Abstract
We examined the efficiency of detection of immunoglobulin M (IgM) antibodies to a 35-kDa antigen (P35) of Toxoplasma gondii for serodiagnosis of acute infection in pregnant women. A double-sandwich enzyme-linked immunosorbent assay (ELISA) with recombinant P35 antigen (P35-IgM-ELISA) was used for this purpose. On the basis of the clinical history and the combination of results from the toxoplasma serological profile (Sabin-Feldman dye test, conventional IgM and IgA ELISAs, and the differential agglutination test), the patients were classified into three groups: group I, status suggestive of recently acquired infection; group II, status suggestive of infection acquired in the distant past; group III, status suggestive of persisting IgM antibodies. Eighteen (90.0%) of 20 serum samples from group I patients were positive by the P35-IgM-ELISA, whereas none of the 33 serum samples from group II patients were positive. Only 4 (25.0%) of 16 serum samples from group III patients were positive by the P35-IgM-ELISA, whereas all these serum samples were positive by the conventional IgM ELISA. These results indicate that demonstration of IgM antibodies against P35 by the P35-IgM-ELISA is more specific for the acute stage of the infection than demonstration of IgM antibodies by the ELISA that uses a whole-lysate antigen preparation. Studies with sera obtained from four pregnant women who seroconverted (IgG and IgM antibodies) during pregnancy revealed that two of them became negative by the P35-IgM-ELISA between 4 and 6 months after seroconversion, whereas the conventional IgM ELISA titers remained highly positive. The P35-IgM-ELISA appears to be useful for differentiating recently acquired infection from those acquired in the distant past in pregnant women.
Detection of recently acquired infection with Toxoplasma gondii is important in pregnant women for prevention of transmission of the infection to their fetuses (11, 13, 18). In the United States, there is no systematic serological screening program for pregnant women. In contrast, in France and Austria, sera for testing are obtained at regular intervals throughout gestation from women who are seronegative when first tested. In the United States, a decision as to whether a woman was recently infected, thereby placing her fetus at risk of congenital infection, is often made from serological test results obtained with a single sample of serum. The presence of immunoglobulin G (IgG) antibodies in a single sample of serum does not indicate whether the infection was acquired prior to or during gestation. Therefore, the presence of IgG antibodies in a pregnant woman leads to additional serological testing to attempt to determine when the infection occurred (13). Of the recommended additional serological tests, those that demonstrate the presence of IgM antibodies are the most frequently used. However, since IgM antibodies may remain detectable for more than 1 year after the initial infection (2, 9, 16, 17), demonstration of these antibodies cannot be used to conclude that the infection is recently acquired. Because accurate diagnosis of recently acquired infection in pregnant women is crucial for clinical management of both the mother and her fetus, it is important to establish better diagnostic methods to distinguish recently acquired infections from those that occurred prior to conception.
In our attempt to determine whether a recently acquired infection with T. gondii could be differentiated from an infection acquired in the distant past with a single sample of serum from pregnant women, we recently found that IgG antibodies to a 35-kDa antigen (P35) of the parasite are detectable in the sera of most pregnant women with a toxoplasma serological profile (TSP) consistent with a recently acquired infection (6). This was not the case for sera from most of pregnant women with a serological profile consistent with an infection acquired in the distant past (6). In the present study, we examined whether demonstration of IgM antibodies to P35 in sera would be a better indicator of recently acquired infection in pregnant women than demonstration of IgM antibodies by the conventional enzyme-linked immunosorbent assay (ELISA) that uses a whole-lysate antigen preparation.
MATERIALS AND METHODS
Serum samples.
All sera used in the study were from pregnant women. The sera were divided into three groups. The serological tests used to classify these sera were the Sabin-Feldman dye test (DT) (14), IgM ELISA (10), IgA ELISA (15), and the differential agglutination (AC/HS) test (3). DT detects IgG antibodies to T. gondii by using live tachyzoites. The IgM ELISA is preformed with microtiter plates coated with anti-human IgM antibodies which bind to IgM antibodies in sample sera, followed by incubation with lysate antigens of tachyzoites and the alkaline phosphatase-labeled F(ab)2 fraction of rabbit anti-T. gondii IgG antibodies. The AC/HS test compares the titers obtained with formalin-fixed tachyzoites (HS antigen) with those obtained with acetone- or methanol-fixed tachyzoites (AC antigen) to determine whether infection was acquired recently or in the more distant past. Samples in group I were from 20 women with a TSP consistent with a recently acquired infection (acute profile) (7): high DT titers (>400 in most cases), positive IgM ELISA and IgA ELISA titers, and acute patterns by the AC/HS test. Samples in group II were from 33 women with a TSP consistent with an infection acquired in the distant past (chronic profile) (7): low DT titers (≤200 in most cases), negative IgM ELISA and IgA ELISA titers, and chronic patterns in the AC/HS test. Samples in group III were from 16 women with a TSP suggestive of persisting IgM antibodies: low DT titers, positive IgM ELISA titers, negative IgA ELISA titers, and chronic patterns in the AC/HS test.
Double-sandwich ELISA for detection of IgM antibodies to P35 antigen of T. gondii (P35-IgM-ELISA).
Anti-P35 IgM antibodies were detected by IgM ELISA (10), with modifications. Each well of microtiter plates (Nunc, Roskilde, Denmark) was coated with 100 μl of goat anti-human IgM antibodies (Caltag, Burlingame, Calif.) diluted in 0.1 M carbonate buffer (pH 9.8). After incubation at 4°C overnight, the plates were washed three times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T), and each well was postcoated with 200 μl of 5% normal calf serum (NCS; Sigma, St. Louis, Mo.) in PBS at 37°C for 2 h. The plates were then washed, and 100 μl of test or control serum diluted 1:100 in 5% NCS in PBS-T (NCS-PBS-T) was applied to each well. Triplicate wells were used for each sample. The plates were incubated at 37°C for 1 h and washed, and then 100-μl samples of recombinant P35 antigen (6a), at a concentration of 4 μg/ml in NCS-PBS-T, were added to the experimental wells. Since the recombinant antigen is a fusion protein with glutathione S-transferase (GST) (6), 100-μl samples of nonrecombinant GST proteins at the same concentration were added to the control wells. After incubation at 4°C for 18 h, the plates were washed again. Rabbit anti-GSP IgG antibodies (obtained by immunizing a rabbit with nonrecombinant GST) were diluted in NCS-PBS-T, and 100 μl was added to each well. After incubation at 4°C for 3 h and washing, 100 μl of alkaline phosphatase-conjugated goat anti-rabbit IgG (Caltag) diluted in NCS-PBS-T was added to each well. The plates were incubated at 4°C for 3 h and washed, and then 100 μl of 0.05 M carbonate buffer (pH 9.8) containing 1 mM MgCl2 and phosphatase substrate (Sigma) was added to each well. The optical densities at 410 nm were measured with an automatic ELISA reader (Dynatech Laboratories, Chantilly, Va.) after 1 h of incubation at room temperature. Each sample was run in triplicate wells. The results for each serum sample were determined by taking the mean value of the absorbency readings for the triplicate wells. The readings for the wells with P35 fusion proteins were normalized by subtraction of the readings for the wells with control GST proteins. The ELISA titers for the test sera were expressed as relative ratios of their readings to the reading for a positive control serum sample.
RESULTS
P35-IgM-ELISA with sera from pregnant women with either acute or chronic TSPs.
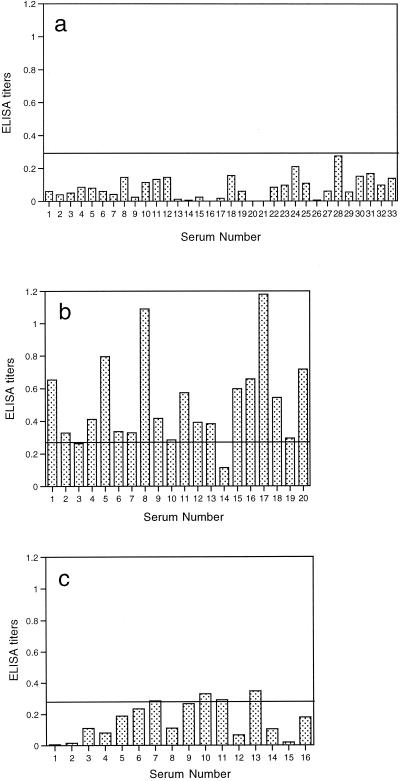
A total of 20 serum samples from group I (acute TSP) and 33 serum samples from group II (chronic TSP) were examined individually by the P35-IgM-ELISA. The ELISA titers for 93.9% (31 of 33) of the samples from group II were <0.2 (Fig. 1a). A cutoff value (i.e., 0.28) was determined on the basis of the mean value plus three standard deviations of the titers in sera from group II. None of the sera in group II had titers above this cutoff value (Fig. 1a). In contrast, 18 (90.0%) of 20 serum samples from group I had titers higher than the cutoff value (Fig. 1b). These results indicate that the P35-IgM-ELISA is effective in distinguishing sera from patients with an acute TSP from those from patients with a chronic TSP. The results of the assay were highly reproducible both within and between test runs. The variations of the readings among triplicate wells for each sample were less than 10% of the mean value of the readings. The results of two separate tests with seven samples (two negative and five positive serum samples, including a low-positive serum sample with a titer of 0.29) were reproducible for each sample.
FIG. 1.
Results of P35-IgM-ELISA with sera from pregnant women. (a) Results for 33 serum samples from those with a TSP consistent with a chronic infection (group II). (b) Results for 20 serum samples from those with a TSP consistent with a recently acquired infection (group I). (c) Results for 16 serum samples from those with a TSP suggestive of persisting IgM antibodies (group III). The horizontal line in each panel indicates the cutoff value of 0.28 (mean plus three standard deviations) obtained from the results noted with sera from group II individuals.
P35-IgM-ELISA with sera from pregnant women with a TSP suggestive of persisting IgM antibodies.
As mentioned above, the persistence of IgM antibodies after the initial infection has made the serodiagnosis of acute infection difficult (2, 9, 16, 17). We examined whether IgM antibodies to P35 are detectable in sera with persisting IgM antibodies. Sixteen serum samples from group III were tested by the P35-IgM-ELISA. The results of the TSP for all these sera are shown in Table 1. Only 4 (25.0%) of them had P35-IgM-ELISA titers above the cutoff value (Fig. 1c). These results indicate that the majority (75.0%) of serum samples with persisting IgM antibodies do not have detectable levels of IgM antibodies to P35.
TABLE 1.
Results of TSP for sera from patients in group III
| Serum sample no. | Serological test titer
|
|||
|---|---|---|---|---|
| DTa | IgM ELISAb | IgA ELISAc | AC/HSd | |
| 1 | 25 | 3.0 | 0.3 | <50/200 |
| 2 | 25 | 2.8 | 1.3 | <50/200 |
| 3 | 400 | 2.7 | 0.4 | <50/400 |
| 4 | 75 | 3.0 | 0.4 | <50/100 |
| 5 | 400 | 6.2 | 0.5 | <50/100 |
| 6 | 150 | 4.8 | 0.2 | <50/3200 |
| 7 | 25 | 2.5 | 0.2 | <50/800 |
| 8 | 12 | 6.4 | 0.2 | <50/100 |
| 9 | 25 | 2.6 | NDe | 50/400 |
| 10 | 100 | 3.8 | 0.2 | <50/1600 |
| 11 | 25 | 2.6 | 0.1 | <50/400 |
| 12 | 100 | 2.6 | 1.1 | <50/100 |
| 13 | 400 | 4.9 | 0.8 | <50/1600 |
| 14 | 25 | 5.5 | 0.2 | <50/200 |
| 15 | 25 | 5.1 | 0.3 | 50/100 |
| 16 | 50 | 5.7 | 0.2 | 100/1600 |
Titers equal to or higher than 4 are positive.
Titers equal to or higher than 2.0 are positive.
Titers equal to or higher than 2.1 are positive.
See reference 3 for interpretation.
ND, not done.
P35-IgM-ELISA with sera from pregnant women who had seroconverted during pregnancy.
In order to compare changes in IgM antibody titers between the P-35-IgM-ELISA and other conventional toxoplasma IgM ELISAs after the initial infection, we applied sequential serum samples from pregnant women who had seroconverted during pregnancy to these tests. Each of 10 serum samples obtained from four individuals within 2 months after they had seroconverted was positive by both the P35-IgM-ELISA and the conventional IgM ELISA (Table 2). Sera obtained between 4 and 6 months after seroconversion were also available from each of these patients. Whereas two of the four serum samples were negative by the P35-IgM-ELISA, all four were positive by the conventional IgM ELISA (Table 2). These results suggest that, following the initial infection, the titers of IgM antibodies detectable by the P35-IgM-ELISA decline earlier than the titers of IgM antibodies detectable by the conventional IgM ELISA.
TABLE 2.
Results of serological tests for toxoplasma antibodies in sera from pregnant women who seroconverted during pregnancy
| Patient no. and date (day/mo/yr) | Serological test titer
|
||
|---|---|---|---|
| DTa | IgM ELISAb | P35-IgM-ELISAc | |
| Patient 1 | |||
| 19/08/96 | <2 | 0.1 | NDd |
| 18/12/96 | 20 | 6.4 | 0.31 |
| 02/01/97 | 100 | 7.3 | 0.34 |
| 01/04/97 | 200 | 5.2 | 0.15 |
| Patient 2 | |||
| 30/11/96 | <2 | ND | ND |
| 25/01/97 | 10 | 6.8 | 0.93 |
| 22/02/97 | 400 | 6.1 | 0.62 |
| 22/03/97 | 800 | 5.3 | 0.47 |
| 16/07/97 | 800 | 4.6 | 0.36 |
| Patient 3 | |||
| 24/05/97 | <2 | 0.0 | ND |
| 27/06/97 | 20 | 4.9 | 0.42 |
| 03/07/97 | 100 | 5.0 | 0.44 |
| 03/12/97 | 100 | 2.5 | 0.01 |
| Patient 4 | |||
| 25/10/97 | <2 | 0.1 | ND |
| 27/11/97 | 20 | 8.1 | 1.87 |
| 04/12/97 | 100 | 7.3 | 1.63 |
| 20/03/98 | 100 | 5.0 | 0.77 |
Titers equal to or higher than 4 are positive.
Titers equal to or higher than 2.0 are positive.
Titers equal to or higher than 0.28 are positive.
ND, not done.
DISCUSSION
To provide proper clinical care for pregnant women, it is important to have a rapid and accurate method for diagnosis to distinguish recently acquired infections from those acquired in the distant past. Although recombinant T. gondii antigens have been evaluated for their ability to detect IgM antibodies (1, 4, 5, 8), it remains unclear which antigen of the parasite is effective for detection of acute stage-specfic IgM antibodies. The present study demonstrated that IgM antibodies to the P35 antigen of T. gondii could be detected by ELISA in 90.0% (18 of 20) of serum samples from pregnant women with an acute TSP but not in those (0 of 33) with a chronic TSP. Thus, the P35-IgM-ELISA has proved to be effective in differentiating an acute from a chronic TSP; the sensitivity and specificity for the acute stage of the infection were 90.0 and 100%, respectively.
Potasman et al. (12) previously reported that a 35-kDa antigen of T. gondii is recognized in immunoblots by IgM antibodies in sera from patients with acute infection. Recently, Aubert et al. (1) reported the efficiencies of six recombinant T. gondii antigens, including P35, for detection of IgM antibodies in human serum samples. In their studies, the sensitivity of an ELISA for the detection of P35 IgM antibodies in sera from individuals with acute infection was 46.1% (41 of 89 samples); this sensitivity is far lower than that of our P35-IgM-ELISA (90.0%). Since they used a direct ELISA (coating of the wells with P35) in their studies (1), a competition in binding to P35 antigen occurs between IgM and IgG antibodies in their test. In the present study, a double-sandwich ELISA (coating of the wells with anti-IgM antibodies) was used. Therefore, the competition between IgM and IgG antibodies in binding to P35 did not occur. The presence or absence of competition between these two immunoglobulin classes may explain the differences in the sensitivities of in the ELISAs in their study and in ours.
Although demonstration of toxoplasma IgM antibodies has been used to assist in the diagnosis of acute infection, as mentioned above, such antibodies may remain detectable for too long a period to make this possible (2, 9, 16, 17). The present study demonstrated that only 25.0% (4 of 16) of serum samples with results suggestive of persisting IgM antibodies (group III) were positive by the P35-IgM-ELISA, whereas all of those were positive by the conventional IgM ELISA with a whole-lysate antigen preparation. These results suggest that IgM antibodies to P35 antigen persist for a briefer period after the initial infection than IgM antibodies to other T. gondii antigens present in the lysate antigen preparation. The results of the P35-IgM-ELISA for sequential serum samples from seroconverters further support this possibility. For two of four seroconverters, P35-IgM-ELISA titers became negative for sera obtained between 4 and 6 months after seroconversion, whereas conventional IgM-ELISA titers remained highly positive for all these patients. The early disappearance of IgM antibodies to P35 after infection may explain the negative results for 10% of sera from patients with an acute TSP (group I); these sera might have been obtained from the patients later than 4 months after infection.
We recently reported that anti-P35 IgG antibodies were detectable in 85.3% of serum samples from pregnant women with an acute TSP but only 8% of serum samples from women with a chronic TSP (6). In the present study, for three of four seroconverters, the first samples after their seroconversion were positive for P35 IgM antibodies but negative for P35 IgG antibodies. In addition, for one of the seroconverters, the latest sample (obtained 4 months after seroconversion) was negative for P35 IgM antibodies but positive for P35 IgG antibodies. These results suggest that P35 IgM antibodies become detectable in serum earlier than P35 IgG antibodies after infection and that the IgM antibodies disappear earlier than the IgG antibodies. Thus, P35 IgM antibodies appear to be more specific for the early stage of infection than the IgG antibodies. Detection of anti-P35 IgM antibodies by the P35-IgM-ELISA appears to provide valuable information for the serodiagnosis of T. gondii infection in pregnant women to help to distinguish recently acquired infections from those acquired in the distant past.
ACKNOWLEDGMENT
This work was supported by U.S. Public Health Service grant AI04717.
REFERENCES
- 1.Aubert D, Maine G T, Villena I, Hunt J C, Howard L, Sheu M, Brojanac S, Chovan L E, Nowlan S F, Pinon J M. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 2000;38:1144–1150. doi: 10.1128/jcm.38.3.1144-1150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candolfi E, Ramirez R, Hadju M-P, Shubert C, Remington J S. The Vitek immunodiagnostic assay for detection of immunoglobulin M toxoplasma antibodies. Clin Diagn Lab Immunol. 1994;1:401–405. doi: 10.1128/cdli.1.4.401-405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannemann B R, Vaughan W C, Thulliez P, Remington J S. Differential aggulutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J Clin Microbiol. 1990;28:1928–1933. doi: 10.1128/jcm.28.9.1928-1933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harning D, Spenter J, Metsis A, Vuust J, Petersen E. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin Diagn Lab Immunol. 1996;3:355–357. doi: 10.1128/cdli.3.3.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson A M, Roberts H, Tenter A M. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J Med Microbiol. 1992;37:404–409. doi: 10.1099/00222615-37-6-404. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Maine G, Suzuki Y, Araujo F G, Galvan G, Remington J S, Parmley S. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J Clin Microbiol. 2000;38:179–184. doi: 10.1128/jcm.38.1.179-184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Li S, Galvan G, Araujo F G, Suzuki Y, Remington J S, Parmley S. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin Diagn Lab Immunol. 2000;7:781–787. doi: 10.1128/cdli.7.5.781-787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liesenfeld O, Press C, Montoya J G, Gill R, Isaac-Renton J L, Hedman K, Remington J S. False-positive results of immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Plateria Toxo IgM Test. J Clin Microbiol. 1997;35:174–178. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin V, Arcavi M, Santillan G, Amendoeira M R, De Souza Neves E, Griemberg G, Guarnera E, Garberi J C, Angel S O. Detection of human Toxoplasma-specific immunoglobulin A, M, and G with a recombinant Toxoplasma gondii Rop2 protein. Clin Diagn Lab Immunol. 1998;5:627–631. doi: 10.1128/cdli.5.5.627-631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naot Y, Guptil D R, Remington J S. Duration of IgM antibodies to Toxoplasma gondii after acute acquired toxoplasmosis. J Infect Dis. 1982;145:770. doi: 10.1093/infdis/145.2.770. [DOI] [PubMed] [Google Scholar]
- 10.Naot Y, Remington J S. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use of diagnosis of acute acquired toxoplasmosis. J Infect Dis. 1980;142:757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- 11.Pelloux H, Brun E, Vernet G, Marcillat S, Jolivet M, Guergour D, Hidalgo H, Fleuret A, Ambroise-Thomas P. Determination of anti-Toxoplasma immunoglobulin G avidity: adaptation to the vidas system (bioMerieux) Diagn Microbiol Infect Dis. 1998;32:69–73. doi: 10.1016/s0732-8893(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 12.Potasman I, Araujo F G, Desmonts G, Remington J S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986;154:650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- 13.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 140–267. [Google Scholar]
- 14.Sabin A B, Feldman H A. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoan parasite (Toxoplasma) Science. 1948;108:660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 15.Stepick-Biek P, Thulliez P, Araujo F G, Remington J S. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990;162:270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- 16.van Loon A M, van der Logt J T M, Heessen F W A, van der Veen J. Enzyme-linked immunosorbent assay that uses labeled antigen for detection of immunoglobulin M and A antibodies in toxoplasmosis: comparison with indirect immunofluorescence and double-sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 1983;17:997–1004. doi: 10.1128/jcm.17.6.997-1004.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson M, Remington J S, Clavet C, Varney G, Press C, Ware D The FDA Toxoplasmosis Ad Hoc Working Group. Evaluation of six commercial kits for the detection of human immunoglobulin M antibodies to Toxoplasma gondii. J Clin Microbiol. 1997;35:3112–3115. doi: 10.1128/jcm.35.12.3112-3115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong S Y, Remington J S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–862. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]