Abstract
Atrial fibrillation (AF) is the most commonly diagnosed arrhythmia, and ECG remains the gold standard for diagnosing AF. Wrist-worn technologies are appealing for their ability to passively process near-continuous pulse signals. The clinical application of wearable devices is controversial. Our systematic review and meta-analysis qualitatively and quantitatively analyze available literature on wrist-worn wearable devices (Apple Watch, Samsung, and KardiaBand) and their sensitivity and specificity in detecting AF compared to conventional methods. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed, yielding nine studies (n = 1,581). Observational studies assessing the sensitivity and specificity of wrist-worn wearables in detecting AF in patients with and without a history of AF were included and analyzed using a fixed-effect model with an inverse-variance method. In patients with a history of AF, the overall sensitivity between device groups did not significantly differ (96.83%; P = 0.207). Specificity significantly differed between Apple, Samsung, and KardiaBand (99.61%, 81.13%, and 97.98%, respectively; P<0.001). The effect size for this analysis was highest in the Samsung device group. Two studies (n = 796) differentiated cohorts to assess device sensitivity in patients with known AF and device specificity in patients with normal sinus rhythm (NSR) (sensitivity: 96.02%; confidence intervals (CI) 93.85%-97.59% and specificity: 98.82%; CI:97.46%-99.57%). Wrist-worn wearable devices demonstrate promising results in detecting AF in patients with paroxysmal AF. However, more rigorous prospective data is needed to understand the limitations of these devices in regard to varying specificities which may lead to unintended downstream medical testing and costs.
Keywords: detection, ecg, specificity, sensitivity, accuracy, wearables, atrial fibrillation
Introduction and background
Atrial fibrillation (AF) is the most commonly diagnosed arrhythmia in clinical practice [1]. It is estimated that 2.3 million adults in the United States are burdened by AF, and as the population ages that number is expected to increase to 5.6 million by 2050 [1]. The consequences of AF, including thromboembolic events, stroke, and heart failure, are well documented. These consequences of disease progression account for the significant impact on morbidity, mortality, and healthcare costs [1]. Therefore, AF is not only a devastating clinical problem but also a public health and economic burden.
While AF typically presents with palpitations, dyspnea, chest pain, and fatigue, it is estimated that a 10-40% incidence of AF is asymptomatic [2]. Subclinical or unrecognized AF presents with the same risks as symptomatic AF and has critical implications when first manifesting at the time of acute stroke. The relationship between arrhythmia and stroke is perplexing; however, reports from the Framingham Study have demonstrated that the concomitant presentation of stroke with newly diagnosed AF suggests that cardiac emboli may be an important cause of stroke [3-4]. Furthermore, the temporal relationship between AF and stroke highlights the importance of prophylactic measures for stroke prevention [4]. Early detection of both clinical and subclinical AF allows for early preventative measures, which would improve health outcomes.
Interpretation of a 12-lead electrocardiogram (ECG) by a trained cardiologist or heart rhythm specialist is the gold standard for detecting AF [5]. The 2014 guidelines from the American Heart Association/American Stroke Association recommend screening for AF with pulse assessments during routine clinical visits and subsequent 12-lead ECGs among individuals who demonstrate an irregular pulse [6-7]. The guidelines highlight the advantages of active screening in patients >65, however, they lack recommendations on frequency [6-7]. Similarly, the US Preventive Services Task Force (USPSTF) published a statement that the current evidence is insufficient to evaluate the benefit of screening for AF with ECG [8]. The problem is that too many uncertainties exist to warrant routine ECG testing for all patients, especially those who are not high-risk. Current research points to the low prevalence and high costs as significant contributors to such screening challenges [9]. One challenge is that ambulatory ECG monitoring, ranging from 12 hours to 14 days, is only marginally representative of a patient’s experience due to the unpredictable and sporadic nature of AF [10].
While it is clear that more evidence is needed to illustrate the advantages of early screening protocols, studies have demonstrated that active screening for undiagnosed AF has proven to be effective starting at an age of 40 years [11]. Furthermore, screening with ECG can identify patients with asymptomatic AF [12]. Therefore, early detection leads to potentially reducing the risk of stroke and heart failure in patients with AF.
An increasing number of individuals use commercially available wearable technology, which has paralleled innovation in the mobile health (mHealth) space. mHealth has been an avenue for expanding AF detection beyond traditional cardiac telemetry. Currently, mHealth technology utilizes electrocardiographic or photoplethysmographic (PPG) signal processing to detect AF [13]. While ECG remains the gold standard for AF detection, these novel technologies are appealing for their ability to passively process near-continuous pulse signals [13]. PPG and similar technology offer an inexpensive and non-invasive means for continuous monitoring throughout the cardiac cycle. Developing the accuracy of wearable technology has the potential to eliminate some of the challenges observed with conventional screening methods for AF detection.
Objective
Observational clinical studies measuring the accuracy of wearable devices in detecting AF demonstrate promising outcomes. This novel development has garnered significant interest in the field of cardiology over the past five years due to the recent FDA clearance of multiple mobile technologies for AF detection [13]. However, the reported accuracy of wrist-worn wearable technologies is inconsistent across the literature.
To date, there are several reviews that look at the use of wearable devices for the detection of AF [14-15]. Only one systematic review focused on the sensitivity and specificity of wearable devices in detecting AF [15]. Therefore, we conducted a systematic review and meta-analysis to compare the accuracy of the most recent wrist-worn wearable devices in detecting AF. Our objective was to qualitatively and quantitatively analyze the available literature on wrist-worn wearable devices and their sensitivity and specificity in detecting AF compared to conventional methods.
Review
Methods
This systematic review and meta-analysis was conducted using the PICO (Patient, Intervention, Comparator, and Outcome) method and followed the framework outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16].
Search strategy
A comprehensive search of several databases from each database’s inception to July 27th, 2020, English language, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from two authors (S.B and W.W). Controlled vocabulary supplemented with keywords was used to search for data collection accuracy of wearables and their efficacy in predicting outcomes in AF.
Study selection criteria
Studies were included for review if they met the following criteria: involve human subjects, collect EKG data, assess the accuracy of wrist-worn wearables, diagnose atrial fibrillation, and published within the past five years (2016-2020), due to the increase in digital health technologies since the FDA approval for the AliveCor Kardia device as the first wearable technology to detect AF [17]. Studies were excluded based on the following predefined criteria: non-English language, pediatric population, mobile apps, and data that lacked sensitivity and specificity statistics.
Quality assessment
The quality of outcomes was assessed using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) methodology, while studies’ quality of evidence was assessed using the modified Newcastle-Ottawa Scale [18,19].
Data extraction
Eligible studies were pooled according to the aforementioned inclusion and exclusion criteria. Data extraction from articles, tables, and figures was pulled by one reviewer (S.B) and accuracy of data entry was verified by a second reviewer (W.W). Data collected included: study author, year of publication, type of device used, sample size, number of recorded events, method of AF verification, true positive, true negative, false positive, false negative, specificity, and sensitivity (Table 1).
Table 1. Summary of Data Extraction and Study Characteristics.
SR: sinus rhythm, AF: atrial fibrillation, ECG: electrocardiogram, ICM: implantable cardiac monitor, TP: true positive, TN: true negative, FP: false positive, FN: false negative
| Study | Year | Device Group | Sample Size (n) | Female (n) | Recorded Events | Past Medical History | Method of AF Verification | TP | TN | FP | FN | Sensitivity | Specificity |
| Seshadri et al. [20] | 2020 | Apple | 50 | 284 | Undergone cardiac surgery | Telemetry | 81 | 200 | 0 | 3 | 96.40% | 100% | |
| Apple, Inc [21] | 2018 | Apple | 588 | 479 | 301 AF 287 SR | 12-lead ECG | 236 | 238 | 1 | 4 | 98.30% | 99% | |
| Tison et al. [22] | 2018 | Apple | 51 | 8 | 51 | AF | 12-lead ECG | 40 | 9 | 1 | 1 | 97.56% | 90% |
| Wasserlauf et al. [23] | 2019 | KB | 24 | 9 | 82 | Paroxysmal AF | ICM recording | 80 | N/A | N/A | 2 | 97.56% | N/A |
| Bumgarner et al. [24] | 2018 | KB | 100 | 17 | 169 | AF | ECG | 63 | 37 | 7 | 5 | 92.65% | 97.57% |
| Rajakariar et al. [25] | 2020 | KB | 200 | 43 | 191 (9/200 no analysis) | 38 AF 162 SR | 12-lead ECG | 47 | 113 | 28 | 3 | 94% | 80.14% |
| Dorr et al. [26] | 2018 | Samsung | 508 | 225 | 508 | 271 AF 237 SR | Cardiologist interpretation of iECG from kardiamobile | 222 | 266 | 5 | 15 | 93.67% | 98.15% |
| Ding et al. [27] | 2019 | Samsung | 40 | 8 | 314 | 9 AF 30 SR | 7-lead Holter monitor | 54 | 254 | 5 | 1 | 98.20% | 98.07% |
| Bashar et al. [28] | 2019 | Samsung | 20 | 242 | 8AF 12 SR | 7-lead Holter monitor | 50 | 185 | 5 | 2 | 96.15% | 97.37% |
Statistical analysis
A meta-analysis of diagnostic test specificity and sensitivity was conducted, with results represented as effect sizes (ES) with corresponding 95% confidence intervals (CIs). A fixed-effect model with an inverse-variance method was used [29-31]. Heterogeneity between groups represents the statistical difference between the three groups in their respective outcomes. Funnel plots were created to assess publication bias within studies. Statistical analysis was done using STATA 16.0 (Stata-Corp 2020. STATA Statistical Software: Release 15. College Station, TX: StataCorp LP) and its “metan” and “metafunnel” packages. A P-value <0.05 was considered significant.
Results
Search Results
Our search strategy yielded a total of 2113 unique articles. After removal of 1263 articles that were published prior to 2016 and 39 articles that concerned a pediatric population, inclusion/exclusion criteria were applied to abstracts of the remaining 814 articles. This resulted in 28 articles that underwent full-text analysis, of which nine met the predefined eligibility criteria and were included in the qualitative and quantitative synthesis (Figure 1) [20-28].
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) search strategy flowchart of the present systematic review and meta-analysis.
AF: atrial fibrillation
Characteristics of studies
The total number of studies included was nine, with three regarding Apple devices, three regarding KardiaBand (KB), and three regarding Samsung devices [20-28]. In total, 1629 patients were included in this meta-analysis and an average of 259 instances were recorded per study. Mean age of all studies was 70.2. Percentage of females across all studies was 29.18% (Table 1).
Sensitivity
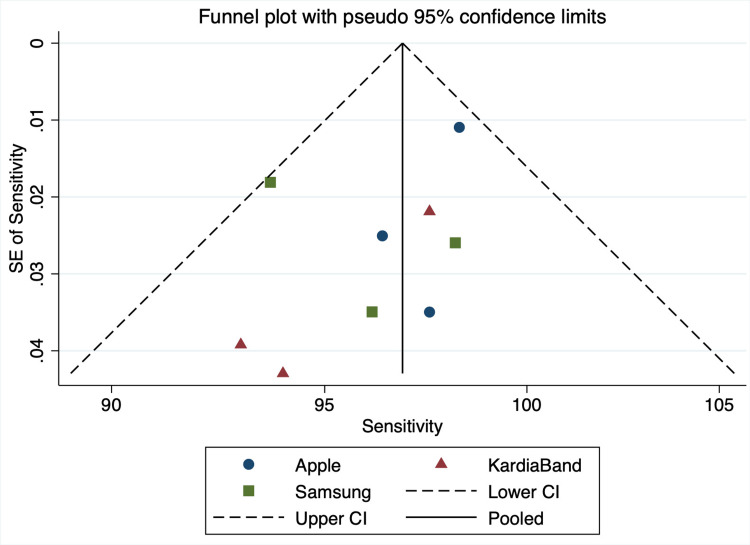
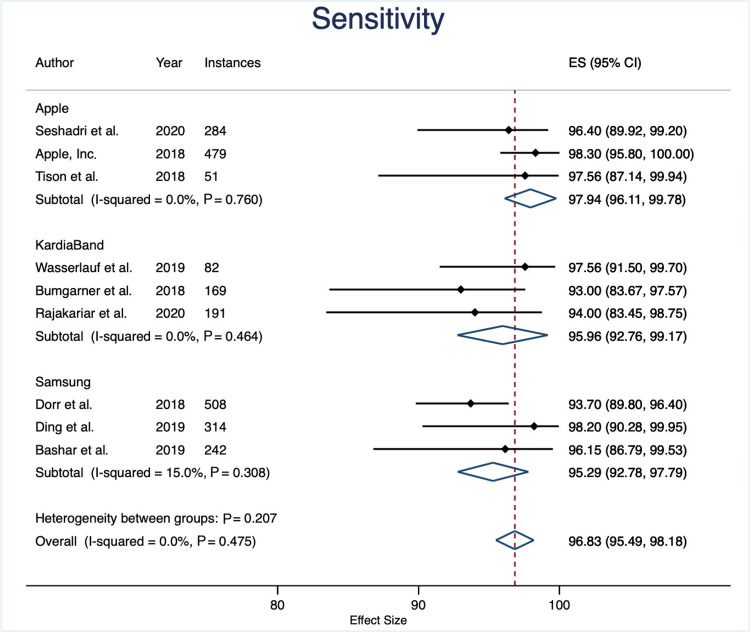
The overall sensitivity between device groups was not statistically significant (P = 0.276). Apple devices had an average sensitivity of 97.9% (95% CI: 96.1% to 99.7%), KB with 96.9% (95% CI: 94.7% to 99.2%), and Samsung devices with 95.5% (95% CI: 93.1% to 97.8%). Overall sensitivity across all devices was 97.0% (95% CI: 95.8% to 98.2%) (Figures 2-3).
Figure 2. Sensitivity Funnel Plot.
SE: standard error, CI: confidence interval
Figure 3. Sensitivity Forest Plot.
Specificity
The overall specificity between device groups was statistically significant (specificity: 99.02%; P<0.001). Apple devices had an average specificity of 99.61% (95% CI: 98.9% to 100.32%), KB with 81.13% (95% CI: 75.19% to 87.08%), and Samsung devices with 97.96% (95% CI: 96.71% to 99.22%). Overall sensitivity across all devices was 99.02% (95% CI: 98.41% to 99.63%) (Figures 4-5).
Figure 4. Specificity Funnel Plot.
SE: standard error, CI: confidence interval
Figure 5. Specificity Forest Plot.
Quality of evidence
Based on the GRADE approach, the certainty assessment was found to be high for sensitivity and specificity (Table 2). None of the studies compared two devices; each study assessed the accuracy of a single wearable device, which resulted in a serious indirectness assessment. Other considerations include one study from Apple, Inc, which was not published in a peer-reviewed journal. Quality of evidence was found to be satisfactory across all studies [18,19] (Table 3).
Table 2. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Assessment of Quality of Evidence.
CI: confidence interval
| Certainty Assessment | Number of Instances | Relative Effect (95% CI) | Certainty | |||||||||
| Number of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Apple | Apple + KardiaBand | Samsung | |||
| Sensitivity | ||||||||||||
| 9 | Observational Studies | Not serious | Not serious | Serious | Not serious | Not serious | 814 | 368 | 1064 | Apple: 97.23 (95.43, 99.03) versus Apple + KB: 96.94 (94.71-99.16) versus Samsung: 95.47 (93.10, 97.84) | ◯◯◯⨁ HIGH | |
| Specificity | ||||||||||||
| 9 | Observational Studies | Not serious | Not serious | Serious | Not serious | Not serious | 814 | 368 | 1064 | Apple: 92.98 (91.95, 94.02) versus Apple + KB: 68.09 (65.20-70.98) versus Samsung: 93.10 (92.34, 93.87) | ◯◯◯⨁ HIGH | |
Table 3. Newcastle-Ottawa Scale (NOS) for Assessing Quality of Included Studies.
| Author Year | Representativeness of the Cohort | Ascertainment of Exposure | Outcome of Interest | Comparability of Cohorts | Assessment of Outcome | Adequate Follow-up Duration | Adequacy of Follow-up of Cohorts |
| Apple | |||||||
| Seshadri et al. 2020 [20] | * | * | * | N/A | * | * | |
| Apple, Inc 2018 [21] | * | * | * | N/A | * | * | |
| Tison et al. 2018 [22] | * | * | * | N/A | * | * | |
| Apple + KardiaBand | |||||||
| Wasserlauf et al. 2019 [23] | * | * | * | N/A | * | * | |
| Bumgarner et al. 2018 [24] | * | * | * | N/A | * | * | |
| Rajakariar et al. 2020 [25] | * | * | * | N/A | * | * | |
| Samsung | |||||||
| Dorr et al. 2018 [26 ] | * | * | * | N/A | * | * | |
| Ding et al. 2019 [27] | * | * | * | N/A | * | * | |
| Bashar et al. 2019 [28] | * | * | * | N/A | * | * | |
Discussion
Summary of Results
This meta-analysis compared the sensitivity and specificity of three wrist-worn wearable devices, Apple Watch, KardiaBand accessory, and Samsung, in their ability to detect AF. The main finding of this study is that wrist-worn wearable technology offers a sensitive method to detect AF, compared to standard of care telemetry (overall sensitivity: 96.99; CI: 95.77 to 98.20). Our results also indicate that the sensitivity was sustained across all three devices (Apple Watch sensitivity: 97.92, CI: 96.09 to 99.74; KB sensitivity 96.94, CI 94.71 to 99.16; Samsung sensitivity 95.47, CI: 93.10 to 97.840).
However, this research demonstrates that specificity differs significantly between device groups (overall specificity: 99.02%; P<0.001). A specificity funnel plot revealed that there might be some publication bias with regards to the KardiaBand studies. Furthermore, a specificity forest plot revealed a wide confidence interval for one of the included Apple studies. This forest plot also demonstrated that the mean of both KardiaBand studies fell short of the overall average (Figure 4).
Clinical significance and future directions
This study demonstrates clinical significance with regards to specificity between device groups, indicating that there is some discrepancy between how these device groups interpret “normal sinus rhythm” (NSR) or “not normal sinus rhythm.” This type of diagnostic information can add value as a screening tool for patients who are either at risk for AF or patients who have had a stroke and are seeking to understand whether it may have been of cardiac origin.
The majority of the studies included patients with some form of cardiac medical history, such as AF. It is important to test these devices in patients who were never diagnosed with AF. This will provide more accurate information on the potential for these devices to be used as a diagnostic screening tool.
Limitations
There are limitations that should be considered with this study. First, an indirect comparison was performed for this meta-analysis. Second, given the sample sizes, a small number of true negatives and false positives may have influenced the specificity. With this in mind, a number of false positives may have artificially inflated the specificity. Lastly, one of the studies included in this analysis was from Apple, Inc but it was not published in a peer-reviewed journal.
Conclusions
In conclusion, this research demonstrates that wrist-worn wearable devices offer promising results in detecting AF in patients with paroxysmal AF. However, caution is needed in all three devices regarding the use of this technology to detect NSR in patients with and without a history of AF. This research suggests that more rigorous prospective data is needed to understand the limitations of these devices in regard to varying specificities which may lead to unintended downstream medical testing and costs.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Atrial fibrillation: the current epidemic. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Significance and management strategies for patients with asymptomatic atrial fibrillation. Majos E, Dabrowski R. J Atr Fibrillation. 2015;7:1169. doi: 10.4022/jafib.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newly diagnosed atrial fibrillation and acute stroke. The Framingham Study. Lin HJ, Wolf PA, Benjamin EJ, Belanger AJ, D'Agostino RB. Stroke. 1995;26:1527–1530. doi: 10.1161/01.str.26.9.1527. [DOI] [PubMed] [Google Scholar]
- 4.Duration of atrial fibrillation and imminence of stroke: the Framingham study. Wolf PA, Kannel WB, McGee DL, Meeks SL, Bharucha NE, McNamara PM. Stroke. 1983;14:664–667. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- 5.Screening for atrial fibrillation: sensitivity and specificity of a new methodology. Lewis M, Parker D, Weston C, Bowes M. Br J Gen Pract. 2011;61:38–39. doi: 10.3399/bjgp11X548956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. January CT, Wann LS, Alpert JS, et al. Circulation. 2014;130:0–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Meschia JF, Bushnell C, Boden-Albala B, et al. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force Recommendation Statement. Curry SJ, Krist AH, Owens DK, et al. JAMA. 2018;320:478–484. doi: 10.1001/jama.2018.10321. [DOI] [PubMed] [Google Scholar]
- 9.Downsides of detecting atrial fibrillation in asymptomatic patients. Mandrola J, Foy A. https://www.ncbi.nlm.nih.gov/pubmed/30874403. Am Fam Physician. 2019;99:354–355. [PubMed] [Google Scholar]
- 10.Transforming the care of atrial fibrillation with mobile health. Turakhia MP, Kaiser DW. J Interv Card Electrophysiol. 2016;47:45–50. doi: 10.1007/s10840-016-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Effectiveness of screening for atrial fibrillation and its determinants. A meta-analysis. Petryszyn P, Niewinski P, Staniak A, et al. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0213198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US Preventive Services Task Force. Jonas DE, Kahwati LC, Yun JD, Middleton JC, Coker-Schwimmer M, Asher GN. JAMA. 2018;320:485–498. doi: 10.1001/jama.2018.4190. [DOI] [PubMed] [Google Scholar]
- 13.Emerging technologies for identifying atrial fibrillation. Ding EY, Marcus GM, McManus DD. Circ Res. 2020;127:128–142. doi: 10.1161/CIRCRESAHA.119.316342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Data management and wearables in older adults: a systematic review. Alharbi M, Straiton N, Smith S, Neubeck L, Gallagher R. Maturitas. 2019;124:100–110. doi: 10.1016/j.maturitas.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Accuracy of mHealth devices for atrial fibrillation screening: systematic review. Giebel GD, Gissel C. JMIR Mhealth Uhealth. 2019;7:0. doi: 10.2196/13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Ann Intern Med. 2009;151:264–269. [PMC free article] [PubMed] [Google Scholar]
- 17.B. Z. 510(k) Premarket notification for KardiaMobile (K140933) B. Z. 510(k) Premarket Notification for KardiaMobile (K140933). Published online August 2014. https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140933.pdf 2014
- 18.Grading quality of evidence and strength of recommendations. Atkins D, Best D, Briss PA, et al. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Wells G. http://www ohri ca/programs/clinical_epidemiology/oxford asp 2001
- 20.Accuracy of Apple Watch for detection of atrial fibrillation. Seshadri DR, Bittel B, Browsky D, Houghtaling P, Drummond CK, Desai MY, Gillinov AM. Circulation. 2020;141:702–703. doi: 10.1161/CIRCULATIONAHA.119.044126. [DOI] [PubMed] [Google Scholar]
- 21.Using Apple Watch for arrhythmia detection. https://www.apple.com/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf Apple, Inc. Using Apple watch for arrhythmia detection. Published online December. 2018
- 22.Passive detection of atrial fibrillation using a commercially available smartwatch. Tison GH, Sanchez JM, Ballinger B, et al. JAMA Cardiol. 2018;3:409–416. doi: 10.1001/jamacardio.2018.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smartwatch performance for the detection and quantification of atrial fibrillation. Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Circ Arrhythm Electrophysiol. 2019;12:0. doi: 10.1161/CIRCEP.118.006834. [DOI] [PubMed] [Google Scholar]
- 24.Smartwatch algorithm for automated detection of atrial fibrillation. Bumgarner JM, Lambert CT, Hussein AA, et al. J Am Coll Cardiol. 2018;71:2381–2388. doi: 10.1016/j.jacc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Accuracy of a smartwatch based single-lead electrocardiogram device in detection of atrial fibrillation. Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L, Teh AW. Heart. 2020;106:665–670. doi: 10.1136/heartjnl-2019-316004. [DOI] [PubMed] [Google Scholar]
- 26.The WATCH AF Trial: SmartWATCHes for detection of atrial fibrillation. Dörr M, Nohturfft V, Brasier N, et al. JACC Clin Electrophysiol. 2019;5:199–208. doi: 10.1016/j.jacep.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Accuracy and usability of a novel algorithm for detection of irregular pulse using a smartwatch among older adults: observational study. Ding EY, Han D, Whitcomb C, et al. JMIR Cardio. 2019;3:0. doi: 10.2196/13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smartwatch based atrial fibrillation detection from photoplethysmography signals. Bashar SK, Han D, Ding E, Whitcomb C, McManus DD, Chon KH. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:4306–4309. doi: 10.1109/EMBC.2019.8856928. [DOI] [PubMed] [Google Scholar]
- 29.A basic introduction to fixed-effect and random-effects models for meta-analysis. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 30.Metan: fixed- and random-effects meta-analysis. Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC. Stata J. 2008;8:3–28. [Google Scholar]
- 31.Borenstein M, Hedges L, Rothstein H. 2007. Meta-analysis: fixed effect vs. random effects. [DOI] [PubMed] [Google Scholar]