Abstract
Plant functional traits can predict community assembly and ecosystem functioning and are thus widely used in global models of vegetation dynamics and land–climate feedbacks. Still, we lack a global understanding of how land and climate affect plant traits. A previous global analysis of six traits observed two main axes of variation: (1) size variation at the organ and plant level and (2) leaf economics balancing leaf persistence against plant growth potential. The orthogonality of these two axes suggests they are differently influenced by environmental drivers. We find that these axes persist in a global dataset of 17 traits across more than 20,000 species. We find a dominant joint effect of climate and soil on trait variation. Additional independent climate effects are also observed across most traits, whereas independent soil effects are almost exclusively observed for economics traits. Variation in size traits correlates well with a latitudinal gradient related to water or energy limitation. In contrast, variation in economics traits is better explained by interactions of climate with soil fertility. These findings have the potential to improve our understanding of biodiversity patterns and our predictions of climate change impacts on biogeochemical cycles.
Subject terms: Macroecology, Biogeography, Ecophysiology, Plant ecology
The authors investigate the broad-scale climatological and soil properties that co-vary with major axes of plant functional traits. They find that variation in plant size is attributed to latitudinal gradients in water or energy limitation, while variation in leaf economics traits is attributed to both climate and soil fertility including their interaction.
Main
Plant functional traits have proved useful in identifying life history strategies1,2 for predicting plant community assembly3,4 and for assessing the impact of vegetation composition and diversity on ecosystem functioning5,6. Consequently, vegetation models including coupled climate–vegetation models benefit from a better representation of plant trait variation to adequately analyse terrestrial biosphere dynamics under global change6,7. Today, in combination with advanced gap-filling techniques8, databases of plant traits have sufficient coverage to allow quantitative analyses of plant form and function at the global scale9. Analysing six fundamental traits, Díaz and colleagues10 revealed that essential patterns of form and function across the plant kingdom can be captured by two main axes. The first reflects the size spectrum of whole plants and plant organs. The second axis corresponds to the ‘leaf economics spectrum’11 emerging from the necessity for plants to balance leaf persistence against plant growth potential. The concept of a global spectrum of plant form and function has since been investigated from various perspectives12–14. It has been shown, for instance, that orthogonal axes of variation in size and economics traits emerge even in the extreme tundra biome13 or at the scale of plant communities12. However, it remains unclear whether the two axes remain dominant for extended sets of traits or when differentiating among growth forms. A particular knowledge gap is what environmental controls determine these two axes of plant form and function.
There is ample evidence that large-scale variation of individual plant traits is related to environmental gradients. Early plant biogeographers suggested that climate and soils together shape plant form and function15–17 but could not propose a more precise theoretical framework describing these fundamental relationships. Over the last decades, examples have thus accumulated without an overall framework in which to place them13,18,19. For instance, tree height depends on water availability20,21 while leaf economics traits depend on soil properties, especially soil nutrient supply, as well as on climatic conditions reflected in precipitation18,22,23. Leaf size, leaf dark respiration rate, specific leaf area (SLA), leaf N and P concentration, seed size and wood density, all show broad-scale correlations with climate or soil22,24–27. It has also been reported that many of these traits show latitudinal patterns24–27. Generalizing such insights is, however, not trivial, as soil properties partly mirror climate gradients, as a consequence of long-term soil formation through weathering, leaching and accumulation of organic matter—processes related to temperature and precipitation28; however, climate-independent features reflecting geology and surface morphology also contribute to soil fertility28. Soil may furthermore buffer climate stresses; for example, by alleviating water deficit in periods of low precipitation29.
Combining the insights suggests that the global spectrum of plant traits reveals two internally correlated orthogonal groups and that many plant traits are individually linked to environmental gradients, we expect that both trait groups should closely follow gradients of climate and soil properties. Here, we investigate to what extent the major dimensions underpinning the global spectrum of plant form and function can be attributed to global gradients of climate and soil conditions; and to what extent these factors can jointly or independently explain the global spectrum of form and function.
We compiled and analysed a dataset of 17 functional traits with a sufficient number of records in the TRY database9 to characterize the main ecoregions of the world30, that is, environmentally homogeneous areas with distinct biota (Extended Data Fig. 1). The dataset is based on 225,206 georeferenced observations comprising records of 20,655 species. The trait data were complemented with 21 climate variables and 107 soil variables (Methods; Supplementary Tables 1 and 2). Trait–environment relationships were analysed for species medians aggregated to ecoregions using ridge regression31, a robust method (Supplementary Figs. 1–3) suitable to deal with high-dimensional, unbalanced and collinear predictors in combination with hierarchical partitioning32 (Methods).
Extended Data Fig. 1. Map of Ecoregion data.
Map of ecoregions (30) included in this study (n=220).
Results
Our main analysis is based on median trait values of plant species per ecoregion. The rationale is that species presence indicates how the trait space can be realized in a given environment. Spatial aggregation is a suitable means to increase the detectability of global trait patterns (Supplementary Fig. 3), as described in earlier studies, where traits have been binned by temperature classes33 or for different altitudinal ranges22. Extreme outliers, for instance towering trees such as the Californian Sequoia (Sequoiadendron sempervirens), may still exist far away from the equator, where precipitation is sufficiently high20 but their influence is outweighed in our approach by an increasing fraction of small-statured herbaceous species from tropical to temperate and boreal regions.
Orthogonal axes and trait clusters
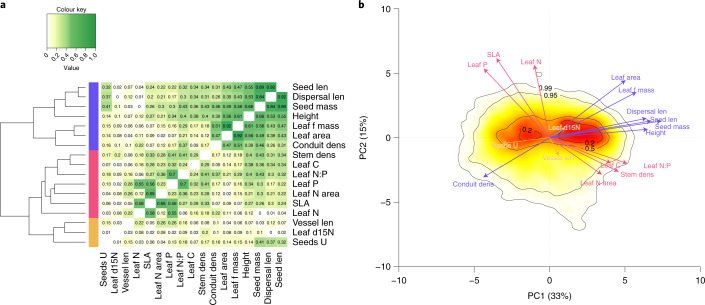
To understand whether the axes of variation identified for the grouping of six traits10 also hold for the extended set of 17 traits, we cluster their trait–trait correlations (Fig. 1a and Supplementary Fig. 4) and further represent these relations on the basis of their principal components (PCA; Methods). This analysis supports the clear distinction of size versus economics traits identified by Díaz and colleagues10. The group of size traits contains two subclusters. The first includes height and seed size traits: plant height (height), seed mass, seed length and dispersal unit length (dispersal length). The second subset contains traits that are linked through plant hydraulic scaling relationships34 and contrasts high conduit density (that is, number of conduits per sapwood cross-sectional area) with high leaf area and leaf fresh mass (leaf f mass). Economics traits represent dry mass and nutrient investments in plant tissues, and the rate and duration of returns on those investments11. They are represented by leaf nitrogen content per leaf area (leaf N area), leaf nitrogen (leaf N), phosphorus (leaf P) and carbon (leaf C) content per dry mass, leaf N to P ratio (leaf N:P) and SLA. Stem specific density (stem density) takes an intermediate position (Fig. 1b) but more closely clusters with this set of economics traits (Fig. 1a), suggesting a syndrome of traits promoting slow to fast nutrient and carbon processing at the whole-plant level35–37. Furthermore, we identify a third group of traits that appear to be only weakly correlated with any other trait. This third group contains seed number per reproduction unit (seeds U), leaf δ15N (leaf d15N) and vessel element length (vessel length). The first two principal components (PC) of the PCA on the trait data represent 48% of the overall variation (Supplementary Fig. 5). PC1 is determined by size traits and accounts for 33% of the variance; PC2 is determined by economics traits and accounts for 15% of the variance (Fig. 1b and Supplementary Fig. 6). These two main axes remain clearly identifiable when the analysis is conducted separately for woody and non-woody species (Supplementary Figs. 7 and 8). The remaining PCs each account for less than 10% of variance (PC3 = 9.36%). In the following, we focus on the two groups of size and economics traits (Supplementary Fig. 5).
Fig. 1. Previously identified global axes of variation in size and economics traits hold for an extended trait set (n = 36,197 species per ecoregion median).
The set of 17 investigated traits (Supplementary Table 5) can be primarily divided into size and economics traits, which load differently onto the two PC axes describing their global distribution. a, Heatmap of covariation. Trait correlations are indicated using absolute Pearson correlation coefficients, with green shades indicating high absolute correlation and yellow shades indicating low absolute correlation. On the left, the distance tree of traits derived from hierarchical clustering is illustrated. Three resulting groups are: (1) size-related traits (blue) consisting of conduit density (conduit dens), leaf area, leaf fresh mass (leaf f mass), plant height (height), seed mass, dispersal unit length (dispersal len) and seed length (seed len); (2) economics traits (red) comprising SLA, leaf N content per area (leaf N area), leaf N, P and C concentrations, leaf N/P ratio (leaf N:P) and stem specific density (stem dens); and (3) a third (yellow) consisting of the number of seeds per reproduction unit (seeds U), leaf δ15N (leaf d15N) and vessel element length (vessel len). b, The first two PCs of the PCA. Arrow tips refer to the loading of the traits (Supplementary Fig. 6). Contour lines delineate the colour scale that corresponds to the kernel density of species (dense, red to sparse, light yellow; 20%, 50%, 95% and 99% of all species). PC1 explains 33% of trait variation and PC2 15% (Supplementary Fig. 5).
Latitudinal trait variation
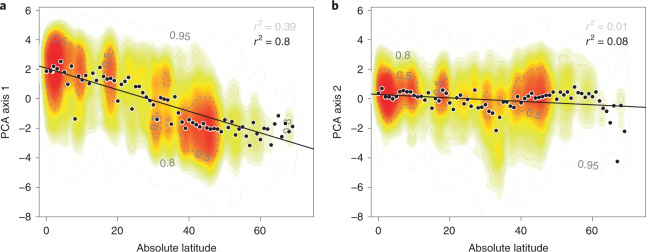
As an investigation of broad-scale gradients among size and economics traits, we analyse latitudinal gradients of the first (PC1) and second (PC2) principal components. PC1—representing primarily size-related traits—shows a strong linear latitudinal signal (on the basis of species: r2(PC1) = 0.37, at the ecoregion level r2(PC1aggregated) = 0.84; Fig. 2a). By contrast, the axis representing primarily economic traits, PC2, shows little response to latitude (on the basis of species: r2(PC2) = 0.01, at the ecoregion level r2(PC2aggregated) = 0.08; Fig. 2b, for woody non-woody species Supplementary Fig. 9), except for a dip at 35° and declining sharply at 60° where the species density also drops (but see Supplementary Fig. 10 for comparison to an independent dataset from arctic latitudes which shows the same pattern). Latitudinal gradients are known to be strongly related to climate, due to the distribution of solar energy and general atmospheric circulation patterns. Therefore, we propose that those climate (and soil) aspects that co-vary with latitude consistently determine size traits, while they have little effect on economics traits, which are more strongly affected by latitude-independent soil (and climate) effects (Supplementary Fig. 11).
Fig. 2. Size traits, not economics traits vary with latitude: the PC1 of the PCA on 17 plant traits shows a clear latitudinal gradient while PC2 does not (n = 36,197 species per ecoregion median).
Contour lines delineate the colour scale that corresponds to the kernel density of species (dense, red to sparse, light yellow; 5%, 95%, 99% quantiles). Mean estimates aggregated at 1° absolute latitude are indicated as black dots. The line refers to a linear model (ordinary least squares). a, PC1 representing mainly size traits (conduit density, leaf area, leaf fresh mass, plant height, seed mass, dispersal unit length, seed length) regressed against absolute latitude. Linear model: r2 = 0.38 without bins; r2 = 0.84 aggregated at 1° absolute latitude. b, PC2 representing mainly economics traits (leaf N, leaf N per area, leaf P, leaf N:P ratio, SLA, leaf C, stem density) regressed against absolute latitude. Linear model: r2 = 0.01 without bins; r2 = 0.08 aggregated at 1° absolute latitude.
Climate and soil: joint and independent effects
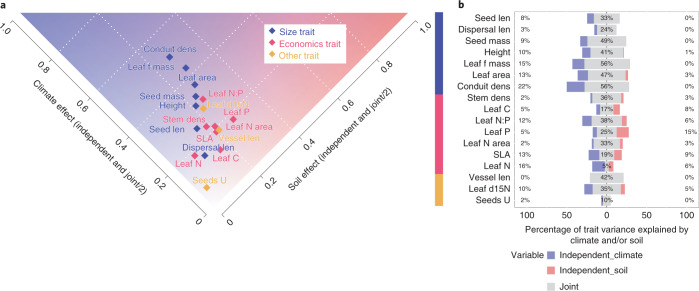
The differences in latitudinal relationships between the two PC axes support the hypothesis that different environmental factors should drive variation within the separate groups of size versus economics traits. We assess the joint and independent effects of climate and soil on trait variability (ridge regression, RR; Table 1 and Fig. 3). Overall, size traits are better explained (RR; r2 = 0.55; maximum r2 = 0.78 for conduit density; Table 1) than are economics traits (RR; r2 = 0.40; maximum r2 = 0.55 for leaf N:P ratio; Table 1). We find a substantial joint effect of climate and soil variables—in every case larger than either unique effect—which reflects strong interactions between specific climate and soil predictors (RR with hierarchical partitioning (HP); Fig. 3b and Supplementary Fig. 12). However, we also observe independent effects of climate and soil (RR with HP; Fig. 3 and Table 1). The independent climate effects are observed across traits but size traits tend to be better explained by the independent climate effects than are economics traits. In contrast, independent soil predictors are relevant for all economics traits but not size traits (apart from a small contribution to leaf area). We interpret these results as evidence for the importance of both joint and independent effects of climate and soil variables for whole-plant strategies2,37,38 which we show here at the global scale along with a dichotomous tendency of a stronger imprint of climate factors on size traits and of soil conditions on economics traits (Fig. 3, Supplementary Figs. 13–38 and Supplementary Table 3). We propose that the dominance of joint effects implies that interactions between soil and climate properties are of primary importance in plant trait ecology; as opposed to trait syndromes being defined by single environmental variables in isolation.
Table 1.
Showing for each trait the variance explained (r2) by ridge regression models for 220 ecoregions and the independent effects for climate and soil listed from hierarchical partitioning that, respectively, add up with the joint effect to the variance explained by climate or soil
| Traita | Group | Explained varianceb by soil and climate (r2) | Soil (independent effectb) (r2) | Climate (independent effectb) (r2) | Joint effectb (r2) |
|---|---|---|---|---|---|
| Ridge regression model | Hierarchical partitioning | Hierarchical partitioning | Hierarchical partitioning | ||
| Seed length | Size | 0.4 | –0.01 | 0.08 | 0.33 |
| Dispersal length | Size | 0.26 | –0.01 | 0.03 | 0.24 |
| Seed mass | Size | 0.57 | –0.01 | 0.09 | 0.49 |
| Height | Size | 0.52 | 0.01 | 0.1 | 0.41 |
| Leaf f mass | Size | 0.72 | 0 | 0.15 | 0.56 |
| Leaf area | Size | 0.63 | 0.03 | 0.13 | 0.47 |
| Conduit density | Size | 0.77 | –0.01 | 0.22 | 0.56 |
| Stem density | Economics | 0.41 | 0.03 | 0.02 | 0.36 |
| Leaf C | Economics | 0.29 | 0.08 | 0.05 | 0.17 |
| Leaf N:P | Economics | 0.55 | 0.06 | 0.12 | 0.38 |
| Leaf P | Economics | 0.45 | 0.15 | 0.05 | 0.25 |
| Leaf N area | Economics | 0.39 | 0.03 | 0.02 | 0.33 |
| SLA | Economics | 0.41 | 0.09 | 0.13 | 0.19 |
| Leaf N | Economics | 0.26 | 0.06 | 0.16 | 0.05 |
| Vessel length | Other | 0.4 | 0 | –0.03 | 0.42 |
| Leaf d15N | Other | 0.51 | 0.05 | 0.1 | 0.35 |
| Seeds U | Other | 0.1 | –0.02 | 0.02 | 0.1 |
aFor full versions of traits, see main text. Mean values; minimum and maximum values from different cross-validation runs in Supplementary Table 8. Negative values indicate a reduction of explained variance when respective variables are added to the ridge regression model.
Fig. 3. Climate and soil variables explain up to 77% of variance in size and economics traits.
Hierarchical partitioning32 identifies the contribution of climate and soil variables to explain each trait (n = 220, ecoregional median trait: blue, size; red, economics; yellow, other). The joint effect is the fraction explained by both climate and soil together, and is split equally among them. The independent effect is the fraction of r2 explained exclusively by either soil or climate variables. a, Tilted x–y plot of the soil versus climate variables to explain a trait. The axes show the sum of the respective joint and independent effect (hierarchical partitioning). The colours reflect the strength of: the independent effect of climate plus its share of the joint effect (r2; purple); and soils' independent effect plus its share of the joint effect (peach). The sum of both axes equals the total r2 explained by climate and soil; in cases where soil showed a negative independent effect only the climate-independent effect is shown (and vice versa but see Table 1). b, Percentage variation explained by climate (purple, percentages on the left), soil (peach, percentages on the right) and jointly (grey, percentages in the middle) for trait groups— size, economics and other. Total bar length = total r2 explained by climate and soil; in cases where soil showed a negative independent effect only the climate-independent effect is shown (and vice versa but see Table 1). For leaf area, climate and soil jointly explain 47%, the independent climate effect explains an additional 13% of the variance, while soil explains 3%, totalling 63% of variance explained. For trait abbreviations see Fig. 1.
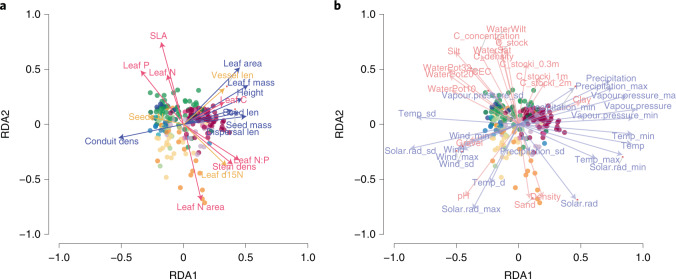
We next ask how the climate and soil datasets are interdependent and which predictors add the most relevant information. For this purpose, we related all traits to environmental variables in a redundancy analysis (RDA; Methods; Fig. 4). The RDA again identifies two main axes of size and economics traits (Fig. 4a), which are now shown together with the environmental variables that co-vary linearly with those traits (Fig. 4b and Supplementary Fig. 39). The first RDA axis corresponds to size traits (Fig. 4a) and represents an axis of water and energy (for example, precipitation, vapour pressure and temperature; Fig. 4b). Two attributes of soil texture important for water retention—the fraction of gravel and clay—also vary along this axis. The second RDA axis corresponds to economics traits (Fig. 4a) co-varying with an axis of soil variables generally associated with soil fertility (that is, soil texture (silt versus sand), water holding capacity, carbon concentration and stocks), as well as the climate variable mean solar radiation (Fig. 4b).
Fig. 4. RDA of traits reveals the relationships of climate and soil factors associated with trait distributions (n = 220, ecoregion median, only topsoil layer variables included; variance explained: RDA1 = 63%, RDA2 = 18%).
a,b, The output of the RDA is split into two plots: traits (a), where arrows are coloured according to trait groups (blue, size traits; red, economics traits; yellow, other traits; arrow length and point positions scaled to fit the plot); and environmental factors (b), where arrows are coloured according to predictor group (climate, blue and soil, red variables; arrow length and point positions scaled to fit the plot). In a and b, points represent ecoregions and are coloured according to biome (red, tropics; green, temperate; yellow, desert; orange, Mediterranean; dark blue, tundra). Climate variable abbreviations are composed of the variable (average if not stated differently) and a suffix. Variables are: Solar.rad, solar radiation; Vapour.pressure, vapour pressure; Wind, average wind speed; Temp, temperature; Precipitation, precipitation. Suffixes are: no suffix, mean of respective variable; d, diurnal range; min, annual minimum of the respective variable; sd, seasonality of respective variable. Soil variable abbreviations (all topsoil) are: Density, soil density (kg/m3); pH, pH value; Sand, sand fraction (vol%); Silt, silt fraction (vol%); Clay, clay fraction (vol%); C_concentration, organic carbon concentration; C_density, organic carbon density; CEC, cation exchange capacity; C_stock, soil organic carbon stock at depth 0.00 m; C_stocki, organic carbon stock for depth intervals—0.3m (0–0.30 m), 1m (0–1 m), 2m (0–2 m); WaterWilt, available soil water capacity (volumetric fraction) until wilting point; WaterSat, saturated water content; WaterPot, available soil water capacity for moisture potentials—10 (–10 kPa;pF 2.0), 20 (–20 kPa;pF 2.3), 32 (–31.6 kPa;pF 2.5). For trait abbreviations see Fig. 1.
Discussion
This study shows that the proposed global spectrum of plant form and function fits well to a substantially extended trait space compared to the original study10, with seven traits that capture the whole-plant size spectrum and seven traits that capture the leaf economic spectrum and only three traits that do not fall along these dimensions (Fig. 1b). One explanation could be that the varying fraction of woody and non-woody species would drive these patterns. However, we showed that these two main trait groups remain clearly identifiable when the analysis is conducted separately, yet with fewer samples, for woody and non-woody species (Supplementary Fig. 8).
However, we cannot discard the possibility that additional traits may add relevant axes of trait variation. For example, our study does not include carbon fixation rates39 or fire adaptation traits40, nor does it include any root traits—representing an essential gap to be filled at the global scale41. The respective data are too scarce to yet be integrated with global datasets. If such data were available they would have the potential to fundamentally change our perception of global plant form and function, and their relation to ecosystem functioning.
Variation in size traits, represented by PC1 in Fig. 1b, shows a clear latitudinal gradient (Fig. 2b). In contrast, variation in economics traits (represented by PC2) does not show a latitudinal trend. Only a dip is apparent at around 35° (absolute), in addition to a decrease at high latitudes above 60° (absolute) where available data become increasingly limited. However, comparison to a recent arctic dataset indicates that this decrease in variation at high latitudes reflects available observations (see Supplementary Fig. 10 for a comparison to independent data). These patterns might represent a response to nutrient limitation and drought42,43 in water-scarce and nutrient-scarce deserts and Mediterranean regions (Supplementary Fig. 40) or boreal and arctic areas characterized by short growing periods slowing down mineralization. The dip at ~35° indeed can be related to low water availability (Supplementary Fig. 41). At high latitudes, cold winters and short growing seasons constrain plant height13 and require on average more conservative nutrient-use strategies (like evergreen leaves) and protection against frost damage than the global mean, despite the high functional diversity in economics traits observed at these latitudes13. Additional datasets may shed more light on specific conditions, for example see Bjorkman et al.19. Future studies should quantify how individual stressors, for example radiative stress or water stress, relate to global patterns of trait variation.
The climate and soil factors used in this analysis explain up to 77% of observed trait variation—a high fraction given that trait variation is widely known to be determined also by other factors such as biotic interactions (for example, soil biota) and anthropogenic effects or disturbances and local effects such as those of microclimate12,44–46. Recent findings on how different trait groups vary with the environment indicate that size and economics traits vary differently13 and in particular respond differently to climate and soil19.
Our analyses reveal a dominant joint effect of climate and soil drivers on trait variation—as already suggested by a number of earlier studies18,19,22 but not yet quantified globally.
The orthogonality of the two main dimensions of plant trait variation suggests that different aspects of climate and soil variables are relevant to explain plant trait patterns at the global scale (Supplementary Figs. 11–39). While latitude-related variables (mainly climate) explain size traits, variables that share less explanatory power with latitude (mainly soil) explain economics traits (Supplementary Table 4 and Supplementary Fig. 11). The RDA presented in Fig. 4 (Supplementary Fig. 39) provides some insight on the nature of these climate–soil interactions. The first RDA axis, which describes variation in size traits, resembles a latitudinal gradient. On one extreme end, ample water supply from high and frequent precipitation, abundant water vapour and constant rates of high solar radiation meet the fundamental requirements of plant physiology—water, sunlight and warm temperatures. Additionally, these conditions promote weathering of soil minerals but also microbial activity, contributing to fast turnover rates of organic matter supporting nutrient provisioning28,47; in brief, they represent conditions that allow plants to grow fast and tall in the race for light. Large vessels supporting large leaves promote high rates of water transport and thus growth, which is only possible because of the small risk of embolism under these benign water conditions43. The high carbon gains can be invested in large fruits and seeds (seed mass, seed length and dispersal unit length). Further along this gradient, the above-mentioned plant requirements become limited: water supply and temperatures are reduced and slow metabolic rates aboveground and belowground. In ecoregions of the boreal and desert biomes, conduit diameter is constrained by the risk of cavitation during freeze–thaw cycles43 and water scarcity, amplified by little water holding capacity of gravel-rich soils. Our analysis thus indicates that size traits appear to be related to a latitudinal gradient of climatic favorability for plant growth determined by water and light availability.
Important correlates of water and nutrient availability are associated with the second RDA axis, describing variation in economics traits. Traits associated with an acquisitive strategy are related to indicators of soil fertility, most importantly silt and organic matter concentration as well as pH (refs. 18,28). Soil pH is intermediate between the two axes, as might be expected given that pH reflects both broad-scale climate variation (especially aridity47) and a variety of processes related to nutrient availability and soil microbial communities18,48–50. Silt forms the substrate of our most fertile soils as its structure is able to retain water against gravitation (unlike sand) but renders it accessible to plants under drought conditions28,51 (unlike clay). The high fertility is associated with a high concentration of organic matter, which has a high cation exchange capacity especially under high pH (ref. 47). On the opposite end of the gradient, sandy soils require adaptations to both water and nutrient limitation. The trait configuration at the conservative end of the economics traits (low SLA, high tissue density and high organ longevity) represents an adaptation to both11,37. Various processes exist that lead to variation in the soil characteristics underlying the second RDA axis independent of latitude18—for example, sandstone as a geological substrate giving rise to sandy soils exists from the tropics to the arctic28,51. However, different climate variables related to solar radiation, temperature and precipitation, which influence long- and short-term soil development processes directly and indirectly via soil biology28,51,52, are related to this axis. Variation in economic traits is most probably the evolutionary response to exploiting this partly climate-independent edaphic niche axis.
Size traits are on average explained better than economics traits by the environmental variables considered in this study. The lower fraction of explained variance for economics traits could have several causes. Firstly, data on soil factors that are likely to be very important, such as soil nitrogen and phosphorus availability18,23, are not yet available at a global scale. Secondly, economics traits show relatively more within-site variation than across-site variation in comparison to size traits (Supplementary Fig. 42), probably because economics traits vary more than size traits within one plant; for example, leaf N per area and SLA vary with age and light availability53. Thirdly, soil heterogeneity within ecoregions—both abiotic and biotic—may weaken the relationship between economics traits and environmental variables12,54,55. Reasons for small-scale soil variation are, for example, topography, soil age and thus fertility56 but also abundance of microbial communities and mycorrhiza that interact with climate, pH, soil properties and also plant traits50. Trait–environment relationships due to smaller scale variation require well-resolved soil data. However, we note that soil physics and chemistry explain a large portion of variance along the trait PC axis three (which itself explains slightly less than 10% of variance in the PCA (9.36%); Supplementary Figs. 5, 6 and 38). We expect that with improved soil datasets and a higher resolution, the joint control of climate and soil on trait variation will probably appear even stronger and more evenly distributed between the two groups of driver variables.
Our analysis can serve as reference for model developments that increasingly consider plant functional traits as part of vegetation dynamics under climate change44.
Individual plants and their trait syndromes are considered to be viable only within specific environmental conditions2. Therefore trait–environment relationships should be scale-independent. However, different plant strategies can be successful under given environmental conditions, which in addition are often confounded by small-scale variation. In analyses to date, trait–environment relationships become more apparent for aggregations higher than the community scale12, where most of the small-scale variation is averaged out. In addition the difference between potential and actual vegetation is suggested to explain some of this gap13. Dynamic global vegetation models predict individual plant processes well but fail to produce reliable forecasts with a changing environment44. Deciphering at which spatial and temporal scale, or conditions, actual vegetation is representative of potential vegetation may advance our understanding of community assembly and necessary model complexity.
Trait–environment correlations identified in our study should not be confounded with causality. Yet, the ubiquitous importance of climate variables for explaining current differences in trait expression at ecoregion scale, suggests that trait shifts will occur with climate change. Trait shifts are constrained by available trait combinations in addition to other constraints such as species dispersal. For example, our results indicate that plant size increases with temperature so long as sufficient water is available (Fig. 4 and Supplementary Figs. 19, 20 and 21), in line with the finding that species become larger and large species are more prevalent at warmer and wetter sites in the tundra19. Global change is also reflected by soil degradation. Changes in soil parameters can be considered to also correspond with trait shifts, especially for economics traits. Human-induced soil degradation has many facets: often fertile topsoil is lost or toxic substances accumulate; rooting is impeded and altered by artificial fertilizers; while soil formation takes millenia57. The trait shifts may thus be similarly complex and depend on the extent and type of soil degradation. For example, in areas of wind and water erosion, species that tolerate lower nutrient availability may be more successful and this may be reflected in lower leaf nutrient contents (Fig. 4 and Supplementary Fig. 30). The fertilization of nutrient-poor grasslands, for example resulting from agricultural run-off, may shift these areas from more conservative to more competitive species with higher leaf nutrient contents.
Plants as a whole need to balance both size and economics traits. To sustain human livelihoods, it may be important to understand the local expression of trait shifts and their global consequences for biodiversity when viable trait combinations change.
In conclusion, the insights extracted here advance our understanding of broad-scale plant functional patterns. In particular, we highlight the combination of independent and particularly joint effects of climate and soil on trait variation, an interaction that has to date been neglected because few studies include both in a single analysis, at the global scale as we have done here. In doing so, we identify an important gap in knowledge: what is the nature of climate–soil interactions that drive whole-plant trait variation and what distinguishes the majority of climate and soil factors having joint effects on plant traits from those with independent effects? These are the sorts of questions that require answers to increase our capacity to predict plant functional diversity in a changing environment. Such predictive power would contribute to a sound basis for assessing long-term feedbacks between global environmental change and the terrestrial biosphere, helping to constrain parameters of global coupled climate–vegetation models. Humans are currently modifying both climatic and edaphic conditions at the global scale. Climate envelope models used to predict vegetation shifts must be complemented by drivers related to large-scale anthropogenic alterations of soil conditions resulting, for example, from land-use change, atmospheric nitrogen deposition, fertilization, liming and salinization. Our global analysis provides an essential context for finer-scale studies to directly tackle questions of biological processes and mechanisms at landscape and community scales.
Methods
We extracted data on 17 plant functional traits from a gap-filled version of TRY database9 (Supplementary Table 5; www.try-db.org, accession date July 2017, request no. 3282) which includes published literature11,58–101,101–310. Quality control was conducted according to the published protocol of TRY.9, 311 Traits with z-score > 4 were excluded and those with z-score > 3 were checked for plausibility. Before this, missing data were imputed using a Bayesian hierarchical probabilistic matrix factorization (BHPMF) algorithm8,312 for an extended dataset, derived from TRY (Supplementary Table 6). Imputation was done to be able to include the maximum number of species in our analyses. Then the 17 traits were selected among the traits with the largest total number of entries. The data were attributed to ecoregions30 (Supplementary Table 7 and Extended Data Fig. 1) and aggregated to species median values. The imputed values were calculated using the whole dataset at the individual record level. BHPMF calculates the imputations from 1,000 Gibbs sampler (Markov chain Monte Carlo) imputations by taking the mean of every twentieth imputation of these 1,000 ‘versions’, after the first 200 are removed. Then the species median was calculated at the ecoregion level. We excluded observations that were not georeferenced because we could not attribute them to ecoregions. According to TRY regulations, data from experimental treatments (for example, fertilization) or from botanic gardens were also excluded. In total, we included 225,206 observations from 20,655 global unique species (36,197 unique species to ecoregion combinations). Throughout this study we used one of two aggregation levels: either species median per ecoregion (ER)30 resulting in unique species values per ecoregion (termed A1, n = 36,197 with n = 20,655 globally unique species) or the aggregation to median ecoregions calculated from median species per ecoregion (termed A2, n = 220). R was used for all analyses and figures313.
Hierarchical probabilistic matrix factorization
Description
BHPMF decomposes or factorizes probabilistically a matrix (probabilistic matrix factorization, PMF314) using information contained within different hierarchical levels (here, taxonomy) within a Bayesian framework8. The underlying premise of BHPMF is to gap-fill (or more accurately, to predict) traits of an individual plant using trait–trait correlations as well as intraspecific and interspecific trait variability.8. Using a Gibbs sampler (a Markov Chain Monte Carlo algorithm), BHPMF also provides a prediction confidence in the form of standard deviations which is a per-value estimate of uncertainty in trait predictions8. BHPMF can fill gaps if there is at least one value per row (species) and column (trait).
Implementation
The largest possible dataset was retrieved at the time when study was conducted, including 172 traits of 652,957 individuals (Supplementary Table 6). For data preparation before BHPMF, all individual-level trait data were firstly log-transformed and secondly normalized via zlog transformation (). Log transformation was chosen to achieve a closer-to-normal distribution of values per trait311,313. This transformation is considered necessary because a given difference for small trait values (absolute value) is likely to be physiologically more relevant than the same difference (absolute value) for large trait values.
BHPMF internally splits the datasets randomly into a training dataset (80%), a test dataset (10%) and a validation dataset (10%).
The training dataset is used during training of latent vectors, while the test data are tested against to improve the latent vectors, and finally the validation dataset serves as the basis for calculation of the root mean square error (RMSE) and stopping the optimization of latent vectors within BHPMF8. The validation dataset ensures ongoing amelioration of the model performance during the training process and stops the process after five consecutive iterations with stable RMSE. The test dataset is used only on the lowest taxonomic level (individuals × traits). BHPMF was run with a maximum of 1,000 iterations, whereas the first 200 were discarded during the ‘burn-in’ phase, as predictions of these iterations are likely to be influenced by the initialization of BHPMF rather than being part of the probability density distribution to be sampled by BHPMF. To avoid autocorrelation, only every twentieth iteration was used to calculate the resulting trait values. The mean of these predictions result in the final trait values used as the output. Compared to the original data, the imputed values are similar in terms of trait–trait correlation, according to the Procrustes test provided in ref. 10.
Trait clustering
To define groups of correlated traits, we clustered species’ traits (species median per ecoregion, A1) on the basis of absolute pairwise Pearson correlation coefficients using a hierarchical clustering algorithm (‘complete linkage clustering’). Variables were transformed into distances previous to the clustering. Hierarchical clustering then attributes variables (here, traits) to groups of least distance and highest similarity. Traits were more like each other if they exhibited similar correlation patterns with all other traits. We set a distance between traits of 1 as the threshold for defining trait clusters. We used the R package ‘stats’ function ‘hclust’ included in R (ref. 315).
PCA
Values for all 17 traits (unique species per ecoregion, A1) were natural log-transformed and then projected onto components (PCA). We used the R package FactoMineR316 that scales data internally. After the PCA (A1), we extracted the variance explained (Fig. 1b and Supplementary Fig. 5) and respective loadings for the first five principle components (Fig. 1b and Supplementary Fig. 6), which are significant according to the number of axes to keep estimated using a sequential Bonferroni procedure (R package ade4 (refs. 317–321), function testdim). For the analysis (ridge regression package ‘glmnet’322,323) for Fig. 3, all environmental variables (climate and soil) were first reduced with this package to 20 PCs.
Environmental variables
To represent climate conditions we used 21 variables derived from WorldClim at a resolution of 1 km for temperature, precipitation, vapour pressure, solar radiation and wind (Supplementary Table 1). To characterize soil conditions we used 107 variables derived from the ISRIC data product ‘SoilGrids’324–326 (https://soilgrids.org/ through ISRIC—WDC Soils). ‘SoilGrids’ provides global predictions of 17 fundamentally different soil characteristics (some for seven depths, that is 0, 5, 15, 30, 60, 100, 200 cm; Supplementary Table 2) at a resolution of 1 km. SoilGrids are publicly accessible environmental data (Creative Commons Attribution 4.0 International), with a collection of georeferenced soil profile data and are managed in World Soil Information Service324.
Aggregation of traits and environmental variables to ecoregions
To determine trait–environment relationships, we aggregated trait as well as environmental data to regions, here ecoregions30 (ecoregion aggregation A2, see also above; Supplementary Table 7). Ecoregions are environmentally homogeneous areas, nested within biogeographic realms (defined by refs. 327,328) and biomes (modified after refs. 329,330 but see ref. 30). As a first estimate, ecoregions are distinct biotas328,331 defined by the physiognomy of the prevailing climatic climax vegetation30. These areas of distinct biotas, areas of relatively uniform flora or fauna, are next subset into provinces with substantial differences of vegetation on the basis of a selection of plants and animals, maps and expert knowledge30,331. At global scale Olson et al.30 defined 867 ecoregions. Ecoregions were chosen as the scale of aggregation for their high signal-to-noise ratio and the ability to correct for sampling bias. While the grid scale has higher spatial resolution, it lacks estimates of species richness (equivalent of Kier species richness174) and is not as well explained by the climate and soil (Supplementary Fig. 3) and distribution of grids is globally uneven (Extended Data Fig. 1) in comparison to ecoregions. The global sampling distribution is recognized to show a bias towards Europe9, which is even more pronounced in the lower level data (grid scale) than in the more aggregated one (Extended Data Fig. 1). Our method accounts for this oversampling and reproduces a stable pattern, even when species in oversampled ecoregions are deleted (Supplementary Fig. 2).
For each of the 867 ecoregions, we calculated the median ecoregion aggregate trait value from the median trait values of all species identified in each region. For further analyses, we only used regions with >20 species and a representation of >1% of the estimated species richness of the ecoregion30. Preliminary tests with different selection criteria (for example, number of species and inclusion or exclusion of 1% of species richness estimate by Kier et al.174) showed that lower numbers of species per ecoregion result in weaker explained variance, while stricter rules reduced the number of ecoregions. These selection criteria serve as a quality control because ecoregions with poor representation of species richness are excluded, as we can expect the regression to the mean to be stronger with more species data. A total of 220 ecoregions met these criteria and were included in the analysis. On average, these ecoregion-level trait values were based on 164 species-level trait medians (with a maximum of 1,245 species in Tapajós-Xingu moist forests; Supplementary Table 7). In total, we aggregated 36,197 median species trait values to ecoregions. These ecoregions cover the global latitudinal gradient (Fig. 2) as well as a substantial fraction of the geographic space (Extended Data Fig. 1). To aggregate environmental variables to ecoregions, we associated each trait observation with its corresponding values of climate and soil variables. Then, we averaged over all values within one ecoregion. Thus, the selected environmental variables represent averages that are weighted by the number and locations of trait observations within ecoregions.
Model building
Ecoregion trait values (natural log-transformed, A2) were related to all environmental variables using ridge regression31, which is a well-established linear regression method that is suitable to deal with a large number of collinear predictors and uneven numbers of predictors for climate and soil. We used the R package ‘glmnet’322,323. From aggregating trait values to ecoregion medians we obtain 220 samples for each trait. The environmental predictors of climate and soil were reduced to 20 each by means of a PCA. In addition, the environmental predictors show relatively high collinearity, thus duplicated information. Ridge regression addresses collinearity among predictors by shrinking (regularizing) regression coefficients according to a penalty on the L2 norm of the vector of regression coefficients. The regularization parameter lambda was obtained via tenfold cross validation. The variance explained was derived from an iterative holdout set (tenfold cross validation), that is, prediction of 90% of randomly sampled ecoregions for inclusion in model building and then predicting the remaining 10% of the data to evaluate the quality of the models. The final model predicts the remaining 10% of unused ecoregions. This prediction-loop was repeated until all ER trait values are predicted, that is, resulting in different linear models. Repeated r2: the r2 is the squared correlation of predicted versus original ER trait values. This procedure was repeated 50 times and the explained variances’ (r2) mean, minimum and maximum were calculated. For the purpose of defining how much of the explained variance is due to independent and joint information in the data streams, we used hierarchical partitioning32. Model outputs (r2) of all repetitions (n = 50, if not indicated differently) were used as input (ridge regression, partial least squares (PLS) with and without PCA, random forest).
Redundancy analysis
To relate trait–trait covariation to trait–environment covariation, we performed a redundancy analysis (R package ‘vegan’). Ecoregion-aggregated traits (A2) were normalized and natural log-transformed. Scaled climate and soil variables were used as predictors. To decrease the factor that quantifies collinearity (variance inflation factor, vif), only the topsoil layer was selected (Fig. 4). For Supplementary Fig. 39, additional model tuning based on vif, with the exclusion of two variables with vif > 20, led to a model with vif < 10, which can be considered low cocorrelation.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary Figs. 1–42 and Tables 1–8.
Acknowledgements
The study was supported by the TRY initiative on plant traits (http://www.try-db.org). The TRY database is hosted at the Max Planck Institute for Biogeochemistry (MPI BGC, Germany) and supported by Future Earth and the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig. We would like to thank all PIs contributing to the TRY database, whose efforts allowed this analysis. In detail, we thank: J.H.C. Cornelissen, R. Milla, W. Cornwell, K. Kramer, S. Gachet, Ingolf Kühn, P. Poschlod, M. Scherer, J. Pausas, B. Sandal, K. Verheyen, J. Penuelas, N. Soudzilovskaia, P. Reich, J. Fang, S. Harrison, R. Gallagher, B. Hawkins, B. Finegan, J. Powers, F. Lenti, S. Higgins, B. Medlyn, H. Ford, V. Pillar, M. Bahn, E. Sosinski, T. He, B. Cerabolini, J. Cavender-Bares, I. J. Wright, F. Louault, B. Amiaud, G. Gonzalez-Melo, P. Adler, F. Schurr, J. Craine, Y. Niinemets, A. Zanne, H. Jactel, M. Harze, R. Montgomery, C. Römermann, T. Hickler, A. Pahl, M. Dainese, D. Kirkup, J. Dickie, W. Hattingh, P. Higuchi, T. Domingues, A. Araujo, M. Williams, C. Price, B. Shipley, L. Sack, B. Schamp, W. Han, Y. Onoda, K. Fleischer, J.P. Wright, G. Guerin, F. de Vries, D.D. Baldocchi, J. Kattge, B. Blonder, K. Brown, D. Campetella, G. Frechet, Q. Read, N. G. Swenson, V. Lanta, E. Weiher, M. Leishman, A. Siefert, M. Spasojevic, R. Jackson, J. Messier, S. J. Wright, D. Craven, J. Molofsky, P. Meir, E. Forey, A. Totte, C. Frenette Dussault, O. Atkin, F. Koike, D. Laughlin, S. Burrascano, K. Ollerer, N. Gross, A. Madhur, P. Begonna, B. Bond-Lamberty, B. von Holle, W. Green, B. Yguel, A. C. Malhado, P. Manning, G. Zotz, E. Lamb, J. Fagundez, Z. Wang, S. Diaz, C. Byun, W. Bond, B. Enquist, C. Baraloto, P. Manning, M. Kleyer, W. Ozinga, J. Ordonez, J. Lloyd, H. Poorter, E. Garnier, F. Valladares, C. Pladevall, G. Freschet, M. Moretti, H. Kurokawa, V. Minden, A. Demey, F. Férnandez-Méndez, J. Butterfield, T. Domingu, E. Swaine, L. Poorter, S. Shiodera, T. Chapin, M. Beckmann, J.A. Gutierrez, M. Mencuccini, S. Jansen, and N. J. B. Kraft. We appreciate the discussions at the MPI BGC. We thank F. Fazayeli for preparing the gap-filled trait data. We thank F. Gans and U. Weber for preparing ancillary data and B. Ahrens for pointing out some soil data availability. We acknowledge Environmental Systems Research Institute (ESRI) and its licensor(s) for the Geodata product of the Missions Database ‘ArcWorld Supplement’ (GMI), published by Global Mapping International and originated from Global Mapping International for producing Extended Data Fig. 1 and Supplementary Fig. 7 and available in ArcGIS software by ESRI. ArcGIS and ArcMap are the intellectual property of ESRI and are used herein under license. For more information about ESRI software, please visit www.esri.com. The authors affiliated with the MPI BGC acknowledge funding by the European Union’s Horizon 2020 project BACI under grant agreement no. 640176. We are thankful to the data providers for the SoilGrids, hosted by ISRIC. J.S.J. acknowledges the International Max Planck Research School for global biogeochemical cycles. J.S.J., M.E.S. and M.C.S. acknowledge support from the University of Zurich University Research Priority Program on Global Change and Biodiversity. P.B.R., M.E.S. and M.C.S. acknowledge membership in the US NSF 20-508 BII-Implementation project, ‘The causes and consequences of plant biodiversity across scales in a rapidly changing world’. M.E.S. acknowledges the NOMIS grant of Remotely Sensing Ecological Genomics that funds J.S.J. and M.C.S. C.W. acknowledges the support of the Max Planck Society via its fellowship programme. N.R. was funded by a research grant from Deutsche Forschungsgemeinschaft DFG (RU 1536/3-1). K.K. was supported by the project Resilient Forests (KB-29-009-003) of the Dutch Ministry of Economic Affairs. The trait data supplied were co-funded by the EU-FP7-KBBE project: BACCARA—Biodiversity and climate change, a risk analysis (project ID 226299). I.W. acknowledges support from the Australian Research Council (DP170103410). J.P. acknowledges financial support from the European Research Council Synergy grant ERC-SyG-2013-610028 IMBALANCE-P. N.A.S. is financed by a VIDI grant (016.161.318) issued by the Netherlands Organization of Scientific Research. The data V.M. provided were funded by II. Oldenburgischer Deichband and the Wasserverbandstag e.V. (NWS 10/05). We thank M. Kleyer for his critical input. P.H. and V.D.P. have been supported by CNPq (grant nos 369617/2017-2 and 307689/2014-0, respectively). C.B. was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1C1B6005351). A.G.G. was funded by FONDECYT grant nos 11150835 and 1200468. V.O. thanks Russian science foundation (RSF, 19-14-00038) for financial support.
Extended data
Author contributions
B.R., C.W., J.K., J.S.J. and M.D.M. were responsible for conceptualization. B.R., C.W., J.S.J. and M.D.M. developed the methodology. J.S.J. undertook the formal analysis, validation, visualization, investigation and project administration. B.R., C.R., C.W., F.L., F.S., I.W., J.H.C.C., J.K., J.S.J., K.K., M.D.M., M.R., N.R., P.B.R., P.v.B., R.R., S.D., S.D.S., Ü.N. and W.N.H. wrote the original draft. J.K. and J.S.J. carried out data curation. A.G.M., A.G.G., B.E.L.C., C.B., C.R., C.W., E.S., E.R.W., F.L., G.C., H.J., I.W., J.M.C., J.H.C.C., J.P., K.K., M.A., M.B., N.K., N.A.S., P.H., P.B.R., P.v.B., S.D., T.H., Ü.N., V.M., V.O., V.D.P. and W.N.H. obtained resources. A.G.M., A.G.G., B.E.L.C., B.R., C.B., C.R., C.W., E.S., E.R.W., F.L., F.S., G.C., H.J., I.W., J.M.C., J.S.J., J.H.C.C., J.P., K.K., M.A., M.B., M.C.S., M.D.M., M.R., M.E.S., N.K., N.R., N.A.S., P.H., P.B.R., P.v.B., S.D., S.D.S., T.H., Ü.N., V.M., V.O., V.D.P. and W.N.H. wrote, reviewed and edited the manuscript. B.R., C.W., J.K., M.C.S., M.D.M., M.E.S. and N.R. undertook supervision. C.W., J.K., M.R. and M.E.S. were involved in project administration and funding. B.R., J.S.J., S.D.S. and R.R. ran the software.
Data availability
Plant trait data were accessed from the TRY database (https://try-db.org, request no. 3282, date accessed July 2017, see also Extended Data Fig. 1). All TRY data required to reproduce this analysis, and the corresponding R scripts, are provided in an open TRY File Archive (https://www.try-db.org/TryWeb/Data.php). Climate data WorldClim are publicly available via https://www.worldclim.org/ (accessed May 2018). Soil data, namely SoilGrids (https://soilgrids.org/, accessed June 2018) are publicly available. Ecoregion information30 shapefiles are publicly available (accessed January 2014, Sciencebase.gov), The estimate of species richness per ecoregion174 is publicly available (accessed January 2014, databasin.org. Data for this study can be accessed on Github (https://github.com/juliajoswig/ Repo_ClimateSoil_TraitSpectrum). For Extended Data Fig. 1 and Supplementary Fig. 7, the Geodata product of the Missions Database ‘ArcWorld Supplement’ (GMI) was used.
Code availability
The code is available on Github (https://github.com/juliajoswig/Repo_ClimateSoil_TraitSpectrum).
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Ecology & Evolution thanks Haydn J.D. Thomas, Vanessa Buzzard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41559-021-01616-8.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-021-01616-8.
References
- 1.Westoby M. A leaf–height–seed (LHS) plant ecology strategy scheme. Plant Soil. 1998;199:213–227. [Google Scholar]
- 2.Kraft NJB, et al. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015;29:592–599. [Google Scholar]
- 3.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. [Google Scholar]
- 5.Musavi T, et al. Potential and limitations of inferring ecosystem photosynthetic capacity from leaf functional traits. Ecol. Evol. 2016;6:7352–7366. doi: 10.1002/ece3.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheiter S, Langan L, Higgins SI. Next-generation dynamic global vegetation models: learning from community ecology. New Phytol. 2013;198:957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- 7.Van Bodegom PM, Douma JC, Verheijen LM. A fully traits-based approach to modeling global vegetation distribution. Proc. Natl Acad. Sci. USA. 2014;111:13733–13738. doi: 10.1073/pnas.1304551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrodt F, et al. BHPMF—a hierarchical Bayesian approach to gap-filling and trait prediction for macroecology and functional biogeography. Glob. Ecol. Biogeogr. 2015;24:1510–1521. [Google Scholar]
- 9.Kattge J, et al. TRY plant trait database—enhanced coverage and open access. Glob. Change Biol. 2020;26:119–188. doi: 10.1111/gcb.14904. [DOI] [PubMed] [Google Scholar]
- 10.Díaz S, et al. The global spectrum of plant form and function. Nature. 2015;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- 11.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 12.Bruelheide H, et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018;2:1906–1917. doi: 10.1038/s41559-018-0699-8. [DOI] [PubMed] [Google Scholar]
- 13.Thomas, H. J. et al. Global plant trait relationships extend to the climatic extremes of the tundra biome. Nat. Commun.11, 1351 (2020). [DOI] [PMC free article] [PubMed]
- 14.Kong, D. et al. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun.10, 2203 (2019). [DOI] [PMC free article] [PubMed]
- 15.Schimper, A. Plant-Geography Upon A Physiological Basis (Clarendon Press, 1903).
- 16.Warming, E. Oecology Of Plants (Oxford, 1909).
- 17.Raunkiær, C. in Life Forms of Plants and Statistical Plant Geography, 4-16 (Clarendon Press, 1934).
- 18.Maire V, et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015;24:706–717. [Google Scholar]
- 19.Bjorkman AD, et al. Plant functional trait change across a warming tundra biome. Nature. 2018;562:57–62. doi: 10.1038/s41586-018-0563-7. [DOI] [PubMed] [Google Scholar]
- 20.Olson ME, et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl Acad. Sci. USA. 2018;115:7551–7556. doi: 10.1073/pnas.1721728115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moles AT, et al. Global patterns in plant height. J. Ecol. 2009;97:923–932. [Google Scholar]
- 22.Ordoñez JC, et al. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009;18:137–149. [Google Scholar]
- 23.Simpson AH, Richardson SJ, Laughlin DC. Soil–climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Glob. Ecol. Biogeogr. 2016;25:964–978. [Google Scholar]
- 24.Wright IJ, et al. Global climatic drivers of leaf size. Science. 2017;357:917–921. doi: 10.1126/science.aal4760. [DOI] [PubMed] [Google Scholar]
- 25.Atkin OK, et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015;206:614–636. doi: 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- 26.Asner GP, Knapp DE, Anderson CB, Martin RE, Vaughn N. Large-scale climatic and geophysical controls on the leaf economics spectrum. Proc. Natl Acad. Sci. USA. 2016;113:E4043–E4051. doi: 10.1073/pnas.1604863113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moles AT, et al. Global patterns in seed size. Glob. Ecol. Biogeogr. 2007;16:109–116. [Google Scholar]
- 28.Blume, H.-P. et al. Soil Science 1st edn.(Springer, Berlin-Heidelberg, 2016).
- 29.Seneviratne SI, et al. Investigating soil moisture–climate interactions in a changing climate: a review. Earth-Sci. Rev. 2010;99:125–161. [Google Scholar]
- 30.Olson DM, et al. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience. 2001;51:933–938. [Google Scholar]
- 31.Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning (Springer, 2008).
- 32.Chevan A, Sutherland M. Hierarchical partitioning. Am. Stat. 1991;45:90–96. [Google Scholar]
- 33.Reich PB, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl Acad. Sci. USA. 2004;101:11001–11006. doi: 10.1073/pnas.0403588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corner EJH. The Durian theory or the origin of the modern tree. Ann. Bot. 1949;XIII:367–414. [Google Scholar]
- 35.Pietsch KA, et al. Global relationship of wood and leaf litter decomposability: the role of functional traits within and across plant organs. Glob. Ecol. Biogeogr. 2014;23:1046–1057. [Google Scholar]
- 36.FloresâMoreno H, et al. Robustness of trait connections across environmental gradients and growth forms. Glob. Ecol. Biogeogr. 2019;28:1806–1826. [Google Scholar]
- 37.Chapin FS. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980;11:233–260. [Google Scholar]
- 38.Vitousek, P. Nutrient Cycling and Limitation: Hawai’i as a Model System (Princeton Univ. Press, 2004).
- 39.Shipley B, Vile D, Garnier E, Wright IJ, Poorter H. Functional linkages between leaf traits and net photosynthetic rate: reconciling empirical and mechanistic models. Funct. Ecol. 2005;19:602–615. [Google Scholar]
- 40.He T, Belcher CM, Lamont BB, Lim SL. A 350-million-year legacy of fire adaptation among conifers. J. Ecol. 2016;104:352–363. [Google Scholar]
- 41.Bergmann J, Ryo M, Prati D, Hempel S, Rillig MC. Root traits are more than analogues of leaf traits: the case for diaspore mass. New Phytol. 2017;216:1130–1139. doi: 10.1111/nph.14748. [DOI] [PubMed] [Google Scholar]
- 42.Aerts R. The advantages of being evergreen. Trends Ecol. Evol. 1995;10:402–407. doi: 10.1016/s0169-5347(00)89156-9. [DOI] [PubMed] [Google Scholar]
- 43.Zanne AE, et al. Functional biogeography of angiosperms: life at the extremes. New Phytol. 2018;218:1697–1709. doi: 10.1111/nph.15114. [DOI] [PubMed] [Google Scholar]
- 44.Franklin O, et al. Organizing principles for vegetation dynamics. Nat. Plants. 2020;6:444–453. doi: 10.1038/s41477-020-0655-x. [DOI] [PubMed] [Google Scholar]
- 45.Legay N, et al. Contribution of above- and below-ground plant traits to the structure and function of grassland soil microbial communities. Ann. Bot. 2014;114:1011–1021. doi: 10.1093/aob/mcu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grime JP. Vegetation classification by reference to strategies. Nature. 1974;250:26–31. [Google Scholar]
- 47.Slessarev EW, et al. Water balance creates a threshold in soil pH at the global scale. Nature. 2016;540:567–569. doi: 10.1038/nature20139. [DOI] [PubMed] [Google Scholar]
- 48.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc. Natl Acad. Sci. USA. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinsabaugh RL, Follstad Shah JJ. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012;43:313–343. [Google Scholar]
- 50.de Vries FT, et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 2012;15:1230–1239. doi: 10.1111/j.1461-0248.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 51.Zech, W., Schad, P. & Hintermaier-Erhard, G. Böden der Welt—Ein Bildatlas (Springer Spectrum, 2014).
- 52.Rosenberg, E. et al. (eds) The Prokaryotes: Prokaryotic Communities and Ecophysiology 4th edn. (Springer-Verlag, 2013).
- 53.Niinemets Ã. Leaf age dependent changes in within-canopy variation in leaf functional traits: a meta-analysis. J. Plant Res. 2016;129:313–338. doi: 10.1007/s10265-016-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler EE, et al. Mapping local and global variability in plant trait distributions. Proc. Natl Acad. Sci. USA. 2017;114:E10937–E10946. doi: 10.1073/pnas.1708984114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freschet GT, et al. Global to community scale differences in the prevalence of convergent over divergent leaf trait distributions in plant assemblages. Global Ecol. Biogeogr. 2011;20:755–765. [Google Scholar]
- 56.Yemefack M, Rossiter DG, Njomgang R. Multi-scale characterization of soil variability within an agricultural landscape mosaic system in southern Cameroon. Geoderma. 2005;125:117–143. [Google Scholar]
- 57.Oldeman, L., Hakkeling, R. & Sombroek, W. Global Assessment of Soil Degradation (GLASOD): World Map of the Status of Human-induced Soil Degradation (United Nations Environment Programme, 1991).
- 58.Ackerly DD, Cornwell WK. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecol. Lett. 2007;10:135–145. doi: 10.1111/j.1461-0248.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 59.Adler, P. B. A Comparison of Livestock Grazing Effects on Sagebrush Steppe, USA, and Patagonian Steppe, Argentina. PhD thesis (Colorado State University, 2003).
- 60.Adler PB, Milchunas DG, Lauenroth WK, Sala OE, Burke IC. Functional traits of graminoids in semi-arid steppes: a test of grazing histories. J. Appl. Ecol. 2004;41:653–663. [Google Scholar]
- 61.Adriaenssens, S. Dry deposition and canopy exchange for temperate tree species under high nitrogen deposition. PhD thesis, Ghent Univ. (2012).
- 62.Atkin OK, Schortemeyer M, McFarlane N, Evans JR. The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia. 1999;120:544–554. doi: 10.1007/s004420050889. [DOI] [PubMed] [Google Scholar]
- 63.Atkin OK, Westbeek M, Cambridge ML, Lambers H, Pons TL. Leaf respiration in light and darkness (a comparison of slow- and fast-growing Poa species) Plant Physiol. 1997;113:961–965. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auger, S. L’Importance de la Variabilité Interspécifique des Traits Fonctionnels par Rapport à la Variabilité Intraspécifique Chez les Jeunes Arbres en Forêt Mature. MSc thesis (Université de Sherbrooke, 2012).
- 65.Bahn, M. et al. in Land-Use Changes in European Mountain Ecosystems. ECOMONT—Concept and Results (eds Cernusca, A. et al.) 247–255 (Blackwell Wissenschaft, 1999).
- 66.Baker TR, et al. Do species traits determine patterns of wood production in Amazonian forests? Biogeosciences. 2009;6:297–307. [Google Scholar]
- 67.Bakker C, Van Bodegom PM, Nelissen HJM, Ernst WHO, Aerts R. Plant responses to rising water table and nutrient management in calcareous dune slacks. Plant Ecol. 2006;185:19–28. [Google Scholar]
- 68.Bakker C, Rodenburg J, van Bodegom PM. Effects of Ca- and Fe-rich seepage on P availability and plant performance in calcareous dune soils. Plant Soil. 2005;275:111–122. [Google Scholar]
- 69.Baraloto C, et al. Decoupled leaf and stem economics in rainforest trees. Ecol. Lett. 2010;13:1338–1347. doi: 10.1111/j.1461-0248.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- 70.Baraloto C, et al. Functional trait variation and sampling strategies in species-rich plant communities. Funct. Ecol. 2010;24:208–216. [Google Scholar]
- 71.Beckmann M, Hock M, Bruelheide H, Erfmeier A. The role of UV-B radiation in the invasion of Hieracium pilosella—a comparison of German and New Zealand plants. Environ. Exp. Bot. 2012;75:173–180. [Google Scholar]
- 72.Blanco CC, Sosinski EE, dos Santos BRC, da Silva MA, Pillar VD. On the overlap between effect and response plant functional types linked to grazing. Community Ecol. 2007;8:57–65. [Google Scholar]
- 73.Blonder B, et al. The shrinkage effect biases estimates of paleoclimate. Am. J. Bot. 2012;99:1756–1763. doi: 10.3732/ajb.1200062. [DOI] [PubMed] [Google Scholar]
- 74.Blonder B, Violle C, Enquist BJ. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. J. Ecol. 2013;101:981–989. [Google Scholar]
- 75.Blonder B, et al. Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants. 2015;7:plv049. doi: 10.1093/aobpla/plv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blonder B, Violle C, Bentley LP, Enquist BJ. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett. 2011;14:91–100. doi: 10.1111/j.1461-0248.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- 77.Bocanegra-González K.T., Fernández-Méndez, F. & David Galvis-Jiménez, J. Funtional groups of tres in secondary forests of the bajo calima region (Buenaventura, Colombia) Boletín CientífiCo Centro de Museos Museo de Historia natura19, (2015).
- 78.Bodegom PMV, Kanter MD, Aerts CBR. Radial oxygen loss, a plastic property of dune slack plant species. Plant Soil. 2005;271:351–364. [Google Scholar]
- 79.Bond-Lamberty CWB, Gower ST. Above- and belowground biomass and sapwood area allometric equations for six boreal tree species of northern Manitoba. Can. J. For. Res. 2002;32:1441–1450. [Google Scholar]
- 80.Bond-Lamberty CWB, Gower ST. Leaf area dynamics of a boreal black spruce fire chronosequence. Tree Physiol. 2002;22:993–1001. doi: 10.1093/treephys/22.14.993. [DOI] [PubMed] [Google Scholar]
- 81.Bond-Lamberty CWB, Gower ST. The use of multiple measurement techniques to refine estimates of conifer needle geometry. Can. J. For. Res. 2003;33:101–105. [Google Scholar]
- 82.Bond-Lamberty CWB, Gower S. Net primary production and net ecosystem production of a boreal black spruce fire chronosequence. Glob. Change Biol. 2004;10:473–487. [Google Scholar]
- 83.Bragazza L. Conservation priority of Italian alpine habitats: a floristic approach based on potential distribution of vascular plant species. Biodivers. Conserv. 2009;18:2823–2835. [Google Scholar]
- 84.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 85.Briemle, G., Nitsche, S. & Nitsche, L. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 203–225 (Bundesamt für Naturschutz, 2002).
- 86.Brown, K. et al. Assessing natural resource use by forest-reliant communities in Madagascar using functional diversity and functional redundancy metrics. PLoS ONE10.1371/journal.pone.0024107 (2011). [DOI] [PMC free article] [PubMed]
- 87.Burrascano S, et al. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 2015;16:244–253. [Google Scholar]
- 88.Butterfield BJ, Briggs JM. Regeneration niche differentiates functional strategies of desert woody plant species. Oecologia. 2011;165:477–487. doi: 10.1007/s00442-010-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Byun C, de Blois S, Brisson J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J. Ecol. 2013;101:128–139. [Google Scholar]
- 90.Campbell C, et al. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytol. 2007;176:375–389. doi: 10.1111/j.1469-8137.2007.02183.x. [DOI] [PubMed] [Google Scholar]
- 91.Campetella G, et al. Patterns of plant trait–environment relationships along a forest succession chronosequence. Agric. Ecosyst. Environ. 2011;145:38–48. [Google Scholar]
- 92.Carswell FE, et al. Photosynthetic capacity in a central Amazonian rain forest. Tree Physiol. 2000;20:179–186. doi: 10.1093/treephys/20.3.179. [DOI] [PubMed] [Google Scholar]
- 93.Castro-Diez P, Puyravaud JP, Cornelissen JHC, Villar-Salvador. P. Stem anatomy and relative growth rate in seedlings of a wide range of woody plant species and types. Oecologia. 1998;116:57–66. doi: 10.1007/s004420050563. [DOI] [PubMed] [Google Scholar]
- 94.Castro-Diez P, Puyravaud JP, Cornelissen JHC. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia. 2000;124:476–486. doi: 10.1007/PL00008873. [DOI] [PubMed] [Google Scholar]
- 95.Cavender-Bares AKJ, Miles B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:109–122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Cavender-Bares LSJ, Savage J. Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol. 2007;27:522–620. doi: 10.1093/treephys/27.4.611. [DOI] [PubMed] [Google Scholar]
- 97.Cerabolini BEL, et al. Can CSR classification be generally applied outside Britain? Plant Ecol. 2010;210:253–261. [Google Scholar]
- 98.Chave J, et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y, Han W, Tang L, Tang Z, Fang J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography. 2011;36:178–184. [Google Scholar]
- 100.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 101.Choat B, Sack L, Holbrook NM. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytol. 2007;175:686–698. doi: 10.1111/j.1469-8137.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- 102.Coomes DA, Heathcote S, Godfrey ER, Shepherd JJ. Scaling of xylem vessels and veins within the leaves of oak species. Biol. Lett. 2008;4:302–306. doi: 10.1098/rsbl.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia. 2001;129:611–619. doi: 10.1007/s004420100752. [DOI] [PubMed] [Google Scholar]
- 104.Cornelissen JHC. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 1996;84:573–582. [Google Scholar]
- 105.Cornelissen JHC, Diez PC, Hunt. R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996;84:755–765. [Google Scholar]
- 106.Cornelissen JHC, Werger MJA, Castro-Diez P, van Rheenen JWA, Rowland AP. Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia. 1997;111:460–469. doi: 10.1007/s004420050259. [DOI] [PubMed] [Google Scholar]
- 107.Cornelissen JHC, et al. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol. 1999;143:191–200. [Google Scholar]
- 108.Cornelissen JHC. A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia. 1999;118:248–255. doi: 10.1007/s004420050725. [DOI] [PubMed] [Google Scholar]
- 109.Cornelissen JHC, Aerts R, Cerabolini B, Werger MJA, van der Heijden. MGA. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia. 2001;129:611–619. doi: 10.1007/s004420100752. [DOI] [PubMed] [Google Scholar]
- 110.Cornelissen JHC, et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct. Ecol. 2004;18:779–786. [Google Scholar]
- 111.Cornelissen JHC, et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Veg. Sci. 2003;14:311–322. [Google Scholar]
- 112.Cornelissen JHC, Diez PC, Hunt R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996;84:755. [Google Scholar]
- 113.Cornelissen JHC, et al. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol. 1999;143:191–200. [Google Scholar]
- 114.Schwilk DW, Cornwell WK, Ackerly. DD. A trait-based test for habitat filtering: convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 115.Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009;79:109–126. [Google Scholar]
- 116.Cornwell WK, Bhaskar R, Sack L, Cordell S, Lunch CK. Adjustment of structure and function of Hawaiian Metrosideros polymorpha at high vs. low precipitation. Funct. Ecol. 2007;21:1063–1071. [Google Scholar]
- 117.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 118.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 119.Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC. Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology. 2005;86:12–19. [Google Scholar]
- 120.Craine JM, et al. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia. 2011;165:1109–1117. doi: 10.1007/s00442-011-1938-8. [DOI] [PubMed] [Google Scholar]
- 121.Craine JM, et al. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change. 2012;3:63–67. [Google Scholar]
- 122.Craven D, et al. Between and within-site comparisons of structural and physiological characteristics and foliar nutrient content of 14 tree species at a wet, fertile site and a dry, infertile site in Panama. For. Ecol. Manag. 2007;238:335–346. [Google Scholar]
- 123.Craven D, et al. Seasonal variability of photosynthetic characteristics influences growth of eight tropical tree species at two sites with contrasting precipitation in Panama. For. Ecol. Manag. 2011;261:1643–1653. [Google Scholar]
- 124.Dainese M, Bragazza L. Plant traits across different habitats of the Italian alps: a comparative analysis between native and alien species. Alpine Bot. 2012;122:11–21. [Google Scholar]
- 125.de Araujo, A. et al. LBA-ECO CD-02 C and N Isotopes in Leaves and Atmospheric CO2, Amazonas, Brazil (ORNL DAAC, 2012); http://daac.ornl.gov
- 126.de Vries FT, Bardgett RD. Plant community controls on short-term ecosystem nitrogen retention. New Phytol. 2016;210:861–874. doi: 10.1111/nph.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Demey A, et al. Nutrient input from hemiparasitic litter favors plant species with a fast-growth strategy. Plant Soil. 2013;371:53–66. [Google Scholar]
- 128.Diaz S, et al. The plant traits that drive ecosystems: evidence from three continents. J. Veg. Sci. 2004;15:295–304. [Google Scholar]
- 129.Domingues, T. F., Berry, J. A., Martinelli, L. A., Ometto, J. P. H. B. & Ehleringer, J. R. Parameterization of canopy structure and leaf-level gas exchange for an eastern Amazonian tropical rain forest (Tapajós National Forest, Pará, Brazil). Earth Interact.10.1175/EI149.1 (2005).
- 130.Domingues TF, Martinelli LA, Ehleringer JR. Ecophysiological traits of plant functional groups in forest and pasture ecosystems from eastern Amazônia, Brazil. Plant Ecol. 2007;193:101–112. [Google Scholar]
- 131.Domingues TF, et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 2010;33:959–980. doi: 10.1111/j.1365-3040.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- 132.Duarte LdS, Carlucci MB, Hartz SM, Pillar VD. Plant dispersal strategies and the colonization of Araucaria forest patches in a grassland–forest mosaic. J. Veg. Sci. 2007;18:847–858. [Google Scholar]
- 133.DunbarâCo S, Sporck MJ, Sack L. Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int. J. Plant Sci. 2009;170:61–75. [Google Scholar]
- 134.Durka, W. In BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 75–91 (Bundesamt für Naturschutz, 2002).
- 135.Durka, W. In BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 57–74 (Bundesamt für Naturschutz, 2002).
- 136.Durka, W. In BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 133–175 (Bundesamt für Naturschutz, 2002).
- 137.Medlyn BE, Jarvis PG. Design and use of a database of model parameters from elevated [CO2] experiments. Ecol. Model. 1999;124:69–83. [Google Scholar]
- 138.Everwand G, Fry EL, Eggers T, Manning P. Seasonal variation in the capacity for plant trait measures to predict grassland carbon and water fluxes. Ecosystems. 2014;17:1095–1108. [Google Scholar]
- 139.Fazayeli, F., Banerjee, A., Kattge, J., Schrodt, F. & Reich, P. B. Uncertainty quantified matrix completion using Bayesian Hierarchical Matrix factorization. In Proc. 13th International Conference on Machine Learning and Applications (eds Ferri, C. et al.) 312–317 (International Conference on Machine Learning and Applications (ICMLA), 2014).
- 140.Fagúndez J, Izco J. Seed morphology of the European species of Erica L. sect. Arsace Salisb. ex Benth. (Ericaceae) Acta Bot. Gall. 2010;157:45–54. [Google Scholar]
- 141.Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 2000;88:964–977. [Google Scholar]
- 142.Fortunel C, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- 143.Frainer A, McKie BG. Shifts in the diversity and composition of consumer traits constrain the effects of land use on stream ecosystem functioning. Adv. Ecol. Res. 2015;52:169–200. [Google Scholar]
- 144.Frenette-Dussault C, Shipley B, Léger J-F, Meziane D, Hingrat Y. Functional structure of an arid steppe plant community reveals similarities with Grime’s C-S-R theory. J. Veg. Sci. 2011;23:208–222. [Google Scholar]
- 145.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Evidence of the plant economics spectrum in a subarctic flora. J. Ecol. 2010;98:362–373. [Google Scholar]
- 146.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits? New Phytol. 2010;186:879–889. doi: 10.1111/j.1469-8137.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- 147.Fry EL, Power SA, Manning P. Trait-based classification and manipulation of plant functional groups for biodiversity–ecosystem function experiments. J. Veg. Sci. 2013;25:248–261. [Google Scholar]
- 148.Fyllas NM, et al. Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences. 2009;6:2677–2708. [Google Scholar]
- 149.Gachet S, Véla E, Tatoni T. BASECO: a floristic and ecological database of Mediterranean French flora. Biodivers. Conserv. 2005;14:1023–1034. [Google Scholar]
- 150.Gallagher RV, Leishman MR. A global analysis of trait variation and evolution in climbing plants. J. Biogeogr. 2012;39:1757–1771. [Google Scholar]
- 151.Garnier E, et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Ann. Bot. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Givnish TJ, Montgomery RA, Goldstein G. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. Am. J. Bot. 2004;91:228–246. doi: 10.3732/ajb.91.2.228. [DOI] [PubMed] [Google Scholar]
- 153.Guerin GR, Wen H, Lowe AJ. Leaf morphology shift linked to climate change. Biol. Lett. 2012;8:882–886. doi: 10.1098/rsbl.2012.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gutiérrez AG, Huth A. Successional stages of primary temperate rainforests of Chiloé Island, Chile. Perspect. Plant Ecol. Evol. Syst. 2012;14:243–256. [Google Scholar]
- 155.Guy AL, Mischkolz JM, Lamb EG. Limited effects of simulated acidic deposition on seedling survivorship and root morphology of endemic plant taxa of the Athabasca sand dunes in well-watered greenhouse trials. Botany. 2013;91:176–181. [Google Scholar]
- 156.Han W, et al. Floral, climatic and soil pH controls on leaf ash content in China’s terrestrial plants. Glob. Ecol. Biogeogr. 2011;21:376–382. [Google Scholar]
- 157.Han W, Fang J, Guo D, Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005;168:377–385. doi: 10.1111/j.1469-8137.2005.01530.x. [DOI] [PubMed] [Google Scholar]
- 158.Hao G-Y, Sack L, Wang A-Y, Cao K-F, Goldstein G. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct. Ecol. 2010;24:731–740. [Google Scholar]
- 159.He J-S, et al. A test of the generality of leaf trait relationships on the Tibetan plateau. New Phytol. 2006;170:835–848. doi: 10.1111/j.1469-8137.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 160.Hickler, T. Plant Functional Types and Community Characteristics along Environmental Gradients on Öland’s Great Alvar (Sweden). Masters thesis (University of Lund, 1999).
- 161.Hoof, J., Sack, L., Webb, D. T. & Nilsen, E. T. Contrasting structure and function of pubescent and glabrous varieties of Hawaiian Metrosideros polymorpha (Myrtaceae) at high elevation. Biotropica40, 113–118 (2008).
- 162.Husson, A. F., Josse, J., Le, S., Mazet, J. & Husson, M. F. Package ‘FactoMineR’ (CRAN, 2017).
- 163.Jacobs B, et al. Unraveling the Phylogeny of Heptacodium and Zabelia (Caprifoliaceae): An Interdisciplinary Approach. Syst. Bot. 2011;36:231–252. [Google Scholar]
- 164.Jansen S, Decraene LPR, Smets E. On the wood and stem anatomy of Monococcus echinophorus (Phytolaccaceae s.l.) Syst. Geogr. Plants. 2000;70:171. [Google Scholar]
- 165.Jansen S, et al. Contributions to the wood anatomy of the Rubioideae (Rubiaceae) J. Plant Res. 2001;114:269–289. [Google Scholar]
- 166.Jansen S, Piesschaert F, Smets E. Wood anatomy of Elaeagnaceae, with comments on vestured pits, helical thickenings, and systematic relationships. Am. J. Bot. 2000;87:20. [PubMed] [Google Scholar]
- 167.Jansen S, Robbrecht E, Beeckman H, Smets E. Gaertnera and Pagamea: genera within the Psychotrieae or constituting the tribe Gaertnereae? A wood anatomical and palynological approach. Bot. Acta. 1996;109:466–476. [Google Scholar]
- 168.S. J, E. R, H. B, Smets E. Comparative wood anatomy of African Coffeae (Rubiaceae-Rubioideae) Belg. J. Bot. 1997;130:47–58. [Google Scholar]
- 169.Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 2009;15:976–991. [Google Scholar]
- 170.Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Funct. Ecol. 2006;20:21–30. [Google Scholar]
- 171.Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 172.Kew, R. B. G. Seed Information Database—SID (Kew, 2008); http://data.kew.org/sid/
- 173.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013;27:1254–1261. [Google Scholar]
- 174.Kier G, et al. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005;32:1107–1116. [Google Scholar]
- 175.Kirkup D, Malcolm P, Christian G, Paton A. Towards a digital African flora. Taxon. 2005;54:457. [Google Scholar]
- 176.Kleyer M, et al. The LEDA traitbase: a database of life-history traits of the northwest European flora. J. Ecol. 2008;96:1266–1274. [Google Scholar]
- 177.Klotz, S. & Kühn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 119-126 (Bundesamt für Naturschutz, 2002).
- 178.Klotz, S. & Kühn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 241–246 (Bundesamt für Naturschutz,2002).
- 179.Klotz, S. & Kühn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 273–281 (Bundesamt für Naturschutz, 2002).
- 180.Klotz, S. & Kühn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 197–201 (Bundesamt für Naturschutz, 2002).
- 181.Koike F. Plant traits as predictors of woody species dominance in climax forest communities. J. Veg. Sci. 2001;12:327–336. [Google Scholar]
- 182.Kraft NJB, Ackerly DD. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010;80:401–422. [Google Scholar]
- 183.Kraft NJB, Valencia R, Ackerly DD. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- 184.Krumbiegel, A. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 93–118 (Bundesamt für Naturschutz, 2002).
- 185.Kühn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 47–56 (Bundesamt für Naturschutz, 2002).
- 186.Kuhn I, Durka W, Klotz S. Biolflor—a new plant-trait database as a tool for plant invasion ecology. Divers. Distrib. 2004;10:363–365. [Google Scholar]
- 187.Kühn, I. & Klotz, S. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 227–239 (Bundesamt für Naturschutz, 2002).
- 188.Kurokawa H, Nakashizuka T. Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology. 2008;89:2645–2656. doi: 10.1890/07-1352.1. [DOI] [PubMed] [Google Scholar]
- 189.Laughlin DC, Fulé PZ, Huffman DW, Crouse J, Laliberté E. Climatic constraints on trait-based forest assembly. J. Ecol. 2011;99:1489–1499. [Google Scholar]
- 190.Laughlin DC, Leppert JJ, Moore MM, Sieg CH. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2009;24:493–501. [Google Scholar]
- 191.Lens F. Comparative wood anatomy of Epacrids (Styphelioideae, Ericaceae s.l.) Ann. Bot. 2003;91:835–856. doi: 10.1093/aob/mcg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lens F, Baas P, Jansen S, Smets E. A search for phylogenetically informative wood characters within Lecythidaceae s.l. Am. J. Bot. 2007;94:483–502. doi: 10.3732/ajb.94.4.483. [DOI] [PubMed] [Google Scholar]
- 193.Lens F, Dressler S, Jansen S, van Evelghem L, Smets E. Relationships within balsaminoid Ericales: a wood anatomical approach. Am. J. Bot. 2005;92:941–953. doi: 10.3732/ajb.92.6.941. [DOI] [PubMed] [Google Scholar]
- 194.Lens F, Eeckhout S, Zwartjes R, Smets E, Janssens SB. The multiple fuzzy origins of woodiness within Balsaminaceae using an integrated approach: where do we draw the line? Ann. Bot. 2011;109:783–799. doi: 10.1093/aob/mcr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lens F, Endress ME, Baas P, Jansen S, Smets E. Vessel grouping patterns in subfamilies Apocynoideae and Periplocoideae confirm phylogenetic value of wood structure within Apocynaceae. Am. J. Bot. 2009;96:2168–2183. doi: 10.3732/ajb.0900116. [DOI] [PubMed] [Google Scholar]
- 196.Lens F, Groeninckx I, Smets E, Dessein S. Woodiness within the Spermacoceae–Knoxieae alliance (Rubiaceae): retention of the basal woody condition in Rubiaceae or recent innovation? Ann. Bot. 2009;103:1049–1064. doi: 10.1093/aob/mcp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lens F, Jansen S, Caris P, Serlet L, Smets E. Comparative wood anatomy of the primuloid clade (Ericales s.l.) Syst. Bot. 2005;30:163–183. [Google Scholar]
- 198.Lens F, Jansen S, Robbrecht E, Smets E. Wood anatomy of the Vangueriaea (Ixoroideae-Rubuaceae), with special emphasis on some geofrutices. IAWA J. 2000;21:443–455. [Google Scholar]
- 199.Lens F, et al. The wood anatomy of the polyphyletic Icacinaceae s.l., and their relationships within asterids. Taxon. 2008;57:525–552. [Google Scholar]
- 200.Lens F, Kron KA, Luteyn JL, Smets E, Jansen S. Comparative wood anatomy of the blueberry tribe (Vaccinieae, Ericaceae s.l) Ann. Missouri Bot. Gard. 2004;91:566–592. [Google Scholar]
- 201.Lens F, Smets E, Jansen S. Comparative wood anatomy of Andromedeae s.s., Gaultherieae, Lyonieae and Oxydendreae (Vaccinioideae, Ericaceae s.l.) Bot. J. Linn. Soc. 2004;144:161–179. [Google Scholar]
- 202.Lens F, Smets E, Melzer S. Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytol. 2011;193:12–17. doi: 10.1111/j.1469-8137.2011.03888.x. [DOI] [PubMed] [Google Scholar]
- 203.Lens F, et al. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2010;190:709–723. doi: 10.1111/j.1469-8137.2010.03518.x. [DOI] [PubMed] [Google Scholar]
- 204.Li H, Liang Y, Xu Q, Cao D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta. 2009;648:77–84. doi: 10.1016/j.aca.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 205.Louault F, Pillar VD, Aufrèère J, Garnier E, Soussana JF. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Veg. Sci. 2005;16:151–160. [Google Scholar]
- 206.Loveys BR, et al. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 2003;9:895–910. [Google Scholar]
- 207.Malhado ACM, et al. Drip-tips are associated with intensity of precipitation in the Amazon rain forest. Biotropica. 2012;44:728–737. [Google Scholar]
- 208.Malhado ACM, et al. Spatial trends in leaf size of Amazonian rainforest trees. Biogeosciences. 2009;6:1563–1576. [Google Scholar]
- 209.Malhado ACM, et al. Spatial distribution and functional significance of leaf lamina shape in Amazonian forest trees. Biogeosciences. 2009;6:1577–1590. [Google Scholar]
- 210.Malhado ACM, et al. Are compound leaves an adaptation to seasonal drought or to rapid growth? Evidence from the Amazon rain forest. Glob. Ecol. Biogeogr. 2010;19:852–862. [Google Scholar]
- 211.Manning P, Houston K, Evans T. Shifts in seed size across experimental nitrogen enrichment and plant density gradients. Basic Appl. Ecol. 2009;10:300–308. [Google Scholar]
- 212.Markesteijn L, Poorter L, Paz H, Sack L, Bongers F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011;34:137–148. doi: 10.1111/j.1365-3040.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 213.Martin RE, Asner GP, Sack L. Genetic variation in leaf pigment, optical and photosynthetic function among diverse phenotypes of Metrosideros polymorpha grown in a common garden. Oecologia. 2007;151:387–400. doi: 10.1007/s00442-006-0604-z. [DOI] [PubMed] [Google Scholar]
- 214.McDonald PG, Fonseca CR, Overton JM, Westoby M. Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Funct. Ecol. 2003;17:50–57. [Google Scholar]
- 215.McKenna MF, Shipley B. Interacting determinants of interspecific relative growth: empirical patterns and a theoretical explanation. Écoscience. 1999;6:286–296. [Google Scholar]
- 216.Medlyn BE, et al. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ. 1999;22:1475–1495. [Google Scholar]
- 217.Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 218.Meir P, et al. Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ. 2002;25:343–357. [Google Scholar]
- 219.Meir P, Levy PE, Grace J, Jarvis PG. Photosynthetic parameters from two contrasting woody vegetation types in West Africa. Plant Ecol. 2007;192:277–287. [Google Scholar]
- 220.Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26:163–182. [Google Scholar]
- 221.Meng T-T, et al. Responses of leaf traits to climatic gradients: Adaptive variation versus compositional shifts. Biogeosciences. 2015;12:5339–5352. [Google Scholar]
- 222.Messier J, McGill BJ, Enquist BJ, Lechowicz MJ. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography. 2016;40:685–697. [Google Scholar]
- 223.Messier J, McGill BJ, Lechowicz MJ. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 2010;13:838–848. doi: 10.1111/j.1461-0248.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- 224.Meziane D, Shipley B. Interacting components of interspecific relative growth rate: constancy and change under differing conditions of light and nutrient supply. Funct. Ecol. 1999;13:611–622. [Google Scholar]
- 225.Milla R, Reich PB. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. 2011;107:455–465. doi: 10.1093/aob/mcq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Minden V, Andratschke S, Spalke J, Timmermann H, Kleyer M. Plant trait–environment relationships in salt marshes: deviations from predictions by ecological concepts. Perspect. Plant Ecol. Evol. Syst. 2012;14:183–192. [Google Scholar]
- 227.Minden V, Kleyer M. Testing the effect–response framework: key response and effect traits determining above-ground biomass of salt marshes. J. Veg. Sci. 2011;22:387–401. [Google Scholar]
- 228.Mischkolz, J. M. Selecting and Evaluating Native Forage Mixtures for the Mixed Grass Prairie. Msc thesis (University of Saskatchewan, 2013).
- 229.Moretti M, Legg C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography. 2009;32:299–309. [Google Scholar]
- 230.Müller SC, Overbeck GE, Pfadenhauer J, Pillar VD. Plant functional types of woody species related to fire disturbance in forest–grassland ecotones. Plant Ecol. 2006;189:1–14. [Google Scholar]
- 231.Nakahashi CD, Frole K, Sack L. Bacterial leaf nodule symbiosis in Ardisia (Myrsinaceae): does it contribute to seedling growth capacity? Plant Biol. 2005;7:495–500. doi: 10.1055/s-2005-865853. [DOI] [PubMed] [Google Scholar]
- 232.Niinemets U. Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999;144:35–47. [Google Scholar]
- 233.Niinemets U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–469. [Google Scholar]
- 234.Ogaya R, Peñuelas J. Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003;50:137–148. [Google Scholar]
- 235.Ogaya R, Penuelas J. Contrasting foliar responses to drought in Quercus ilex and Phillyrea latifolia. Biol. Plant. 2006;50:373–382. [Google Scholar]
- 236.Ogaya R, Peñuelas J. Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol. 2006;189:291–299. [Google Scholar]
- 237.Ogaya R, Peñuelas J. Changes in leaf δ13C and δ15N for three Mediterranean tree species in relation to soil water availability. Acta Oecol. 2008;34:331–338. [Google Scholar]
- 238.Onoda Y, et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011;14:301–312. doi: 10.1111/j.1461-0248.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 239.Ordoñez JC, et al. Leaf habit and woodiness regulate different leaf economy traits at a given nutrient supply. Ecology. 2010;91:3218–3228. doi: 10.1890/09-1509.1. [DOI] [PubMed] [Google Scholar]
- 240.Otto, B. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 177–196 (Bundesamt für Naturschutz, 2002).
- 241.Overbeck GE, Müller SC, Pillar VD, Pfadenhauer J. Fine-scale post-fire dynamics in southern Brazilian subtropical grassland. J. Veg. Sci. 2005;16:655–664. [Google Scholar]
- 242.Overbeck GE, Pfadenhauer J. Adaptive strategies in burned subtropical grassland in southern Brazil. Flora. 2007;202:27–49. [Google Scholar]
- 243.Baas P, Smets E, Jansen S. Vegetative anatomy and effinities of Dirachma socotrana (Dirachmaceae) Syst. Bot. 2001;26:231–241. [Google Scholar]
- 244.Pakeman RJ, et al. Impact of abundance weighting on the response of seed traits to climate and land use. J. Ecol. 2008;96:355–366. [Google Scholar]
- 245.Pakeman RJ, Lep J, Kleyer M, Lavorel S, Garnie E. Relative climatic, edaphic and management controls of plant functional trait signatures. J. Veg. Sci. 2009;20:148–159. [Google Scholar]
- 246.Papanastasis M, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- 247.Patiño S, et al. Branch xylem density variations across the Amazon basin. Biogeosciences. 2009;6:545–568. [Google Scholar]
- 248.Paula S, et al. Fire-related traits for plant species of the Mediterranean basin. Ecology. 2009;90:1420–1420. [Google Scholar]
- 249.Paula S, Pausas JG. Burning seeds: germinative response to heat treatments in relation to resprouting ability. J. Ecol. 2008;96:543–552. [Google Scholar]
- 250.Peco B, de Pablos I, Traba J, Levassor C. The effect of grazing abandonment on species composition and functional traits: the case of Dehesa grasslands. Basic Appl. Ecol. 2005;6:175–183. [Google Scholar]
- 251.Peñuelas J, et al. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob. Change Biol. 2009;16:2171–2185. [Google Scholar]
- 252.Peñuelas J, et al. Higher allocation to low cost chemical defenses in invasive species of Hawaii. J. Chem. Ecol. 2010;36:1255–1270. doi: 10.1007/s10886-010-9862-7. [DOI] [PubMed] [Google Scholar]
- 253.Petter G, et al. Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra- and interspecific trait variability, and taxonomic signals. Funct. Ecol. 2015;30:188–198. [Google Scholar]
- 254.Pierce S, Brusa G, Sartori M, Cerabolini BEL. Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Ann. Bot. 2012;109:1047–1053. doi: 10.1093/aob/mcs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pierce S, Brusa G, Vagge I, Cerabolini BEL. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013;27:1002–1010. [Google Scholar]
- 256.Pierce S, Ceriani RM, De Andreis R, Luzzaro A, Cerabolini B. The leaf economics spectrum of Poaceae reflects variation in survival strategies. Plant Biosyst. 2007;141:337–343. [Google Scholar]
- 257.Pierce S, Luzzaro A, Caccianiga M, Ceriani RM, Cerabolini B. Disturbance is the principal α-scale filter determining niche differentiation, coexistence and biodiversity in an alpine community. J. Ecol. 2007;95:698–706. [Google Scholar]
- 258.Pillar VD, Sosinski EE. An improved method for searching plant functional types by numerical analysis. J. Veg. Sci. 2003;14:323–332. [Google Scholar]
- 259.Powers JS, Tiffin P. Plant functional type classifications in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Funct. Ecol. 2010;24:927–936. [Google Scholar]
- 260.Prentice IC, et al. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol. 2010;190:169–180. doi: 10.1111/j.1469-8137.2010.03579.x. [DOI] [PubMed] [Google Scholar]
- 261.Preston KA, Cornwell WK, DeNoyer JL. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytol. 2006;170:807–818. doi: 10.1111/j.1469-8137.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 262.Price CA, Enquist BJ. Scaling mass and morphology in leaves: an extention of the WBE model. Ecology. 2007;88:1132–1141. doi: 10.1890/06-1158. [DOI] [PubMed] [Google Scholar]
- 263.Price CA, Enquist BJ, Savage VM. A general model for allometric covariation in botanical form and function. Proc. Natl Acad. Sci. USA. 2007;104:13204–13209. doi: 10.1073/pnas.0702242104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pyankov VI, Kondratchuk AV, Shipley B. Leaf structure and specific leaf mass: the alpine desert plants of the Eastern Pamirs, Tadjikistan. New Phytol. 1999;143:131–142. [Google Scholar]
- 265.Quero JL, et al. Relating leaf photosynthetic rate to whole-plant growth: drought and shade effects on seedlings of four Quercus species. Funct. Plant Biol. 2008;35:725. doi: 10.1071/FP08149. [DOI] [PubMed] [Google Scholar]
- 266.Quested HM, et al. Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology. 2003;84:3209–3221. [Google Scholar]
- 267.Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis–nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- 268.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 269.Auger S, Shipley B. Inter-specific and intra-specific trait variation along short environmental gradients in an old-growth temperate forest. J. Veg. Sci. 2012;24:419–428. [Google Scholar]
- 270.Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ’hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003;26:1343–1356. [Google Scholar]
- 271.Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- 272.Sack L, Melcher PJ, Liu WH, Middleton E, Pardee T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am. J. Bot. 2006;93:829–839. doi: 10.3732/ajb.93.6.829. [DOI] [PubMed] [Google Scholar]
- 273.Sack L, Tyree MT, Holbrook NM. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005;167:403–413. doi: 10.1111/j.1469-8137.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 274.Sanda V., Bita-Nicolae, C. D. & Barabas, N. The Flora of Spontaneous and Cultivated Cormophytes from Romania (in Romanian) (Editura Ion Bacău, 2003).
- 275.Sandel B, Corbin JD, Krupa M. Using plant functional traits to guide restoration: a case study in California coastal grassland. Ecosphere. 2011;2:art23. [Google Scholar]
- 276.Sardans J, Penuelas J, Ogaya R. Drought-induced changes in C and N stoichiometry in a Quercus ilex Mediterranean forest. For. Sci. 2008;54:513–522. [Google Scholar]
- 277.Sardans, J., Peñuelas, J., Prieto, P. & Estiarte, M. Changes in Ca, Fe, Mg, Mo, Na, and S content in a Mediterranean shrubland under warming and drought. J. Geophys. Res.10.1029/2008jg000795 (2008).
- 278.Scherer-Lorenzen M, Schulze E, Don A, Schumacher J, Weller E. Exploring the functional significance of forest diversity: a new long-term experiment with temperate tree species (biotree) Perspect. Plant Ecol. Evol. Syst. 2007;9:53–70. [Google Scholar]
- 279.Schurr FM, et al. Colonization and persistence ability explain the extent to which plant species fill their potential range. Global Ecol. Biogeogr. 2007;16:449–459. [Google Scholar]
- 280.Schwallier R, et al. Evolution of wood anatomical characters in Nepenthes and close relatives of Caryophyllales. Ann. Bot. 2017;119:1179–1193. doi: 10.1093/aob/mcx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schweingruber FH, Poschlod P. Growth rings in herbs and shrubs: life span, age determination and stem anatomy. Forest Snow Landsc. Res. 2005;79:195–415. [Google Scholar]
- 282.Scoffoni C, Pou A, Aasamaa K, Sack L. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant Cell Environ. 2008;31:1803–1812. doi: 10.1111/j.1365-3040.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 283.Shiodera S, Rahajoe JS, Kohyama T. Variation in longevity and traits of leaves among co-occurring understorey plants in a tropical montane forest. J. Trop. Ecol. 2008;24:121–133. [Google Scholar]
- 284.Shipley B. The use of above-ground maximum relative growth rate as an accurate predictor of whole-plant maximum relative growth rate. Funct. Ecol. 1989;3:771. [Google Scholar]
- 285.Shipley B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Funct. Ecol. 2002;16:682–689. [Google Scholar]
- 286.Shipley B, Lechowicz MJ. The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in 40 wetland species. Écoscience. 2000;7:183–194. [Google Scholar]
- 287.Shipley B, Parent M. Germination responses of 64 wetland species in relation to seed size, minimum time to reproduction and seedling relative growth rate. Funct. Ecol. 1991;5:111. [Google Scholar]
- 288.Shipley B, Vu T-T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002;153:359–364. [Google Scholar]
- 289.Spasojevic MJ, Suding KN. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. J. Ecol. 2012;100:652–661. [Google Scholar]
- 290.Swaine, E. K. Ecological and Evolutionary Drivers of Plant Community Assembly in a Bornean Rain Forest. PhD Thesis (University of Aberdeen, 2007).
- 291.Trefflich, A., Klotz, S. & Kuhn, I. in BIOLFLOR—Eine Datenbank mit Biologisch-ökologischen Merkmalen zur Flora von Deutschland (eds Klotz, S. et al.) 127–131 (Bundesamt für Naturschutz, 2002).
- 292.Tucker SS, Craine JM, Nippert JB. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere. 2011;2:art48. [Google Scholar]
- 293.Ciocarlan, V. The Illustrated Flora of Romania. Pteridophyta et Spermatopyta (in Romanian) (Editura Ceres, 2009).
- 294.van Bodegom PM, Sorrell BK, Oosthoek A, Bakker C, Aerts R. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology. 2008;89:193–204. doi: 10.1890/07-0390.1. [DOI] [PubMed] [Google Scholar]
- 295.Vergutz, L. et al. A Global Database of Carbon and Nutrient Concentrations of Green and Senesced Leaves (ORNL DAAC, 2012); 10.3334/ORNLDAAC/1106 [DOI]
- 296.Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012;82:205–220. [Google Scholar]
- 297.Vile, D. Significations Fonctionnelle et Ecologique des Traits des Especes Vegetales: Exemple dans une Succession Post-cultural Méditerranéenne et Generalisations. PhD thesis (University of Montpellier II, 2005).
- 298.Von Holle B, Simberloff D. Testing Fox’s assembly rule: does plant invasion depend on recipient community structure? Oikos. 2004;105:551–563. [Google Scholar]
- 299.Williams, M., Shimabukuro, Y. E. & Rastetter, E.B. LBA-ECO CD-09 Soil and Vegetation Characteristics, Tapajos National Forest, Brazil (ORNL DAAC, 2012); 10.3334/ORNLDAAC/1104 [DOI]
- 300.Willis CG, et al. Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography. 2010;33:565–577. [Google Scholar]
- 301.Wilson KB, Baldocchi DD, Hanson PJ. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 2000;20:565–578. doi: 10.1093/treephys/20.9.565. [DOI] [PubMed] [Google Scholar]
- 302.Wirth, C. & Lichstein, J. W. in Old-Growth Forests: Function, Fate and Value (eds Wirth, C. et al.) 81–113 (Springer, 2009).
- 303.Wohlfahrt G, et al. Inter-specific variation of the biochemical limitation to photosynthesis and related leaf traits of 30 species from mountain grassland ecosystems under different land use. Plant Cell Environ. 1999;22:1281–1296. [Google Scholar]
- 304.Wright IJ, et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann. Bot. 2007;99:1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wright JP, Sutton-Grier A. Does the leaf economic spectrum hold within local species pools across varying environmental conditions? Funct. Ecol. 2012;26:1390–1398. [Google Scholar]
- 306.Wright SJ, et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology. 2010;91:3664–3674. doi: 10.1890/09-2335.1. [DOI] [PubMed] [Google Scholar]
- 307.Xu L, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 2003;23:865–877. doi: 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]
- 308.Yguel B, et al. Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecol. Lett. 2011;14:1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 309.Zanne, A. E. et al. Global Wood Density Database (EOL, 2009); https://opendata.eol.org/dataset/dde86ffb-7741-44a1-acf2-808b3dd6bc97/resource/d1e2b018-a7ce-444b-ac8a-ac43b2355cc9/download/archive
- 310.Zanne AE, et al. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010;97:207–215. doi: 10.3732/ajb.0900178. [DOI] [PubMed] [Google Scholar]
- 311.Kattge V, et al. TRY - a global database of plant traits. Global Change Biol. 2011;9:2905–2935. [Google Scholar]
- 312.Shan, H. et al. Gap Filling in the Plant Kingdom—Trait Prediction Using Hierarchical Probabilistic Matrix Factorization (ICML, 2012); http://arxiv.org/abs/1206.6439
- 313.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
- 314.Salakhutdinov, R. & Mnih, A. Probabilistic matrix factorization. In Proc. 20th International Conference on Neural Information Processing Systems (eds Platt, J. C. et al.) 1257–1264 (Curran Associates Inc., 2007).
- 315.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
- 316.Lê S, Josse J, Husson F. FactoMineR: a package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. [Google Scholar]
- 317.Dray S, Dufour A-B. The ade4 package: implementing the duality diagram for ecologists. J. Stat. Softw. 2007;22:1–20. [Google Scholar]
- 318.Bougeard S, Dray S. Supervised multiblock analysis in R with the ade4 package. J. Stat. Softw. 2018;86:1–17. [Google Scholar]
- 319.Chessel D, Dufour A-B, Thioulouse J. The ade4 package—I: one-table methods. R News. 2004;4:5–10. [Google Scholar]
- 320.Dray S, Dufour A-B, Chessel D. The ade4 package—II: two-table and K-table methods. R News. 2007;7:47–52. [Google Scholar]
- 321.Thioulouse, J. et al. Multivariate Analysis of Ecological Data with ade4 (Springer, 2018).
- 322.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 323.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J. Stat. Softw. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Batjes NH, Ribeiro E, van Oostrum A. Standardised soil profile data to support global mapping and modelling (WoSIS snapshot 2019) Earth Syst. Sci. Data. 2020;12:299–320. [Google Scholar]
- 325.Hengl T, et al. SoilGrids1km—global soil information based on automated mapping. PLoS ONE. 2014;9:e105992. doi: 10.1371/journal.pone.0105992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Arrouays D, et al. Soil legacy data rescue via GlobalSoilMap and other international and national initiatives. GeoResJ. 2017;14:1–19. doi: 10.1016/j.grj.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Richard, P. & Pielou, E. C. Biogeography (John Wiley & Sons, 1979).
- 328.Udvardy, M. D. F. A Classification of the Biogeographical Provinces of the World (International Union for Conservation of Nature and Natural Resources, 1975).
- 329.Dinerstein, E. et al. A Conservation Assessment of the Terrestrial Ecoregions of Latin America and the Caribbean (The World Bank, 1995).
- 330.Ricketts, T. H. et al. Terrestrial Ecoregions of North America: A Conservation Assessment (Island Press, 1999).
- 331.Dasmann, R. F. A System for Defining and Classifying Natural Regions for Purposes of Conservation: A Progress Report (IUCN, 1973).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–42 and Tables 1–8.
Data Availability Statement
Plant trait data were accessed from the TRY database (https://try-db.org, request no. 3282, date accessed July 2017, see also Extended Data Fig. 1). All TRY data required to reproduce this analysis, and the corresponding R scripts, are provided in an open TRY File Archive (https://www.try-db.org/TryWeb/Data.php). Climate data WorldClim are publicly available via https://www.worldclim.org/ (accessed May 2018). Soil data, namely SoilGrids (https://soilgrids.org/, accessed June 2018) are publicly available. Ecoregion information30 shapefiles are publicly available (accessed January 2014, Sciencebase.gov), The estimate of species richness per ecoregion174 is publicly available (accessed January 2014, databasin.org. Data for this study can be accessed on Github (https://github.com/juliajoswig/ Repo_ClimateSoil_TraitSpectrum). For Extended Data Fig. 1 and Supplementary Fig. 7, the Geodata product of the Missions Database ‘ArcWorld Supplement’ (GMI) was used.
The code is available on Github (https://github.com/juliajoswig/Repo_ClimateSoil_TraitSpectrum).