Abstract
Background
Portosystemic collateral vessels are a sign of portal hypertension in liver cirrhosis. Esophageal collateral veins (ECVs) are one major type of portosystemic collateral vessels, which increase the recurrence of esophageal varices and bleeding after variceal eradication. However, the risk factors for ECVs were still unclear.
Methods
We retrospectively screened cirrhotic patients who had contrast-enhanced computed tomography (CT) images to evaluate ECVs and upper gastrointestinal endoscopic reports to evaluate gastroesophageal varices at our department. Univariate and multivariate logistic regression analyses were performed to explore the independent risk factors for ECVs. Odds ratios (ORs) were calculated. Subgroup analyses were performed in patients with and without previous endoscopic variceal therapy which primarily included endoscopic variceal ligation (EVL) and endoscopic injection sclerotherapy (EIS).
Results
Overall, 243 patients were included, in whom the prevalence of ECVs was 53.9%. The independent risk factors for ECVs were hepatitis C virus infection (OR = 0.250, p = 0.026), previous EVL (OR = 1.929, p = 0.044), platelet (OR = 0.993, p = 0.008), and esophageal varices needing treatment (EVNTs) (OR = 2.422, p = 0.006). The prevalence of ECVs was 60.8% (73/120) in patients undergoing EVL, 50% (10/20) in those undergoing EIS, and 47.5% (48/101) in those without previous endoscopic variceal therapy. The independent risk factors for ECVs were the use of nonselective beta-blockers (OR = 0.294, p = 0.042) and EVNTs (OR = 3.714, p = 0.006) in subgroup analyses of patients with and without previous endoscopic variceal therapy, respectively.
Conclusions
The presence of ECVs should be closely associated with the severity of portal hypertension in liver cirrhosis. Risk of ECVs might be increased by previous EVL.
1. Introduction
Esophageal varices (EVs) are the most common collaterals in advanced cirrhosis that are located inside the esophageal lumen [1]. Bleeding from EVs remarkably increases the risk of mortality [2, 3]. The recommendations on management of EVs bleeding are clearly given by the current practice guidelines and consensus, and the most commonly recommended approach is endoscopic variceal therapy, such as endoscopic variceal ligation (EVL) and endoscopic injection sclerotherapy (EIS) [3, 4].
Esophageal collateral veins (ECVs) refer to the portosystemic collateral vessels outside the esophageal lumen [5]. Individual studies and meta-analyses by our and other teams suggest a high prevalence of ECVs in patients with portal hypertension and a remarkable impact of ECVs on the recurrence of EVs [6–9]. However, the risk factors for developing ECVs remained unclear. On the other hand, it seems that the incidence of ECVs would be different between patients who underwent EVL and EIS [10, 11]. In the present study, we aimed to explore the risk factors for developing ECVs in cirrhotic patients with and without endoscopic esophageal variceal therapy.
2. Materials and Methods
2.1. Patients
In this single-center retrospective study, we screened cirrhotic patients who underwent both contrast-enhanced computed tomography (CT) and upper gastrointestinal endoscopy between December 2014 and May 2019. The data were derived from our prospectively established database collecting cirrhotic patients admitted to our department. This study was approved by the medical ethical committee of our hospital, and the approval number was k (2019) 35.
The inclusion criteria were as follows: (1) patients were diagnosed with cirrhosis according to the medical history, clinical features, laboratory, and/or imaging results and (2) both endoscopic examinations and contrast-enhanced CT scans were performed at their admissions. Patients whose contrast-enhanced CT images were not well preserved were excluded.
2.2. Data Collection
We collected the data as follows: age, sex, etiology of liver diseases, hepatic encephalopathy (HE), gastrointestinal bleeding (GIB), ascites, history of GIB, history of endoscopic variceal therapy, endoscopic variceal therapy approaches including EVL and EIS, interval between previous endoscopic variceal therapy and present contrast-enhanced CT scans, red blood cell, hemoglobin, white blood cell (WBC), platelet (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase (AKP), γγ-glutamine transferase (GGT), blood urea nitrogen, creatinine (Cr), sodium, potassium, prothrombin time (PT), activated partial thromboplastin time, and international normalized ratio (INR). EVL was performed by ligation device MBL-6-F (Wilson-Cook Medical Inc., NC, USA). The drug used during EIS was polycinnamyl alcohol (Shaanxi TIANYU Pharmaceutical Co., Ltd., Shaanxi, China). We also recorded the use of nonselective beta-blockers (NSBBs) within 1 month before admission. The Child-Pugh and model for end stage of liver disease (MELD) scores were calculated as follows [12, 13].
Child-Pugh score = ALB score + TBIL score + INR score + ascites score + HE score. MELD score = 9.57 × ln [Cr (µmol/L) × 0.011] + 3.78 × ln [TBIL (µmol/L) × 0.058] + 11.2 × ln(INR) + 6.43.
2.3. Evaluation of ECVs on Contrast-Enhanced CT
The presence of ECVs on contrast-enhanced CT images was evaluated by two observers (QL and XQ). We were blind to endoscopic findings before analyzing the CT images. ECVs were defined as enhanced dilated vascular shadow surrounding the esophagus at the portal vein phases of contrast-enhanced CT images (Figure 1).
Figure 1.
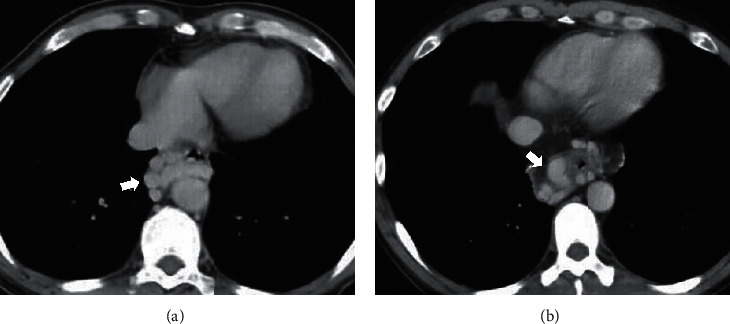
Esophageal collateral veins on contrast-enhanced CT scans.
2.4. Evaluation of EVs on Endoscopy
As for the patients who underwent endoscopic variceal therapy, the endoscopic findings regarding EVs would be extracted, if endoscopic examinations were performed after contrast-enhanced CT scans during the same hospitalizations; by contrast, the endoscopic findings would not be extracted, if endoscopic examinations were performed before contrast-enhanced CT scans during the same hospitalizations. As for the patients who did not undergo endoscopic variceal therapy, the endoscopic findings regarding EVs would be extracted regardless of the order of contrast-enhanced CT and endoscopy. EVs needing treatment (EVNTs) include moderate and severe EVs that are diagnosed according to the Chinese consensus regarding management of gastroesophageal varices [4]. In details, they were defined as follows: (1) straight or slightly tortuous EVs with red color (RC) signs; (2) serpentine tortuous uplifted EVs with RC signs with or without RC signs; or (3) beaded, nodular, or tumor-like EVs with or without RC signs.
2.5. Statistical Analyses
Continuous variables were expressed as mean ± standard deviation and median (range) and compared by using Mann–Whitney U test. Categorical variables were expressed as frequencies and percentages and compared by Chi-square tests. A two-sided p < 0.05 was considered statistically significant. Univariate and multivariate logistic regression analyses were used to identify the risk factors of developing ECVs. Variables with a p < 0.1 in univariate analysis were included in multivariate analyses. Only one of the variables with collinearity was selected in multivariate analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Subgroup analyses were performed in patients with and without history of endoscopic variceal therapy. Statistical analyses were performed using the SPSS software version 20.0 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Patients
A total of 243 cirrhotic patients who underwent contrast-enhanced CT and upper gastrointestinal endoscopy at the same hospitalization were included (Table 1). Among them, 71.2% (173/243) of patients were male and 28.8% (70/243) were female. Hepatitis B virus infection and alcohol abuse were the major etiologies. One hundred and forty-one patients underwent endoscopic variceal therapy, of whom 121 and 20 underwent EVL and EIS as the last endoscopic variceal therapeutic approach, respectively. The interval between last endoscopic variceal therapy and CT could not be calculated in 4 patients due to the lack of specific date. The information regarding use of NSBBs was available in 192 patients, of whom 16.1% (31/192) took NSBBs within 1 month before admission. The prevalence of ECVs on contrast-enhanced CT scans was 53.9% (131/243).
Table 1.
Characteristics of patients.
| Variables | No. Pts | Mean ± SD Median (range) or frequency (percentage) |
|---|---|---|
| Age (years) | 243 | 55.11 ± 10.39 |
| Sex (male) | 243 | 173 (71.2%) |
|
| ||
| Etiology of liver diseases | ||
| Hepatitis B virus infection | 243 | 103 (42.4%) |
| Hepatitis C virus infection | 243 | 19 (7.8%) |
| Alcohol abuse | 243 | 92 (37.9%) |
| Drug related | 243 | 21 (8.6%) |
| Autoimmune liver diseases | 243 | 17 (7.0%) |
|
| ||
| Clinical presentations at admission | ||
| Hepatic encephalopathy | 243 | 5 (2.1%) |
| Gastrointestinal bleeding | 243 | 95 (39.1%) |
| Ascites (no/mild/moderate-severe) | 243 | 112 (46.1%)/89 (36.6%)/42 (17.3%) |
|
| ||
| History | ||
| History of gastrointestinal bleeding | 243 | 161 (66.3%) |
| History of endoscopic variceal therapy | 243 | 141 (58.0%) |
| EVL as last endoscopic variceal therapeutic approach | 243 | 121 (49.8%) |
| EIS as last endoscopic variceal therapeutic approach | 243 | 20 (8.2%) |
| Interval between last endoscopic variceal therapy and CT (days) | 137 | 313.60 ± 371.29 190.00 (1.00–1676.00) |
| NSBBs within 1 month before admission | 192 | 31 (16.1%) |
|
| ||
| Laboratory data | ||
| Red blood cell (1012/L) | 243 | 3.63 ± 0.90 3.73 (1.51–9.92) |
| Hemoglobin (g/L) | 243 | 102.97 ± 28.41 103.00 (28.00–181.00) |
| White blood cell (109/L) | 243 | 4.22 ± 3.00 3.40 (0.70–21.60) |
| Platelet (109/L) | 243 | 102.79 ± 82.54 79.00 (15.00–681.00) |
| Total bilirubin (µmol/L) | 243 | 26.20 ± 26.62 17.90 (5.60–216.50) |
| Albumin (g/L) | 243 | 34.67 ± 6.21 35.20 (14.20–71.40) |
| Alanine aminotransferase (U/L) | 243 | 29.57 ± 28.99 22.14 (4.23–332.50) |
| Aspartate aminotransferase (U/L) | 243 | 41.28 ± 34.30 30.78 (9.63–376.35) |
| Alkaline phosphatase (U/L) | 243 | 111.77 ± 88.64 90.06 (24.35–983.93) |
| γ-Glutamyl transpeptidase (U/L) | 243 | 91.81 ± 213.01 33.93 (7.49–1779.18) |
| Blood urea nitrogen (mmol/L) | 243 | 5.72 ± 2.60 5.23 (1.86–20.15) |
| Creatinine (µmol/L) | 243 | 64.27 ± 17.27 61.71 (27.95–178.55) |
| Potassium (mmol/L) | 243 | 3.89 ± 0.42 3.91 (2.42–5.87) |
| Sodium (mmol/L) | 243 | 138.79 ± 3.11 139.10 (118.00–147.70) |
| Prothrombin time (seconds) | 243 | 16.31 ± 2.50 15.80 (12.50–28.00) |
| Activated partial thromboplastin time (seconds) | 243 | 40.66 ± 5.54 40.10 (19.80–71.30) |
| International normalized ratio | 243 | 1.33 ± 0.28 1.27 (0.94–2.77) |
| Child-Pugh score | 243 | 6.72 ± 1.65 6.00 (5.00–13.00) |
| Child-Pugh class (A/B/C) | 243 | 133 (54.7%)/93 (38.3%)/17 (7.0%) |
| MELD score | 243 | 6.46 ± 4.48 5.58 (−2.13–27.42) |
| EVs on endoscopy (no/yes/unknown) | 211a | 34 (16.1%)/177 (72.8%) 0 (0.0%) |
| EVNTs on endoscopy (no/yes/unknown) | 211a | 104 (49.3%)/106 (50.2%)/1 (0.5%)b |
| ECVs on CT (no/yes/unknown) | 243 | 110 (45.3%)/131 (53.9%)/2 (0.8%)c |
Notes: aAs for the patients who underwent endoscopic variceal therapy, only EVs on endoscopy performed after CT during the same hospitalizations were evaluated; as for the patients who did not undergo endoscopic variceal therapy, EVs on endoscopy performed during the same hospitalizations were evaluated, regardless of the order of CT and endoscopy; bEVNTs could not be evaluated due to the absence of detailed grade of EVs in their endoscopic reports; cECVs could not be evaluated because the venous vessels were not obviously enhanced. Pts, patients; SD, standard deviation; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy; CT, computed tomography; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
3.2. Overall Comparison between ECVs and No ECVs Groups
Patients with ECVs had significantly lower proportion of hepatitis C virus (HCV) infection (p = 0.011) and levels of WBC (p < 0.0001), PLT (p < 0.0001), AKP (p = 0.014), and GGT (p = 0.042) and higher proportions of EVs (p < 0.0001), EVNTs (p = 0.002), and previous EVL (p = 0.044) and levels of INR (p = 0.005), PT (p = 0.005), and MELD score (p = 0.017) than those without ECVs (Table 2).
Table 2.
Comparison between ECVs and no ECVs groups.
| Variables | ECVs | No ECVs | p value | ||
|---|---|---|---|---|---|
| No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
||
| Age (years) | 131 | 55.60 ± 10.78 | 110 | 54.55 ± 10.00 | 0.475 |
| Sex (male/female) | 131 | 96 (73.3%)/35 (26.7%) | 110 | 76 (69.1%)/34 (30.9%) | 0.473 |
|
| |||||
| Etiology of liver diseases | |||||
| Hepatitis B virus infection | 131 | 61 (46.6%) | 110 | 42 (38.2%) | 0.190 |
| Hepatitis C virus infection | 131 | 5 (3.8%) | 110 | 14 (12.7%) | 0.011 |
| Alcohol abuse | 131 | 52 (39.7%) | 110 | 40 (36.4%) | 0.596 |
| Drug related | 131 | 11 (8.4%) | 110 | 9 (8.2%) | 0.952 |
| Autoimmune liver diseases | 131 | 7 (5.3%) | 110 | 9 (8.2%) | 0.378 |
|
| |||||
| Clinical presentations at admission | |||||
| Hepatic encephalopathy | 131 | 1 (0.8%) | 110 | 4 (3.6%) | 0.269 |
| Gastrointestinal bleeding | 131 | 56 (42.7%) | 110 | 38 (34.5%) | 0.193 |
| Ascites (no/mild/moderate-severe) | 131 | 54 (41.2%)/52 (39.7%)/25 (19.1%) | 110 | 56 (50.9%)/37 (33.6%)/17 (15.5%) | 0.321 |
|
| |||||
| History | |||||
| History of gastrointestinal bleeding | 131 | 89 (67.9%) | 110 | 71 (64.5%) | 0.579 |
| History of endoscopic variceal therapy | 131 | 83 (63.4%) | 110 | 57 (51.8%) | 0.071 |
| EVL as last endoscopic variceal therapeutic approach | 131 | 73 (55.7%) | 110 | 47 (42.7%) | 0.044 |
| EIS as last endoscopic variceal therapeutic approach | 131 | 10 (7.6%) | 110 | 10 (9.1%) | 0.683 |
| Interval between last endoscopic variceal therapy and CT (days) | 83 | 322.35 ± 399.43 188.00 (1.00–1644.00) |
53 | 303.47 ± 328.70 201.00 (3.00–1676.00) |
0.598 |
| NSBBs within 1 month before admission | 111 | 15 (13.5%) | 79 | 16 (20.3%) | 0.215 |
|
| |||||
| Laboratory data | |||||
| Red blood cell (1012/L) | 131 | 3.64 ± 0.96 3.70 (1.59–9.92) |
110 | 3.60 ± 0.82 3.76 (1.51–5.05) |
0.964 |
| Hemoglobin (g/L) | 131 | 100.87 ± 27.68 101.00 (28.00–181.00) |
110 | 105.80 ± 29.28 106.00 (32.00–159.00) |
0.152 |
| White blood cell (109/L) | 131 | 3.73 ± 2.90 3.20 (0.70–21.60) |
110 | 4.80 ± 3.03 4.30 (1.00–20.80) |
<0.0001 |
| Platelet (109/L) | 131 | 86.65 ± 76.13 68.00 (15.00–681.00) |
110 | 120.42 ± 85.34 91.00 (23.00–470.00) |
<0.0001 |
| Total bilirubin (µmol/L) | 131 | 26.14 ± 25.71 18.50 (5.60–215.30) |
110 | 26.45 ± 27.96 17.60 (6.20–216.50) |
0.614 |
| Albumin (g/L) | 131 | 34.77 ± 6.47 35.30 (14.20–71.40) |
110 | 34.55 ± 5.97 35.15 (19.00–50.60) |
0.766 |
| Alanine aminotransferase (U/L) | 131 | 25.77 ± 15.90 20.95 (4.23–99.13) |
110 | 33.98 ± 39.01 24.33 (4.47–332.50) |
0.119 |
| Aspartate aminotransferase (U/L) | 131 | 37.47 ± 24.15 28.62 (9.63–151.35) |
110 | 45.59 ± 43.11 32.52 (9.74–376.35) |
0.057 |
| Alkaline phosphatase (U/L) | 131 | 100.08 ± 62.67 84.94 (24.35–399.34) |
110 | 124.22 ± 109.93 94.61 (31.00–983.93) |
0.014 |
| γ-Glutamyl transpeptidase (U/L) | 131 | 76.67 ± 193.57 28.60 (9.64–1779.18) |
110 | 107.93 ± 234.39 41.05 (7.49–1680.03) |
0.042 |
| Blood urea nitrogen (mmol/L) | 131 | 5.65 ± 2.48 5.16 (1.88–20.15) |
110 | 5.82 ± 2.77 5.29 (1.86–18.83) |
0.897 |
| Creatinine (µmol/L) | 131 | 65.57 ± 18.07 62.30 (36.39–178.55) |
110 | 62.74 ± 16.30 58.67 (27.95–112.58) |
0.223 |
| Potassium (mmol/L) | 131 | 3.90 ± 0.42 3.91 (2.70–5.87) |
110 | 3.87 ± 0.43 3.97 (2.42–4.96) |
0.950 |
| Sodium (mmol/L) | 131 | 138.72 ± 2.62 139.00 (127.00–147.70) |
110 | 138.84 ± 3.65 139.55 (118.00–145.20) |
0.194 |
| Prothrombin time (seconds) | 131 | 16.52 ± 2.17 16.20 (12.50–23.10) |
110 | 16.06 ± 2.85 15.20 (12.60–28.00) |
0.005 |
| Activated partial thromboplastin time (seconds) | 131 | 40.86 ± 5.01 40.20 (30.30–58.10) |
110 | 40.39 ± 6.17 39.90 (19.80–71.30) |
0.400 |
| International normalized ratio | 131 | 1.35 ± 0.25 1.31 (1.01–2.56) |
110 | 1.30 ± 0.31 1.22 (0.94–2.77) |
0.005 |
| Child-Pugh score | 131 | 6.77 ± 1.61 6.00 (5.00–12.00) |
110 | 6.69 ± 1.71 6.00 (5.00–13.00) |
0.534 |
| Child-Pugh class (A/B/C) | 131 | 71 (54.2%)/51 (38.9%)/9 (6.9%) | 110 | 60 (54.5%)/42 (38.2%)/8 (7.3%) | 0.988 |
| MELD score | 131 | 6.93 ± 4.21 6.66 (0.03–24.73) |
110 | 5.93 ± 4.77 5.09 (−2.13–27.42) |
0.017 |
| EVs on endoscopyb | 110 | 103 (93.6%) | 99 | 72 (72.7%) | <0.0001 |
| EVNTs on endoscopyb | 109c | 65 (59.6%) | 99 | 39 (39.4%) | 0.002 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 2 patients;bas for the patients who underwent endoscopic variceal therapy, only EVs on endoscopy performed after CT during the same hospitalizations were evaluated; as for the patients who did not undergo endoscopic variceal therapy, EVs on endoscopy performed during the same hospitalizations were evaluated, regardless of the order of CT and endoscopy;cEVNTs could not be evaluated due to the absence of detailed grade of EVs in their endoscopic reports. Pts, patients; SD, standard deviation; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy; CT, computed tomography; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
Prevalence of ECVs in patients who underwent EVL and EIS and those who did not undergo endoscopic variceal therapy was 60.8%, 50%, and 47.5%, respectively.
Univariate logistic regression analyses showed that HCV, previous EVL, WBC, PLT, ALT, AST, AKP, MELD score, EVs, and EVNTs were significantly associated with ECVs. Because there is a collinearity between WBC and PLT, only PLT was included in the multivariate analyses. Because there is a collinearity between ALT and AST, only ALT was included in the multivariate analyses. Because there is a collinearity between EVs and EVNTs, only EVNTs were included in the multivariate analyses. Multivariate logistic regression analyses showed that HCV (OR = 0.250, 95% CI = 0.074–0.846, p = 0.026), previous EVL (OR = 1.929, 95% CI = 1.016–3.661, p = 0.044), PLT (OR = 0.993, 95% CI = 0.988–0.998, p = 0.008), and EVNTs (OR = 2.422, 95% CI = 1.297–4.522, p = 0.006) were independently associated with ECVs (Table 3).
Table 3.
Univariate and multivariate analysis for risk factors for ECVs.
| Variables | No. Ptsa | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| Age (Years) | 241 | 1.010 | 0.985–1.035 | 0.436 | |||
| Sex (male) | 241 | 1.227 | 0.701–2.148 | 0.474 | |||
|
| |||||||
| Etiology of liver diseases | |||||||
| Hepatitis B virus infection | 241 | 1.411 | 0.843–2.363 | 0.191 | |||
| Hepatitis C virus infection | 241 | 0.272 | 0.095–0.782 | 0.016 | 0.250 | 0.074–0.846 | 0.026 |
| Alcohol abuse | 241 | 1.152 | 0.683–1.943 | 0.596 | |||
| Drug related | 241 | 1.029 | 0.410–2.581 | 0.952 | |||
| Autoimmune liver diseases | 241 | 0.634 | 0.228–1.760 | 0.381 | |||
|
| |||||||
| Clinical presentations at admission | |||||||
| Hepatic encephalopathy | 241 | 0.204 | 0.022–1.851 | 0.158 | |||
| Gastrointestinal bleeding | 241 | 1.415 | 0.838–2.388 | 0.194 | |||
| Ascites | 241 | 1.479 | 0.887–2.464 | 0.133 | |||
|
| |||||||
| History | |||||||
| History of gastrointestinal bleeding | 241 | 1.164 | 0.681–1.989 | 0.597 | |||
| History of endoscopic variceal therapy | 241 | 1.608 | 0.960–2.693 | 0.071 | |||
| EVL alone as last endoscopic variceal therapeutic approach | 241 | 1.687 | 1.012–2.814 | 0.045 | 1.929 | 1.016–3.661 | 0.044 |
| EIS alone as last endoscopic variceal therapeutic approach | 241 | 0.826 | 0.331–2.065 | 0.683 | |||
| Interval between last endoscopic variceal therapy and CT (days) | 136 | 1.000 | 0.999–1.001 | 0.772 | |||
| NSBBs within 1 month before admission | 190 | 0.615 | 0.284–1.332 | 0.218 | |||
|
| |||||||
| Laboratory data | |||||||
| Red blood cell (1012/L) | 241 | 1.060 | 0.798–1.408 | 0.687 | |||
| Hemoglobin (g/L) | 241 | 0.994 | 0.985–1.003 | 0.181 | |||
| White blood cell (109/L) | 241 | 0.876 | 0.793–0.968 | 0.010 | |||
| Platelet (109/L) | 241 | 0.994 | 0.990–0.998 | 0.003 | 0.993 | 0.988–0.998 | 0.008 |
| Total bilirubin (µmol/L) | 241 | 1.000 | 0.990–1.009 | 0.928 | |||
| Albumin (g/L) | 241 | 1.006 | 0.966–1.048 | 0.777 | |||
| Alanine aminotransferase (U/L) | 241 | 0.987 | 0.975–1.000 | 0.043 | 0.989 | 0.973–1.004 | 0.152 |
| Aspartate aminotransferase (U/L) | 241 | 0.992 | 0.983–1.001 | 0.084 | |||
| Alkaline phosphatase (U/L) | 241 | 0.996 | 0.992–1.000 | 0.047 | 1.000 | 0.996–1.004 | 0.966 |
| γ-Glutamyl transpeptidase (U/L) | 241 | 0.999 | 0.998–1.001 | 0.274 | |||
| Blood urea nitrogen (mmol/L) | 241 | 0.976 | 0.886–1.075 | 0.623 | |||
| Creatinine (µmol/L) | 241 | 1.010 | 0.994–1.026 | 0.209 | |||
| Potassium (mmol/L) | 241 | 1.227 | 0.670–2.244 | 0.508 | |||
| Sodium (mmol/L) | 241 | 0.988 | 0.910–1.072 | 0.763 | |||
| Prothrombin time (seconds) | 241 | 1.078 | 0.970–1.198 | 0.162 | |||
| Activated partial thromboplastin time (seconds) | 241 | 1.015 | 0.970–1.063 | 0.517 | |||
| International normalized ratio | 241 | 2.007 | 0.765–5.262 | 0.157 | |||
| Child-Pugh score | 241 | 1.030 | 0.883–1.202 | 0.707 | |||
| MELD score | 241 | 1.053 | 0.992–1.117 | 0.088 | 1.061 | 0.987–1.140 | 0.110 |
| EVs on endoscopyb | 209 | 5.518 | 2.279–13.358 | <0.0001 | |||
| EVNTs on endoscopyb | 208c | 2.273 | 1.304–3.962 | 0.004 | 2.422 | 1.297–4.522 | 0.006 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 2 patients; bas for the patients who underwent endoscopic variceal therapy, only EVs on endoscopy performed after CT during the same hospitalizations were evaluated; as for the patients who did not undergo endoscopic variceal therapy, EVs on endoscopy performed during the same hospitalizations were evaluated, regardless of the order of CT and endoscopy; cEVNTs could not be evaluated due to the absence of detailed grade of EVs in their endoscopic reports. CI, confidence interval; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy; CT, computed tomography; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
3.3. Subgroup Analysis in Patients Who Underwent Endoscopic Variceal Therapy
Compared with those without ECVs, patients with ECVs had significantly lower proportion of HCV (p = 0.010) and levels of WBC (p = 0.036), PLT (p = 0.014), AST (p = 0.045), AKP (p < 0.0001), and GGT (p = 0.008) and higher levels of Cr (p = 0.010) and MELD score (p = 0.018) (Table 4).
Table 4.
Comparison of patients with previous endoscopic variceal therapy between ECVs and no ECVs groups.
| Variables | ECVs | No ECVs | p value | ||
|---|---|---|---|---|---|
| No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
||
| Age (Years) | 83 | 57.38 ± 10.38 | 57 | 54.44 ± 10.53 | 0.150 |
| Sex (male) | 83 | 64 (77.1%) | 57 | 36 (63.2%) | 0.073 |
|
| |||||
| Etiology of liver diseases | |||||
| Hepatitis B virus infection | 83 | 44 (53.0%) | 57 | 23 (40.4%) | 0.141 |
| Hepatitis C virus infection | 83 | 2 (2.4%) | 57 | 9 (15.8%) | 0.010 |
| Alcohol abuse | 83 | 31 (37.3%) | 57 | 17 (29.8%) | 0.357 |
| Drug related | 83 | 3 (3.6%) | 57 | 4 (7.0%) | 0.608 |
| Autoimmune liver diseases | 83 | 4 (4.8%) | 57 | 7 (12.3%) | 0.196 |
|
| |||||
| Clinical presentations at admission | |||||
| Hepatic encephalopathy | 83 | 0 (0.0%) | 57 | 2 (3.5%) | 0.164 |
| Gastrointestinal bleeding | 83 | 32 (38.6%) | 57 | 15 (26.3%) | 0.132 |
| Ascites (no/mild/moderate-severe) | 83 | 36 (43.4%)/36 (43.4%)/11 (13.3%) | 57 | 27 (47.4%)/23 (40.4%)/7 (12.3%) | 0.897 |
|
| |||||
| History | |||||
| History of gastrointestinal bleeding | 83 | 72 (86.7%) | 57 | 55 (96.5%) | 0.051 |
| EVL as last endoscopic variceal therapeutic approach | 83 | 73 (88.0%) | 57 | 47 (82.5%) | 0.361 |
| EIS as last endoscopic variceal therapeutic approach | 83 | 10 (12.0%) | 57 | 10 (17.5%) | 0.361 |
| Interval between last endoscopic variceal therapy and CT (Days) | 83 | 322.35 ± 399.43 188.00 (1.00–1644.00) |
53 | 303.47 ± 328.70 201.00 (3.00–1676.00) |
0.598 |
| NSBBs within 1 month before admission | 76 | 15 (19.7%) | 43 | 15 (34.9%) | 0.068 |
|
| |||||
| Laboratory data | |||||
| Red blood cell (1012/L) | 83 | 3.70 ± 0.80 3.78 (1.77–5.49) |
57 | 3.69 ± 0.78 3.84 (1.51–4.94) |
0.973 |
| Hemoglobin (g/L) | 83 | 102.88 ± 25.40 104.00 (46.00–161.00) |
57 | 104.30 ± 26.21 106.00 (32.00–153.00) |
0.722 |
| White blood cell (109/L) | 83 | 3.54 ± 1.95 3.30 (1.20–11.90) |
57 | 4.43 ± 2.65 3.80 (1.30–15.20) |
0.036 |
| Platelet (109/L) | 83 | 86.96 ± 64.64 68.00 (15.00–457.00) |
57 | 115.98 ± 80.36 86.00 (23.00–448.00) |
0.014 |
| Total bilirubin (µmol/L) | 83 | 22.00 ± 13.42 17.10 (7.60–78.20) |
57 | 21.99 ± 14.41 17.60 (8.80–92.60) |
0.916 |
| Albumin (g/L) | 83 | 35.41 ± 6.59 35.60 (21.50–71.40) |
57 | 34.95 ± 4.95 35.20 (23.10–45.60) |
0.620 |
| Alanine aminotransferase (U/L) | 83 | 22.97 ± 10.76 20.95 (6.78–54.73) |
57 | 27.21 ± 18.56 21.93 (4.52–113.78) |
0.346 |
| Aspartate aminotransferase (U/L) | 83 | 33.51 ± 19.23 27.81 (9.63–130.22) |
57 | 37.23 ± 18.36 31.28 (17.22–118.28) |
0.045 |
| Alkaline phosphatase (U/L) | 83 | 88.02 ± 49.41 79.00 (24.35–351.33) |
57 | 120.61 ± 68.41 98.96 (45.45–466.34) |
<0.0001 |
| γ-Glutamyl transpeptidase (U/L) | 83 | 44.40 ± 54.54 23.49 (9.64–357.32) |
57 | 84.54 ± 221.41 39.14 (7.49–1680.03) |
0.008 |
| Blood urea nitrogen (mmol/L) | 83 | 5.93 ± 2.87 5.33 (1.88–20.15) |
57 | 5.81 ± 2.43 5.28 (1.88–14.69) |
0.966 |
| Creatinine (µmol/L) | 83 | 64.26 ± 12.94 62.12 (36.39–108.80) |
57 | 58.78 ± 14.12 56.99 (34.51–109.21) |
0.010 |
| Potassium (mmol/L) | 83 | 3.92 ± 0.37 3.86 (3.34–5.87) |
57 | 3.94 ± 0.42 3.99 (2.76–4.96) |
0.289 |
| Sodium (mmol/L) | 83 | 138.66 ± 2.11 138.70 (133.40–147.70) |
57 | 138.78 ± 3.41 139.50 (127.50–143.80) |
0.087 |
| Prothrombin time (seconds) | 83 | 16.23 ± 1.84 16.10 (13.50–22.50) |
57 | 15.97 ± 2.32 15.40 (12.80–25.20) |
0.160 |
| Activated partial thromboplastin time (seconds) | 83 | 40.13 ± 4.42 39.70 (33.30–54.10) |
57 | 40.58 ± 5.93 39.60 (32.80–71.30) |
0.966 |
| International normalized ratio | 83 | 1.33 ± 0.23 1.30 (1.06–2.56) |
57 | 1.30 ± 0.25 1.23 (0.98–2.41) |
0.212 |
| Child-Pugh score | 83 | 6.51 ± 1.27 6.00 (5.00–11.00) |
57 | 6.47 ± 1.35 6.00 (5.00–11.00) |
0.809 |
| Child-Pugh class (A/B/C) | 83 | 48 (57.8%)/34 (41.0%)/1 (1.2%) | 57 | 32 (56.1%)/22 (38.6%)/3 (5.3%) | 0.366 |
| MELD score | 83 | 6.33 ± 3.51 6.66 (0.48–15.42) |
57 | 5.15 ± 4.24 4.28 (−1.32–19.38) |
0.018 |
| EVs on endoscopyb | 62 | 57 (91.9%) | 46 | 39 (84.8%) | 0.242 |
| EVNTs on endoscopyb | 62 | 27 (43.5%) | 46 | 12 (26.1%) | 0.062 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 1 patient; bas for the patients who underwent endoscopic variceal therapy, only EVs on endoscopy performed after CT during the same hospitalizations were evaluated; as for the patients who did not undergo endoscopic variceal therapy, EVs on endoscopy performed during the same hospitalizations were evaluated, regardless of the order of CT and endoscopy. Pts, patients; SD, standard deviation; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy; CT, computed tomography; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
Univariate logistic regression analyses showed that sex, HCV, history of GIB, NSBBs, WBC, PLT, AKP, Cr, MELD score, and EVNTs were significantly associated with ECVs. Because there is a collinearity between WBC and PLT, only PLT was included in the multivariate analyses. Because there is a collinearity between Cr and MELD score, only MELD score was included in the multivariate analyses (Table 5). Multivariate logistic regression analyses showed that the use of NSBBs (OR = 0.294, 95% CI = 0.091–0.957, p = 0.042) was independently associated with ECVs (Table 5).
Table 5.
Univariate and multivariate analysis for risk factors for ECVs in patients with previous endoscopic variceal therapy.
| Variables | No. Ptsa | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| Age (years) | 140 | 1.028 | 0.994–1.062 | 0.107 | |||
| Sex (male) | 140 | 1.965 | 0.935–4.130 | 0.075 | 1.171 | 0.312–4.391 | 0.815 |
|
| |||||||
| Etiology of liver diseases | |||||||
| Hepatitis B virus infection | 140 | 1.668 | 0.843–3.300 | 0.142 | |||
| Hepatitis C virus infection | 140 | 0.132 | 0.027–0.635 | 0.012 | NA | NA | 0.999 |
| Alcohol abuse | 140 | 1.403 | 0.682–2.885 | 0.358 | |||
| Drug related | 140 | 0.497 | 0.107–2.310 | 0.372 | |||
| Autoimmune liver diseases | 140 | 0.362 | 0.101–1.299 | 0.119 | |||
|
| |||||||
| Clinical presentations at admission | |||||||
| Hepatic encephalopathy | 140 | NA | NA | 0.999 | |||
| Gastrointestinal bleeding | 140 | 1.757 | 0.841–3.671 | 0.134 | |||
| Ascites | 140 | 1.175 | 0.597–2.313 | 0.641 | |||
|
| |||||||
| History | |||||||
| History of gastrointestinal bleeding | 140 | 0.238 | 0.051–1.118 | 0.069 | NA | NA | 0.999 |
| EVL alone as last endoscopic variceal therapeutic approach | 140 | 1.553 | 0.601–4.016 | 0.364 | |||
| EIS alone as last endoscopic variceal therapeutic approach | 140 | 0.644 | 0.249–1.665 | 0.364 | |||
| Interval between last endoscopic variceal therapy and CT (days) | 136 | 1.000 | 0.999–1.001 | 0.772 | |||
| NSBBs within 1 month before admission | 119 | 0.459 | 0.197–1.068 | 0.071 | 0.294 | 0.091–0.957 | 0.042 |
|
| |||||||
| Laboratory data | |||||||
| Red blood cell (1012/L) | 140 | 1.028 | 0.670–1.578 | 0.899 | |||
| Hemoglobin (g/L) | 140 | 0.998 | 0.985–1.011 | 0.747 | |||
| White blood cell (109/L) | 140 | 0.840 | 0.717–0.984 | 0.031 | |||
| Platelet (109/L) | 140 | 0.994 | 0.989–0.999 | 0.027 | 0.993 | 0.983–1.003 | 0.157 |
| Total bilirubin (µmol/L) | 140 | 1.000 | 0.976–1.025 | 0.997 | |||
| Albumin (g/L) | 140 | 1.013 | 0.956–1.073 | 0.654 | |||
| Alanine aminotransferase (U/L) | 140 | 0.979 | 0.955–1.004 | 0.102 | |||
| Aspartate aminotransferase (U/L) | 140 | 0.990 | 0.972–1.008 | 0.258 | |||
| Alkaline phosphatase (U/L) | 140 | 0.989 | 0.982–0.997 | 0.004 | 0.997 | 0.989–1.006 | 0.536 |
| γ-Glutamyl transpeptidase (U/L) | 140 | 0.996 | 0.990–1.002 | 0.204 | |||
| Blood urea nitrogen (mmol/L) | 140 | 1.018 | 0.896–1.156 | 0.784 | |||
| Creatinine (µmol/L) | 140 | 1.033 | 1.005–1.062 | 0.022 | |||
| Potassium (mmol/L) | 140 | 0.867 | 0.361–2.080 | 0.749 | |||
| Sodium (mmol/L) | 140 | 0.983 | 0.867–1.115 | 0.794 | |||
| Prothrombin time (seconds) | 140 | 1.066 | 0.899–1.264 | 0.461 | |||
| Activated partial thromboplastin time (seconds) | 140 | 0.983 | 0.920–1.050 | 0.609 | |||
| International normalized ratio | 140 | 1.725 | 0.392–7.590 | 0.471 | |||
| Child-Pugh score | 140 | 1.020 | 0.785–1.324 | 0.885 | |||
| MELD score | 140 | 1.088 | 0.990–1.196 | 0.079 | 1.232 | 0.982–1.544 | 0.071 |
| EVs on endoscopyb | 108 | 2.046 | 0.605–6.915 | 0.249 | |||
| EVNTs on endoscopyb | 108 | 2.186 | 0.955–5.001 | 0.064 | 2.931 | 0.879–9.780 | 0.080 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 1 patient; bas for the patients who underwent endoscopic variceal therapy, only EVs on endoscopy performed after CT during the same hospitalizations were evaluated; as for the patients who did not undergo endoscopic variceal therapy, EVs on endoscopy performed during the same hospitalizations were evaluated, regardless of the order of CT and endoscopy. CI, confidence interval; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy; CT, computed tomography; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins; NA, not available.
3.4. Subgroup Analysis in Patients Who Did Not Undergo Endoscopic Variceal Therapy
Compared with those without ECVs, patients with ECVs had significantly lower levels of WBC (p = 0.002) and PLT (p = 0.003) and higher proportions of EVs (p < 0.0001) and EVNTs (p = 0.002) and levels of INR (p = 0.007) and PT (p = 0.010) (Table 6).
Table 6.
Comparison of patients without previous endoscopic variceal therapy between ECVs and no ECVs groups.
| Variables | ECVs | No ECVs | p value | ||
|---|---|---|---|---|---|
| No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
No. Ptsa | Mean ± SD Median (range) or frequency (percentage) |
||
| Age (Years) | 48 | 52.52 ± 10.88 | 53 | 54.67 ± 9.51 | 0.348 |
| Sex (male) | 48 | 32 (66.7%) | 53 | 40 (75.5%) | 0.329 |
|
| |||||
| Etiology of liver diseases | |||||
| Hepatitis B virus infection | 48 | 17 (35.4%) | 53 | 19 (35.8%) | 0.964 |
| Hepatitis C virus infection | 48 | 3 (6.2%) | 53 | 5 (9.4%) | 0.824 |
| Alcohol abuse | 48 | 21 (43.8%) | 53 | 23 (4.34%) | 0.971 |
| Drug related | 48 | 8 (16.7%) | 53 | 5 (9.4%) | 0.278 |
| Autoimmune liver diseases | 48 | 3 (6.2%) | 53 | 2 (3.8%) | 0.909 |
|
| |||||
| Clinical presentations at admission | |||||
| Hepatic encephalopathy | 48 | 1 (2.1%) | 53 | 2 (3.8%) | 1.000 |
| Gastrointestinal bleeding | 48 | 24 (50.0%) | 53 | 23 (43.4%) | 0.506 |
| Ascites (no/mild/moderate-severe) | 48 | 18 (37.5%)/16 (33.3%)/14 (29.2%) | 53 | 29 (54.7%)/14 (26.4%)/10 (18.9%) | 0.209 |
|
| |||||
| History | |||||
| History of gastrointestinal bleeding | 48 | 17 (35.4%) | 53 | 16 (30.2%) | 0.576 |
| NSBBs within 1 month before admission | 35 | 0 (0.0%) | 36 | 1 (2.8%) | 1.000 |
|
| |||||
| Laboratory data | |||||
| Red blood cell (1012/L) | 48 | 3.56 ± 1.20 3.51 (1.59–9.92) |
53 | 3.51 ± 0.87 3.71 (1.91–5.05) |
0.897 |
| Hemoglobin (g/L) | 48 | 97.40 ± 31.20 96.00 (28.00–181.00) |
53 | 107.42 ± 32.45 106.00 (37.00–159.00) |
0.103 |
| White blood cell (109/L) | 48 | 4.05 ± 4.06 3.15 (0.70–21.60) |
53 | 5.19 ± 3.38 4.5.0 (1.00–20.80) |
0.002 |
| Platelet (109/L) | 48 | 86.10 ± 93.50 66.50 (26.00–681.00) |
53 | 125.19 ± 90.92 98.00 (30.00–470.00) |
0.003 |
| Total bilirubin (µmol/L) | 48 | 33.30 ± 37.83 20.95 (5.60–215.30) |
53 | 31.24 ± 37.02 19.30 (6.20–216.50) |
0.324 |
| Albumin (g/L) | 48 | 33.68 ± 6.16 33.65 (14.20–45.10) |
53 | 34.11 ± 6.92 34.60 (19.00–50.60) |
0.916 |
| Alanine aminotransferase (U/L) | 48 | 30.61 ± 21.43 21.24 (4.23–99.13) |
53 | 41.26 ± 52.10 28.57 (4.47–332.50) |
0.395 |
| Aspartate aminotransferase (U/L) | 48 | 44.33 ± 29.87 32.20 (15.35–151.35) |
53 | 54.57 ± 58.09 38.96 (8.74–376.35) |
0.589 |
| Alkaline phosphatase (U/L) | 48 | 120.94 ± 76.83 94.56 (33.00–399.34) |
53 | 128.10 ± 142.35 83.00 (31.00–983.93) |
0.395 |
| γ-Glutamyl transpeptidase (U/L) | 48 | 132.48 ± 305.66 41.52 (11.42–1779.18) |
53 | 133.09 ± 247.23 41.56 (8.23–1283.03) |
0.903 |
| Blood urea nitrogen (mmol/L) | 48 | 5.16 ± 1.52 4.99 (2.31–9.53) |
53 | 5.83 ± 3.12 5.29 (1.86–18.83) |
0.799 |
| Creatinine (µmol/L) | 48 | 67.84 ± 24.56 63.03 (37.66–178.55) |
53 | 66.99 ± 17.50 64.75 (27.95–112.58) |
0.572 |
| Potassium (mmol/L) | 48 | 3.87 ± 0.50 3.94 (2.70–5.19) |
53 | 3.79 ± 0.43 3.95 (2.42–4.64) |
0.452 |
| Sodium (mmol/L) | 48 | 138.84 ± 3.35 139.75 (127.00–143.40) |
53 | 138.92 ± 3.92 139.60 (118.00–145.20) |
0.984 |
| Prothrombin time (seconds) | 48 | 17.03 ± 2.60 16.45 (12.50–23.10) |
53 | 16.17 ± 3.34 15.20 (12.60–28.00) |
0.010 |
| Activated partial thromboplastin time (seconds) | 48 | 42.13 ± 5.72 41.90 (30.30–58.10) |
53 | 40.20 ± 6.47 40.10 (19.80–55.30) |
0.134 |
| International normalized ratio | 48 | 1.40 ± 0.27 1.33 (1.01–2.07) |
53 | 1.31 ± 0.36 1.20 (0.94–2.77) |
0.007 |
| Child-Pugh score | 48 | 7.23 ± 1.99 7.00 (5.00–12.00) |
53 | 6.92 ± 2.02 6.00 (5.00–13.00) |
0.350 |
| Child-Pugh class (A/B/C) | 48 | 23 (47.9%)/17 (35.4%)/8 (16.75) | 53 | 28 (52.8%)/20 (37.7%)/5 (9.4%) | 0.554 |
| MELD score | 48 | 7.99 ± 5.09 6.72 (0.03–24.73) |
53 | 6.78 ± 5.19 6.09 (−2.13–27.42) |
0.163 |
| EVs on endoscopy | 48 | 46 (95.8%) | 53 | 33 (62.3%) | <0.0001 |
| EVNTs on endoscopy | 47b | 39 (80.9%) | 53 | 27 (50.9%) | 0.002 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 1 patient; bEVNTs could not be evaluated due to the absence of detailed grade of EVs in their endoscopic reports. Pts, patients; SD, standard deviation; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
Univariate logistic regression analyses showed that ascites, PLT, EVs, and EVNTs were significantly associated with ECVs. Because there is a collinearity between EVs and EVNTs, only EVNTs were included in the multivariate analyses (Table 7). Multivariate logistic regression analyses showed that the presence of EVNTs (OR = 3.714, 95% CI = 1.469–9.391, p = 0.006) was independently associated with ECVs (Table 7).
Table 7.
Univariate and multivariate analysis for risk factors for ECVs in patients without previous endoscopic variceal therapy.
| Variables | No. Ptsa | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| Age (years) | 101 | 0.979 | 0.941–1.018 | 0.291 | |||
| Sex (male) | 101 | 1.538 | 0.646–3.661 | 0.330 | |||
|
| |||||||
| Etiology of liver diseases | |||||||
| Hepatitis B virus infection | 101 | 0.981 | 0.434–2.218 | 0.964 | |||
| Hepatitis C virus infection | 101 | 0.640 | 0.145–2.834 | 0.557 | |||
| Alcohol abuse | 101 | 1.014 | 0.462–2.230 | 0.971 | |||
| Drug related | 101 | 1.920 | 0.582–6.334 | 0.284 | |||
| Autoimmune liver diseases | 101 | 1.700 | 0.272–10.635 | 0.571 | |||
|
| |||||||
| Clinical presentations at admission | |||||||
| Hepatic encephalopathy | 101 | 0.543 | 0.048–6.181 | 0.622 | |||
| Gastrointestinal bleeding | 101 | 1.304 | 0.595–2.858 | 0.507 | |||
| Ascites | 101 | 2.014 | 0.908–4.465 | 0.085 | 1.488 | 0.628–3.527 | 0.367 |
|
| |||||||
| History | |||||||
| History of gastrointestinal bleeding | 101 | 1.268 | 0.551–2.917 | 0.576 | |||
| NSBBs within 1 month before admission | 71 | NA | NA | 1.000 | |||
|
| |||||||
| Laboratory data | |||||||
| Red blood cell (1012/L) | 101 | 1.042 | 0.712–1.526 | 0.832 | |||
| Hemoglobin (g/L) | 101 | 0.99 | 0.978–1.003 | 0.119 | |||
| White blood cell (109/L) | 101 | 0.911 | 0.805–1.031 | 0.141 | |||
| Platelet (109/L) | 101 | 0.994 | 0.987–1.000 | 0.054 | 0.995 | 0.990–1.001 | 0.092 |
| Total bilirubin (µmol/L) | 101 | 1.001 | 0.991–1.012 | 0.781 | |||
| Albumin (g/L) | 101 | 0.990 | 0.932–1.051 | 0.737 | |||
| Alanine aminotransferase (U/L) | 101 | 0.992 | 0.979–1.005 | 0.219 | |||
| Aspartate aminotransferase (U/L) | 101 | 0.995 | 0.985–1.005 | 0.292 | |||
| Alkaline phosphatase (U/L) | 101 | 0.999 | 0.996–1.003 | 0.756 | |||
| γ-Glutamyl transpeptidase (U/L) | 101 | 1.000 | 0.999–1.001 | 0.991 | |||
| Blood urea nitrogen (mmol/L) | 101 | 0.891 | 0.750–1.059 | 0.191 | |||
| Creatinine (µmol/L) | 101 | 1.002 | 0.983–1.021 | 0.839 | |||
| Potassium (mmol/L) | 101 | 1.505 | 0.635–3.564 | 0.353 | |||
| Sodium (mmol/L) | 101 | 0.994 | 0.892–1.107 | 0.912 | |||
| Prothrombin time (seconds) | 101 | 1.102 | 0.963–1.262 | 0.158 | |||
| Activated partial thromboplastin time (seconds) | 101 | 1.054 | 0.986–1.127 | 0.121 | |||
| International normalized ratio | 101 | 2.543 | 0.705–9.174 | 0.154 | |||
| Child-Pugh score | 101 | 1.080 | 0.887–1.315 | 0.444 | |||
| MELD score | 101 | 1.048 | 0.969–1.134 | 0.243 | |||
| EVs on endoscopy | 101 | 13.939 | 3.046–63.783 | 0.001 | |||
| EVNTs on endoscopy | 100b | 4.066 | 1.646–10.044 | 0.002 | 3.714 | 1.469–9.391 | 0.006 |
Notes: aECVs could not be evaluated because the venous vessels were not obviously enhanced in 1 patient;bEVNTs could not be evaluated due to the absence of detailed grade of EVs in their endoscopic reports. CI, confidence interval; NSBBs, nonselective beta-blockers; MELD, model for end stage of liver disease; EVs, esophageal varices; ECVs, esophageal collateral veins.
4. Discussion
The present study showed that HCV infection, a low PLT count, presence of EVNTs, and previous EVL were independently associated with ECVs in cirrhosis.
EVL and EIS were the common endoscopic variceal therapy approaches for controlling variceal hemorrhage and preventing from first or recurrent bleeding from high-risk varices. Current guidelines recommend EVL as the preferred endoscopic therapy because EVL may be superior to EIS in terms of complications and patients' outcomes [14, 15]. In details, a meta-analysis showed that patients who underwent EVL might have significantly higher variceal elimination rate and lower rebleeding rate than those who underwent EIS [16]. However, the choice of endoscopic variceal therapy might influence the presence of ECVs [8, 10]. The present study also reported that the prevalence of ECVs was different between patients with and without history of endoscopic esophageal variceal therapy, and it was higher in patients who underwent EVL than those who underwent EIS (60.8% versus 50%). This could be explained that EVL only achieved superficial eradication of EVs through a mechanical constriction, but EIS could act on submucosal tissues through a chemical reaction, which would reduce the number and size of ECVs and even obliterate ECVs completely [11, 17, 18].
NSBBs are recommended as another first-line therapy for preventing variceal bleeding in patients with high-risk varices because it could significantly reduce portal pressure [15, 19]. Besides, a previous study has confirmed that NSBBs could slow the development of ECVs and reduce the size of ECVs [20]. Similarly, the present study demonstrated that patients with history of endoscopic variceal therapy who adhered to the use of NSBBs had a lower risk of developing ECVs.
EVs and ECVs, the common types of portosystemic collateral veins, were one of the common consequences of portal hypertension [1, 21]. According to their location with the esophagus, ECVs can be classified as para-esophageal veins (para-EVs), peri-esophageal veins (peri-EVs), and perforating veins (PVs) [22]. Endoscopic color Doppler ultrasonography demonstrates a blood flow communication between para-EVs or peri-EVs and EVs through PVs [23]. Therefore, it is readily understood that EVNTs are more likely to be accompanied with ECVs. On the other hand, a low PLT count has been widely considered as an indicator for severity of hypersplenism and portal hypertension in cirrhosis [24]. In details, PLT count is a major component of PLT count to spleen diameter ratio (PSR) [25] and Baveno VI criteria [15, 26], which are two important indexes for evaluating EVNTs. The present study also found that a low PLT count was associated with ECVs, which further suggested that the presence of ECVs should be in parallel with the severity of portal hypertension.
Most previous studies have suggested that the presence of ECVs may be associated with the recurrence of EVs [9]. However, it is possible that portal pressure can be reduced by ECVs as collateral vessels. Especially if para-ECVs were not connected with esophageal varices through PVs, they would decrease the risk of variceal recurrence after endoscopic variceal therapy [27]. Therefore, the association of ECVs with recurrence of EVs should be further explored.
There were several limitations in our study. First, this was a single-center retrospective study, in which selection bias and data missing were inevitable. Second, we employed CT scans, but not endoscopic ultrasonography. Thus, the types of ECVs could not be accurately classified. Third, CT images are not ideal in a few patients, in whom ECVs and EVs were not clearly distinguished on CT scans. Fourth, ECVs mostly appear as irregular blood vessel clusters on CT scans, so we cannot measure the diameter of ECVs and record the changes of ECVs.
In conclusion, the presence of ECVs was closely associated with the severity of portal hypertension indicated by lower PLT count and EVNTs. Additionally, EVL might induce the development of ECVs; by comparison, EIS might be more effective for eliminating ECVs. Further prospective studies should be needed to confirm this finding.
Acknowledgments
The authors are indebted to our study team for establishing and updating our prospective database, including Han Deng, Jing Li, Ran Wang, Yingying Li, Qianqian Li, Kexin Zheng, Zhaohui Bai, Xiangbo Xu, Le Wang, and Fangfang Yi, of whom all had worked for our study group. This work was partially supported by the Natural Science Foundation of Liaoning Province (20180530057).
Abbreviations
- EVs:
Esophageal varices
- EVL:
Endoscopic variceal ligation
- EIS:
Endoscopic injection sclerotherapy
- ECVs:
Esophageal collateral veins
- CT:
Computed tomography
- HE:
Hepatic encephalopathy
- GIB:
Gastrointestinal bleeding
- WBC:
White blood cell
- PLT:
Platelet
- TBIL:
Total bilirubin
- ALB:
Albumin
- AKP:
Alkaline phosphatase
- GGT:
γ-Glutamine transferase
- Cr:
Creatinine
- PT:
Prothrombin time
- INR:
International normalized ratio
- NSBBs:
Nonselective beta-blockers
- MELD:
Model for end stage of liver disease
- EVNTs:
Esophageal varices needing treatment
- RC:
Red color
- ORs:
Odds ratios
- CIs:
Confidence intervals
- HCV:
Hepatitis C virus
- para-EVs:
Para-esophageal veins
- peri-EVs:
Peri-esophageal veins
- PVs:
Perforating veins
- PSR:
Platelet count to spleen diameter ratio.
Contributor Information
Hongyu Li, Email: 13309887041@163.com.
Xingshun Qi, Email: xingshunqi@126.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The abstract was published in the Asian Pacific Association for the Study of the Liver (APASL) 2020 Conference as a poster presentation. Please see the following link: https://link.springer.com/content/pdf/10.1007/s12072-020-10030-4.pdf.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Qianqian Li, Xiaozhong Guo, and Ji Feng equally contributed to this work.
References
- 1.Garcia-Tsao G., Bosch J. Management of varices and variceal hemorrhage in cirrhosis. New England Journal of Medicine . 2010;362(9):823–832. doi: 10.1056/nejmra0901512. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G., Sanyal A. J., Grace N. D., Carey W. D. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. American Journal of Gastroenterology . 2007;102(9):2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G., Abraldes J. G., Berzigotti A., Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology . 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Society of Gastroenterology, Chinese Society of Hepatology, Chinese Society of Endoscopy, Association C. M. Consensus on prevention and treatment for gastroesophageal varices and variceal hemorrhage in liver cirrhosis. Chinese Journal of Digestive Diseases . 2008;28:551–558. [Google Scholar]
- 5.Bandali M. F., Mirakhur A., Lee E. W., et al. Portal hypertension: imaging of portosystemic collateral pathways and associated image-guided therapy. World Journal of Gastroenterology . 2017;23(10):1735–1746. doi: 10.3748/wjg.v23.i10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masalaite L., Valantinas J., Stanaitis J. The role of collateral veins detected by endosonography in predicting the recurrence of esophageal varices after endoscopic treatment: a systematic review. Hepatology International . 2014;8(3):339–351. doi: 10.1007/s12072-014-9547-3. [DOI] [PubMed] [Google Scholar]
- 7.Masalaite L., Valantinas J., Stanaitis J. Endoscopic ultrasound findings predict the recurrence of esophageal varices after endoscopic band ligation: a prospective cohort study. Scandinavian Journal of Gastroenterology . 2015;50(11):1322–1330. doi: 10.3109/00365521.2015.1043640. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J., Zhang Y., Li P., et al. The endoscopic ultrasound probe findings in prediction of esophageal variceal recurrence after endoscopic variceal eradication therapies in cirrhotic patients: a cohort prospective study. BMC Gastroenterology . 2019;19(1):p. 32. doi: 10.1186/s12876-019-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q.-Q., Li H.-Y., Bai Z.-H., Philips C. A., Guo X.-Z., Qi X.-S. Esophageal collateral veins in predicting esophageal variceal recurrence and rebleeding after endoscopic treatment: a systematic review and meta-analysis. Gastroenterology Report . 2020;8(5):355–361. doi: 10.1093/gastro/goaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo G.-H., Lai K.-H., Cheng J.-S., Huang R.-L., Wang S.-J., Chiang H.-T. Prevalence of paraesophageal varices and gastric varices in patients achieving variceal obliteration by banding ligation and by injection sclerotherapy. Gastrointestinal Endoscopy . 1999;49(4):428–436. doi: 10.1016/s0016-5107(99)70038-6. [DOI] [PubMed] [Google Scholar]
- 11.Kume K., Yamasaki M., Watanabe T., Yoshikawa I., Otsuki M., Harada M. Mild collateral varices and a fundic plexus without perforating veins on EUS predict endoscopic non-recurrence of esophageal varices after EVL. Hepato-Gastroenterology . 2011;58:798–801. [PubMed] [Google Scholar]
- 12.Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery . 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Kamath P. S., Kim W. R. The model for end-stage liver disease (MELD) Hepatology . 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 14.Hwang J. H., Shergill A. K., Acosta R. D., et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointestinal Endoscopy . 2014;80(2):221–227. doi: 10.1016/j.gie.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R., Baveno V. I. F. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology . 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Dai C., Liu W. X., Jiang M., Sun M. J. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World Journal of Gastroenterology . 2015;21(8):2534–2541. doi: 10.3748/wjg.v21.i8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitano S., Terblanche J., Kahn D., Bornman P. C. Venous anatomy of the lower oesophagus in portal hypertension: practical implications. British Journal of Surgery . 1986;73:525–531. doi: 10.1002/bjs.1800730704. [DOI] [PubMed] [Google Scholar]
- 18.Irisawa A., Obara K., Sato Y., et al. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointestinal Endoscopy . 1999;50(3):374–380. doi: 10.1053/ge.1999.v50.97777. [DOI] [PubMed] [Google Scholar]
- 19.Villanueva C., Albillos A., Genescà J., et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet . 2019;393(10181):1597–1608. doi: 10.1016/s0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 20.Liao W.-C., Chen P.-H., Hou M.-C., et al. Endoscopic ultrasonography assessment of para-esophageal varices predicts efficacy of propranolol in preventing recurrence of esophageal varices. Journal of Gastroenterology . 2015;50(3):342–349. doi: 10.1007/s00535-014-0970-y. [DOI] [PubMed] [Google Scholar]
- 21.Sanyal A. J., Bosch J., Blei A., Arroyo V. Portal hypertension and its complications. Gastroenterology . 2008;134(6):1715–1728. doi: 10.1053/j.gastro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Tajiri T., Yoshida H., Obara K., et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition) Digestive Endoscopy . 2010;22(1):1–9. doi: 10.1111/j.1443-1661.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 23.Sato T., Yamazaki K., Toyota J., et al. Evaluation of the alternate blood flow in esophageal variceal patients by endoscopic color Doppler ultrasonography. Digestive Endoscopy . 2004;16(3):208–212. doi: 10.1111/j.1443-1661.2004.00350.x. [DOI] [Google Scholar]
- 24.Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver International . 2017;37(6):778–793. doi: 10.1111/liv.13317. [DOI] [PubMed] [Google Scholar]
- 25.Giannini E., Botta F., Borro P., Risso D., Romagnoli P., Fasoli A. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut . 2003;52(8):1200–1205. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustin S., Pons M., Maurice J. B., et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology . 2017;66(6):1980–1988. doi: 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- 27.Irisawa A., Obara K., Bhutani M. S., et al. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. Journal of Gastroenterology and Hepatology . 2003;18(3):309–314. doi: 10.1046/j.1440-1746.2003.02956.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


