Abstract
Background
Inflammation is associated with end-organ disease and mortality for people with human immunodeficiency virus (PWH). Ruxolitinib, a Jak 1/2 inhibitor, reduces systemic inflammation for individuals without human immunodeficiency virus (HIV) and HIV reservoir markers ex vivo. The goal of this trial was to determine safety and efficacy of ruxolitinib for PWH on antiretroviral therapy (ART).
Methods
AIDS Clinical Trials Group (ACTG) A5336 was an open-label, multisite, randomized controlled trial (RCT). Participants were randomly assigned (2:1) using centralized software to ruxolitinib (10 mg twice daily) plus stable ART for 5 weeks vs ART alone, stratified by efavirenz use. Eligible participants were suppressed on ART for ≥2 years, without comorbidities, and had >350 CD4+ T cells/µL. Primary endpoints were premature discontinuation, safety events, and change in plasma interleukin 6 (IL-6). Secondary endpoints included other measures of inflammation/immune activation and HIV reservoir.
Results
Sixty participants were enrolled from 16 May 2016 to 10 January 2018. Primary safety events occurred in 2.5% (1 participant) for ruxolitinib and 0% for controls (P = .67). Three participants (7.5%) prematurely discontinued ruxolitinib. By week 5, differences in IL-6 (mean fold change [FC], 0.93 vs 1.10; P = .18) and soluble CD14 (mean FC, 0.96 vs 1.08; relative FC, 0.96 [90% confidence interval {CI}, .90–1.02]) levels for ruxolitinib vs controls was observed. Ruxolitinib reduced CD4+ T cells expressing HLA-DR/CD38 (mean difference, –0.34% [90% CI, –.66% to –.12%]) and Bcl-2 (mean difference, –3.30% [90% CI, –4.72% to –1.87%]).
Conclusions
In this RCT of healthy, virologically suppressed PWH on ART, ruxolitinib was well-tolerated. Baseline IL-6 levels were normal and showed no significant reduction. Ruxolitinib significantly decreased markers of immune activation and cell survival. Future studies of Jak inhibitors should target PWH with residual inflammation despite suppressive ART.
Clinical Trials Registration
Keywords: HIV, inflammation, Jak inhibitors, immune activation, reservoir
A low dose of ruxolitinib for only 5 weeks can safely reduce important biomarkers of inflammation, T-cell activation, immune dysregulation, cellular lifespan, and intestinal translocation/inflammation/homing while increasing expression of the interleukin 7 receptor for healthy people with human immunodeficiency virus who are virologically suppressed on antiretroviral therapy.
Janus kinase (Jak) and signal transducer and activator of transcription (STAT) inhibitors have revolutionized the treatment of several inflammatory, autoimmune, and neoplastic conditions [1]. Ruxolitinib, an orally bioavailable Jak 1/2 inhibitor approved for the treatment of myelofibrosis and polycythemia vera, reduced inflammatory biomarkers for these and other conditions under investigation [2–4].
Although antiretroviral therapy (ART) is an effective strategy for treating people with human immunodeficiency virus (PWH), systemic inflammation and human immunodeficiency virus (HIV)–related immunologic impairment can persist for some individuals, particularly if ART initiation is delayed [5]. Chronic inflammation is thought to drive several consequences for PWH on ART including increased end-organ damage [6–9], less robust immunologic reconstitution [10, 11], and enhanced susceptibility of myeloid and lymphoid cells to HIV infection while promoting viral production and proliferation of infected cells [12, 13]. Many of the cytokines induced by Jak-STAT signaling are central to the pathogenesis of HIV including tumor necrosis factor alpha (TNF-α) and interleukin (IL)–6. It is from these observations that ruxolitinib was first considered as a potential therapeutic option for HIV-1 disease [14]. Therefore, the objectives of this clinical trial were to (1) assess the safety/tolerability and (2) determine the immunologic and viral reservoir activity of ruxolitinib for PWH virologically suppressed on ART.
METHODS
Study Design
AIDS Clinical Trials Group (ACTG) A5336 was a phase 2a multicenter, randomized (2:1), open-label, parallel-arm study conducted in 14 academic sites across the United States that enrolled PWH (18–74 years of age) who were virologically suppressed for ≥2 years on ART containing a nonnucleoside reverse transcriptase inhibitor or an integrase strand transfer inhibitor not requiring cobicistat (Supplementary Methods). Full details of the study are given at https://clinicaltrials.gov/ct2/show/NCT02475655.
Participants
Given that this was the first-in-class trial of Jak inhibitors for PWH, regulatory requirements restricted enrollment to healthy individuals and required participants to have >350 CD4+ T cells/µL and plasma HIV-1 RNA below the limit of quantification. Participants must have been on stable ART ≤12 weeks of entry and could not have an ongoing or previous comorbidity except well-controlled hypertension (see Supplementary Table 1 for eligibility criteria). This trial was approved by the institutional review boards of each institution, and all participants provided written informed consent.
Randomization and Masking
Remaining on their ART regimen, participants were randomized (2:1) in an open-label fashion using a centralized computer system maintained by the ACTG Data Management Center using permuted blocks to treatment with ruxolitinib 10 mg orally twice daily for 5 weeks or no study treatment (controls), and was stratified by whether the participant was on an efavirenz (EFV)–containing regimen in order to ensure an adequate number of EFV-treated participants for pharmacokinetic analyses.
Procedures
Participants assigned to ruxolitinib were followed for 7 weeks off-treatment; controls were followed on-study for 12 weeks. At preentry, entry, and weeks 4, 5, 10, and 12, soluble markers of immune activation and inflammation in the peripheral blood were measured by high-sensitivity enzyme-linked immunosorbent assay (ELISA) for IL-6, and standard ELISA and microarray analysis for soluble CD14 (sCD14), TNF-α, IL-1β, IL-7, IL-10, IL-15, IL-18, and transforming growth factor–β1/2/3. At entry, and weeks 2, 5, and 12, peripheral blood mononuclear cell (PBMC) indices of immune activation, homing, cycling, and survival, including HLA-DR/CD38, CD25, CD127, Bcl-2, Ki-67, and α4β7, as well as lymphocyte subsets, were determined using flow cytometry and microarray panels (messenger RNA and protein expression). Low-level HIV viremia was quantified using the integrase single-copy assay (iSCA) [15]. Levels of cell-associated HIV-1 RNA (CA-RNA) and total HIV-1 DNA in total PBMCs were quantified at entry and at weeks 5 and 12 as previously described [16].
Outcomes
Primary tolerability and safety outcomes included premature ruxolitinib discontinuation and occurrence of predefined safety events (Supplementary Data). The primary efficacy endpoint was change in plasma IL-6 levels through week 5. Secondary endpoints included changes in other plasma, cell surface, and HIV reservoir measures.
Statistical Analysis
Inflating for 20% loss to follow-up or exclusion from primary efficacy analyses, with 60 participants, there was 80% power to detect a 0.20 log10 pg/mL difference between arms for change in IL-6 from baseline to week 4/5, using a 2-sided t test with 10% type I error (Supplementary Methods). Safety and tolerability analyses included all participants randomized to ruxolitinib who took at least 1 dose and all participants randomized to the control arm. The proportion of participants who experienced at least 1 primary safety milestone was compared between arms using a 1-sided mid-P Fisher exact test with 5% type I error.
Efficacy analyses were evaluated among a restricted subset of participants (see Supplementary Data for exclusions). Continuous measures were summarized with means, standard deviations (SDs), and confidence intervals (CIs). Mean changes were compared between arms using 2-sided t tests with a 10% type I error. To reduce variability and maximize statistical power, the average of 2 timepoints was used in analyses. Soluble markers of inflammation were log10 transformed, with changes shown as fold change (FC) and differences between arms as relative fold change (rFC).
Total HIV-1 DNA copies/106 CD4+ T cells were normalized by the CD4% from the same date and log10 transformed. Analytic lower limits were determined for each marker with values below limit imputed as half the distance from zero. HIV-1 RNA levels by iSCA were categorized as above or below assay limit of detection (0.4 copies/mL). Log-binomial regression with log-link, fitted with generalized estimating equations, compared the proportion of participants with detectable HIV-1 RNA by iSCA between arms with 10% type I error. P values were presented at their nominal level and interval widths were not adjusted for multiple comparisons; therefore, inferences may not be reproducible. Missing data were rare and were considered ignorable in analyses. All analyses were conducted using SAS version 9.4 software.
The study was reviewed by an independent study monitoring committee at 6-month intervals and was registered with ClinicalTrials.gov (NCT02475655).
RESULTS
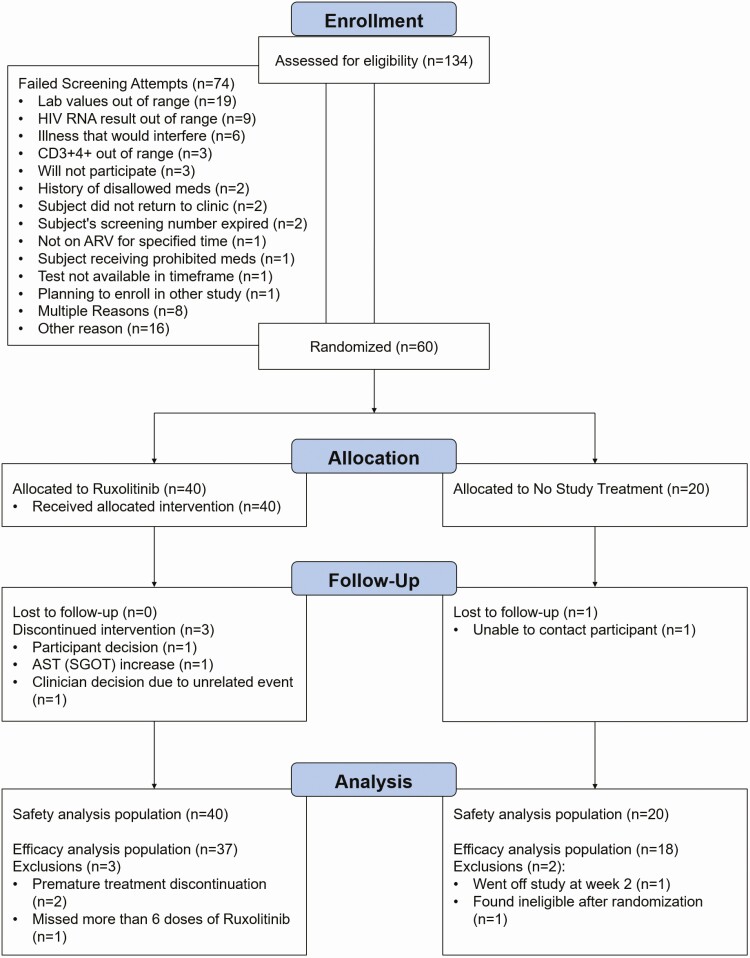
In total, 60 participants (40 on ruxolitinib and 20 controls) were enrolled from 16 May 2016 to 10 January 2018 (Figure 1). Participant characteristics at entry were balanced in both arms (Table 1). The median age was 49 (interquartile range [IQR], 37–54) years, 80% (n = 48) male, 50% (n = 29) black non-Hispanic, 36% (n = 21) white non-Hispanic, and 10% (n = 6) Hispanic. Median CD4 count was 791 (IQR, 622–972) cells/µL and 98% (n = 59) had plasma viral load <40 copies/mL.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Abbreviations: ARV, antiretroviral; AST, aspartate aminotransferase; HIV, human immunodeficiency virus; SGOT, serum glutamic-oxaloacetic transaminase.
Table 1.
Participant Baseline Characteristics
| Characteristic | Total (N = 60) | Study Arm | |
|---|---|---|---|
| Ruxolitinib (n = 40) | Controls (n = 20) | ||
| Age, y, median (IQR) | 49.0 (36.5–54.0) | 49 (45–54) | 43.5 (31.0–54.0) |
| Sex | |||
| Male | 48 (80) | 32 (80) | 16 (80) |
| Female | 12 (20) | 8 (20) | 4 (20) |
| Race/ethnicity | |||
| White non-Hispanic | 21 (36) | 14 (37) | 7 (35) |
| Black non-Hispanic | 29 (50) | 19 (50) | 10 (50) |
| Hispanic (any race) | 6 (10) | 4 (11) | 2 (10) |
| >1 race | 2 (3) | 1 (3) | 1 (5) |
| IV drug history | |||
| Never | 52 (87) | 33 (83) | 19 (95) |
| Previously | 8 (13) | 7 (18) | 1 (5) |
| Entry CD4 count, cells/μL, median (IQR) | 791 (622–972) | 798 (628–973) | 737 (610–930) |
| Entry CD8 count, cells/μL, median (IQR) | 644 (490–852) | 704 (483–842) | 629 (496–852) |
| Entry CD4/CD8 ratio, median (IQR) | 1.3 (0.9–1.6) | 1.3 (0.9–1.6) | 1.3 (0.8–1.6) |
| Nadir CD4 cell count, cells/μL | |||
| ≤50 | 3 (5) | 3 (8) | 0 (0) |
| 51–100 | 3 (5) | 2 (5) | 1 (5) |
| 101–200 | 10 (18) | 7 (18) | 3 (16) |
| 201–500 | 31 (54) | 21 (55) | 10 (53) |
| >500 | 10 (18) | 5 (13) | 5 (26) |
| HIV-1 RNA, copies/mL | |||
| <40 | 59 (98) | 39 (98) | 20 (100) |
| 114 | 1 (2) | 1 (3) | 0 (0) |
| ART regimen | |||
| TDF/FTC/EFV | 20 (33) | 14 (35) | 6 (30) |
| ABC/3TC/DTG | 13 (22) | 8 (20) | 5 (25) |
| TAF/FTC/RPV | 7 (12) | 3 (8) | 4 (20) |
| TAF/FTC + DTG | 5 (8) | 4 (10) | 1 (5) |
| TDF/FTC + RAL | 5 (8) | 4 (10) | 1 (5) |
| TAF/FTC + RAL | 4 (7) | 2 (5) | 2 (10) |
| TDF/FTC + DTG | 3 (5) | 3 (8) | 0 (0) |
| TDF/FTC/RPV | 2 (3) | 1 (3) | 1 (5) |
| ABC/3TC + DTG | 1 (2) | 1 (3) | 0 (0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; IV, intravenous; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Three participants (7.5%) prematurely discontinued ruxolitinib (Supplementary Table 3). One participant (2.5%) on ruxolitinib had a primary safety event, which was reported by site investigators as probably not related to ruxolitinib. This participant was diagnosed with Escherichia coli pyelonephritis at week 1 that resolved in 1 day without interruption of ruxolitinib. There was no difference in the proportion of participants with primary safety events between arms (P = .67). Complete safety data are shown in Supplementary Tables 4 and 5.
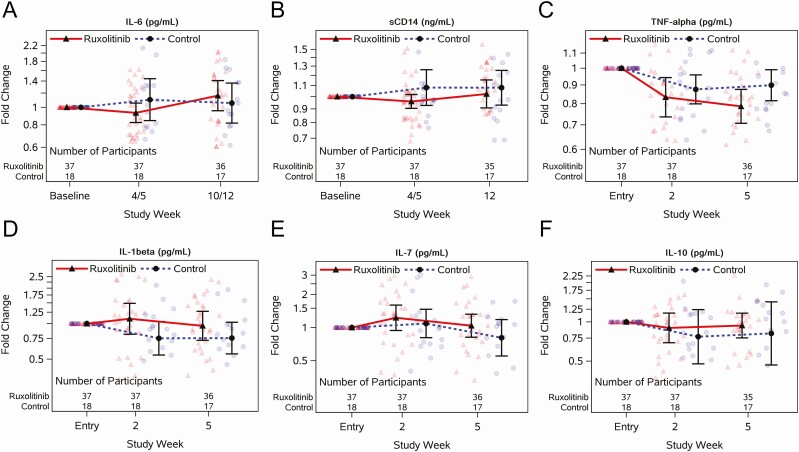
Baseline IL-6 levels were not elevated for any participants (Supplementary Table 6). There was no significant difference between arms in IL-6 FC from baseline to week 4/5 (mean rFC, 0.85 [90% CI, .69–1.04]; P = .18) or week 10/12 (rFC, 1.10 [90% CI, .84–1.44]) (Figure 2A, Supplementary Table 12). In contrast, there was a significant relative decrease in sCD14 at week 4/5 (mean FC, 0.96 [90% CI, .90–1.02] for ruxolitinib vs 1.08 [90% CI, .93–1.26] for controls; rFC, 0.89 [90% CI, .80–.99]) (Figure 2B). There was a trend toward a greater reduction in TNF-α for participants who received ruxolitinib compared with controls (rFC, 0.88 [90% CI, .76–1.01]) and no difference between arms for IL-1β, IL-7, or IL-10 (Figure 2C–F, Supplementary Table 13) at week 5.
Figure 2.
Longitudinal jitter plots of changes in plasma cytokines with or without 5 weeks of ruxolitinib for individuals virologically suppressed on antiretroviral therapy. Interleukin (IL)–6 (A), soluble CD14 (sCD14; B), tumor necrosis factor alpha (TNF-α; C), IL-1β (D), IL-7 (E), and IL-10 (F) measured at preentry and entry, and weeks 2, 4, 5, 10, and 12, depending on the marker (see x-axis); baseline is the average of preentry and entry, week 4/5 is the average of weeks 4 and 5, and week 10/12 is the average of weeks 10 and 12. Black circles and triangles represent the geometric mean, and error bars represent 95% confidence intervals. Jittered data points are shown as transparent circles and triangles.
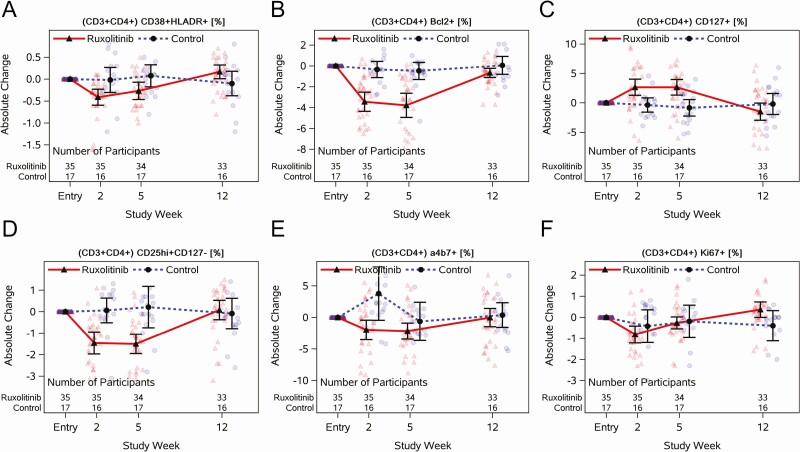
HLA-DR/CD38 (Figure 3A, Supplementary Table 15), a marker of immune activation, significantly decreased on CD4+ T cells by week 2 with ruxolitinib compared to controls (mean difference [md], –0.39% [90% CI, –.66% to –.12%]), and persisted while on ruxolitinib. The proportion of CD4+ T cells expressing the cell survival marker Bcl-2 (Figure 3B, Supplementary Table 15) also significantly decreased by week 2 and remained lower at 5 weeks for participants who received ruxolitinib compared to controls (md, –3.30% [90% CI, –4.72% to –1.87%]). The proportion of CD4+ T cells expressing the IL-7 receptor α-chain, CD127 (Figure 3C, Supplementary Table 15), significantly increased for participants who received ruxolitinib compared to controls (week 5: md, 3.49% [90% CI, 1.75%–5.22%]). The proportion of CD4+ T cells that were regulatory T cells, CD25Hi+/CD127– (Figure 3D, Supplementary Table 15), significantly decreased at weeks 2 and 5 for participants on ruxolitinib compared to controls (week 5: md, –1.71% [90% CI, –2.46% to –.97%]). The proportion of CD4+ T cells expressing the gut homing marker, α4β7 (Figure 3E, Supplementary Table 15), significantly decreased at 2 and 5 weeks for participants on ruxolitinib, but the difference between arms was largely driven by increases at week 2 in controls. Finally, there were no differences between arms in the proportion of CD4+ T cells expressing the cycling marker Ki67 (Figure 3F, Supplementary Table 15). Total CD8+ T-cell and monocyte results are provided in Supplementary Tables 7–9.
Figure 3.
Longitudinal jitter plots of changes in cell surface markers with or without 5 weeks of ruxolitinib for individuals virologically suppressed on antiretroviral therapy. Percentage of CD4+CD38+HLA-DR+ (A), CD4+Bcl2+ (B), CD4+CD127+ (C), CD4+CD25Hi+CD127– (D), CD4+α4β7+ (E), and CD4+Ki 67+ measured at entry and at weeks 2, 5, and 12. Black circles and triangles represent the mean, and error bars represent 95% confidence intervals. Jittered data points are shown as transparent circles and triangles.
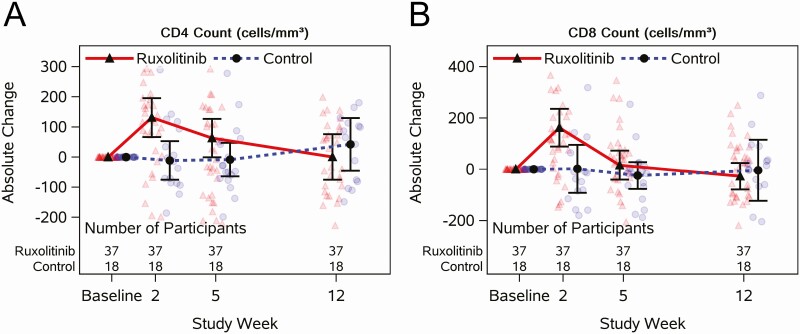
At week 2, there was a greater increase in CD4+ T-cell counts for ruxolitinib (mean, 131 cells/µL) than controls (mean, –11 [SD, 129] cells/µL) (md, 142 [90% CI, 58–227] cells/µL; Figure 4A, Supplementary Table 14). Increased CD4 counts persisted in the ruxolitinib arm through week 5 but returned to baseline levels by week 12. One participant on ruxolitinib experienced a confirmed CD4+ T-cell decline >50% below the entry level in the absence of an ART interruption at week 12 (1530 to 654 cells/µL). Likewise, CD8+ T-cell counts increased significantly (md, 160 [90% CI, 59–261] cells/µL) by 2 weeks for ruxolitinib (mean, 162 [SD, 220] cells/µL) compared to controls (mean, 2 [SD, 188] cells/µL), and returned to baseline levels by week 12 (Figure 4B, Supplementary Table 14).
Figure 4.
Longitudinal jitter plots of changes in T-cell subsets with or without 5 weeks of ruxolitinib for individuals virologically suppressed on antiretroviral therapy. CD4 absolute count (A) and CD8 absolute count (B) measured at preentry and entry, and weeks 2, 5, and 12; baseline is the average of preentry and entry. Black circles and triangles represent the mean and error bars represent 95% confidence intervals. Jittered data points are shown as transparent circles and triangles.
The absolute neutrophil count decreased more for participants on ruxolitinib compared to controls from entry to week 4/5 (md, –651 [90% CI, –1110 to –192] cells/µL); however, the absolute change in the ruxolitinib arm was not significant (mean, –251 [95% CI, –506 to 4] cells/µL). During this same period, the hemoglobin levels for the ruxolitinib arm also declined (mean, 0.65 [SD, 0.71] g/dL; md, –0.55 [90% CI, –.87 to –.23] g/dL), whereas the platelet counts increased (mean, 32 435 [SD, 39 771]/µL; md, 27 832 [90% CI, 9849–45 815]/μL) compared to controls. All hematological levels for participants on ruxolitinib returned to baseline levels by week 12.
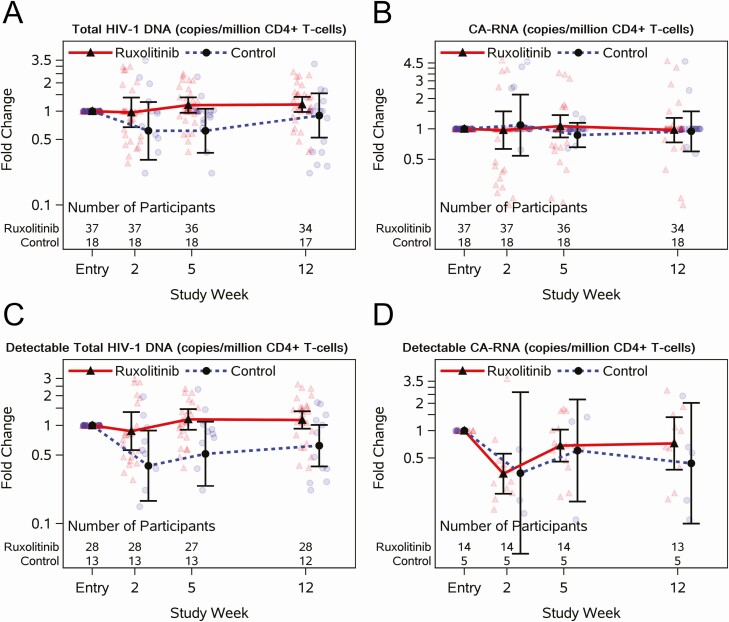
Overall, 3 participants on ruxolitinib had documented low-level HIV-1 RNA viremia (above the lower limit of quantification). At entry, 30% (11 of 37) of ruxolitinib and 50% (9 of 18) of control participants had HIV-1 RNA <0.4 copies/mL by iSCA. Similar proportions were observed at week 5 (34% [12 of 35] and 61% [11 of 18], respectively) and at week 12 (26% [9 of 35] and 56% [10 of 18], respectively). Participants on ruxolitinib compared to controls had a similar likelihood of having HIV-1 RNA <0.4 by iSCA at week 5 (relative risk, 0.98 [90% CI, .65–1.49]). Total HIV-1 DNA per million CD4+ T cells increased modestly in the ruxolitinib arm at week 5 compared to entry (mean FC, 1.16 [90% CI, .96–1.41]; Figure 5A), but differences between arms were driven by decreases in the controls (mean FC, 0.62 [90% CI, .36–1.06]). Comparable trends were observed when examining changes among those with detectable total DNA at entry (Figure 5C). Changes in CA-RNA/106 CD4+ T cells among all participants (Figure 5B) and those with a detectable baseline CA-RNA (Figure 5D) did not differ between arms at weeks 2, 5, and 12.
Figure 5.
Longitudinal jitter plots of changes in human immunodeficiency virus (HIV) reservoir markers with or without 5 weeks of ruxolitinib for individuals virologically suppressed on antiretroviral therapy. Total HIV-1 DNA (A) and cell-associated RNA (B) copies per million CD4+ T cells measured at entry and weeks 2, 5, and 12. Among participants who were detectable at entry for each respective marker, total HIV-1 DNA (C) and cell-associated RNA (D) copies per million CD4+ T cells measured at entry and at weeks 2, 4, and 12. Black circles and triangles represent the geometric mean, and error bars represent 95% confidence intervals. Jittered data points are shown as transparent circles and triangles.
DISCUSSION
This trial represents the first-in-class investigation of Jak 1/2 inhibitors for PWH, and demonstrates safety, tolerability, and initial insights into potential anti-inflammatory effects. To date, no therapy has been substantially effective at reducing systemic inflammation for individuals on ART. Ruxolitinib is a promising anti-inflammatory agent that appears to affect many pathways associated with the development of comorbidities and disease progression in treated HIV disease. Given that ruxolitinib has been Food and Drug Administration–approved for >9 years and has a favorable safety and pharmacokinetic profile, we conducted a first-in-HIV study. As a precaution, the study was restricted to a healthy population, thereby limiting the inclusion of participants with high baseline inflammatory biomarkers. For PWH with normal CD4+ T cells and no major comorbidities, we found that 5 weeks of 10 mg twice-daily ruxolitinib was safe and well-tolerated, but significant reductions in IL-6 levels were not observed. We did observe that ruxolitinib decreased biomarkers associated with poor outcomes for PWH including T-cell activation, immune dysregulation, and inflammation (CD3+/CD4+/HLA-DR+/CD38+, CD3+/CD4+/CD25Hi+/CD127–), cellular lifespan (CD3+/CD4+/BCL2+), and intestinal translocation/inflammation/homing (CD3+/CD4+/α4β7+, IL-18, sCD14). Collectively, these data support future studies of Jak inhibitors for PWH in order to identify ideal populations and the dose and duration of treatment required to affect clinical outcomes.
Safety events were rare and did not differ between arms. Decreases in absolute neutrophil count and hemoglobin for participants receiving ruxolitinib has been well-described in myelofibrosis clinical trials [17, 18]. Platelets also characteristically increased, as is observed within the first month of treatment in other conditions, though this is usually more prominent for those with baseline thrombocytopenia. A recent case report of a patient with HIV who developed secondary hemophagocytic lymphohistiocytosis was successfully treated with ruxolitinib and did not have any adverse events [19].
Among inflammatory biomarkers associated with mortality and serious clinical events for PWH, IL-6 has been the most predictive [20]. Plasma IL-6 levels can fluctuate significantly within individuals; therefore, we averaged 2 measures at baseline and follow-up in this proof-of-concept trial. Although there was a trend toward a reduction, most participants had baseline levels that were not elevated, which would make robust decreases unlikely. On the other hand, sCD14 tends to be much more stably elevated over time—even in those with high CD4+ T-cell counts [21]—and has also been strongly associated with mortality for PWH [22]. Jak-STAT inhibition could reduce sCD14 through a reduction in TNF-α. Bacterial lipopolysaccharide triggers TNF-α secretion, which then upregulates transcription of CD14. In participants receiving ruxolitinib, TNF-α showed a trend toward reduction, which could have led to the decrease in sCD14 levels.
Absolute CD4 and CD8 counts significantly increased for individuals receiving ruxolitinib in contrast to myelofibrosis patients [23]. This could represent redistribution as there was no evidence of cellular proliferation (Ki67 expression did not increase in the treated arm). Moreover, there was a decrease in α4β7 and chemokine receptor expression, implying a migration of cells from the gut to the periphery. Conversely, regulatory T cells decreased as observed in myelofibrosis [24]. Regulatory T cells likely serve a dual role in HIV infection by reducing inflammation while impairing viral clearance and shifting infected cells toward a resting state. Longer studies are needed to better understand how these factors impact the HIV reservoir in stable, ART-treated individuals.
Despite the relative health of the participants in this trial, low dose of ruxolitinib, and short duration of treatment, CD38/HLA-DR cell surface expression on CD4+ T cells markedly declined for participants on ruxolitinib. CD38+/HLA-DR+ cells are a hallmark of immune activation and dysregulation despite suppressive ART. Persistent elevation is associated with comorbid disease and mortality [25]. To date, no therapy beyond ART has demonstrated a sustained effect on this immune activation biomarker. In an RCT for PWH on at least 6 months of suppressive ART, there was a 6.7% reduction of CD38/HLA-DR expression on CD8+ T cells after 8 weeks of valganciclovir treatment [26]. It is unclear if either of these changes would provide a clinically meaningful benefit.
Overall, HIV virological measures did not differ between arms; however, CA-RNA changes correlated with CD4+/CD38+/HLA-DR+ changes (Supplementary Data) as previously described, underscoring the interplay between immune activation and reservoir dynamics [27]. Marked changes in CD4+ T-cell counts and associated activation/survival markers during ruxolitinib treatment were not accompanied by changes in HIV-1 DNA, unspliced CA-RNA, or residual viremia, suggesting that ruxolitinib-associated perturbations in T-cell homeostasis were not specific to latently HIV-infected cells. We cannot, however, exclude a selective effect of ruxolitinib on the replication-competent HIV reservoir. Importantly, cell survival marker Bcl-2, which is transcriptionally regulated by the Jak-STAT pathway, demonstrated a significant decrease of expression in CD4+ T cells for ruxolitinib-treated participants. In previous in vitro studies, antagonism of Bcl-2 using venetoclax demonstrated a reduction of the HIV reservoir following reactivation [28] and homeostatic proliferation [29], selectively killing HIV-infected cells by reversing the intrinsic ability of HIV to block apoptotic pathways. This suggests a benefit of combining Jak inhibitors with latency reversal agents. Other studies have demonstrated a significant decrease in the frequency of T cells harboring integrated HIV-DNA using various Jak-STAT inhibitors, which correlated with a decrease in Bcl-2 [13]. Recent modeling data suggest a novel mechanism for preventing viral replication of SARS-CoV-2 through the inhibition of Numb-associated kinases [30] independent of its proven anti-inflammatory benefit for hospitalized patients with COVID-19 (Emergency Use Authorization approval of baricitinib for hospitalized COVID-19 patients) [31, 32]. In particular, the adaptor-associated kinase plays a critical role in receptor-mediated endocytosis of SARS-CoV-2, and BMP-2–inducible kinase is involved in clathrin-coated vesicle-associated endocytosis for HIV. These data highlight that direct and indirect-acting antiviral activity of Jak 1/2 inhibitors across viral infections is a likely shared mechanistic underpinning that confers efficacy beyond the anti-inflammatory effects. These data suggest that multiple mechanisms impacted by Jaki (Jak inhibitors) could be leveraged to mitigate clinical disease from viral infections, and in particular the HIV reservoir if treated over a longer duration and in a less restricted population. The success of baricitinib for COVID-19, coupled with the body of in vitro, in vivo (murine), and ex vivo studies toward HIV-1, favorable pharmacokinetic profile (daily dosing and renal clearance avoiding hepatically metabolized drug interactions), and safety record (approved in children ≥2 years old) warrants consideration for baricitinib for additional longer-duration studies for HIV-1. It is important to note that not all Jaki are created equal, and individual safety and efficacy profiles must be carefully considered in the space of viral infections.
There were limitations based upon rigidity of the trial design. The eligibility criteria restricted participation to healthy PWH as manifested by high CD4 counts and normal CD4/CD8 ratios at baseline. The participants failed to have elevated biomarkers at baseline and therefore have minimal potential for a reduction in plasma cytokines and cell surface markers associated with immune activation and inflammation. Additionally, the ruxolitinib dose selected for this trial was relatively low, representing the starting dose for polycythemia vera and graft-vs-host disease or the myelofibrosis dose for individuals with thrombocytopenia. Finally, the duration of treatment was relatively brief, considering that the time required to observe a decline in biomarkers of inflammation and HIV persistence could require 3–6 months.
After 5 weeks of ruxolitinib, healthy, virologically suppressed PWH experienced a reduction in specific markers of inflammation and immune activation/dysregulation associated with clinical disease. There were no significant safety events, and ruxolitinib was well-tolerated. These findings provide a rationale for longer-duration studies to examine the role of Jak inhibitors to reduce systemic inflammation and HIV persistence for PWH who are virologically suppressed on ART. Ultimately, these studies can inform strategies to mitigate age-related diseases such as neurocognitive disorder, cardiovascular disease, and frailty while providing insight into a functional HIV cure.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. V. C. M., C. M., C. G., S. G. D., M. M. L., A. T., P. W. H., R. T., C. F., S. H., C. D. R., R. F. S., and J. J. L. developed the concept and designed the study. M. M. L., E. T. O., A. T., P. W. H., and R. P. S. provided study material or participants. C. M. and A. K. verified the data and performed the statistical analyses. V. C. M., C. M., C. G., and J. J. L. wrote the initial draft of the manuscript. All authors provided critical comments and editing, contributed to the data interpretation, and reviewed the analyses of this manuscript and approved its final version.
Acknowledgments. We are grateful to the participants, clinical staff, and study team members at the participating sites for their generous contributions to this work and especially the AIDS Clinical Trials Group (ACTG), Statistical and Data Management Center, participating Clinical Research Sites, and specialty laboratories including the Brigham and Women’s Hospital, Harvard Medical School, Case Western Reserve University, and Emory University School of Medicine. The following reagent was obtained through the AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): Anti-Human α4-β7 integrin monoclonal (Act-1) (catalog number 11718) from Dr A. A. Ansari.
Financial support. This work was supported by the NIAID/NIH (grant numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701 to V. C. M.); the National Institute of Mental Health (grant number R01-MH-116695 to R. F. S.); and the Emory Center for AIDS Research (grant number P30AI050409 to V. C. M. and R. F. S.). R. F. S. provided funding support for the study drug.
Potential conflicts of interest. V. C. M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV. M. M. L. has received a competitive research grant award (to the institution) from Gilead and has consulted for Eli Lilly. C. F. received consultation fees unrelated to the current work from Merck, Mylan Pharmaceuticals, and ViiV Healthcare. S. D. reports grants from Gilead and Merck, and personal fees from Abbvie, Biotron, Eli Lilly, Enochian Biosciences, and ImmunoCore, outside the submitted work. C. F. also reports expert witness services to Gilead, outside the submitted work. A. T. reports grants from the ACTG. A. T. also reports grants from Merck and personal fees from Gilead Sciences and EBSCO/DynaMed, outside the submitted work. P. H. reports grants from Gilead and personal fees from Gilead, ViiV, Janssen, and Biotron, outside the submitted work. J. L. reports grants and personal fees from ViiV Pharmaceuticals, outside the submitted work. C. M. reports grants from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases, outside the submitted work. R. F. S. and C. G. have served as unpaid consultants for Eli Lilly. In addition, R. F. S. owns shares in Eli Lilly and Incyte, the manufacturers of baricitinib and ruxolitinib, respectively. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. T Virtanen A, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs 2019; 33:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bose P, Verstovsek S. Updates in the management of polycythemia vera and essential thrombocythemia. Ther Adv Hematol 2019; 10:2040620719870052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park H, Yu DA, Kwon O. Janus kinase inhibitors: an innovative treatment for alopecia areata. J Dermatol 2019; 46:724–30. [DOI] [PubMed] [Google Scholar]
- 4. Masarova L, Bose P, Verstovsek S. The rationale for immunotherapy in myeloproliferative neoplasms. Curr Hematol Malig Rep 2019; 14:310–27. [DOI] [PubMed] [Google Scholar]
- 5. Marcus JL, Chao CR, Leyden WA, et al. . Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duprez DA, Kuller LH, Tracy R, et al. ; INSIGHT SMART Study Group . Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 2009; 207:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borges ÁH, Silverberg MJ, Wentworth D, et al. ; INSIGHT SMART; ESPRIT; SILCAAT Study Groups . Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tien PC, Choi AI, Zolopa AR, et al. . Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunt PW, Martin JN, Sinclair E, et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 11. Dunham RM, Vujkovic-Cvijin I, Yukl SA, et al. . Discordance between peripheral and colonic markers of inflammation during suppressive ART. J Acquir Immune Defic Syndr 2014; 65:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chomont N, El-Far M, Ancuta P, et al. . HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gavegnano C, Brehm JH, Dupuy FP, et al. . Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog 2017; 13:e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, Schinazi RF. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob Agents Chemother 2014; 58:1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cillo AR, Vagratian D, Bedison MA, et al. . Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malnati MS, Scarlatti G, Gatto F, et al. . A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc 2008; 3:1240–8. [DOI] [PubMed] [Google Scholar]
- 17. Al-Ali HK, Griesshammer M, le Coutre P, et al. . Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica 2016; 101:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verstovsek S, Mesa RA, Gotlib J, et al. ; COMFORT-I Investigators . Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol 2017; 10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galvez Acosta S, Javalera Rincon M. Ruxolitinib as first-line therapy in secondary hemophagocytic lymphohistiocytosis and HIV infection. Int J Hematol 2020; 112:418–21. [DOI] [PubMed] [Google Scholar]
- 20. Tenorio AR, Zheng Y, Bosch RJ, et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wada NI, Jacobson LP, Margolick JB, et al. . The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parampalli Yajnanarayana S, Stübig T, Cornez I, et al. . JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol 2015; 169:824–33. [DOI] [PubMed] [Google Scholar]
- 24. Massa M, Rosti V, Campanelli R, Fois G, Barosi G. Rapid and long-lasting decrease of T-regulatory cells in patients with myelofibrosis treated with ruxolitinib. Leukemia 2014; 28:449–51. [DOI] [PubMed] [Google Scholar]
- 25. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt PW, Martin JN, Sinclair E, et al. . Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cockerham LR, Siliciano JD, Sinclair E, et al. . CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One 2014; 9:e110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cummins NW, Sainski AM, Dai H, et al. . Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol 2016; 90:4032–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cummins NW, Sainski-Nguyen AM, Natesampillai S, Aboulnasr F, Kaufmann S, Badley AD. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J Virol 2017; 91:e00012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stebbing J, Phelan A, Griffin I, et al. . COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020; 20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Titanji BK, Farley MM, Mehta A, et al. . Use of baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis 2021; 72:1247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalil AC, Patterson TF, Mehta AK, et al. . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2020; 384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.