Abstract
One of the strategies of the World Health Organization End Tuberculosis (TB) was to reduce the catastrophic costs incurred by TB-affected families to 0% by 2020.Catastrophic cost is defined by the total cost related to TB management exceeding 20% of the annual pre-TB household income. This study aimed to estimate the pooled proportion of TB affected households who incurred catastrophic costs. We searched PubMed, SciELO, Scopus, Embase, Google Scholar, ProQuest, SAGE, and Web of Science databases according to Preferred Reporting Items of the Systematic Reviews and Meta-Analysis (PRISMA) guidelines till November 20, 2020. Eligible studies were identified and data on catastrophic costs due to TB were extracted. We performed a meta-analysis to generate the pooled proportion of patients with TB facing catastrophic costs. From 5114 studies identified, 29 articles were included in the final analysis. The pooled proportion of patients faced catastrophic costs was (43%, 95% CI [34–51]). Meta-regression revealed that country, drug sensitivity, and Human immune-deficiency Virus (HIV) co-infection were the main predictors of such costs. Catastrophic costs incurred by drug sensitive, drug resistant, and HIV co-infection were 32%, 81%, and 81%, respectively. The catastrophic costs incurred were lower among active than passive case findings (12% vs. 30%). Half (50%) of TB-affected households faced catastrophic health expenditure at 10% cut-off point. The financial burden of patients seeking TB diagnosis and treatment continues to be a worldwide impediment. Therefore, the End TB approach should rely on socioeconomic support and cost-cutting initiatives.
PROSPERO registration: CRD42020221283.
Subject terms: Diseases, Health care
Introduction
Tuberculosis (TB) infection is one of the top ten causes of death, with more than one million deaths worldwide in 20191. According to the 2020 World Health Organization (WHO) report, Africa region had the highest incidence of TB (220/105), followed by the South-East Asian region (211/105), the East Mediterranean region (112/105), and Western Pacific region (93/105)2. At a country-based level, the number of reported new cases was the highest in India (26%), followed by Indonesia (8.5%), China (8.4%), Philippines (6.0%), Pakistan (5.7%), Nigeria (4.4%), Bangladesh and South Africa (3.6% for each)3.
On September 26, 2018, the WHO’s End TB Strategy was set to reduce TB incidence and deaths by 90% and 95%, respectively, and to find TB missing cases by active case finding (ACF) instead of passive case finding (PCF). The ACF refers to systematic identification and screening of people with presumptive TB among high-risk groups, using rapidly used screening tools or tests. On contrary, PCF entails visiting health services for diagnosis4,5.
The WHO also recommended that all patients with TB or their families should not be impeded by catastrophic costs incurred due to TB to complete their treatment6. Catastrophic costs are the total direct and indirect costs that reach or exceed 20% of the pre-TB-patient or household’s annual income6. Direct costs represent either the medical cost (consultation fees, diagnostic tests, and treatment) or nonmedical cost (transportation, accommodation, and increased food needs). Indirect costs include lost wages due to unemployment, time spent away from work, and associated loss of productivity. Moreover, patients incur huge costs in the pre-treatment phase to cover consultations and laboratory tests, symptomatic treatment, antibiotics trial, and hospitalization7. An important segment of the financial hardship is dissaving, which means reduced financial strength of a household, or engaging the household in damaging financial coping strategies. This reduces the financial capacity and their ability to cope with the financial shocks and casts them into the poverty trap8. Dissaving can take many forms, such as availing a loan, taking children out of education, selling assets, and reducing consumption to below basic needs to cope with health-related expenditure7–9.
Such expenses can impede their access and adherence to treatment, affect health outcomes, increase the risk of disease transmission, and add to the household`s economic burden. These added expenses were exaggerated by the coronavirus disease (COVID-19) pandemic10. Patients with TB incur expenses that, on average, equal half of their yearly income in some low- and middle-income countries. Moreover, TB disproportionately affects the lowest section of society. The poverty-aggravating consequences of TB are, thus, most severe for those who are already vulnerable11. Catastrophic costs are affected by several factors, such as patient age and sex, socioeconomic status, Human immuno-deficiency virus (HIV) co-infection, and being infected with multidrug-resistant TB (MDR-TB) that does not respond to at least isoniazid and rifampicin11,12.
The WHO has developed a cost survey of patients with TB to properly assess the total costs and proportion of patients facing catastrophic costs. This tool provides a standardized methodology for cross-sectional surveys in TB affected countries13. Many studies have used this cost survey to report catastrophic costs, catastrophic health expenditure, or hardship in financing faced by patients with TB14–16. Some studies calculated the catastrophic costs incurred for drug sensitive, MDR, or HIV co-infection16–18. Other studies have estimated these costs by adopting different case finding strategies (ACF versus PCF)19,20. In response to the reported catastrophic costs, the Global TB Program endorses social protection initiatives as cash transfers, food assistance, disability grants, and health insurance. These initiatives were run in parallel to the Universal health coverage initiatives11,21,22. Data on the pooled prevalence of TB patients suffering from catastrophic costs has not been aggregated through meta-analysis. We therefore conducted this systematic review to estimate the pooled proportion of patients with TB who incured catastrophic costs and identify the predictors of these costs among patients and their households.
Method
We performed this systematic review and meta-analysis according to the Preferred Reporting Items of the Systematic Reviews and Meta-Analyses (PRISMA) guidelines23. Our research protocol was registered in PROSPERO (registration no. CRD42020221283).
Data source and search strategy
We searched EMBASE, Scopus, EBSCO, MEDLINE central/PubMed, ProQuest, SciELO, SAGE, Web of Science, and Google Scholar databases for articles without timeframe, geographical or language restrictions till November 20, 2020 by two authors (ShA & NZ). RMG and SA re-ran the data-base search to check the search strategy and number of citations reported. In addition, they checked the number of citations exported to the reference manager. Highly focused and sensitive search strategies were developed by RMG after approval of PubMed Help Disk. The search terms include “tuberculosis “OR “Mycobacterium tuberculosis” OR “Koch’s disease” AND “catastrophic cost”. (Supplementary Table 1). We searched reference lists from included publications by hand and contacted researchers who are expertise in these surveys to assist in identifying any relevant publications.
Study selection and eligibility criteria
We included observational studies that reported the proportion of patients suffering from catastrophic costs during the intensive (first 2 or 8 months of treatment in drug sensitivity (DS) or MDR, respectively), or the continuation phases of TB treatment.
Four authors (AME, ShA, NZ, and EE) independently screened titles and abstracts for relevant studies. We excluded non-observational studies, case reports, editorial, reviews, letters, and studies that did not report income because the catastrophic costs could not be calculated or when catastrophic costs were not calculated at the individual level (when the total direct and indirect costs incurred by all patients divided by the total income).
Two authors (AME, HE) independently assessed the retrieved abstracts and the full texts of these studies to determine eligibility according to the inclusion criteria. Disagreements were resolved through discussion and consensus, or through consultation with a third reviewer (SA), who solved these differences based on study judgements.
Data extraction and analysis
Three authors (RMG, AME, HE) extracted the following data from eligible studies: country, study design, population criteria (age, sex, drug sensitive/resistant), treating facility (public/private sector) strategy of case finding (ACF/PCF), tool used to identify the catastrophic costs, and the catastrophic total costs and its determinants at different cut-off points.
The outcomes and definitions
The primary outcome was the proportion of patients with TB and their households who incurred catastrophic costs. It was defined as the total direct and indirect costs because of TB reaching or exceeding 20% of the patient’s or household’s pre-treatment annual income6. Additionally, we addressed the main predictors of catastrophic costs and different coping strategies. Finally, we assessed the catastrophic costs among patients according to their drug sensitivity as DS or MDR (with or without HIV) and strategy of case finding (ACF versus PCF).
Secondary outcomes were the proportion of the direct costs to the total costs of TB treatment among DS or MDR, with or without HIV, catastrophic health expenditure (CHE; defined as the direct costs that reach or exceed 40% of patient’s capacity to pay or 10% of their household income)24, and the different coping strategies.
Study quality assessment
The Newcastle–Ottawa Scale was used to classify the quality of studies as very good studies (9–10 points), good studies (7–8 points), satisfactory studies (5–6 points), and unsatisfactory studies (0–4 points)25.
Publication bias
We assessed publication bias by visual inspection of funnel plots and Egger’s regression test.
Statistical analysis
Owing to the heterogeneity between studies, the proportion of catastrophic costs among patients with TB was pooled using the random effects model. Owing to the heterogeneity between studies, the proportion of catastrophic costs among patients with TB was pooled using the random effects model26.
Assessment of heterogeneity
Heterogeneity was assessed using the chi-square test on n-1 degrees of freedom, with an alpha of 0.05 considered for statistical significance and Cochrane-I-squared (I2) statistic. I2 values were classified as follows: 0 to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50 to 90%, may represent substantial heterogeneity; 75% to 100%, considerable heterogeneity27. Sources of heterogeneity, for identifying the possible effect modifiers on the pooled analyses, were explored using the following techniques:
Find-out outliers: If the study's confidence interval does not coincide with that of the pooled effect, it is considered an outlier. The size of the outlier has a substantial effect, and it deviates considerably from the overall effect. High-sampling-error studies vary significantly from the pooled result. However, because the confidence intervals of such studies are wide, there is a greater chance that the confidence intervals may overlap with one of the pooled effects. This basic outlier elimination technique is implemented using the find outliers function (dmetar) package. It seeks outlying studies in a (meta) item, eliminates them, and then recalculates the result (Supplementary Figure 1).
Sensitivity analysis: We used the metafor R tool to conduct a leave-one-out sensitivity analysis. In this method, we recalculate the meta-analysis results K times; each time excluding one study. The influence () function includes a set of leave-one-out diagnostic tests that help identify of influential studies. This analysis also includes a categorization of what is regarded as influential. We used I2 to sort the studies in the plot. We identified studies with the highest heterogeneity and the final heterogeneity after excluding these studies (Supplementary Figure 2). We also created a Baujat plot, which compares the total heterogeneity contribution of each study to its effect on the aggregated outcome26,28 (Supplementary Figure 3).
Graphic Display of Heterogeneity (GOSH) plots29: we fit the same meta-analysis model to all possible subsets of our included studies. In contrast to the leave-one-out method, we did not only fit K models, but also modelled for all 2 k − 1possible study combinations (Supplementary Figure 4).
Subgroup analysis
We categorized the catastrophic costs at 20% for ACF and PCF patients, according to the country where the studies were conducted (inside/outside) India.
Meta-regression
We studied the impact of the country where the survey was conducted (high versus low incidence of TB)30, quality of the study, sex, and population criteria (DS, drug resistant with or without HIV) on the size effect of studies to explain the substantial heterogeneity.
Results
Search results
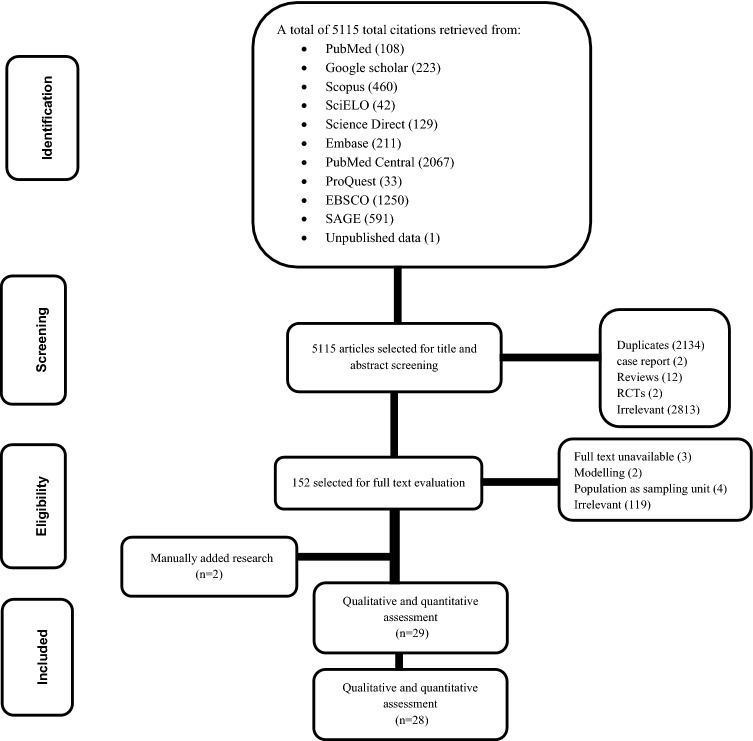
Figure 1 showed the flow diagram of the selection process. The database search yielded a total of 5114 potentially relevant articles. After title and abstract screening, we excluded 2134 duplicates (1922 by Endnote, 212 manually), 2813 irrelevant articles, 12 reviews, 2 randomized control trials, and 2 case reports. Overall, 152 articles were eligible for full text screening. Two additional citations were found through manual search. Qualitative analyses included 29 articles; one study was omitted owing to its unsatisfactory quality. Finally, 28 studies were included in quantitative analysis. The inter-rater agreements for title and abstract screening, inclusion, quality assessment were κ = 0.8, 0.95, and 0.8, respectively.
Figure 1.
PRISMA flow-charts of studies included in meta-analysis of catastrophic costs among patients with tuberculosis.
Study characteristics
Among the 29 studies included in the qualitative and quantitative analyese; six studies were from India, five were from China, four were from Indonesia, two were from Uganda and South Africa, and one was from Egypt, Zimbabwe, Nepal, Lao PDR, Ghana, Pakistan, Vietnam, Cambodia, Peru, and Cavite. Most studies were conducted at public health sectors, except for the study by Prasanna, et al.31, and four studies were conducted at both private and public sectors14,19,32,33. Only one study did not specify whether patients were treated under private or governmental sector34. The tools used for estimation of the cost survey were either WHO-TB cost survey tool6,17,32,33,34–43, the adapted WHO tool to Indonesian context14,34, structured questionnaire19,44–46, pre-coded interview scheduled20, tool of stop TB partnership15,31,47, headcount tool48, Lumley T. survey16, or TB coalition tool18. However, two studies did not mention the tool used49,50.
Quality assessment
Paper quality was very good in one study38 and good in thirteen studies6,18,19,31–34,36,37,41,42,44,45. Fourteen studies were of satisfactory quality14–17,20,35,39,40,43,45–48,50 and one was unsatisfactory49 (Table 1).
Table 1.
Studies that addressed catastrophic costs included in systematic review analysis.
| Author, Year/Country | Study design | Population criteria study duration | Study setting | Sample size/Sex/Age | Tool used in cost estimation | Catastrophic costs (cut-off point) Predictors of CC | Quality interpretation |
|---|---|---|---|---|---|---|---|
| Shewade, 2018/ India19 | Community-based cohort study |
Sputum + ve pulmonary TB ACF&PCF 3/2016 – 2/2017 |
Both public and private sectors |
N = 465 Male: 66% Age (years): 42 ± 17 |
Structured questionnaire |
ACF:10.3% PCF: 11.5% (20%) Predictors: not mentioned |
8 (Good) |
| Muniyandi, 2020/ India33 | Community based cross-sectional study |
Pulmonary and extrapulmonary TB patients registered in NTCP 2/2017 -3/2018 |
Both public and private sectors |
N = 384 Male:67% Mean age 38.4 ± 16 |
WHO-TB cost survey |
31% (20%) Predictors: Lower socioeconomic segments |
7 (Good) |
| Wingfield, 2016/ Peru44 | Community-based Prospective cohort study |
Any patient treated with the Peruvian NTCP DS & MDR 2/2014 – 8/2014 |
Public sector |
N = 876 Male: 59% Age ≥ 15 years |
Questionnaire |
39% (20%) Predictors: Inadequate nutrition, severe TB, hidden costs and adherence |
7 (Good) |
| Muniyandi, 2019/ India20 | Community-based cross-sectional study |
ACF vs PCF 10/2016—3/2018 |
Public sector |
N = 336 Male :77% Age ≥ 15 years |
Pre-coded interview schedule |
PCF:29% ACF:9% (20%) Predictors: not mentioned |
5 (Satisfactory) |
| Fuady, 2020/ Indonesia14 | Hospital-based cohort study |
Treatment duration ≥ 1 month or completed treatment since < 1 month DS only 7–9/ 2016 |
Both public and private sectors |
N = 252 Male: 54% Age ≥ 18 years |
Tool adapted according to the Indonesian context |
46% (10%) 38% (15%) 33% (20%) 26% (25%) 22% (30%) 17% (35%) Predictors: treatment duration, and additional visits |
5 (Satisfactory) |
| Mullerpattan, 2018/ India49 | Hospital-based cross-sectional study |
Drug resistant-TB, hospitalized patients 8/2015 – 2/2016 |
Private sector |
N = 50 Male: 30% Mean age = 30 years |
Not mentioned |
68% (20%) 78% (10%) Predictors: not mentioned |
3 (Unsatisfactory) |
| Lu, 2020/ China45 | Both community and hospital- based cross-sectional study |
Culture-confirmed DS pulmonary TB 12/2014 – 12/2015 |
Public sector |
N = 248 Male:54.9% Mean Age = 34 (IQR 26–49) |
Standardized questionnaire |
22.2% (20%) Predictors: not mentioned |
6 (Satisfactory) |
| Prasanna, 2018/ India31 | Both community and hospital-based Mixed methods |
Both newly diagnosed and previously treated TB patients registered for treatment under NTCP DS 1/12/2016—31/1/2017 |
Private sector |
N = 102 Male: 69% All ages |
Estimate TB, Patient’s Costs developed by the Poverty SWC of the Stops Partnership |
49% (10%) 32% (20%) Predictors: Age, HIV status and Hospitalization |
8 (Good) |
| Fuady, 2018/ Indonesia34 | Primary health care centers linked to NTCP cross-sectional survey |
Patients treated 1 month or finished treatment since < 1 month Not Extra-pulmonary TB TB vs MDR-TB (poor vs non poor) 7–9/2016 |
Not mentioned |
N = 346 (282 TB—64 MDR) Male: 55% Age: ≥ 18 years |
Adapted Bahasa Indonesia version |
DS 36% [Poor 43%, Non poor 25%] MDR-TB 83% (20%) Predictors: Travel costs, food / nutritional supplementation costs and income loss |
8 (Good) |
| Yang, 2020/ China36 | Both community and hospital- based cross sectional study |
Pulmonary TB confirmed by sputum culture Rifampicin sensitive, MDR 9–10/2018 |
Public sector |
N = 672 Male:64.3% Median age = 41 years |
WHO-TB cost survey |
46% (15%) 37.1% (20%) 30.2% (25%) Predictors: Age, Senior school or above, minimum living security household, employment status, household economic status, patient delay, medical care outside the city, hospitalization, MDR |
8 (Good) |
| Chittamany,2020/Lao PDR37 | Hospital-based Cross-sectional study |
TB patients on treatment in intensive (> 14 days) or continuation phase People treated under NTCP, Pulmonary and extra-pulmonary, HIV, MDR-TB 12/2018- 1/2019 & 5–6/2019 |
Public sector |
N = 848 Male:59.7% Mean age = 50.4 years |
WHO-TB cost survey |
Total 62.6% DS-TB 62.2%, DR-TB 86.7%, TB -HIV Co-inf. 81.1%, at (20%) Predictors: Food & nutritional supplements, income loss, treatment phase and educational status |
8 (Good) |
| Viney, 2019/ Indonesia38 | Hospital- based cross- sectional study |
Any patient received treatment ≥ 2 weeks 10/2016 – 3/2017 |
Public sector |
N = 457 Male: 50.6% Age = 32 years (IQR 22–52) |
WHO-TB cost surveys |
83% (20%) Predictors: Income loss & nutritional supplements, travel and medical costs after diagnosis |
9 (Very good) |
| Wang, 2020/ China48 | Hospital-based cross-sectional study |
TB-MDR finished 1 year of treatment MDR-TB 1–8/ 2018 |
Public sector |
N = 161 Male:68.9% Age = 36 years (IQR 26–48) |
Headcount tool |
87% (20%) Predictors: Low household income, absence of students in a family, LOS, male gender, job and productivity loss |
5 (Satisfactory) |
| Muttamba, 2020/ Uganda35 | Hospital-based cross-sectional study |
Started treatment ≥ 2 weeks DS & MDR-TB 2017 |
Public sector |
N = 1178 Male:62.7% All ages |
WHO-TB cost surveys |
53% (20%) Predictors: Transport, symptom relieving medications, food and loss of income |
5 (Satisfactory) |
| Pedrazzoli, 2018/ Ghana39 | Hospital-based cross- sectional study |
Patients started treatment ≥ 2weaks DS & DR-TB, HIV 2016 |
Public sector |
N = 691 Male:67.3% Median age = 41 years (IQR 29–52) |
WHO-TB cost surveys |
64.1% (20%) Predictors: Income loss, DR-TB & nutritional supplements |
5 (Satisfactory) |
| Xu, 2019/China46 | Hospital-based cross-sectional study |
DS, pulmonary TB, under NTCP 3–6/ 2017 |
Public sector |
N = 1147 Male:70.7% Median age = 51 years (IQR 12- 89) |
Structured questionnaire |
11.7% (20%) Predictors: Region, residence and insurance |
6 (Satisfactory) |
| Ikram, 2020/ Pakistan40 | Hospital-based cross-sectional study |
TB- patients diagnosed > 3 months Pulmonary & DS, without HIV, hepatitis, nor DM |
Public sector |
N = 400 Male:47% Median age = 30 years (IQR 22–49 .50) |
WHO-TB cost surveys |
67% (20%) Predictors: Availability of paid sick leave, number of follow up visits and job loss |
5 (Satisfactory) |
| Nhung, 2018/ Viet Nam32 | Community-based cross-sectional study |
(DS-TB & MDR-TB) including children Started treatment at least 2 weeks All ages DS & MDR-TB 7–10/2016 |
Both public and private sectors |
N = 735 Male:75.9% Median age = 47 years (IQR 35–58) |
WHO-TB cost surveys |
Total 63%, 48%, 35% MDR 98%, 98%, 39%, DS 59.6%, 43% 30% COP:(20%), (30%), (40%) Predictors: Purchase special foods, travel, nutritional supplements, and accommodation |
7 (Good) |
| Morishita, 2016/ Cambodia50 | Both hospital and community-based cross-sectional comparative study |
New pulmonary TB patients without unfavorable treatment outcomes & retreatment ACF vs PCF 2012 -2013 |
Public sector |
N = 208 (108 ACF + 100 PCF) Male: 51.9% ACF: 48.1% PCF: 56% Median age: ACF = 55 (IQR 43.8–68) PCF = 52.5 (IQR 45–62.3) |
– |
ACF 54.6% 36.1% 24.1% 17.6% PCF 63% 45% 34% 21% COP: (10%) (20%) (30%) (40%) Predictors: Time spent for travel, waiting, consultation and hospitalization |
6 (Satisfactory) |
| McAllister, 2020/ Indonesia41 | Hospital-based cross-sectional study |
Newly diagnosed pulmonary TB patients 10/2017 – 1/2019 |
Both public and private sectors |
N = 69 Male:49.25% Age: ≥ 18 years |
WHO-TB cost surveys |
38.6% (10%) 26.5% (20%) 21.7% (25%), Predictors: not mentioned |
7 (Good) |
| Tomeny, 2020/Cavite17 | Hospital-based cross-sectional study |
DS-TB vs MDR-TB 5–8/2016 |
Both public and private sectors |
N = 194 Male:66% Age: ≥ 16 years |
WHO-TB cost surveys |
DS-TB 28% (20%) MDR-TB 80% (20%), Predictors: Travel, accommodation, and nutritional supplement |
6 (Satisfactory) |
| Stracker, 2019/ South Africa6 | Hospital-based cross-sectional study |
2 months after diagnosis, transferred patients from other health care facilities to study clinics for treatment 10/ 2017–1/2018 |
Public sector |
N = 237 Male:54% Age: ≥ 18 years |
WHO-TB cost surveys |
28% (20%) Predictors: Transport, treatment, income loss and time lost in seeking care |
8 (Good) |
| Ruan, 2016/China16 | Hospital-based cross-sectional study |
MDR-TB 6–8/2012 |
Public sector |
N = 73 Male: 48% All ages |
Lumley T. Survey |
78% (20%) Predictors: tests, nutrition, transportation, accommodation and time loss |
6 (Satisfactory) |
| Mudzengi, 2017/ South Africa18 | Hospital-based cross-sectional study |
Diagnosed 3–5 month prior to the interview TB, HIV, or Both 4–10/ 2013 |
Public sector |
N = 454 Male:36% Age: ≥ 18 years |
TB Coalition tool |
Total 60% (10%) TB/HIV 79% 67% 65% 64% 61% TB only: 55% 53% 47% 47% 45% HIV only: 72% 60% 55% 52% 49% COP: (5%), (10%),(15%), (20%), (25%) |
7 (Good) |
| Gurung, 2019/ Nepal42 | Hospital-based cross-sectional study |
New and relapsed patients with TB (ACF vs PCF) 4–10/2013 |
Public sector |
N = 99 Male:71% Age: ≥ 18 years |
WHO-TB cost surveys |
Total 52% PCF 61% ACF 44% (20%) Predictors: gender, age, disease category (new, relapse), poverty line, dissaving, financial and social impact |
7 (Good) |
| Walctt, 2020/ Uganda15 | Hospital-based retrospective cohort study |
Spoke Luganda or English, confirmed active pulmonary TB Newly diagnosed TB 7–9/2017 |
Public sector |
N = 224 Male:60.2% age: ≥ 18 years |
Adapted version of Tool to Estimate Patients' Cost (stop TB partnership) |
41.8% (20%) Predictors: Hospitalization, experience of coping costs, low-income status, age, education, HIV, unemployment, and female gender |
6 (Satisfactory) |
| Rupani, 2020/ India51 | Cross-sectional study |
Patients not previously treated DS pulmonary TB 1/2019 |
Public sector |
N = 458 Male:70% Median age = 35 (IQR 23–50) |
WHO-TB cost surveys |
14% (10%) 7% (15%) 4% (20%) Predictors: not mentioned |
7 (Good) |
| Timire, 2020/ Zimbabwe43 | Hospital-based cross-sectional study |
Patients with DS or MDR TB 23/7–31/-8 2018 |
Public sector |
N = 900 Male:56% Mean age: 36.9 ± 14.7 |
WHO-TB cost surveys |
80% (20%) Predictors: Gender, Age, TB type, treatment phase, treatment delay HIV status, breadwinner, income quintile, and location of health facility |
5 (Satisfactory) |
| Gadallah, 2018/ Egypt47 | Hospital-based. prospective cohort study |
New TB patients attending TBMUs for starting their treatment 1–6/2019 |
Public sector |
N = 257 Male:61.9% Mean age: 38.3 ± 14.8 years |
WHO-TB cost surveys |
22.6% (10%) 24.1% (20%) 6.6% (30%) Predictors: Age, gender, unemployment, crowding index, governorates, income, |
5 (Satisfactory) |
ACF Active case finding, PCF Passive case finding, SP Smear Positive, TB Tuberculosis, DS Drug sensitive, HIV Human immunodeficiency virus, LOS Length of stay, MDR Multi-Drug Resistant, NTCP National TB Control Program, SWC Sub-Working Group, TBMU Tuberculosis medical unit.
Publication bias
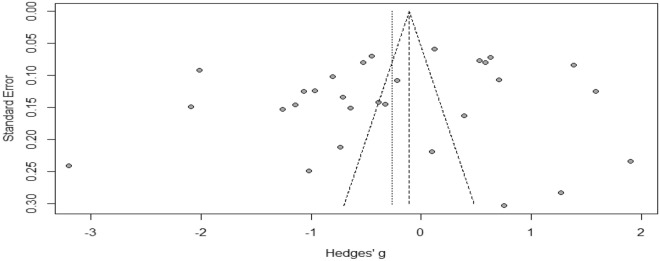
Figure 2 showed that there was no publication bias as the funnel plot was symmetric. In addition, Eggers’ test was not significant [t = − 1.188, p = 0.24].
Figure 2.
Funnel plot of studies included in the estimation of the proportion of tuberculosis patients and their households who faced catastrophic costs at a cut-off point of 20%.
Primary outcome
Catastrophic costs at cut-off point 20%
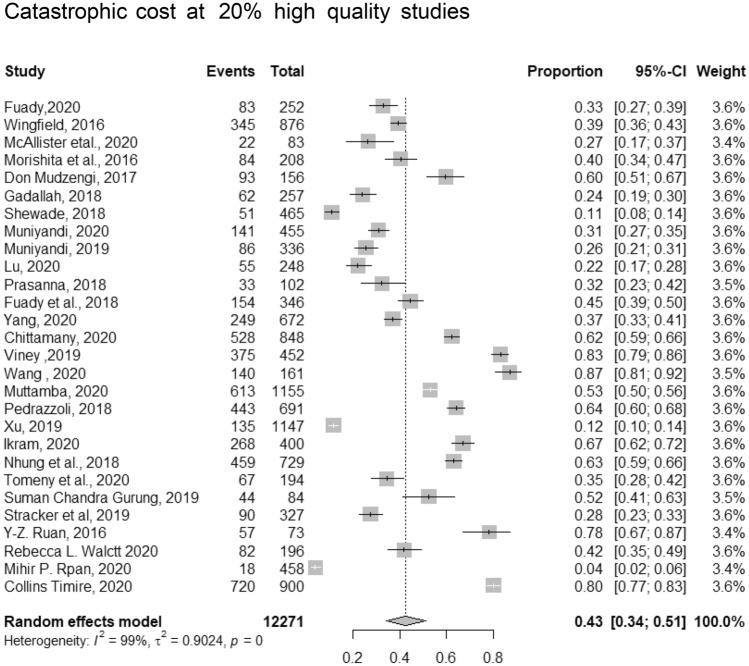
The pooled proportion of catastrophic costs among 11,750 TB patients included in 28 studies at cut-off point of 20% was (43%, 95% CI [34–51]). The in between-study heterogeneity variance was estimated at ^τ2 = 0.90, p < 0.01 with an I2 value of = 99% (Fig. 3). We conducted meta-regression to identify the cause of this substantial heterogeneity. The predictors were sex, country where the study was conducted (had high incidence vs. none)30, DS (DS or MDR ± HIV), and quality of the study. The model was significant (p < 0.0127, R2 = 51.57%). This model explained more than 50% of the reported heterogeneity. The identified significant predictors were country (high vs low incidence) (β = − 0.194, p = 0.04) and type of patients regarding drug sensitivity (DS or MDR) and HIV co-infection (β = 0.289, p = 0.026).
Figure 3.
Pooled proportion of catastrophic costs incurred by TB patient and their household at a cut-off point of 20%.
Predictors of catastrophic costs
The main predictors of catastrophic costs were food and nutritional supplements37–39, travel and transportation35,36,50, age category31,36,44, employment status34,36,40,44,48, the socioeconomic status15,34,36,44,47,48,52, MDR or HIV positive31,36,39,52, male gender44,47,48, and duration of hospitalization15,31,36,48,50.
Coping strategy
To balance the enormous financial burden the families encountered by TB, they may adopt some coping strategies as borrowing money, availing loans, pledging gold and jewels, bringing their children out of schools, or selling assets32,50. All these approaches referred to as “dissaving” which is at the core of the hardship financing dilemma.
Pooled proportion of catastrophic costs at cut-off point of 20% among different TB affected patients
Pooled proportion of catastrophic costs at cut-off point of 20% among TB drug sensitive
The pooled proportion of patients facing catastrophic costs was (39%, 95% CI [28–51]), and the reported heterogeneity was 99%. After removing the outliers32,36–40,43,45,46, the pooled proportion of 11 studies that recruited 3492 patients dropped to (32%, 95% [CI 29–35]). The pooled proportion of DS-TB patients facing catastrophic costs ranged from (24%, 95% CI [19–30]) in the study by Gadallah et al.47 to (42%, 95% CI [35–49]) in the study by Walctt et al.15 The heterogeneity of the included studies was as follows: I2 = 70%, p < 0.01 (Table 2).
Table 2.
Pooled proportion of catastrophic costs at 20% among drug sensitive.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Fuady, 2020 | 83 | 252 | 0.33 | [0.27–0.39] | 9.30% |
| Wingfield, 2016 | 295 | 783 | 0.38 | [0.34–0.41] | 12.20% |
| McAllister, 2020 | 22 | 83 | 0.27 | [0.17–0.37] | 5.10% |
| Gadallah, 2018 | 62 | 257 | 0.24 | [0.19–0.30] | 8.80% |
| Muniyandi, 2020 | 141 | 455 | 0.31 | [0.27–0.35 | 10.90% |
| Prasanna, 2018 | 33 | 102 | 0.32 | [0.23–0.42] | 6.20% |
| Fuady, 2018 | 101 | 282 | 0.36 | [0.30–0.42] | 9.80% |
| Yang, 2020 | 197 | 586 | 0.34 | [0.30–0.38] | 11.50% |
| Tomeny , 2020 | 47 | 169 | 0.28 | [0.21–0.35] | 7.70% |
| Stracker, 2019 | 90 | 327 | 0.28 | [0.23–0.33] | 9.80% |
| Walctt, 2020 | 82 | 196 | 0.42 | [0.35–0.49] | 8.80% |
| Random effect model | 1153 | 3492 | 0.32 | [0.29–0.35] | |
| I2 = 70% | |||||
Pooled proportion according to TB drug resistant
With a heterogeneity of 92%, the pooled proportion of TB affected household of MDR patients facing catastrophic costs among 1879 patients was (78%, 95% CI [86–86%]). After excluding the outliers32,36,53, the pooled proportion of patients facing catastrophic costs among 524 patients with MDR reached (81%, 95% CI [76–86]), I2 = 46%. The highest proportion (90%) was reported by Collin et al.45, while the lowest proportion (70%) was reported by Yang et al.36 (Table 3).
Table 3.
Pooled proportion of catastrophic costs at 20% among drug resistant.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Fuady, 2018 | 53 | 64 | 0.83 | [0.71–0.91] | 11.60% |
| Yang, 2020 | 39 | 56 | 0.7 | [0.56–0.81] | 12.90% |
| Chittamany, 2020 | 26 | 30 | 0.87 | [0.69–0.96] | 6.60% |
| Wang, 2020 | 140 | 161 | 0.87 | [0.81–0.92] | 15.00% |
| Pedrazzoli, 2018 | 50 | 66 | 0.76 | [0.64–0.85] | 13.10% |
| Tomeny,2020 | 20 | 25 | 0.8 | [0.59–0.93] | 7.30% |
| Collins Timire, 2020 | 44 | 49 | 0.9 | [0.78–0.97] | 7.80% |
| Ruan, 2016 | 57 | 73 | 0.78 | [0.67–0.87] | 13.20% |
| Random effect model | 429 | 1375 | 0.81 | [0.76–0.83] | |
| I2 = 46% | |||||
Pooled proportion of TB-HIV co-infected patients facing catastrophic costs at cut-off point of 20%
The pooled proportion of the 796 TB patients with HIV facing catastrophic costs at cut-off point of 20% was (76%, 95% CI [65–85%]), with a heterogeneity of 88%. After conducting leave-one out sensitivity analysis, the study by Mudzengi et al.18 was removed. The heterogeneity dropped to 0% and the pooled proportion of patients facing catastrophic costs has increased to (81%, 95% CI [78–84]) (Table 4).
Table 4.
Pooled proportion of catastrophic costs at 20% among TB and HIV infected patients.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Chittamany, 2020 | 100 | 123 | 0.81 | [0.73–0.88] | 17.80% |
| Timire, 2020 | 450 | 557 | 0.81 | [0.77–0.84] | 82.20% |
| Random effect model | 550 | 680 | 0.81 | [0.78–0.84] | |
| I2 = 0% | |||||
Pooled proportion of TB facing catastrophic costs at cut-off point of 20% through ACF
The proportion of patients facing catastrophic costs among the 491 patients exposed to ACF ranged from (9%, 95% CI [7–15%]) to (62%, 95% CI [45–77%]). After subgroup analysis based on the country where the ACF was implemented (inside/outside India), the pooled proportion was (10%, 95% CI [7–14%]), I2 = 0%) inside India and (48%, 95% CI [25–72%]), I2 86% outside India. The difference in proportion of patients with TB incurring catastrophic costs at 20% was significant across the studied groups (p < 0.001) (Table 5).
Table 5.
Pooled proportion of catastrophic costs at 20% among during active case finding after sub-group analysis.
| Study | Event | Total | Proportion | 95%CI |
|---|---|---|---|---|
| Inside India | ||||
| Muniyandi, 2019 | 10 | 110 | 0.09 | [0.5–0.16] |
| Shewade, 2018 | 24 | 234 | 0.10 | [0.7–0.15] |
| Fixed effect model | 34 | 342 | 0.1 | [0.07–0.14] |
| Heterogeneity I2 = 0% | ||||
| Outside India | ||||
| Morishita, 2016 | 39 | 108 | 0.36 | [0.27–0.46] |
| Gurung, 2019 | 24 | 39 | 0.61 | [0.45–0.77] |
| Fixed effect model | 63 | 247 | 0.26 | [0.25–0.72] |
| I2 = 86.3% | ||||
| In-between groups P < 0.0001 | ||||
Pooled proportion of patients with TB facing catastrophic costs during PCF
The proportion of patients facing catastrophic costs among 638 patients during PCF ranged from (12%, 95% CI [8–17%]) to (45%, 95% CI [35–55%]). The pooled proportion was (30%, 95% CI [17–48%]), I2 = 94%. We further subdivided the studies according to the studied country (inside/outside) India. The pooled proportion of TB households facing catastrophic costs outside India was (45%, 95% CI [37–53%]), I2 = 0% while inside India (19%, 95% CI [7–41%]), I2 = 95% (Table 6). After subgrouping the included studies according to the country where PCF was used (inside India/outside India), the difference in proportion of TB patients facing catastrophic costs at 20% was not significant across the studied groups (p = 0.063).
Table 6.
Pooled proportion of catastrophic costs at cut-off point of 20% among during passive case finding after sub-group analysis.
| Study | Event | Total | Proportion | 95%CI |
|---|---|---|---|---|
| Morishita, 2016 | 45 | 100 | 0.45 | [0.35–0.55] |
| Gurung, 2019 | 20 | 45 | 0.44 | [0.30–0.60] |
| I2 = 0% | ||||
| Shewade, 2018 | 27 | 231 | 0.12 | [0.0–0.17] |
| Muniyandi, 2019 | 76 | 262 | 0.29 | [0.24–0.35] |
| I2 = 95.2 | ||||
| In between groups P = 0.06 | ||||
Secondary outcomes
Proportion of direct costs to the total costs
Direct to total costs among drug sensitive
The proportion of the mean direct costs to the mean total costs were addressed in six studies; the pooled proportion of direct to total costs at catastrophic costs of 20% were not calculated because of high heterogeneity. The proportion was variable; Tomeny et al.17 and Timire et al.52 reported that catastrophic costs were 41% and 43%, respectively. However, a higher proportion (52%) was reported by Chittamany et al.37 and Nhung et al.32. Two other extreme values were reported by Fuady et al.34 and Muttamba et al.35 (33% and 65%, respectively).
Direct to total costs among multidrug resistance
The proportion of the mean direct costs to the mean total costs at cut-off point of 20% was addressed in seven studies ranged from 26% in Chittamany et al.37 to 93% in Yang et al.36. Low proportions were observed in the studies of Fuady et al.34, Tomeny et al.17, and Timire et al.52 with proportion of 32%, 34% and 49% respectively, while a high proportion was reported by Muttamba et al. (66%)35, and by Nhung et al. (68%)32. The pooled proportion of mean direct to total costs was difficult to assess because of the substantial heterogeneity which was not explained even after a meta-regression analysis.
Pooled proportion of direct costs to total costs in the case of ACF
The pooled proportion of the mean direct costs to the mean total costs was addressed in four studies and was (25%, 95% CI [16–37%]), I2 = 83%. After conducting leave one out sensitivity analysis, the study of Gurung et al.42, was removed, the pooled proportion dropped to (29%, 95% C1 [20–41%]), I2 = 55%. (Table 7).
Table 7.
Pooled proportion of direct to total costs at catastrophic costs of 20% among active case finding.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Morishita, 2016 | 110.5 | 399 | 0.28 | [0.23–0.32] | 57.70% |
| Shewade, 2018 | 12 | 4.5 | 0.8 | 0.28–0.99] | 4.90% |
| Muniyandi, 2019 | 18 | 69 | 0.26 | [0.16–0.38] | 37.40% |
| Random effect model | 140.5 | 427.5 | 0.29 | [0.20–0.41] | |
| I2 = 55% | |||||
Pooled proportion of direct costs to total costs in case of passive case finding (PCF)
The pooled proportion of the mean direct costs to the mean total costs was addressed in four studies19,20,42,50 and was (37%, 95% C1 [31–42%]), I2 = 0% (Table 8).
Table 8.
Pooled proportion of direct to total costs at catastrophic costs of 20% among passive case finding.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Morishiita, 2016 | 206 | 535 | 0.39 | [0.34–0.43] | 33.60% |
| Shewade, 2018 | 26.9 | 28.4 | 0.94 | 0.98–0.90] | 4.20% |
| Muniyandi, 2019 | 79 | 227 | 0.35 | [0.29–0.41] | 30.10% |
| Gurung, 2019 | 131.74 | 325.3 | 0.45 | [0.35–0.46] | 32.10% |
| Random effect model | 443.64 | 1115.7 | 0.37 | [0.31–0.42] | |
| I2 = 0% | |||||
Proportion of direct costs to total costs in the case of HIV and TB co-infection
The proportion of the direct costs to the total costs were addressed in two studies. Mudzengi et al.18 showed that the proportion of mean direct costs to the mean total costs was 30% among HIV and TB co-infection patients, while a higher proportion (59%) was reported by Chittamany et al.37. We couldn’t pool these studies because of the unexplained heterogeneity.
Proportion of mean direct costs to total costs
The pooled proportion of the mean direct costs to the mean total costs was addressed in 13 studies, which ranged from 4 to 87% (Supplementary Table 2).
CHE at 10% & capacity to pay at 40%
There were six studies calculated the CHE 10% and the capacity to pay (CTP) 40%.
Pooled proportion of CHE at 10%
The pooled proportion of the CHE at 10% was addressed in three studies. The pooled proportion of TB patients who incurred CHE was (45%, 95% CI [35–56%]), I2 = 93%. After leave one out sensitivity analysis, Fuady et al.41 was excluded, and the heterogeneity decreased to reach I2 = 28% and the pooled proportion has increased to (50%, 95% CI [47–54%]) (Table 9).
Table 9.
Pooled proportion of Catastrophic Health Expenditure at 10%.
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Lu, 2020 | 132 | 248 | 0.53 | [0.47–0.60] | 26.80% |
| Muttamba, 2020 | 567 | 1155 | 0.49 | [0.46–0.52] | 73.20% |
| Random effect model | 699 | 1403 | 0.5 | [0.47–0.54] | |
| I2 = 28% | |||||
Pooled proportion of capacity to pay (CTP) at 40%
Three studies measured the CHE in relation to CTP. The pooled proportion of TB patients who face CHE was (63%, 95% CI [40–80%]), I2 = 96%. After conducting the sensitivity analysis, the heterogeneity was found to be = 0%, while the pooled proportion increased to (70%, 95% CI [64–76%]) (Table 10).
Table 10.
Catastrophic Health Expenditure (Capacity to Pay at 40%).
| Study | Event | Total | Proportion | 95%CI | Weight |
|---|---|---|---|---|---|
| Wang, 2020 | 110 | 161 | 0.68 | [0.61–0.5] | 71.30% |
| Ruan, 2016 | 54 | 73 | 0.74 | [0.62–0.84] | 28.70% |
| Random effect model | 164 | 234 | 0.7 | [0.64–0.76] | |
| Heterogeneity I2 = 0% | |||||
Discussion
Our meta-analysis showed that the proportion of patients facing catastrophic costs at a cut-off point of 20% was 43%; (32%, 95% CI (29–35)) among DS and (80%, 95% CI [74–85%]) among MDR). Patients with TB co-infected with HIV faced the highest catastrophic costs (81%, 95% CI [78–84]). Catastrophic costs were variable according to the strategy of case finding; ACF = (12%, 95% CI [9–16%]) versus PCF (42%, 95% CI [35–50%]). Among drug sensitive and drug resistant TB, the proportion of direct costs to the total costs ranged from 33 to 65%17,32,34,35,37,52 and 26–93%17,32,34–37,52 respectively. ACF incurred lower catastrophic costs than PCF (29%, 95% CI [20–41%]) versus (37%, 95% C1 [34–40%]). The direct to the total costs among TB and HIV co-infected patients ranged from 30%18 to 59%37. The CHE was (50%, 95% CI [47–54%]), and (70%, 95% CI [64–76%]) at 10% of household’s yearly income and 40% of their CTP, respectively.
Catastrophic costs
The costs incurred by TB on some patients may be catastrophic and minimal for others. This is based on the household annual income. In the current study, we included 28 studies that addressed catastrophic costs among patients with TB at different thresholds points (30%, 25%, 20%, 10%, and 5%). Despite the absence of robust evidence on the sensitivity of the cut-off point at 20% to reflect the catastrophic costs, regardless of whether patients are drug sensitive or resistant; Fuady et al.14 established 15% and 30% as more consistent cut-off points for treatment adherence and success, respectively. In the current study, the proportion of TB-household patients facing catastrophic costs was 39%, which was considered very high compared with the targeted sustained developmental goals in 2020 (0%), thus more efforts and activities should be directed to reduce these costs. Diagnosis and treatment are provided free in many of the included countries under the umbrella pool of NTP; however, the treatment related expenditure is still very high. Yadav et al.54 illustrated that even with free services for TB care, 21.3% of the patients were exposed to hardship financing, thus recommending more innovative ways to increase the supported coverage of TB treatment in the countries. The study also suggests the use of hardship financing as an index to measure the effectiveness of TB control program. It is crucial to decrease the burden of catastrophic costs among patients with TB, as it results in poorer treatment outcome. Patients suffering from catastrophic costs had 2–4 times higher odds of treatment failure than those who do not14. This could be explained by the reduced access to treating health facility and treatment completion. Regarding the coping costs, the majority of household’s resort to different coping strategies to deal with the increased out-of-pocket costs and to compensate for the consequences of income loss. Those coping strategies include selling a property or livestock, taking loans, pledging jewels, dropping their children out of school, and cutting down their consumption to below basic needs11. Despite pooling of these studies’ outcome yielded substantial heterogeneity, the current study found that 51.57% of heterogeneity was mainly because of two predictors; the first was that some studies estimated catastrophic costs of DS and patients with MDR with or without HIV together. This factor played a major role in the heterogeneity, as it was clear that the catastrophic costs were dramatically higher among patients with HIV. The second predictor was the classification of the country where the study was conducted30. Two-thirds of the new cases of TB are reported in eight countries of the world, with India being the highest, followed by Indonesia, China, the Philippines, Pakistan, Nigeria, Bangladesh and South Africa. Consequently, we sub-grouped the studies according to the country where they were conducted; countries with high versus low TB incidence. In meta-regression analysis, the country where the study was conducted was the second major determinant of the different size effect.
The reported high incidence of catastrophic costs in many countries raised the need for social protection interventions. The most common social protection intervention is the cash transfer or cash assistance, which has already been implemented in many countries across the world, either conditionally or unconditionally55. Thus, it is supposed that the household can get better access to treatment and food. Other social protection interventions include disability grants, food baskets (food assistance), food or travel vouchers and social insurance11. Many countries have implemented reimbursement programs to help patients with TB to cope with the disease costs. However, these programs prioritize poorer and MDR56. The effect of this intervention is questionable. At a cut-off point of 20%, two studies have applied and calculated a catastrophic costs before and after reimbursement. Lue et al.45 reported a minimum change in the proportion of catastrophic costs; before reimbursement, the catastrophic costs were (22%) and declined to 19% after the reimbursement. In contrast, Fuady et al.57 showed a higher change in the proportion of catastrophic costs after the reimbursement. The intervention program effectively decreased catastrophic costs from 44 to 13%. Regarding cash transfer, Wingfield et al.53 reported that the proportion of TB households suffering from catastrophic costs was 30% and 42% among intervention and control groups, respectively. These findings indicate that this social support is not enough to mitigate the impact of TB. Consequently, households of TB patients should receive sufficient financial support that covers indirect costs (job lost) and direct costs (transportation, food, accommodation)58. Such social support should be proportionate to the income lost because of the high variability of the pre-treatment income. We speculate that developing newer treatment guidelines for TB of a shorter duration would be beneficial. At the bottom, providing free medication is insufficient to prevent the catastrophic costs. TB patients should receive transport vouchers, reimbursement schemes, and food assistance to reduce or compensate for such catastrophic costs. Furthermore, decentralization of patient supervision (including directly observed therapy), for example, through community-based or workplace-based treatment59, can reduce transport costs and income loss for patients60.
As expected, the catastrophic costs among MDR were higher than among DS, as DS patients receive treatment for shorter duration (6 months only), while MDR treatment extends to 24 months. Additional cost is incurred by MDR patients, such as the cost related to prolonged work absenteeism, need for daily injection, exposure to more side effects, and need for more investigation61.
Direct costs to total costs
The mean total direct costs to the mean total costs were lower than the mean indirect costs among drug sensitive patients, HIV co-infected patients, while it was higher among drug resistant patients. This finding is essential to be considered when reimbursement strategies are implemented. Stakeholders should know which part of patient costs should be compensated. The direct costs dropped significantly if the ACF strategy was adopted instead of the PCF (29% to 37%) respectively.
Determinant of catastrophic costs
Recognizing the determinants of catastrophic costs could provide an insight into approaches for mitigating catastrophic costs among the vulnerable TB patients and their households. The epidemiological consequence of TB is directly related to a country's socioeconomic profile. TB vulnerability is determined by biological variables (e.g., malnutrition, HIV infection, and age) and social factors (e.g., poor housing conditions, high population density, inhumane working conditions, and a lack of access to health services). Under many circumstances, numerous vulnerabilities occur simultaneously62. In this study, the main determinants of catastrophic costs were income loss as an impact of being diseased and food and nutritional supplements other than the patients’ regular diet habit37–39. In addition, travel and transportation affected the direct non-medical costs, thus increased the suffering of patients with TB35. Age also affected the proportion of patients with TB suffering from catastrophic costs, whether young47 or old36. Additionally, Kirubi et al.63 found that delayed treatment initiation was a major predictor of catastrophic costs. Approximately 24% of individuals with catastrophic expenses waited for more than four weeks following the onset of symptoms to start treatment. Severe symptoms, prolonged hospitalization, more expensive non-TB medication, or even more frequent visits to the facilities may explain why delayed treatment initiation was related. Health and social protection investments have minimized the negative health effects of TB. Moreira et al.62 emphasized the relevance of public social protection programs in mitigating the consequences of TB indicators in the pursuit of TB elimination.
Catastrophic health expenditure
Out of the 28 studies, only six studies have been included with a clear measurement of the CHE (at cut-off point of 10% of their income and at cut-off point of 40% of their CTP). It was clear that many studies ignored CHE, despite its importance to understand the impact of these costs on treatment outcomes45. Two studies assessed the effect of reimbursements intervention on the CHE. Xiang et al.64 reported a 8% reduction in CHE, however, this reduction was not statistically significant. Similarly, Zhou et al.65 reported that the effect of reimbursement on CHE was minimal; only 12% reduction in CHE was achieved. To decrease the catastrophic expenditures national health financing systems must be designed and implemented, not only to allow people to access services when they are needed but also to protect households from financial catastrophe, by reducing out-of-pocket spending. Eventually, prepayment mechanisms should be developed, for instance, social health insurance, tax-based financing of health care, or some mix of prepayment mechanisms such as efficient reimbursement or cash intervention66.
Strengths and limitations of the study
Our study has several strengths and limitations. The strengths include the wide sensitive search strategy and multiple studied outcomes. The limitations of this study was that different cut-off points were established by different studies to estimate the proportion of the households facing catastrophic costs using different tools. Second, a major challenge was that different studies estimated catastrophic costs due to TB, regardless of drug sensitivity (DS, MDR), co-infection with HIV, case finding strategy (ACF, and PCF). Third, all studies included subjects with confirmed TB. Costs for those ill patients with undiagnosed TB may add much to the already estimated values. Fourth, many of the included studies used the WHO cost survey tool which included patients only treated in the NTP, omitting patients treated in private sectors who represent a considerable proportion. Fifth, owing to the observational nature of the studies included, there was a risk of recall bias as well as a chance of reporting inaccurate significant relationships due to confounders. Sixth, the degree of heterogeneity across the studies was likewise substantial; so, the random effects method was used to generate the pooled data. In addition, we adopted several techniques to overcome it like meta-regression and subgroup analysis. Finally, the quality of a meta-analysis is determined by the quality of the included research; here, the quality of the majority of the included studies was graded as satisfactory or good.
Conclusion
Regarding future global policy, our study provides an evidence for the high proportion of TB patients who are still facing catastrophic costs despite the free TB treatment policy. The proportion of patients facing catastrophic costs varies according to the type of TB, which is the lowest among DS, higher in MDR, and the highest among those with concomitant infection with HIV. Patients exposed to ACF incurred lower costs than those exposed to PCF. The direct costs (medical &non-medical) related to TB is not the only major contributor to the catastrophic costs, but indirect costs (Job and productivity lost) also represent a major contributor that should not be ignored. Overall, this study paves the way to effective cost mitigation in the context of the End TB Strategy. Effective management of the predictors of catastophic costs will eventually contribute to better community, clinical, and financial outcomes. It is clear that the global health system must make more efforts to achieve the zero catastrophic costs for TB by 2030. Future research should assess the effectiveness of reimbursement for TB patients on the reduction of the proportion of patients who face catastrophic costs. Furthermore, in an attempt to reduce the costs incurred by TB patients, researchers should develop more reliable diagnostic tools to reduce patients' need for frequent visits to healthcare facilities. They also have to study the impact of educational programs on TB patients' compliance with the prescribed medicine to lower the retreatment rates. Finally, NTP should monitor the financial and social status of patients treated and intervene as early as possible to protect them from incurring these catastrophic costs.
Supplementary Information
Acknowledgements
We would like to thank Dr. Samia laokri and Dr. Charlesbatt, Dr. Ramy shaaban, Dr. Suzan, and Dr. Ahmed Mandil (EMRO-WHO) for their great help, guidance, and support that helped us to accomplish this work.
Author contributions
R.M.G.: Grant holder, conceptualized and designed the study, database search, full text screening, data analysis, writing manuscript. H.E.S.: Revision of tittle & abstract, revision of the full text screening and data extraction, data analysis and writing manuscript. S.A.: Database search with full text screening, data extraction, writing manuscript and references manager. A.M.E.: Full text screening, data extraction, data analysis and writing manuscript. H.K.: Database search with full text screening. N.Z.: Database search with full text screening, data extraction and writing manuscript. E.A.: Full text screening, data extraction and writing manuscript. E.E.: Full text screening, data extraction and writing manuscript. S.A.: Final decision of the title & abstract screening with the full text screening and writing manuscript.
Funding
This research was funded by the WHO Eastern Mediterranean Regional Office / TDR Grant Number SGS 20-28.
Data availability
Data are available upon request by contacting the first author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-04345-x.
References
- 1.World Health Organization. 10 facts on tuberculosis. 2020 Oct 14, 2020 2021 Feb 20th]; Available from: https://www.who.int/news-room/facts-in-pictures/detail/tuberculosis.
- 2.Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- 3.World Health Organization. Tuberculosis. 2020 [cited 2021 Feb 19]; Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- 4.World Health Organization, Systematic screening for active tuberculosis: principles and recommendations. 2013: World Health Organization. [PubMed]
- 5.Singh M, et al. Are treatment outcomes of patients with tuberculosis detected by active case finding different from those detected by passive case finding? J. Glob. Infect. Dis. 2020;12(1):28. doi: 10.4103/jgid.jgid_66_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stracker N, et al. Risk factors for catastrophic costs associated with tuberculosis in rural South Africa. Int. J. Tuberc. Lung Dis. 2019;23(6):756–763. doi: 10.5588/ijtld.18.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lönnroth K, et al. Beyond UHC: monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014;11(9):e1001693. doi: 10.1371/journal.pmed.1001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madan J, et al. What can dissaving tell us about catastrophic costs? Linear and logistic regression analysis of the relationship between patient costs and financial coping strategies adopted by tuberculosis patients in Bangladesh, Tanzania and Bangalore, India. BMC Health Serv. Res. 2015;15(1):1–8. doi: 10.1186/s12913-015-1138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, Global status report on alcohol and health 2018. 2019: World Health Organization.
- 10.Hogan, A. et al.Report 19: the potential impact of the COVID-19 epidemic on HIV, TB and malaria in low-and middle-income countries. 2020.
- 11.Tanimura T, et al. Financial burden for tuberculosis patients in low-and middle-income countries: A systematic review. Eur. Respir. J. 2014;43(6):1763–1775. doi: 10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Oganization. Tuberculosis: Multidrug-resistant tuberculosis (MDR-TB). 2018 [cited 2021 Feb 19]; Available from: https://www.who.int/news-room/q-a-detail/tuberculosis-multidrug-resistant-tuberculosis-(mdr-tb).
- 13.World Health Organization . Tuberculosis Patient Cost Surveys: A Handbook. WHO; 2017. [Google Scholar]
- 14.Fuady A, et al. Catastrophic costs due to tuberculosis worsen treatment outcomes: A prospective cohort study in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2020;2:2. doi: 10.1093/trstmh/traa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walcott RL, et al. There’s no such thing as a free TB diagnosis: Catastrophic TB costs in Urban Uganda. Glob. Public Health. 2020;2:1–12. doi: 10.1080/17441692.2020.1724313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Y, et al. The affordability for patients of a new universal MDR-TB coverage model in China. Int. J. Tuberc. Lung Dis. 2016;20(5):638–644. doi: 10.5588/ijtld.15.0413. [DOI] [PubMed] [Google Scholar]
- 17.Tomeny E, et al. Patient-cost survey for tuberculosis in the context of patient-pathway modelling. Int. J. Tuberculosis Lung Dis. 2020;24(4):420–427. doi: 10.5588/ijtld.19.0206. [DOI] [PubMed] [Google Scholar]
- 18.Mudzengi D, et al. The patient costs of care for those with TB and HIV: a cross-sectional study from South Africa. Health Policy Plann. 2017;32(4):48–56. doi: 10.1093/heapol/czw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shewade HD, et al. Active case finding among marginalised and vulnerable populations reduces catastrophic costs due to tuberculosis diagnosis. Glob. Health Action. 2018;11(1):1494897. doi: 10.1080/16549716.2018.1494897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muniyandi M, et al. Catastrophic costs due to tuberculosis in South India: Comparison between active and passive case finding. Trans. R. Soc. Trop. Med. Hyg. 2020;114(3):185–192. doi: 10.1093/trstmh/trz127. [DOI] [PubMed] [Google Scholar]
- 21.Rudgard WE, et al. Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low-and middle-income countries: An economic modelling study. PLoS Med. 2017;14(11):e1002418. doi: 10.1371/journal.pmed.1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingfield T, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med. 2014;11(7):e1001675. doi: 10.1371/journal.pmed.1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tricco AC, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . Distribution of Health Payments and Catastrophic Expenditures Methodology. World Health Organization; 2005. [Google Scholar]
- 25.Moskalewicz A, Oremus M. No clear choice between newcastle-ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J. Clin. Epidemiol. 2020;120:94–103. doi: 10.1016/j.jclinepi.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Harrer, M. et al.Doing meta-analysis in R: a hands-on guide. PROTECT Lab Erlangen, 2019.
- 27.Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 28.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008;37(5):1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 29.Olkin I, Dahabreh IJ, Trikalinos TA. GOSH–a graphical display of study heterogeneity. Res. Synth. Methods. 2012;3(3):214–223. doi: 10.1002/jrsm.1053. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Tuberculosis (TB) 2021 [cited 2021 6 February ]; Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis#:~:text=Global%20impact%20of%20TB&text=In%202019%2C%2087%25%20of%20new,Nigeria%2C%20Bangladesh%20and%20South%20Africa.
- 31.Prasanna T, et al. Catastrophic costs of tuberculosis care: A mixed methods study from Puducherry, India. Glob. Health Action. 2018;11(1):1477493. doi: 10.1080/16549716.2018.1477493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nhung N, et al. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int. J. Tuberc. Lung Dis. 2018;22(9):983–990. doi: 10.5588/ijtld.17.0859. [DOI] [PubMed] [Google Scholar]
- 33.Muniyandi M, et al. Association of tuberculosis with household catastrophic expenditure in South India. JAMA Netw. Open. 2020;3(2):e1920973–e1920973. doi: 10.1001/jamanetworkopen.2019.20973. [DOI] [PubMed] [Google Scholar]
- 34.Fuady A, et al. Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infect. Dis. Poverty. 2018;7(1):3. doi: 10.1186/s40249-017-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muttamba W, et al. Households experiencing catastrophic costs due to tuberculosis in Uganda: Magnitude and cost drivers. BMC Public Health. 2020;20(1):1–10. doi: 10.1186/s12889-020-09524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang T, et al. Factors associated with catastrophic total costs due to tuberculosis under a designated hospital service model: a cross-sectional study in China. BMC Public Health. 2020;20(1):1–13. doi: 10.1186/s12889-020-09136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chittamany P, et al. First national tuberculosis patient cost survey in Lao People’s Democratic Republic: Assessment of the financial burden faced by TB-affected households and the comparisons by drug-resistance and HIV status. PLoS ONE. 2020;15(11):e0241862. doi: 10.1371/journal.pone.0241862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viney K, et al. Four of five tuberculosis patients experience catastrophic costs related to TB diagnosis and care in Timor-Leste. Int. J. Tuberc. Lung Dis. 2019;23(11):1191–1197. doi: 10.5588/ijtld.18.0765. [DOI] [PubMed] [Google Scholar]
- 39.Pedrazzoli D, et al. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop. Med. Int. Health. 2018;23(8):870–878. doi: 10.1111/tmi.13085. [DOI] [PubMed] [Google Scholar]
- 40.Ikram A, et al. Is tuberculosis treatment truly free? A study to identify key factors contributing to the catastrophic cost of TB care in Pakistan. J. Tuberc. Res. 2020;8(4):181–198. [Google Scholar]
- 41.McAllister SM, et al. Out-of-pocket costs for patients diagnosed with tuberculosis in different healthcare settings in Bandung, Indonesia. Am. J. Trop. Med. Hyg. 2020;103(3):1057–1064. doi: 10.4269/ajtmh.19-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurung SC, et al. The role of active case finding in reducing patient incurred catastrophic costs for tuberculosis in Nepal. Infect. Dis. Poverty. 2019;8(1):99. doi: 10.1186/s40249-019-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins D, et al. Can patients afford the cost of treatment for multidrug-resistant tuberculosis in Ethiopia? Int. J. Tuberc. Lung Dis. 2018;22(8):905–911. doi: 10.5588/ijtld.17.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wingfield T, et al. Beyond pills and tests: Addressing the social determinants of tuberculosis. Clin. Med. 2016;16(6):s79. doi: 10.7861/clinmedicine.16-6s-s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, et al. Catastrophic costs of tuberculosis care in a population with internal migrants in China. BMC Health Serv. Res. 2020;20(1):1–9. doi: 10.1186/s12913-020-05686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu C-H, et al. Inequity in catastrophic costs among tuberculosis-affected households in China. Infect. Dis. Poverty. 2019;8(1):46. doi: 10.1186/s40249-019-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohsen Gadallah W. Assessing household catastrophic total cost of Tuberculosis and their determinants in Egypt: A cohort prospective study. Ain Shams University, Editor. 2019;2:1477493. [Google Scholar]
- 48.Wang Y, et al. Household financial burden among multidrug-resistant tuberculosis patients in Guizhou province, China: A cross-sectional study. Medicine. 2020;99:28. doi: 10.1097/MD.0000000000021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullerpattan JB, et al. Catastrophic costs of treating drug resistant TB patients in a tertiary care hospital in India. Indian J. Tuberc. 2019;66(1):87–91. doi: 10.1016/j.ijtb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Morishita F, et al. Mitigating financial burden of tuberculosis through active case finding targeting household and neighbourhood contacts in Cambodia. PLoS ONE. 2016;11(9):e0162796. doi: 10.1371/journal.pone.0162796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rupani MP, et al. Costs incurred by patients with drug-susceptible pulmonary tuberculosis in semi-urban and rural settings of Western India. Infect. Dis. Poverty. 2020;9(1):1–8. doi: 10.1186/s40249-020-00760-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timire, C. et al.Catastrophic costs among tuberculosis patients in Zimbabwe: a national health facility-based survey. 2020. [DOI] [PMC free article] [PubMed]
- 53.Wingfield T, et al. The economic effects of supporting tuberculosis-affected households in Peru. Eur. Respir. J. 2016;48(5):1396–1410. doi: 10.1183/13993003.00066-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yadav J, John D, Menon G. Out of pocket expenditure on tuberculosis in India: Do households face hardship financing? Indian J. Tuberc. 2019;66(4):448–460. doi: 10.1016/j.ijtb.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Boccia D, et al. Towards cash transfer interventions for tuberculosis prevention, care and control: Key operational challenges and research priorities. BMC Infect. Dis. 2016;16(1):1–12. doi: 10.1186/s12879-016-1529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saqib SE, Ahmad MM, Amezcua-Prieto C. Economic burden of tuberculosis and its coping mechanism at the household level in Pakistan. Soc. Sci. J. 2018;55(3):313–322. [Google Scholar]
- 57.Fuady A, et al. Effect of financial support on reducing the incidence of catastrophic costs among tuberculosis-affected households in Indonesia: eight simulated scenarios. Infect. Dis. Poverty. 2019;8(1):1–14. doi: 10.1186/s40249-019-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuady A, et al. Effect of financial support on reducing the incidence of catastrophic costs among tuberculosis-affected households in Indonesia: Eight simulated scenarios. Infect. Dis. Poverty. 2019;8(1):10. doi: 10.1186/s40249-019-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinanovic E, et al. Cost and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int. J. Tuberc. Lung Dis. 2003;7(9):S56–S62. [PubMed] [Google Scholar]
- 60.Datiko DG, Lindtjørn B. Cost and cost-effectiveness of smear-positive tuberculosis treatment by Health Extension Workers in Southern Ethiopia: a community randomized trial. PLoS ONE. 2010;5:2. doi: 10.1371/journal.pone.0009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Organization, W.H. and W.E.C.o. Malaria, WHO Expert Committee on Malaria: twentieth report. 2000: World Health Organization.
- 62.Moreira ASR, Kritski AL, Carvalho ACC. Social determinants of health and catastrophic costs associated with the diagnosis and treatment of tuberculosis. J. Bras. Pneumol. 2020;46:2. doi: 10.36416/1806-3756/e20200015. [DOI] [PubMed] [Google Scholar]
- 63.Kirubi B, et al. Determinants of household catastrophic costs for drug sensitive tuberculosis patients in Kenya. Infect. Dis. Poverty. 2021;10(1):1–15. doi: 10.1186/s40249-021-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiang L, et al. The impact of the new cooperative medical scheme on financial burden of tuberculosis patients: Evidence from six counties in China. Infect. Dis. Poverty. 2016;5(1):8. doi: 10.1186/s40249-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou C, et al. The effect of NCMS on catastrophic health expenditure and impoverishment from tuberculosis care in China. Int. J. Equity Health. 2016;15(1):172. doi: 10.1186/s12939-016-0463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization . Designing Health Financing Systems to Reduce Catastrophic Health Expenditure. World Health Organization; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request by contacting the first author.