Abstract
Fibromuscular dysplasia (FMD) is a non-atherosclerotic vascular disease that may involve medium-sized muscular arteries throughout the body. The majority of FMD patients are women. Although a variety of genetic, mechanical, and hormonal factors play a role in the pathogenesis of FMD, overall, its cause remains poorly understood. It is probable that the pathogenesis of FMD is linked to a combination of genetic and environmental factors. Extensive studies have correlated the arterial lesions of FMD to histopathological findings of arterial fibrosis, cellular hyperplasia, and distortion of the abnormal architecture of the arterial wall. More recently, the vascular phenotype of lesions associated with FMD has been expanded to include arterial aneurysms, dissections, and tortuosity. However, in the absence of a string-of-beads or focal stenosis, these lesions do not suffice to establish the diagnosis. While FMD most commonly involves renal and cerebrovascular arteries, involvement of most arteries throughout the body has been reported. Increasing evidence highlights that FMD is a systemic arterial disease and that subclinical alterations can be found in non-affected arterial segments. Recent significant progress in FMD-related research has led to improve our understanding of the disease’s clinical manifestations, natural history, epidemiology, and genetics. Ongoing work continues to focus on FMD genetics and proteomics, physiological effects of FMD on cardiovascular structure and function, and novel imaging modalities and blood-based biomarkers that can be used to identify subclinical FMD. It is also hoped that the next decade will bring the development of multi-centred and potentially international clinical trials to provide comparative effectiveness data to inform the optimal management of patients with FMD.
Keywords: Fibromuscular dysplasia, Research, Proteomic, Genomic, Spontaneous dissection
Graphical Abstract
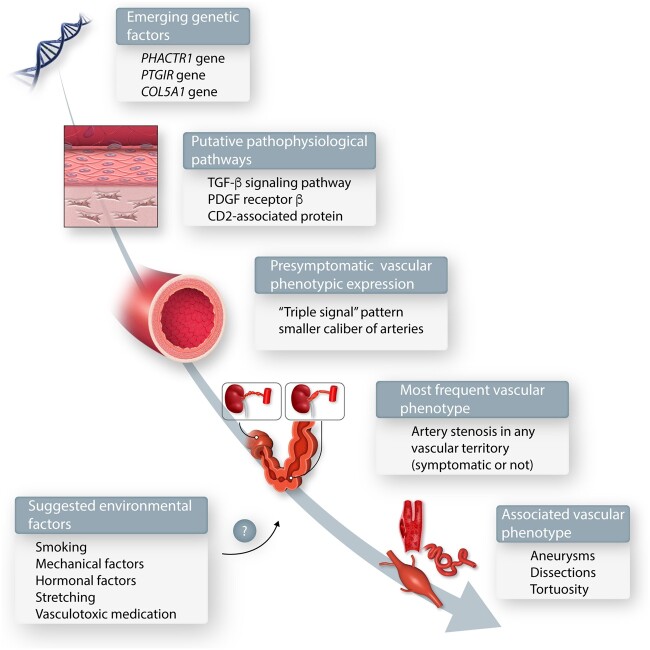
Current understanding of FMD.
Current understanding of FMD.
1. Introduction and aim of the review
Fibromuscular dysplasia (FMD) is a non-atherosclerotic vascular disease that may involve medium-sized muscular arteries throughout the body (Table 1). The frequency of FMD in the general population is not known. Though FMD may occur in men, the majority of FMD patients are women (82–95% in contemporary registries) (Table 2).1,2 FMD can occur at any age, including children and elderly patients, though presentation in young and middle adulthood is most common. Mean age at diagnosis of FMD is estimated as 43–53 years across different registries (Table 2). The pathogenesis of FMD remains largely unknown, but is probably multifactorial including genetic and environmental factors. However, it is not thought to be an inflammatory process (i.e. vasculitis) and it is also not known to involve the venous system. The angiographic features of FMD include the classical multifocal (string-of-beads) lesions as well as tubular or focal stenosis (now grouped under the generic term ‘focal’)1,2 (Figure 1 and Table 1). These findings may be associated with the clinical manifestations of the disease depending on the stenosis-induced ischaemia of the vascular territory predominantly involved. While FMD most commonly involves the renal (66–91%), cerebrovascular (25–80%), and less often mesenteric (14–21%) and lower extremity (10–45%) arteries, involvement of most arteries throughout the body has been reported (Table 2).
Table 1.
Consensus points from the International FMD Consensus—Definitions and Nomenclature for FMD. Adapted from Refs1,2
|
|
|
Table 2.
Comparison of selected data from the US, French (ARCADIA), European/International FMD registries and ARCADIA-POL study
| Registry | US Registry for FMD (USA)1 | European/International Registry3 | ARCADIA (France, Belgium)4 | ARCADIA-POL (Poland)5 |
|---|---|---|---|---|
| Number of patients analysed, n | 1885 | 1022 | 469 | 232 |
| No. of centres evaluating patients | 13 | 46 | 16 | 1a |
| Women (%) | 95 | 82 | 84 | 83 |
| BMI (kg/m2) | 26 | 25 | 24 | 25 |
| Family history of FMD (%) | 5.4 | 3.0 | 2.4 | 2.6 |
| Age at diagnosis of FMD (years) | 53 | 46 | 53 | 43 |
| Hypertension (%) | 67 | 86 | 77 | 91 |
| Office blood pressure (mmHg) | 132/75 | 140/85 | 139/83 | 134/83 |
| Age at hypertension diagnosis (years) | 45 | 37 | No data | 36 |
| Multifocal FMD (%) | 95c | 72 | 92 | 82 |
| Multivessel FMD (%) | 55.1 | 57.4 | 48.0 | 30.2 |
| FMD lesions distribution | ||||
| Renal (%)b | 66 | 91 | 79 | 88 |
| Cerebrovascular (including extracranial) (%)b | 80 | 63 | 50 | 25 |
| Mesenteric (%)b | 15 | 21 | 17 | 14 |
| Lower extremity (%)b | 45 | 31 | 15 | 10 |
| FMD-associated vascular complications | ||||
| Dissections (%) | 28 | 6 | 16 | 13 |
| Aneurysms (%) | 23 | 22 | 26 | 31 |
BMI, body mass index; FMD, fibromuscular dysplasia; IQR, interquartile range.
A total of 32 centres are actively recruiting patients and referring them to one national center.
Rate in patients in whom evaluation was performed.
Excluding patients with undetermined/missing FMD phenotype, in addition 2% of patients had both multifocal and focal FMD.
Figure 1.
Multifocal (A) and focal (B) lesion of FMD of the renal arteries.
Extensive prior studies have correlated the arterial lesions of FMD to histopathological findings of arterial fibrosis, cellular hyperplasia, and distortion of the abnormal architecture of the arterial wall.6 More recently, the vascular phenotype of lesions associated with FMD has been expanded to include arterial aneurysms, dissections, and tortuosity. However, in the absence of a string-of-beads or focal stenosis, these lesions do not suffice to establish the diagnosis.1,2,7 The scope of this review is to present an outline of recent advances and ongoing progress in the understanding of FMD and related diseases, made since publication of the International FMD Consensus1,2 or not extensively covered in this document, from bench to bedside. A comprehensive summary of clinical management based on the International FMD Consensus1,2 can be found in Supplementary Table S1.
2. Progress in research on FMD—multicentre registries
2.1 US registry for FMD
The US Registry for FMD, launched in 2009,8 currently includes nearly 3000 patients from 17 specialized FMD clinical centres (Figure 2 and Table 2). It is coordinated by the University of Michigan’s Michigan Cardiovascular Outcomes Research and Reporting Program and funded by the patient advocacy organization the FMD Society of America (www.fmdsa.org). New centres have been incrementally added across the USA to increase geographical, racial/ethnic, and clinical diversity of the Registry population. Key reports of the Registry published since 2012 include: description of the most common clinical manifestations of FMD, including hypertension, headache,9 and pulsatile tinnitus;8 the finding of cerebrovascular (carotid and vertebral) involvement of FMD being as common as renal involvement;8 a report of a high prevalence of aneurysms and dissections among patients with FMD;10,11 a description of a higher prevalence of aneurysms and dissections in men with FMD,12 and of a more benign vascular phenotype in patients diagnosed ≥65 years old.13
Figure 2.
National and international registries evaluating patients with FMD: The United States Registry for FMD (green), The French-Belgian ARCADIA network (orange/blue), Polish ARCADIA-POL Registry (red), and the European/International FMD Registry (blue).
2.2 ARCADIA registry
The French-Belgian ARCADIA Registry4 represents the ultimate development of a series of French achievements on FMD mostly produced by the Hypertension Excellence Centre of the Hôpital Européen Georges Pompidou (Paris, France).14,15 It includes expert centres from 16 university hospitals in France and Belgium (Figure 2). The ARCADIA registry is at the origin of the landmark ARCADIA study4—which provided definitive evidence of a high prevalence of multivessel FMD based on systematic state-of-the art imaging of brain-to-pelvis main arterial beds (by CT- or MR-angiography) with centralized image reading in 469 patients (Table 2). It also provided the backbone for the PROFILE study, looking at the incidence of novel FMD localizations and complications during a 3 year-follow-up in patients who had been fully examined at baseline. The results of the PROFILE study are expected to be published in 2021.
2.3 ARCADIA-POL study
The Polish study ARCADIA-POL initiated in January 2015 at the National Institute of Cardiology in Warsaw currently includes more than 300 patients with confirmed FMD referred by 32 centres (Figure 2). All patients are examined by head to pelvis CT angiography and benefit from an exhaustive work-up during a 5 day hospitalization. As of now, the main achievement of the ARCADIA-POL study was to document a high prevalence of previously undetected FMD lesions affecting clinical decisions using this systematic approach in the first 232 patients (Table 2).5 Other contributions include evaluation of structural and functional changes in the heart and kidneys of patients with FMD,16,17 and description of prevalence and characteristics of spontaneous cervical artery dissections18 and visceral FMD.19
2.4 European/International FMD registry
The European/International FMD registry was launched in Brussels in December 20153,20 and endorsed by the European Society of Hypertension (ESH) in 2016. It forms the nucleus of the wider European/International FMD Registry and Initiative (FEIRI).3 As of now, it enrolled about 1800 patients from 47 centres in 23 countries, also including Argentina, China, and Japan (Figure 2D). Currently, the main achievements of the European/International FMD Registry are (i) detailed analysis of the first 1000 patients included in the Registry, allowing characterization of distinct patient profiles according to FMD subtype, age and gender and identification of novel predictors of multivessel disease, aneurysms, and dissections3 (Table 2) and (ii) first-time detailed assessment of the prevalence and nature of FMD-related complications during pregnancy.21
3. Genetics of FMD
While both sporadic and familial forms of FMD exist, symptomatic FMD in relatives is reported only in a minority of cases (<5%). In some families, autosomal dominant inheritance with incomplete penetrance has been suggested.22,23 Traditional, family-based genetic studies have been challenging to conduct due to the relatively low frequency of well-characterized multiplex pedigrees and incomplete penetrance (∼0.5).23–26 FMD is currently thought to have at least a partially complex genetic basis, due to the influence of common genetic variants. Additionally, studies on genetic background of FMD are difficult due to the likely high prevalence of asymptomatic FMD patients (∼3–6%)8,27 and the influence of potential environmental modifiers (e.g. female hormones, lifetime mechanical stress, and tobacco smoking).
Using a genome-wide association study approach, a single nucleotide polymorphism (SNP) rs9349379-A, in the Phosphatase and Actin Regulator 1 (PHACTR1) gene has been identified as a common genetic risk variant. The protein encoded by this gene is a member of the phosphatase and actin regulator family of proteins. This family member can bind actin and regulate the reorganization of the actin cytoskeleton. It plays a role in tubule formation and endothelial cell survival.28 The associated SNP confers an odds ratio of ∼1.4 for FMD.29 It is located within an intron of the PHACTR1 gene and is associated with PHACTR1 transcript expression levels in dermal fibroblasts.29 The same variant has been associated with spontaneous coronary artery dissection (SCAD) and expression QTL and colocalization analyses in this context have supported an effect of this SNP on PHACTR1 expression in vascular tissues.30,31 Additional data suggest that this SNP is located at the site of an enhancer in vascular tissue, where it has been shown to regulate PHACTR1 and endothelin-1 (EDN-1) expression.32 The potential involvement of EDN-1 is especially intriguing as it has several known vascular effects on arterial tone and remodelling. However, at this time, the precise pathological changes in vascular function resulting from the PHACTR1 risk variant have yet to be defined.33 Notably, this risk locus has pleiotropic associations, with SCAD, carotid artery dissection, hypertension, and migraine headache, as well as an inverse association with coronary atherosclerotic arterial disease and related myocardial infarction30,31,34–39 (Figure 3). Among individuals with FMD, a recent analysis of a genetic risk score for SCAD identified increased risk of SCAD among individuals with FMD, supporting that while these phenotypes overlap clinically and genetically at loci such as rs9349379, there are distinct genetic risk factors as well.30 It is noteworthy that FMD, SCAD, and related diseases all have a higher prevalence in women, typically manifesting at younger ages than the timeframe of disease manifestations from atherosclerotic arterial disease,43 which suggests possible shared genetic and non-genetic risks.
Figure 3.
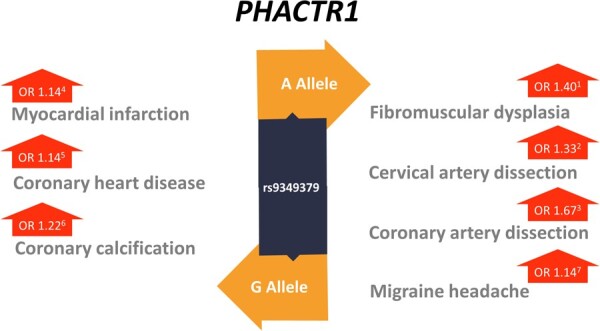
Association of PHACTR1 variant with FMD, spontaneous cervical or coronary artery dissection, coronary artery diseases, and related diseases. (i) Warchol-Celinska et al.—updated analysis of previously published data by Kiando et al.40 based on 1283 FMD cases and 4193 controls;41 (ii) Debette et al.—based on 1393 SCeAD cases and 14 446 controls;38 (iii) Adlam et al.31—based on 1055 SCAD cases and 7190 controls; (iv) Nikpay et al.36 based on 43 171 cases and 127 176 controls; (v) Gupta et al.32 based on Nikpay et al.36 and UK Biobank, 65 262 cases and 231 381 controls; (vi) O’Donnell et al.;42 and (vii) Gupta et al.32 based on Anttila et al.39 and UK Biobank, 7995 cases and 116 409 controls. OR, odds ratio; FMD, fibromuscular dysplasia; SCAD, spontaneous coronary artery dissection; SCeAD, spontaneous cervical artery dissection.
Notably, in the European/International FMD Registry, the overall prevalence of atherosclerotic lesions reported by the investigators was 17% (171/1012).3 While, expectedly, it was almost double in a subset of 122 patients aged 65 or older (33%), this prevalence remains surprisingly low for age. As a matter of comparison, in the Cardiovascular Health Study, including 5888 participants aged ≥65 years, the prevalence of carotid atherosclerotic plaques was 77%.44 In agreement with the lower prevalence of cardiovascular events in elderly patients from the US Registry,13 this may reflect survival or inclusion biases. Alternatively, it may support the belief that patients with FMD are somehow protected from atherosclerosis.
A number of rare, exonic coding genetic variants have been implicated in FMD. Exome sequencing has identified loss-of-function variants in PTGIR, which encodes for the prostaglandin I2 receptor.45 Recently, a recurrent novel variant in the collagen type V alpha 1 chain COL5A1 gene, c.1540G>A, p.(Gly514Ser), resulting in the substitution of a glycine residue with a serine residue at position 514 of the protein, has been associated with a phenotype encompassing multifocal FMD, arterial dissections, aneurysms, and tortuosity.46 This finding is the first clinically actionable genetic finding for adult multifocal FMD. The phenotype is notable for involvement of the external iliac artery and coeliac artery with arterial aneurysms and dissections, and carotid artery tortuosity was observed as well. Spontaneous arterial dissections were seen in individuals harbouring this variant in the coronary, carotid, coeliac, and iliac arteries. Clinical presentations occurred in the fourth or fifth decades of life, and proposed management was similar to that for vascular Ehlers–Danlos syndrome due to collagen type III alpha 1 chain (COL3A1) pathogenic variants, including consideration of surveillance angiographic imaging, blood pressure monitoring and control, consideration of pregnancy risk, avoidance of contact sports, and surgical precautions. The recurrent COL5A1 variant is rare, as it was not observed in genetic population databases, such as gnomAD. Further, the variant was found to exist on a shared ancestral haplotype, supporting a ‘founder effect’ that would implicate additional individuals in the population harbouring the variant. Finally, additional individuals with multifocal FMD also harbouring genetic variants in the COL5A1 gene that were predicted to be deleterious by in silico analysis were predisposed to arterial dissections.46 Whether the additional variants in this gene are pathogenic or may be acting as modifiers of FMD remains to be clarified.
Future directions of FMD genetic investigation include comprehensive genome-wide analyses of genetic variation including both rare, high-impact alleles hypothesized to underlie familial forms of FMD, as well as common genetic variation contributing to a complex genetic architecture of FMD. Cutting-edge genetic technologies have recently made these types of investigations feasible, and accordingly, these studies are now underway to provide urgently needed genetic information for FMD.
4. Molecular studies in FMD
Over the last decades, increasing evidence has emerged that altered Transforming growth factor-β (TGF-β) signalling plays a crucial role in the pathogenesis of connective tissue disorders. A first observation was made in a mouse model of Marfan syndrome (MFS), a multisystemic disorder characterized by aortic aneurysm/dissection, lens dislocation, and skeletal overgrowth. TGF-β neutralizing antibodies administered to a MFS mouse model demonstrated that increased TGF-β signalling was driving the multisystemic aspects of the disease in addition to the historically known structural deficiency of fibrillin-1, an extracellular matrix protein that is mutated in MFS.47,48 The important role of TGF-β signalling pathway was further supported by the identification of mutations in the genes encoding for components of this pathway, affecting the cytokines (TGFB2/3), the receptors (TGFBR1/2), and the downstream effectors (SMAD2/3) in a new aortic aneurysmal syndrome, now called Loeys–Dietz syndrome (LDS).49–51
Clinical studies in patients with FMD indicated that 30–57% of patients have angiographical manifestations in multiple arterial beds.8 In addition, some FMD patients may also have connective tissue features beyond the arterial pathology, including low bone density, joint laxity, scoliosis, early onset arthritis, and degenerative spine disease.52 Based on these observations, FMD is increasingly considered as a systemic disease with some overlap with known connective tissue disorders, including MFS, LDS, and Ehlers–Danlos syndrome. As altered TGF-β signalling was already involved in these conditions, a role for TGF-β in the pathogenesis of FMD was hypothesized.52 In a small cohort of 38 FMD patients, it was shown that both circulating TGF-β1 and TGF-β2 plasma levels were increased.52 In fibroblasts of FMD patients, no effect on downstream TGF-β pathway effectors could be demonstrated, even when stimulated with TGF-β1, TGF-β2, or bone morphogenetic protein 4.52 Overall, these findings suggest that activation of the TGF-β pathway is not a primary cause of FMD. This is also confirmed by the fact that mutational screening of TGF-β pathway related genes in FMD patients has yielded very few mutations. In the cohort of Ganesh et al.,52 no mutations were found in TGFBR1/2, TGFB2, and SMAD3 in 47 patients. In another series of 35 patients with FMD who underwent genetic evaluation, 2 variants (p.Thr204Ile & p. Tyr429His) were identified in TGFBR1.53 Both patients had multiple cervical artery dissections and thoracic aortic aneurysm as well as more classical multifocal FMD lesions, so may represent LDS variants. No familial segregation was done for these two variants. A recent study54 analysed all known pathogenic TGF-β signalling pathway genes in a cohort of 179 SCAD patients (with or without FMD) and 102 patients with FMD only. Whereas a mutational burden was identified in the SCAD cohort in the known LDS genes, the latter was not found in the FMD only cohort. Notably, in most cases, the distinction between FMD and MFS, LDS and Ehlers–Danlos syndrome is easy to make based on the presence or absence of typical string-of-beads arterial lesions. Though common pathogenic mechanisms are postulated, these are clearly separate entities.
Recently, researchers uncovered a possible link between FMD and mutations in a second pathway, driven by platelet-derived growth factor (PDGF) receptor β (encoded by the PDGFRB gene). PDGFRB is highly expressed in vascular mural cells and drives their migration and proliferation in angiogenesis.55 PDGFRB gain-of-function mutations cause myofibroma, classified as a benign pericytic tumour made of myofibroblastic cells.56,57 The development of multiple myofibroma defines infantile myofibromatosis, a disease that may become life threatening when internal organs are involved. Most of the mutant receptors identified in myofibromatosis are sensitive to tyrosine kinase inhibitors, such as imatinib in vitro, which was successfully tested in several severely affected patients.56,58
In 2010, Brasseur et al.59 reported myofibromatosis also involving renal arteries in a young patient with severe hypertension, multiple myofibromas and aneurysms of the renal and iliac arteries. Sequencing tumour DNA revealed an acquired PDGFRB p. D850V substitution,57 which was also present in the renal artery lesion (Dachy and Demoulin, personal communication). This mutation constitutively activates the receptor signalling in the absence of ligand.57 Recently, somatic activating mutations were also identified in four out of six cases of cerebral fusiform aneurysms.60 Interestingly, several of these PDGFRB variants were identical to those found in myofibroma. The association between PDGFRB and aneurysms was further confirmed in patients with Kosaki overgrowth syndrome, another rare connective tissue disease caused by germline PDGFRB variants. Two patients were reported with fusiform aneurysms of the basilar artery, resulting in a lethal haemorrhage at the age of 20, and stroke, respectively.61 In another report, saccular aneurysms of coronary arteries and arterial tortuosity were diagnosed in a patient with Kosaki syndrome who suddenly died at the age of 19.62 Altogether, these studies confirm that overactivation of PDGF receptor β causes aneurysms and, possibly, other vessels anomalies. Nevertheless, whether PDGFB and PDGFRB genes are involved in the pathophysiology of classic FMD remains to be demonstrated.
5. Proteomics and transcriptomics of FMD
It has been known for several decades that FMD involves changes in the composition of the arterial wall.63,64 However, unlike diseases such as atherosclerosis where samples from affected vessels can be obtained via surgery, the vast majority of patients with FMD are treated conservatively or with catheter-based therapy (e.g. angioplasty). As a result, FMD vascular tissue samples are very rarely obtained. As a more novel line of investigation, there have been increasing efforts directed towards leveraging blood and dermal fibroblast samples to gain insights on the pathophysiology of FMD. Notably, dermal fibroblasts can be readily obtained from a small (2–3 mm) skin biopsy using outgrowth tissue culture techniques over 4–8 weeks, and it is intuitive that FMD involves alterations in fibroblast cells. Indeed, as mentioned, Ganesh et al.52 found elevated plasma TGF-β and inflammatory marker levels, and increased TGF-β secretion in dermal fibroblast cell lines from subjects with FMD compared to age- and gender-matched controls.52
More recently, Olin et al.65 published the first report from the DEFINE-FMD study, a functional multi-omics systems biology study aiming to decipher the molecular and genetic basis of FMD. Of relevance, systems biology approaches leverage highly tractable and informative methods66 that have been used with success for understanding coronary artery and other vascular diseases.67,68 In their study, Olin et al.65 evaluated plasma levels of 981 proteins and 31 lipid sub-classes in women with multifocal FMD and matched healthy controls. Using separate discovery and validation cohorts with a combined total of 113 FMD cases and 128 controls, they identified and successfully validated a signature of 37 plasma proteins and 10 lipid sub-classes with differential abundance in FMD patients vs. controls. Using systems biology approaches, they showed that the genetic locus of one of these ‘FMD signature proteins’, CD2-associated protein (CD2AP), was independently associated with risk of having FMD (P=0.0003) and that it is widely expressed by endothelial cells in medium-large-sized human arteries (Figure 4). In addition, machine learning trained on the discovery cohort plasma protein and lipid data were used to develop a test for FMD. When independently applied to the validation cohort, the test showed promising proof-of-principle for its ability to diagnose the presence of FMD. Accordingly, an accompanying editorial suggested that while these exciting findings require further validation, the potential clinical relevance of a blood-based test for FMD may include the ability to: (i) predict the presence of silent FMD in relatives of patients with symptomatic FMD; (ii) support or rule out the diagnosis of FMD in patients with suggestive arterial features but without the pathognomonic ‘string-of-beads’; and (iii) predict progression of the disease.69
Figure 4.
FMD signature proteins. CD2AP was associated with FMD and was expressed by endothelial cells. Immune-fluorescence staining for CD2AP is performed on adult human non-FMD samples from renal artery (A), internal mammary artery (B), and aorta (C). Endothelial cells are identified by staining for CD31 (green), while CD2AP is shown in red. Nuclei are stained with DAPI (blue). Scale bar—25 µm. Inset panels on the right, represent a magnified view of the area in the respective dashed squares, show endothelial cells at higher enlargement. M—tunica media; L—lumen. Reproduced with permission from Olin et al.65
DEFINE-FMD has now enrolled over 400 subjects and while unpublished, additional studies using the fibroblast samples from this study appear equally promising. Again using systems biology approaches but on this occasion applied to transcriptomic (i.e. gene expression) data from fibroblasts, the investigators have reportedly identified a gene regulatory network that is of importance in FMD, which has led to their developing mouse model of this disease.70
6. Immunological and inflammatory aspects of FMD
The current definition of FMD emphasizes its fibrotic, non-atherosclerotic, and non-inflammatory nature. This last statement is based on the early observations of relative paucity of inflammatory cells in advanced FMD lesions at a time where vascular samples were available. However, in view of the critical role of inflammation in triggering vascular fibrosis, which has emerged in recent years, including the role of key cytokines, such as TGF-β, IL-17, and IL-9 in the regulation of fibrosis,52,71 it may be hypothesized that inflammation is involved at the early stages of FMD. In a recent study, angiotensin II was reported to lead to severe perivascular and vascular fibrosis in the absence of T cells and T cell-dependent cytokines. Moreover, FMD patients showed features of increased inflammatory biomarkers including tumour necrosis factor alpha, C-reactive protein, monocyte chemoattractant protein 1, serum amyloid A protein, intercellular adhesion molecule 1, vascular cell adhesion molecule 1 and IL-8, as well as clearly profibrotic TGF-β compared to control subjects and patients with MFS.52 Finally, the proteomic signature of FMD confirmed that number of top newly identified FMD-specific proteins are inflammatory.65 These include CCL11 (eotaxin), fibroblast growth factor 19, or GRB2 related adaptor protein 2. Interestingly similar sets of mechanisms are linked with immune and inflammatory pathogenesis of hypertension itself.72 Recent studies using optical coherence tomography demonstrate that vascular segments affected by coronary FMD display features usually associated with vascular inflammation, such as multiple areas of patchy or diffuse intimal, medial or adventitial abnormalities, and features of macrophage infiltration.73 Further mechanistic studies will be critical to provide detailed insights into the role of inflammation in the pathogenesis of FMD.
7. New and revisited risk factors for FMD
As discussed above, although a variety of genetic, mechanical, and hormonal factors may play a role in the pathogenesis of FMD, overall, its cause remains poorly understood. It is probable that the pathogenesis of FMD is linked to a combination of genetic and environmental factors e.g. smoking, mechanical factors, stretching, and maybe vasculotoxic medications, such as fluoroquinolones.
Tobacco smoking may be a potential pathogenic factor associated with FMD. Following early observation of Nicholson et al.74, Savard et al.14 showed that the proportions of current smokers (30% vs. 18%) or ever smokers (50% vs. 37%) were much higher in FMD patients as compared to matched hypertensive controls (Figure 5). Moreover, the prevalence of current smoking was much higher in patients with focal than with multifocal lesions (50% vs. 26%, respectively), possibly reflecting the difference in sex distribution (female: male ratio, 2:1 and 5:1, respectively). Current smoking was also associated with a more aggressive course among patients with multifocal FMD. In the US Registry, history of smoking was associated with a significantly higher rate of aneurysm detection in patients with FMD (25% vs. 19% in never smokers) (Figure 5) as well as an increased prevalence of major vascular events (43% vs. 37%, respectively).75
Figure 5.
Frequency of smoking in FMD patients in Savard et al., Dobrowolski et al., and O’Connor et al. studies.14,75,76 HT, hypertensive; FMD, fibromuscular dysplasia.
Contrasting with these two studies, Dobrowolski et al. documented no difference in the rate of ever smokers among FMD patients as compared to two matched control groups—the first from the general population and the second consisting of hypertensive subjects (Figure 5). Moreover, smoking was neither associated with clinical characteristics of patients with FMD nor with extent or complications of the disease.76 In summary, data linking smoking with the pathogenesis of FMD are equivocal and further studies are needed to elucidate this potential relationship.
The use of fluoroquinolones is associated with thoracic and abdominal aortic aneurysms or dissections.77,78 Also, the use of fluoroquinolones was associated with a more than two-fold increased risk of spontaneous cervical artery dissection.79 However, a recent study did not provide evidence for an excess of risk of intracranial aneurysm or dissection with fluoroquinolone use.80 Experimental studies have demonstrated several molecular mechanisms responsible for fluoroquinolone-associated collagen toxicity, including increased matrix metalloproteinases (MMPs) activity and collagen degradation, as well as decreased activity of tissue inhibitors of MMPs.81,82 Although there are no data on the role of fluoroquinolones in the pathogenesis of FMD and associated dissection, while waiting for specific evidence, patients with FMD should follow the advice of the FDA that ‘fluoroquinolones should not be used in patients at increased risk [of aortic ruptures or dissections] unless there are no other treatment options available’.83
8. Arterial structure and function in FMD
Increasing evidence highlights that FMD is a systemic disease, and that subclinical alterations can be found in non-affected arterial segments, such as the ‘triple signal’ pattern in the common carotid artery22,84 (Figure 6), or the presence of reduced arterial diameter and impaired smooth muscle cell function in the brachial artery.85 Triple signal was associated with increased stiffness of the vascular wall of the common carotid artery85 and may represent the ultrasound signature of outer media fibrosis observed in renal biopsies.22 However, carotid triple signal has limitations: it is less FMD-specific than previously thought, being also associated with atherosclerosis and traditional risk factors,85,86 and it has not been validated in independent cohorts yet. Indeed, subclinical abnormalities seem to be more evident in the muscular medium-sized arteries (usually spared by atherosclerosis but specifically affected by FMD), according to a ‘muscular-to-elastic’ gradient.85
Figure 6.
Subclinical ultrasound FMD features in non-affected districts include disarray of the arterial wall. Normal common carotid artery wall (standard ultrasound) (A) and normal radial artery (UHF-US) (B) are characterized by one and two echogenic interfaces, respectively (white arrows). In FMD patients, common findings are the presence of additional echogenic interfaces (yellow arrows), either in the posterior wall of the carotid artery (the so-called triple signal) (C) or in the radial artery (D).
The recently introduced technique of ultra-high-frequency ultrasound (UHF-US) allows imaging of superficial tissues (2 cm depth max), making it possible to study arterial wall thickness and ultrastructure of medium-sized and small-sized arteries (lumen down to 300 μm) in vascular diseases and therefore has the potential to overcome these limitations.87–89
In 2017, the first research programme for non-invasive vascular phenotyping by UHF-US in diseases from the FMD-spectrum, the FUCHSIA study—‘Very high-Frequency Ultrasonography for arterial phenotyping in patients with Cervico-Cerebral Artery Dissection (CCeAD), Hypertension, Spontaneous Coronary Artery Dissection (SCAD) and FibroMuscular Dysplasia (FMD)’—was launched in four different countries (Italy, UK, France, and Belgium). Preliminary results from the Italian cohort showed an altered ultrastructure in carotid and radial walls of patients with FMD, with thickening and disarray of wall layers in comparison to healthy controls90 (Figure 6).
In the next years, the FUCHSIA research plan includes replication in independent validation cohorts; integration of the radiomics—machine learning approach for classification; and correlation of vascular phenotypes with disease characteristics and clinical outcomes. Coupling with other omics techniques is also advisable,69 for a more accurate diagnosis and risk stratification, as well as for a better understanding of the pathophysiology of FMD.
9. Renal haemodynamics in FMD
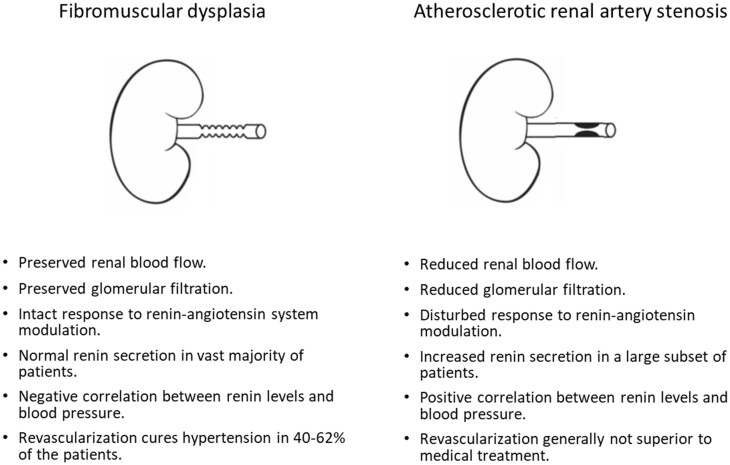
Until recently, little was known on the impact of multifocal renal artery FMD on the kidney. Most knowledge was based on experiments in animal models with clipping of the renal artery and studies in patients with atherosclerotic renal artery stenosis (ARAS). Recent studies in patients with renal artery FMD, however, shed a new light on the kidney in FMD and suggested important differences with ARAS and animal models.
First of all, it has been proposed that in multifocal FMD the intrarenal vasculature is relatively intact. Renal blood flow (RBF) was indeed measured in 64 patients with multifocal FMD (prior to balloon angioplasty and without the use of antihypertensive drugs) using the 133Xenon washout method. RBF in the affected kidney was comparable to that in the unaffected contralateral kidney.91 Moreover, the global RBF in multifocal FMD was comparable to that in matched patients with essential hypertension, and significantly higher than that in patients with ARAS.91,92
The ARCADIA-POL study evaluated intrarenal haemodynamic patterns by Doppler-ultrasonography in 153 patients with renal artery FMD.17 Among FMD patients with non-significant renal FMD, the renal resistive index (RRI) was comparable to that in patients with essential hypertension and healthy normotensives. Also, no difference in RRI was found between patients with multifocal and focal FMD. However, in FMD patients with significant stenosis, post-stenotic intrarenal RRI was significantly lower as compared to FMD patients without significant stenosis, hypertensive controls, and normotensive subjects.17 Likewise, lower RRI was measured in kidneys with FMD with a significant stenosis as compared to those without, along with significant differences in other intra-renal duplex ultrasound parameters.
Since the post-stenotic flow pattern is associated with the compliance of the post-stenotic vessel wall, a lower RRI may reflect a high compliance. This may explain the finding of a lower RRI in patients with FMD with significant stenosis.
Finally, microvascular function appears to be preserved in kidneys with multifocal FMD. Global renal function is generally in the normal range (mean eGFR in the European FMD registry was 92 mL/min/1.73 m2)3 and does not differ between the affected and unaffected kidney in patients with unilateral FMD if the renal artery stenosis is not severe.91 However, this may depend on the severity of stenosis.93 Moreover, in patients with multifocal FMD enrolled in the ARCADIA-POL study, RRI was not correlated with renal function or albuminuria.17 Finally, intrarenal infusion of vasoactive substances in kidneys with FMD results in a haemodynamic response that is comparable to that in controls,91 while this response is severely decreased in ARAS (Figure 7).94,95
Figure 7.
Kidney in FMD: tentative differences between FMD and ARAS. Adapted from Ref.95
As a whole, it appears that the presence of a string-of-beads does not significantly impair renal perfusion or intrarenal microvascular function in most patients, which is in sharp contrast to ARAS. In the short term, this remains true even in the presence of a unilateral tight stenosis because of compensatory mechanisms in the contralateral kidney.93 Presumably, the lower exposition to other pro-atherosclerotic and nephrotoxic factors (diabetes and hypercholesterolemia) and the shorter duration of hypertension, may also contribute to preserve the kidney tissue from glomerular and tubular damage and fibrosis, as well as intrarenal vascular compliance. Possibly, altered haemodynamics caused by the string-of-beads may also protect the kidneys from hypertensive damage. These differences in renal microvasculature may explain why revascularization is usually unsuccessful in ARAS96,97 while it frequently has a blood pressure-lowering effect in FMD.98
Renal perfusion is relatively preserved in patients with multifocal FMD and thus renin secretion is not markedly increased compared to patients with essential hypertension. Moreover, there is no renin secretion gradient between the affected and unaffected kidney in patients with unilateral multifocal FMD.91,92 However, interpretation of these studies4,17,95,99 is complex since in the absence of trans-stenotic pressure gradient measurements, a proportion of patients may have non-significant renal artery stenosis associated with essential hypertension.
In contrast, in patients with focal FMD, the impact on the kidney appears similar to that of ARAS and renal artery clipping in animal models: renal perfusion and glomerular filtration are lower in kidneys with focal FMD and renin secretion is increased in the affected kidneys.99 Hence, it appears that the haemodynamic impact of focal FMD lesions on the kidney is more severe, resulting in a more classic pattern of renovascular hypertension. This may in turn explain the more often severe clinical presentation and higher success rate of balloon angioplasty in patients with focal FMD compared to multifocal FMD. Work regarding renal haemodynamics and haemodynamic assessment of renal artery FMD is ongoing. In 2019, the International FMD Consensus proposed a standardized clinical protocol for haemodynamic assessment of renal arteries based on trans-stenotic pessure gradient measurements prior to and following angioplasty, which requires additional validation.1,2
10. Neurological manifestations of FMD
The most frequent presenting symptoms of neurological FMD are headaches and pulsatile tinnitus.4,8,100 Headaches (mostly migraine type and tension type) were reported by 70% of patients with FMD.4,8,9 The underlying pathophysiology of headaches in FMD patients remains vague and potential mechanisms comprise changes in cerebrovascular flow—labile hypertension, hyper- or hypoperfusion, neurovascular dysregulation or dysautonomia, structural injury—dissection, microtrauma, or enhanced pain sensitivity.101 Although headaches are commonly reported by FMD patients, irrespective of involved vascular beds, the presence of headaches is significantly more frequent in FMD patients with a history of cervical or intracranial artery dissection or intracranial aneurysm.9 Pulsatile tinnitus is reported in 40% of patients with neurological FMD and may be associated with the presence of cervical artery dissection.8,9
Patients with FMD have a high prevalence of cervical (carotid and vertebral) artery dissection and intracranial saccular aneurysms.10,102 The most frequent complications of neurological FMD are, in decreasing order, transient ischaemic attack (TIA) (8–53%) or ischaemic stroke (8–35%), subarachnoid haemorrhage or unruptured aneurysm (3–49%), and intracerebral haemorrhage (6–13%).102
In FMD patients, TIA or ischaemic stroke can be a consequence of: haemodynamic mechanism (hypoperfusion from cervical or intracranial arterial stenosis or occlusion—Supplementary Figure S2A), emboli (thrombosis in the area of a stenosis or dilation), or thrombosis (lacunar stroke, thrombosis of small, perforating arteries due to secondary hypertension). However, TIA/ischaemic stroke appear to occur primarily in the presence of associated cervical artery dissection, due to artery-to-artery thromboembolism or cerebral hypoperfusion (Supplemental Figure S2B). Subarachnoid haemorrhage mainly results from the rupture of an intracranial aneurysm and less commonly from dissecting vertebral artery into the intracranial segment.102 Intracerebral haemorrhage is rare in patients with FMD but may be due to the rupture of an intracranial aneurysm, a dissection or the presence of hypertensive microangiopathy.102
FMD is the most common underlying vasculopathy at the origin of cervical artery dissection, and a recent systematic review of patients with cervical artery dissection estimated the presence of FMD lesions to be 16%.103 Also, in a recent small study of 43 patients with spontaneous cervical artery dissection who underwent brain-to-pelvis CT-angio evaluations, FMD was found in 39.5% of patients.18
The prevalence of cervical artery dissection has been estimated to be 21% in all FMD patients, with an increased prevalence of carotid (16%) as opposed to vertebral (5%) artery dissection.104 Furthermore, among patients with a neurological presentation leading to the diagnosis of FMD, the prevalence of cervical artery dissection is even higher, being 27%.4 Cervical arteries remain the main location of arterial dissections in FMD patients, with cervical artery dissection accounting for up to 65% of all dissections.104 Of note, among FMD patients with a cervical artery dissection, the presence of multiple cervical artery dissections is not uncommon and estimated to be up to 37%.8,10
The ARCADIA registry has identified male sex, age >50years, history of migraine or hypertension and involvement of ≥3 vascular beds with FMD lesions to be independent risk factors for cervical artery dissection in patients with FMD (Arnaud et al., Journal of American Heart Association, in press).
Intracranial saccular aneurysms are mainly unruptured and diagnosed incidentally on imaging rather than discovered after rupture. The prevalence of intracranial aneurysms in patients with FMD is higher (7%) than in the general population (<5%).105 In the US Registry, 13% of women with FMD had at least one intracranial aneurysm and 4% more than one. Of interest, 29% of these aneurysms were of size ≥5 mm, which is the threshold commonly judged to classify intracranial aneurysms as of high risk of rupture.106 Also, there was no difference in the rate of intracranial aneurysms between patients presenting with renal and cervical FMD.11 It remains unclear whether the risk of rupture of intracranial aneurysm is higher in FMD patients that in the general population (<1%/year), because of the shortage of longitudinal data in patients with FMD with intracranial aneurysm.
Information on risk of long-term progression of FMD and occurrence of non-specific lesions (aneurysm, dissection etc.) is scarce. Longitudinal data from the US FMD registry suggest that FMD is a non-progressive disease, with no or rare extension of FMD lesions in initially involved arteries and no FMD involvement in previously unaffected arteries.104
Similarly, patients with cervico-encephalic FMD do not appear to have an increased risk of FMD progression and occurrence of non-specific lesions (e.g. recurrent cervical artery dissection).104 Indeed, in 146 patients with multifocal FMD with follow-up cervical imaging, none had development of FMD in a previously unaffected artery and none had FMD progression in previously involved artery after a mean follow-up of 35 months (range 5–153 months).104 No patient had a new aneurysm and three (2%) had a new cervical artery dissection during follow-up. Interestingly, all three patients with a new cervical artery dissection during follow-up already had multifocal FMD lesions on the same cervical artery at baseline, one had a previous contralateral cervical artery dissection and another a previous coronary artery dissection.
Arterial diaphragms are thin translucent endoluminal webs localized in the carotid (especially in the posterolateral side of the carotid bulb) or vertebral (V3 segment or ostium) artery and correspond to a linear defect on angiography that does not change or disappear after change of the patient’s head position (Supplemental Figure S2C).107–109 This entity has been reported to share similar histological findings with FMD, with anatomical specimens showing intimal fibroplasia without atheromatous or inflammatory lesions in almost all cases.102,110,111 Several authors estimate that this could be the predominant form of FMD in Black populations and have classified it as ‘atypical FMD’.112
Carotid webs have been reported in middle-aged adults, predominantly women, of African or Afro-Caribbean ethnicity, presenting with a TIA or ischaemic stroke in the carotid artery territory ipsilateral to the web, with no other causes identified after a comprehensive work-up. Patients with carotid webs have no typical multifocal or focal FMD lesions, no aneurysm or dissection, and no atherosclerotic risk factors.107–109,112 Carotid webs could be more frequent than previously thought in patients who suffered an ischaemic stroke. A review of consecutive patients with ischaemic stroke indeed reported a 1.2% rate of carotid web (0.7% ipsilateral to the stroke),108 while a subanalysis of patients with ischaemic stroke due to intracranial large-vessel occlusion included in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischaemic Stroke in the Netherlands (MR CLEAN trial) showed a 2.7% frequency (2.5% ipsilateral to the stroke).113
Cerebral ischaemia is thought to be mediated by an embolic mechanism because of stasis upstream to the web or within an associated aneurysmal bulb or from focal dissection, and a strong association between carotid webs and ischaemic stroke has been reported in a population-based case–control study.112
11. Heart and FMD
11.1 Heart morphology and function in FMD
Contrary to atherosclerotic RAS, data on the relationship between renal FMD and left ventricular morphology and function are limited.114–116
In the ARCADIA-POL study, LV morphology and function of 144 FMD patients were compared to those in a matched control group. No significant differences in left ventricular morphology and systolic function were found between FMD patients and controls or between subjects with multifocal and focal FMD, even in case of significant RAS.16
In contrast, early alterations in left ventricular diastolic function (lower e’ and higher E/e’) were found in patients with multifocal FMD compared to both patients with focal FMD and controls.16 These results may be explained by the older age of subjects with multifocal FMD.
In summary, there is no evidence that FMD is associated with alteration in LV morphology and function over and above BP levels.
11.2 Coronary arteries in FMD
The coronary phenotype in patients with FMD may include the presence of excessive tortuosity, the intravessel or multivessel symmetry sign, corkscrew appearance, dissection, aneurysm, distal tapering or long, smooth narrowing in relation with either a dissection or an intramural haematoma within the coronary artery.1,2,117
The most common feature of coronary phenotype in FMD is coronary tortuosity, however, the diagnostic criteria applied across studies are non-uniform and arbitrary (Supplementary Figure S1).118–120
Coronary tortuosity has been reported in all of 32 examined patients with extra-coronary FMD in one study.73 Similarly in another study five patients with resistant hypertension, exertional chest pain, and significant coronary tortuosity were diagnosed with FMD of renal and/or cervical arteries.121 Corkscrew appearance and multivessel symmetrical tortuosity were also associated with extra-coronary FMD in SCAD patients.120 On the other hand, coronary tortuosity defined as ≥3 bends above 45° may be found in 39.1% of consecutive patients undergoing coronary angiography due to chest pain whereas it may be found in 12.5% when tortuosity is defined as ≥2 bends above 180°.118,119 These results should therefore be interpreted with caution due to limited number of patients and potential selection bias.
Clinical presentation of coronary artery alterations observed in FMD ranges across a wide spectrum from asymptomatic, through exertional chest pain, to rarely life threatening, such as myocardial infarction or sudden cardiac death mostly due to SCAD. More severe coronary tortuosity is linked to an increased risk of recurrent coronary dissection in SCAD.120 Coronary tortuosity may increase transcoronary gradient leading to myocardial ischaemia and increased local shear stress, which may facilitate dissections.122–124
11.3 SCAD and FMD
SCAD is a cause of acute coronary syndromes, which, like FMD, afflicts predominantly young to middle-aged women. Following the first report of extra-coronary arteriopathies in SCAD-survivors,125 a range of vascular abnormalities have been described including aneurysms, dissections, focal stenoses, and aortic root dilation but the commonest finding is an appearance indistinguishable from the typical radiological manifestations of multifocal FMD.126,127 To date, there has been no histological confirmation that these appearances arise from the same changes at a cellular level as those described in classical FMD128 but it is now widely accepted that the radiological findings in SCAD represent true multifocal FMD rather than a phenocopy.129,130
It remains uncertain if all other vascular abnormalities seen in SCAD are part of the FMD-spectrum or if FMD is just one of a range of SCAD-associated arteriopathies. Whilst coronary arterial wall abnormalities have been described from intravascular imaging in SCAD,73,131 typical multifocal FMD-like coronary appearances are not generally seen. Recent genetic studies have shown SCAD and FMD share common risk variants including the PHACTR1 locus31 but with hints that the effect size may be different between patients presenting primarily with SCAD compared with those with FMD. Common genetic variants associated with general forms of SCAD are also associated with SCAD risk among individuals with FMD,30 suggesting that the two diseases are not one and the same. Likewise, the contribution of causal rare genetic variants may be different. Further work is continuing to understand at a molecular genetic level to what extent SCAD and FMD relate.54
Reports of the prevalence of FMD in SCAD have ranged widely depending on modality and completeness of imaging and the permissiveness of the definition used but it is likely this affects between 30% and 50% of SCAD-survivors with the commonest affected vascular beds being the cervical, renal, and ilio-femoral vessels.129 On the other side, SCAD may occur in patients with FMD but the incidence appears low in follow-up reports from international FMD registries (2.7% of patients in the US Registry for FMD).10 So, whilst there is clinical overlap between these conditions, a significant proportion of patients with SCAD have no evidence of FMD when screened using cross-sectional brain-to-pelvis imaging and most patients with FMD will never have SCAD.
Importantly in most SCAD patients, findings of multifocal FMD appear benign and non-progressive in the short to medium term, with, low reported rates of non-coronary major adverse cardiovascular events in SCAD-survivors.129,130 For example, there have been no reports to date of renovascular hypertension requiring intervention developing in a SCAD-survivor identified on screening to have renal multifocal FMD. Longer follow-up data however, are of considerable importance in this younger population and are currently limited.
It seems clear that SCAD and FMD are closely related pathologies. However, clinical, radiological and genetic data suggest they are overlapping disorders rather than a single disease. Whilst initial progress has been made, further international collaborative research is needed to better understand the pathophysiological relationship and the best clinical management of these important conditions.
12. Visceral phenotype in FMD
Visceral artery FMD (VA FMD) is an uncommon manifestation of FMD, with presentations varying from incidentally discovered to symptomatic or even life-threatening disorder.3,4,8,19,132
Its prevalence among FMD patients is estimated at 15–20% (range 9–48.7%), based on various cohorts and comprehensiveness of vascular screening.132
In FMD patients enrolled in ARCADIA-POL who underwent a full brain-to-pelvis vascular screening, the prevalence of VA FMD was 13.8%.19 Most cases of VA FMD were asymptomatic. However, in five patients (15.6%), VA FMD presented as abdominal emergencies e.g. rupture of a hepatic artery aneurysm or acute thrombosis of mesenteric artery.
In this series, VA FMD affected most frequently the coeliac trunk (83.1%). Other arteries were affected less frequently: superior mesenteric (25%), splenic (9.1%), common hepatic (6.3%), and inferior mesenteric (6.3%). Of interest, 42 aneurysms in visceral arteries were found in 20 patients with VA FMD (62.5% of this cohort). Moreover, patients with VA FMD were characterized by lower BMI as compared to patients with only renal FMD, healthy controls and patients with resistant essential hypertension.19
Furthermore, compared to matched controls, FMD patients were characterized by significantly smaller diameters of all visceral arteries irrespectively of the VA FMD presence.19 In line with the aforementioned findings of Bruno et al. in brachial arteries, this anatomical difference may represent a novel phenotypic expression of FMD.19 This observation nevertheless requires confirmation in larger cohorts and other vascular beds.19,85
13. Pregnancy-related complications
Exogenous or endogenous female hormones have been reported as potential risk factors for FMD133,134 but no strong evidence of a clear association with FMD has yet been identified. In a small renal histological study, an intense progesterone receptor expression in the nuclei of smooth muscle cells of the renal arteries was detected in six patients with FMD but not in three control subjects, suggesting that progesterone may play a role in the pathogenesis of FMD, but further studies are needed to confirm these findings.135
Until recently, data on pregnancy-related complications in patients with FMD were mostly based on the results of a single retrospective study136 reporting a very high risk of superimposed pre-eclampsia in a small cohort of patients subsequently revascularized for FMD-related renal artery stenosis. Furthermore, an increased risk of worsening of hypertension status and of vascular complications (aneurysms rupture or arterial dissection) during the peri-partum period has been postulated in FMD patients according to several case reports and expert opinion.137–140
Very recently, the nature and prevalence of complications occurring in FMD patients during pregnancy or within 3 months postpartum were extensively assessed in the European/Internal FMD Registry.21 Out of 237 women with FMD, 40% experienced pregnancy-related complications. Compared with women from the general population,141–143 patients with FMD had a substantially higher prevalence of gestational hypertension (25% vs. 3.6–9.1%) and preterm birth (20% vs. 5.5–12.2%), while pre-eclampsia was only moderately increased (7.5% vs. 1.6–4%) (Figure 8). When compared to women with primary hypertension,142,144,145 both prevalence of preterm birth and, especially, of pre-eclampsia was lower in women with FMD (20% vs. 22.7–33.3% and 7.5% vs. 22–25.9%, respectively). Other maternal placental syndromes, such as abruptio placentae and intra-uterine foetal death, as well as severe vascular complications (arterial dissection and/or aneurysm rupture) were rare (Figure 8). Finally, only <5% of women with FMD developed resistant hypertension during pregnancy.
Figure 8.
Pregnancy-related complications in patients subsequently diagnosed with FMD enrolled in the European/International FMD Registry.21 Gestational hypertension—25%, 0%, and 9.1%; preterm birth—20.1%, 33.3%, and 12.2%; pre-eclampsia—7.5%, 25.9%, and 4.0%, for patients with FMD, chronic hypertensives, women from the general population, respectively.
Since complicated pregnancies were more frequent in younger patients with renal FMD and a higher rate of renal artery revascularizations,21 the higher prevalence of pregnancy-related complications compared to the general population may be due to the severity of renovascular hypertension. Still, other less well-studied factors, such as increased inflammation, endothelial dysfunction, changes in cytokines (i.e. TGF-β1 and -β2), and changes in the VGEF/PlGF balance or female hormones levels may account for this increased risk. Further studies are needed in order to replicate these results and dissect the underlying mechanisms.
14. 2019 FMD consensus
In 2019, the First International Consensus on FMD was published simultaneously in the Journal of Hypertension and Vascular Medicine.1,2 This document built upon 2014 scientific statements from the USA and Europe, with a writing committee commissioned in 2017 by the Working Group ‘Hypertension and the Kidney’ of the European Society of Hypertension (ESH) and the Society for Vascular Medicine (SVM).6,146 The goal of the International Consensus document was to create a single, up-to-date expert consensus on adult FMD that would replace the two 2014 documents. In addition to European and American specialists representing the ESH and SVM, the writing committee included experts from other regions of the world and representatives of patient advocacy groups. The document includes 13 consensus-based points covering multiple aspects of FMD with the acknowledgement that there was generally limited level I data available on which to make recommendations. As explained, the intent of the writing committee was that the document, ‘including identification of research priorities, will lead to future high-quality research efforts, additional observational studies, and randomized controlled trials, and that these data will be incorporated into a future international guideline document’. Important clinical consensus points in the document address brain-to-pelvis imaging for patients with FMD, the prescription of antiplatelet therapy for patients with FMD in the absence of contraindication, and the use of a standardized protocol for catheter-based renal angiography and angioplasty that incorporates haemodynamic assessment of translesional renal artery pressure gradients.1,2 In addition to the consensus points and research priorities (Table 3), the document includes a summary of FMD mimics, comparison of data in the US and European FMD registries, and a review of international patient advocacy efforts. The co-published document has been widely read with more than 22 000 views since its publication.
Table 3.
|
|
|
|
|
|
|
|
|
|
15. New directions in FMD research
The past decade has seen significant progress in FMD-related research, which has led to improved understandings of the disease’s clinical manifestations, natural history, epidemiology, and genetics. An imaging-based taxonomy has been developed and an International Consensus has been published, which includes guidance on the diagnostic approach and management of patients with FMD.1,2 Ongoing work described above continues to focus on FMD genetics and proteomics, physiological effects of FMD on cardiovascular structure and function, and novel imaging modalities and blood-based biomarkers that can be used to identify subclinical FMD. As outlined in the International Consensus, there are a number of high priority research areas within our field that can hopefully be addressed in the years to come through ongoing and future international collaborations (Table 3). In addition to these priorities, exploration of non-vascular manifestations of FMD, including those potentially involving the connective tissues and musculoskeletal system, should be undertaken.52,147 It is also hoped that the next decade will bring the development of multi-centred and potentially international clinical trials to provide comparative effectiveness data to inform the optimal management of patients with FMD and guide future clinical practice guidelines with level I evidence.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Conception and design of the work: A.P., P.D., A.P., and A.J.
Drafting of the work: A.P., P.D., H.L.G., D.A., R.M.B., M.B., J.-B.D., S.K.G., T.G., M.J., J.C.K., M.K., B.L., M.P., M.P., P.V.d.N., D.J.L.V.T., E.W.-C., A.P., and A.J.
Critical revision for important intellectual content: J.W.O., M.A., P.B., d.P.L., and E.T.
Conflict of interest: This paper was handled by deputy editor Karlheinz Peter.
Funding
This work was supported by Grant of The National Institute of Cardiology no. 2.40/III/19, Poland to P.D, E.W.C., A.P and A.J., National Heart, Lung and Blood Institute/National Institutes of Health (R01HL139672, R01HL122684, R01HL086694), Frankel Cardiovascular Center, and the University of Michigan Taubman Institute to S.K.G., by National Institutes of Health (R01HL130423, R01HL135093, R01HL148167-01A1) and New South Wales health grant to J.C.K., by grants from Beat SCAD, the British Heart Foundation (PG/13/96/3060) and the NIHR Leicester Biomedical Research Centre to DA.
Supplementary Material
References
- 1. Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, Bruno RM, de Leeuw P, Fendrikova-Mahlay N, Froehlich J, Ganesh SK, Gray BH, Jamison C, Januszewicz A, Jeunemaitre X, Kadian-Dodov D, Kim ES, Kovacic JC, Mace P, Morganti A, Sharma A, Southerland AM, Touze E, van der Niepen P, Wang J, Weinberg I, Wilson S, Olin JW, Plouin PF. First International Consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med 2019;24:164–189. [DOI] [PubMed] [Google Scholar]
- 2. Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, Bruno RM, De Leeuw P, Fendrikova-Mahlay N, Froehlich J, Ganesh SK, Gray BH, Jamison C, Januszewicz A, Jeunemaitre X, Kadian-Dodov D, Kim ESH, Kovacic JC, Mace P, Morganti A, Sharma A, Southerland AM, Touze E, Van der Niepen P, Wang J, Weinberg I, Wilson S, Olin JW, Plouin PF, Working Group ‘Hypertension and the Kidney’ of the European Society of Hypertension (ESH) and the Society for Vascular Medicine (SVM). First international consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens 2019;37:229–252. [DOI] [PubMed] [Google Scholar]
- 3. Pappaccogli M, Di Monaco S, Warchol-Celinska E, Lorthioir A, Amar L, Aparicio LS, Beauloye C, Bruno RM, Chenu P, de Leeuw P, De Backer T, Delmotte P, Dika Z, Gordin D, Heuten H, Iwashima Y, Krzesinski JM, Kroon AA, Mazzolai L, Poch E, Sarafidis P, Seinturier C, Spiering W, Toubiana L, Van der Niepen P, van Twist D, Visona A, Wautrecht JC, Witowicz H, Xu J, Prejbisz A, Januszewicz A, Azizi M, Persu A. The European/International Fibromuscular Dysplasia Registry and Initiative (Feiri)- clinical phenotypes and their predictors based on a cohort of one thousand patients. Cardiovasc Res 2020;117:950–959. [DOI] [PubMed] [Google Scholar]
- 4. Plouin P-F, Baguet J-P, Thony F, Ormezzano O, Azarine A, Silhol F, Oppenheim C, Bouhanick B, Boyer L, Persu A, Hammer F, Gosse P, Mounier-Vehier C, Le Hello C, Jeunemaitre X, Azizi M, Amar L, Chatellier G, Mousseaux E, Touzé E, ARCADIA Investigators. High prevalence of multiple arterial bed lesions in patients with fibromuscular dysplasia: the ARCADIA registry (Assessment of Renal and Cervical Artery Dysplasia). Hypertension 2017;70:652–658. [DOI] [PubMed] [Google Scholar]
- 5. Warchol-Celinska E, Prejbisz A, Dobrowolski P, Klisiewicz A, Kadziela J, Florczak E, Michalowska I, Jozwik-Plebanek K, Kabat M, Kwiatek P, Nazarewski S, Madej K, Rowinski O, Swiatlowski L, Peczkowska M, Hanus K, Talarowska P, Smolski M, Kowalczyk K, Kurkowska-Jastrzebska I, Stefanczyk L, Wiecek A, Widecka K, Tykarski A, Stryczynski L, Litwin M, Hoffman P, Witkowski A, Szczerbo-Trojanowska M, Januszewicz M, Januszewicz A. Systematic and multidisciplinary evaluation of fibromuscular dysplasia patients reveals high prevalence of previously undetected fibromuscular dysplasia lesions and affects clinical decisions: the ARCADIA-POL study. Hypertension 2020;75:1102–1109. [DOI] [PubMed] [Google Scholar]
- 6. Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT, Lookstein RA, Lumsden AB, Newburger JW, Rundek T, Sperati CJ, Stanley JC, American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Functional Genomics and Translational Biology; American Heart Association Council for High Blood Pressure Research; American Heart Association Council on the Kidney in Cardiovascular Disease; American Heart Association Stroke Council. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048–1078. [DOI] [PubMed] [Google Scholar]
- 7. Olin JW. Expanding clinical phenotype of fibromuscular dysplasia. Hypertension 2017;70:488–489. [DOI] [PubMed] [Google Scholar]
- 8. Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, Jaff MR, Kim ES, Mace P, Matsumoto AH, McBane RD, Kline-Rogers E, White CJ, Gornik HL. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012;125:3182–3190. [DOI] [PubMed] [Google Scholar]
- 9. Wells BJ, Modi RD, Gu X, Bumpus SM, Swan K, Froehlich JB, Gray BH, Southerland AM, Kim ES, Fendrikova Mahlay N, Gupta K, Weinberg I, Bacharach M, Gornik HL, Olin JW. Clinical associations of headaches among patients with fibromuscular dysplasia: a Report from the US Registry for Fibromuscular Dysplasia. Vasc Med 2020;25:348–350. [DOI] [PubMed] [Google Scholar]
- 10. Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, Gray BH, Jaff MR, Kim ES, Mace P, Sharma A, Kline-Rogers E, White C, Olin JW. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for FMD. J Am Coll Cardiol 2016;68:176–185. [DOI] [PubMed] [Google Scholar]
- 11. Lather HD, Gornik HL, Olin JW, Gu X, Heidt ST, Kim ESH, Kadian-Dodov D, Sharma A, Gray B, Jaff MR, Chi YW, Mace P, Kline-Rogers E, Froehlich JB. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the US Registry for fibromuscular dysplasia. JAMA Neurol 2017;74:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim ESH, Olin JW, Froehlich JB, Gu X, Bacharach JM, Gray BH, Jaff MR, Katzen BT, Kline-Rogers E, Mace PD, Matsumoto AH, McBane RD, White CJ, Gornik HL. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States registry for fibromuscular dysplasia. J Am Coll Cardiol 2013;62:2026–2028. [DOI] [PubMed] [Google Scholar]
- 13. Bagh I, Olin JW, Froehlich JB, Kline-Rogers E, Gray B, Kim ESH, Sharma A, Weinberg I, Wells BJ, Gu X, Gornik HL. Association of multifocal fibromuscular dysplasia in elderly patients with a more benign clinical phenotype: data from the US Registry for fibromuscular dysplasia. JAMA Cardiol 2018;3:756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 2013;61:1227–1232. [DOI] [PubMed] [Google Scholar]
- 15. Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, Plouin PF. Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation 2012;126:3062–3069. [DOI] [PubMed] [Google Scholar]
- 16. Dobrowolski P, Januszewicz M, Klisiewicz A, Prejbisz A, Warchoł-Celińska E, Michałowska I, Florczak E, Kożuch K, Hanus K, Aniszczuk-Hybiak A, Witowicz H, Witkowski A, Kądziela J, Kabat M, Madej K, Nazarewski S, Tykarski A, Stryczyński Ł, Szczerbo-Trojanowska M, Światłowski Ł, Kosiński P, Widecka K, Januszewicz A, Hoffman P. Echocardiographic assessment of left ventricular morphology and function in patients with fibromuscular dysplasia: the ARCADIA-POL study. J Hypertens 2018;36:1318–1325. [DOI] [PubMed] [Google Scholar]
- 17. Januszewicz M, Januszewicz A, Michalowska I, Klisiewicz A, Dobrowolski P, Warchol-Celinska E, Jozwik-Plebanek K, Witkowski A, Kadziela J, Kowalczyk K, Ziebka J, Talarowska P, Kabat M, Florczak E, Pregowska-Chwala B, Tykarski A, Stryczynski L, Stefanczyk L, Litwin M, Widecka K, Adamczak M, Szczerbo-Trojanowska M, Hoffman P, Wiecek A, Prejbisz A. Association of intrarenal blood flow with renal function and target organ damage in hypertensive patients with fibromuscular dysplasia: the ARCADIA-POL study. Pol Arch Intern Med 2019;129:234–241. [DOI] [PubMed] [Google Scholar]
- 18. Talarowska P, Dobrowolski P, Klisiewicz A, Kostera-Pruszczyk A, Członkowska A, Kurkowska-Jastrzębska I, Gąsecki D, Warchoł-Celińska E, Światłowski Ł, Florczak E, Januszewicz M, Michałowska I, Józwik-Plebanek K, Szczudlik P, Błażejewska-Hyżorek B, Protasiewicz M, Odrowąż-Pieniążek P, Tekieli Ł, Michel-Rowicka K, Hanus K, Widecka K, Sołtysiak M, Tykarski A, Stryczyński Ł, Szczerbo-Trojanowska M, Hoffman P, Prejbisz A, Januszewicz A. High incidence and clinical characteristics of fibromuscular dysplasia in patients with spontaneous cervical artery dissection: the ARCADIA-POL study. Vasc Med 2019;24:112–119. [DOI] [PubMed] [Google Scholar]
- 19. Warchoł-Celińska E, Pieluszczak K, Pappaccogli M, Soplińska A, Prejbisz A, Dobrowolski P, Klisiewicz A, Kądziela J, Falkowski A, Śmigielski W, Florczak E, Jóźwik-Plebanek K, Michałowska I, Kabat M, Zgorzelski C, Madej K, Nazarewski S, Smólski M, Olewnik Ł, Litwin M, Szczerbo-Trojanowska M, Zieniewicz K, Drygas W, Rowiński O, Witkowski A, Adlam D, Van der Niepen P, Persu A, Januszewicz A, Januszewicz M. Dissecting visceral fibromuscular dysplasia reveals a new vascular phenotype of the disease: a report from the ARCADIA-POL study. J Hypertens 2020;38:737–744. [DOI] [PubMed] [Google Scholar]
- 20. Persu A, Van der Niepen P, Touze E, Gevaert S, Berra E, Mace P, Plouin PF, Jeunemaitre X, Working Group “Hypertension and the Kidney” of the European Society of Hypertension and the European Fibromuscular Dysplasia Initiative. Revisiting fibromuscular dysplasia: rationale of the European fibromuscular dysplasia initiative. Hypertension 2016;68:832–839. [DOI] [PubMed] [Google Scholar]
- 21. Pappaccogli M, Prejbisz A, Ciurică S, Bruno RM, Aniszczuk-Hybiak A, Bracalente I, De Backer T, Debiève F, Delmotte P, Di Monaco S, Jarraya F, Gordin D, Kosiński P, Kroon AA, Maas AHEM, Marcon D, Minuz P, Montagud-Marrahi E, Pasquet A, Poch E, Rabbia F, Stergiou GS, Tikkanen I, Toubiana L, Vinck W, Warchoł-Celińska E, Van der Niepen P, de Leeuw P, Januszewicz A, Persu A, on behalf of the European/International Fibromuscular Dysplasia Registry and Initiative (FEIRI) and the Working Group “Hypertension and the Kidney” of the ESH. Pregnancy-related complications in patients with fibromuscular dysplasia: a report from the European/International Fibromuscular Dysplasia Registry. Hypertension 2020;76:545–553. [DOI] [PubMed] [Google Scholar]
- 22. Perdu J, Boutouyrie P, Bourgain C, Stern N, Laloux B, Bozec E, Azizi M, Bonaiti-Pellie C, Plouin PF, Laurent S, Gimenez-Roqueplo AP, Jeunemaitre X. Inheritance of arterial lesions in renal fibromuscular dysplasia. J Hum Hypertens 2007;21:393–400. [DOI] [PubMed] [Google Scholar]
- 23. Mettinger KL, Ericson K. Fibromuscular dysplasia and the brain. I. Observations on angiographic, clinical and genetic characteristics. Stroke 1982;13:46–52. [DOI] [PubMed] [Google Scholar]
- 24. Gladstien K, Rushton AR, Kidd KK. Penetrance estimates and recurrence risks for fibromuscular dysplasia. Clin Genet 1980;17:115–116. [DOI] [PubMed] [Google Scholar]
- 25. Rushton AR. The genetics of fibromuscular dysplasia. Arch Intern Med 1980;140:233–236. [PubMed] [Google Scholar]
- 26. Major P, Genest J, Cartier P, Kuchel O. Hereditary fibromuscular dysplasia with renovascular hypertension. Ann Intern Med 1977;86:583. [DOI] [PubMed] [Google Scholar]
- 27. McKenzie GA, Oderich GS, Kawashima A, Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 2013;24:1477–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. https://www.ncbi.nlm.nih.gov/gene/221692. (14 September 2020, date last accessed).
- 29. Kiando SR, Tucker NR, Castro-Vega L-J, Katz A, D’Escamard V, Tréard C, Fraher D, Albuisson J, Kadian-Dodov D, Ye Z, Austin E, Yang M-L, Hunker K, Barlassina C, Cusi D, Galan P, Empana J-P, Jouven X, Gimenez-Roqueplo A-P, Bruneval P, Hyun Kim ES, Olin JW, Gornik HL, Azizi M, Plouin P-F, Ellinor PT, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet 2016;12:e1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saw J, Yang M-L, Trinder M, Tcheandjieu C, Xu C, Starovoytov A, Birt I, Mathis MR, Hunker KL, Schmidt EM, Jackson L, Fendrikova-Mahlay N, Zawistowski M, Brummett CM, Zoellner S, Katz A, Coleman DM, Swan K, O’Donnell CJ, Zhou X, Li JZ, Gornik HL, Assimes TL, Stanley JC, Brunham LR, Ganesh SK, Million Veteran Program. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nat Commun 2020;11:4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adlam D, Olson TM, Combaret N, Kovacic JC, Iismaa SE, Al-Hussaini A, O'Byrne MM, Bouajila S, Georges A, Mishra K, Braund PS, d'Escamard V, Huang S, Margaritis M, Nelson CP, de Andrade M, Kadian-Dodov D, Welch CA, Mazurkiewicz S, Jeunemaitre X, Wong CMY, Giannoulatou E, Sweeting M, Muller D, Wood A, McGrath-Cadell L, Fatkin D, Dunwoodie SL, Harvey R, Holloway C, Empana JP, Jouven X, Olin JW, Gulati R, Tweet MS, Hayes SN, Samani NJ, Graham RM, Motreff P, Bouatia-Naji N, CARDIoGRAMPlusC4D Study Group. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol 2019;73:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta RM, Hadaya J, Trehan A, Zekavat SM, Roselli C, Klarin D, Emdin CA, Hilvering CRE, Bianchi V, Mueller C, Khera AV, Ryan RJH, Engreitz JM, Issner R, Shoresh N, Epstein CB, de Laat W, Brown JD, Schnabel RB, Bernstein BE, Kathiresan S. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell 2017;170:522–533. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Musunuru K. Confirmation of causal rs9349379- PHACTR1 expression quantitative trait locus in human-induced pluripotent stem cell endothelial cells. Circ Genom Precis Med 2018;11:e002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, Gaget S, Korner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen AL, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AI, Montpetit A, Polychronakos C, Weill J, Coin LJ, Asher J, Elliott P, Jarvelin MR, Visvikis-Siest S, Balkau B, Sladek R, Balding D, Walley A, Dina C, Froguel P. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 2009;41:157–159. [DOI] [PubMed] [Google Scholar]
- 35. Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Consortium D, Consortium C, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Mu TC, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wellcome Trust Case CC, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ, The CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJF, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, Grarup N, Sim X, Barnes DR, Witkowska K, Staley JR, Tragante V, Tukiainen T, Yaghootkar H, Masca N, Freitag DF, Ferreira T, Giannakopoulou O, Tinker A, Harakalova M, Mihailov E, Liu C, Kraja AT, Fallgaard Nielsen S, Rasheed A, Samuel M, Zhao W, Bonnycastle LL, Jackson AU, Narisu N, Swift AJ, Southam L, Marten J, Huyghe JR, Stancakova A, Fava C, Ohlsson T, Matchan A, Stirrups KE, Bork-Jensen J, Gjesing AP, Kontto J, Perola M, Shaw-Hawkins S, Havulinna AS, Zhang H, Donnelly LA, Groves CJ, Rayner NW, Neville MJ, Robertson NR, Yiorkas AM, Herzig KH, Kajantie E, Zhang W, Willems SM, Lannfelt L, Malerba G, Soranzo N, Trabetti E, Verweij N, Evangelou E, Moayyeri A, Vergnaud AC, Nelson CP, Poveda A, Varga TV, Caslake M, de Craen AJ, Trompet S, Luan J, Scott RA, Harris SE, Liewald DC, Marioni R, Menni C, Farmaki AE, Hallmans G, Renstrom F, Huffman JE, Hassinen M, Burgess S, Vasan RS, Felix JF, Consortium C-HF, Uria-Nickelsen M, Malarstig A, Reily DF, Hoek M, Vogt T, Lin H, Lieb W, EchoGen C, Traylor M, Markus HF, Consortium M, Highland HM, Justice AE, Marouli E, Consortium G, Lindstrom J, Uusitupa M, Komulainen P, Lakka TA, Rauramaa R, Polasek O, Rudan I, Rolandsson O, Franks PW, Dedoussis G, Spector TD, Consortium EP-I, Jousilahti P, Mannisto S, Deary IJ, Starr JM, Langenberg C, Wareham NJ, Brown MJ, Dominiczak AF, Connell JM, Jukema JW, Sattar N, Ford I, Packard CJ, Esko T, Magi R, Metspalu A, de Boer RA, van der Meer P, van der Harst P, Lifelines Cohort S, Gambaro G, Ingelsson E, Lind L, de Bakker PI, Numans ME, Brandslund I, Christensen C, Petersen ER, Korpi-Hyovalti E, Oksa H, Chambers JC, Kooner JS, Blakemore AI, Franks S, Jarvelin MR, Husemoen LL, Linneberg A, Skaaby T, Thuesen B, Karpe F, Tuomilehto J, Doney AS, Morris AD, Palmer CN, Holmen OL, Hveem K, Willer CJ, Tuomi T, Groop L, Karajamaki A, Palotie A, Ripatti S, Salomaa V, Alam DS, Shafi Majumder AA, Di Angelantonio E, Chowdhury R, McCarthy MI, Poulter N, Stanton AV, Sever P, Amouyel P, Arveiler D, Blankenberg S, Ferrieres J, Kee F, Kuulasmaa K, Muller-Nurasyid M, Veronesi G, Virtamo J, Deloukas P, Wellcome Trust Case Control C, Elliott P, Understanding Society Scientific G, Zeggini E, Kathiresan S, Melander O, Kuusisto J, Laakso M, Padmanabhan S, Porteous D, Hayward C, Scotland G, Collins FS, Mohlke KL, Hansen T, Pedersen O, Boehnke M, Stringham HM, Consortium E-C, Frossard P, Newton-Cheh C, Consortium Cecbp Tobin MD, Nordestgaard BG, Mahajan A, Morris AP, Tomaszewski M, Samani NJ, Saleheen D, Asselbergs FW, Lindgren CM, Danesh J, Wain LV, Butterworth AS, Howson JM, Munroe PB, Consortium TDG, Go TDC, Exome BPC, Consortium CHDE, Caulfield MJ. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet 2016;48:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Debette S, Kamatani Y, Metso TM, Kloss M, Chauhan G, Engelter ST, Pezzini A, Thijs V, Markus HS, Dichgans M, Wolf C, Dittrich R, Touze E, Southerland AM, Samson Y, Abboud S, Bejot Y, Caso V, Bersano A, Gschwendtner A, Sessa M, Cole J, Lamy C, Medeiros E, Beretta S, Bonati LH, Grau AJ, Michel P, Majersik JJ, Sharma P, Kalashnikova L, Nazarova M, Dobrynina L, Bartels E, Guillon B, van den Herik EG, Fernandez-Cadenas I, Jood K, Nalls MA, De Leeuw FE, Jern C, Cheng YC, Werner I, Metso AJ, Lichy C, Lyrer PA, Brandt T, Boncoraglio GB, Wichmann HE, Gieger C, Johnson AD, Bottcher T, Castellano M, Arveiler D, Ikram MA, Breteler MM, Padovani A, Meschia JF, Kuhlenbaumer G, Rolfs A, Worrall BB, International Stroke Genetics C, Ringelstein EB, Zelenika D, Tatlisumak T, Lathrop M, Leys D, Amouyel P, Dallongeville J, CADISP Group. Common variation in PHACTR1 is associated with susceptibility to cervical artery dissection. Nat Genet 2015;47:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimaki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schurks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Farkkila M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PAF, Montgomery GW, Martin NG, Borck G, Gobel H, Heinze A, Heinze-Kuhn K, Williams FMK, Hartikainen AL, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga JJ, Vink JM, Heikkila K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor B, Trabzuni D, North American Brain Expression C, Consortium UKBE, Rossin E, Lage K, Jacobs SBR, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin MR, Zwart JA, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg A, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman D, Palotie A, North American Brain Expression Consortium. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet 2013;45:912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiando SR, Tucker N, Katz A, Tréard C, D’Escamard V, Castro-Vega LJ, Ye Z, Smith CY, Austin E, Barlasina C, Cusi D, Galan P, Empana J-P, Jouven X, Brunval P, Olin JW, Gornik H, Plouin P-F, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic J, Jeunemaitre X, Bouatia-Naji N. Abstract 15370: genetic study identifies common variation in PHACTR1 to associate with fibromuscular dysplasia (Best of Basic Science Abstract). Circulation 2015;132:A15370. [Google Scholar]
- 41. Warchol-Celinska E, Berrandou T, Prejbisz A, Georges A, Dupre D, Januszewicz M, Florczak E, Jozwik-Plebanek K, Dobrowolski P, Smigielski W, Drygas W, Kadziela J, Witkowski A, Kabat M, Szczerbo-Trojanowska M, Pappaccogli M, Persu A, Jeunemaitre X, Januszewicz A, Bouatia-Naji N. Genetic study of PHACTR1 and fibromuscular dysplasia, meta-analysis and effects on clinical features of patients: the ARCADIA-POL study. Hypertension 2020;76:e4–e7. [DOI] [PubMed] [Google Scholar]
- 42. O'Donnell CJ, Kavousi M, Smith AV, Kardia SLR, Feitosa MF, Hwang S-J, Sun YV, Province MA, Aspelund T, Dehghan A, Hoffmann U, Bielak LF, Zhang Q, Eiriksdottir G, van Duijn CM, Fox CS, de Andrade M, Kraja AT, Sigurdsson S, Elias-Smale SE, Murabito JM, Launer LJ, van der Lugt A, Kathiresan S, Krestin GP, Herrington DM, Howard TD, Liu Y, Post W, Mitchell BD, O'Connell JR, Shen H, Shuldiner AR, Altshuler D, Elosua R, Salomaa V, Schwartz SM, Siscovick DS, Voight BF, Bis JC, Glazer NL, Psaty BM, Boerwinkle E, Heiss G, Blankenberg S, Zeller T, Wild PS, Schnabel RB, Schillert A, Ziegler A, Münzel TF, White CC, Rotter JI, Nalls M, Oudkerk M, Johnson AD, Newman AB, Uitterlinden AG, Massaro JM, Cunningham J, Harris TB, Hofman A, Peyser PA, Borecki IB, Cupples LA, Gudnason V, Witteman JCM, CARDIoGRAM Consortium. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011;124:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672–1677. [DOI] [PubMed] [Google Scholar]
- 44. Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation 2007;116:32–38. [DOI] [PubMed] [Google Scholar]
- 45. Georges A, Albuisson J, Berrandou T, Dupre D, Lorthioir A, D'Escamard V, Di Narzo AF, Kadian-Dodov D, Olin JW, Warchol-Celinska E, Prejbisz A, Januszewicz A, Bruneval P, Baranowska AA, Webb TR, Hamby SE, Samani NJ, Adlam D, Fendrikova-Mahlay N, Hazen S, Wang Y, Yang ML, Hunker K, Combaret N, Motreff P, Chedid A, Fiquet B, Plouin PF, Mousseaux E, Azarine A, Amar L, Azizi M, Gornik HL, Ganesh SK, Kovacic JC, Jeunemaitre X, Bouatia-Naji N. Rare loss-of-function mutations of PTGIR are enriched in fibromuscular dysplasia. Cardiovasc Res 2021;117:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richer J, Hill HL, Wang Y, Yang M-L, Hunker KL, Lane J, Blackburn S, Coleman DM, Eliason J, Sillon G, D’Agostino M-D, Jetty P, Mongeon F-P, Laberge A-M, Ryan SE, Fendrikova-Mahlay N, Coutinho T, Mathis MR, Zawistowski M, Hazen SL, Katz AE, Gornik HL, Brummett CM, Abecasis G, Bergin IL, Stanley JC, Li JZ, Ganesh SK. Novel recurrent COL5A1 genetic variant is associated with a dysplasia-associated arterial disease exhibiting dissections and fibromuscular dysplasia. ATVB 2020;40:2686–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003;33:407–411. [DOI] [PubMed] [Google Scholar]
- 48. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–281. [DOI] [PubMed] [Google Scholar]
- 50. Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788–798. [DOI] [PubMed] [Google Scholar]
- 51. Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, Bjeda D, Oswald G, Elias AF, Levy HP, Anderlid BM, Yang MH, Bongers EM, Timmermans J, Braverman AC, Canham N, Mortier GR, Brunner HG, Byers PH, Van Eyk J, Van Laer L, Dietz HC, Loeys BL. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 2012;44:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, Tong L, Yang ML, Hunker K, Sloper L, Kuo S, Raza R, Milewicz DM, Francomano CA, Dietz HC, Van Eyk J, McDonnell NB. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-beta expression and connective tissue features. FASEB J 2014;28:3313–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poloskey SL, Kim E, Sanghani R, Al-Quthami AH, Arscott P, Moran R, Rigelsky CM, Gornik HL. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012;17:371–378. [DOI] [PubMed] [Google Scholar]
- 54. Verstraeten A, Perik M, Baranowska AA, Meester JAN, Van Den Heuvel L, Bastianen J, Kempers M, Krapels IPC, Maas A, Rideout A, Vandersteen A, Sobey G, Johnson D, Fransen E, Ghali N, Webb T, Al-Hussaini A, de Leeuw P, Delmotte P, Lopez-Sublet M, Pappaccogli M, Sprynger M, Toubiana L, Van Laer L, Van Dijk FS, Vikkula M, Samani NJ, Persu A, Adlam D, Loeys B, Collaborators of the European/International Fibromuscular Dysplasia Registry and Initiative (FEIRI) Enrichment of rare variants in Loeys-Dietz syndrome genes in spontaneous coronary artery dissection but not in severe fibromuscular dysplasia. Circulation 2020;142:1021–1024. [DOI] [PubMed] [Google Scholar]
- 55. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dachy G, de Krijger RR, Fraitag S, Theate I, Brichard B, Hoffman SB, Libbrecht L, Arts FA, Brouillard P, Vikkula M, Limaye N, Demoulin JB. Association of PDGFRB mutations with pediatric myofibroma and myofibromatosis. JAMA Dermatol 2019;155:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arts FA, Sciot R, Brichard B, Renard M, de Rocca Serra A, Dachy G, Noel LA, Velghe AI, Galant C, Debiec-Rychter M, Van Damme A, Vikkula M, Helaers R, Limaye N, Poirel HA, Demoulin JB. PDGFRB gain-of-function mutations in sporadic infantile myofibromatosis. Hum Mol Genet 2017;26:1801–1810. [DOI] [PubMed] [Google Scholar]
- 58. Pond D, Arts FA, Mendelsohn NJ, Demoulin JB, Scharer G, Messinger Y. A patient with germ-line gain-of-function PDGFRB p.N666H mutation and marked clinical response to imatinib. Genet Med 2018;20:142–150. [DOI] [PubMed] [Google Scholar]
- 59. Brasseur B, Chantrain CF, Godefroid N, Sluysmans T, Anslot C, Menten R, Clapuyt P, Dupont S, Vermylen C, Brichard B. Development of renal and iliac aneurysms in a child with generalized infantile myofibromatosis. Pediatr Nephrol 2010;25:983–986. [DOI] [PubMed] [Google Scholar]
- 60. Karasozen Y, Osbun JW, Parada CA, Busald T, Tatman P, Gonzalez-Cuyar LF, Hale CJ, Alcantara D, O'Driscoll M, Dobyns WB, Murray M, Kim LJ, Byers P, Dorschner MO, Ferreira M Jr. Somatic PDGFRB activating variants in fusiform cerebral aneurysms. Am J Hum Genet 2019;104:968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Foster A, Chalot B, Antoniadi T, Schaefer E, Keelagher R, Ryan G, Thomas Q, Philippe C, Bruel AL, Sorlin A, Thauvin-Robinet C, Bardou M, Luu M, Quenardelle V, Wolff V, Woodley J, Vabres P, Lim D, Igbokwe R, Joseph A, Walker H, Jester A, Ellenbogen J, Johnson D, Rooke B, Moss C, Cole T, Faivre L. Kosaki overgrowth syndrome: a novel pathogenic variant in PDGFRB and expansion of the phenotype including cerebrovascular complications. Clin Genet 2020;98:19–31. [DOI] [PubMed] [Google Scholar]
- 62. Zarate YA, Boccuto L, Srikanth S, Pauly R, Ocal E, Balmakund T, Hinkle K, Stefans V, Schaefer GB, Collins RT III. Constitutive activation of the PI3K-AKT pathway and cardiovascular abnormalities in an individual with Kosaki overgrowth syndrome. Am J Med Genet A 2019;179:1047–1052. [DOI] [PubMed] [Google Scholar]
- 63. Sottiurai VS, Fry WJ, Stanley JC. Ultrastructure of medial smooth muscle and myofibroblasts in human arterial dysplasia. Arch Surg 1978;113:1280–1288. [DOI] [PubMed] [Google Scholar]
- 64. Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg 1975;110:561–566. [DOI] [PubMed] [Google Scholar]
- 65. Olin JW, Di Narzo AF, d'Escamard V, Kadian-Dodov D, Cheng H, Georges A, King A, Thomas A, Barwari T, Michelis KC, Bouchareb R, Bander E, Anyanwu A, Stelzer P, Filsoufi F, Florman S, Civelek M, Debette S, Jeunemaitre X, Bjorkegren JLM, Mayr M, Bouatia-Naji N, Hao K, Kovacic JC. A plasma proteogenomic signature for fibromuscular dysplasia. Cardiovasc Res 2020;116:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bjorkegren JLM, Kovacic JC, Dudley JT, Schadt EE. Genome-wide significant loci: how important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. J Am Coll Cardiol 2015;65:830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michelis KC, Nomura-Kitabayashi A, Lecce L, Franzén O, Koplev S, Xu Y, Santini MP, D'Escamard V, Lee JTL, Fuster V, Hajjar R, Reddy RC, Chikwe J, Stelzer P, Filsoufi F, Stewart A, Anyanwu A, Björkegren JLM, Kovacic JC. CD90 identifies adventitial mesenchymal progenitor cells in adult human medium- and large-sized arteries. Stem Cell Reports 2018;11:242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zeng L, Talukdar HA, Koplev S, Giannarelli C, Ivert T, Gan LM, Ruusalepp A, Schadt EE, Kovacic JC, Lusis AJ, Michoel T, Schunkert H, Bjorkegren JLM. Contribution of gene regulatory networks to heritability of coronary artery disease. J Am Coll Cardiol 2019;73:2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bruno RM, Mischak H, Persu A. Multi-omics applied to fibromuscular dysplasia: first steps on a new research avenue. Cardiovasc Res 2020;116:4–5. [DOI] [PubMed] [Google Scholar]
- 70. Kovacic JC. International symposium revisiting fibromuscular dysplasia & related vascular diseases. Belgian Royal Academy of Medicine, Brussels, Belgium, 2018. Abstract and Lecture title: “What can we learn from the fibroblast? The DEFINE study”.
- 71. Nosalski R, Siedlinski M, Denby L, McGinnigle E, Nowak M, Cat AND, Medina-Ruiz L, Cantini M, Skiba D, Wilk G, Osmenda G, Rodor J, Salmeron-Sanchez M, Graham G, Maffia P, Graham D, Baker AH, Guzik TJ. T-cell-derived miRNA-214 mediates perivascular fibrosis in hypertension. Circ Res 2020;126:988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 73. Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016;133:1548–1559. [DOI] [PubMed] [Google Scholar]
- 74. Nicholson J, Alderman M, Pickering T, Teichman S, Sos T, Laragh J. Cigarette smoking and renovascular hypertension. Lancet 1983;322:765–766. [DOI] [PubMed] [Google Scholar]
- 75. O’Connor S, Gornik HL, Froehlich JB, Gu X, Gray BH, Mace PD, Sharma A, Olin JW, Kim ESH. Smoking and adverse outcomes in fibromuscular dysplasia: U.S. Registry Report. J Am Coll Cardiol 2016;67:1750–1751. [DOI] [PubMed] [Google Scholar]
- 76. Dobrowolski P, Januszewicz M, Witowicz H, Warchoł-Celińska E, Klisiewicz A, Skrzypczyńska-Banasik U, Kabat M, Kowalczyk K, Aniszczuk-Hybiak A, Florczak E, Witkowski A, Tykarski A, Widecka K, Szczerbo-Trojanowska M, Śmigielski W, Drygas W, Michałowska I, Hoffman P, Prejbisz A, Januszewicz A. Prevalence of smoking and clinical characteristics in fibromuscular dysplasia. The ARCADIA-POL study. Blood Press 2019;28:49–56. [DOI] [PubMed] [Google Scholar]
- 77. Maumus-Robert S, Berard X, Mansiaux Y, Tubert-Bitter P, Debette S, Pariente A. Short-term risk of aortoiliac aneurysm or dissection associated with fluoroquinolone use. J Am Coll Cardiol 2019;73:875–877. [DOI] [PubMed] [Google Scholar]
- 78. Lee C-C, Lee M-tG, Hsieh R, Porta L, Lee W-C, Lee S-H, Chang S-S. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol 2018;72:1369–1378. [DOI] [PubMed] [Google Scholar]
- 79. Del Zotto E, Pezzini A. Use of fluoroquinolones and the risk of spontaneous cervical artery dissection. Eur J Neurol 2019;26:1028–1031. [DOI] [PubMed] [Google Scholar]
- 80. Maumus-Robert S, Debette S, Bérard X, Mansiaux Y, Tubert-Bitter P, Pariente A. Risk of intracranial aneurysm and dissection and fluoroquinolone use. Stroke 2020;51:994–997. [DOI] [PubMed] [Google Scholar]
- 81. Guzzardi D, Teng G, Svystonyuk D, Kang S, Park D, Belke D, Turnbull J, Fedak P. Fluoroquinolone induces human aortic fibroblast-mediated extracellular matrix dysregulation. Can J Cardiol 2017;33:S38–S39. [Google Scholar]
- 82. Corps AN, Harrall RL, Curry VA, Fenwick SA, Hazleman BL, Riley GP. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1? in human tendon-derived cells. Arthritis Rheum 2002;46:3034–3040. [DOI] [PubMed] [Google Scholar]
- 83. Born-Frontsberg E, Reincke M, Rump LC, Hahner S, Diederich S, Lorenz R, Allolio B, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Endres S, Quinkler M, for the participants of the German Conn’s Registry. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn's Registry. J Clin Endocrinol Metab 2009;94:1125–1130. [DOI] [PubMed] [Google Scholar]
- 84. Boutouyrie P, Gimenez-Roqueplo AP, Fine E, Laloux B, Fiquet-Kempf B, Plouin PF, Jeunemaitre X, Laurent S. Evidence for carotid and radial artery wall subclinical lesions in renal fibromuscular dysplasia. J Hypertens 2003;21:2287–2295. [DOI] [PubMed] [Google Scholar]
- 85. Bruno RM, Marais L, Khettab H, Lorthioir A, Frank M, Jeunemaitre X, Laurent S, Boutouyrie P, Azizi M. Deep vascular phenotyping in patients with renal multifocal fibromuscular dysplasia. Hypertension 2019;73:371–378. [DOI] [PubMed] [Google Scholar]
- 86. Ryabikov A, Malyutina S, Halcox J, Nikitin Y, Marmot M, Bobak M. Prevalence and predictors of carotid wall triple line pattern in a general population sample. Arterioscler Thromb Vasc Biol 2011;31:1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sundholm JKM, Pettersson T, Paetau A, Alback A, Sarkola T. Diagnostic performance and utility of very high-resolution ultrasonography in diagnosing giant cell arteritis of the temporal artery. Rheumatol Adv Pract 2019;3:rkz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang BB, Zhou YJ, Du J, Yang SW, Wang ZJ, Shen H, Zhou ZM. Comparison of very-high-frequency ultrasound assessment of radial arterial wall layers after first and repeated transradial coronary procedures. J Geriatr Cardiol 2017;14:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dangardt F, Charakida M, Chiesa S, Bhowruth D, Rapala A, Thurn D, Schaefer F, Deanfield J, Shroff R. Intimal and medial arterial changes defined by ultra-high-frequency ultrasound: response to changing risk factors in children with chronic kidney disease. PLoS One 2018;13:e0198547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bruno RM, Lascio ND, Guarino D, Vitali S, Rossi P, Caramella D, Taddei S, Ghiadoni L, Faita F. Abstract P509: identification of radial vascular wall abnormalities by very-high frequency ultrasound in patients with fibromuscular dysplasia: the Fuchsia study. Hypertension 2017;70:AP509. [Google Scholar]
- 91. van Twist DJ, Houben AJ, de Haan MW, de Leeuw PW, Kroon AA. Renal hemodynamics and renin-angiotensin system activity in humans with multifocal renal artery fibromuscular dysplasia. J Hypertens 2016;34:1160–1169. [DOI] [PubMed] [Google Scholar]
- 92. van Twist DJ, Houben AJ, de Haan MW, de Leeuw PW, Kroon AA. Pathophysiological differences between multifocal fibromuscular dysplasia and atherosclerotic renal artery stenosis. J Hypertens 2017;35:845–852. [DOI] [PubMed] [Google Scholar]
- 93. La Batide-Alanore A, Azizi M, Froissart M, Raynaud A, Plouin PF. Split renal function outcome after renal angioplasty in patients with unilateral renal artery stenosis. J Am Soc Nephrol 2001;12:1235–1241. [DOI] [PubMed] [Google Scholar]
- 94. Van Twist D, Houben A, De Haan M, Mostard G, De Leeuw P, Kroon A. Angiotensin-(1-7)-induced renal vasodilation is reduced in human kidneys with renal artery stenosis. J Hypertens 2014;32:2428–2432. [DOI] [PubMed] [Google Scholar]
- 95. van Twist DJL, de Leeuw PW, Kroon AA. Renal artery fibromuscular dysplasia and its effect on the kidney. Hypertens Res 2018;41:639–648. [DOI] [PubMed] [Google Scholar]
- 96. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D'Agostino RB Sr, Dworkin LD, CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014;370:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009;150:840–848. [DOI] [PubMed] [Google Scholar]
- 98. Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension 2010;56:525–532. [DOI] [PubMed] [Google Scholar]
- 99. van Twist DJL, de Heer PWM, Houben A, de Haan MW, de Leeuw PW, Kroon AA. Differences in renal hemodynamics and renin secretion between patients with unifocal and multifocal fibromuscular dysplasia. J Hypertens 2018;36:1729–1735. [DOI] [PubMed] [Google Scholar]
- 100. Touzé E, Southerland AM, Boulanger M, Labeyrie PE, Azizi M, Bouatia-Naji N, Debette S, Gornik HL, Hussain SM, Jeunemaitre X, Joux J, Kirton A, Le Hello C, Majersik JJ, Mocco J, Persu A, Sharma A, Worrall BB, Olin JW, Plouin PF. Fibromuscular dysplasia and its neurologic manifestations: a systematic review. JAMA Neurol 2019;76:217–226. [DOI] [PubMed] [Google Scholar]
- 101. O'Connor SC, Poria N, Gornik HL. Fibromuscular dysplasia: an update for the headache clinician. Headache 2015;55:748–755. [DOI] [PubMed] [Google Scholar]
- 102. Touze E, Oppenheim C, Trystram D, Nokam G, Pasquini M, Alamowitch S, Herve D, Garnier P, Mousseaux E, Plouin PF. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke 2010;5:296–305. [DOI] [PubMed] [Google Scholar]
- 103. Guglielmi V, Visser J, Arnold M, Sarikaya H, van den Berg R, Nederkoorn PJ, Leys D, Calvet D, Kloss M, Pezzini A, Tatlisumak T, Schilling S, Debette S, Coutinho JM. Triple and quadruple cervical artery dissections: a systematic review of individual patient data. J Neurol 2019;266:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kadian-Dodov D, Goldfinger JZ, Gustavson S, Olin JW. Natural history of cervical artery fibromuscular dysplasia and associated neurovascular events. Cerebrovasc Dis 2018;46:33–39. [DOI] [PubMed] [Google Scholar]
- 105. Cloft HJ, Kallmes DF, Kallmes MH, Goldstein JH, Jensen ME, Dion JE. Prevalence of cerebral aneurysms in patients with fibromuscular dysplasia: a reassessment. J Neurosurg 1998;88:436–440. [DOI] [PubMed] [Google Scholar]
- 106. Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A, Torner JC, Derdeyn CP, Raabe A, Mocco J, Korja M, Abdulazim A, Amin-Hanjani S, Al-Shahi Salman R, Barrow DL, Bederson J, Bonafe A, Dumont AS, Fiorella DJ, Gruber A, Hankey GJ, Hasan DM, Hoh BL, Jabbour P, Kasuya H, Kelly ME, Kirkpatrick PJ, Knuckey N, Koivisto T, Krings T, Lawton MT, Marotta TR, Mayer SA, Mee E, Pereira VM, Molyneux A, Morgan MK, Mori K, Murayama Y, Nagahiro S, Nakayama N, Niemelä M, Ogilvy CS, Pierot L, Rabinstein AA, Roos YBWEM, Rinne J, Rosenwasser RH, Ronkainen A, Schaller K, Seifert V, Solomon RA, Spears J, Steiger H-J, Vergouwen MDI, Wanke I, Wermer MJH, Wong GKC, Wong JH, Zipfel GJ, Connolly ES, Steinmetz H, Lanzino G, Pasqualin A, Rüfenacht D, Vajkoczy P, McDougall C, Hänggi D, LeRoux P, Rinkel GJE, Macdonald RL. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 2015;85:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Choi PM, Menon BK, Demchuk AM. Carotid web and stroke. Eur J Neurol 2014;21:e53. [DOI] [PubMed] [Google Scholar]
- 108. Choi PM, Singh D, Trivedi A, Qazi E, George D, Wong J, Demchuk AM, Goyal M, Hill MD, Menon BK. Carotid webs and recurrent ischemic strokes in the Era of CT angiography. AJNR Am J Neuroradiol 2015;36:2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lenck S, Labeyrie MA, Saint-Maurice JP, Tarlov N, Houdart E. Diaphragms of the carotid and vertebral arteries: an under-diagnosed cause of ischaemic stroke. Eur J Neurol 2014;21:586–593. [DOI] [PubMed] [Google Scholar]
- 110. Joux J, Chausson N, Jeannin S, Saint-Vil M, Mejdoubi M, Hennequin JL, Deschamps L, Smadja D, Olindo S. Carotid-bulb atypical fibromuscular dysplasia in young Afro-Caribbean patients with stroke. Stroke 2014;45:3711–3713. [DOI] [PubMed] [Google Scholar]
- 111. Kubis N, Von LD, Petitjean C, Brouland JP, Guichard JP, Chapot R, Mikol J, Woimant F. Thrombotic carotid megabulb: fibromuscular dysplasia, septae, and ischemic stroke. Neurology 1999;52:883–886. [DOI] [PubMed] [Google Scholar]
- 112. Joux J, Boulanger M, Jeannin S, Chausson N, Hennequin JL, Molinie V, Smadja D, Touze E, Olindo S. Association between carotid bulb diaphragm and ischemic stroke in young Afro-Caribbean patients: a population-based case-control study. Stroke 2016;47:2641–2644. [DOI] [PubMed] [Google Scholar]
- 113. Compagne KCJ, van Es A, Berkhemer OA, Borst J, Roos Y, van Oostenbrugge RJ, van Zwam WH, Majoie C, Marquering HA, Dippel DWJ, van der Lugt A, Emmer BJ, MR Clean Trial Investigators. Prevalence of carotid web in patients with acute intracranial stroke due to intracranial large vessel occlusion. Radiology 2018;286:1000–1007. [DOI] [PubMed] [Google Scholar]
- 114. Catena C, Verheyen N, Pilz S, Kraigher-Krainer E, Tomaschitz A, Sechi LA, Pieske B. Plasma aldosterone and left ventricular diastolic function in treatment-naive patients with hypertension: tissue-Doppler imaging study. Hypertension 2015;65:1231–1237. [DOI] [PubMed] [Google Scholar]
- 115. Cuspidi C, Dell’Oro R, Sala C, Tadic M, Gherbesi E, Grassi G, Mancia G. Renal artery stenosis and left ventricular hypertrophy: an updated review and meta-analysis of echocardiographic studies. J Hypertens 2017;35:2339–2345. [DOI] [PubMed] [Google Scholar]
- 116. Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Left ventricular morphology and function in patients with atherosclerotic renovascular disease. Jasn 2005;16:2746–2753. [DOI] [PubMed] [Google Scholar]
- 117. Michelis KC, Olin JW, Kadian-Dodov D, d'Escamard V, Kovacic JC. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol 2014;64:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Groves SS, Jain AC, Warden BE, Gharib W, Beto RJ 2nd. Severe coronary tortuosity and the relationship to significant coronary artery disease. W V Med J 2009;105:14–17. [PubMed] [Google Scholar]
- 119. Li Y, Shen C, Ji Y, Feng Y, Ma G, Liu N. Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS One 2011;6:e24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, Vrtiska TJ, Prasad M, Rihal CS, Hayes SN, Gulati R. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656–662. [DOI] [PubMed] [Google Scholar]
- 121. van Twist DJL, de Leeuw PW, Kroon AA. Coronary tortuosity: a clue to the diagnosis of fibromuscular dysplasia? Am J Hypertens 2017;30:776–780. [DOI] [PubMed] [Google Scholar]
- 122. Zegers ES, Meursing BTJ, Zegers EB, Oude Ophuis AJ. Coronary tortuosity: a long and winding road. Neth Heart J 2007;15:191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li Y, Shi Z, Cai Y, Feng Y, Ma G, Shen C, Li Z, Liu N. Impact of coronary tortuosity on coronary pressure: numerical simulation study. PLoS One 2012;7:e42558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xie X, Wang Y, Zhu H, Zhou H, Zhou J. Impact of coronary tortuosity on coronary blood supply: a patient-specific study. PLoS One 2013;8:e64564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv 2005;64:138–145. [DOI] [PubMed] [Google Scholar]
- 126. Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012;5:134–137. [DOI] [PubMed] [Google Scholar]
- 127. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 128. Harrison EG Jr, McCormack LJ. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc 1971;46:161–167. [PubMed] [Google Scholar]
- 129. Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ, American Heart Association Council On Peripheral Vascular D, Council On Clinical C, Council On C, Stroke N, Council On G, Precision M, Stroke C, American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jackson R, Al-Hussaini A, Joseph S, van Soest G, Wood A, Macaya F, Gonzalo N, Cade J, Caixeta A, Hlinomaz O, Leinveber P, O’Kane P, García-Guimaraes M, Cortese B, Samani NJ, Escaned J, Alfonso F, Johnson T, Adlam D. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc Imaging 2019;12:2475–2488. [DOI] [PubMed] [Google Scholar]
- 132. Van der Niepen P, van Tussenbroek F, Devos H, Debing E, Di Monaco S, Goffette P, Astarci P, Persu A. Visceral fibromuscular dysplasia: from asymptomatic disorder to emergency. Eur J Clin Invest 2018;48:e13023. [DOI] [PubMed] [Google Scholar]
- 133. Sang CN, Whelton PK, Hamper UM, Connolly M, Kadir S, White RI, Sanders R, Liang KY, Bias W. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension 1989;14:472–479. [DOI] [PubMed] [Google Scholar]
- 134. Silhol F, Radix W, Courbieres B, Cornand D, Vaisse B, Sarlon-Bartoli G. Fibromuscular dysplasia exposes to early natural impregnation with progesterone. J Med Vasc 2017;42:392–394. [DOI] [PubMed] [Google Scholar]
- 135. Silhol F, Sarlon-Bartoli G, Daniel L, Bartoli JM, Cohen S, Lepidi H, Piquet P, Bartoli MA, Vaïsse B. Intranuclear expression of progesterone receptors in smooth muscle cells of renovascular fibromuscular dysplasia: a pilot study. Ann Vasc Surg 2015;29:830–835. [DOI] [PubMed] [Google Scholar]
- 136. Vance CJ, Taylor RN, Craven TE, Edwards MS, Corriere MA. Increased prevalence of preeclampsia among women undergoing procedural intervention for renal artery fibromuscular dysplasia. Ann Vasc Surg 2015;29:1105–1110. [DOI] [PubMed] [Google Scholar]
- 137. Berra E, Dominiczak AF, Touyz RM, Pierard S, Hammer F, Rossi GP, Micali RG, Staessen JA, Bursztyn M, Kahan T, Persu A. Management of a pregnant woman with fibromuscular dysplasia. Hypertension 2018;71:540–547. [DOI] [PubMed] [Google Scholar]
- 138. Cunningham TK, Draper H, Rajesh U. Management of a pregnancy with underlying fibromuscular dysplasia with a history of stroke and carotid artery dissection. J Obstet Gynaecol 2019;39:417–419. [DOI] [PubMed] [Google Scholar]
- 139. Khan F, Ghani AR, Mackenzie L, Matthew A, Sarwar U, Klugherz B. A rare presentation of fibromuscular dysplasia: postpartum vascular catastrophe and brief literature review. J Investig Med High Impact Case Rep 2017;5:2324709617719917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Prejbisz A, Dobrowolski P, Kosiński P, Bomba-Opoń D, Adamczak M, Bekiesińska-Figatowska M, Kądziela J, Konopka A, Kostka-Jeziorny K, Kurnatowska I, Leszczyńska-Gorzelak B, Litwin M, Olszanecka A, Orczykowski M, Poniedziałek-Czajkowska E, Sobieszczańska-Małek M, Stolarz-Skrzypek K, Szczepaniak-Chicheł L, Szyndler A, Wolf J, Wielgoś M, Hoffman P, Januszewicz A. Management of hypertension in pregnancy: prevention, diagnosis, treatment and longterm prognosis. Kardiol Pol 2019;77:757–806. [DOI] [PubMed] [Google Scholar]
- 141. Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, Gokhale M, Kotelchuck M, Melve KK, Langridge A, Morris C, Morris JM, Nassar N, Norman JE, Norrie J, Sorensen HT, Walker R, Weir CJ. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open 2011;1:e000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Boisrame T, Sananes N, Fritz G, Boudier E, Aissi G, Favre R, Langer B. Placental abruption: risk factors, management and maternal-fetal prognosis. Cohort study over 10 years. Eur J Obstet Gynecol Reprod Biol 2014;179:100–104. [DOI] [PubMed] [Google Scholar]
- 144. Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, MacPherson C, Landon M, Miodovnik M, Paul R, Meis P, Dombrowski M. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1998;339:667–671. [DOI] [PubMed] [Google Scholar]
- 145. Chappell LC, Enye S, Seed P, Briley AL, Poston L, Shennan AH. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension 2008;51:1002–1009. [DOI] [PubMed] [Google Scholar]
- 146. Persu A, Giavarini A, Touze E, Januszewicz A, Sapoval M, Azizi M, Barral X, Jeunemaitre X, Morganti A, Plouin PF, de Leeuw P, ESH Working Group Hypertension and the Kidney. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens 2014;32:1367–1378. [DOI] [PubMed] [Google Scholar]
- 147. O’Connor S, Kim ES, Brinza E, Moran R, Fendrikova-Mahlay N, Wolski K, Gornik HL. Systemic connective tissue features in women with fibromuscular dysplasia. Vasc Med 2015;20:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.