Abstract
A member of the Enterobacteriaceae initially identified as Kluyvera cryocrescens by the MicroScan Gram-Negative Combo 13 panel caused an outbreak of nosocomial infections in four patients (pneumonia, n = 2; urinary tract infection, n = 1; wound infection, n = 1) and urinary tract colonization in one patient. When the strains were tested by the Enteric Reference Laboratory of the Centers for Disease Control and Prevention, biochemical results were most compatible with Yersinia intermedia, Kluyvera cryocrescens, and Citrobacter farmeri but identification scores were low and test results were discrepant. However, when the biochemical test profile was placed in the computer database as a new organism, all strains were identified as the organism with high identification scores (0.999968 to 0.999997) and no discrepant test results. By 16S rRNA sequence analysis the organism clustered most closely with, but was distinct from, Citrobacter farmeri and Citrobacter amalonaticus. Based on its unique biochemical profile and rRNA sequence, this organism is designated Enteric Group 137. Restriction endonuclease analysis and taxonomic antibiograms of strains causing the outbreak demonstrated a single clone of Enteric Group 137, and antibiotic susceptibility testing revealed the presence of extended-spectrum β-lactamase (ESBL) resistance. Enteric Group 137 appears to be a new opportunistic pathogen that can serve as a source of ESBL resistance in the hospital.
Gram-negative bacilli that belong to the family Enterobacteriaceae continue to be the most frequently recovered bacterial isolates from clinical specimens. Thirty named genera are now recognized in the family (7). Although most clinically significant isolates belong to 20 or 25 species that have been well known for many years (7), new species are being continually discovered. Commercial microbial identification systems (kits) using miniaturized biochemical reactions and computer-based algorithms are widely utilized in clinical microbiology laboratories. Commercial kits generally provide accurate identification of the common species of Enterobacteriaceae but are problematic with newly described organisms. It is important for the clinical laboratory to recognize lower-probability computer-generated identifications with discrepant reactions for the Enterobacteriaceae.
We recently encountered an outbreak of an unusual strain of Enterobacteriaceae isolated from five patients. It was initially identified by the MicroScan Gram-Negative Combo 13 panel as Kluyvera cryocrescens with a probability of 86.9% with citrate as negative and 90.0% with citrate as positive and a computer-flagged discrepant result for sorbitol fermentation. Extensive biochemical and physiological testing of this organism by the Enteric Reference Laboratory of the Centers for Disease Control and Prevention (CDC) and rRNA sequence analysis revealed it to be a new organism in the family Enterobacteriaceae. In addition, restriction endonuclease analysis and taxonomic antibiograms demonstrated the clonal nature of strains causing our hospital outbreak, and antibiotic susceptibility testing detected the presence of extended-spectrum β-lactamase (ESBL) resistance. The clinical, biochemical, physiological, and genetic properties of this new member of the Enterobacteriaceae that we have named Enteric Group 137 are described in this report.
CASE REPORTS
Patient 1.
A 56-year-old man with a history of severe pneumonia and pulmonary embolus was admitted to the Lakeside Division of Veterans Administration Chicago Health Care System (VA Lakeside) 3 March 1997 after 5 days of fever, shortness of breath, productive cough, and chest pain. Within 2 days he developed respiratory failure and was transferred to the Medicine and Surgery Intensive Care Unit. He received treatment for pneumonia with multiple antimicrobial agents, including aztreonam, gentamicin, ciprofloxacin, trimethoprim-sulfamethoxazole, erythromycin, and vancomycin. In addition to respiratory distress, his course was notable for catheter-associated Torulopsis glabrata fungemia, which was treated with fluconazole, and renal failure. On 31 March 1997 a strain of Enterobacteriaceae (strain 1) (Table 1) was recovered by culture on eosin-methylene blue and sheep blood agar of a purulent sputum (>25 leukocytes/microscopic field at ×100 magnification) positive by Gram's stain for gram-negative bacilli. The organism was identified as K. cryocrescens by the MicroScan Gram-Negative Combo 13 panel. A few urease-negative yeast species were also recovered in the sheep blood agar culture. He remained on antifungal and broad-spectrum antibacterial therapy until he died 10 April 1997. Autopsy examination confirmed that acute neutrophilic and organizing bronchopneumonia contributed to his death. A species of Enterobacteriaceae identified by the Gram-Negative Combo 13 panel as K. cryocrescens was isolated in autopsy cultures of lung, as was Pseudomonas aeruginosa.
TABLE 1.
Strains of Enteric Group 137 at VA Lakeside during 1997
| Strain | No. assigned by:
|
Human source | |
|---|---|---|---|
| ATCCa | CDC | ||
| 1 | BAA-68 | 2410-97 | Sputum |
| 2 | BAA-69 | 2411-97 | Urine |
| 3 | BAA-70 | 2416-97 | Sputum |
| 4 | BAA-71 | 2427-97 | Urine |
| 5 | BAA-72 | 2429-97 | Wound |
ATCC, American Type Culture Collection.
Patient 2.
A 47-year-old man had advanced multiple sclerosis with quadriparesis, hyperreflexic bladder, and recurrent urinary tract infection. Between 4 September and 14 October 1996 he was a hospital patient at the VA Lakeside for cellulitis and urinary tract infection and was in the Medicine and Surgery Intensive Care Unit from 6 to 9 October. He was readmitted to VA Lakeside 28 March 1997 from an outpatient clinic after 2 days of fever and diaphoresis. His urine was purulent, and cultures of two separate urine specimens were positive on 31 March 1997 for a strain of Enterobacteriaceae (strain 2) (Table 1) identified by the Gram-Negative Combo 13 panel as K. cryocrescens. The organism was a pure isolate with each urine specimen. It was present at more than 100,000 organisms/ml of urine by quantitative culture of one specimen using sheep blood agar and 16,000 organisms/ml of urine by quantitative culture of the other specimen. The isolate from both urine specimens demonstrated growth with a metallic green sheen in primary culture on eosin-methylene blue agar. Treatment was initiated with imipenem and amikacin. His urinary tract infection resolved within 2 weeks. He was discharged from the hospital 19 May 1997.
Patient 3.
A 68-year-old man with recurrent adenocarcinoma of the lung was admitted to VA Lakeside 15 April 1997 for right upper lobectomy. Following the lobectomy he developed fever and pneumonia and was placed in the Medicine and Surgery Intensive Care Unit where he was treated with vancomycin, ticarcillin-clavulanate, and gentamicin and showed improvement. Within 2 weeks fever and neutrophilic leukocytosis recurred, with pneumonic infiltrates in the middle and lower lobes of his right lung. On 15 May 1997 a strain of Enterobacteriaceae (strain 3) (Table 1) was recovered in a culture of a purulent sputum that was positive by Gram's stain for gram-negative bacilli. The organism was identified by the Gram-Negative Combo 13 panel as K. cryocrescens. The sputum culture was also positive for Proteus mirabilis and Pseudomonas aeruginosa. His fever and leukocytosis resolved upon treatment with imipenem and amikacin. He was discharged 2 August 1997.
Patient 4.
A 64-year-old man was admitted to VA Lakeside 19 July 1997 because of progressive lethargy. A craniotomy was performed for resection of a craniopharyngeoma and placement of a ventriculoperitoneal shunt. Multiple revisions of his shunt were necessary because of hydrocephalus. He developed a persistent shunt infection with methicillin-resistant Staphylococcus aureus that was complicated by a pelvic abscess. Aspiration of the abscess tested positive in a culture for methicillin-resistant S. aureus and Pseudomonas aeruginosa. He was treated with vancomycin and gentamicin and then vancomycin, tobramycin, and ceftazidime. After 2 months in the hospital, most of which was spent in the Medicine and Surgery Intensive Care Unit, his urine was positive (23 September 1997; 18,000 organisms/ml) for a strain of Enterobacteriaceae (strain 4) (Table 1) identified by the Gram-Negative Combo 13 panel as K. cryocrescens. Candida albicans was also recovered in the culture at 13,000 organisms/ml of urine. His antibiotic treatment was not altered, and subsequent cultures were negative. He was discharged 17 December 1997.
Patient 5.
A 65-year-old man with prostate carcinoma was admitted to VA Lakeside 23 September 1997 for radical prostatectomy and removal of pelvic lymph nodes. His postoperative course in the Medicine and Surgery Intensive Care Unit was complicated by surgical wound infection with dehiscence, and he was initially given ampicillin-sulbactam and then piperacillin, both in combination with gentamicin. He was discharged 1 month after surgery to be readmitted 31 October 1997 with fever, chills, and periumbilical abdominal pain. He was initially treated with ticarcillin-clavulanate and ciprofloxacin. A surgical wound specimen showing gram-negative bacilli by Gram's stain was positive in culture on 3 November 1997 for a strain of Enterobacteriaceae (strain 5) (Table 1) identified by the Gram-Negative Combo 13 panel as K. cryocrescens. The wound culture was also positive for growth of Pseudomonas aeruginosa. Treatment was changed to imipenem and amikacin. The operative wound site was debrided, and a left thigh graft was successfully placed. He was discharged 24 November 1997.
MATERIALS AND METHODS
Clinical laboratory bacteriologic methods.
Isolates were obtained from five patients at VA Lakeside in Chicago, Ill., during 1997. Five strains of a species of Enterobacteriaceae identified by the MicroScan Gram-Negative Combo 13 panel (Dade International, West Sacramento, Calif.) as K. cryocrescens were studied (Table 1). Computer identification based on reactions with the Gram-Negative Combo 13 panel was obtained by the MicroScan data management system, versions 20.55 and 20.60. Identifications by this system are reported as percent probabilities based on the frequency of positive reactions with the following interpretations: most probable, 95 to 99.9%; very probable, 90 to 94.9%; probable, 85 to 89.9%; low selectivity, 60 to 84.9%, with additional confirmatory tests to be set up; questionable, below 60% (MicroScan Biotype Codebook for Aerobic Gram-Negative Bacilli; Baxter Diagnostics, Deerfield, Ill.). Cytochrome oxidase activity was determined using N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (Sigma Chemical Company, St. Louis, Mo.). Other tests performed were growth and reactivity on MacConkey agar and Levine modified eosin-methylene blue agar, Simmons citrate agar, and purple fermentation broth containing sorbitol (Becton Dickinson Microbiology Systems, Sparks, Md.).
CDC laboratory methods and media.
The media and methods utilized in the Enteric Reference Laboratory of the CDC have evolved over the laboratory's 50-year history (6, 8). Commercial dehydrated media were used whenever possible and were prepared according to instructions given by the manufacturer. All incubations were at 36 ± 1°C unless otherwise noted, and all testing was done with subcultures picked from a single colony (single-colony pick) using the originally submitted culture. The strains were studied with biochemical tests normally used to characterize Enterobacteriaceae (6, 7, 8, 12), and the tests were read at days 1, 2, 5, and 7. Because of variation in results with the five strains, tests were repeated for the following: citrate utilization (Simmons), urea hydrolysis, arginine dihydrolase, malonate utilization, α-methyl-d-glucoside fermentation, and tartrate fermentation (Jordan). A detailed description of media and methods can be found in previous publications (7, 12).
Biochemical test results were analyzed with two different computer programs, GEORGE and STRAIN MATCHER (8). GEORGE compares the test strain with over 150 named taxa (genera, species, subspecies, biogroups, and enteric groups) in the family Enterobacteriaceae. It lists 24 different mathematical scores that indicate how well the test strain fits the organisms in the database that are the closest biochemical matches. It also lists the biochemical tests that are incompatible with the organism chosen as the best biochemical fit. It is based on the “normalized-likelihood” method previously described (8, 9, 15). Computer analysis of the first two strains is illustrated in Tables 4 and 5. The computer analysis for the next three strains was very similar (examples not shown). STRAIN MATCHER does a “strain-by-strain” analysis and compares the test strain to over 11,000 individual strains in the database. The final printout from STRAIN MATCHER lists the 60 strains that are the closest biochemical matches of the test strain (6).
TABLE 4.
Examples of the original analysis when the biochemical results for strains 1 and 2 were run in the computer program GEORGE (version 99A), which did not contain Enteric Group 137 in its databasea
| Strain | Program choiceb | Identification score
|
|
|---|---|---|---|
| Raw | Normalized | ||
| 1c | K. cryocrescens | 0.000001 | 0.842321 |
| Y. intermedia | 0.00000026 | 0.150232 | |
| K. ascorbata | 0.0000000074 | 0.004256 | |
| 2d | C. farmeri | 0.00000023 | 0.922658 |
| K. cryocrescens | 0.000000015 | 0.059466 | |
| Y. intermedia | 0.0000000026 | 0.010606 | |
Only the first set of identification scores is given (out of the 12 sets actually done), but it is the most important.
The species listed first is the best identification.
Test results incompatible with K. cryocrescens (expected percent positive at day 1 or 2): gas from glucose (95%), citrate (Simmons) (80%), and malonate utilization (86%). The strain had a negative reaction in each test.
Test results incompatible with C. farmeri (expected percent positive, strain reaction): gas from glucose (99%, negative), salicin fermentation (7%, positive), esculin hydrolysis (1%, positive), lactose fermentation (13%, positive).
TABLE 5.
Examples of the analysis when the biochemical results for strains 1 and 2 were run in a revised version of the program GEORGE (version 99B) after Enteric Group 137 had been added to the databasea
| Strain | Program choice | Identification score
|
|
|---|---|---|---|
| Raw | Normalized | ||
| 1 | Enteric Group 137 | 0.058847 | 0.999971 |
| K. cryocrescens | 0.000001 | 0.000025 | |
| Y. intermedia | 0.00000026 | 0.000004 | |
| 2 | Enteric Group 137 | 0.058847 | 0.999996 |
| C. farmeri | 0.00000023 | 0.000004 | |
| K. cryocrescens | 0.000000015 | 0.25 × 10−6 | |
Only the first set of identification scores is given (out of 12 actually done), but it is the most important. No tests were incompatible with Enteric Group 137 for either strain.
Antibiograms were determined by the disk diffusion method of Bauer et al. (2) as a taxonomic and epidemiological tool rather than to obtain information for antibiotic usage with infections. A standard taxonomic set of 12 antibiotics used in the Enteric Reference Laboratory of the CDC since 1972 for testing cultures of Enterobacteriaceae and Vibrionaceae was utilized.
Crude DNA isolation for 16S rRNA sequencing.
Bacteria were cultured on Trypticase soy agar. A loopful of bacterial cells was harvested and suspended in 100 μl of lysis buffer (50 mM Tris-HCl [pH 7.6], 1 mM EDTA, 0.5% Tween 20, 200 μg of proteinase K/ml) and incubated at 55°C for 2 h. The proteinase K was then inactivated by heating to 95°C for 10 min.
Amplification of 16S rRNA cistrons by PCR and purification of PCR products.
The 16S rRNA cistrons were amplified with bacterial universal primers F24 and F25 (Table 2). PCR was performed in thin-walled tubes with a Perkin-Elmer 9700 Thermocycler. One microliter of the DNA template was added to a reaction mixture (50-μl final volume) containing 20 pmol of each primer, 40 nmol of deoxynucleoside triphosphates, and 1 U of Taq 2000 polymerase (Stratagene, La Jolla, Calif.) in buffer containing Taqstart antibody (Sigma Chemical Co.). In a hot-start protocol, samples were preheated at 95°C for 8 min followed by amplification using the following conditions: denaturation at 95°C for 45 s, annealing at 60°C for 45 s, and elongation at 72°C for 1.5 min, with an additional 5 s for each cycle. A total of 30 cycles were followed by a final elongation step at 72°C for 10 min. The results of PCR amplification were examined by electrophoresis in a 1% agarose gel. DNA was stained with ethidium bromide and visualized under short-wavelength UV light.
TABLE 2.
Oligonucleotide primers used for PCR amplification and sequencing of strain 1 Enteric Group 137 16S rDNA
| No. | IDc | Sequence (5′–3′)a | Positionb | Orientation |
|---|---|---|---|---|
| 1 | F24 | GAGTTTGATYMTGGCTCAG | 9–27 | Forward |
| 2 | F23 | ACGGGAGGCAGCAGY | 344–358 | Forward |
| 3 | F22 | RCTGCTGCCTCCCGT | 344–358 | Reverse |
| 4 | F15 | TTACCGCGGCTGCTG | 519–533 | Reverse |
| 5 | F16 | TAGATACCCYGGTAGTCC | 789–806 | Forward |
| 6 | F17 | CCGTCWATTCMTTTGAGTTT | 907–926 | Reverse |
| 7 | F18 | GCAACGAGCGCAACC | 1099–1113 | Forward |
| 8 | F20 | CCATTGTARCACGTGTG | 1226–1242 | Reverse |
| 9 | F25 | AAGGAGGTGWTCCARCC | 1525–1541 | Reverse |
Base codes are standard International Union of Biochemistry codes for bases and ambiguity.
Numbering based on E. coli.
ID, identification.
16S rRNA sequencing.
Purified DNA from PCR was sequenced using an ABI prism cycle sequencing kit (BigDye terminator cycle sequencing kit with AmpliTaq DNA polymerase FS; Perkin-Elmer). The primers in Table 2 were used for sequencing. Quarter dye chemistry was used with 80 μM primers and 1.5 μl of PCR product in a final volume of 20 μl. Cycle sequencing was performed using an ABI 9700 with 25 cycles of denaturation at 96°C for 10 s and annealing and extension at 60°C for 4 min. Sequencing reactions were run on an ABI 377 DNA sequencer.
16S rRNA data analysis.
Sequence data were entered and aligned using RNA, a microcomputer program set for data entry, editing, sequence alignment, secondary structure comparison, similarity matrix generation, and dendrogram construction for 16S rRNA, written in Microsoft QuickBasic for use with PC-compatible computers (18). The database contains over 2,000 sequences obtained by the laboratory of one of us (F. E. Dewhirst) and over 1,000 obtained from GenBank. Sequences were first checked by BLAST analysis versus all entries in GenBank (1). Sequences for related organisms not already in the database were downloaded and added. Dendrograms were constructed by the neighbor-joining method (19).
Restriction endonuclease analysis.
Genomic DNA was prepared by a guanidinium thiocyanate extraction method for gram-negative bacilli (22). DNA from each strain was quantitatively measured by absorption spectroscopy. DNA (3 μg) was restricted using PvuII and EcoRI enzymes (Gibco BRL) separately. Each mixture was incubated at 37°C for 1 h. Electrophoresis was performed in 0.6% agarose gel at 40 V for 24 h. Gels were stained with SYBR green nucleic acid stain (FMC Bioproducts) according to the manufacturer's instructions and photographed. Photographs were compared visually to determine the degree of DNA relatedness of isolates. Relatedness was classified as identical if no band differences were detected, similar if 10% or fewer bands differed, and different if more than 10% of the DNA bands differed (4).
Plasmid analysis.
Extrachromosomal DNA was extracted by an alkaline lysis method (20). Electrophoresis was performed using 0.8% agarose gel. Gels were stained with SYBR green nucleic acid stain and photographed. A 1-kb DNA molecular weight standard (Gibco BRL) was used to estimate band sizes.
β-Lactamase IEF.
Isolates were prepared using the method of Huovinen (13). Strains were grown overnight on Mueller-Hinton agar (Difco) containing 25 μg of ampicillin (Sigma)/ml. Bacterial cells were suspended in 0.5 ml of buffer (90 mM Na2HPO4, 10.4 mM NaH2PO4) (pH 7.8), sonicated for 30 s at 8-μm amplitude, and centrifuged for 10 min at 13,000 × g at 4°C. The supernatant was transferred to a fresh tube and stored at −20°C until utilized. Extracts were tested for the presence of β-lactamase activity by application of 50 μl of supernatant to a Cefinase disc (Becton Dickinson Microbiology Systems) and noting a color change from yellow to red. The β-lactamase isoelectric point (pI) was determined with the PhastSystem and PhastGel IEF3-9 media of Pharmacia. PhastGel sample applicators were used to load 1 μl of extract onto the gel. Gel separation was performed using a programmed isoelectric focusing (IEF) method (Pharmacia) that included prefocusing, application, and protein migration. The β-lactamase bands were detected utilizing nitrocefin (Becton Dickinson Microbiology Systems). Nitrocefin (500 μg/ml) was applied to a PVDF-Plus transfer membrane (Micron Separations, Inc.) precut to the size of the gel. The gel was covered with the nitrocefin-soaked membrane, and β-lactamase bands were developed. A pI value was determined for each β-lactamase by reference to a pI ladder created using strains of ESBL-containing Escherichia coli and Klebsiella pneumoniae obtained from the CDC (TEM-12, pI 5.25, NCCLS E-3; TEM-10, pI 5.6, NCCLS K-4; SHV-4, pI 7.8, NCCLS E-7; SHV-5, pI 8.2, NCCLS E-8).
Antimicrobial susceptibility testing.
Susceptibility testing was performed by broth microdilution and agar dilution methods. Broth microdilution MICs were determined by inoculation of MicroScan Gram Negative Combo 13 panels, incubation for 18 h at 35°C in an air incubator, and measurement of MICs by the autoScan-4 (Baxter). Agar dilution MICs were measured as recommended by National Committee for Clinical Laboratory Standards (NCCLS) document M7-A4. MIC interpretive standards for susceptible, intermediate, and resistant were utilized as defined by NCCLS document M100-S9.
Measurement of clavulanate effect.
A confirmatory test for ESBL production was performed based on the ratio of ceftazidime to ceftazidime-clavulanate MICs. Ceftazidime and ceftazidime-clavulanate MICs were determined using the agar dilution method, and results were interpreted as recommended by NCCLS document M7-A4. Isolates were tested against concentrations of ceftazidime (Eli Lilly) in the range of 0.5 to 64 μg/ml and against the same concentration range of ceftazidime in the presence of 4.0 μg of clavulanate (SmithKline Beecham Pharmaceuticals)/ml. Strains of ESBL-containing E. coli and K. pneumoniae were utilized as controls (NCCLS E-3, E-8, and K-4).
Nucleotide sequence accession number.
The GenBank accession number for the 16S rRNA sequence of strain 1 (Table 1) is AF208013.
RESULTS
Clinical microbiology laboratory evaluation.
Positive reactions for glucose fermentation and nitrate reduction and a negative reaction for cytochrome oxidase placed this gram-negative bacillus in the family Enterobacteriaceae. All isolates were strong lactose fermenters as demonstrated by the metallic green sheen of growth on Levine modified eosin-methylene blue agar and formation of intensely red, pitted colonies on MacConkey agar. The following reaction results were obtained using the MicroScan Gram-Negative Combo 13 panel, with the percentage of positive reactions for K. cryocrescens indicated by MicroScan given in parentheses: positive reactions for raffinose (95%), rhamnose (99%), arabinose (99%), melibiose (99%), sucrose (50%), o-nitrophenol-β-d-galactopyranoside (99%), esculin (95%), ornithine (90%), and indole (95%); negative reactions for inositol (1%), adonitol (1%), lysine (1%), arginine (1%), urea (1%), H2S (1%), tryptophan deaminase (1%), and Voges-Proskauer (5%).
Utilization of citrate as a sole carbon source, positive for 75% of strains of K. cryocrescens by the Gram-Negative Combo 13 panel and 80% by Simmons citrate agar (7), was variable with four of the five strains. These four strains (strains 1, 3, 4, and 5) demonstrated growth in citrate-containing broth or agar after 24 h in 62% of individual tests (n = 13 tests). One strain (strain 2) was consistently citrate negative after 24 h (n = 6 tests). With a positive citrate reaction the computer-calculated probability of identification as K. cryocrescens was “very probable” at 90.0% (MicroScan biotype 7711121-2); with a negative citrate reaction the calculated probability was “probable” at 86.9% (MicroScan biotype 7711125-2). With a positive citrate reaction, the probability of Kluyvera ascorbata was 9.6% and that of the Yersinia enterocolitica group was 0.3%. With a negative citrate reaction the probability of E. coli was 7.0%, that of the Y. enterocolitica group was 3.6%, and that of K. ascorbata was 2.3%. These were the only organisms listed by the MicroScan data management system.
All five strains fermented sorbitol, as indicated by the Gram-Negative Combo 13 panel, and this positive reaction was flagged as discrepant for K. cryocrescens by the MicroScan data management system. Only 5% of strains of K. cryocrescens ferment sorbitol with the Gram-Negative Combo 13 panel. All of the isolates were also positive for sorbitol fermentation by the tube method with purple broth containing sorbitol.
CDC biochemical profiles.
Table 3 is a summary of the biochemical reactions for the five strains. Most of the tests were either 100% positive or 100% negative, but variation existed for six tests: citrate utilization, urea hydrolysis, arginine dihydrolase, malonate utilization, α-methyl-d-glucoside fermentation, and tartrate fermentation (Jordan). Variable results were obtained with these six tests in each of two different CDC laboratories. The six tests done with the same lot number of media by the same person at the same time still showed variation in results. These six tests were thus considered to give variable results as reflected in the final percentages, which are a composite of all individual test results (Table 3). Consequently for each strain there are two biochemical profiles for “original profile” and “repeat profile.”
TABLE 3.
Biochemical reactions of the five Enteric Group 137 strainsa
| Test | Cumulative % positive on day:
|
Reaction for strain 1b | ||
|---|---|---|---|---|
| 1 | 2 | 7 | ||
| Indole production | 100 | +2 | ||
| Methyl red | 100 | +2 | ||
| Voges-Proskauer (O'Meara) | 0 | − | ||
| Citrate utilization (Simmons)* | 0 | 0 | 70 | +5 |
| H2S on triple-sugar iron agar | 0 | 0 | 0 | − |
| H2S on peptone iron agar | 0 | 0 | 0 | − |
| Urea hydrolysis (Christensen)* | 0 | 70 | 80 | +2 |
| Phenylalanine deaminase | 0 | − | ||
| Lysine decarboxylase (Moeller) | 0 | 0 | 0 | − |
| Arginine dihydrolase (Moeller)* | 0 | 20 | 90 | +3 |
| Ornithine decarboxylase (Moeller) | 100 | 100 | 100 | + |
| Motility | 100 | 100 | 100 | + |
| Gelatin hydrolysis (20–22°C) | 0 | 0 | 0 | − |
| KCN test (% resistant to cyanide) | 100 | 100 | 100 | + |
| Malonate utilization* | 0 | 0 | 20 | − |
| d-Glucose: acid production | 100 | 100 | 100 | + |
| d-Glucose: gas production | 0 | 0 | 0 | − |
| Acid production from: | ||||
| Adonitol | 0 | 0 | 0 | − |
| l-Arabinose | 100 | 100 | 100 | + |
| d-Arabitol | 0 | 0 | 0 | − |
| Cellobiose | 100 | 100 | 100 | + |
| Dulcitol | 0 | 0 | 0 | − |
| Erythritol | 0 | 0 | 0 | − |
| d-Galactose | 100 | 100 | 100 | + |
| Glycerol | 100 | 100 | 100 | + |
| myo-Inositol | 0 | 0 | 0 | − |
| Lactose | 100 | 100 | 100 | + |
| Maltose | 100 | 100 | 100 | + |
| d-Mannitol | 100 | 100 | 100 | + |
| d-Mannose | 100 | 100 | 100 | + |
| Melibiose | 100 | 100 | 100 | + |
| α-Methyl-d-glucoside* | 10 | 80 | 100 | +2–3 |
| Raffinose | 100 | 100 | 100 | + |
| l-Rhamnose | 100 | 100 | 100 | + |
| Salicin | 100 | 100 | 100 | + |
| d-Sorbitol | 100 | 100 | 100 | + |
| Sucrose | 100 | 100 | 100 | + |
| Trehalose | 100 | 100 | 100 | + |
| d-Xylose | 100 | 100 | 100 | + |
| Esculin hydrolysis | 100 | 100 | 100 | + |
| Mucate fermentation | 100 | 100 | 100 | + |
| Tartrate fermentation (Jordan)* | 50 | 50 | 50 | +7 |
| Acetate utilization | 60 | 100 | 100 | + |
| Lipase (corn oil) | 0 | 0 | 0 | − |
| DNase (25°C) | 0 | 0 | 0 | − |
| Nitrate reduction to nitrite | 100 | + | ||
| Oxidase | 0 | − | ||
| ONPGc test | 80 | 100 | 100 | + |
| Yellow pigment production (25°C) | 0 | 0 | 0 | − |
| Citrate utilization (Christensen) | 30 | 90 | 100 | +2 |
| Tyrosine clearing | 0 | 0 | 0 | − |
Tests were done at the CDC with standard methods as previously described. Symbols: −, negative at end of the appropriate incubation time; + (no superscript), positive at 24 h. Superscripts give the day the reaction became positive if it was delayed (indole production, methyl red, and Voges-Proskauer tests are done only at 48 h). For most tests the percentages were based on one observation per strain, but for six tests (∗) final percentages were based on composite results after the strains were retested.
Strain 1 (ATCC BAA-68) is designated as the reference strain of Enteric Group 137 and will become the designated type strain if Enteric Group 137 is given a scientific name in the future.
ONPG, o-nitrophenyl-β-d-galactopyranoside.
The five original and five repeat biochemical profiles underwent analysis by the computer program GEORGE. Three different organisms were listed based on the best biochemical fit as the program's first choice: Yersinia intermedia was listed six times, K. cryocrescens was listed twice, and Citrobacter farmeri was listed twice. Each of these 10 first choices had a low raw identification score and, in addition, had two to four tests listed as incompatible with the identification. This computer analysis is illustrated in Table 4. As second-choice organisms (Table 4), Y. intermedia was listed three times and K. cryocrescens was listed seven times. Six different organisms were listed as the third choice including all three of the species mentioned above. The conclusion from this computer analysis was that the five strains had biochemical similarity to several species but differed in their overall biochemical profiles from all organisms included in the program's database. However, when Enteric Group 137 was defined and added to the program as a new organism (taxon), the identification scores improved dramatically (the lowest score was 0.999968 and the highest was 0.999997) and there were no tests listed as being incompatible with Enteric Group 137. The results for strains 1 and 2 in the revised computer analysis are illustrated in Table 5.
Computer analysis of the biochemical profiles was also obtained by program STRAIN MATCHER. For all five strains (both original and repeat profiles) this program usually listed strains of C. farmeri as the closest biochemical matches; C. farmeri was listed 176 times in the 200 closest matches. Strains of Enterobacter cloacae were listed 14 times, but other organisms, i.e., K. cryocrescens (five strains), Enterobacter species (two strains), Citrobacter youngae (two strains), and Y. intermedia (one strain), were rarely listed. The analysis indicated that the CDC collection did not have any strains that were identical to this new organism or that would be identified as Enteric Group 137 based on reanalysis of the data.
16S rRNA sequence.
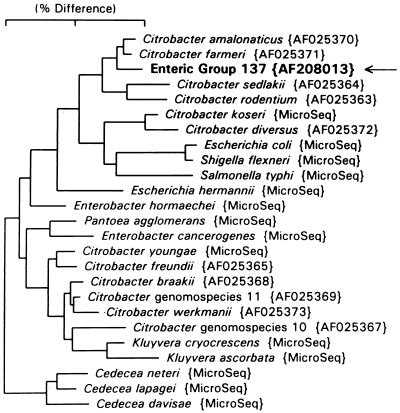
The essentially complete (1,520-base) sequence of this organism was obtained and compared to sequences in the GenBank database. The sequence was 99.5% similar to that of C. farmeri and 99.3% similar to that of Citrobacter amalonaticus. Citrobacter 16S rRNA sequences not already in our database were downloaded from GenBank. A 16S rRNA-based neighbor-joining tree constructed from all named Citrobacter species and genomospecies and selected enteric reference organisms is shown in Fig. 1. This organism clusters most closely with C. farmeri and C. amalonaticus.
FIG. 1.
Phylogenetic tree based on 16S rRNA sequence comparison. The marker bar represents 2% difference. GenBank accession numbers are listed in braces. Microseq, sequences from the PE Applied Biosystems MicroSeq sequence database. Distance is measured by adding the horizontal distances connecting any two species.
Nomenclature proposal: Enteric Group 137.
Based on its unique biochemical reaction pattern and its position in the dendrogram based on 16S rRNA sequencing, we propose Enteric Group 137 as a new and distinct group of Enterobacteriaceae. A complete phenotypic description is given in Table 3. Strain 1 (Table 1) is designated the reference strain. We recommend that this strain be elevated in status as the type strain if Enteric Group 137 is given a scientific name in the future.
Molecular epidemiology.
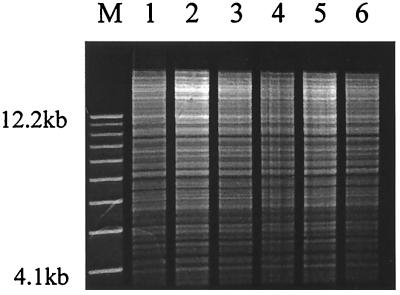
All strains of Enteric Group 137 had identical restriction endonuclease analysis patterns with genomic DNA treated by EcoRI (Fig. 2) and PvuII. A single band >12 kb in size was detected for all isolates using plasmid DNA agarose gel electrophoresis. In addition, each isolate contained four distinct β-lactamases, as shown by IEF, with pI values of 8.2, 8.0, 7.4, and 5.6. These results indicate infection of the five patients by a clonal strain of Enteric Group 137 having identical genomic DNA and β-lactamase enzymes and containing identical plasmid DNA profiles.
FIG. 2.
Restriction endonuclease analysis of EcoRI-generated DNA digest patterns of Enteric Group 137. Lane M, 1-kb DNA molecular weight ladder (Gibco BRL); lane 1, first urine isolate, strain 2; lane 2, second urine isolate, strain 2; lane 3, sputum isolate, strain 1; lane 4, sputum isolate, strain 3; lane 5, urine isolate, strain 4; lane 6, wound isolate, strain 5.
Taxonomic antibiograms.
Taxonomic antibiograms were performed for the five strains of Enteric Group 137 by the disk diffusion method. All strains showed no growth inhibition by nalidixic acid (30 μg), sulfadiazine (250 μg), gentamicin (10 μg), penicillin (10 U), ampicillin (10 μg), carbenicillin (100 μg), and cephalothin (30 μg). Similar zones of inhibition were observed with colistin (10 μg) (12 to 14 mm), streptomycin (10 μg) (10 to 16 mm), kanamycin (30 μg) (13 to 18 mm), tetracycline (30 μg) (10 to 19 mm), and chloramphenicol (30 μg) (9 to 13 mm). The essentially identical taxonomic antibiograms obtained with all strains of Enteric Group 137 suggest their clonal nature.
Antibiotic susceptibility patterns.
Broth microdilution testing with β-lactam antibiotics demonstrated categorical resistance of all strains of Enteric Group 137 to the extended-spectrum cephalosporin drug ceftazidime, with measured MICs of >16 μg/ml, and to the monobactam drug aztreonam, with measured MICs of >16 μg/ml. The strains were susceptible to the cephamycin antibiotic cefotetan and the carbapenem drug imipenem, with MICs of ≤4 μg/ml observed for all five strains with both antibiotics. Broth microdilution testing also revealed resistance to ampicillin, with measured MICs of >16 μg/ml, to piperacillin, with MICs of >64 μg/ml, and the narrow-spectrum cephalosporin cephalothin, with MICs of >16 μg/ml.
Except for cefoxitin, agar dilution testing with these β-lactam antibiotics also gave identical results with all strains of Enteric Group 137 for both categorical susceptibility or resistance and measured MICs. The strains were resistant to ampicillin, with measured MICs of >128 μg/ml, to piperacillin, with MICs of >64 μg/ml, and to a narrow-spectrum cephalosporin (cefazolin), with MICs of >8 μg/ml. All strains were susceptible to ampicillin in the presence of the β-lactamase inhibitor sulbactam, with measured MICs of 16 μg/ml, and to piperacillin in the presence of tazobactam, with MICs of ≤8 μg/ml. Strains from four patients demonstrated intermediate susceptibility to the cephamycin cefoxitin, with measured MICs of 16 μg/ml, while the strain from one patient was resistant, with MICs of >16 μg/ml. As observed with broth microdilution testing, all strains were resistant to ceftazidime, with measured MICs of >64 μg/ml and to aztreonam, with MICs of >16 μg/ml, while they were susceptible to a carbapenem (meropenem), with measured MICs of ≤1 μg/ml.
Ceftazidime resistance, when accompanied by susceptibility to the carbapenem drugs imipenem and meropenem and by susceptibility to the cephamycins cefotetan and (with the exception of one strain) cefoxitin, suggests ESBL resistance (16). When strains were tested in the presence of 4 μg of clavulanate/ml, the measured MIC of ceftazidime decreased from >64 to 8 μg/ml, a concentration decrease greater than twofold. All strains of Enteric Group 137 demonstrated ceftazidime to ceftazidime-clavulanate MIC ratios of ≥16. The effect of clavulanate on the ceftazidime MIC phenotypically confirms the presence of ESBL resistance.
DISCUSSION
The first isolates of Enteric Group 137 submitted to the Enteric Bacteriology Laboratories at CDC were suspected of being E. coli based on their biochemical reactions, especially strong lactose fermentation with eosin-methylene blue and MacConkey agar. E. coli is the most common species of Enterobacteriaceae isolated in clinical microbiology laboratories. In addition, the isolates of Enteric Group 137 possess ESBL resistance, common in E. coli but less common for rare species of Enterobacteriaceae (8). For these reasons the isolates were evaluated initially by the E. coli laboratory at the CDC. However, they were shown to be negative in the phoE PCR test for E. coli, which ruled out this identification (7). Isolates were then completely characterized, and found to be closest to C. farmeri, K. cryocrescens, and Y. intermedia in their biochemical reactions. It was initially difficult to identify the isolates using their biochemical profiles. Since they were very similar to each other in their phenotypic characteristics and different from all named species of Enterobacteriaceae, one of us (J.J.F.) designated them Enteric Group 137 and considered this a possible new group of Enterobacteriaceae of uncertain taxonomic position. When Enteric Group 137 was added to the database in the computer program GEORGE as a new organism, identification scores were definitive for this organism.
Enteric Group 137 is 99.5% similar to C. farmeri and 99.3% similar to C. amalonaticus by 16S rRNA sequence analysis and could be a distinct biogroup of one of these two species. C. farmeri would be the most likely choice because of its closer biochemical similarity. Another possibility is that the organism represents a new species closely related to C. farmeri and C. amalonaticus. Figure 1 indicates that Citrobacter species fall into three different 16S rRNA clusters. The bottom cluster (Fig. 1) includes the type species Citrobacter freundii, and the species C. youngae, C. braakii, C. werkmanii, Citrobacter genomospecies 10, and Citrobacter genomospecies 11. The top cluster (Fig. 1) contains C. farmeri, C. amalonaticus, C. sedlakii, C. rodentium, and Enteric Group 137 (AF208013). The middle cluster (Fig. 1) contains C. koseri (C. diversus). By 16S rRNA analysis, the genus Kluyvera is close to the Citrobacter freundii cluster. It may be desirable to propose an alternative classification in the future that splits the genus Citrobacter into two or more genera. The Citrobacter species in the top two clusters might not remain in a redefined genus Citrobacter. For this reason we propose the name Enteric Group 137 until further studies including DNA-DNA hybridization (3) have resolved the relationship of Enteric Group 137 to C. farmeri and C. amalonaticus and the issue of subdividing the genus Citrobacter.
The drug resistance profile of these organisms is notable. They were found to possess ESBLs, plasmid-mediated enzymes that confer resistance to β-lactams containing an oxyimino group including cefotaxime, ceftizoxime, ceftazidime, and aztreonam. The cephamycins, including cefotetan and cefoxitin, remain relatively active against ESBL-producing gram-negative bacteria in vitro, as do the carbapenems, although the clinical relevance of this in vitro activity is unclear (11, 17). The first ESBL-producing gram-negative bacilli were discovered in Germany in 1983 (14). Since then, ESBL production among gram-negative bacilli has become a global concern, and nosocomial outbreaks of these organisms are well documented. Outbreaks can occur through either the dissemination of a single clone or, because these enzymes are encoded by plasmids, through dissemination among various members of the family Enterobacteriaceae. Enteric Group 137, involved in this outbreak, most likely acquired its ESBL resistance from other Enterobacteriaceae.
We suggest that Enteric Group 137 is a possible new opportunistic pathogen in the family Enterobacteriaceae. Four of our five patients (patients 1, 2, 3, and 5) had signs and symptoms indicating infection by this new organism, including urinary tract infection (patient 2), pneumonia (patients 1 and 3), and surgical wound infection (patient 5). The urinary tract infection was associated with pure cultures of Enteric Group 137, whereas the cases of pneumonia and surgical wound infection yielded cultures positive for Enteric Group 137, Pseudomonas aeruginosa (patients 1, 3, and 5), and Proteus mirabilis (patient 5). Isolates of Enteric Group 137 were susceptible to imipenem and amikacin, and signs and symptoms of infection resolved in three patients treated by imipenem and amikacin (patients 2, 3, and 5). The patient whose death was associated with pneumonia and had sputum cultures positive for Enteric Group 137 (patient 1) was not treated with a carbapenem. One patient (patient 4) was apparently colonized with Enteric Group 137. The DNA restriction and β-lactamase patterns, plasmid profiles, and taxonomic antibiograms of the Enteric Group 137 strains indicate a nosocomial outbreak due to dissemination of a single clone. All five patients were admitted to the Medicine and Surgery Intensive Care Unit of VA Lakeside during 1997, and it is likely that nosocomial acquisition of the Enteric Group 137 clone occurred by contact spread in the Intensive Care Unit.
Although ESBL production has been reported in other Enterobacteriaceae including Citrobacter species, it has been found predominately in Klebsiella species and E. coli (5, 10, 21). Because of strong lactose fermentation by Enteric Group 137 on eosin-methylene blue and MacConkey agar, positive methyl red and indole reactions, and variable citrate reactions, this organism could be misidentified as a lysine-negative strain of E. coli by clinical laboratories using only a basic set of reactions. ESBL production among E. coli strains is not unusual and would therefore not alert microbiologists to a new organism. Surveillance for ESBL-producing gram-negative bacilli is not a routine practice at most institutions, including ours at the time of this outbreak. Thus, infection or colonization by Enteric Group 137 having ESBL resistance would not be readily detected. This would be especially problematic in Enteric Group 137 infections associated with multiple gram-negative etiologies, as occurred with patients 1, 3, and 5. It is possible that large reservoirs of Enteric Group 137 are present unrecognized in hospitals. It is hoped that this report will stimulate others to isolate and identify this new organism so that more can be learned about its ecology and role in human infections.
ACKNOWLEDGMENTS
We thank technologists and technicians of the Microbiology Laboratories of the Veterans Administration Chicago Health Care System Lakeside Division and Dixie Peters of the Molecular Epidemiology Laboratory of the Northwestern Memorial Hospital for expert technical assistance.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang M J, Zhang Z, Miller W, Lipton D J. GappedBLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer A W, Kirby W M M, Sherris J C, Turck M. Antimicrobic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 3.Brenner D J, Grimont P A, Steigerwalt A G, Fanning G R, Ageron E, Riddle C F. Classification of Citrobacter by DNA hybridization: designation of Citrobacter farmeri sp. nov., Citrobacter youngae sp. nov., Citrobacter braakii sp. nov., Citrobacter werkmanii sp. nov., Citrobacter dedlakii sp. nov., and three unnamed Citrobacter genomospecies. Int J Syst Bacteriol. 1993;43:645–658. doi: 10.1099/00207713-43-4-645. [DOI] [PubMed] [Google Scholar]
- 4.Clabots C R, Johnson S, Bettin K M, Mathie P A, Mulligan M E, Schaberg D R, Peterson L R, Gerding D N. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J Clin Microbiol. 1993;31:1870–1875. doi: 10.1128/jcm.31.7.1870-1875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Harrif-Heraud Z, Arpin C, Benliman S, Quentin C. Molecular epidemiology of a nosocomial outbreak due to SHV-4-producing strains of Citrobacter diversus. J Clin Microbiol. 1997;35:2561–2567. doi: 10.1128/jcm.35.10.2561-2567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewing W H. Edwards and Ewing's identification of Enterobacteriaceae. New York, N.Y: Elsevier Science Publishing Co., Inc.; 1986. [Google Scholar]
- 7.Farmer J J., III . Enterobacteriaceae: introduction and identification. In: Murray P R, Barron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1999. pp. 442–458. [Google Scholar]
- 8.Farmer J J, III, Davis B R, Hickman-Brenner F W, McWhorter A, Huntley-Carter G P, Asbury M A, Riddle C, Wathen-Grady H G, Elias C, Fanning G R, Steigerwalt A G, O'Hara C M, Morris G K, Smith P B, Brenner D J. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman R B, Bruce D, MacLowry J, Brenner V. Computer-assisted identification of bacteria. Am J Clin Pathol. 1973;60:395–403. doi: 10.1093/ajcp/60.3.395. [DOI] [PubMed] [Google Scholar]
- 10.Gniadkowsk M, Palucha A, Grzesiowski P, Hryniewicz W. Outbreak of ceftazidime-resistant Klebsiella pneumoniae in a pediatric hospital in Warsaw, Poland: clonal spread of the TEM-47 extended-spectrum β-lactamase (ESBL)-producing strain and transfer of a plasmid carrying the SHV-5-like ESBL-encoding gene. Antimicrob Agents Chemother. 1998;42:3079–3085. doi: 10.1128/aac.42.12.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold H S, Moellering R C. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 12.Hickman F W, Farmer J J., III Salmonella typhi: identification antibiograms, serology, and bacteriophage typing. Am J Med Technol. 1978;44:1149–1159. [PubMed] [Google Scholar]
- 13.Huovinen S. Rapid isoelectric focusing of plasmid-mediated β-lactamase with Pharmacia PhastSystem. Antimicrob Agents Chemother. 1988;32:1730–1732. doi: 10.1128/aac.32.11.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole, and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 15.LaPage S P, Bascomb S, Willcox W R, Curtis M A. Identification of bacteria by computer: general aspects and perspectives. J Gen Microbiol. 1973;77:273–290. doi: 10.1099/00221287-77-2-273. [DOI] [PubMed] [Google Scholar]
- 16.Livermore D M, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996;38:409–424. doi: 10.1093/jac/38.3.409. [DOI] [PubMed] [Google Scholar]
- 17.Meyer K S, Urgan C, Eagan J A, Berger B J, Rahal J J. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med. 1993;119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Paster B J, Dewhirst F E. Phylogeny of Campylobacter wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol. 1988;38:56–62. [Google Scholar]
- 19.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.25–1.28. [Google Scholar]
- 21.Shannon K, Stapleton P, Xiang X, Johnson A, Beattie H, El Bakri R, Cookson B, French G. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains causing nosocomial outbreaks of infection in the United Kingdom. J Clin Microbiol. 1998;36:3105–3110. doi: 10.1128/jcm.36.10.3105-3110.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley Interscience; 1987. pp. 2.4.1–2.4.5. [Google Scholar]