Abstract
Objective
Demonstrate the ability of a novel steerable distal chip endoscope to traverse the Eustachian tube and provide diagnostic quality images of the human middle ear.
Patients
Three cadaveric temporal bone specimens were used in this work.
Intervention
Diagnostic transeustachian endoscopy of the middle ear was performed.
Main Outcome Measure
Diagnostic image quality.
Results
A novel 1.62mm steerable endoscope successfully cannulated the Eustachian tube of 3 human cadaveric temporal bone specimens to reveal intact middle ear anatomy with high optical clarity.
Conclusions
A steerable endoscope can be designed to traverse the human Eustachian tube and provide diagnostic quality images of middle ear anatomy.
Keywords: Endoscopy, Eustachian Tube, Otology, Device Development
Introduction
The modern era of otologic surgery began 100 years ago when microscopes were first employed to better visualize the mastoid and middle ear cavities. Since that time, key improvements in optical technology, especially the binocular otomicroscope as used by Zeiss in the 1950’s and the otologic endoscopes developed by Harold Hopkins in the 1960’s, have driven the field forward.1 The most prominent challenge that these technologies overcame were the small size and complex geometry of the middle ear. Endoscopes offered the additional benefit of a wider field of view, with the ability to see around ledges and into bony pockets otherwise obscured.2 Surgical access to the middle ear is performed either through a mastoidectomy or through a transcanal approach, both of which can offer a limited view of different regions of the middle ear space. Without mastoidectomy or elevation of a tympanomeatal flap, diagnostic views of the middle ear and its anatomy are limited by the tympanic membrane and its surrounding bony anatomy.
A less invasive method of visualizing the middle ear could have diagnostic utility for a number of conditions, including cholesteatoma, adhesive middle ear disease, otosclerosis and displaced ossicular prostheses. Over the past 20 years, there have been multiple efforts to develop transeustachian endoscopes both for examining the Eustachian tube itself for conditions like patulous Eustachian tube or Eustachian tube dysfunction,3,4 as well as for examining the middle ear.5-9 There have generally been two major limitations of this prior work; (1) an inability to traverse the Eustachian tube due to diameter and steerability concerns, and (2) insufficient optical quality due to the use of fiberoptic endoscopes.
The Eustachian tube is a 3-4cm long tube that extends from the anterior epitympanum to the nasopharynx and is divided into bony and cartilaginous portions. The bony Eustachian tube has the narrowest fixed diameter and spans a length of 1.4cm.10 The average narrowest portion of the bony Eustachian tube is 1.94mm with a standard deviation of 0.54mm.11 The cartilaginous and proximal bony portions of the tube are generally straight and there is a 27-degree distal curve in the tube as it enters the anterior middle ear.10,12,13 This curvature has proven problematic for non-steerable endoscopes, as it has prevented entry into the middle ear space, limited the view of the middle ear, and broken optical fibers.9
Optical quality in a fiberoptic endoscope is proportional to the number of optical fibers, which in turn is directly limited by the diameter of the endoscope. Small (<2mm) fiberoptic cables have a resolution limit of 3-6000 pixels, which is insufficient for otologic diagnostic purposes. The inclusion of a steering mechanism in a flexible endoscope further restricts the size constraints of the optical fiber bundle.
Distal chip endoscopes can outperform traditional fiberoptic endoscopes in terms of lighting, depth of field, and optical clarity. Until recently, distal chip cameras were too large to fit within the diameter of the Eustachian tube. Complementary metal oxide semiconductor (CMOS) based cameras can now be made at sizes of less than 2mm. Prior work by our group has yielded a steering mechanism for needle-sized devices, which could be utilized to navigate the Eustachian tube.14,15 This work combines a high resolution CMOS camera with a steering mechanism that allows for an endoscope to traverse the Eustachian tube, including the curvature of the bony portion as it enters the middle ear, while simultaneously providing diagnostic-quality optical resolution.16 This work will demonstrate the potential of this technology in human temporal bone specimens, and represents an early step towards clinical validation of this technology.
Materials and Methods
The transeustachian endoscope is steerable thanks to a pull-wire embedded in the endoscope shaft. This is similar to that seen in other flexible endoscopes, however due to size constraints this endoscope has a single-direction of flexion. It returns to a straight configuration due to the superelastic nature of the metal (Nitinol) that comprises the shaft of the endoscope. This mechanism has been pioneered in prior work by our group and has been reduced in size until the present model with an outer diameter of 1.62mm (Figure 1).14,15 The insertion cannula shaft measures 125mm in length, with an additional 20mm of extendable steerable tip (Figure 1). The CMOS camera is embedded in the tip of the endoscope and can provide resolution of greater than 1 megapixel.
Figure 1:
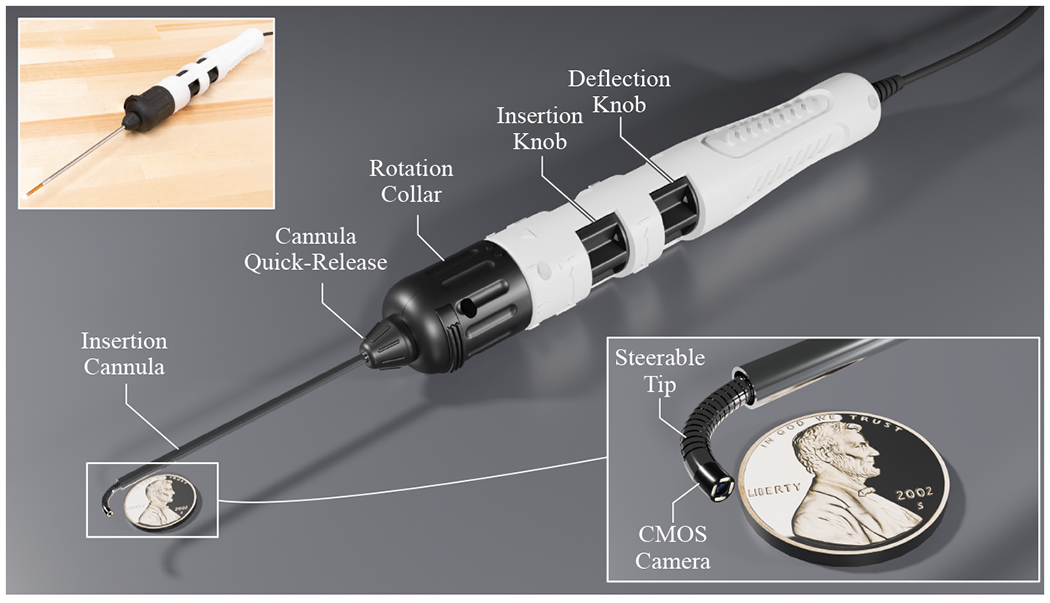
Schematic view of steerable transeustachian endoscope with handle, with inlaid close-up view of the steerable portion of the endoscope (penny for scale)
3 de-identified formalin-preserved human temporal bone specimens were used for this experimentation. Specimen 1 was a right ear, and Specimen 2 and 3 were left ears. Prior to each insertion of the endoscope, an Acclarent® Eustachian tube dilating balloon (Johnson and Johnson, New Brunswick, NJ) was utilized to perform dilation of the cartilaginous Eustachian tube at 10 atm of pressure for 1 minute. Video samples were collected of each endoscopy, including insertion of the endoscope into the Eustachian tube as well as multiple directions of flexion of the camera tip once inside the middle ear space. No live patients, animals, or subjects were utilized in this work, and as such it was exempt from the IRB/IACUC review process.
Results
Early attempts at endoscopy were marred by collapse of the cartilaginous Eustachian tube as well as accumulation of detritus on the camera tip. The process of Eustachian tube balloon dilation allowed for debris dispersion and cannulation of each specimen’s Eustachian tube without obstructing camera view. Specimen 1 allows for a clear view of the mesotympanum, hypotympanum, and epitympanum, as well as the ossicular chain (Figure 2, Supplemental Video 1). Specimen 2 shows similar anatomic range to Specimen 1, albeit with impaired depth of field and some problems with gain of the image compromising clarity (Figure 3, Supplemental Video 2). Specimen 3 showed less hypotympanic range of view and did not enable view to the level of the middle ear tegmen, but otherwise showed comparable anatomy to Specimens 1 and 2 (Figure 4, Supplemental Video 3). All three specimens showed diagnostic-quality images of much of the ossicular chain, tympanic membrane, mesotympanum, anterior epitympanum, and hypotympanum. Areas with limited visualization in all 3 specimens include the stapes footplate, sinus tympani, incudomalleolar joint and mastoid antrum.
Figure 2:






Specimen 1, Right Ear – (a) Mesotympanum (Labeled), (b) Mesotympanum (Unlabeled), (c) Epitympanum (Labeled), (d) Epitympanum (Unlabeled), (e) Hypotympanum (Labeled), (f) Hypotympanum (Unlabeled) [Labels: TM = Tympanic Membrane, m = Malleus, t = Tensor Tympani, i = Incus, s = Stapes, st = Stapedial Tendon, ps = Prussak Space, et = Epitympanum, ht = Hypotympanum]
Figure 3:







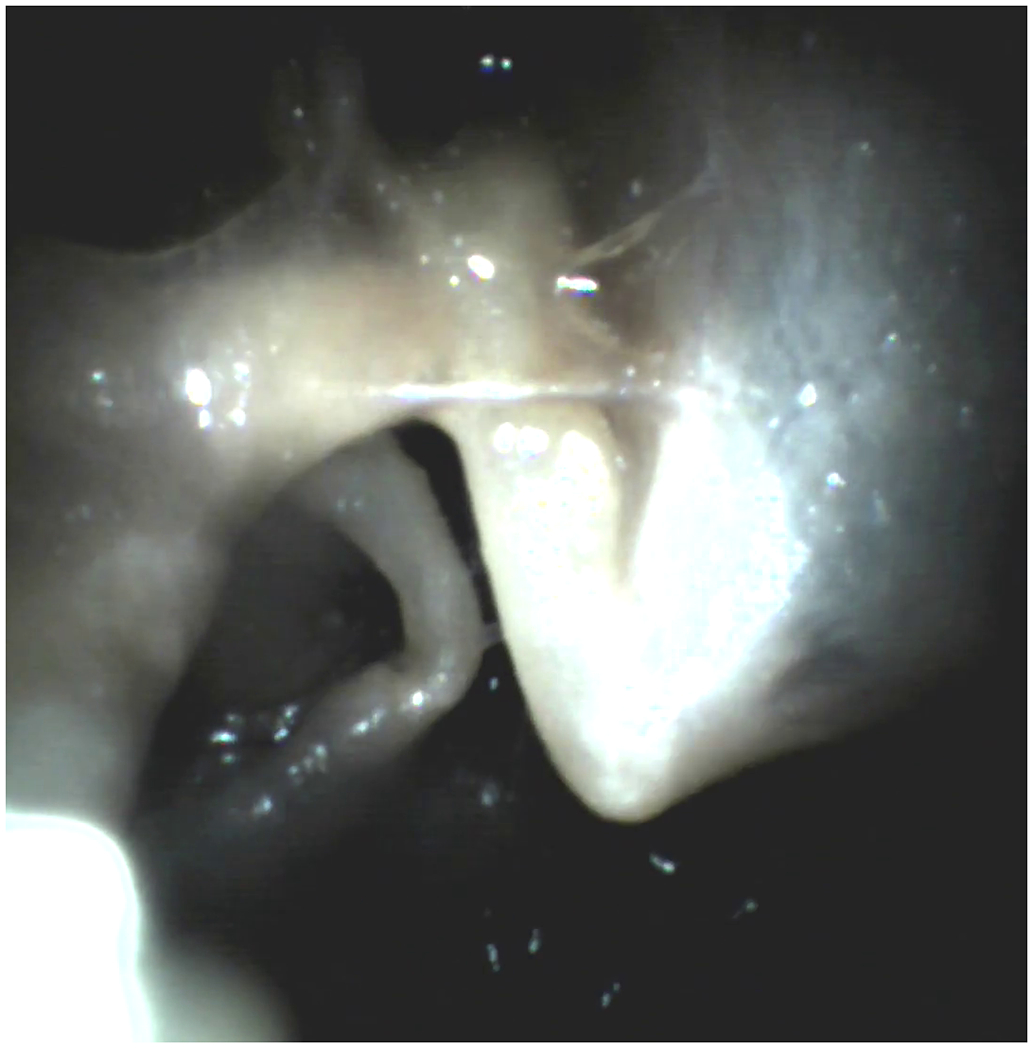
Specimen 2, Left Ear – (a) Mesotympanum (Labeled), (b) Mesotympanum (Unlabeled), (c) Epitympanum (Labeled), (d) Epitympanum (Unlabeled), (e) Hypotympanum (Labeled), (f) Hypotympanum (Unlabeled), (g) Ossicular Chain Closeup (Labeled), (h) Ossicular Chain Closeup (Unlabeled) [Labels: TM = Tympanic Membrane, m = Malleus, t = Tensor Tympani, i = Incus, s = Stapes, ps = Prussak Space, et = Epitympanum, ht = Hypotympanum]
Figure 4:


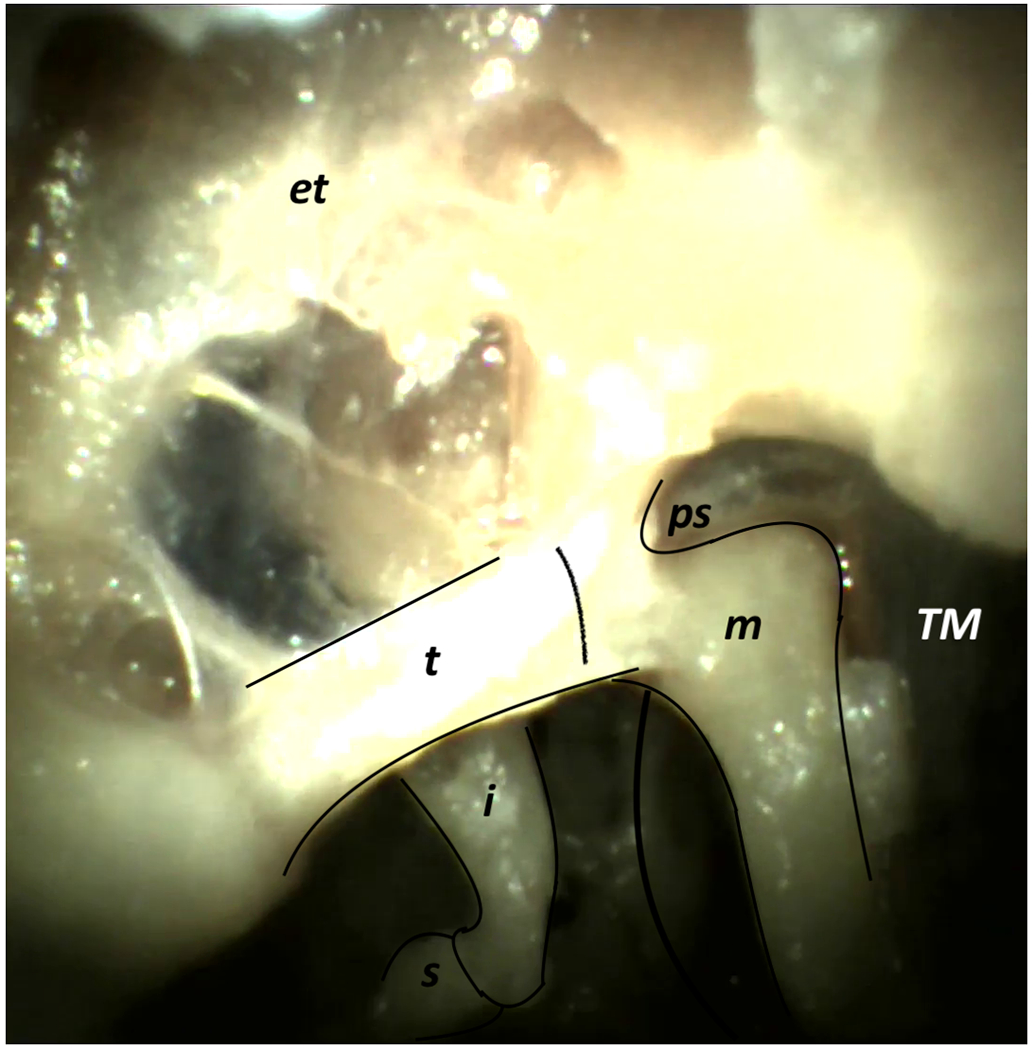





Specimen 3, Left Ear – (a) Mesotympanum (Labeled), (b) Mesotympanum (Unlabeled), (c) Epitympanum (Labeled), (d) Epitympanum (Unlabeled), (e) Hypotympanum (Labeled), (f) Hypotympanum (Unlabeled), (g) Ossicular Chain Closeup (Labeled), (h) Ossicular Chain Closeup (Unlabeled) [Labels: TM = Tympanic Membrane, m = Malleus, t = Tensor Tympani, i = Incus, s = Stapes, ps = Prussak Space, et = Epitympanum, ht = Hypotympanum]
Discussion
This proof-of-concept work demonstrates a first step in implementing this technology in human specimens. The optical quality provided by the high-resolution CMOS camera tip alongside the narrow diameter enabled by the enclosed steering mechanism allow this to be the first endoscope capable of traversing the Eustachian tube and providing diagnostic quality images for both static assessment of disease (e.g., recurrence of cholesteatoma) as well as dynamic assessment of ossicular chain mobility (e.g. otosclerosis or displaced ossicular prosthesis). As expected based on the normal orientation of the middle ear, the transeustachian approach provides an excellent view of much of the ossicular chain, tympanic membrane, epitympanum, mesotympanum, and hypotympanum, but a limited view of the facial recess and stapes footplate (due to obstruction by the cochlear promontory), Prussak Space (due to obstruction by the anterior malleolar ligament), and incudomalleolar joint (due to obstruction by the tensor tympani and malleus neck and head). These areas of worst visibility are important for diagnosing cholesteatoma and otosclerosis and may limit the applications of this technology.
Prior work has calculated that the average narrowest point of the Eustachian tube is 1.94mm in diameter with a standard deviation of 0.54mm.11 This can be extrapolated to expect that, assuming a normal distribution, a 1.62mm endoscope should be able to traverse 72% of human Eustachian tubes. Prior research suggests that patients with chronic middle ear disease may have narrower Eustachian tubes than the general population, limiting the proportion of navigable Eustachian tubes by an unknown degree.8 This work also utilized a steerable endoscope with a straight insertion cannula (Figure 1) in isolated temporal bone specimens with a wholly intact Eustachian tube complex but no other preserved nasal anatomy. The next version of the endoscope will include a prebuilt curvature to permit Eustachian tube cannulation in whole-head cadaveric specimens. Future iterations of the CMOS camera tip may enable further narrowing of the endoscope, which could improve the safety profile and increase the number of eligible patients to include those with pathologically narrow Eustachian tubes. Further refinement of the steering mechanism as well as narrowing of the endoscope tip may also allow for the endoscope to penetrate deeper into the middle ear to view other important anatomy, such as the stapes footplate and Prussak Space. Navigation of the endoscope around the ossicular chain certainly could expose a patient to a risk of ossicular chain disruption, although this was not seen in the cadaveric trials performed for this study. The risks associated with cannulation of the eustachian tube in the manner described above are unclear, and further study will be needed to clarify the safety profile of this device. Future models could also consider a lens clearance mechanism to bypass the problems posed by mucous and debris in the Eustachian tube and to limit entry and exit attempts required in each specimen.
Conclusions
Important questions remain regarding the safety profile and the tolerability of this intervention in awake patients, as well as the ability to predict anatomic contraindications prior to attempting transeustachian endoscopy. These questions will be addressed in future work. As it currently stands, this proof-of-concept work demonstrates the ability of a novel steerable distal chip endoscope to traverse the human Eustachian tube and provide diagnostic quality images of the middle ear and its contents.
Supplementary Material
Supplemental Video Figure 1: Transeustachian endoscopy of Specimen 1, Right Ear
Supplemental Video Figure 2: Transeustachian endoscopy of Specimen 2, Left Ear
Supplemental Video Figure 3: Transeustachian endoscopy of Specimen 3, Left Ear
Acknowledgments
The authors would like to acknowledge Enable, Inc. for assisting in the design, development, and fabrication of the steerable endoscope presented in this work. The authors thank the National Institutes of Health (NIH) for Grant R21 DC016153-01A1 which supported the work described in this paper. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institutes of Health.
Conflicts of Interest and Source of Funding:
No conflicts of interest to note. The authors thank the National Institutes of Health (NIH) for Grant R21 DC016153-01A1, which supported the work described in this paper. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institutes of Health.
Footnotes
Work performed at Vanderbilt University Medical Center
References
- 1.Thomassin JM. The History and Development of Endoscopic Ear Surgery, Abstracts for the 10th International Conference on Cholesteatoma. J Laryngol Otol 2016; 130:S46–7. [Google Scholar]
- 2.Bennett ML, Zhang D, Labadie RF, et al. Comparison of Middle Ear Visualization With Endoscopy and Microscopy. Otol Neurotol 2016; 37:362–6. [DOI] [PubMed] [Google Scholar]
- 3.Poe DS, Abou-Halawa A, Abdel-Razek O. Analysis of the Dysfunctional Eustachian Tube by Video Endoscopy. Otol Neurotol 2001; 22:590–5. [DOI] [PubMed] [Google Scholar]
- 4.Poe DS, Silvola J, Pyykko I. Balloon Dilation of the Cartilaginous Eustachian Tube. Otolaryngol Head Neck Surg 2011; 144:563–9. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H, Yamaguchi H, Cheng S, et al. Direct observation of the tympanic cavity by superfine fiberscope. Nippon Jibiinkoka Gakkai Kaiho 1989;92:233–8 [DOI] [PubMed] [Google Scholar]
- 6.Karhuketo TS, Puhakka HJ. Middle Ear Imaging via the Eustachian Tube with a Superfine Fiberoptic Videomicroendoscope. ORL J Otorhinolaryngol Relat Spec 1998;60:30–4. [DOI] [PubMed] [Google Scholar]
- 7.Klug C, Fabinyi B, Tschabitscher M. Endoscopy of the Middle Ear Through the Eustachian Tube: Anatomic Possibilities and Limitations. Am J Otol 1999; 20:299–303. [PubMed] [Google Scholar]
- 8.Linstrom CJ, Silverman CA, Rosen A, et al. Eustachian Tube Endoscopy in Patients With Chronic Ear Disease. Laryngoscope 2000; 110:1884–9. [DOI] [PubMed] [Google Scholar]
- 9.Edelstein DR, Magnan J, Parisier SC, et al. Microfiberoptic evaluation of the middle ear cavity. Am J Otol 1994;15:50–5. [PubMed] [Google Scholar]
- 10.Djeric D, Savic D Anatomical Variations and Relations of the Bony Portion of the Eustachian Tube. Acta Otolaryngol. 1985. 99:543–50. [DOI] [PubMed] [Google Scholar]
- 11.Paltura C, Can TS, Yilmaz BK, et al. Eustachian tube diameter: Is it associated with chronic otitis media development? Am J Otolaryngol 2017;38:414–16. [DOI] [PubMed] [Google Scholar]
- 12.Janzen-Senn I, Schuon RA, Tavassol F, et al. Dimensions and position of the Eustachian tube in Humans. PLoS One 2020; 15(5): e0232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takasaki K, Takahashi H, Miyamoto I, et al. Measurement of angle and length of the eustachian tube on computed tomography using the multiplanar reconstruction technique. Laryngoscope 2007;117:1251–1254. [DOI] [PubMed] [Google Scholar]
- 14.Swaney PJ, York PA, Gilbert HB, et al. Design, fabrication, and testing of a needle-sized wrist for surgical instruments. J Med Device 2017;11(1):0145011–0145019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichera L, Dillon NP, Zhang D, et al. Through the Eustachian Tube and Beyond: A New Miniature Robotic Endoscope to See Into the Middle Ear. IEEE Robot Autom Lett 2017;2(3):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gafford J, Freeman M, Fichera L, et al. Eyes in Ears: A Miniature Steerable Digital Endoscope for Trans-Nasal Diagnosis of Middle Ear Disease. Ann Biomed Eng 2020; 49(1):219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video Figure 1: Transeustachian endoscopy of Specimen 1, Right Ear
Supplemental Video Figure 2: Transeustachian endoscopy of Specimen 2, Left Ear
Supplemental Video Figure 3: Transeustachian endoscopy of Specimen 3, Left Ear


