Abstract
STUDY OBJECTIVE:
To examine the effectiveness of endometrial sampling for preoperative detection of uterine leiomyosarcoma in women undergoing hysterectomy, identify factors associated with missed diagnosis, and compare outcomes of patients who had preoperative versus missed diagnosis.
DESIGN:
Retrospective cohort study using linked data from the New York Statewide Planning and Research Cooperative System and New York State Cancer Registry in 2003–2015.
SETTING:
Inpatient and outpatient encounters at civilian hospitals and ambulatory surgery centers in New York State.
PATIENTS:
Women with uterine leiomyosarcoma who underwent a hysterectomy and a preoperative endometrial sampling within 90 days before the hysterectomy.
INTERVENTION:
Endometrial sampling.
MEASUREMENT:
Detection of leiomyosarcoma before hysterectomy (preoperative diagnosis) versus after hysterectomy (postoperative diagnosis).
MAIN RESULTS:
A total of 79 patients with uterine leiomyosarcoma met sample eligibility criteria. Of these patients, 46 (58.2%) were diagnosed preoperatively and 33 (41.8%) were diagnosed postoperatively. Patients in the two groups did not differ significantly in age, race/ethnicity, bleeding symptoms, and comorbidities assessed. In multivariable regression analysis, women who had endometrial sampling performed with hysteroscopy (compared to without hysteroscopy) had a higher likelihood of preoperative diagnosis (adjusted risk ratio [aRR]=3.03, 95% confidence interval [CI]: 1.43–6.42). Patients with localized stage (versus distant stage) or tumor size >11 centimeters (versus <8 centimeters) were less likely to be diagnosed preoperatively (aRR=0.50, 95% CI: 0.28–0.89; and aRR=0.54, 95% CI: 0.30–0.99; respectively). Supracervical hysterectomy was not performed in any of the patients whose leiomyosarcoma was diagnosed preoperatively, compared to 21.2% of patients who were diagnosed postoperatively (p=.002).
CONCLUSION:
Endometrial sampling detected leiomyosarcoma preoperatively in 58.2% of patients. Use of hysteroscopy with endometrial sampling improved preoperative detection of leiomyosarcoma by three-fold. Patients with missed diagnosis had a higher risk of undergoing suboptimal surgical management at the time of their index surgery.
Keywords: Leiomyosarcoma, Endometrial sampling, Hysterectomy, Hysteroscopy
Précis
For women with leiomyosarcoma undergoing hysterectomy, preoperative endometrial sampling failed to detect 41.8% of the leiomyosarcomas; missed diagnosis increased patients’ risk of undergoing supracervical hysterectomy.
INTRODUCTION
Uterine leiomyomas or fibroids are benign smooth muscle tumors of the uterus, affecting 70% of white women and 84% of African American women by age 50 [1]. Patients can have abnormal bleeding, pelvic pressure/pain, and infertility up to 30% of the time [2–4]. Symptomatic fibroids account for up to 40% of all hysterectomies performed for benign indications in the U.S. [5].
Leiomyosarcoma (LMS) is a rare but aggressive malignant tumor that often mimics the appearance of benign leiomyomas and can be challenging to distinguish preoperatively. LMS has a particularly poor prognosis, with a 5-year survival rate ranging from 13.1% for stage IV disease to 55.4% for stage I disease [6]. The risk of unexpected LMS can be up to 13 per 10,000 surgeries for presumed fibroids [7].
Choice of hysterectomy approach (supracervical versus total hysterectomy) and tissue extraction method (intact versus morcellated in pieces) can affect LMS prognosis [8]. Therefore, it is important to diagnose LMS preoperatively in order to inform appropriate surgical planning. Yet preoperative diagnosis of LMS remains a major challenge. Although we are aware of some risk factors for LMS (e.g., postmenopausal age, Black race, history of pelvic radiation, extended tamoxifen use, and solitary, rapidly increasing tumors with atypical features) [9–13], there is currently no reliable diagnostic test to differentiate LMS from benign leiomyomas [8]. Endometrial sampling is the most widely used tool for preoperative evaluation of uterine malignancy [14]. However, even though it can detect over 90% of epithelial endometrial carcinomas, evidence on the predictive value of preoperative endometrial sampling to identify LMS is sparse [8].
The primary objective of this study was to examine the frequency of missed LMS diagnosis in patients undergoing preoperative endometrial sampling prior to hysterectomy and the characteristics of patients who had missed diagnosis. Our secondary objective was to examine the association between missed diagnosis and patient outcomes, including use of inappropriate surgical approach and survival.
MATERIALS AND METHODS
Data Sources and Sample
This study used data from the New York Statewide Planning and Research Cooperative System (SPARCS) [15], linked to data from the New York State Cancer Registry (NYSCR) [16]. The SPARCS has rigorous procedures in place (such as edit checks and audit review) to ensure high quality of data, and the NYSCR has been awarded the North American Association of Central Cancer Registries (NAACCR) Gold-level certification for cancer data quality for cancer cases diagnosed since 1998 [15, 16]. The SPARCS captures all inpatient stays and outpatient encounters (including ambulatory surgery, emergency department visits, and outpatient service visits) that occur at civilian hospitals and ambulatory surgery centers in New York State regardless of payer. Data linkage was performed based on a unique personal identifier and date of birth. The linked data provided sociodemographic and clinical information for all women age 18 years or older who underwent an inpatient or outpatient hysterectomy from October 1, 2003 through September 30, 2015 in the SPARCS database, along with cancer registry information on diagnosis of LMS and vital status through December 31, 2015. This study was approved by the Yale Human Investigation Committee.
We limited our sample to women with LMS who underwent a hysterectomy and an endometrial sampling within 90 days before the date of hysterectomy. Patients who had a pre-existing diagnosis of LMS prior to the date of endometrial sampling, had other cancers prior to the diagnosis of LMS, or had an LMS diagnosed more than three months after hysterectomy (which might not have been present at the time of endometrial sampling) were excluded (Figure 1). Hysterectomies were identified using the International Classification of Diseases Ninth Revision (ICD-9) procedure codes and Current Procedural Terminology (CPT) codes.
Figure 1.
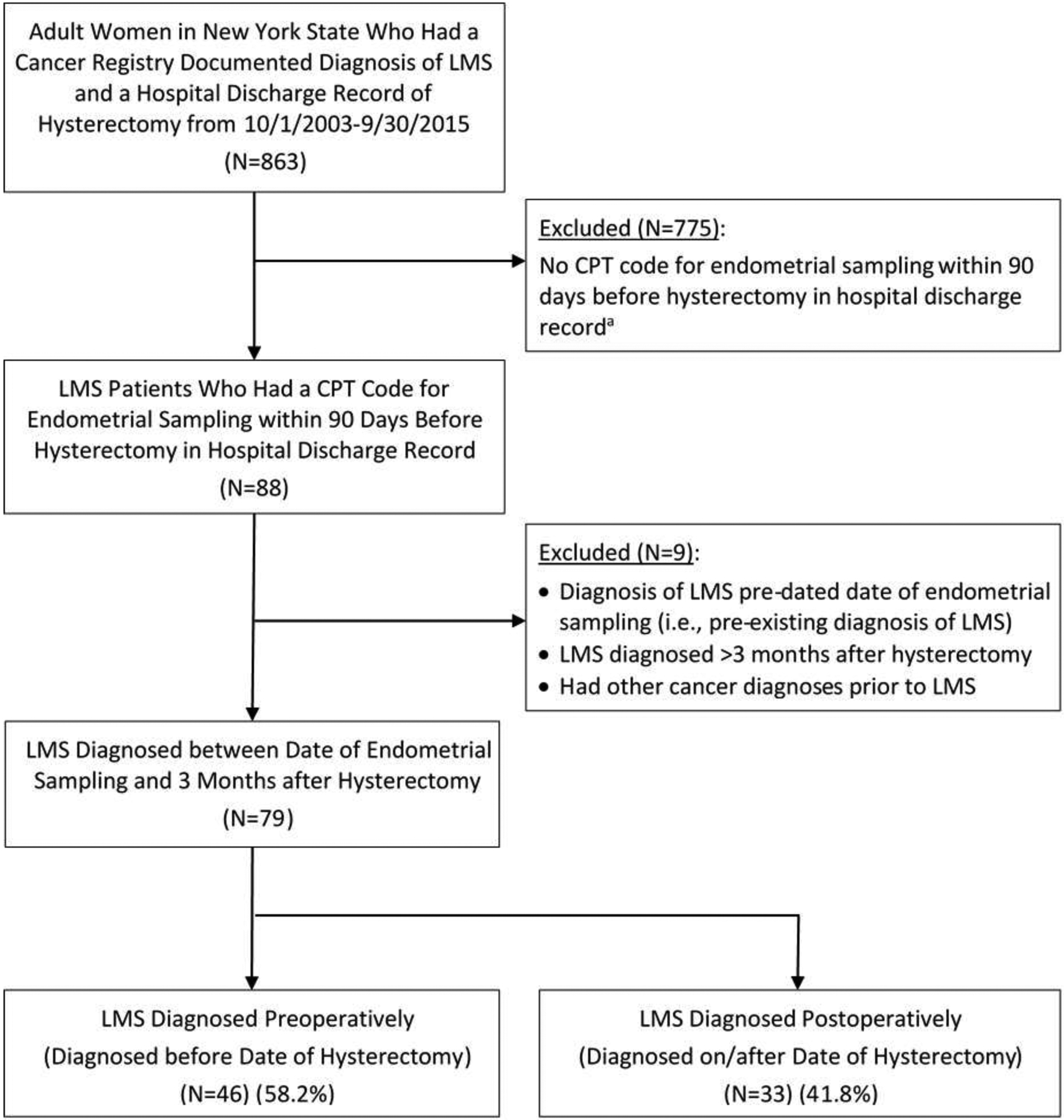
Sample selection diagram
CPT = Current Procedural Terminology; ICD = International Classification of Diseases; LMS = leiomyosarcoma.
a. Some of these patients might have received endometrial sampling outside of hospitals which we were not able to capture.
Endometrial sampling was identified by CPT codes and included endometrial biopsy (58100 or 58110), dilation and curettage (D&C) (58120), and hysteroscopic endometrial biopsy (with or without D&C) (58558). As the SPARCS data are based on inpatient and outpatient hospital encounters, endometrial sampling included in our analysis reflect those performed in the hospital setting. Diagnosis of LMS was determined based on the International Classification of Diseases for Oncology Third Edition (ICD-O-3) histology code 8890, 8891 and 8896 in conjunction with site code 54.x and 55.x and behavioral code for malignancy (excluding in situ disease).
Measures
We categorized each patient into one of the following two groups: LMS diagnosed before the date of hysterectomy (preoperative diagnosis) and LMS diagnosed on/after the date of hysterectomy (postoperative diagnosis). This was determined by comparing the date of LMS diagnosis (as documented in cancer registry) and date of hysterectomy procedure (as documented in hospital discharge record).
For each patient in the sample, we documented age, race/ethnicity, type of insurance, cancer stage, grade, tumor size, and receipt of chemotherapy and radiation therapy, and date and cause of death using cancer registry data. Cancer stage was categorized as localized, regional, or distant by consolidating information from the American Joint Committee on Cancer stage variable and the Surveillance, Epidemiology, and End Results Program stage variable (using the more advanced stage if there was discrepancy).
We distinguished whether the endometrial sampling was performed with hysteroscopy (CPT code 58558) versus without hysteroscopy (CPT codes 58100, 58110, or 58120) based on CPT codes. Presence of uterine leiomyoma and abnormal bleeding at the time of endometrial sampling, use of other diagnostic procedures, comorbid conditions, hysterectomy approach, and surgical outcomes were measured based on diagnosis and procedure codes in the patient’s longitudinal hospital discharge records. For abnormal bleeding, postmenopausal bleeding was distinguished from premenopausal menorrhagia or frequent menstruation. Additional diagnostic procedures included pelvic magnetic resonance imaging and transvaginal ultrasound performed during the 90-day preoperative period. Hysterectomy approach was categorized as total/radical hysterectomy versus supracervical hysterectomy. Measures of surgical outcomes included whether blood transfusion occurred, length of hospital stay, whether re-operation was performed within a four-month period after hysterectomy (for staging, debulking, or removal of the cervix if supracervical hysterectomy was performed initially), and readmission in the four-month period after hysterectomy.
We also measured all-cause and disease-specific survival for each patient. Date and cause of death were documented in the cancer registry based on New York State death certificate, U.S. National Death Index, and/or Social Security Death Index. Patients without recorded death in these information sources were presumed alive. We calculated all-cause survival as the time (in months) between the date of hysterectomy (as documented in hospital discharge record) and date of death (if a patient died) or 12/31/2015 (if a patient was alive). We calculated disease-specific survival as the time (in months) between the date of hysterectomy and the date of death (if a patient died of corpus uteri cancer) or 12/31/2015. If a patient died of other causes, her date of death was used as the date of censoring for disease-specific survival. For the purpose of this study, we used date of hysterectomy (instead of date of diagnosis) as the starting date for calculating survival time to avoid bias against patients whose LMS was missed preoperatively.
Statistical Analysis
We compared characteristics and outcomes of patients whose LMS was diagnosed preoperatively versus women whose LMS was diagnosed postoperatively. Chi square test (or Fisher’s exact test when the expected number of cases was less than 5 for at least 25% of the cells) was used for categorical variables and Wilcoxon rank sum test was used for continuous variables. We performed a multivariable regression to examine the association between use of hysteroscopy at the time of endometrial sampling (versus without hysteroscopy) and the likelihood of preoperative diagnosis of LMS, while adjusting for other patient and tumor characteristics (age, race/ethnicity, cancer stage, cancer grade, tumor size, and symptoms). Since patients undergoing hysteroscopy tended to have more extensive sampling via a D&C (rather than simple endometrial biopsy), we performed a sensitivity analysis comparing the likelihood of preoperative diagnosis between patients who underwent endometrial sampling with hysteroscopy and patients who underwent D&C without hysteroscopy.
All-cause mortality and disease-specific mortality between the two groups were compared using Kaplan-Meier curves with log-rank test. We further examined the association between timing of diagnosis (preoperative versus postoperative) and mortality risk using Cox proportional hazards models, while adjusting for patient age, race/ethnicity, cancer stage, grade, tumor size, and number of comorbidities. Proportional hazard assumptions were assessed using the supremum test. P values less than 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 79 patients with LMS met our sample eligibility criteria (Figure 1). Of these patients, 60 (75.9%) underwent endometrial sampling with hysteroscopy, and 19 (24.1%) underwent endometrial sampling without hysteroscopy. Of the 79 patients, 46 (58.2%) were diagnosed before the date of hysterectomy, and 33 (41.8%) were diagnosed on or after the date of hysterectomy.
In bivariate analysis, patients diagnosed postoperatively did not differ significantly from those diagnosed preoperatively in cancer stage, grade, age, bleeding symptoms, or comorbidities (Table 1). However, tumor size was larger among patients who were diagnosed postoperatively than those diagnosed preoperatively (median: 11 versus 9 centimeters, p=.04). A higher proportion of patients who underwent endometrial sampling with hysteroscopy were diagnosed preoperatively (40 out of 60 patients, i.e., 66.7%) compared to those who underwent sampling without hysteroscopy (6 out of 19 patients, i.e., 31.6%) (p=.007) (Table 1).
TABLE 1.
Patient characteristics by timing of leiomyosarcoma diagnosis (N=79)
| Patient Characteristics | Diagnosed Preoperatively (N=46) | Diagnosed Postoperatively (N=33) | P Value |
|---|---|---|---|
| Age, years | .27 | ||
| 18–54 | 14 (50.0%) | 14 (50.0%) | |
| ≥55 | 32 (62.7%) | 19 (37.3%) | |
| Race/ethnicity | .31 | ||
| Non-Hispanic white | 29 (63.0%) | 17 (37.0%) | |
| Other/unknown | 17 (51.5%) | 16 (48.5%) | |
| Primary payer | .83 | ||
| Private insurance | 29 (59.2%) | 20 (40.8%) | |
| Other/unknown | 17 (56.7%) | 13 (43.3%) | |
| Cancer stage | .71 | ||
| Localized | 25 (54.3%) | 21 (45.7%) | |
| Regional | 11 (64.7%) | 6 (35.3%) | |
| Distant | 10 (62.5%) | 6 (37.5%) | |
| Tumor grade | .16 | ||
| 1–2 | 2 (100.0%) | 0 (0.0%) | |
| 3 | 11 (47.8%) | 12 (52.2%) | |
| 4 | 23 (69.7%) | 10 (30.3%) | |
| Unknown | 10 (47.6%) | 11 (52.4%) | |
| Tumor size (in centimeters),a mean±SD, median (interquartile range) | 8.6±4.6, 9 (6–11) | 11.2±5.0, 11 (8.5–14.5) | .04 |
| Presence of a diagnosis code for leiomyoma | .09 | ||
| Yes | 15 (46.9%) | 17 (53.1%) | |
| No | 31 (66.0%) | 16 (34.0%) | |
| Postmenopausal bleeding | .12 | ||
| Yes | 29 (65.9%) | 15 (34.1%) | |
| No | 17 (48.6%) | 18 (51.4%) | |
| Premenopausal menorrhagia or frequent menstruation | .33 | ||
| Yes | 6 (46.2%) | 7 (53.8%) | |
| No | 40 (60.6%) | 26 (39.4%) | |
| Diabetes | .73 | ||
| Yes | 6 (66.7%) | 3 (33.3%) | |
| No | 40 (57.1%) | 30 (42.9%) | |
| Hypertension | .09 | ||
| Yes | 18 (72.0%) | 7 (28.0%) | |
| No | 28 (51.9%) | 26 (48.1%) | |
| Obesity | 1.00 | ||
| Yes | 2 (66.7%) | 1 (33.3%) | |
| No | 44 (57.9%) | 32 (42.1%) | |
| Additional MRI/transvaginal ultrasound evaluation in 90-day preoperative periodb | .64 | ||
| Yes | 3 (75.0%) | 1 (25.0%) | |
| No | 43 (57.3%) | 32 (42.7%) | |
| Type of endometrial sampling | .007 | ||
| With hysteroscopy | 40 (66.7%) | 20 (33.3%) | |
| Without hysteroscopy | 6 (31.6%) | 13 (68.4%) |
MRI = Magnetic resonance imaging; SD = standard deviation.
N=18 patients had missing data on tumor size.
Limited to MRI/transvaginal ultrasound documented in hospital discharge records.
After adjusting for other patient and tumor characteristics, patients whose endometrial sampling involved hysteroscopy were more likely to be diagnosed preoperatively (adjusted risk ratio = 3.03, 95% CI: 1.43–6.42) compared to patients whose endometrial sampling was performed without hysteroscopy (Table 2). In addition, patients with localized stage were less likely than those with distant stage to be diagnosed preoperatively (adjusted risk ratio = 0.50, 95% CI: 0.28–0.89). Patients with a tumor size larger than 11 centimeters were less likely than those with a tumor size smaller than 8 centimeters to be diagnosed preoperatively (adjusted risk ratio = 0.54, 95% CI: 0.30–0.99).
TABLE 2.
Association between use of hysteroscopy and likelihood of preoperative diagnosis of leiomyosarcoma, after adjusting for other patient and tumor characteristics
| Patient and Tumor Characteristics | Adjusted Risk Ratio | 95% Confidence Interval |
|---|---|---|
| Type of endometrial sampling | ||
| With hysteroscopy | 3.03 | (1.43, 6.42) |
| Without hysteroscopy | Reference | |
| Age | ||
| 18–54 years | Reference | |
| ≥55 years | 1.00 | (0.64, 1.54) |
| Race/Ethnicity | ||
| Non-Hispanic White | 1.00 | (0.66, 1.50) |
| Other/Unknown | Reference | |
| Cancer stage | ||
| Localized | 0.50 | (0.28, 0.89) |
| Regional | 0.60 | (0.35, 1.05) |
| Distant | Reference | |
| Cancer grade | ||
| 1–3 | Reference | |
| 4 | 1.35 | (0.90, 2.03) |
| Unknown | 1.06 | (0.61, 1.84) |
| Tumor size | ||
| <8 cm | Reference | |
| 8–11 cm | 0.82 | (0.49, 1.38) |
| >11 cm | 0.54 | (0.30, 0.99) |
| Unknown | 1.36 | (0.82, 2.24) |
| Presence of a diagnosis code for leiomyoma | ||
| Yes | 0.92 | (0.62, 1.36) |
| No | Reference | |
| Postmenopausal bleeding | ||
| Yes | 1.13 | (0.70, 1.81) |
| No | Reference | |
| Premenopausal menorrhagia/frequent Menstruation | ||
| Yes | 0.78 | (0.42, 1.47) |
| No | Reference |
Similar results were found in our sensitivity analysis comparing patients who underwent endometrial sampling with hysteroscopy versus patients who underwent D&C without hysteroscopy. Specifically, 66.7% of the patients who underwent endometrial sampling with hysteroscopy versus 28.6% of the patients who underwent D&C without hysteroscopy were diagnosed preoperatively (p=0.009). The corresponding adjusted risk ratio for preoperative diagnosis was 3.56 (95% CI: 1.84–6.90) in multivariable regression analysis accounting for other patient and tumor characteristics.
All patients who were diagnosed preoperatively underwent a total/radical hysterectomy at the index surgery (Table 3). In contrast, 7 of the 33 patients (21.2%) who were diagnosed postoperatively underwent a supracervical hysterectomy (p=.002), and three of them later underwent a second surgery for removal of the cervix uteri or staging. Among the seven patients whose LMS was missed preoperatively and underwent a supracervical hysterectomy, four had localized stage while three had regional or distant stage. Five of them received or were recommended to receive chemotherapy as part of their first course treatment, and one received radiation therapy. There was no significant difference in length of stay between patients diagnosed preoperatively and postoperatively. However, a higher proportion of patients who were diagnosed postoperatively had blood transfusion, compared to those who were diagnosed preoperatively (42.4% versus 17.4%, p=.01). The proportion of women receiving chemotherapy or radiation therapy was similar between those diagnosed preoperatively and postoperatively.
TABLE 3.
Patient outcomes by timing of leiomyosarcoma diagnosis (N=79)
| Patient Outcomes | Diagnosed Preoperatively (N=46) | Diagnosed Postoperatively (N=33) | P Value |
|---|---|---|---|
| Type of hysterectomy | .002 | ||
| Total/radical hysterectomy | 46 (100.0%) | 26 (78.8%) | |
| Supracervical hysterectomy | 0 (0%) | 7 (21.2%) | |
| Length of stay (days), mean±SD, median (interquartile range) | 4.4±6.6, 3 (2–5) | 4.2±2.6, 4 (2–5) | .32 |
| Blood transfusion | .01 | ||
| Yes | 8 (17.4%) | 14 (42.4%) | |
| No | 38 (82.6%) | 19 (57.6%) | |
| Reoperation (within 4 months after hysterectomy) | .07 | ||
| Yes | 0 (0%) | 3 (9.1%) | |
| No | 46 (100.0%) | 30 (90.9%) | |
| Re-admission (within 4 months after hysterectomy) | .78 | ||
| Yes | 14 (30.4%) | 11 (33.3%) | |
| No | 32 (69.6%) | 22 (66.7%) | |
| Radiation therapya | .75 | ||
| Yes | 7 (15.9%) | 4 (12.1%) | |
| No | 37 (84.1%) | 29 (87.9%) | |
| Chemotherapyb | .87 | ||
| Yes | 21 (45.7%) | 14 (43.8%) | |
| No | 25 (54.3%) | 18 (56.3%) | |
| Mortality risk | |||
| All-cause mortality | |||
| Unadjusted, hazard ratio (95% CI) | Reference | 1.15 (0.61–2.17) | .66 |
| Adjusted,c hazard ratio (95% CI) | Reference | 0.87 (0.41–1.85) | .72 |
| Disease-specific mortality | |||
| Unadjusted, hazard ratio (95% CI) | Reference | 1.16 (0.54–2.52) | .70 |
| Adjusted,c hazard ratio (95% CI) | Reference | 0.65 (0.24–1.75) | .39 |
CI = confidence interval; SD = standard deviation.
N=2 patients had missing data.
N=1 patient had missing data.
After adjustment for patient age, race/ethnicity, cancer stage, grade, tumor size, and number of comorbidities.
The median overall survival of the 79 patients in the sample was 50 months (95% CI: 31–109). There was no significant difference in unadjusted survival between women diagnosed preoperatively and postoperatively (p value for log-rank test = 0.66 for all-cause mortality and 0.70 for disease-specific mortality) (Figure 2). After adjusting for patient clinical risk factors, the risk of all-cause mortality (adjusted hazard ratio = 0.87, 95% CI: 0.41–1.85) and disease-specific mortality (adjusted hazard ratio = 0.65, 95% CI: 0.24–1.75) did not differ significantly for patients who were diagnosed postoperatively versus preoperatively (Table 3).
Figure 2.
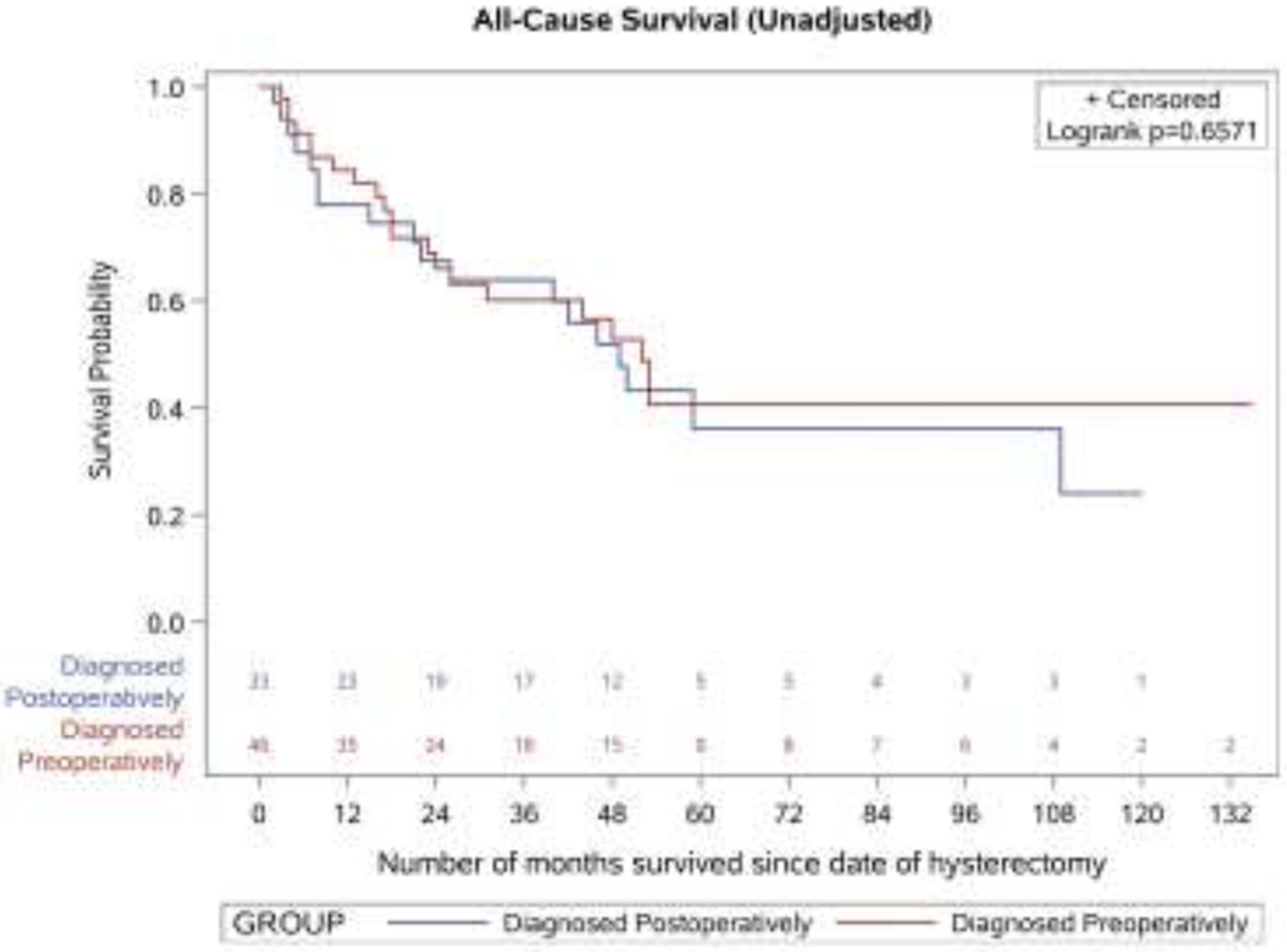
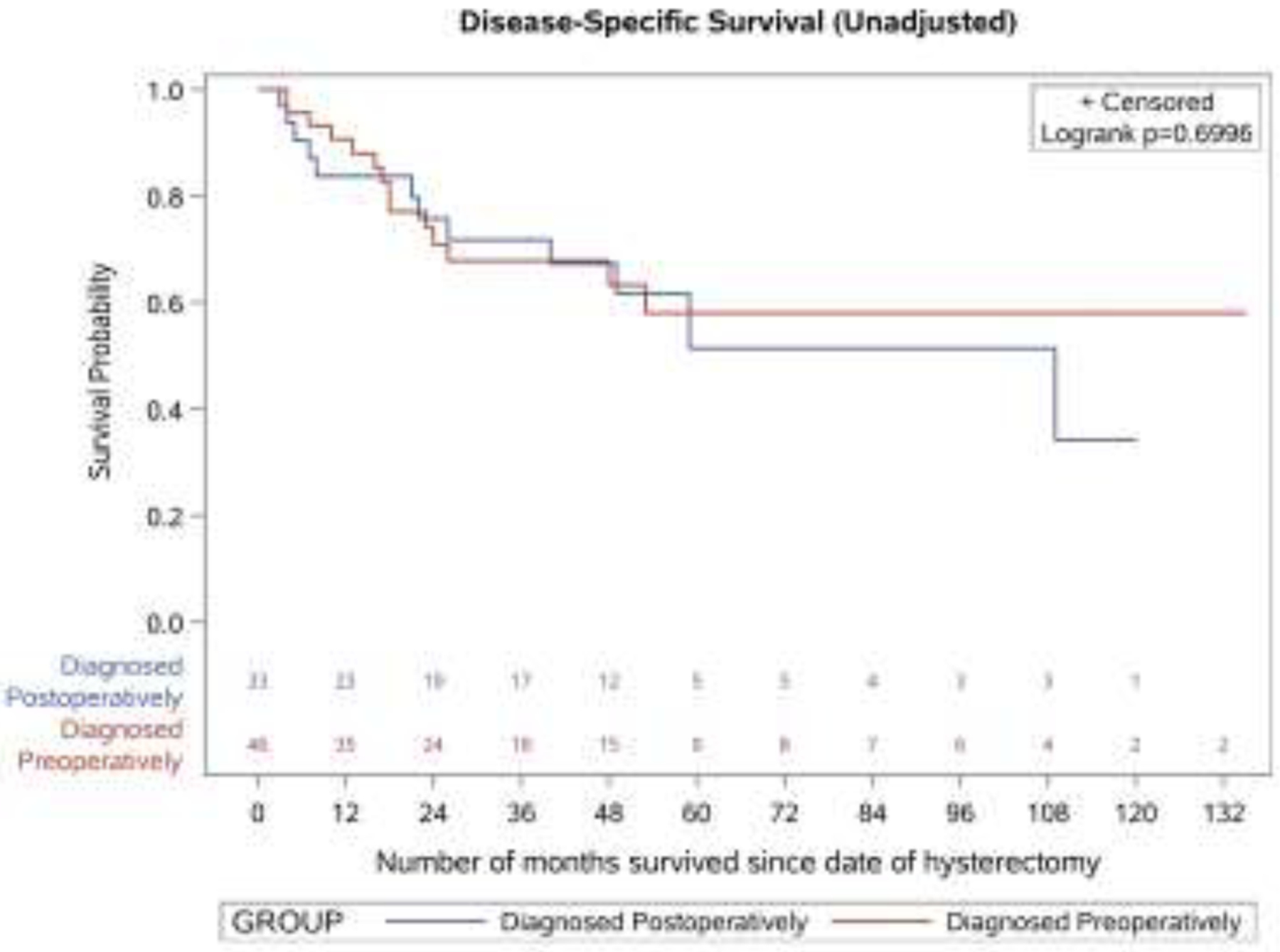
Unadjusted survival outcomes by timing of leiomyosarcoma diagnosis
A. All-cause survival
B. Disease-specific survival
DISCUSSION
Among women with LMS undergoing hysterectomy, we showed that endometrial sampling was able to diagnose 58.2% of them preoperatively. Use of hysteroscopy during endometrial sampling improved LMS detection, whereas early cancer stage and large tumor size were associated with a lower likelihood of detection. When missed preoperatively, 21.2% of the patients underwent a supracervical hysterectomy, which is inappropriate for surgical management of LMS.
LMS, an aggressive subtype of uterine sarcoma, contributes to a significant proportion of uterine cancer deaths [6]. Uterine sarcoma arises from the deep muscle layer of the uterus and is generally considered inaccessible using standard endometrial sampling. However, endometrial sampling is the most readily available tool for preoperative evaluation and detection of uterine malignancy, and it is important to understand its clinical utility in detecting uterine sarcoma. Yet empirical data have been sparse and were limited to the experience from a few selected institutions. These studies reported that preoperative endometrial biopsy using Pipelle biopsy or traditional uterine curettage detected only 35.3%−66.7% of patients with LMS [17–19]. Our study extends this literature by presenting more generalizable data using a statewide database and showed that preoperative endometrial sampling detected LMS in 58.2% of the patients.
Moreover, our study is among the first to demonstrate the benefit of hysteroscopy for the detection of LMS. Previous research showed that D&C alone failed to detect intrauterine pathology in 62.5% of patients [20] and blind endometrial sampling with curettage missed 42% of endometrial polyps and 27% of premalignant/malignant endometrial lesions [21]. Hysteroscopy has since been established to increase the accuracy of endometrial sampling for detecting endometrial pathology (e.g., atypical hyperplasia and endometrial cancer), and has now replaced blind D&C as a reference standard [22, 23]. Nonetheless, the additional benefit of hysteroscopy for the detection of LMS, which is a deep muscle tumor, has not been previously shown. Our study addressed this important gap and demonstrated that hysteroscopy in addition to biopsy or D&C improved the likelihood of LMS detection by three-fold.
Patients whose LMS diagnosis was missed preoperatively had significantly larger tumors compared to those whose LMS was diagnosed preoperatively, even after adjusting for other tumor characteristics. Although we could not determine the location of the index tumor in relation to the endometrial cavity from our dataset, the association between larger tumor size and missed diagnosis may reflect the greater difficulty in adequately sampling the lining of the uterus when larger size tumors are present. Large tumors often distort the endometrial cavity making it difficult to visualize and navigate the pipelle or other instrument to sample the lining. In addition, a higher proportion (20.0%) of patients with large tumors (>11 centimeter) in our sample had “fibroid” without bleeding abnormality, compared to 10.5% of patients with small tumors (<8 centimeters). It is possible that LMS patients with larger tumors in our sample had less “concerning” symptoms and hence endometrial sampling was less vigorously attempted. These potential reasons should be closely examined in future studies to inform opportunities for improvement.
Our study confirmed that preoperative detection of LMS resulted in appropriate management and improved patients’ surgical outcome. Patients whose LMS was diagnosed preoperatively had a lower risk of blood transfusion compared to patients whose diagnosis was missed. This may reflect appropriate surgical planning and better coordinated intraoperative management for cases who were diagnosed preoperatively. In contrast, for patients whose diagnosis was missed preoperatively, unexpected finding or suspicion of malignancy might increase the complexity of the surgery, such as possible involvement of intraoperative frozen section procedure, prolonged operative time, and increased blood loss. Unfortunately, empirical data in this area have been sparse and warrant close attention in future research. In addition, 21.2% of the patients whose LMS was missed preoperatively ended up with a subtotal hysterectomy and some required a second procedure for the removal of the cervix or staging. A subtotal hysterectomy may result in a higher risk of residual disease and inadvertent dissemination of disease if morcellation is performed for tissue extraction. Several studies have shown worse prognosis of patients with LMS if they underwent power morcellation [24–26]. The lack of association between correct preoperative diagnosis and mortality risk in our study was likely due to our relatively small sample size and short follow-up period. Future research with a larger sample and longer follow-up would be helpful. Moreover, suboptimal index surgery (e.g., supracervical hysterectomy) and a need for re-operation can adversely affect other measures of prognosis, such as recurrence and quality of life, which are also important outcomes for cancer patients and should be evaluated in future studies as well.
Strengths of this study include the utilization of a statewide database that is linked to a cancer registry and the longitudinal nature of the data. These allowed for reliable identification of LMS, inclusion of a diverse sample of patients, and tracking of subsequent surgical and cancer-related outcomes. However, we recognize several limitations of this study. First, we relied on hospital-based data. Although these data captured endometrial sampling performed in hospital-based outpatient clinics, we could not evaluate the effectiveness of endometrial sampling performed by physicians in office-based settings outside of hospitals. Likewise, we may have missed concomitant tests or imaging studies received by patients outside the hospitals, leading to a potential overestimation of the effectiveness of endometrial sampling in diagnosing LMS. Second, our data lacked granularity in some clinical information (e.g., patient symptoms) and provider characteristics (e.g., experience), which limited our ability in identifying factors that might affect the effectiveness of endometrial sampling in detecting LMS. We also lacked information about the exact technique or process of how specimens were collected at the time of a hysteroscopic procedure. Further research to elucidate the best practice of using hysteroscopy to assist endometrial sampling would provide additional insights. Third, although we included statewide data from 12 years, LMS is rare and we focused on LMS patients who underwent a hospital-based endometrial sampling preoperatively. This resulted in a relatively small sample size, limiting our statistical power in some analysis. Finally, our data came from a single state and our findings may not be generalizable to other regions in the country.
CONCLUSION
For women with LMS undergoing hysterectomy, preoperative endometrial sampling missed LMS diagnosis in over 40% of cases. However, use of hysteroscopy with the endometrial sampling increased likelihood of preoperative detection by three-fold. When the suspicion level is high for LMS, judicious use of endometrial sampling with hysteroscopy in the preoperative workup and selection of total (instead of supracervical) hysterectomy may help improve patient outcomes.
ACKNOWLEDGEMENT:
We would like to thank colleagues at the New York Statewide Planning and Research Cooperative System (SPARCS) for their assistance with data acquisition.
Conflicts of Interest:
Dr. Desai is an employee of CooperSurgical Inc. with an adjunct faculty appointment with Yale University. Dr. Wright has served as a consultant for Tesaro and Clovis Oncology and received research funding from Merck. Dr. Gross has received grant funding for research distinct from this project from the National Comprehensive Cancer Network (NCCN) Foundation (Pfizer/Astra-Zeneca), Genentech, and Johnson & Johnson, as well as funding from Flatiron, Inc. for travel to and speaking at a scientific conference. The other authors had no conflict of interest to declare.
Funding/Support:
This project was supported by grant number R01HS024702 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The New York State Cancer Registry was supported by the State of New York and by cooperative agreement 6NU58DP006309 awarded to the New York State Department of Health by the Centers for Disease Control and Prevention (CDC) and by Contract 75N91018D00005 (Task Order 75N91018F00001) from the National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services.
Role of the Funding Source:
The funders had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conference Presentation: Preliminary results of this study were presented at the American Association of Gynecologic Laparoscopists virtual global congress, November 6-14, 2020.
Disclaimer: This publication was produced from raw data purchased from or provided by the New York State Department of Health (NYSDOH). However, the conclusions derived, and views expressed herein are those of the author(s) and do not reflect the conclusions or views of NYSDOH. NYSDOH, its employees, officers, and agents make no representation, warranty or guarantee as to the accuracy, completeness, currency, or suitability of the information provided here.
Date and Number of IRB: Yale University Human Investigation Committee Protocol#: 1604017569; Date of Approval: April 19, 2016 (original application) and April 10, 2019 (latest renewal)
REFERENCES
- 1.Sparic R, Mirkovic L, Malvasi A, Tinelli A. Epidemiology of uterine myomas: a review. Int J Fertil Steril. 2016; 9(4): 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol 2016;59(1):30–52. [DOI] [PubMed] [Google Scholar]
- 3.David M, Pitz CM, Mihaylova A, Siedentopf F. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol 2016;199:137–140. [DOI] [PubMed] [Google Scholar]
- 4.Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet 2012;29(08):703–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 2013;122(2 Pt 1):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seagle BL, Sobecki-Rausch J, Strohl AE, Shili A, Grace A, Shahabi S. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gyn Oncol 2017; 145(1):61–70. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of Uterine Fibroids. Comparative Effectiveness Review No. 195. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2015-00003-I.) AHRQ Publication No. 17(18)-EHC028- EF. Rockville, MD: Agency for Healthcare Research and Quality; December 2017. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-195-uterine-fibroids-final-revision.pdf. Accessed April 20, 2020. [PubMed] [Google Scholar]
- 8.Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol 2017;145(1):208–216. [DOI] [PubMed] [Google Scholar]
- 9.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112(4): 820–830. [DOI] [PubMed] [Google Scholar]
- 10.Halaska MJ, Haidopoulos D, Guyon F, Morice P, Zapardiel I, Kesic V. European Society of Gynecological Oncology Statement on fibroid and uterine morcellation. Int J Gynecol Cancer. 2016;27(1):189–192. [DOI] [PubMed] [Google Scholar]
- 11.Brohl AS, Li L, Andikyan V, et al. Age-specific risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. Oncologist. 2015;20(4):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amant F, Van den Bosch T, Vergote I, et al. Morcellation of uterine leiomyomas: a plea for patient triage. Lancet Oncol. 2015;16(15):1454–1456. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014;210(4):368.e1–368.e8. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121(4):891–896. [DOI] [PubMed] [Google Scholar]
- 15.New York State Department of Health. Statewide Planning and Research Cooperative System (SPARCS). Available at: https://www.health.ny.gov/statistics/sparcs/. Accessed January 21, 2021.
- 16.New York State Department of Health. NYS Cancer Registry and Cancer Statistics. Available at: https://www.health.ny.gov/statistics/cancer/registry/. Accessed January 21, 2021.
- 17.Leibsohn S, d’Ablaing G, Mishell DR Jr, Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162(4):968–974. [DOI] [PubMed] [Google Scholar]
- 18.Hinchcliff EM, Esselen KM, Watkins JC, et al. The role of endometrial biopsy in the preoperative detection of uterine leiomyosarcoma. J Minim Invasive Gynecol. 2016; 23(4):567–572. [DOI] [PubMed] [Google Scholar]
- 19.Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol 2008;110(1):43–48. [DOI] [PubMed] [Google Scholar]
- 20.Bettocchi S, Ceci O, Vicino M, Marello F, Impedovo L, Selvaggi L. Diagnostic inadequacy of dilatation and curettage. Fertil Steril 2001;75(4):803–805. [DOI] [PubMed] [Google Scholar]
- 21.Ergenoglu AH, Hortu I, Taylan E, et al. Can we rely on blind endometrial curettage for complete removal of focal intrauterine lesion? A prospective clinical study. J Gynecol Obstet Hum Rep. 2020;49(4):101696. [DOI] [PubMed] [Google Scholar]
- 22.ACOG Committee Opinion, Number 800. The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology. Obstet Gynecol. 2020;135(3):e138–e148. [DOI] [PubMed] [Google Scholar]
- 23.van Hanegem N, Prins MM, Bongers MY, Opmeer BC, Sahota DS, Mol BW, Timmermans A. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147–55. [DOI] [PubMed] [Google Scholar]
- 24.Raine-Bennett T, Tucker LY, Zaritsky E, et al. Occult uterine sarcoma and leiomyosarcoma: Incidence of and survival associated with morcellation. Obstet Gynecol. 2016;127(1):29–39. [DOI] [PubMed] [Google Scholar]
- 25.Raspagliesi F, Maltese G, Bogani G, et al. Morcellation worsens survival outcomes in patients with undiagnosed uterine leiomyosarcomas: A retrospective MITO group study. Gynecol Oncol. 2017;144(1):90–95. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Lin H, Wright JD, et al. Association between power morcellation and mortality in women with unexpected uterine cancer undergoing hysterectomy or myomectomy. J Clin Oncol. 2019;37(35):3412–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]


