Abstract
It is well established that the vasculature plays a crucial role in maintaining oxygen and nutrients supply to the heart. Increasing evidence further suggests that the microcirculation has additional roles in supporting a healthy microenvironment. Heart failure is well known to be associated with changes and functional impairment of the microvasculature. The specific ablation of protective signals in endothelial cells in experimental models is sufficient to induce heart failure. Therefore, restoring a healthy endothelium and microcirculation may be a valuable therapeutic strategy to treat heart failure. This review article will summarize the current understanding of the vascular contribution to heart failure with reduced or preserved ejection fraction. Novel therapeutic approaches including next generation pro-angiogenic therapies and non-coding RNA therapeutics, as well as the targeting of metabolites or metabolic signalling, vascular inflammation and senescence will be discussed.
Keywords: Non-coding RNAs, MicroRNAs, Angiogenesis, Microcirculation
1. Introduction
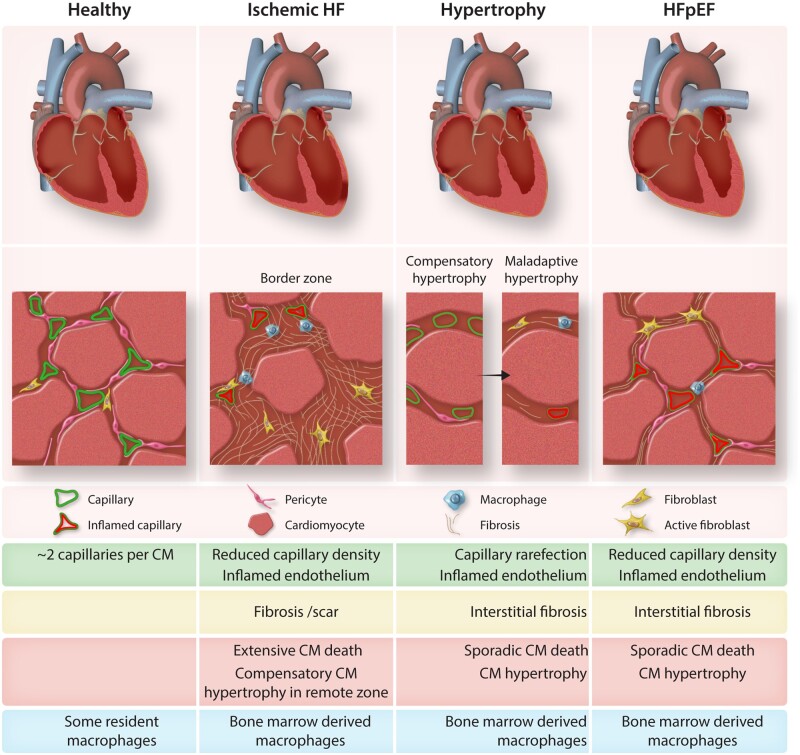
Every organ in the human body has its own vasculature specialized for the specific needs of each organ.1,2 Historically the function of the vasculature has been described to be the transport of oxygen and nutrients to all tissues and to carry away the products of cellular metabolism in order to maintain cellular homeostasis.3 In recent years, it has been recognized that the endothelium actively controls its microenvironment regulating different processes like organ development, homeostasis, and tissue regeneration.4 The vasculature in the heart, the coronary vasculature, receives its name from the latin word corona, meaning crown, because of the resemblance of its structure to a radiant crown. The heart is a highly vascularized organ, every cardiomyocyte is located in close proximity to a capillary5 and endothelial and associated mural cells are the most abundant cell types in the heart6,7 (Figure 1).
Figure 1.
Compared characteristics of the microvasculature in different pathological diseases. Ischaemic HF shows a reduced capillary density with inflamed endothelium. There is massive cardiomyocyte death plus a hypertrophic compensatory effect on the remote zone. Furthermore, there is invasion of bone marrow-derived macrophages. First, there is a compensatory hypertrophy characterized by an increase of capillary density and cardiomyocyte increased size followed by a maladaptive hypertrophy is characterized by capillary rarefaction and an inflamed endothelium, interstitial fibrosis and the presence of bone marrow-derived macrophages. Moreover, the cardiomyocytes are hypertrophic and there is sporadic cardiomyocyte death. Finally, HFpEF is also characterized by reduced capillary density and inflamed endothelium. This is accompanied by interstitial fibrosis, sporadic cardiomyocyte death, and hypertrophy. HFpEF hearts also show the presence of bone marrow-derived macrophages.
The question whether the endothelium might be a potential therapeutic target in cardiac disease is an old one. The Greek philosopher Aristotle already proposed in classic times that blood vessels are the frame around which the rest of the organism is built.8 Heart failure (HF) patients are characterized by systemic vasoconstriction and reduced peripheral perfusion and the therapeutic benefits from intervening with vascular tone are known long ago.9 Treatments based on the release of nitric oxide (NO) have been used in cardiac disease for a long time,10 in particular, nitroglycerine has been in clinical use for over 100 years.11 Recently, guanylate cyclase activators trials support that activation of the NO down-stream signalling is of therapeutic benefit in HF.12 Another vasculature-oriented treatment are angiotensin-converting enzyme inhibitors, which inhibit the breakdown of bradykinin, which can then stimulate NO release.13
In this review, we will discuss how the vasculature interacts with different types of HF cause by myocardial infarction, maladaptive hypertrophy and the age-associated HF with preserved ejection fraction (HFpEF) (Figure 1). We will primarily focus on small vessels, and refer the readers to other review articles regarding coronary artery disease, which obviously is the cause of ischaemicHF. Based on the insights into the adaptive and maladaptive signals, we will provide a summary of possible therapeutic strategies to target the vasculature in cardiovascular disease.
2. The vasculature of the heart—a brief introduction
Vascular development is organ specific14 and in the heart, historically, the epicardium was considered the source of cardiac capillaries. Epicardial cells were thought to perform epithelial to mesenchymal transition, invade the developing myocardium and give rise to the coronary capillaries.15–19 More recent studies performed using lineage tracing have now shown that only a subset of the proepicardium gives rise to a small proportion of the endothelial cells of the capillaries. The sinus venosus provides endothelial progenitors to form the cardiac capillaries in the lateral free walls of the ventricles and the septum during development,20–22 while endocardial cells are converted to capillary endothelial cells in the inner ventricular wall postnatally23 in a myocardial secreted VEGF-dependent manner.24 All these findings show that there are—at least—three different cellular sources of coronary endothelium. Interestingly, capillary density in the healthy heart is regionally different; the number of epimyocardial capillaries is >21% as compared to endocardial capillary density.25
Blood vessels are formed by two biological processes;26 vasculogenesis is the development of de novo blood vessels by the differentiation of angioblasts into endothelial cells,27,28 while angiogenesis is the growth of new blood vessels from pre-existing vessels via sprouting.29–31 There are a variety of signals implicated in correct vasculogenesis. FGF-receptor tyrosine kinases, and in particular FGF4, are required for the induction of the mesoderm.32,33 Gene knock out experiments demonstrated a pivotal role of VEGF, VEGFR2 (KDR), and VEGFR1 (FLT1) in embryonic vasculogenesis.34–37 Furthermore, cell adhesion molecules, such as VE-Cadherin, PECAM-1, and Fibronectin, also play important roles during de novo blood vessel formation.28,38
In order to perform sprouting angiogenesis, endothelial cells need to degrade the basal membrane, a process mediated by different proteinases. Proteinases also release matrix-bound angiogenic growth factors FGF, VEGF, and TGF-β .39 An integrated feedback loop between the VEGF and Notch signalling pathways regulates the endothelial cell determination between ‘tip’, the migratory endothelial cell that guides the sprout, and ‘stalk’, the proliferative endothelial cells that supports vascular growth (for details see References40,41). Ischaemia-induced neovascularization of the heart additionally involves clonally expanded VE-cadherin-expressing endothelial cells.42,43Myocardial-secreted FGF and VEGF regulate coronary endothelial cell fate and vascular assembly.44,45 Furthermore, retinoic acid and VEGF are required for the stabilization of the primitive network during development.46 Disruption of the processes described above result in coronary congenital defects that may persist after birth and can affect cardiovascular health (for review see References47) The estimated prevalence of these anomalies ranges from 0.21% to 5.79% depending on the diagnostic tools48 and they can be associated with shunting, ischaemia, and sudden death.49,50
There is growing evidence of a number of angiocrine signals that have effects on cardiac remodelling.51 Examples are NRG-1 that binds and activates Erbb receptors in cardiomyocytes promoting cardiomyocyte proliferation and growth52,53 or Apelin and its receptor APJ regulating the myocardial response to infarction and cardiomyocyte hypertrophy.54,55 Endothelin-1, that is also produced by endothelial cells in the heart, regulates cardiomyocyte contractility and can induce cardiac remodelling.56 Endothelial cells and cardiomyocytes also have direct cell–cell contact. Connexins 37, 40, and 43 are expressed both in endothelial cells and cardiomyocytes and there is evidence that they are important for the spatial organization and survival of the cardiac muscle cells.57 Although it has been suggested that they might mediate communication between endothelial cells and cardiomyocytes this needs to be further studied (reviewed in References51,58).
Understanding the molecular mechanisms of cardiac vascular morphogenesis and the interaction between endothelial cells and cardiomyocytes is crucial to develop therapeutic strategies.48
3. The vasculature in heart failure with reduced ejection fraction
Many studies demonstrated that heart failure with reduced ejection fraction (HFrEF) is associated with impaired coronary flow reserve and microvascular perfusion.59 Reduced perfusion and microvascular dysfunction can be primarily caused by narrowing or occlusion of the coronary arteries leading to reduced blood supply to the myocardium (Figure 1). However, already in the 90s it was proposed that the endothelium may also be a therapeutic target in dilative cardiomyopathy and in patients without coronary artery disease.9 Risk factors, such as metabolic syndromes and diabetes, as well as hypertension, can affect the coronary microcirculation.59 A pig study recently confirmed a direct impact of diabetes on cardiac microvascular dysfunction and capillary rarefaction.60 Maladaptive hypertrophy as it occurs after pressure overload also is associated with reduced vessel density.61,62 Thus, microvascular dysfunction can occur in the absence of obstructive epicardial coronary artery disease in the context of cardiomyopathies or risk factors.59,63
Impaired perfusion was detected in HF patients of different aetiologies by various techniques including the measurement of coronary flow reserve, magnetic resonance imaging, positron emission tomography, single-photon emission computed tomography, or contrast echo in humans and by assessing capillary density by histology. However, surprisingly little is known regarding the structural and molecular changes that occur in the microcirculation in HF. In experimental models, acute myocardial infarction (with or without reperfusion) or aortic banding induces an initial increase in capillary density, which is mainly mediated by hypoxia-induced augmentation of angiocrine signals (e.g. VEGF) in cardiomyocytes that induces a pro-angiogenic response.61 However, at later time points, capillary density is reduced leading to a mismatch of oxygen supply to the hypertrophic myocardium61,62 (Figure 1). Interestingly, a detailed histological study of vessel morphology in rats after aortic banding and ischaemia/reperfusion followed with aortic debanding describes that the coronary vasculature volume increased in this HF model.64 This study also reports striking effects on capillary morphology: whereas in control hearts, capillaries were uniformly arranged with a linear orientation and consistent shape, they exhibit irregular arrangements, significant augmentation of diameter and a curvy, distorted, inconsistent shape in HF. They found extremely narrow capillary branches (<3 µm) that appear to bridge between larger capillaries and contribute to the increased microvascular density in this study. Since these small capillaries likely do not allow erythrocytes to pass through, it is unlikely they contribute to cardiac tissue oxygenation. The results of this study suggest that a careful assessment of vascular structures is very important and that just counting of capillary density or area may not necessarily correlate with perfusion or the provision of appropriate microenvironmental factors.
Recent studies additionally show that the vasculature participates not only in the regulation of local blood perfusion by also controls the metabolic exchange between the blood and tissues.65 The metabolic requirements of the heart in order to fulfil its pumping function are immense.66 In a healthy heart, most of the energetic requirements of the heart are fulfilled by fatty acid metabolism but the heart also can use other sources for generation of ATP.67 During HF, but also during ageing, cardiomyocytes have been described to present a metabolic shift, from fatty acids to glucose.68Altered nutrient delivery from endothelial cells to the cardiomyocytes might play a role as one potential cause for these metabolic changes in HF. Thus, specific inhibition of endothelial Notch signalling pathway impairs fatty acid delivery to the cardiomyocytes and induces metabolic and vascular remodelling in the heart.69 Treatment of mice with Delta-like 4 neutralizing antibodies impaired fractional shortening and ejection fraction by reducing the expression of CD36 and FABP4 and the increased expression of ANGPTL4, an inhibitor of lipoprotein lipase.70 Furthermore, vascular Eph/ephrin signalling controls the function of caveolae and lipid transport. Loss of functions analysis revealed that caveolae are required for CD36 traffic to the membrane and fatty acid uptake by endothelial cells.71 CD36 is required in the endothelial cells for the uptake of circulating fatty acids into muscle tissue.72 Failure to do so results in dilated cardiomyopathy-like defects: reduced ejection fraction and increased diastolic and systolic volumes.71 These studies situate the vasculature in a central position regulating cardiac metabolism and, thus, protecting it against HF. However, given that the heart is considered as a ‘metabolic omnivore’ and can use multiple sources to produce ATP,67 further studies are essential to provide more insights whether endothelial nutrient transport can directly influence cardiomyocyte metabolism and how this may contribute to cardiomyocyte failure.
The cause of endothelial microcirculatory dysfunction in HFrEF is diverse. Impaired capillary growth in the infarct and border zone even after appropriate reperfusion may be one primary cause particularly in aged and diabetic patients. The underlying coronary artery disease, however, is also associated with induction of reactive oxygen species (ROS), reduces NO bioavailability and inflammatory activation in the microvasculature.59,73 Microvascular rarefaction may occur under conditions of continuous stress exposed by risk factors (such as diabetes)60 or the noxious environment of the scar tissue. The shedding of the vascular endothelial glycocalyx, which is the fragile inner layer of the endothelium composed by a network of different glycosaminoglycans and proteoglycans via activation of matrix metalloproteases,74,75 may further contribute to the impairment of endothelial function.
The occurrence of microcirculatory dysfunction may not necessarily be causally related to the development of HF and it may represent a consequence or an epiphenomenon: cardiomyocyte death and dysfunction may lead to fibrosis and impairment of cardiomyocyte–endothelial communication pathways, which subsequently may induce endothelial cell dysfunction. In this situation, simply reverting endothelial dysfunction to restore oxygen supply may not be sufficient to rescue the dysfunctional cardiomyocytes. Thus, an approach to target the cell intrinsic cardiomyocyte dysfunction and a restoration of the overall metabolic milieu and paracrine environment may be required to heal the failing heart. However, microvascular dysfunction may contribute to a vicious cycle by further promoting cardiac inflammation and limiting local oxygen or nutrient supply. This may further deteriorate cardiac tissue homeostasis, and as such treating the cardiac microvasculature may be, independent of it being a cause or a consequence of the cardiac disease, a valuable therapeutic approach to reduce progression of HF. In this sense, recovering microvasculature function might be of especial interest for patients that present a stunned or hibernating myocardium.76
4. The vasculature in HFpEF
HFpEF is becoming the predominant form of HF in ageing societies.77 Pre-clinical and clinical evidence support an important link between coronary microcirculatory dysfunction and HFpEF.78,79 Clinically, several studies showed a high prevalence of impaired coronary microvascular dysfunction in patients with HFpEF.80,81 Specifically, the PROMIS-HFpEF study showed an impaired coronary flow in HFpEF patients.80 Histological analysis of autopsies confirmed a significant reduction of capillary density in subepicardial, midmyocardial, subendocardial, and papillary muscle of patients with HFpEF.81
A recent experimental study further showed that cardiac microvascular endothelial cells regulate the relaxation profile of cardiomyocytes.82 Experimentally, various studies suggest that impaired endothelial function induced by reduced endothelial NO bioavailability plays a causal role in HFpEF. Reduced NO availability results in reduced PKG activity in cardiomyocytes and contributes to the development of cardiac hypertrophy.83 The microvasculature in the HFpEF myocardium shows an increased expression of adhesion molecules, migration of activated leucocytes and elevated levels of active oxygen species.84 Interestingly, the protective effects of the cardiac microvasculature on the relaxation profile of cardiomyocytes is lost when endothelial cells are exposed to pro-inflammatory cytokines82 supporting a critical role of vascular inflammation in the pathogenesis of HFpEF. Thereby, inflammation and endothelial dysfunction with impaired NO–sGC–cGMP signalling axis cause a reduction of the activity of the down-stream kinases PKG and PKA.85 These alterations lead to an excess of diastolic Ca2+ and sensitivity to it by troponin C and hypophosphorylation of titin. This leads to myocardial delayed relaxation and increased stiffness. The final consequence of a deficient NO–cGMP signalling pathway is a concentrically remodelled left ventricle with diastolic dysfunction.85 A crucial and causal role of lack of NO in the pathogenesis of HFpEF is supported by Shiattarella et al., who demonstrated that inhibition of constitutive NOS using N(omega)-nitro-L-arginine methyl ester in combination with high fat diet induces many of the clinical features of HFpEF in mice.86 However, attempts to augment NO by either increasing its synthesis or bioavailability by interfering with ROS had have not yet materialized in clinically effective therapies.87
5. Therapeutic strategies
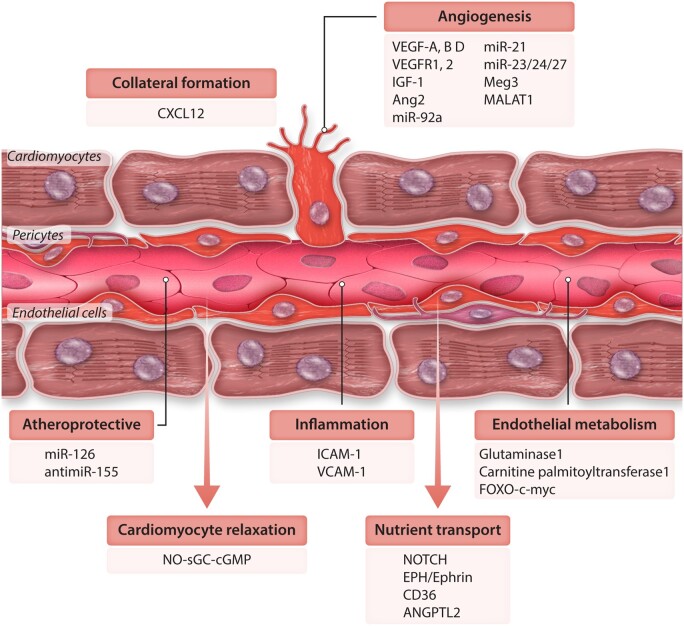
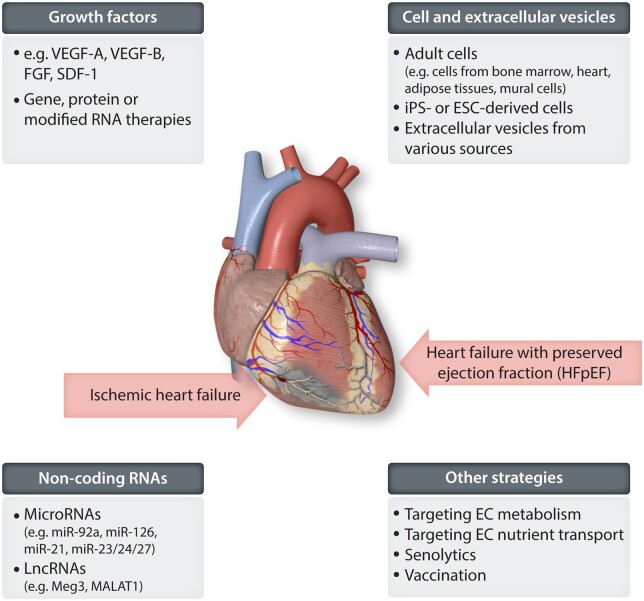
Although ample evidence supports a critical role of the vasculature and particularly the endothelium in controlling cardiac function, therapeutic approaches are so far spare. The field had been suffering from failures of pro-angiogenic gene therapies and limited clinical success of cell therapeutic approaches. However, increasing understanding of the processes of regulating vessel growth and new insights regarding ‘angiocrine’ signals that mediate protective and pro-regenerative functions of endothelial cells open new avenues for therapeutic approaches (Figures 2 and 3).
Figure 2.
Schematic of the different mechanisms regulating vascular homeostasis in the heart and the molecular players involved in them. There mechanism specific for the maintenance of vascular homeostasis like the endothelial metabolism, angiogenesis and the formation of collaterals, or the signals involved in atherosclerosis and inflammation. Other mechanisms like nutrient transport or the effect on cardiomyocyte relaxation have an effect directly on cardiomyocytes.
Figure 3.
Graphical scheme of the different strategies to improve microcirculatory dysfunction in the HF.
5.1 Growth factors
The potential of therapeutic angiogenesis has been discussed already in the last two decades.88,89 Several studies have been using pro-angiogenic factors with the objective to induce neo vascularization in patients with ischaemic heart disease. VEGF-A has been tested in different clinical trials using different delivery systems, adenoviruses or naked plasmid, injected intramyocardially or via catheter directly into the coronary arteries.90–93 Unfortunately, the results of these trials have been disappointing.26,92 Nonetheless, a recent study has revealed the importance of correct dosing as high mitogenic stimulation of the endothelial cells can arrest angiogenesis,94 thus, it might be important to reconsider those clinical trials and define the correct dosing and delivery method(s). VEGF-D induces both angiogenesis and lymph angiogenesis. It has been tested in patients with refractory angina pectoris and it increases the myocardial perfusion.95–97 Angiogenic VEGF-B has been shown to induce the expression of genes related to myocardial contraction and metabolism98 protecting the muscular cells from apoptosis and ischaemic damage in mice.99 Despite its promising perspectives, high doses of VEGF-B can induce ventricular arrhythmias100 and thus, as with VEGF-A, dose and delivery method will be crucial for the development of potential clinical trials. Intracoronary adenoviral gene transfer of FGF4 has been shown to improve cardiac perfusion in post-menopausal women although it had no effect on men.101,102 Furthermore, FGF is a mediator of the physiological repair of the glycocalyx.103 Enhancing this repairing signal would be a potential therapeutic approach to be explored in cardiac disease.
Where viral and plasmid gene therapies have had limited success,104 mRNA therapy with transcripts prepared using naturally occurring modifications might be more successful. Modified mRNA with N1-methylpseudouridine and further optimization by purification and capping105 enhances translation106 and has improved cardiac delivery.107 A single injection of modified VEGF-A mRNA in the cardiac apex of mice after myocardial infarction increased myocardial capillary density, reduced infarct size, and significantly improved survival even after a year.108 Modified mRNA has also been tested in large animal models. Carlsson et al. have shown that human VEGF mRNA injections into the swine heart improves cardiac function when given 1 week post-myocardial infarction.109 In addition, injection of modified IGF-1 modified mRNA has been shown to reduce apoptosis in the infarct border zone.110
Alternative to overexpression of angiogenic and endothelial-protective factors, one may envision inhibiting harmful mediators of vascular dysfunction. A prominent example is angiopoeitin-2, which is induced by cardiac injury and mediates pericyte detachment, vascular leakage, increased adhesion molecular expression, and degradation of the glycocalyx.111 Inhibition of angiopoietin-2 by gene deletion or by blocking antibodies preserved cardiac function.111
Finally, collateral induction may augment cardiac tissue perfusion, particularly in patients with vessel occlusions.112Historically activated monocytes and monocyte-derived growth factors were considered as therapeutic approaches.113,114 A recent study by Das et al. have shown that collateral induction improves neonatal regeneration after myocardial infarction in a CXCL12-CXCR4 dependent manner.115 A single CXCL12 injection at the time of infarction induced collateral formation 14 days after the ischaemic damage.115 Although the mechanisms of collateral formation in the adult human heart remain unclear, to induce arteriogenesis in combination with angiogenesis is a potential interesting approach to treat ischaemicHF.
5.2 Pro-angiogenic cell therapies
Cell therapies aiming at restoring the vasculature by delivering progenitor cells came into the focus in 1997, when Asahara and Isner described circulating cells expressing endothelial and haematopoietic markers. Meanwhile various cell populations isolated from the circulating blood or the bone marrow but also of other sources (e.g. fat tissue) have been experimentally and clinically been used in patients with cardiovascular disease (for review see References116,117) Although promising in many experimental studies and in patients with refractory angina,118 the overall clinical success has been limited. This may be due to multiple issues including challenges in cell delivery, which is even more complicated in a chronic disease state, such as HFpEF or HFrEF, which ultimately would require repetitive treatment. Autologous cell therapy is additionally compromised by the impaired functionality of cells derived from elderly and diseased patients.119,120 The new discovery of a high incidence of mutations in haematopoietic stem cells driving clonal expansion in elderly patients with cardiovascular disease and HF (up to 20–30%) may have additional impact.121,122 Since such mutations are associated with profound alterations in inflammatory and other signatures, autologous cells of carriers with such mutations may have different (likely impaired) functions.123,124 Recent larger scale clinical trials using bone marrow-derived mononuclear cells for the treatment of acute myocardial infarction (the BAMI trial) failed to enrol sufficient patients to finally clarify the potential of bone marrow mononuclear cells.125 A next generation of vessel forming cells may include iPS-derived endothelial cells, for which several protocols have been developed.126 However, such strategies may be more likely to be successful in combination with tissue engineering.
Mural cells, such as pericytes, may also have a reparative potential in the heart.127 Pericytes cover the capillaries, which grant them a privileged position to control and modulate the vasculature. Transplantation of different populations of pericytes has been shown to increase cardiac function and increased vascularization in infarcted mice but also in large animal models like the swine.127,128 But all these approaches rely on transplantation of external pericytes and their capacity to adapt to the environment they are transplanted into. To understand the molecular mechanisms that govern pericyte biology in the heart, and the response to injury from the local cardiac pericytes will be crucial to develop pericyte-based therapies or to therapeutically modulate pericyte functions and phenotypes in the future.
5.3 Non-coding RNAs
The advent of deep sequencing technologies led to the identification of a considerable amount of non-coding RNA transcripts, which are increasingly recognized for their functions in controlling endothelial and vascular functions.129,130 MicroRNAs have already been studied for a decade and several microRNA were shown to either protect or harm endothelial cells.130 In the context of vascular functions in the heart, examples included miR-92a, which can be targeted by antimiRs to augment neovascularization in mice and pigs.131,132 Moreover, miR-126 was shown to improve endothelial cell functions, promote vessel growth and prevents atherosclerotic lesion formation.133,134 Other examples include miR-21, which impairs pro-angiogenic cell functions and augments fibrosis,135 and members of the miR-23/24/27 cluster, which regulates angiogenesis and endothelial apoptosis in cardiac ischaemia.136 Some miRNAs were already further considered for pre-clinical development. AntimiR approaches against miR-92a were shown to be safe and efficient in a recent human Phase I study.137 AntimiRs directed against miR-155 (cobomarsen), which might also provide an atheroprotective effect, are currently applied in patients with multiple haematological malignancies.138
Other non-coding RNAs, such as circular RNAs, YRNAs, or long non-coding RNAs (lncRNAs), are currently gaining increasing attention (for review see References129,130). Among the many angiogenesis-regulating lncRNAs, the lncRNA RNAs Meg3 may be an interesting candidate to therapeutic development. The inhibition of this age-induced lncRNA reduces endothelial senescence and improves neovascularization in the context of aging.139 It is also particularly highly expressed in fibroblasts and GapmeR-mediated inhibition of Meg3-reduced cardiac fibrosis140 and cardiac hypertrophy.141 The combination of vasculoprotective and anti-fibrotic effects may be advantageous in the context of HFpEF.
Another well studied lncRNA is MALAT1, which is induced by hypoxia and is known to be important for vascularization.142It controls vascular and cardiac inflammation.143,144 However, a therapeutic approach would require overexpression, which is particularly challenging due to the excessive length of the lncRNA. Understanding its molecular mechanism of actions, however, might lead to the identification of down-stream signals, which might be easier accessible.
5.4 Extracellular vesicles
Extracellular vesicles and particularly the <100 nm small exosomes gained increasing attention for augmenting vascular repair. These vesicles come in different sizes and flavours depending on the cellular origin and the way they are released in response to physiological stimuli or cell death. Their putative therapeutic activity including the increase in angiogenesis has been shown in many different mice models.145,146 It is believed that the pro-angiogenic activity might be due to the delivery of growth factors, mRNAs or non-coding RNAs. Particularly the transport of pro-angiogenic microRNAs was shown to induce vessel growth and improve cardiac function (e.g.147) A potential disadvantage of endogenously derived extracellular vesicles is the lack of specificity and the complex cargo. This may be circumventing by the engineering of recombinant vesicles that can be loaded with a defined mixture of molecules and might be linked to specific adaptor to control delivery.148As a first step, targeting inflamed endothelial cells was reported by using leucocyte-inspired biodegradable particles that selectively adhere to inflamed endothelium.149
5.5 Targeting vascular inflammation
Endothelial cells of patients with HF are characterized by increased expression of vascular adhesion molecules [e.g. E-selectinand intercellular adhesion molecule-1 (ICAM1)],150 which promotes adhesion and invasion of pro-inflammatory cells into the heart.151 Since recent studies suggest that invasion of bone marrow-derived inflammatory cells, particularly monocytes, replace tissue-resident reparative macrophages, and thereby contribute to chronic inflammation and HF, targeting of monocyte adhesion may be a strategy to prevent chronic inflammation. Indeed, deletion of ICAM1 reduced infiltration of immune cells including T-cells and improved cardiac function in experimental models of HF.152 In addition, systemic anti-inflammatory therapies, most prominently TNFalpha inhibitors, were developed and tested in patients withHF. However, anti-TNF antibodies as well as other general anti-inflammatory strategies (e.g. pentoxifyllineand methodextrate) revealed mixed results (for summary of clinical studies see References153). Additional approaches include more specific targeting of inflammatory mediators to prevent endothelial activation. For example, targeting myeloperoxidase, which is released by neutrophils and profoundly augments vascular inflammation and dysfunction, was shown to prevent ischaemic HF.154 In addition, preventing the shedding or restoration of the protective endothelial glycocalix, as it occurs during HF,155 may ameliorate vascular inflammation and preserve endothelial functions, such as NO release. Heparanase inhibition156 or sulodexide, a mixture of heparin and dermatan sulphate,157 which have been shown to preserve endothelial glycocalix in different diseases, or growth factors (such as FGF or anti-angiopoietin-2)103,111 may be useful to restore endothelial cell functions also in the context of HF.
5.6 Targeting endothelial metabolism for vessel normalization
The importance of endothelial metabolism for proper endothelial cell functioning and the role of endothelial cells in nutrient transport and the metabolic control of tissues have been increasingly recognized in the last years.158Interestingly, several metabolic pathways have been identified as targets to prevent pathological angiogenesis. Inhibition of carnitine palmitoyltransferase 1, a regulator of fatty acid oxidation, or glutaminase 1, which hydrolyses glutamine into ammonia and glutamate, both impaired angiogenesis.159,160 Likewise, silencing of asparagine synthetase reduces vessel sprouting in vitro.160 The transcription factor FOXO, which regulate various targets including the inhibition of c-myc, controls endothelial quiescence.161 Whether modulations of these pathways can be used to augment or normalize cardiac microvasculature is currently unknown.
Endothelial metabolism can also be a target in metabolic disease, such as hyperglycaemia. Hyperglycaemia can trigger the production of ROS162 that can then uncouple eNOS.163 Because glycolytic intermediates feed into the pentose phosphate pathway, it was proposed that increasing the pentose phosphate pathway and away from glycolysis would reduce the levels of the damaging metabolites and be protective upon hyperglycaemia.65
Metabolites of arachidonic acid or other polyunsaturated fatty acids are long known for their vascular effects. For example, coronary endothelial function is controlled by thromboxane A2, prostacyclin, and prostaglandin H2.73 Recent studies now identified additional lipid metabolites that control vascular functions. Hu et al. demonstrate that the inhibition of the soluble epoxide hydrolase, which reduces the formation of the diol 19,20-dihydroxydocosapentaenoic acid improves vessel integrity by reducing pericyte loss and breakdown of endothelial barrier function in the retina.164 It might be interesting to employ these new insights in the context of the cardiac vasculature during aging orHF.
Finally, first studies in mice showed that endothelial cell specific modulation of Notch or EphB4 signalling leads to altered nutrient supply and cardiac dysfunction.70,71 Although evidence for a dysfunctional metabolic nutrient supply by endothelial cells in human HF is so far spares, one may speculate that controlling endothelial nutrient transport capacity may be used as future therapeutic option.
5.7 Others
5.7.1 Targeting endothelial cell senescence
Senescence is a protective response from the organisms against stress that limits the proliferation of aged non-functional cells.165 However, senescent cells accumulate in fibrotic regions166 and there is increasing accumulative evidence that senescence is closely related to cardiovascular disease.167,168 Indeed, endothelial cell senescence is associated with an augmented dysfunction and vascular inflammation.169 Recent studies further demonstrated that endothelial senescence contributes to HFpEF.170
Senolytics, which selectively target senescent cells have been shown to reverse pathological changes in post-infarction remodelling and HF,171,172 Also the genetic senolytic model, which allows the inducible elimination of p16INK4a senescent cells reduced the size of fibrotic area in the heart of old mice.168 Of note, these approaches not only target endothelial cells but also cardiomyocytes and other mural cells, which may together contribute to the therapeutic benefits.
5.7.2 Vaccination
Therapeutic vaccines for non-infectious diseases are currently in development for the treatment of various disorders including cardiovascular diseases, such as hypertension or atherosclerosis. Vaccination may be an attractive therapeutic strategy because of its high specificity and potential long-term effects. Although not yet directly explored for the treatment of the vasculature in HF, one may consider to apply some of the recent strategies shown to be effective in pulmonary arterial hypertension.173,174 This study used a passive vaccination approach to inhibit ET-1 signalling by targeting a 10-amino acid peptide sequence in the second extracellular loop-domain of the G-protein coupled ETA receptor.173 The inhibitory effects were similar to a clinically used pharmacological approach.
6. Conclusions
The cardiac vasculature plays a crucial role in maintaining oxygen and nutrient supply to the cardiac tissue and supports a healthy microenvironment. HF is well known to be associated with changes and functional impairment of the microvasculature. Experimental studies with specific ablation of protective signals in endothelial cells demonstrate that the induction of microcirculatory dysfunction is sufficient to induceHF. Therefore, the restoration of a healthy endothelium and microcirculation represent a valuable therapeutic strategy. Even if a sole targeting of the microcirculation may not reverse HF, it may prevent the further progression of the disease. Examples for such potential interventions are described above and include growth factors, cells, biologicals, non-coding RNAs, and others. Since HF comes in different flavours and not all HF patients will primarily suffer from microcirculatory dysfunction, the challenge will be to define the subgroup of patients that will likely profit from such treatments. Precision imaging and/or biomarkers will be necessary for the successful clinical development of microcirculation targeting therapies.
Conflict of interest: S.D. received a research grant of Servier, is on the scientific advisory board of miRagen Therapeutics and has a patent on miR-92a.
Funding
S.D. is supported by the Deutsche Forschungsgemeinschaft (DFG) (SFB1366) and the European Research Council (ERC) (Advanced grant (Angiolnc).
References
- 1. Aird WC. Phenotypic heterogeneity of the endothelium.Circ Res Am Heart Assoc 2007; 100: 158–173. [DOI] [PubMed] [Google Scholar]
- 2. Aird WC. Phenotypic heterogeneity of the endothelium.Circ Res Am Heart Assoc 2007; 100: 174–190. [DOI] [PubMed] [Google Scholar]
- 3. Witzleb E. Functions of the vascular system.In: Schmidt RF, Thews G (eds). Human Physiology.Berlin, Heidelberg: Springer; 1989. p480–542. [Google Scholar]
- 4. Rafii S, Butler JM, Ding B-S.. Angiocrine functions of organ-specific endothelial cells. Nature 2016; 529: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 2003; 83: 59–115. [DOI] [PubMed] [Google Scholar]
- 6. Hsieh PCH, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol 2006; 68: 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nees S, Weiss DR, Senftl A, Knott M, Förch S, Schnurr M, Weyrich P, Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J PhysiolHeart Circ Physiol 2012; 302: H69–H84. [DOI] [PubMed] [Google Scholar]
- 8. Crivellato E, Nico B, Ribatti D. Contribution of endothelial cells to organogenesis: a modern reappraisal of an old Aristotelian concept.J Anat 2007; 211: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drexler H. Endothelium as a therapeutic target in heart failure.Circ Am Heart Assoc 1998; 98: 2652–2655. [DOI] [PubMed] [Google Scholar]
- 10. Macdonald P, Schyvens C, Winlaw D. The role of nitric oxide in heart failure.Drugs Aging 1996; 8: 452–458. [DOI] [PubMed] [Google Scholar]
- 11. Murrell W. Nitroglycerin as a remedy for angina pectoris.Lancet 1879; 113: 80–81. [Google Scholar]
- 12. Jia X, Al Rifai M, Liu J, Agarwala A, Gulati M, Virani SS. Highlights of studies in cardiovascular disease prevention presented at the 2020 American College of Cardiology Annual Scientific Session. Curr Atheroscler Rep 2020; 22: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mombouli JV, Illiano S, Nagao T, Scott-Burden T, Vanhoutte PM. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ Res 1992; 71: 137–144. [DOI] [PubMed] [Google Scholar]
- 14. Potente M, Mäkinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 2017; 18: 477–494. [DOI] [PubMed] [Google Scholar]
- 15. Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad SciUSA 1992; 89: 9504–9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell 2012; 22: 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pérez-Pomares JM, Macías D, García-Garrido L, Muñoz-Chápuli R.. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail–chick chimera study. Dev Biol 1998; 200: 57–68. [DOI] [PubMed] [Google Scholar]
- 18. Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von GA, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008; 454: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cano E, Carmona R, Ruiz-Villalba A, Rojas A, Chau Y-Y, Wagner KD, Wagner N, Hastie ND, Muñoz-Chápuli R, Pérez-Pomares JM. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio–venous connections.Proc Natl Acad Sci USA 2016; 113: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Red-Horse K, Ueno H, Weissman IL, Krasnow MA.. Coronary arteries form by developmental reprogramming of venous cells. Nature 2010; 464: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, Zhong TP, Yang X, Yang Z, Yan Y, Baldini A, Sun Y, Lu J, Schwartz RJ, Evans SM, Gittenberger-de Groot AC, Red-Horse K, Zhou B. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res 2013; 23: 1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivelä R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, Jacobs AH, Epstein JA, Stankunas K, Alitalo K, Red-Horse K. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 2014; 141: 4500–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Yan Y, Yang X, Zhong TP, Pu WT, Zhou B. De novo formation of a distinct coronary vascular population in neonatal heart. Science 2014; 345: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang C-P, Zhou B. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 2012; 151: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoker ME, Gerdes AM, May JF. Regional differences in capillary density and myocyte size in the normal human heart.Anat Rec 1982; 202: 187–191. [DOI] [PubMed] [Google Scholar]
- 26. Kolte D, McClung JA, Aronow WS, Chapter 6 - Vasculogenesis and angiogenesis. In: Aronow WS, McClung JA (eds). Translational Research in Coronary Artery Disease.Boston: Academic Press; 2016. p49–65. [Google Scholar]
- 27. Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells.Development 1998; 125: 725–732. [DOI] [PubMed] [Google Scholar]
- 28. Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995; 11: 73–91. [DOI] [PubMed] [Google Scholar]
- 29. Folkman J. Tumor angiogenesis: therapeutic Implications. N Engl J Med 1971; 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 30. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8: 464–478. [DOI] [PubMed] [Google Scholar]
- 31. Potente M, Gerhardt H, Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis.Cell 2011; 146: 873–887. [DOI] [PubMed] [Google Scholar]
- 32. Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in xenopus embryos. Cell 1991; 66: 257–270. [DOI] [PubMed] [Google Scholar]
- 33. Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science 1995; 267: 246–249. [DOI] [PubMed] [Google Scholar]
- 34. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435–439. [DOI] [PubMed] [Google Scholar]
- 35. Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW.. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439–442. [DOI] [PubMed] [Google Scholar]
- 36. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995; 376: 62–66. [DOI] [PubMed] [Google Scholar]
- 37. Fong G-H, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995; 376: 66–70. [DOI] [PubMed] [Google Scholar]
- 38. George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO.. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin.Development 1993; 119: 1079–1091. [DOI] [PubMed] [Google Scholar]
- 39. Carmeliet P. Angiogenesis in health and disease.Nat Med 2003; 9: 653–660. [DOI] [PubMed] [Google Scholar]
- 40. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 2013; 3: a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mack JJ, Iruela-Arispe ML. NOTCH regulation of the endothelial cell phenotype.Curr Opin Hematol 2018; 25: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manavski Y, Lucas T, Glaser SF, Dorsheimer LenaGünther StefanBraun T, Rieger MA, Zeiher Andreas M, Boon Reinier A, Dimmeler S. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ Res 2018; 122: 670–677. [DOI] [PubMed] [Google Scholar]
- 43. Li Z, Solomonidis EG, Meloni M, Taylor RS, Duffin R, Dobie R, Magalhaes MS, Henderson BEP, Louwe PA, D’Amico G, Hodivala-Dilke KM, Shah AM, Mills NL, Simons BD, Gray GA, Henderson NC, Baker AH, Brittan M. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J 2019; 40: 2507–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res 2006; 98: 947–953. [DOI] [PubMed] [Google Scholar]
- 45. Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui C, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev 2006; 20: 1651–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Azambuja AP, Portillo-Sánchez V, Rodrigues MV, Omae SV, Schechtman D, Strauss BE, Costanzi-Strauss E, Krieger JE, Perez-Pomares JM, Xavier-Neto J. Retinoic acid and VEGF delay smooth muscle relative to endothelial differentiation to coordinate inner and outer coronary vessel wall morphogenesis. Circ Res 2010; 107: 204–216. [DOI] [PubMed] [Google Scholar]
- 47. Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: the normal and the abnormal. WJR 2016; 8: 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pérez-Pomares JM, de la Pompa JL, Franco D, Henderson D, Ho SY, Houyel L, Kelly RG, Sedmera D, Sheppard M, Sperling S, Thiene G, van den Hoff M, Basso C. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology—a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res 2016; 109: 204–216. [DOI] [PubMed] [Google Scholar]
- 49. Corrado D, Thiene G, Cocco P, Frescura C. Non-atherosclerotic coronary artery disease and sudden death in the young. Br Heart J 1992; 68: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 2010; 96: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 51. Talman V, Kivelä R. Cardiomyocyte-endothelial cell interactions in cardiac remodeling and regeneration.Front Cardiovasc Med 2018; 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 2015; 4: e05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation.Nat Cell Biol 2015; 17: 627–638. [DOI] [PubMed] [Google Scholar]
- 54. Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature 2012; 488: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang W, McKinnie SMK, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das Subhash K, Ratnadeep B, Brent M, Vijay K, Penninger JM, Zamaneh K, Vederas JC, Murray Allan G, Oudit Gavin Y. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia‐reperfusion injury: therapeutic potential of synthetic Apelin analogues.JAHA 2013; 2: e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drawnel FM, Archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth.Br J Pharmacol 2013; 168: 296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization. Circulation 2004; 110: 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johnson RD, Camelliti P. Role of non-myocyte gap junctions and connexin hemichannels in cardiovascular health and disease: novel therapeutic targets? IJMS 2018; 19: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020; 116: 806–816. [DOI] [PubMed] [Google Scholar]
- 60. Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz K-L, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C. Diabetes mellitus–induced microvascular destabilization in the myocardium. J Am Coll Cardiol 2017; 69: 131–143. [DOI] [PubMed] [Google Scholar]
- 61. Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005; 115: 2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007; 446: 444–448. [DOI] [PubMed] [Google Scholar]
- 63. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2014; 35: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen J, Yaniz-Galende E, Kagan HJ, Liang L, Hekmaty S, Giannarelli C, Hajjar R. Abnormalities of capillary microarchitecture in a rat model of coronary ischemic congestive heart failure.Am J Physiol Heart Circ Physiol 2015; 308: H830–H840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Potente M, Carmeliet P. The link between angiogenesis and endothelial metabolism.Annu Rev Physiol 2017; 79: 43–66. [DOI] [PubMed] [Google Scholar]
- 66. Kolwicz SC, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes.Circ Res 2013; 113: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter H-G, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ, American Heart Association Council on Basic Cardiovascular Sciences. Assessing cardiac metabolism: a scientific statement from the American Heart Association.Circ Res 2016; 118: 1659–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms.Am J Med Sci 1999; 318: 36–42. [DOI] [PubMed] [Google Scholar]
- 69. Taylor J, Fischer A. Endothelial cells dictate cardiac fuel source. Aging 2019; 11: 1083–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis E-M, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Gröne H-J, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018; 137: 2592–2608. [DOI] [PubMed] [Google Scholar]
- 71. Luxán G, Stewen J, Díaz N, Kato K, Maney SK, Aravamudhan A, Berkenfeld F, Nagelmann N, Drexler HC, Zeuschner D, Faber C, Schillers H, Hermann S, Wiseman J, Vaquerizas JM, Pitulescu ME, Adams RH. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. Elife 2019; 8: e45863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Son N-H, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu S-Q, Scerbo D, Chang HR, Sun F, Bagdasarov S, Drosatos K, Yeh ST, Mullick AE, Shoghi KI, Gumaste N, Kim K, Huggins L-A, Lhakhang T, Abumrad NA, Goldberg IJ. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest 2018; 128: 4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J 2017; 38: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, OudeEgbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline.Microcirculation 2009; 16: 657–666. [DOI] [PubMed] [Google Scholar]
- 76. Colbert RW, Holley CT, Stone LH, Crampton M, Adabag S, Garcia S, Iaizzo PA, Ward HB, Kelly RF, McFalls EO. The recovery of hibernating hearts lies on aspectrum: from bears in nature to patients with coronary artery disease. J Cardiovasc Trans Res 2015; 8: 244–252. [DOI] [PubMed] [Google Scholar]
- 77. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Crea F, Bairey MC, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017; 38: 473–477. [DOI] [PubMed] [Google Scholar]
- 79. D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol 2019; 10: 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, Beussink-Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan L-M, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Juni RP, Kuster DWD, Goebel M, Helmes M, Musters RJP, Velden J, van derKoolwijk P, Paulus WJ, van Hinsbergh VWM. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC Basic Transl Sci 2019; 4: 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Heerebeek Loek H, Nazha F-P, Inês L-M, Adelino F, Begieneman MPV, Bronzwaer Jean GF, van der Velden Jolanda S, Ger JM, Laarman GJ, Somsen A, Verheugt Freek WA, Niessen Hans WM, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012; 126: 830–839. [DOI] [PubMed] [Google Scholar]
- 84. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 85. Lim SL, Lam CSP. Breakthrough in heart failure with preserved ejection fraction: are we there yet? Korean J Intern Med 2015; 31: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019; 568: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sairam T, Patel AN, Subrahmanian M, Gopalan R, Pogwizd SM, Ramalingam S, Sankaran R, Rajasekaran NS. Evidence for a hyper-reductive redox in a sub-set of heart failure patients.J Transl Med 2018; 16: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999; 5: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 89. Ylä-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 2007; 49: 1015–1026. [DOI] [PubMed] [Google Scholar]
- 90. Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, Bøtker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyöngyösi M, Sylvén C. Direct intramyocardial plasmid vascular endothelial growth factor-A165gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol 2005; 45: 982–988. [DOI] [PubMed] [Google Scholar]
- 91. Marja H, Juha H, Mikko S, Joachim S, Antti H, Antti K, Esko V, Hanna M, Esa K, Sakari S, Outi N, Arto R, Keijo P, Markku SN, Markku L, Seppo Y-H. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia. Circulation 2003; 107: 2677–2683. [DOI] [PubMed] [Google Scholar]
- 92. Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, Siega AD, Bilodeau L, Burton JR, Proulx G, Radhakrishnan S. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther 2009; 17: 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kaminsky SM, Quach L, Chen S, Pierre-Destine L, Van de Graaf B, Monette S, Rosenberg JB, De BP, Sondhi D, Hackett NR, Mezey JG, Rosengart TK, Crystal RG. Safety of direct cardiac administration of AdVEGF-All6A+, a replication-deficient adenovirus vector cDNA/genomic hybrid expressing all three major isoforms of human vascular endothelial growth factor, to the ischemic myocardium of rats. Hum Gene Ther Clin Dev 2013; 24: 38–46. [DOI] [PubMed] [Google Scholar]
- 94. Pontes-Quero S, Fernández-Chacón M, Luo W, Lunella FF, Casquero-Garcia V, Garcia-Gonzalez I, Hermoso A, Rocha SF, Bansal M, Benedito R. High mitogenic stimulation arrests angiogenesis. Nat Commun 2019; 10: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rosengart TK, Bishawi MM, Halbreiner MS, Fakhoury M, Finnin E, Hollmann C, Shroyer AL, Crystal RG.. Long-term follow-up assessment of a phase 1 trial of angiogenic gene therapy using direct intramyocardial administration of an adenoviral vector expressing the VEGF121 cDNA for the treatment of diffuse coronary artery disease. Hum Gene Ther 2012; 24: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hassinen I, Hartikainen J, Antti H, Antti K, Antti S, Juhani K, Minna H, Hanna M, Marja H, Pyry T, Tommi H, Seppo Y-H.. Abstract 11987: adenoviral Intramyocardial VEGF-D gene transfer increases myocardial perfusion in refractory angina patients. Circulation 2015; 132: A11987–A11987. [Google Scholar]
- 97. Hartikainen J, Hassinen I, Hedman A, Kivelä A, Saraste A, Knuuti J, Husso M, Mussalo H, Hedman M, Rissanen TT, Toivanen P, Heikura T, Witztum JL, Tsimikas S, Ylä-Herttuala S. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur Heart J 2017; 38: 2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Terhi K, Maija B, Ollila Hanna M, Tuulikki S-L, Erkki L, Hanna L, Riikka K, Teemu H, Mari M, Michael J, Karri P, Andersson Leif C, Mervaala E, Hassinen Ilmo E, Seppo Y-H, Matej O, Kari A. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res 2008; 103: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lähteenvuo JE, Lähteenvuo Markku T, Antti K, Carolina R, Annelie F, Joakim K, Tommi H, Rissanen Tuomas T, Elisa V, Petra K, Berndt E, Peter C, Kari A, Ulf E, Seppo Y-H.. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1– and neuropilin receptor-1–dependent mechanisms. Circulation 2009; 119: 845–856. [DOI] [PubMed] [Google Scholar]
- 100. Lähteenvuo J, Hätinen O-P, Kuivanen A, Huusko J, Paananen J, Lähteenvuo M, Nurro J, Hedman M, Hartikainen J, Laham-Karam N, Mäkinen P, Räsänen M, Alitalo K, Rosenzweig A, Ylä-Herttuala S, Ylä-Herttuala S. Susceptibility to cardiac arrhythmias and sympathetic nerve growth in VEGF-B overexpressing myocardium. Mol Ther 2020; 28: 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol 2007; 50: 1038–1046. [DOI] [PubMed] [Google Scholar]
- 102. Kaski JC, Consuegra-Sanchez L. Evaluation of ASPIRE trial: a Phase III pivotal registration trial, using intracoronary administration of Generx (Ad5FGF4) to treat patients with recurrent angina pectoris. Expert Opin Biol Ther 2013; 13: 1749–1753. [DOI] [PubMed] [Google Scholar]
- 103. Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, Ford JA, Picon MA, Stearman RS, Lin L, Liu X, Han X, Linhardt RJ, Schmidt EP. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol 2017; 56: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cannatà A, Ali H, Sinagra G, Giacca M. Gene therapy for the heart lessons learned and future perspectives. Circ Res 2020; 126: 1394–1414. [DOI] [PubMed] [Google Scholar]
- 105. Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA vaccines protect against Zika virus infection. Cell 2017; 168: 1114–1125.E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Svitkin YV, Cheng YM, Chakraborty T, Presnyak V, John M, Sonenberg N. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res 2017; 45: 6023–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sultana N, Magadum A, Hadas Y, Kondrat J, Singh N, Youssef E, Calderon D, Chepurko E, Dubois N, Hajjar RJ, Zangi L. Optimizing cardiac delivery of modified mRNA. Mol Ther 2017; 25: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zangi L, Lui KO, von GA, Ma Q, Ebina W, Ptaszek LM, Später D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe DM, Li RA, Wagers AJ, Rossi DJ, Pu WT, Chien KR. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol 2013; 31: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Carlsson L, Clarke JC, Yen C, Gregoire F, Albery T, Billger M, Egnell A-C, Gan L-M, Jennbacken K, Johansson E, Linhardt G, Martinsson S, Sadiq MW, Witman N, Wang Q-D, Chen C-H, Wang Y-P, Lin S, Ticho B, Hsieh PCH, Chien KR, Fritsche-Danielson R. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol Ther Methods Clin Dev 2018; 9: 330–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang C-L, Leblond A-L, Turner EC, Kumar AH, Martin K, Whelan D, O’Sullivan DM, Caplice NM.. Synthetic chemically modified mRNA-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction.Mol Pharm 2015; 12: 991–996. [DOI] [PubMed] [Google Scholar]
- 111. Lee S-J, Lee C-K, Kang S, Park I, Kim YH, Kim SK, Hong SP, Bae H, He Y, Kubota Y, Koh GY. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J Clin Invest 2018; 128: 5018–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schaper W. Collateral circulation.Basic Res Cardiol 2009; 104: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Apostolakis S, Lip GYH, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res 2010; 85: 649–660. [DOI] [PubMed] [Google Scholar]
- 114. Shahid F, Lip GYH, Shantsila E. Role of monocytes in heart failure and atrial fibrillation.J Am Heart Assoc 2018; 7: e007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, Eskandari A, Hironaka CE, Phansalkar R, Sharma B, Rhee S, Shamskhou EA, Agalliu D, Jesus Perez V, deWoo YJ, Red-Horse K. A unique collateral artery development program promotes neonatal heart regeneration. Cell 2019; 176: 1128–1142.E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Banerjee Monisha N, Roberto B, Hare JM. Clinical studies of cell therapy in cardiovascular medicine. Circ Res 2018; 123: 266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mariann G, Paul HM, Blake Derek J, Enca MR.. Meta-analysis of cell therapy studies in heart failure and acute myocardial infarction.Circ Res 2018; 123: 301–308. [DOI] [PubMed] [Google Scholar]
- 118. Jones DA, Deshan W, Martina C, Hussain Mohsin A, Devanayegi V, Mervyn A, Rathod Krishnaraj S, Andreas B, Anthony M. The impact of cell therapy on cardiovascular outcomes in patients with refractory angina.Circ Res 2019; 124: 1786–1795. [DOI] [PubMed] [Google Scholar]
- 119. Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol 2007; 49: 2341–2349. [DOI] [PubMed] [Google Scholar]
- 120. Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89: e1–e7. [DOI] [PubMed] [Google Scholar]
- 121. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure.JAMA Cardiol 2019; 4: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 2018; 123: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure.JAMA Cardiol 2020; 5: 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mathur A, Arnold R, Assmus B, Bartunek J, Belmans A, Bönig H, Crea F, Dimmeler S, Dowlut S, Fernández-Avilés F, Galiñanes M, Garcia-Dorado D, Hartikainen J, Hill J, Hogardt-Noll A, Homsy C, Janssens S, Kala P, Kastrup J, Martin J, Menasche P, Miklik R, Mozid A, Román JAS, Sanz-Ruiz R, Tendera M, Wojakowski W, Ylä-Herttuala S, Zeiher A. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: rationale and design of the BAMI trial.Eur J Heart Fail 2017; 19: 1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hong X, Le Bras A, Margariti A, Xu Q. Reprogramming towards endothelial cells for vascular regeneration. Genes Dis 2016; 3: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Murray IR, Baily JE, Chen WCW, Dar A, Gonzalez ZN, Jensen AR, Petrigliano FA, Deb A, Henderson NC. Skeletal and cardiac muscle pericytes: functions and therapeutic potential. PharmacolTher 2017; 171: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Alvino VV, Fernández-Jiménez R, Rodriguez-Arabaolaza I, Slater S, Mangialardi G, Avolio E, Spencer H, Culliford L, Hassan S, Sueiro BL, Herman A, Ayaon-Albarrán A, Galán-Arriola C, Sánchez-González J, Hennessey H, Delmege C, Ascione R, Emanueli C, Angelini GD, Ibanez B, Madeddu P. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. J Am Heart Assoc 2018; 7: e006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bär C, Chatterjee S, Pires IF, Rodrigues P, Sluijter JPG, Boon RA, Nevado RM, Andrés V, Sansonetti M, L de W, Ciccarelli M, Hamdani N, Heymans S, Videira RF, Tocchetti CG, Giacca M, Zacchigna S, Engelhardt S, Dimmeler S, Madonna R, Thum T. Non-coding RNAs - update on mechanisms and therapeutic targets from the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc Res 2020; 116: 1805–1819. [DOI] [PubMed] [Google Scholar]
- 130. Jaé N, Dimmeler S. Noncoding RNAs in vascular diseases.Circ Res 2020; 126: 1127–1145. [DOI] [PubMed] [Google Scholar]
- 131. Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009; 324: 1710–1713. [DOI] [PubMed] [Google Scholar]
- 132. Rabea H, Daniela P, Stefanie Z, Ariane F, Wira H, Quan-Fu X, Elisabeth B, Eva vR, Andreas ZM, Kupatt C, Stefanie D. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013; 128: 1066–1075. [DOI] [PubMed] [Google Scholar]
- 133. Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RTA, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 2014; 20: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 2008; 15: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Huang C-K, Bär C, Thum T. miR-21, mediator, and potential therapeutic target in the cardiorenal syndrome. Front Pharmacol 2020; 11: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bang C, Fiedler J, Thum T. Cardiovascular importance of the microRNA-23/27/24 family. Microcirculation 2012; 19: 208–214. [DOI] [PubMed] [Google Scholar]
- 137. Abplanalp WT, Fischer A, John D, Zeiher AM, Gosgnach W, Darville H, Montgomery R, Pestano L, Allée G, Paty I, Fougerousse F, Dimmeler S. Efficiency and target derepression of anti-miR-92a: results of a first in human study. Nucleic Acid Ther 2020; 30: 335–345. [DOI] [PubMed] [Google Scholar]
- 138. Querfeld C, Foss FM, Kim YH, Pinter-Brown L, William BM, Porcu P, Pacheco T, Haverkos BM, DeSimone J, Guitart J, Halwani AS, Eradat HA, Huen A, Schroeder K, Pestano LA, Williams PJ, Cheronis I, Gordon GS, Escolar D, Rubin P, Marshall WS. Phase 1 trial of cobomarsen, an inhibitor of Mir-155, in cutaneous T cell lymphoma. Blood 2018; 132: 2903–2903. [Google Scholar]
- 139. Boon RA, Hofmann P, Michalik KM, Lozano-Vidal N, Berghäuser D, Fischer A, Knau A, Jaé N, Schürmann C, Dimmeler S. Long noncoding RNA Meg3 controls endothelial cell aging and function: implications for regenerative angiogenesis. J Am Coll Cardiol 2016; 68: 2589–2591. [DOI] [PubMed] [Google Scholar]
- 140. Maria-Teresa P, Shashi Kumar G, Janika V, Ariana F, Sabine S, Luise KF, Ankita G, Janet R, Karina Z, Sandor B, Thomas T.. Inhibition of the cardiac fibroblast–enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res 2017; 121: 575–583. [DOI] [PubMed] [Google Scholar]
- 141. Zhang J, Liang Y, Huang X, Guo X, Liu Y, Zhong J, Yuan J. STAT3-induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR-361-5p/HDAC9 axis. Sci Rep 2019; 9: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Michalik KM, Xintian Y, Yosif M, Anuradha D, Martin Z, Thomas B, David J, Yuliya P, Wei C, Shizuka U, Boon RA, Stefanie D. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 2014; 114: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 143. Sebastian C, Michalik Katharina M, Ariane F, Larissa P, Nicolas J, Carla W, Boon RA, Marion M-R, David J, Shizuka U, Christian W, Wolfgang P, Stefan G, Thomas B, Li Daniel Y, Maegdefessel L, Ljubica PM, Ulf H, Oliver S, Andreas Z, Stefanie D. Hematopoietic deficiency of the long noncoding RNA MALAT1 promotes atherosclerosis and plaque inflammation. Circulation 2019; 139: 1320–1334. [DOI] [PubMed] [Google Scholar]
- 144. Gast M, Schroen B, Voigt A, Haas J, Kuehl U, Lassner D, Skurk C, Escher F, Wang X, Kratzer A, Michalik K, Papageorgiou A, Peters T, Loebel M, Wilk S, Althof N, Prasanth KV, Katus H, Meder B, Nakagawa S, Scheibenbogen C, Schultheiss H-P, Landmesser U, Dimmeler S, Heymans S, Poller W. Long noncoding RNA MALAT1-derived mascRNA is involved in cardiovascular innate immunity. J Mol Cell Biol 2016; 8: 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang J-W. Extracellular vesicles in cardiovascular diseases: alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int J Mol Sci 2019; 20: 3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res 2017; 120: 1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 2015; 37: 2415–2424. [DOI] [PubMed] [Google Scholar]
- 148. Murphy DE, Jong OG, deBrouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med 2019; 51: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci USA 2003; 100: 15895–15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction.JACC Heart Fail 2016; 4: 312–324. [DOI] [PubMed] [Google Scholar]
- 151. Gerhardt T, Ley K. Monocyte trafficking across the vessel wall.Cardiovasc Res 2015; 107: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Salvador AM, Nevers T, Velázquez F, Aronovitz M, Wang B, Abadía Molina A, Jaffe IZ, Karas RH, Blanton RM, Alcaide P. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload–induced heart failure.JAHA 2016; 5: e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Riehle C, Bauersachs J. Key inflammatory mechanisms underlying heart failure. Herz 2019; 44: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Askari AT, Brennan M-L, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med 2003; 197: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ajaero CN, Procter NEK, Chirkov YY, Heresztyn T, Arstall MA, McGavigan AD, Frenneaux MP, Horowitz JD. Endothelial dysfunction and glycocalyx shedding in heart failure: insights from patients receiving cardiac resynchronisation therapy. Heart Vessels 2020; 35: 197–206. [DOI] [PubMed] [Google Scholar]
- 156. Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18: 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ESG, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010; 53: 2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Draoui N, P de Z, Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol 2017; 7: 170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schoors S, Bruning U, Missiaen R, Queiroz KCS, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Goddé L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquière B, Lunt SY, Fendt S-M, Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015; 520: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Brüning U, Visnagri A, Yuldasheva N, Goveia J, Cruys B, Brepoels K, Wyns S, Rayport S, Ghesquière B, Vinckier S, Schoonjans L, Cubbon R, Dewerchin M, Eelen G, Carmeliet P. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J 2017; 36: 2334–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Brüning JC, Gerhardt H, Carmeliet P, Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016; 529: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999; 48: 1–9. [DOI] [PubMed] [Google Scholar]
- 163. Naoto S, Tomoya Y, Tomofumi T, Masakazu S, Rio S, Masafumi T, Noriaki E, Akiko F, Toshio H, Kazuhisa I, Takahide N, Mitsuhiro Y, Ken-Ichi H, Seinosuke K. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vas Biol 2008; 28: 1068–1076. [DOI] [PubMed] [Google Scholar]
- 164. Hu J, Dziumbla S, Lin J, Bibli S-I, Zukunft S, Mos J, deAwwad K, Frömel T, Jungmann A, Devraj K, Cheng Z, Wang L, Fauser S, Eberhart CG, Sodhi A, Hammock BD, Liebner S, Müller OJ, Glaubitz C, Hammes H-P, Popp R, Fleming I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 2017; 552: 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS.. The essence of senescence.Genes Dev 2010; 24: 2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 2018; 217: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Shimizu I, Minamino T. Cellular senescence in cardiac diseases.J Cardiol 2019; 74: 313–319. [DOI] [PubMed] [Google Scholar]
- 168. Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019; 18: e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Donato AJ, Morgan RG, Walker AE, Lesniewski LA.. Cellular and molecular biology of aging endothelial cells.J Mol Cell Cardiol 2015; 89: 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gevaert AB, Hadis S, Leloup AJ, Van Hove Cor E, De Meyer Guido RY, Vrints Christiaan J, Katrien L, Van Craenenbroeck Emeline M. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail 2017; 10: e003806. [DOI] [PubMed] [Google Scholar]
- 171. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann‐Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Walaszczyk A, Dookun E, Redgrave R, Tual‐Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 2019; 18: e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Dai Y, Chen X, Song X, Chen X, Ma W, Lin J, Wu H, Hu X, Zhou Y, Zhang H, Liao Y, Qiu Z, Zhou Z. Immunotherapy of endothelin-1 receptor type A for pulmonary arterial hypertension. J Am Coll Cardiol 2019; 73: 2567–2580. [DOI] [PubMed] [Google Scholar]
- 174. Shah PK. Active and passive vaccination for pulmonary arterial hypertension: a novel therapeutic paradigm. J Am Coll Cardiol 2019; 73: 2581–2583. [DOI] [PubMed] [Google Scholar]