Abstract
Background:
Little is known regarding the long-term effects of antiretroviral (ARV) exposure on body composition for people living with HIV (PLWH) since early childhood. This study explores changes in body fat distribution in relation to ARV exposure.
Methods:
We conducted a prospective study of adults with perinatal HIV (n=70) using dual energy X-ray absorptiometry and standard anthropometrics. Trunk-limb fat ratio and waist-hip ratio were compared cross-sectionally to 47 matched controls. Further, changes in body composition and ARV exposure were evaluated longitudinally in a subset of 40 PLWH with a median follow-up of 7 years.
Results:
Cross-sectional comparisons of PLWH to controls revealed significantly higher waist-hip ratio, trunk-limb fat ratio, HOMA-IR, and triglycerides, whereas BMI did not differ. Among PLWH with longitudinal follow-up, the prevalence of overweight increased (27.5% to 52.5%) as did obesity (12.5% to 25%); waist-hip and trunk-limb fat ratios also increased (p<.0001). Changes in waist-hip ratio were positively correlated with longer exposure during follow-up to darunavir (r=0.36; p=.02); whereas, increases in trunk-limb fat ratio were positively correlated with longer exposure to stavudine (r=0.39; p=.01) and didanosine (r=0.39; p=.01), but inversely associated with emtricitabine (r=−0.33; p=.04). Increases in waist-hip ratio were correlated with increases in triglyceride levels (r=0.35; p=.03).
Conclusion:
This study presents strong evidence for persistent and worsening central adiposity in young adults with life-long HIV and extensive ARV exposure. As this cohort ages, continued evaluation of the body composition and metabolic impact of life-long ARV therapy is warranted to optimize long-term health.
Keywords: central obesity, lipodystrophy, perinatal HIV, hyperlipidemia, antiretroviral therapy
Introduction
While the introduction of antiretroviral therapy has dramatically decreased morbidity and mortality associated with HIV/AIDS, antiretroviral medications (ARVs) have also been linked with metabolic complications and alterations in body fat distribution, also known as lipodystrophy.1–3 These unintended medication effects may be especially important for the unique group of individuals who acquired HIV early in life. Persons living with HIV (PLWH) from the earliest generation of children with transfusion-acquired and perinatal transmissions are now adults approaching their fourth decade of life with extensive ARV exposure. Metabolic complications and lipodystrophy contribute to insulin resistance, hyperlipidemia, and endothelial dysfunction, which may increase the risk of cardiovascular disease.3 Therefore, recognizing the long-term impact of ARV exposure in young adults infected with HIV in early childhood is of great importance.
The etiologies of these complications in PLWH are not entirely known but may be associated with a complex interaction between consequences of the virus itself, chronic use of ARVs, and underlying inflammatory processes. Previous studies have demonstrated a link between metabolic abnormalities, lipodystrophy, and most classes of ARVs, including nucleoside reverse transcriptase inhibitors (NRTIs),4, 5 non-nucleoside reverse transcriptase inhibitors (NNRTIs),6 and protease inhibitors (PIs),7, 8 More recently, the advent of integrase strand transfer inhibitors (INSTIs), in particular dolutegravir, has also been associated with considerable weight gain and other related metabolic complications.9–11
While many studies evaluating alterations in body fat distribution among PLWH rely on anthropometric measures (i.e. body mass index, hip and waist circumferences, skinfold thickness), dual-energy X-ray absorptiometry (DEXA) provides a more comprehensive assessment of regional body fat distribution as well as lean body mass and body composition.12 The current study builds on prior findings of body composition and metabolic abnormalities among a cohort of young adults living with HIV acquired in childhood,2 and utilizes DEXA along with other anthropometric evaluations to directly assess the potential long-term associations between ARVs and body composition. Insight into the complications associated with long-term ARV exposure may promote the development of preventive strategies to optimize health outcomes and reduce the risk for cardiometabolic comorbidities in this unique group of individuals with life-long HIV.
Methods
Subjects
Young adults who acquired HIV in early childhood, either perinatally or transfusion-acquired, were enrolled and followed in a prospective, longitudinal, cohort study at the National Institutes of Health (ClinicalTrials.gov NCT01656564). Written informed consent was obtained from participants and/or legal guardians when appropriate. The study was approved by the National Institute of Allergy and Infectious Diseases institutional review board.
PLWH completed annual clinical evaluations between July 2000 and September 2019. Healthy volunteers were prospectively recruited for a single assessment to serve as a comparator group with similar distributions of age, sex, race, and ethnicity to that of existing PLWH cohort. Cross-sectional analysis was performed in healthy controls compared to PLWH at their most recent follow-up visits. The subset of PLWH who had a baseline and at least one follow-up visit that included a body composition evaluation with DEXA were included in longitudinal data analyses.
Study Protocol
Each participant regardless of HIV status completed the following clinical evaluations at their baseline visit: medical history, physical examination, and body composition using anthropometrics and whole-body DEXA. Laboratory determination at each visit included fasting glucose, insulin, lipid profile, and CD4 T-cell count. For PLWH, detailed medical record extraction was completed to obtain lifetime ARV exposure and nadir CD4 T-cell count. Total years of ARV exposure was calculated for each participant with further delineation of duration by medication class and individual agents. PLWH received medical care independent of the study and were evaluated regardless of ARV adherence, total CD4 T-cell count, or viral suppression.
Metabolic Parameters
We used the homeostatic model of insulin resistance (fasting insulin (μU/L) × fasting glucose (mmol/L) / 22.5; HOMA-IR); insulin resistance was defined as a HOMA-IR > 4. Abnormal fasting lipid levels and metabolic syndrome were defined based on the criteria set by the National Cholesterol Education Program Adult Treatment Panel (NCEP- ATP III).13 Hypertriglyceridemia was defined as triglyceride > 150mg/dL, abnormal low-density lipoprotein cholesterol (LDL-c) as ≥ 130mg/ dL, and abnormal high-density lipoprotein cholesterol (HDL-c) as < 40 mg/dL for men or 50 mg/dl for women. Metabolic syndrome (MetS) was defined as having at least three of five abnormal characteristics: waist circumference > 102 cm (men) or 88 cm (women), blood pressure ≥ 130/85 mmHg or taking anti-hypertensive medications, triglyceride > 150 mg/dl or taking a lipid-lowering medication, HDL-c < 40 m/dl (men) or 50 mg/dl (women), and fasting glucose > 100 mg/dl.14
Body Measurements and DEXA
Standard anthropometric measurements were obtained including waist and hip circumferences. Body mass index (BMI) was calculated from height and weight measurements. Whole-body DEXA (Hologic QDR4500A, Marlborough, MA) was completed for all participants at baseline visit and approximately every five years for PLWH who were evaluated longitudinally. Regional and total body fat measures by DEXA were reviewed by a single radiologist for quality, positioning, and technique to provide more accurate detail of regional fat distribution. Waist-hip ratio (waist circumference / hip circumference) was used along with trunk-limb fat ratio (total trunk fat / sum of total fat in all 4 limbs) to quantify the regional distribution of body fat.
Statistical Analyses
Clinical characteristics are summarized using mean ± standard deviation. Non-normally distributed variables are presented as a median with interquartile range (IQR) or log-transformed to approximate a normal distribution. Cross-sectional comparisons were completed between groups using the last available follow-up visit data for PLWH and the one-time visit data for the control group using t-tests and chi-squared likelihood ratio statistics. Longitudinal analyses were conducted using paired t-test for normally distributed and Wilcoxon Signed-rank test for non-normally distributed changes in metabolic parameters and body composition between baseline and follow-up among the subset of PLWH. Mean annual changes in waist circumference, waist-hip ratio, and trunk-limb fat ratio were calculated by dividing the difference of each parameter over the duration of follow-up for every PLWH.
Duration of use for each individual ARV agent was reported as median (IQR). Regression analyses were calculated using data from all participants, regardless of duration of exposure or lack thereof. However, individual ARV agents were included in the analysis only if at least 10 participants had exposure to the agent. In the cross-sectional study, univariate linear regression was conducted to identify potential relationships between body composition and cumulative ARV exposures. In the longitudinal study, univariate linear regressions were initially performed to examine the relationships between changes in body composition and change in duration of ARV exposure during follow-up. ARV classes and individual ARV agents identified as significant in univariate analyses were then entered into multivariate regression models of change in measures of body composition which also included duration of follow-up in the model. Similar analyses were performed for changes in metabolic characteristics, including log-transformed triglycerides and HOMA-IR, to account for changes potentially due to age and duration of follow-up.
All statistical analyses were performed using SAS JMP Statistical Software (Version 14.3, SAS Institute Inc., Cary, NC), using a two-tailed alpha level of 0.05 to establish statistical significance.
Results
Cross-sectional Analysis of PLWH and Controls
PLWH (n=70) who completed at least one evaluation were included in cross-sectional comparison to healthy volunteers (n=47) who were similar in age, sex, race, and ethnicity distributions. Participant demographics and clinical characteristics at their last follow-up are displayed in Table 1. Of the PLWH, 54% (38/70) had HIV RNA < 50 copies/mL and the median (IQR) ARV exposure was 16.2 years (9.0, 20.7) at last follow-up. Of the PLWH for whom nadir CD4 T-cell count was available, 49% (25/51) had a nadir < 200 cells/mm3.
Table 1.
Demographics and clinical characteristics at last follow-up visit
| HIV+ (N=70) | Control (N=47) | p-value | |
|---|---|---|---|
| Age, median (IQR) | 26 (23–29) | 26 (23–28) | .97 |
| Sex, n (%) | .56 | ||
| Male | 29 (41) | 22 (47) | |
| Female | 41 (59) | 25 (53) | |
| Hispanic ethnicity, n (%) | 12 (17) | 9 (19) | .78 |
| Race, n (%) | .79 | ||
| White | 27 (39) | 18 (38) | |
| Black | 35 (50) | 26 (56) | |
| Native American | 2 (3) | 1 (2) | |
| Mixed Race | 6 (9) | 2 (4) | |
| CD4 T-cell count, cells/mm3 | 542 ± 361 | 830 ± 305 | < .0001 |
| CD4 T-cell % | 26 ± 14 | 43 ± 7 | < .0001 |
| Systolic blood pressure, mmHg | 118 ± 13 | 116 ± 10 | .28 |
| Diastolic blood pressure, mmHg | 72 ± 9 | 69 ± 7 | .03 |
| Insulin, μIU/mL | 16 ± 11 | 11 ± 6 | .001 |
| Glucose, mg/dL | 94 ± 38 | 89 ± 6 | .25 |
| HOMA-IR | 3.8 ± 3.1 | 2.5 ± 1.6 | .002 |
| Total cholesterol, mg/dL | 161 ± 36 | 164 ± 30 | .63 |
| Triglyceride, mg/dL | 120 ±102 | 68 ± 38 | .0002 |
| log Triglyceride | 4.57 ± 0.6 | 4.13 ± 0.4 | < .0001 |
| HDL-c, mg/dL | 48 ± 14 | 61 ± 14 | < .0001 |
| LDL-c, mg/dL | 89 ± 29 | 89 ±30 | .97 |
| Metabolic syndrome, n (%) | 9 (13) | 2 (4) | .10 |
| BMI, kg/m2 | 26.8 ± 7.0 | 26.2 ± 4.9 | .57 |
| Height, cm | 164.1 ± 8.8 | 169.6 ± 9.3 | .002 |
| Weight, kg | 71.9 ± 18.0 | 75.3 ± 16.2 | .29 |
| Waist-hip ratio | 0.91 ± 0.1 | 0.84 ± 0.1 | < .0001 |
| Waist circumference, cm | 91.3 ± 15.3 | 87.1 ± 12.2 | .11 |
| Hip circumference, cm | 99.4 ± 14.3 | 103.8 ± 15.4 | .12 |
| Trunk-limb fat ratio | 0.98 ± 0.3 | 0.76 ± 0.2 | < .0001 |
| Trunk fat, kg | 10.3 ± 6.2 | 8.9 ± 4.1 | .16 |
| Limb fat, kg | 11.0 ± 6.7 | 11.8 ± 4.8 | .47 |
| Percentage body fat | 29.7 ± 11.1 | 28.5 ± 8.7 | .52 |
| Percentage trunk fat | 27.9 ± 10.7 | 25.4 ± 8.3 | .16 |
Values are mean ± standard deviation unless indicated.
Abbreviations: HOMA-IR. Homeostatic model of insulin resistance; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; BMI, body mass index
PLWH had significantly lower HDL-c, and higher diastolic blood pressure, fasting insulin, HOMA-IR, and triglyceride levels relative to the control group (Table 1). Prevalence of hypertriglyceridemia (17% vs. 4%; p=.03) and insulin resistance (35% vs 11%; p=.002) were also significantly higher compared to healthy controls. The two groups were similar with respect to systolic blood pressure, fasting glucose, total cholesterol, and LDL-c. Metabolic syndrome was more common among PLWH although this difference was not statistically significant (13% vs. 4%; p=.1).
PLWH were significantly shorter although weight and BMI were not significantly different relative to the control group. The prevalence of obesity (PLWH 20% vs. control 17%) and overweight (PLWH 47% vs. control 51%) in the cross-sectional cohort also did not differ significantly between groups (p>.05). Waist-hip ratio and trunk-limb fat ratio were significantly higher in PLWH relative to controls. The individual components of each ratio, namely waist and hip circumferences as well as trunk and limb fat, were not significantly different between the two groups.
Table 2 presents the correlations between measures of body composition and cumulative ARV exposure by class and individual agent among PLWH. There were modest positive correlations between waist-hip ratio and cumulative exposure to NRTIs, NNRTIs, and PIs. Trunk-limb fat ratio was positively correlated with cumulative NRTI exposure. Neither waist-hip nor trunk-limb fat ratios were associated with cumulative exposure to INSTIs. Analyses within each medication class further revealed that waist-hip ratio was positively correlated with cumulative exposure to tenofovir disoproxil fumarate, nevirapine, and ritonavir; whereas trunk-limb fat ratio was positively correlated with stavudine, tenofovir, and nelfinavir. Complete list of individual agents and the median duration of exposures for each agent may be found in the supplemental table (Supplemental Digital Content 1).
Table 2.
Cross sectional correlations between body composition and cumulative ARV exposure by class and individual agents at last visit in PLWH (N=70)
| Waist-hip ratio, r (p-value) | Trunk-limb fat ratio, r (p-value) | |
|---|---|---|
| NRTI | 0.29 (.01) | 0.31 (.01) |
| Abacavir | 0.004 (.97) | −0.05 (.70) |
| Didanosine | 0.04 (.78) | 0.18 (.15) |
| Emtricitabine | 0.23 (.06) | 0.17 (.15) |
| Lamivudine | 0.06 (.60) | 0.10 (.42) |
| Stavudine | 0.16 (.19) | 0.42 (.0003) |
| Tenofovir DF | 0.36 (.002) | 0.24 (.047) |
| Zidovudine | 0.11 (.41) | −0.09 (.47) |
| NNRTI | 0.24 (.04) | 0.07 (.54) |
| Efavirenz | 0.05 (.70) | −0.05 (.65) |
| Nevirapine | 0.28 (.02) | 0.17 (.17) |
| Rilpivirine | −0.08 (.52) | −0.03 (.79) |
| PI | 0.28 (.02) | 0.13 (.28) |
| Atazanavir | 0.04 (.74) | 0.06 (.61) |
| Darunavir | 0.20 (.10) | −0.05 (.67) |
| Indinavir | 0.09 (.48) | 0.05 (.67) |
| Lopinavir | 0.15 (.22) | 0.03 (.83) |
| Nelfinavir | 0.08 (.52) | 0.35 (.003) |
| Ritonavir | 0.29 (.01) | 0.11 (.36) |
| INSTI | 0.12 (.31) | 0.06 (.61) |
| Elvitegravir | 0.03 (.83) | 0.03 (.83) |
| Dolutegravir | −0.09 (.48) | −0.13 (.27) |
| Raltegravir | 0.16 (.18) | 0.10 (.41) |
Abbreviations: NRTI, nucleotide reverse transcriptase inhibitor; DF, disoproxil fumarate, NNRTI, non-nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor
Longitudinal Follow-up of PLWH
The median duration of follow-up was 7 years (IQR 5, 10 y) for the subset of PLWH (n=40) who completed at least 2 DEXA scans and anthropometric measurements. Mean differences over the follow-up period are presented in Table 3. Total CD4 count and rates of viral suppression did not change significantly during follow-up. Only HDL-c significantly increased while there were no significant changes in total cholesterol, LDL-c, triglycerides, blood pressure, insulin, or HOMA-IR. During the course of follow-up, 10% (4/40) of PLWH received anti-hypertensive medications and 20% (8/40) received lipid-lowering medications. Median duration of ARV exposure during follow-up by class and individual agents are included in Supplemental Table 1.
Table 3.
Longitudinal changes in a subset of HIV+ subjects (N= 40)
| Baseline | Last Visit | p-value | Mean Difference | |
|---|---|---|---|---|
| CD4 T-cell count, cells/mm3 | 603 ± 445 | 633 ± 370 | .62 | 31 ± 409 |
| CD4 T-cell % | 41 ± 91 | 30 ± 13 | .14 | −11 ± 90 |
| HIV RNA < 50 copies/mL, n (%) | 20 (51) | 19 (49) | ||
| Systolic blood pressure, mmHg | 117 ± 13 | 119 ± 12 | .33 | 2.3 ± 11.4 |
| Diastolic blood pressure, mmHg | 69 ± 9 | 72 ± 8 | .07 | 2.4 ± 8.7 |
| Insulin, μIU/mL a | 22 ± 25 | 20.3 ± 13.4 | .79 | −1.9 ± 24.0 |
| Glucose, mg/dL | 90 ± 12 | 92.6 ± 9.9 | .052 | 3.1 ± 15.7 |
| HOMA-IR a | 5.4 ± 8.3 | 4.8 ± 3.6 | .96 | −0.71 ± 8.4 |
| Total cholesterol, mg/dL b | 168 ± 42 | 165 ± 36 | .07 | −2 ± 39 |
| Triglyceride, mg/dL b | 161 ± 141 | 130 ± 109 | .10 | −29 ± 154 |
| log Triglyceride b | 4.85 ± 0.7 | 4.65 ± 0.6 | .06 | −0.19 ± 0.65 |
| HDL-c, mg/dL b | 45 ± 12 | 49 ± 14 | .04 | 4.0 ± 13 |
| LDL-c, mg/dL b | 100 ± 34 | 90 ± 33 | .12 | −9.5 ± 34 |
| Metabolic Syndrome, n (%) | 7 (18) | 6 (15) | .76 | |
| BMI, kg/m2 | 23.4 ± 5.3 | 26.9 ±6.3 | <.0001 | 3.5 ± 3.5 |
| Height, cm | 162.1 ± 11.0 | 164.5 ± 9.0 | .006 | 2.4 ± 5.4 |
| Weight, kg | 62.0 ± 16.9 | 73.0 ± 18.3 | <.0001 | 11.0 ± 10.2 |
| Waist-hip ratio | 0.91 ± 0.1 | 0.93 ± 0.09 | .02 | 0.02 ± 0.07 |
| Waist circumference, cm | 83.5 ± 14.4 | 93.2 ± 17.0 | <.0001 | 9.8 ± 9.5 |
| Hip circumference, cm | 91.6 ± 12.2 | 100.0 ± 14.2 | <.0001 | 8.0 ± 9.6 |
| Trunk-limb fat ratio | 0.99 ± 0.37 | 1.08 ± 0.37 | .002 | 0.09 ±0.24 |
| Trunk fat, kg | 7.1 ± 5.4 | 10.9 ± 6.7 | <.001 | 3.8 ± 3.5 |
| Limb fat, kg | 7.5 ± 5.7 | 10.5 ± 6.4 | <.001 | 3.0 ± 2.8 |
| Percentage body fat | 23.8 ± 11.2 | 29.5 ± 11.6 | <.001 | 5.8 ± 5.7 |
| Percentage trunk fat | 22.3 ± 10.7 | 28.7 ± 11.3 | <.001 | 6.4 ± 6.1 |
Abbreviations: HOMA-IR. Homeostatic model of insulin resistance; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; BMI, body mass index
Values are mean ± standard deviation unless indicated.
Data available for 38 PLWH
Data available for 39 PLWH
Both waist-hip ratio and trunk-limb fat ratio significantly increased from first assessment to last follow-up, along with their individual components: waist circumference, hip circumference, trunk fat, and limb fat. There was a mean (SD) annual increase of 1.24 (1.20) cm for waist circumference, 0.004 (0.01) for waist-hip ratio, and 0.02 (0.04) for trunk-limb fat ratio. Change in duration of general classes of ARVs during follow-up was not significantly related to changes in either waist-hip or trunk-limb fat ratios. However, longer exposure to specific agents throughout follow-up correlated with body composition changes. Increase in waist-hip ratio was positively correlated with greater increase in exposure to darunavir (r=0.36; p=.02). The increase in trunk-limb fat ratio was positively correlated with greater increase in exposure to didanosine (r=0.39; p=.01) and stavudine (r=0.40; p=.01). There was also a significant inverse correlation between the increases in trunk-limb fat ratio and longer exposure to emtricitabine (r=−0.33; p=.04) during follow-up, but not with tenofovir DF (r=−0.22; p=.17). Each of the statistically significant correlations remained significant after adjusting for the duration of follow-up in a multivariate regression (data not shown).
Although metabolic parameters remained relatively stable over time, the increase in waist-hip ratio was positively correlated with the change in log-transformed triglyceride (r=0.35; p=.03, Figure 1), and remained significant after a multivariate regression controlling for follow-up duration. Changes in HOMA-IR were not correlated with changes in either waist-hip ratio (r=0.23, p=.18) or trunk-limb fat ratio (r=0.19, p=.26). The observed changes in BMI were not statistically associated with changes in the duration of ARV agents. There were considerable increases in the prevalence of overweight (27.5% to 52.5%) and obesity (12.5% to 25%) during the follow-up period.
1.
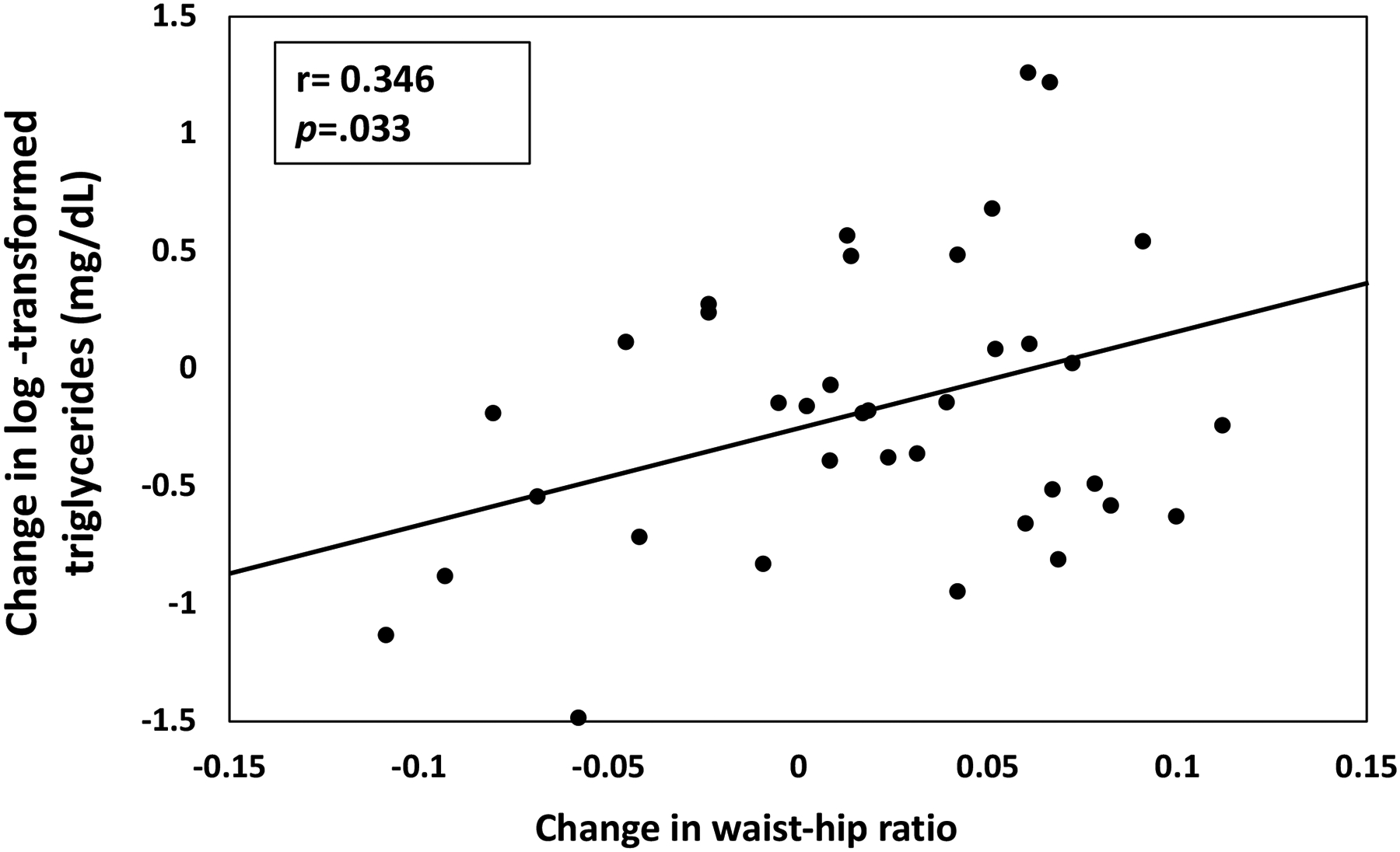
Change in waist-hip ratio plotted against the change in log-transformed triglyceride level (mg/dL) calculated from baseline to last follow-up in 39 PLWH (r=0.35, p=0.03); one participant with history of hyperlipidemia was excluded from analysis.
Discussion
In this natural history study, young adults who acquired HIV in early childhood demonstrated a heightened risk for metabolic disorders compared to those without HIV. Hyperlipidemia and insulin resistance were both strikingly common among PLWH. Body fat distribution also tended to be central with adipose tissue accumulation preferentially in the truncal region, relative to those without HIV. Although BMI and biomarkers for lipid and glucose metabolism did not change significantly between baseline and last follow-up, noticeable increases in waist-hip circumference ratio and trunk-limb fat ratio were observed and may reflect the residual lipodystrophic effects of extensive ARV use since early childhood and adolescence.
The increased prevalence of MetS among individuals with perinatally-acquired HIV has been demonstrated in previous studies. In particular, Auripubul et al. reported 10.6% MetS prevalence among Thai adolescents living with HIV with a mean age of 20 years.15 Arrive et al. also reported a 13.2% and 10.4% prevalence of MetS among French men and women of the same age group, respectively.16 Within our cohort, we identified similar results with a 13% prevalence of MetS among PLWH, which, though not significantly different from the 4% among non-HIV controls may represent a meaningful progression of metabolic disorders among this young adult cohort. Older PLWH, regardless of HIV acquisition history, have been identified to have significantly higher rates of MetS relative to comparator groups.17, 18 For example, in a large cohort of PLWH in Spain, Jerrico et al. found that MetS increased from 5.1% in those under 30 years old to 27% in those aged 50–59 years,18 indicating exacerbation of known age-related increase in risks for cardiovascular disorders among those living with HIV. These findings highlight the need for careful monitoring of individual components of MetS among this cohort, specifically, dyslipidemia, glucose intolerance, and abdominal obesity.
Lipid biomarkers for cardiovascular health normally worsen with age as a result of certain changes in diet and lifestyle.19, 20 We observed the opposite trend during follow-up among PLWH, with a significant increase in HDL-cholesterol with small but non-significant, decreases in log-transformed triglycerides and cholesterol. These changes could be a result of unique factors, including wider availability of dietary and lifestyle guidance as well as closer health monitoring associated with living with a chronic health condition.21 The use of lipid-lowering medications may have also contributed to the observed changes in lipids in the present cohort. Alternatively, it may represent a correction of PI-induced dyslipidemia from baseline with less exposure to certain protease inhibitors in particular.8, 22 We previously reported a 52% prevalence of hypertriglyceridemia in a subset of this cohort during their adolescence.2 In the present study, the rate of hypertriglyceridemia was 17% at the last follow-up visit, although it was still higher relative to controls. The number of PLWH on a PI-based agent decreased from 22/40 to 16/40 over follow-up and those who remained tended to receive ritonavir-boosted agents with more favorable lipid profile effects.23 Additionally, compared to healthy volunteers, we found higher triglycerides and lower HDL-cholesterol in the PLWH, 84% of whom had life-time exposure to either nelfinavir or ritonavir. Although none of the lipid biomarkers correlated with ARV exposure, we found that nelfinavir and ritonavir were correlated with trunk-limb fat ratio and waist-hip ratio, respectively. The observed lipid alterations may be indirectly driven by exposure to these agents through residual central adiposity acquired in childhood and adolescence. Overall, although dyslipidemia tended to improve, 20% of PLWH were receiving lipid-lowering medications during follow-up. PLWH had comparatively worse lipid profiles relative to those without HIV which may be associated with persistent alterations in body composition. As this cohort ages, monitoring for lipid biomarkers with abdominal obesity warrants continued attention to reduce the risk for cardiovascular disorders.
We previously reported that increased waist-hip ratio and longer exposure to stavudine and didanosine were significant predictors of insulin resistance in PLWH youth and adolescents.2 This is in contrast to our present findings in which levels of HOMA-IR did not correlate with waist-hip ratio, trunk-limb fat ratio, or the duration of ARV exposure. However, we found that waist-hip ratio was associated with cumulative exposure to tenofovir, nevirapine, and ritonavir whereas trunk-limb fat ratio was associated with stavudine, tenofovir, and nelfinavir. The individual components of these body composition ratios did not significantly differ in either the cross-sectional or longitudinal analyses. The present finding of persistent central fat distribution may be a carry-over effect from childhood but is now contributing to metabolic complications that are normally seen with aging, even among those without HIV.17
There are limited available normative data for changes in waist-hip ratio and trunk-limb fat ratio in the general population and we did not have a non-HIV comparison group in our longitudinal study. Alternatively, waist circumference provides a comparative point of reference to ascertain whether the changes in body composition among our cohort differ relative to the general population. It is widely demonstrated that changes in waist circumference normally varies by age and sex.24 In a 5-year longitudinal study following a large non-HIV cohort between late adolescence and early adulthood, Orlandi et al. found an annual waist circumference increase of 0.75 cm in men and 0.88 cm in women.25 Considering the 1.24 cm per year increase in waist circumference observed in our cohort, individuals living with HIV from early childhood may have different waist circumference trajectories compared to the non-HIV population within the same age group.
The use of a tenofovir-based regimen among the majority of our cohort could account for some of the observed improvements in insulin resistance and dyslipidemia during follow-up. The TULIP Study Group demonstrated that tenofovir/FTC, when added to PI monotherapy, may have lipid-lowering effects.26 Further, the increased use of tenofovir/FTC over the NRTIs stavudine and didanosine may account for the observed protective effect of greater exposure to emtricitabine on trunk-limb fat ratio during follow-up, considering that the two agents are typically coformulated. Although it was non-significant, longer exposure to tenofovir over follow-up tended to be associated with a lower increase in trunk-limb fat ratio. In a 12 year longitudinal study of HIV-infected adult males, Price et al. also demonstrated that switching from stavudine to abacavir or tenofovir was associated with a reduced likelihood of lipodystrophy.21 We also identified that trunk-limb fat ratio was more strongly correlated with cumulative exposure to stavudine compared to tenofovir. When we analyzed the relationship between change in trunk-limb fat ratio with change in duration of exposure to individual agents over follow-up, continued exposure to didanosine was linked with worsening trunk-limb fat ratio. These relationships remained significant even after adjusting for the duration of follow-up.
Prior literature indicates switching from PI-based therapy to nevirapine-containing regimens may result in a more favorable lipid profile.27–29 While nevirapine use was not significantly associated with lipid levels, we identified a deleterious association between greater exposure to nevirapine and waist-hip ratio. Central obesity has been previously associated with nevirapine-containing regimens in PI-naïve individuals.30 In children, nevirapine-based treatment was also associated with a greater prevalence of central hypertrophy.31 Thus, our finding, although limited by the small sample size and shorter duration of exposures, is in agreement with prior observations of the effects of nevirapine use on central obesity.
One strength of this study is the detailed longitudinal characterization of the clinical and metabolic parameters of a unique cohort of young adults who have lived with HIV throughout most of their lives. Considering the extensive exposure of this group to multiple ARV agents, we present a comprehensive history that includes biomarkers for metabolic disturbances and detailed characterizations of body composition. In combination with our previous observational studies of this cohort describing cardiovascular health32 as well as bone and renal health33, we provide further knowledge that could potentially guide treatment and care for the aging population of PLWH exposed in their early life.
The limited number and short duration of exposures for certain ARVs highlight an important potential limitation. As such, some observations, nevirapine in particular, may be spurious and should be further scrutinized in larger cohort studies as discussed above. Although we did not use a standardized rating system to score lipodystrophy, the use of whole-body DEXA and anthropometrics provide objective quantification of body fat distribution, which, in the case of waist-hip ratio, may be accurate and accessible in resource-limited settings. It is important to note that our findings cannot establish direct causality and that behavioral factors that may impact lipids and body composition such as, dietary habits, exercise, alcohol and tobacco use, were not included in the analyses. Lastly, because the longitudinal component of the study lacks a comparator non-HIV group, the observed alterations in body composition during follow-up may be attributable to normal changes seen in the general population of the same age group.
In conclusion, individuals who acquired HIV in early childhood were more likely to demonstrate central obesity compared with those without HIV. Using DEXA scans and anthropometrics, we measured body fat distribution over an average of 7 years. We also identified associations between body composition and metabolic parameters as well as the duration of exposure to certain ARVs. These findings underscore the long-term persistence and associated risk factors of central adiposity among PLWH who have life-long ARV exposure which may translate to increased metabolic disturbances and enhanced risk of cardiovascular disorders in future decades.
Supplementary Material
Supplemental Digital Content 1 – Supplemental Table 1. ARV Exposure
Source of Support:
This work was supported by the National Institutes of Health through the National Institute of Allergy and Infectious Diseases Intramural Research Program and the National Institutes of Health Clinical Center.
Footnotes
Previous Presentations: The abstract for this paper was presented at the International Workshop on Co-morbidities and Adverse Drug Reactions in HIV held virtually on December 1st 2020.
References
- 1.Beraldo RA, Santos APD, Guimarães MP, et al. Body fat redistribution and changes in lipid and glucose metabolism in people living with HIV/AIDS. Rev Bras Epidemiol. Jul-Sep 2017;20(3):526–536. Redistribuição de gordura corporal e alterações no metabolismo de lipídeos e glicose em pessoas vivendo com HIV/AIDS. doi: 10.1590/1980-5497201700030014 [DOI] [PubMed] [Google Scholar]
- 2.Dimock D, Thomas V, Cushing A, et al. Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. Jun 2011;60(6):874–80. doi: 10.1016/j.metabol.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. Jan 6 2005;352(1):48–62. doi: 10.1056/NEJMra041811 [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Innes S, Geelen SPM, et al. Long-Term Changes of Subcutaneous Fat Mass in HIV-Infected Children on Antiretroviral Therapy: A Retrospective Analysis of Longitudinal Data from Two Pediatric HIV-Cohorts. PLoS One. 2015;10(7):e0120927–e0120927. doi: 10.1371/journal.pone.0120927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly V, Flandre P, Meiffredy V, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;16(18):2447–2454. [DOI] [PubMed] [Google Scholar]
- 6.Arpadi S, Shiau S, Strehlau R, et al. Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy. Archives of Disease in Childhood. 2013;98(4):258–264. doi: 10.1136/archdischild-2012-302633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr A. HIV Protease Inhibitor-Related Lipodystrophy Syndrome. Clinical Infectious Diseases. 2000;30(Supplement_2):S135–S142. doi: 10.1086/313854 [DOI] [PubMed] [Google Scholar]
- 8.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia Among Perinatally HIV-Infected Children Enrolled in the PACTS-HOPE Cohort, 1999–2004: A Longitudinal Analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;41(4):453–460. doi: 10.1097/01.qai.0000218344.88304.db [DOI] [PubMed] [Google Scholar]
- 9.Auclair M, Guénantin A-C, Fellahi S, Garcia M, Capeau J. HIV antiretroviral drugs, dolutegravir, maraviroc and ritonavir-boosted atazanavir use different pathways to affect inflammation, senescence and insulin sensitivity in human coronary endothelial cells. PLoS One. 2020;15(1):e0226924. doi: 10.1371/journal.pone.0226924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484–e25484. doi: 10.1002/jia2.25484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y-W, Hardy H, Pericone CD, Chow W. Real-World Assessment of Weight Change in People with HIV-1 After Initiating Integrase Strand Transfer Inhibitors or Protease Inhibitors. J Health Econ Outcomes Res. 2020;7(2):102–110. doi: 10.36469/jheor.2020.13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101–105. doi: 10.1016/j.bone.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E, Adults ToHBCi. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 14.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. May-Jun 2009;2(5–6):231–237. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurpibul L, Namwongprom S, Sudjaritruk T, Ounjaijean S. Metabolic syndrome, biochemical markers, and body composition in youth living with perinatal HIV infection on antiretroviral treatment. PLoS One. 2020;15(3):e0230707. doi: 10.1371/journal.pone.0230707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrive E, Viard J-P, Salanave B, et al. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS One. 2018;13(11):e0206745–e0206745. doi: 10.1371/journal.pone.0206745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelpi M, Afzal S, Lundgren J, et al. Higher Risk of Abdominal Obesity, Elevated Low-Density Lipoprotein Cholesterol, and Hypertriglyceridemia, but not of Hypertension, in People Living With Human Immunodeficiency Virus (HIV): Results From the Copenhagen Comorbidity in HIV Infection Study. Clinical Infectious Diseases. 2018;67(4):579–586. doi: 10.1093/cid/ciy146 [DOI] [PubMed] [Google Scholar]
- 18.Jericó C, Knobel H, Montero M, et al. Metabolic Syndrome Among HIV-Infected Patients. Prevalence, characteristics, and related factors. 2005;28(1):132–137. doi: 10.2337/diacare.28.1.132 [DOI] [PubMed] [Google Scholar]
- 19.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between Multiple Cardiovascular Risk Factors and Atherosclerosis in Children and Young Adults. New England Journal of Medicine. 1998;338(23):1650–1656. doi: 10.1056/nejm199806043382302 [DOI] [PubMed] [Google Scholar]
- 20.Mofenson LM, Cotton MF. The challenges of success: adolescents with perinatal HIV infection. J Int AIDS Soc. 2013;16(1):18650. doi: 10.7448/IAS.16.1.18650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price J, Hoy J, Ridley E, Nyulasi I, Paul E, Woolley I. Changes in the prevalence of lipodystrophy, metabolic syndrome and cardiovascular disease risk in HIV-infected men. Sex Health. Jun 2015;12(3):240–8. doi: 10.1071/sh14084 [DOI] [PubMed] [Google Scholar]
- 22.Calza L, Manfredi R, Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. Journal of Antimicrobial Chemotherapy. 2004;53(1):10–14. doi: 10.1093/jac/dkh013 [DOI] [PubMed] [Google Scholar]
- 23.Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. Oct 2012;28(10):1184–95. doi: 10.1089/aid.2011.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. Eur J Clin Nutr. Jan 2010;64(1):6–15. doi: 10.1038/ejcn.2009.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlandi SP, Santos LP, Menezes AMB, Wehrmeister FC, Gonçalves H, Assunção MCF. Evolution of total body and regional adiposity from late adolescence to early adulthood in a birth cohort study. Nutrition & Metabolism. 2019/March/25 2019;16(1):21. doi: 10.1186/s12986-019-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos JR, Saumoy M, Curran A, et al. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis. Aug 1 2015;61(3):403–8. doi: 10.1093/cid/civ296 [DOI] [PubMed] [Google Scholar]
- 27.Clotet B, van der Valk M, Negredo E, Reiss P. Impact of nevirapine on lipid metabolism. J Acquir Immune Defic Syndr. Sep 2003;34 Suppl 1:S79–84. doi: 10.1097/00126334-200309011-00012 [DOI] [PubMed] [Google Scholar]
- 28.Negredo E, Ribalta J, Paredes R, et al. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. Aids. Jul 5 2002;16(10):1383–9. doi: 10.1097/00002030-200207050-00010 [DOI] [PubMed] [Google Scholar]
- 29.Petit JM, Duong M, Masson D, et al. Serum adiponectin and metabolic parameters in HIV-1-infected patients after substitution of nevirapine for protease inhibitors. Eur J Clin Invest. Aug 2004;34(8):569–75. doi: 10.1111/j.1365-2362.2004.01379.x [DOI] [PubMed] [Google Scholar]
- 30.Aldeen T, Wells C, Hay P, Davidson F, Lau R. Lipodystrophy associated with nevirapine-containing antiretroviral therapies. AIDS. 1999;13(7):865. [DOI] [PubMed] [Google Scholar]
- 31.Aurpibul L, Puthanakit T, Lee B, Mangklabruks A, Sirisanthana T, Sirisanthana V. Lipodystrophy and metabolic changes in HIV-infected children on non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Antivir Ther. 2007;12(8):1247–54. [PubMed] [Google Scholar]
- 32.Abd-Elmoniem KZ, Unsal AB, Eshera S, et al. Increased coronary vessel wall thickness in HIV-infected young adults. Clin Infect Dis. Dec 15 2014;59(12):1779–86. doi: 10.1093/cid/ciu672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unsal AB, Mattingly AS, Jones SE, et al. Effect of Antiretroviral Therapy on Bone and Renal Health in Young Adults Infected With HIV in Early Life. J Clin Endocrinol Metab. Aug 1 2017;102(8):2896–2904. doi: 10.1210/jc.2017-00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1 – Supplemental Table 1. ARV Exposure


