Abstract
The gp43 glycoprotein is an immune-dominant antigen in patients with paracoccidioidomycosis (PCM). It is protective against murine PCM and is a putative virulence factor. The gp43 gene of Paracoccidioides brasiliensis B-339 is located in a 1,329-bp DNA fragment that includes two exons, a 78-bp intron, and a leader peptide-coding region of 105 bp. Polymorphism in gp43 has been suggested by the occurrence, in the same isolate or among different fungal samples, of isoforms with distinct isoelectric points. In the present study we aligned and compared with a consensus sequence the gp43 precursor genes of 17 P. brasiliensis isolates after sequencing two PCR products from each fungal sample. The genotypic types detected showed 1 to 4 or 14 to 15 informative substitution sites, preferentially localized between 578 and 1166 bp. Some nucleotide differences within individual isolates (noninformative sites) resulted in a second isoelectric point for the deduced protein. The most polymorphic sequences were also phylogenetically distant from the others and encoded basic gp43 isoforms. The three isolates in this group were from patients with chronic PCM, and their DNA restriction patterns were distinct in Southern blots. The nucleotides encoding the inner core of the murine T-cell-protective epitope of gp43 were conserved, offering hope for the development of a universal vaccine.
Paracoccidioidomycosis (PCM) in humans is a systemic granulomatous mycosis caused by Paracoccidioides brasiliensis, a dimorphic fungus. The disease is restricted to Central and South America, where P. brasiliensis has also been isolated from soil and nine-banded armadillos (28). In humans, infection starts by the inhalation of fungal propagules, which reach the pulmonary alveolar epithelium and transform into the parasitic yeast form. Acute PCM and subacute PCM affect members of both sexes, progress rapidly, and disseminate through the lymphatic system, with lymph node hypertrophy and, in severe cases, intense hepatosplenomegaly and involvement of other organs. Chronic forms affect mainly male adults and evolve gradually in the lungs, being associated or not with mucous and skin lesions and clinical involvement of other organs. Severe PCM forms are characterized by inhibition of the protective cellular immunity against the infectious agent, thus allowing fungal growth, with high antigenic load and high titers of specific antibodies, which are, however, not protective (12).
The main antigenic component described in P. brasiliensis is gp43 (24), an exocellular glycoprotein containing a single oligosaccharide chain (2). The open reading frame of the gp43 gene is within a 1,329-bp DNA fragment comprising two exons separated by a 78-bp intron (10). The gene codes for a precursor protein of 416 amino acids, which includes a leader peptide region of 35 residues. Although the protein sequence is similar to those of exo-1,3-β-d-glucanases from Saccharomyces cerevisiae and Candida albicans, the hydrolase activity is absent, probably because the conserved glucanase active site NEP is NKP in gp43, whereas the LEP active site is conserved. gp43 is recognized by most sera from patients with PCM (4, 40), whose antibodies are preferentially directed to conformational peptide epitopes (25, 26). Patients suffering from severe PCM have high antibody titers against gp43, which tend to decrease with successful treatment (4, 19). Reduced and negative antibody responses to P. brasiliensis antigens generally point to a good prognosis and clinical cure (12, 19, 27). Besides eliciting humoral immune responses, gp43 is an immune-dominant antigen for cellular immunity in humans (34) and experimentally infected animals (29). The gp43 T-cell epitope compatible with H-2a, H-2b, and H-2c murine haplotypes has recently been mapped to a 15-mer peptide called P10 (41). In murine PCM, both gp43 and P10 were able to protect against an intratracheal challenge with virulent P. brasiliensis by eliciting a Th1, gamma interferon-mediated response, and genetic vaccination with the gp43 gene was also protective (23). In addition to these properties, gp43 is a receptor for murine laminin and may therefore be a virulence factor (43). From a panel of murine anti-gp43 monoclonal antibodies, one of them was able to modulate infection with P. brasiliensis cells coated with laminin in a hamster intratesticular PCM model (14). The monoclonal antibodies tested recognized about three different conformational peptide epitopes, as suggested by inhibition assays (7, 26), but their localization in the molecule is still unclear. Vaccination with P10 alone did not produce detectable antibody titers in mouse sera (41).
The processed gp43 can be purified from P. brasiliensis B-339 culture medium as a mixture of isoforms (24) with three near but distinct isoelectric points (pIs). In addition, the gp43 pI values varied between 5.8 and 7.2, depending on the isolate studied (22), and in one case it was 8.5. Considering the importance of gp43 in PCM and the multifunctional nature of the molecule, the present study was carried out in order to evaluate its gene polymorphism in a variety of P. brasiliensis isolates from patients suffering from chronic and acute PCM. Two PCR fragments of the precursor genes of 17 isolates, including 1 from soil and another from an armadillo, were completely sequenced and compared.
MATERIALS AND METHODS
P. brasiliensis isolates and growth conditions.
The fungal sources and providers are specified in Table 1. The cultures were maintained at 35°C in modified solid YPD (0.5% Bacto Yeast extract, 0.5% casein peptone, 1.5% dextrose [pH 6.3]). For DNA extraction, the fungal cells were cultivated in modified YPD at 35°C with shaking for 7 to 10 days, checked microscopically for homogeneity, collected by filtration in paper, and kept frozen at −70°C until use. Total DNA was extracted from 10 ml (wet pellet) of powdered yeast cells frozen in liquid nitrogen, as described previously (11).
TABLE 1.
P. brasiliensis isolates analyzed in this study
| Strain name | Collection no. | State and/or country of isolation | Source | Isolated by/provided by: |
|---|---|---|---|---|
| Pb1 | B-339 | Brazil | Chronic PCM | ?/A. Restrepo |
| Pb2 | 1925 | Venezuela | Chronic PCM | M. B. Albornoz/Z. P. Camargo |
| Pb3 | 608 | São Paulo, Brazil | Chronic PCM | M. H. H. Forjaz/T. I. E. Svidzinski |
| Pb4 | 1017 | São Paulo, Brazil | Chronic PCM | M. H. H. Forjaz/T. I. E. Svidzinski |
| Pb5 | Ap | Paraná, Brazil | Chronic PCM | T. I. E. Svidzinski |
| Pb6 | Mg4 | Paraná, Brazil | Chronic PCM | T. I. E. Svidzinski |
| Pb7 | 18 | São Paulo, Brazil | Chronic PCM | ?/Z. P. Camargo |
| Pb8 | 9673 | São Paulo, Brazil | Chronic PCM | E. M. Heins-Vaccari/T. I. E. Svidzinski |
| Pb9 | 924 | São Paulo, Brazil | Chronic PCM | M. H. H. Forjaz/T. I. E. Svidzinski |
| Pb10 | Peru 18749 | Peru | Acute PCM | B. Bustamante/T. I. E. Svidzinski |
| Pb11 | Mg5 | Paraná, Brazil | Acute PCM | T. I. E. Svidzinski |
| Pb12 | Argentina | Argentina | Acute PCM | R. Negroni/T. I. E. Svidzinski |
| Pb13 | SS | Goiás, Brazil | Acute PCM | ?/Z. P. Camargo |
| Pb14 | 470 | São Paulo, Brazil | Acute PCM | M. H. H. Forjaz/T. I. E. Svidzinski |
| Pb15 | Venezuela 350.85 | Venezuela | Acute PCM | M. B. Albornoz/T. I. E. Svidzinski |
| Pb16 | Solo | Venezuela | Soil | M. B. Albornoz/Z. P. Camargo |
| Pb17 | PRT1 | São Paulo, Brazil | Armadillo | E. Bagagli/Z. P. Camargo |
PCR and sequencing conditions.
The gp43 gene precursor (GenBank accession no. U26160) from 16 P. brasiliensis isolates was obtained by PCR with upstream primer 490 (5′-GTCAGATCTATCATGAATTTTAGTTCCTTAAC-3′), which contains a BglII site before the ATG start codon, and downstream primer 491 (5′-ACGTCGACTCACCTGCATCCACCATACTT-3′), which contains a SalI site immediately after the TGA stop codon. In order to prevent polymerase misreading, the PCRs were carried out in fewer cycles, with high DNA but low deoxynucleoside triphosphate (dNTP) concentrations, in 10 mM Tris-HCl (pH 9.0) (100 μl) containing 50 mM KCl, 1.5 mM MgCl2, each dNTP at a concentration of 50 μM, each primer at a concentration of 1 μM, 1 μg of genomic DNA, and 5 U of Taq polymerase (Pharmacia). The reactions were performed in a Pharmacia thermocycler for 20 cycles of 94°C (1 min), 50°C (1 min), and 72°C (2 min), followed by a final extension for 7 min at 72°C. The PCR product migrating at approximately 1.3 kb in ethidium bromide-stained agarose gels was cut, extracted with the Nucleiclean kit (Sigma), and cloned for sequencing in either the pMosBlue T (pMosBlue T kit; Amersham) or the pGEM-T (pGEM-T kit; Promega) vector. The inserts of two independent clones were sequenced by the dideoxynucleotide chain termination reaction described by Sanger et al. (31) and with the T7 Sequencing Kit (Pharmacia), using internal sense and antisense primers designed according to the original gene sequence (10). Base substitutions were confirmed in both strands.
Southern blot hybridization.
Southern blots were carried out as described by Sambrook et al. (30), using high-stringency conditions as already described (10). Total DNAs (10 μg/lane) of different P. brasiliensis isolates were restricted with the EcoRI and BglII enzymes and tested for hybridization with [α-32P]dATP-labeled DNA probes of the gp43 (1,329 bp) and the LON (777-bp) genes of P. brasiliensis (GenBank accession no. AF239178). The fungal LON gene has recently been cloned and sequenced in our laboratory (T. F. Barros and R. Puccia, unpublished data).
Data analysis.
The sequences were analyzed with the EditSeq, Seqman, Megalign, and Protean programs of the Lasergene System (DNAstar Inc.). Phylogenetic analyses were carried out with the computer program Phylip (Phylogeny Inference Package) to build a phylogenetic tree based on maximum likelihood.
RESULTS
We compared the gp43 gene sequences of 17 P. brasiliensis isolates (Table 1), including that originally reported for P. brasiliensis B-339 (10). Besides the human isolates, one isolate from soil (1) and another isolate from an armadillo (3) were also analyzed. Among the isolates listed in Table 1, Pb6 and Pb11 were recent isolates from patients. The others have been kept in culture for different periods of time, but were recently recovered from animal orchitis (39), with the exception of Pb2, Pb16, and Pb17. The precursor gene of each fungal sample was obtained by PCR, and since two cloned fragments (referred to as clones 1 and 2) were sequenced, two gp43 sequences were obtained for each isolate. In addition, the original genomic clone containing the gp43 gene from strain B-339 (10) was submitted to automatic sequencing, showing that there had been some sequencing errors in the previously reported gene. An updated GenBank accession no. U26160 sequence has been used for comparison in the present work, where the amino acids at positions 266, 296, 317, 335, 336, and 376 are L, D, C, P, S, and A, respectively.
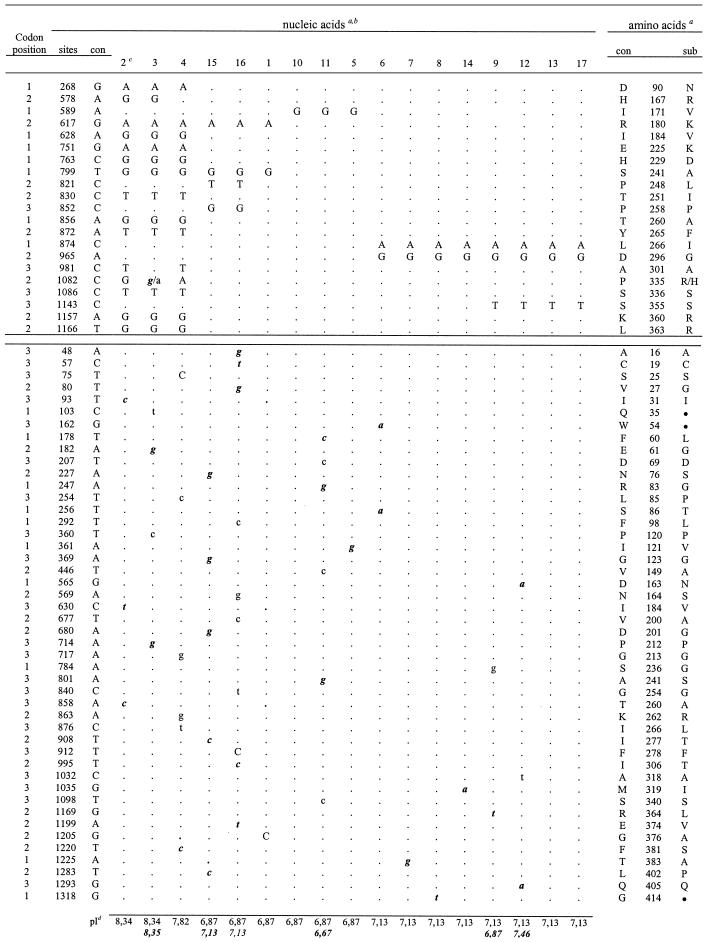
The results are detailed in Table 2, which shows the informative sites (seen in two or more isolates) in the upper section, the noninformative sites (peculiar to one isolate) in the lower section, and the substitutions compared with a consensus sequence generated from the alignment. The corresponding amino acid changes and positions are also shown. The deduced pIs for each sequence corresponding to the processed gp43 are seen on the bottom, where italic bold numbers refer to clone 2. Considering all the DNA sequences obtained, 67 substitution sites were found, with two bases per site, except for site 1082, which was C, G, or A. The informative sites (31.3% of the total) occurred mainly at position 1 or 2 of the codon and were the result of 66% transitions, predominantly of the A↔G type (Table 2). The noninformative sites (Table 2) were the result of transitions (84.8%), with 53.8% being of the C↔T type. In general, the substitutions resulted in nonsynonymous amino acid changes. Noninformative sites 247 (Pb11), 565 (Pb12), 680 (Pb15), and 1169 (Pb9) resulted in amino acid changes responsible for pI changes. Most of the informative sites were between 578 and 1166 bp, while the noninformative sites were scattered along the sequence.
TABLE 2.
Distribution of nucleotide polymorphisms in gp43 gene of P. brasiliensis
Informative and noninformative sites are presented in the upper and lower sections, respectively; con, consensus sequence; sub, substitution; positions are as described elsewhere (10).
Lowercase letters, clone 1 or clone 2 (italics); capital letters, both clones; ●, termination codon.
Numbers indicate P. brasiliensis isolates Pb1 to Pb17.
Nonitalic numbers, clone 1 or both clones 1 and 2; italic numbers, clone 2.
The DNA sequences corresponding to the intron (sites 464 to 541), the glycosylation site (sites 661 to 669), and the exoglucanase activity regions (sites 151 to 159 and 697 to 705) of the gp43 gene were conserved in all isolates (Table 2). Six noninformative sites were detected in the leader peptide-coding region (sites 1 to 105), but four of them resulted in synonymous amino acid changes. The sites (sites 619 to 663) coding for the P10 peptide containing the murine T-cell epitope were altered only in Pb2, Pb3, and Pb4 (sites 628 and 630), but the resulting amino acid changes were not within the inner epitope core (41). Substitutions at positions 103, 162, and 1318 resulted in stop codons. Two substitutions (sites 268 and 565) prompted amino acid replacement to an asparagine, but these were not potential new glycosylation sites.
The gp43 sequences of Pb2, Pb3, and Pb4 were more polymorphic than the others (Table 2), with 13 exclusive informative sites, and 3 (sites 617, 799, and 874) were shared with other isolates. Among the 15 possible amino acid changes in these sequences, 7 were to a positively charged R (in four positions), K, or H, and consequently, the gp43 isoforms were basic (pIs 7.82 to 8.35). The other genotypic groups showed one (in Pb10, Pb11, and Pb5), two (in Pb1, Pb6, Pb7, Pb8, and Pb14), three (in Pb9, Pb12, Pb13, and Pb17), or four (in Pb15 and Pb16) informative sites. In these sequences (Table 2), the deduced gp43 pI values were generally neutral (7.13 or 6.87, with a pI of one 6.67 for one isolate and a pI of 7.46 for one isolate).
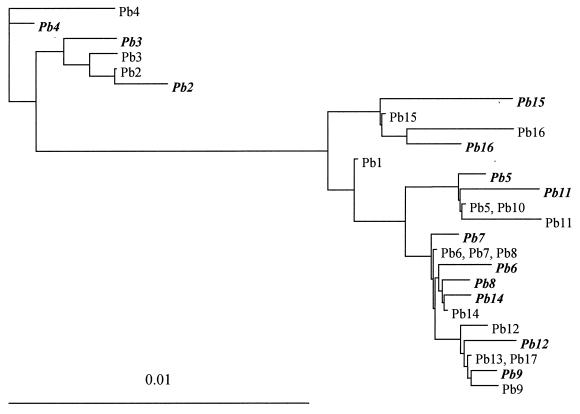
The phylogenetic relationships among the gp43 gene sequences were analyzed by the maximum-likelihood method, generating the tree shown in Fig. 1. The conserved intron and the leader peptide-coding region were disregarded, and so a total of 1,146 bp encoding the processed antigen was used in the analysis (GenBank accession nos. AY005405 to AY005437). Both the informative and noninformative sites were considered; therefore, two sequences can be seen for most isolates. Three distinct clades were observed; one included the gp43 sequences of Pb4, clade 2 had the sequences of Pb2 and Pb3, and clade 3 included the sequences of all the other isolates. Clade 3 was subdivided into four main branches that included the sequences of (i) Pb15 and Pb16; (ii) Pb1 (strain B-339); (iii) Pb5, Pb10, and Pb11; and (iv) all the other isolates. Clades 1 and 2 were phylogenetically distant from clade 3 and grouped the most polymorphic gp43 sequences. In some cases, the two gp43 sequences of the same individual were not each other's closest neighbor (see Pb8, for example).
FIG. 1.
Maximum-likelihood phylogenetic tree resulting from the analysis of 30 gp43 sequences (1,146 bp, encoding the processed antigen) from 17 different P. brasiliensis isolates. The sequences are indicated by the isolate code names (Pb1 to Pb17; Table 1), and clone 2 variants are in italics. Both informative and noninformative sites were considered.
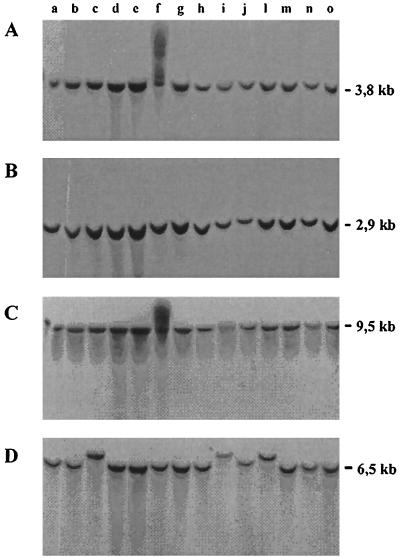
Polymorphism among the P. brasiliensis isolates was also revealed by Southern blot analyses (Fig. 2D) with total DNA digested with BglII and hybridized with a proteinase Lon probe, recently sequenced in our laboratory (Barros and Puccia, unpublished data). In this case, the labeled fragment seen in Pb2, Pb3, Pb4, and Pb10 migrated more slowly than the correspondent fragment (6.5 kb) in the other isolates. No difference in fragment size among isolates was noticed when the proteinase Lon probe was used to label EcoRI-cut DNA or when a gp43 probe was used to label EcoRI- or BglII-cut DNA (Fig. 2A to C).
FIG. 2.
Southern blot analyses with labeled probes of the gp43 (A and B) and proteinase Lon (C and D) genes. Genomic DNA was restricted with EcoRI (A and C) or BglII (B and D). Lanes: a, Pb1; b, Pb7; c, Pb2; d, Pb5; e, Pb14; f, Pb13; g, Pb12; h, Pb9; i, Pb4; j, Pb10; l, Pb3; m, Pb11; n, Pb6; and o, Pb8. Digestion with EcoRI was partial in lane f. In a second experiment with the LON probe and BglII-cut DNAs of Pb1 to Pb17, it was confirmed that the labeled fragment migrated at 6.5 kb for all isolates except isolates Pb2, Pb3, and Pb4 (data not shown).
DISCUSSION
Our data confirm, at the DNA level, the gp43 polymorphism suggested by the expression in vitro of isoforms in different P. brasiliensis isolates (22, 24). By sequencing two PCR fragments of the gp43 gene precursor and comparing the products of 17 fungal isolates, we have been able to recognize sequences containing 1, 2, 3, 4, or 14 to 15 informative substitution sites when the sequences were compared to a consensus sequence. The occurrence of two genotypic forms in a single individual, defined by the noninformative sites characteristic of each PCR fragment, explains the finding of more than one gp43 isoform per isolate (22, 24) and could be due to the presence of many nuclei per cell (18) and to the possible diploid nature (8, 10) of this microorganism.
We have been able to distinguish some gp43 genotypic types among the 17 isolates. In 12 isolates, including isolate B-339, the sequences differed from a consensus sequence at only one to three bases, and they composed three branches with the same origin in a phylogenetic tree. These isolates were from patients with both acute and chronic PCM and from an armadillo. Isolates Pb15 and Pb16 formed a separate branch that was closer to Pb1, and their sequences showed four substitutions at informative sites (bases 617, 799, 821, and 852). Despite their distinct sources (one from soil and another from a patient with acute PCM), isolates Pb15 and Pb16 were both from Venezuela. That might not be coincidental, since by randomly amplified polymorphic DNA (RAPD) analysis with a selected primer, Calcagno et al. (6) were able to cluster P. brasiliensis isolates according to the country of isolation, suggesting that the fungus might be under environmental genetic pressure. Although Pb2, also from Venezuela, was in a genetically distant group in terms of gp43 sequences, it shared two substitution sites (sites 617 and 799) with the Venezuelan group.
By comparing P. brasiliensis isolates from human patients and nine-banded armadillos (Dasypus novemcinctus) by RAPD analysis, Sano et al. (32) have recently proposed that these animals are a source of the fungus in nature and that individual armadillos can be infected with different isolates which are genotypically distinct. The isolates from the spleen, liver, and lymph nodes of one armadillo were investigated for their virulence and a partial sequence of gp43 (33). Among 539 bases, the authors found substitutions at sites 617, 799, 821, and 852 that distinguished the spleen isolate from the others. The gp43 sequence from the armadillo's spleen isolate (33) resembled that of Pb17 (Table 2), originally a spleen isolate from armadillo PRT1 (32). The gp43 gene sequences from the liver and lymph node isolates (33), on the other hand, were similar to the Pb15 and Pb16 gp43 gene sequences (Table 2) when the informative sites are considered. It is noteworthy that the substitution that Sano et al. (33) observed in the gp43 intron was not seen in the sequences analyzed here.
Genetic polymorphism in P. brasiliensis has been detected in the past by RAPD analysis (6, 32, 37) and chromosome analyses (8, 21). It is also known that individual isolates show different degrees of pathogenicity in susceptible inbred mice (15, 33, 35). More recently, the similarities of P. brasiliensis isolates from humans in their ability to invade tissues and cause disease in mice were associated with their genetic background, as evaluated by RAPD analysis (20). In that study, the clinical status of the patients infected with each isolate analyzed was not known and a correlation between genetic group, virulence in mice, and clinical PCM form was not possible, but an association between the severity of disease in human and experimentally infected mice was not evident in an earlier report (36). P. brasiliensis isolates from the liver and lymph nodes were less virulent in mice than another isolate from the spleen of the same armadillo, which was also had a distinct gp43 sequence (33). In the present study, the gp43 sequences of Pb2, Pb3, and Pb4 were phylogenetically distant from the gp43 sequences of other isolates, and the DNAs of these isolates were also polymorphic in Southern blots. It is noteworthy that these samples were all from patients with pulmonary or chronic PCM. It was our special interest to correlate the clinical form of PCM with the gp43 polymorphism for future understanding of the functional regions of the molecule, and the fact that peculiar gp43 genotypic forms were found only in isolates from patients with chronic PCM could be meaningful. The pathogenicity in mice of the isolates studied here is under investigation in our laboratory.
The purified gp43 from isolate 1925 (Pb2) was shown to be less antigenic than other isoforms when tested by a capture enzyme-linked immunosorbent assay with a great variety of sera from patients with PCM (38). However, its reactivity was significantly higher with sera from patients with chronic PCM than from patients with acute PCM. The peculiar gp43 sequences seen in Pb2, Pb3, and Pb4 showed 14 to 15 informative substitution sites, leading to changes with a bias to basic amino acids, especially arginine. Hence, the deduced protein had basic pIs, in contrast to the generally neutral values observed for the other sequences. The existence of a basic gp43 isoform was first reported by Moura-Campos et al. (22) and was found in only one of the eight isolates analyzed. In our analyses, three isolates had basic gp43 isoforms, and all three isolates were from patients with chronic PCM. The amino acid replacements characteristic of these isoforms produced some differences in the antigenic index profile (Protean analysis; Dnastar [data not shown]) that could justify the peculiar antigenicity observed for the Pb2 gp43 (22). We believe that this antigenic pattern could be characteristic of some isolates from patients with chronic PCM, but this is going to be better understood only if the localization of the antibody epitopes on gp43 is unraveled.
Our results suggest that the inner core of the gp43 T-cell epitope HTLAIR (amino acid positions 187 to 192) is conserved among P. brasiliensis isolates. The closest amino acid change detected was Ile to Val, at position 184, which would not affect the hydrophobic nature of the flanking amino acids and the presentation by major histocompatibility complex type II of the 12-amino-acid sequence minimally required for a T-cell response. This is an important finding because gp43 and a 15-amino-acid peptide containing the HTLAIR core (P10) are the only possible vaccines so far reported for PCM, considering their protective effects in mice (41). Although the gp43 epitopes for the human T-cell response have not been mapped yet, the Tepitope computer program points to a high probability of presentation of the P10 peptide sequence by the most frequent human DR haplotypes.
gp43 is highly recognized by sera from patients with PCM and as such should be under strong selective pressure. Our data are in agreement with this assumption because we have shown that the informative sites were not randomly scattered throughout the molecule but, rather, were preferentially localized from nucleotide 578, which is downstream of the conserved intron. The fact that there is a codon preference for amino acids such as Ile (AUC), Pro (CCG), Thr (ACC), and Val (GUC) was also suggestive of a high rate of expression of the glycoprotein, and that was indeed observed to occur in vitro with some isolates (22). The gene sequencing data enabled the construction of a phylogenetic tree characteristic of clonal or asexual organisms (42), which suggests the existence of genetically isolated groups within P. brasiliensis, possibly clonal lineages. In addition, the two genotypic forms of one individual were not always each other's closest neighbor, suggesting that at some point there could have been recombination in the evolution of the fungus (42). None of these assumptions, however, can be conclusive after the study of only one gene (42). The gene sequencing approach, among other genetic analysis techniques, has successfully been used for strain typing and population genetic analysis of medically important fungi like Histoplasma capsulatum (9, 16), Coccidioides immitis (5, 17), and Aspergillus flavus (13). It will be especially interesting to investigate if the P. brasiliensis samples analyzed here will fit into similar genetic groups when other loci are studied.
ACKNOWLEDGMENTS
We thank Z. P. Camargo, A. Restrepo, and T. I. E. Svidzinski for providing fungal isolates. We also thank J. W. Taylor and J. F. Silveira for generous discussions.
This work was supported by FAPESP, CNPq, and PRONEX.
REFERENCES
- 1.Albornoz M B. Isolation of Paracoccidioides brasiliensis from rural soil in Venezuela. Sabouraudia. 1971;9:248–253. [PubMed] [Google Scholar]
- 2.Almeida I C, Neville D C A, Mehlert A, Treumann A, Ferguson M A J, Previato J O, Travassos L R. Structure of the N-linked oligosaccharides of the main diagnostic antigen of the pathogenic fungus Paracoccidioides brasiliensis. Glycobiology. 1996;6:507–515. doi: 10.1093/glycob/6.5.507. [DOI] [PubMed] [Google Scholar]
- 3.Bagagli E, Sano A, Coelho K I, Alquati S, Miyaji M, Camargo Z P, Franco M, Montenegro M R. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus noveminctus) captured in an endemic area of paracoccidioidomycosis. Am J Trop Med Hyg. 1998;58:505–512. doi: 10.4269/ajtmh.1998.58.505. [DOI] [PubMed] [Google Scholar]
- 4.Blotta M H S L, Camargo Z P. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J Clin Microbiol. 1993;31:671–676. doi: 10.1128/jcm.31.3.671-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt A, Dechairo B M, Koenig G L, Carter D A, White T J, Taylor J W. Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Proc Natl Acad Sci USA. 1996;93:770–773. doi: 10.1046/j.1365-294x.1997.00245.x. [DOI] [PubMed] [Google Scholar]
- 6.Calcagno A M, Niño-Vega G, San-Blas F, San-Blas G. Geographic discrimination of Paracoccidioides brasiliensis strains by randomly amplified polymorphic DNA analysis. J Clin Microbiol. 1998;36:1733–1736. doi: 10.1128/jcm.36.6.1733-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo Z P, Gesztesi J L, Saraiva E C O, Taborda C P, Vicentini A P, Lopes J D. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J Clin Microbiol. 1994;32:2377–2381. doi: 10.1128/jcm.32.10.2377-2381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano M I, Cisalpino P S, Galindo I, Ramirez J L, Mortara R A, Silveira J F. Electrophoretic karyotypes and genome sizing of the pathogenic fungus Paracoccidioides brasiliensis. J Clin Microbiol. 1998;36:742–747. doi: 10.1128/jcm.36.3.742-747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter D A, Burt A, Taylor J W, Koenig G L, White T J. Clinical isolates of Histoplasma capsulatum from Indianapolis, Indiana, have a recombining population structure. J Clin Microbiol. 1996;34:2577–2584. doi: 10.1128/jcm.34.10.2577-2584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisalpino P S, Puccia R, Yamauchi L M, Cano M I N, Silveira J F, Travassos L R. Cloning, characterization, and epitope expression of the major diagnostic antigen of Paracoccidioides brasiliensis. J Biol Chem. 1996;271:4553–4560. doi: 10.1074/jbc.271.8.4553. [DOI] [PubMed] [Google Scholar]
- 11.Cisalpino P S, Silveira J F, Travassos L R. RNA and DNA isolation from Paracoccidioides brasiliensis yeast cells: establishment of cDNA and genomic libraries, and PCR amplification. In: Maresca B, Kobayashi G S, editors. Molecular biology of pathogenic fungi, a laboratory manual. New York, N.Y: Telos Press; 1994. pp. 461–467. [Google Scholar]
- 12.Franco M. Host-parasite relationships in paracoccidioidomycosis. J Med Vet Mycol. 1987;25:5–18. doi: 10.1080/02681218780000021. [DOI] [PubMed] [Google Scholar]
- 13.Geiser D M, Pitt J I, Taylor J W. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc Natl Acad Sci USA. 1998;95:388–393. doi: 10.1073/pnas.95.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gesztesi J L, Puccia R, Travassos L R, Vicentini A P, Franco M F, Lopes J D. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 15.Kashino S S, Calich V L, Burger E, Singer-Vermes L M. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia. 1985;92:173–178. doi: 10.1007/BF00437630. [DOI] [PubMed] [Google Scholar]
- 16.Kasuga T, Taylor J W, White T J. Phylogenetic relationships of varieties and geographical groups of the human pathogenic fungus Histoplasma capsulatum Darling. J Clin Microbiol. 1999;37:653–663. doi: 10.1128/jcm.37.3.653-663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koufopanou V, Burt A, Taylor J W. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc Natl Acad Sci USA. 1997;94:5478–5482. doi: 10.1073/pnas.94.10.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen J G, Restrepo B I, Salazar M E, Restrepo A. Nuclear staining of Paracoccidioides brasiliensis conidia. J Med Vet Mycol. 1987;25:343–345. [PubMed] [Google Scholar]
- 19.Mendes-Giannini M J S, Bueno J P, Shikanai-Yasuda M A, Stolf A M S, Masuda A, Amato Neto V, Ferreira A W. Antibody response to the 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am J Trop Med Hyg. 1990;43:200–206. doi: 10.4269/ajtmh.1990.43.200. [DOI] [PubMed] [Google Scholar]
- 20.Molinari-Madlum E E, Felipe M S, Soares C M. Virulence of Paracoccidioides brasiliensis isolates can be correlated to groups defined by random amplified polymorphic DNA analysis. Med Mycol. 1999;37:269–276. [PubMed] [Google Scholar]
- 21.Montoya A E, Moreno M N, Restrepo A, McEwen J G. Electrophoretic karyotype of clinical isolates of Paracoccidioides brasiliensis. Fungal Genet Biol. 1997;21:223–227. doi: 10.1006/fgbi.1996.0955. [DOI] [PubMed] [Google Scholar]
- 22.Moura-Campos M C R, Gesztesi J L, Vicentini A P, Lopes J D, Camargo Z P. Expression and isoforms of gp43 in different strains of Paracoccidioides brasiliensis. J Med Vet Mycol. 1995;33:223–227. doi: 10.1080/02681219580000461. [DOI] [PubMed] [Google Scholar]
- 23.Pinto A R, Puccia R, Diniz S N, Franco M F, Travassos L R. DNA-based vaccination against murine paracoccidioidomycosis using the gp43 gene from Paracoccidioides brasiliensis. Vaccine. 2000;18:3050–3058. doi: 10.1016/s0264-410x(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 24.Puccia R, Schenkman S, Gorin P A J, Travassos L R. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun. 1986;53:199–206. doi: 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puccia R, Travassos L R. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidiodomycosis, histoplasmosis, and Jorge Lobo's disease. J Clin Microbiol. 1991;29:1610–1615. doi: 10.1128/jcm.29.8.1610-1615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puccia R, Travassos L R. The 43-kDa glycoprotein from Paracoccidioides brasiliensis and its deglycosylated form: excretion and susceptibility to proteolysis. Arch Biochem Biophys. 1991;289:298–302. doi: 10.1016/0003-9861(91)90475-x. [DOI] [PubMed] [Google Scholar]
- 27.Restrepo A, Restrepo M, Restrepo F, Aristizábal L H, Moncada L H, Velez H. Immune responses in paracoccidioidomycosis. A controlled study of 16 patients before and after treatment. Sabouraudia. 1978;16:151–163. [PubMed] [Google Scholar]
- 28.Restrepo-Moreno A. Ecology of Paracoccidioides brasiliensis. In: Franco M, Lacaz C S, Restrepo-Moreno A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 121–130. [Google Scholar]
- 29.Rodrigues E G, Travassos L R. Nature of the reactive epitopes in Paracoccidioides brasiliensis polysaccharide antigen. J Med Vet Mycol. 1994;32:77–81. [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequence with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano A, Tanaka R, Yokoyama N, Franco M, Bagagli E, Defaveri J, Montenegro M R, Mikami Y, Miyaji M, Nishimura K. Comparison between human and armadillo Paracoccidioides brasiliensis isolates by random amplified polymorphic DNA analysis. Mycopathologia. 1999;143:165–169. doi: 10.1023/a:1006949113529. [DOI] [PubMed] [Google Scholar]
- 33.Sano A, Defaveri J, Tanaka R, Yokoyama K, Kurita N, Franco M, Coelho K I R, Bagagli E, Montenegro M R, Miyaji M, Nishimura K. Pathogenicities and GP43kDa gene of three Paracoccidioides brasiliensis isolates originated from nine-banded armadillo (Dasypus novemcinctus) Mycopathology. 1999;144:61–65. doi: 10.1023/a:1007024923042. [DOI] [PubMed] [Google Scholar]
- 34.Saraiva E C O, Altemani A, Franco M F, Unterkircher C S, Camargo Z P. Paracoccidioides brasiliensis-gp43 used as paracoccidioidin. J Vet Med Mycol. 1996;34:155–161. doi: 10.1080/02681219680000261. [DOI] [PubMed] [Google Scholar]
- 35.Singer-Vermes L M, Burger E, Franco M F, Di-Bacchi M M, Mendes-Giannini M J, Calich V L. Evaluation of the pathogenicity and immunogenicity of seven Paracoccidioides brasiliensis isolates in susceptible inbred mice. J Med Vet Mycol. 1989;27:71–82. [PubMed] [Google Scholar]
- 36.Singer-Vermes L M, Burger E, Calich V L, Modesto-Xavier L H, Sakamoto T N, Sugizaki M F, Meira D A, Mendes R P. Pathogenicity and immunogenicity of Paracoccidioides brasiliensis isolates in the human disease and in an experimental murine model. Clin Exp Immunol. 1994;97:113–119. doi: 10.1111/j.1365-2249.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares C M, Madlun E E, Silva S P, Pereira M, Felipe M S. Characterization of Paracoccidioides brasiliensis isolates by random amplified polymorphic DNA analysis. J Clin Microbiol. 1995;33:505–507. doi: 10.1128/jcm.33.2.505-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza M C, Gesztezi J L, Souza A R, Moraes J Z, Lopes J D, Camargo Z P. Differences in reactivity of paracoccidioidomycosis sera with gp43 isoforms. J Med Vet Mycol. 1997;35:13–18. doi: 10.1080/02681219780000811. [DOI] [PubMed] [Google Scholar]
- 39.Svidzinski T I, Miranda Neto M H, Santana R G, Fischman O, Colombo A L. Paracoccidioides brasiliensis isolates obtained from patients with acute and chronic disease exhibit morphological differences after animal passage. Rev Inst Med Trop São Paulo. 1999;41:279–283. doi: 10.1590/s0036-46651999000500003. [DOI] [PubMed] [Google Scholar]
- 40.Taborda C P, Camargo Z P. Diagnosis of paracoccidioidomycosis by passive hemagglutination assay for antibody using a purified and specific antigen gp43. J Med Vet Mycol. 1993;31:155–160. doi: 10.1080/02681219380000171. [DOI] [PubMed] [Google Scholar]
- 41.Taborda C P, Juliano M A, Puccia R, Franco M, Travassos L R. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun. 1998;66:786–793. doi: 10.1128/iai.66.2.786-793.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor J W, Geiser D M, Burt A, Koufopanou V. The evolutionary biology and population genetics underlying fungal strain typing. Clin Microbiol Rev. 1999;12:126–146. doi: 10.1128/cmr.12.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicentini A P, Gesztesi J L, Franco M F, Souza W, Moraes J Z, Travassos L R, Lopes J D. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 1994;62:1465–1469. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]