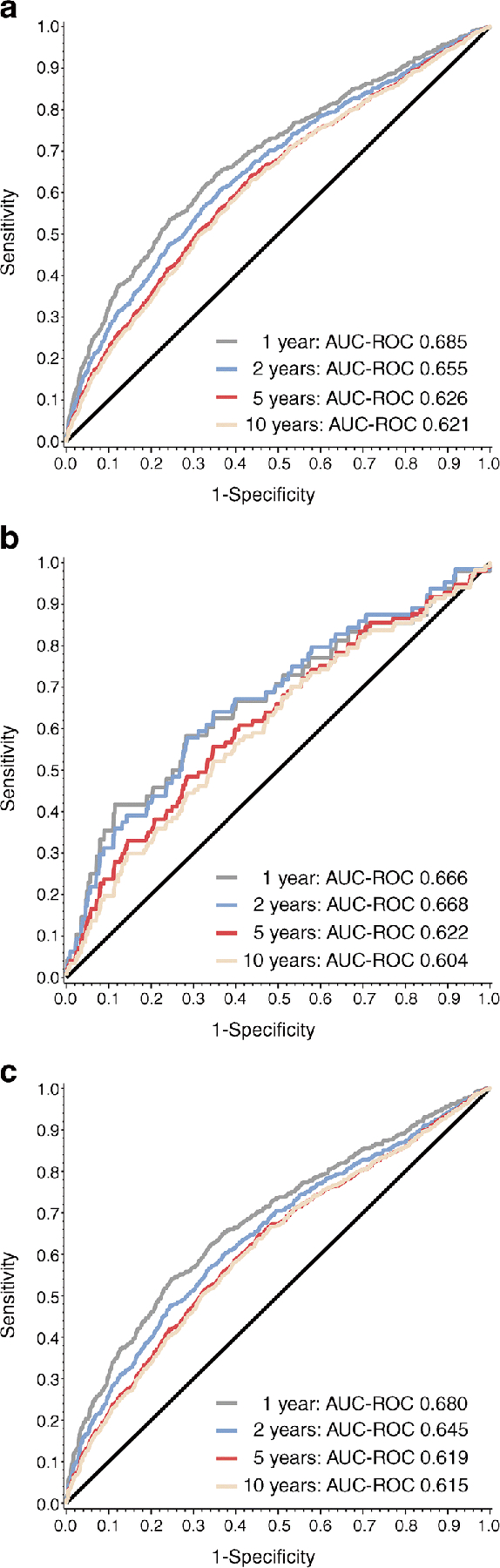
Association of HOMA2-B with type 1 diabetes progression by autoantibody status. AUC-ROC for HOMA2-B by autoantibody status for combined single- and multiple-Aab+ (a), single-Aab+ (b) and multiple-Aab+ (c) participants to assess association with type 1 diabetes development at 1, 2, 5 and 10 years from study entry
Table 4.
Optimal HOMA2-B cut-off values for type 1 diabetes prediction at 1, 2, 5 and 10 years from study entry
| Time to diagnosis | HOMA2-B | Sensitivity (95% CI) | Specificity (95% CI) | Youden’s index |
|---|---|---|---|---|
| All Aab+ | ||||
| 1 year | 69.3 | 0.646 (0.605, 0.687) | 0.644 (0.632, 0.657) | 0.416 |
| 2 years | 70.1 | 0.607 (0.571, 0.643) | 0.637 (0.625, 0.650) | 0.387 |
| 5 years | 76.0 | 0.640 (0.610, 0.670) | 0.564 (0.551, 0.577) | 0.361 |
| 10 years | 76.1 | 0.629 (0.600, 0.657) | 0.564 (0.551, 0.577) | 0.355 |
| Single-Aab+ | ||||
| 1 year | 64.9 | 0.583 (0.444, 0.723) | 0.717 (0.700, 0.734) | 0.418 |
| 2 years | 70.1 | 0.641 (0.523, 0.758) | 0.653 (0.635, 0.671) | 0.418 |
| 5 years | 70.1 | 0.557 (0.458, 0.656) | 0.654 (0.635, 0.672) | 0.364 |
| 10 years | 70.1 | 0.521 (0.431, 0.612) | 0.654 (0.635, 0.672) | 0.341 |
| Multiple-Aab+ | ||||
| 1 year | 67.5 | 0.625 (0.581, 0.668) | 0.658 (0.641, 0.674) | 0.411 |
| 2 years | 69.8 | 0.599 (0.561, 0.637) | 0.630 (0.613, 0.647) | 0.377 |
| 5 years | 72.2 | 0.588 (0.556, 0.619) | 0.601 (0.583, 0.619) | 0.353 |
| 10 years | 72.1 | 0.578 (0.547, 0.608) | 0.607 (0.588, 0.625) | 0.350 |
To determine whether HOMA2-B could further enhance risk stratification within the presymptomatic stages of type 1 diabetes, we next performed ROC analysis for multiple-Aab+ participants with normal glucose tolerance (stage 1 type 1 diabetes) and multiple-Aab+ participants with abnormal glucose tolerance (stage 2 type 1 diabetes) at study entry (Fig. 5). The highest AUC-ROC for both stage 1 and stage 2 participants was for progression to diabetes within 1 year (AUC-ROC 0.595 [95% CI 0.501, 0.689] and AUC-ROC 0.563 [95% CI 0.514, 0.612], respectively). AUC-ROC assessed for disease progression by stage at all time points were less than AUC-ROCs assessed by autoantibody status alone.
Fig. 5.
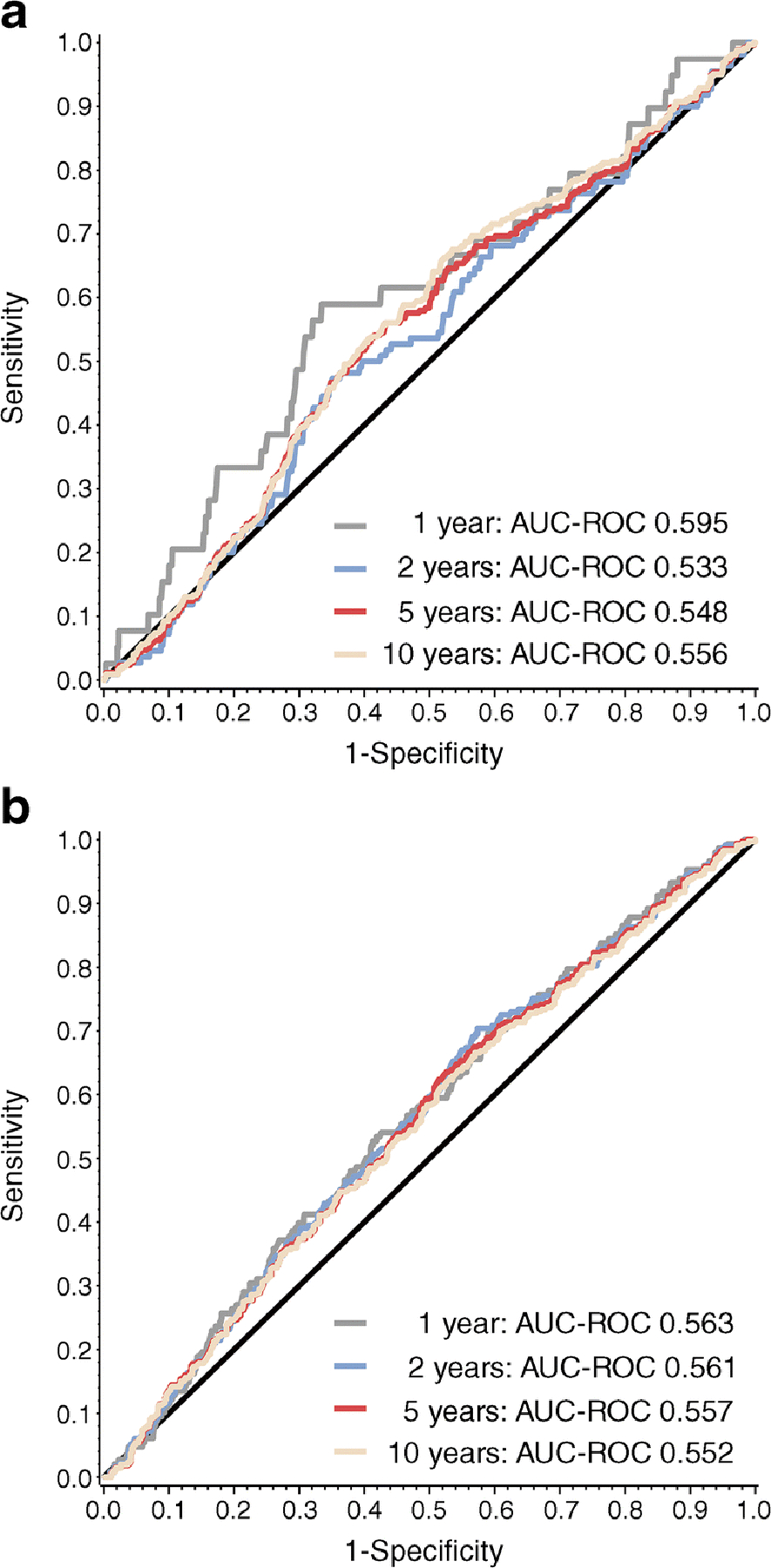
Association of HOMA2-B with type 1 diabetes progression by type 1 diabetes stage. AUC-ROC- for HOMA2-B to assess correlation with type 1 diabetes development at 1, 2, 5 and 10 years from study entry in participants with stage 1 type 1 diabetes (multiple-Aab+ and normal glucose tolerance) (a) and stage 2 type 1 diabetes (multiple-Aab+ and abnormal glucose tolerance) (b). Glucose tolerance was assessed by OGTT at study entry
Finally, to determine whether HOMA2-B could be used in combination with other, single-time-point measures to improve type 1 diabetes risk stratification, we developed AUC-ROCs for HOMA2-B combined with other factors that have been shown to influence progression to type 1 diabetes: HOMA2-S, HLA status and age (Fig. 6). While HOMA2-B and HOMA2-S are correlated (r=−0.421, p<0.001), all factors were significant in these models and, together, the combined models were more strongly correlated to type 1 diabetes progression across 1, 2, 5 and 10 years than HOMA2-B alone. For all groups, AUC-ROC for progression to type 1 diabetes within 1 year was >0.7. AUC-ROCs were higher for the single-Aab+ group, compared with the multiple-Aab+ group, for progression to type 1 diabetes at all time points. AUC-ROC was highest for the single-Aab+ group risk of progression at 2 years (AUC-ROC 0.723 [95% CI 0.652, 0.794]).
Fig. 6.
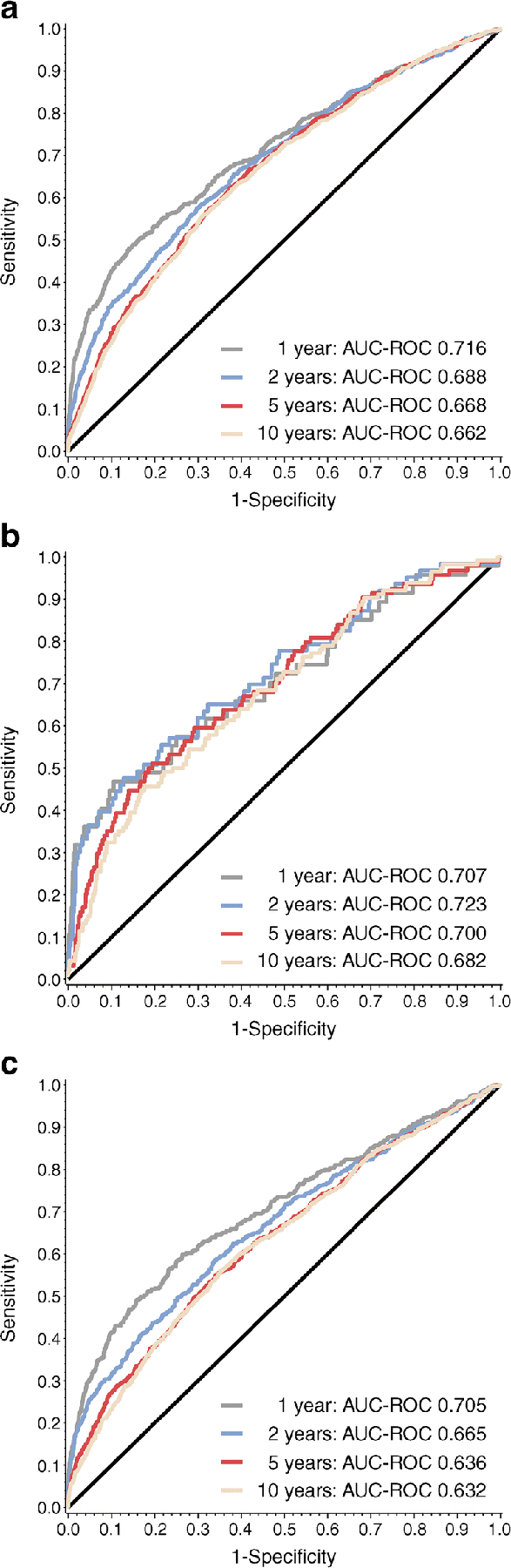
Multivariate analysis strengthens the correlation between HOMA2-B and type 1 diabetes progression. Multivariate logistic regression model, with HOMA2-S, HLA status and age (categorised), to assess the association of HOMA2-B with type 1 diabetes progression for all participants (a), single-Aab+ participants (b) and multiple-Aab+ participants (c)
Discussion
While type 1 diabetes can be predicted by the presence of autoantibodies [1], the time to clinical disease onset can span months to years among Aab+ individuals, and rates of progression to type 1 diabetes vary by modifiers such as age, family history and environmental factors [2, 21]. Data from the Diabetes Prevention Trial – Type 1 (DPT-1) and TrialNet Pathway to Prevention studies show that metabolic impairment and beta cell dysfunction, characterised by the loss of C-peptide and first-phase insulin responses [22, 23], are present several years in advance of a clinical diagnosis in many Aab+ individuals [24]. While the use of stimulatory tests, such as OGTT and IVGTT, and diagnostic models derived from these measures, including Index60 (the composite measure of fasting C-peptide, 60 min C-peptide and 60 min glucose) [25–27], are well-documented predictors of type 1 diabetes risk, these tests are time consuming and labour intensive, rendering them unsuitable for application to larger, population-based screening or intervention efforts. Our analysis shows that the single-time-point measure of beta cell function, HOMA2-B, is associated with type 1 diabetes progression. Our analysis also shows that, within the TrialNet Pathway to Prevention cohort, HOMA2-B: (1) stratifies risk among multiple-Aab+ individuals; and (2) identifies a high-risk subgroup of single-Aab+ participants who have the potential to benefit from more frequent monitoring and/or from intervention trials from which they would have been previously excluded based on autoantibody number alone. Thus, the use of HOMA2-B has the potential to bolster the predictive power of other single-time-point measures that, when used together, could enhance clinical trial efficacy and be used to implement broader screening efforts to identify high-risk individuals.
As a computer-solved, paradigm model of the physiological liver–beta cell feedback loop, HOMA2-B analysis permits the assessment of beta cell function from a single, paired measurement of fasting glucose and insulin or C-peptide values that can be used across multiple populations for assessment of longitudinal changes [28]. Examples include the UK Prospective Diabetes Study Group, which used HOMA2-B to follow beta cell function over a period of 6 years in individuals with type 2 diabetes who were treated with either sulfonylureas, metformin or diet [29], and the Belfast Diet Study, which followed a large cohort of type 2 diabetes patients over 10 years and found that the rate of decline in beta cell function, as measured by HOMA2-B, determined the rate of failure of diet therapy [30]. In islet transplant recipients, HOMA2-B has been used to identify allograft dysfunction and anticipate the loss of insulin independence at 1 year [31]. However, the use of HOMA modelling in type 1 diabetes is limited. Two studies have shown that HOMA-IR could be combined with first-phase insulin response (FPIR) measures (FPIR/HOMA-IR ratio) to predict type 1 diabetes in Aab+ relatives [32, 33]. An analysis of DPT-1 participants demonstrated a negative correlation between islet autoimmunity and HOMA2-B in first-degree relatives with normal glucose tolerance, consistent with decreased beta cell secretory reserve in the early stages of type 1 diabetes [34]. Ours is the first study to demonstrate that HOMA2-B is associated with risk for type 1 diabetes development independently in those with established autoimmunity.
Autoantibody subtypes used alone (IA-2A) [35], or in combination with other markers (HbA1c, BMI, single nucleotide polymorphisms, autoantibody number) [36], have long been shown to be strong predictors of type 1 diabetes risk in first-degree relatives. Jacobsen et al used logistic regression modelling of metabolic and immune variables to predict type 1 diabetes development by age 6 years within a birth cohort of children with high-risk HLA and followed in The Environmental Determinants of Diabetes in Youth (TEDDY) study [36]. The model provided the highest AUC-ROC compared with models based on autoantibodies alone, although the differences in AUC-ROC, sensitivity and specificity between logistic regression modelling and autoantibody status were small. Despite this model lacking the specificity and positive predictive value (0.59 and 0.35, respectively) to support meaningful clinical implementation, it served as an important proof of concept for development of models of combined immunological and metabolic factors to stratify type 1 diabetes risk progression [36]. Therefore, it is likely that HOMA2-B’s greatest strength will be its use in combination with other single time point markers of type 1 diabetes risk. Indeed, here, we have demonstrated that the addition of insulin sensitivity, age and HLA status to a multivariate logistic regression model strengthens the correlation of HOMA2-B with progression to type 1 diabetes.
The capacity to stratify type 1 diabetes risk using a single sample has the potential to influence two major areas in type 1 diabetes research: clinical trial design and population-based screening. Heterogeneity in type 1 diabetes disease progression and clinical phenotype have likely contributed to the equivocal outcomes in many immune intervention trials performed over the last three decades. Historically, only multiple-Aab+ individuals have been included in clinical prevention trials and abnormal glucose tolerance is often also required. While the current analysis confirms that lower HOMA2-B values significantly increase type 1 diabetes risk in multiple-Aab+ individuals, it also reveals a similarly increased and significant risk for a subset of single-Aab+ individuals in the lowest HOMA2-B tertile. When HOMA2-B was assessed for individuals with stage 1 and stage 2 type 1 diabetes, AUC-ROC was highest for type 1 diabetes progression at 1 year in the stage 1 group (individuals with multiple autoantibodies and normal glucose tolerance). Therefore, it is possible that the use of HOMA2-B will uncover high-risk groups that may be overlooked when autoantibody number and glucose tolerance are used to identify clinical trial participants.
At the same time, HOMA2-B’s simplicity and scalability make it an appealing measure for use in larger, epidemiological studies and population-based screening efforts. Studies have demonstrated that individuals who have been screened for type 1 diabetes risk are less likely to present with diabetic ketoacidosis (DKA) and more likely to have a lower HbA1c at diagnosis [37]. Recent publication of the Fr1da study group’s results demonstrated the feasibility of primary-care-based screening for islet autoantibodies after screening over 90,000 children aged 2–5 years in Bavaria, Germany [38]. In this cohort, risk was assessed on the basis of how many autoantibodies were present with or without consideration of underlying genetic risk. The use of a single-time-point measure like HOMA2-B is a cost-effective way to add metabolic information to these strategies of risk assessment and its simplicity permits its use sequentially and over time. It is foreseeable that understanding the association between HOMA2-B and type 1 diabetes progression will foster the development of HOMA2-B ranges that correspond to the tertiles assessed here for further stratification of Aab+ individuals into low, medium and high risk. Combining immunological, genetic and metabolic data may help identify individuals that require additional, dynamic measures of metabolic function, more intensive surveillance or are candidates for intervention trials.
Our study has several limitations. While HOMA2-B has been shown to correlate with well-validated models of beta cell function, its use has not been validated in populations at risk for type 1 diabetes. Future studies are needed to assess whether HOMA2-B can distinguish non-related control individuals from first-degree relatives and Aab+ from autoantibody-negative relatives of individuals with type 1 diabetes. It is encouraging that, when studied in an adult population of first-degree relatives of individuals with type 2 diabetes, HOMA modelling of insulin resistance successfully distinguished normal, non-related individuals from those with a first-degree relative with type 2 diabetes and normal glucose tolerance [39]. As a single-time-point measure, it is unreasonable to expect that the predictive power of HOMA2-B alone will surpass models derived from dynamic testing, such as Index60 [26].
Despite these limitations, our study suggests that simple measures of beta cell function have the capacity to enhance validated risk prediction by autoantibody status. The predictive power of HOMA2-B was bolstered by the addition of other, single-time-point measures (HOMA2-S, HLA status and demographic factors [age]) that have been shown to influence the risk of type 1 diabetes progression. In this way, our results support conclusions drawn by many other biomarker investigations, suggesting that multiple biomarkers will need to be used together to significantly improve power in type 1 diabetes risk prediction. As the heterogeneity of type 1 diabetes development is increasingly recognised, further risk stratification of type 1 diabetes progression by simple, scalable measures, such as HOMA2-B, is essential for effective clinical trial design, meaningful interpretation of clinical trial results, and the implementation of population-based screening.
Supplementary Material
Research in context.
What is already known about this subject?
Islet autoantibodies predict risk of type 1 diabetes, but time to clinical diagnosis of disease is highly variable
Risk stratification of autoantibody-positive (Aab+) individuals has clinical utility and aids in design of prevention trials
HOMA2-B is a simple, one-time-point measurement that has been used extensively to assess beta cell function in individuals with type 2 diabetes but its utility in assessing beta cell function in Aab+ individuals at risk of type 1 diabetes is not known
What is the key question?
Is HOMA2-B associated with risk of type 1 diabetes progression?
What are the new findings?
HOMA2-B identifies a high-risk subgroup of single-Aab+ individuals and stratifies risk for multiple-Aab+ individuals
The addition of single-time-point measures and demographic factors, including insulin sensitivity, age and HLA status, strengthen the correlation between HOMA2-B and progression to type 1 diabetes
How might this impact on clinical practice in the foreseeable future?
HOMA2-B may have utility for stratifying type 1 diabetes risk among Aab+ individuals in order to improve clinical trial efficacy and to support population-level type 1 diabetes screening efforts
Acknowledgements
The authors thank M. Cleves, within the Health Informatics Institute, University of South Florida (Tampa, FL, USA), for assistance with statistical analysis and data preparation. The Graphical Abstract was created with Biorender.com.
Funding
This work was supported by NIH grant R21 DK119800 (CEM), JDRF grant 3-PDF-2019–752-A-N (JLF) and JDRF Australia grant 1-SRA-2020–900 (JMW). We acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085453, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, UC4 DK11700901, U01 DK 106693–02 and the JDRF. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Abbreviations
- Aab+
Autoantibody positive
- AUC-ROC
Area under the receiver operating characteristic curve
- DPT-1
Diabetes Prevention Trial – Type 1
- FPIR
First-phase insulin response
- IA-2
Islet antigen-2
- ICA
Islet cell autoantibody
- mIAA
Micro-insulin autoantibody
- ROC
Receiver operating characteristic
- ZnT8
Zinc transporter 8
Footnotes
A complete list of Type 1 Diabetes TrialNet Study Group members is included in the electronic supplementary material (ESM).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ziegler AG, Rewers M, Simell O, et al. (2013) Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309(23):2473–2479. 10.1001/jama.2013.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsen LM, Bocchino L, Evans-Molina C, et al. (2020) The risk of progression to type 1 diabetes is highly variable in individuals with mulitple autoantibodies following screening. Diabetologia 63(3):588–596. 10.1007/s00125-019-05047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insel RA, Dunne JL, Atkinson MA, et al. (2015) Staging presymptomatic type 1 diabetes: A scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38(10):1964–1974. 10.2337/dc15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold KC, Bundy BN, Long SA, et al. (2019) An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med 381(7):603–613. 10.1056/NEJMoa1902226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skyler JS, Brown D, Chase HP, et al. (2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346(22):1685–1691. 10.1056/NEJMoa012350 [DOI] [PubMed] [Google Scholar]
- 6.Ehlers MR (2016) Strategies for clinical trials in type 1 diabetes. J Autoimmun 71:88–96. 10.1016/j.jaut.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JRPR, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 8.Levy JC, Matthews DR, Hermans MP (1998) Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21(12):2191–2192. 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 9.Emoto M, Nishizawa Y, Maekawa K, et al. (1999) Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 22(5):818–822. 10.2337/diacare.22.5.818 [DOI] [PubMed] [Google Scholar]
- 10.Hermans MP, Levy JC, Morris RJ, Turner RC (1999) Comparison of tests of βcell function across a range of glucose tolerance from normal to diabetes. Diabetes 48(9):1779–1786. 10.2337/diabetes.48.9.1779 [DOI] [PubMed] [Google Scholar]
- 11.Skyler JS, Greenbaum CJ, Lachin JM, et al. (2008) Type 1 diabetes TrialNet - An international collaborative clinical trials network. Ann N Y Acad Sci 1150:14–24. 10.1196/annals.1447.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vehik K, Beam CA, Mahon JL, et al. (2011) Development of autoantibodies in the TrialNet natural history study. Diabetes Care 34(9):1897–1901. 10.2337/dc11-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. (2009) The TrialNet natural history study of the development of type 1 diabetes: Objectives, design, and initial results. Pediatr Diabetes 10(2):97–104. 10.1111/j.1399-5448.2008.00464.x [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Boulware DC, Beam CA, et al. (2012) Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 35(6):1213–8. 10.2337/dc11-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum CJ, Thomas MP, Mcgee PF, et al. (2008) Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 31(10):1966–1971. 10.2337/dc07-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Mahon J, Rafkin L, et al. (2011) A comparison of the baseline metabolic profiles between Diabetes Prevention Trial-Type 1 and TrialNet Natural History Study participants. Pediatr Diabetes 12(2):85–90. 10.1111/j.1399-5448.2010.00662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahon JL, Beam CA, Marcovina SM, et al. (2011) Comparison of two insulin assays for first-phase insulin release in type 1 diabetes prediction and prevention studies. Clin Chim Acta 412(23–24):2128–2131. 10.1016/j.cca.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association (2014) Standards of Medical Care in Diabetes - 2014. Diabetes Care 37(Supplement 1):S14–S80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 19.Shultz EK (1995) Multivariate receiver-operating characteristic curve analysis: Prostate cancer screening as an example. Clin Chem 41(8):1248–1255. 10.1093/clinchem/41.8.1248 [DOI] [PubMed] [Google Scholar]
- 20.Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia M, Ahmed S, Anderson MS, et al. (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43(1):5–12. 10.2337/dc19-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosenko JM, Skyler JS, Herold KC, Palmer JP (2012) The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1. Diabetes 61(6):1331–1337. 10.2337/db11-1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans-Molina C, Sims EK, DiMeglio LA, et al. (2018) β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI insight 3(15):e120877. 10.1172/jci.insight.120877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. (2009) Incident dysglycemia and progression to type 1 diabetes among participants in the diabetes prevention trial-type 1. Diabetes Care 32(9):1603–1607. 10.2337/dc08-2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redondo MJ, Nathan BM, Jacobsen LM, et al. (2021) Index60 as an additional diagnostic criterion for type 1 diabetes. Diabetologia 64(4):836–844. 10.1007/s00125-020-05365-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sosenko JM, Skyler JS, Dimeglio LA, et al. (2015) A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 38(2):271–276. 10.2337/dc14-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan BM, Boulware D, Geyer S, et al. (2017) Dysglycemia and Index60 as Prediagnostic End Points for Type 1 Diabetes Prevention Trials. Diabetes Care 40(11):1494–1499. 10.2337/dc17-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace TM, Levy JC, Matthews DR (2004) Use and Abuse of HOMA Modeling. Diabetes Care 27(6):1487–1495. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 29.Turner RC, Holman RR (1995) Lessons from UK prospective diabetes study. Diabetes Res Clin Pract 28:S151–S157. 10.1016/0168-8227(95)01105-M [DOI] [PubMed] [Google Scholar]
- 30.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR (1998) Beta-cell Deterioration Determines the Onset and Rate of Progression of Secondary Dietary Failure in Type 2 Diabetes Mellitus: the 10-year Follow-up of the Belfast Diet Study. Diabet Med 15:290–296. [DOI] [PubMed] [Google Scholar]
- 31.Gołębiewska JE, Solomina J, Thomas C, et al. (2018) Comparative evaluation of simple indices using a single fasting blood sample to estimate beta cell function after islet transplantation. Am J Transplant 18(4):990–997. 10.1111/ajt.14620 [DOI] [PubMed] [Google Scholar]
- 32.Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP (2007) Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 30(9):2314–2320. 10.2337/dc06-2389 [DOI] [PubMed] [Google Scholar]
- 33.Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC (2004) Insulin resistance is a risk factor for progression to Type 1 diabetes. Diabetologia 47(10):1661–1667. 10.1007/s00125-004-1507-3 [DOI] [PubMed] [Google Scholar]
- 34.Siewko K, Popławska-Kita A, Telejko B, et al. (2014) Prognostic markers for the development of type 1 diabetes in first-degree relatives of diabetic patients. Endokrynol Pol 65(3):176–180. 10.5603/EP.2014.0024 [DOI] [PubMed] [Google Scholar]
- 35.Gorus FK, Decochez K, De Leeuw IH, et al. (2002) IA-2 autoantibodies predict impending Type I diabetes in siblings of patients. Diabetologia 45:1658–1666. 10.1007/s00125-002-0949-8 [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen LM, Larsson HE, Tamura RN, et al. (2019) Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr Diabetes 20(3):263–270. 10.1111/pedi.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narendran P (2019) Screening for type 1 diabetes: are we nearly there yet? Diabetologia 62(1):24–27. 10.1007/s00125-018-4774-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler AG, Kick K, Bonifacio E, et al. (2020) Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany. JAMA 323(4):339–351. 10.1001/jama.2019.21565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa A, Rios M, Casamitjana R, Gomis R, Conget I (1998) High prevalence of abnormal glucose tolerance and metabolic disturbances in first degree relatives of NIDDM patients. A study in Catalonia, a mediterranean community. Diabetes Res Clin Pract 41(3):191–196. 10.1016/S0168-8227(98)00086-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.


