Abstract
Purpose:
Increasing tumor-infiltrating lymphocytes (TILs) is associated with higher rates of pathologic complete response (pCR) to neoadjuvant therapy (NAT) in patients with triple-negative breast cancer (TNBC). However, the presence of TILs does not consistently predict pCR, therefore, the current study was undertaken to more fully characterize the immune cell response and its association with pCR.
Experimental Design:
We obtained pre-treatment core needle biopsies from 105 patients with stage I-III TNBC enrolled in ARTEMIS (NCT02276443) who received NAT from 10/22/2015 through 7/24/2018. The tumor-immune microenvironment was comprehensively profiled by performing T-cell receptor (TCR) sequencing, PD-L1 immunohistochemistry, multiplex immunofluorescence and RNA sequencing on pretreatment tumor samples. The primary endpoint was pathological response to NAT.
Results:
The pCR rate was 40% (42/105). Higher TCR clonality (median=0.2 vs 0.1, p=0.03), PD-L1 positivity (odds ratio: 2.91, p=0.020), higher CD3+:CD68+ ratio (median=14.70 vs 8.20, p=0.0128), and closer spatial proximity of T-cells to tumor cells (median=19.26μm vs 21.94μm, p=0.0169) were associated with pCR. In a multivariable model, the closer spatial proximity of T-cells to tumor cells and PD-L1 expression enhanced prediction of pCR when considered in conjunction with clinical stage.
Conclusion:
In patients receiving NAT for TNBC, deep immune profiling through detailed phenotypic characterization and spatial analysis can improve prediction of pCR in patients receiving NAT for TNBC when considered with traditional clinical parameters.
Keywords: tumor-immune microenvironment, triple-negative breast cancer, spatial analytics, transcriptomic profiling, neoadjuvant therapy
INTRODUCTION
Globally, approximately 170,000 women are diagnosed with triple-negative breast cancer (TNBC) every year (1). In the curative neoadjuvant setting, pathological complete response (pCR) after neoadjuvant therapy (NAT) is associated with excellent long-term outcomes (2). However, 50–60% of patients with TNBC have residual disease (non-pCR) after receiving standard NAT (multi-agent chemotherapy) (2–4) and are at risk of poor long-term outcomes (2). Notably, TNBCs have significantly higher tumor-infiltrating lymphocyte (TIL) infiltration (5,6) compared to non-TNBCs and it is known that pre-treatment TIL infiltration is associated with response to NAT in TNBC (5,7–10), suggesting that the immune system plays a critical role in modulating that response.
Interestingly, the addition of immunotherapy, specifically immune checkpoint blockade, in combination with chemotherapy has recently emerged as a promising therapeutic strategy in the neoadjuvant setting for TNBC (11–13), further underscoring the importance of the anti-tumor immune response in modulating responses to NAT. Although an abundance of TILs is generally associated with higher rates of pCR to NAT in TNBC (7), this relationship is not consistent. Recent data have suggested that a fraction of TILs are merely bystander immune cells with limited anti-tumor activity (14). Specifically, in-depth analysis of the T-cell receptor repertoire of tumor-infiltrating CD8+ T-cells in ovarian and colorectal cancer have demonstrated that only about 10% of intratumoral CD8+ T-cells have the capacity to recognize autologous tumor cells (14). Although similar studies have not been done in TNBC, these data suggest that the intrinsic ability of TILs to recognize tumor cells may be limited to a small fraction of TILs, potentially explaining the inconsistent relationship between TIL infiltration and response to NAT in TNBC. This study was undertaken to more fully characterize the tumor-immune microenvironment (TIME) in order to identify novel predictors of response to NAT in TNBC.
PATIENTS AND METHODS
Patient cohort and clinical variables
Patients with stage I-III TNBC enrolled in the prospective, IRB-approved, clinical study, “A Robust TNBC Evaluation framework to Improve Survival” (ARTEMIS, NCT02276443), were included in this study if they had sufficient tissue for molecular and immune profiling with corresponding germline controls. In the ARTEMIS trial, patients with stage I-III TNBC underwent a pre-treatment core-needle biopsy prior to initiating neoadjuvant doxorubicin and cyclophosphamide (AC). Disease response was monitored with breast imaging (ultrasound and/or magnetic resonance imaging) after two and four cycles of AC. Patients with suboptimal responses to AC, defined as disease progression or a <70% reduction in tumor volume after 4 cycles of AC, were offered the opportunity to enroll in a therapeutic neoadjuvant clinical trial evaluating novel combinations of systemic therapy. Patients not meeting the criteria for suboptimal response to AC were recommended to continue standard of care neoadjuvant therapy with paclitaxel +/− carboplatin (Supplementary Fig. S1). The ARTEMIS study protocol was reviewed by The University of Texas MD Anderson Cancer Center Institutional Review Board and all patients provided informed consent. All study procedures performed were in accordance with ethical standards of the Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. TNBC was defined as breast cancers with <10% estrogen receptor (ER) and progesterone receptor (PR) staining of invasive tumor cells by immunohistochemistry (IHC) that were also negative for the human epidermal growth factor receptor 2 (HER2) according to HER2 testing guidelines from the American Society of Clinical Oncology/College of American Pathologists (15). Age at diagnosis was calculated as the age in years at the time of the first positive breast biopsy. The Nottingham histologic grade was determined by breast pathologists as part of routine clinical care (16). Node positivity was defined as the presence of at least one malignant regional lymph node confirmed by fine-needle aspiration or core needle biopsy at the time of diagnosis. The clinical anatomic stage was determined at the time of diagnosis as per the American Joint Committee on Cancer Staging Manual (8th edition) (17).
TIL quantification
The percentage of stromal TIL infiltration was determined on hematoxylin and eosin (H&E) stained slides by breast pathologists according to standardized methods described by the International TILs Working Group (18).
Immunohistochemistry
Ki-67 was performed as part of routine clinical care in some patients. IHC staining for Ki-67, androgen receptor (AR), vimentin, and PTEN were performed on unstained 4-μm thick tissue sections that had been prepared from a representative paraffin block of the tumor, using the polymeric biotin-free horseradish peroxidase method on the Leica Microsystems Bond III autostainer (Leica Microsystems, Buffalo Grove, IL, USA). The slides were incubated at 60°C for 25 minutes. Heat-induced epitope retrieval was performed with TRIS-EDTA buffer for 20 minutes at 100°C (for Ki-67, PTEN), citrate buffer for 25 minutes at 100°C (for AR), or citrate buffer for 5 minutes at 100°C (for vimentin). Slides were incubated with mouse monoclonal antibodies to Ki-67 (clone MIB-1, Dako; 1:100), AR (clone AR441, Dako, Carpinteria, CA, USA; 1:30), vimentin (clone V9, 1:900, Dako), or PTEN (clone 6H2.1, 1:100, Dako North America Inc., Carpinteria, CA). The Refine Polymer Detection kit was used to detect bound antibody, with 3,3-diaminobenzidine serving as the chromogen (Leica Microsystems). IHC staining for programmed death-ligand 1 (PD-L1) was performed using the PD-L1 IHC 22C3 pharmDx kit (Dako) on the Dako AutostainerLink 48 according to the manufacturer’s instructions. Slides were counterstained with Mayer’s hematoxylin. Results were evaluated with known positive and negative tissue controls. For Ki-67, the percentage of tumor cells with any nuclear staining of any intensity was recorded (low/moderate: ≤35% and high: >35%) (19). For AR, the percentage and intensity of any nuclear staining in tumor cells were recorded (AR negative: <10% and AR positive: ≥10%) (20). For vimentin, positive staining was defined as moderate or strong cytoplasmic staining in ≥50% invasive tumor cells. For PTEN, unequivocal nuclear or cytoplasmic staining of any intensity in any proportion of invasive tumor cells was considered positive, and the stain was considered negative if there was no staining in invasive tumor cells. PD-L1 expression was quantified as the percentage of tumor area occupied by PD-L1 expressing tumor-infiltrating immune cells (PD-L1 negative: <1% and PD-L1 positive: ≥1%). All H&E and IHC stains from each specimen were evaluated by a single breast pathologist. Cases with equivocal results were evaluated by a team of breast pathologists to reach a consensus (L.H., Q.Q.D.).
Multiplex immunofluorescence
Multiplex immunofluorescence (mIF) was performed on 4-μm thick formalin-fixed paraffin-embedded (FFPE) tissue sections using the Opal 7-color Kit (Akoya/PerkinElmer, Waltham, MA) as previously described (21). Briefly, using an automated staining system (BOND-MAX; Leica Microsystems, Vista, CA), tissue sections were stained consecutively with antibodies against the following: pancytokeratin (panCK) AE1/AE3 (dilution 1:100; Dako, Carpinteria, CA), PD-L1 (PD-L1 clone E1L3N, dilution 1:1500; Cell Signaling Technology), CD8 (clone C8/144B, dilution 1:25; Thermo Fisher Scientific, Waltham, MA), CD3 (dilution 1:200; Dako), CD68 (clone PG-M1, dilution 1:50; Dako), and programmed cell death protein 1 (PD-1) (clone EPR48772, dilution 1:3000; Abcam, Cambridge, MA). The Opal 7-color Kit was used to detect antibody staining according to the manufacturer’s instructions. Human tonsil FFPE tissues were also used with and without primary antibodies as positive and negative (autofluorescence) controls, respectively. The stained slides were scanned by a Vectra multispectral microscope using the Vectra 3.0 spectral imaging system (PerkinElmer). After the specimens were scanned at low magnification (x10), five individual fields (669 × 500 μm each) in the tumor area were examined with a Phenochart (Akoya/PerkinElmer) viewer and followed by scanning at high resolution (x20). After imaging, quantification of cell density (cells/mm2) and co-localization of the biomarkers were performed using the inForm software (Akoya/PerkinElmer) and reviewed by subspecialized breast research pathologists (F.Y., X.S., B.S., E.P.). The spatial distribution of individual tumor cells and their surrounding immune cell subpopulations were analyzed using multivariate point pattern analysis (22). Briefly, the distance between each tumor (panCK+) cell and the nearest member of the immune cell subpopulation being analyzed was calculated (nearest neighbor distance). This process was repeated for all tumor cells within each sample and a mean nearest neighbor distance was calculated for each sample.
T-cell receptor sequencing (TCRseq)
Total DNA was extracted from prospectively collected pre-treatment core needle biopsies of primary TNBCs. Amplification and sequencing of the T-cell receptor β (TCRβ) complementarity-determining region 3 (CDR3) sequence was performed using the ImmunoSEQ platform (Adaptive Biotechnologies)(23,24). Clonality scores based on Shannon’s entropy were calculated using the ImmunoSEQ Analyzer software and reported on a scale of 0 to 1, with 0 and 1 indicating a maximally diverse and completely monoclonal T cell population, respectively.
Whole transcriptomic sequencing
RNA was extracted by NORGEN Total RNA Purification Kit (Cat. 37500) (NORGEN BIOTEK CORP), treated with DNase I and purified using AMPure XP beads (Beckman Coulter Life Sciences). cDNA was prepared from the extracted total RNA using the Ovation RNA-Seq System V2 (NuGEN). Up to 200 ng of each cDNA sample was sheared using the E220 Focused-ultrasonicator (Covaris). Library preparation and hybridization were performed on the Sciclone G3 NGSx Workstation (PerkinElmer, Inc.) using SureSelect XT Low Input Reagent Kit with indexes 1–96 (Agilent Technologies) and Agilent SureSelect Human All Exon v.4 probes (Agilent Technologies), respectively. Agilent captures were hybridized as single sample reactions using 500–1000 ng of the prepared library as input. Captured libraries were sequenced on the Illumina NovaSeq 6000 platform for 2 × 150 paired end reads with an 8nt read for indexes using Cycle Sequencing v3 reagents (Illumina). Raw RNA sequence data were processed by an in-house RNAseq data analysis pipeline which, among other tools, uses the STAR aligner (25) to align raw reads to hg19 version of the Human reference genome, featureCounts(26) and RSEM (27) to quantify aligned reads to produce raw and normalized counts, and FastQC to evaluate the quality of raw reads. We estimated the activation of the Hallmarks pathways (28) using ssGSEA (29). Data processing was performed using the BETSY bioinformatics analysis system (30).
Response to NAT and time-to-event endpoints
Response to NAT was determined using the residual cancer burden (RCB) index (31). Patients who experienced disease progression precluding curative surgical resection while receiving neoadjuvant therapy were classified as having RCB-III disease. Event-free survival was defined as the time from diagnosis to disease progression during NAT to an extent that precluded curative surgical resection, distant recurrence (local and/or distant) following definitive surgical resection, or death from any cause, whichever occurred first. Metastasis-free survival (MFS) was defined as the time from diagnosis to the development of distant metastatic disease or death from any cause, whichever occurred first. Overall survival (OS) was defined as the time from diagnosis to death from any cause. All time-to-event endpoints were censored at the time of last known follow up.
Statistical methods
Associations between baseline patient and tumor characteristics and response to NAT were assessed using univariable logistic regression, the Wilcoxon rank-sum test, and Student’s t-test. Associations between TCR clonality and various immune cell populations were examined with Spearman’s rank correlation. Multivariable logistic regression was used to construct predictive models of pCR. Selected variables were fit jointly in a multivariable model. Model fit was assessed using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC). Receiver operating characteristic (ROC) curves were constructed to determine the Area Under the Curve (AUC) for each iteration of the model. Univariable and multivariable Cox regression analyses were used to determine the effect of study covariates on EFS, MFS, and OS. Results were expressed as hazard ratios (HR) and adjusted hazard ratios (aHR), with accompanying 95% confidence intervals (CIs). For all analyses, a two-sided p-value of <0.05 was considered statistically significant. When appropriate, p values were corrected for multiple hypotheses using the Benjamini and Hochberg method for estimating false discovery rates (32). All data were analyzed using R Statistical Software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
A total of 105 patients with stage I-III TNBC were included in this study. The median age at diagnosis was 57.3 years (range: 24.4–77.6 years). Baseline patient, tumor and immune characteristics are summarized in Table 1. All 105 patients were treated with NAT. Of these, 76 (72.4%) received chemotherapy alone (Table 1). The remaining patients were treated with combination chemo- and targeted therapy (n=20, 19.1%) or chemoimmunotherapy (n=9, 8.6%) on clinical trials as part of therapeutic escalation due to suboptimal ultrasonographic response to initial standard NAT with doxorubicin and cyclophosphamide (Table 1). Notably, these clinical trials of therapeutic escalation for patients with suboptimal ultrasonographic response to initial standard NAT are still ongoing and the 29 patients included in this study only represent a subset of patients enrolled in these clinical trials of therapeutic escalation. The pCR rate was 40.0% (42/105). One patient experienced disease progression to such an extent that precluded curative surgical resection and was considered to have a non-pCR. Clinical characteristics associated with pCR are detailed in Supplementary Table S1. Notably, patients with a higher clinical stage at diagnosis (stage III vs. stage I, odds ratio [OR]: 0.10, 95% CI: 0.02–0.54, p=0.008) were less likely to experience a pCR while patients who had tumors with high Ki-67 (Ki-67 high vs. Ki-67 low/moderate, OR: 4.54, 95% CI: 1.41–14.62, p=0.01) were more likely to experience pCR following NAT (Supplementary Table S1).
TABLE 1.
Baseline Characteristics and Neoadjuvant Therapy Received
| Characteristic | Total (N=105) |
|---|---|
| Median age at diagnosis - years (range) | 57.26 (24.44–77.61) |
| Race | |
| White | 70 (66.67) |
| Black | 16 (15.24) |
| Other | 19 (18.10) |
| T category | |
| T1 | 18 (17.14) |
| T2 | 71 (67.62) |
| T3 | 13 (12.38) |
| T4 | 3 (2.86) |
| Nodal Status | |
| Negative | 59 (56.19) |
| Positive | 46 (43.81) |
| TNM Stage | |
| I | 11 (10.48) |
| II | 73 (69.52) |
| III | 21 (20.00) |
| Histologic grade | |
| 1/2 | 15 (14.29) |
| 3 | 90 (85.71) |
| Ki-67 | |
| Low / Moderate | 28 (26.67) |
| High | 77 (73.33) |
| Androgen Receptor | |
| Negative | 74 (70.48) |
| Positive | 31 (29.52) |
| Vimentin | |
| Negative | 91 (86.67) |
| Positive | 14 (13.33) |
| PTEN | |
| Present | 51 (48.57) |
| Absent | 26 (24.76) |
| Indeterminate | 8 (7.62) |
| Missing | 20 (19.05) |
| Stromal TIL | |
| <10% | 38 (36.19) |
| 10–29% | 42 (40.00) |
| ≥ 30% | 25 (23.81) |
| PD-L1 immunohistochemistry (22C3) – Immune Cell | |
| <1% | 78 (74.29) |
| ≥1% | 27 (25.71) |
| PD-L1 immunohistochemistry (22C3) – Combined Positive Score (CPS) | |
| <10 | 92 (87.62) |
| ≥10 | 13 (12.38) |
| Neoadjuvant Therapy Received | |
| AC-Paclitaxel | 68 (64.76) |
| AC-Paclitaxel-Carboplatin | 7 (6.67) |
| AC-Paclitaxel-Carboplatin-Gemcitabine | 1 (0.95) |
| AC-Paclitaxel-Carboplatin-Panitumumab | 6 (5.71) |
| AC-Paclitaxel-Enzalutamide | 8 (7.62) |
| AC-Nab-Paclitaxel-Atezolizumab | 9 (8.57) |
| AC-Liposomal Doxorubicin-Bevacizumab-Temsirolimus/Everolimus | 6 (5.71) |
All values reported as n (%) unless otherwise noted.
AC, Adriamycin-Cyclophosphamide; TIL, tumor-infiltrating lymphocytes
Immune characteristics
Of the 105 patients, 38 (36.2%) had tumors with <10% TIL infiltrate, 43 (41.0%) had tumors with 10–29% TIL, and 24 (22.9%) had tumors with ≥30% TIL. In 98 patients in whom adequate DNA was available to address TCR clonality, the median TCR clonality was 0.17 (range: 0.03–0.24). Of note, patients who experienced a pCR following NAT had tumors with higher TCR clonality at baseline (median TCR clonality: 0.20, range: 0.06–0.24) compared with those who did not (median TCR clonality: 0.10, range: 0.03–0.23, p=0.03, Figure 1A). There was a positive correlation between TCR clonality and CD3+ (Spearman’s rank correlation coefficient [rho]=0.32, p=0.002) and CD3+CD8+ (rho=0.33, p=0.001) cell densities whereas there was a negative correlation between TCR clonality and CD3+PD-1+ (rho=−0.24, p=0.02) and CD3+CD8+PD-1+ (rho=−0.21, p=0.04) cell densities (Figure 1B). Interestingly, among the 102 tumor biospecimens subject to mIF, PD-L1-positive tumor cells were found in 46 samples (45%). In contrast, the rate of PD-L1 positivity by immunohistochemistry (immune cell staining ≥1%) among tumors from these 105 patients was 25.7% (27/105). PD-L1-positive tumors (by immunohistochemistry) had higher median TCR clonality (median TCR clonality=0.20; range: 0.09–0.23) compared with PD-L1-negative tumors (median TCR clonality=0.10; range: 0.03–0.24) (p=0.004, Figure 1C). Of note, patients with PD-L1-positive tumors were more likely to experience pCR than patients with PD-L1-negative tumors (OR: 2.91, 95% CI: 1.18–7.16, p=0.02, Figure 1D). Cell densities of various immune cell populations as quantified by mIF did not reveal any statistically significant associations (Supplementary Table S2).
FIGURE 1. A more clonal T-cell population in pre-treatment tumor tissue is associated with response to neoadjuvant therapy, an immunologically active infiltrate and PD-L1 positivity, which is also associated with response to neoadjuvant therapy.
(A) Pre-treatment T-cell clonality scores in tumor tissue from patients with NAT-sensitive disease (pCR, n=40) and NAT-resistant disease (RCB I-III, n=58). (B) Associations between T-cell clonality and cell densities of CD3+, CD3+CD8+, CD3+PD-1+, and CD3+CD8+PD-1+ cells. (C) PD-L1+ tumors (n=24) have higher in T-cell clonality scores (n=27) compared with PD-L1- tumors (n=78). (D) PD-L1+ tumors are more likely to respond to neoadjuvant therapy compared to PD-L1- tumors.
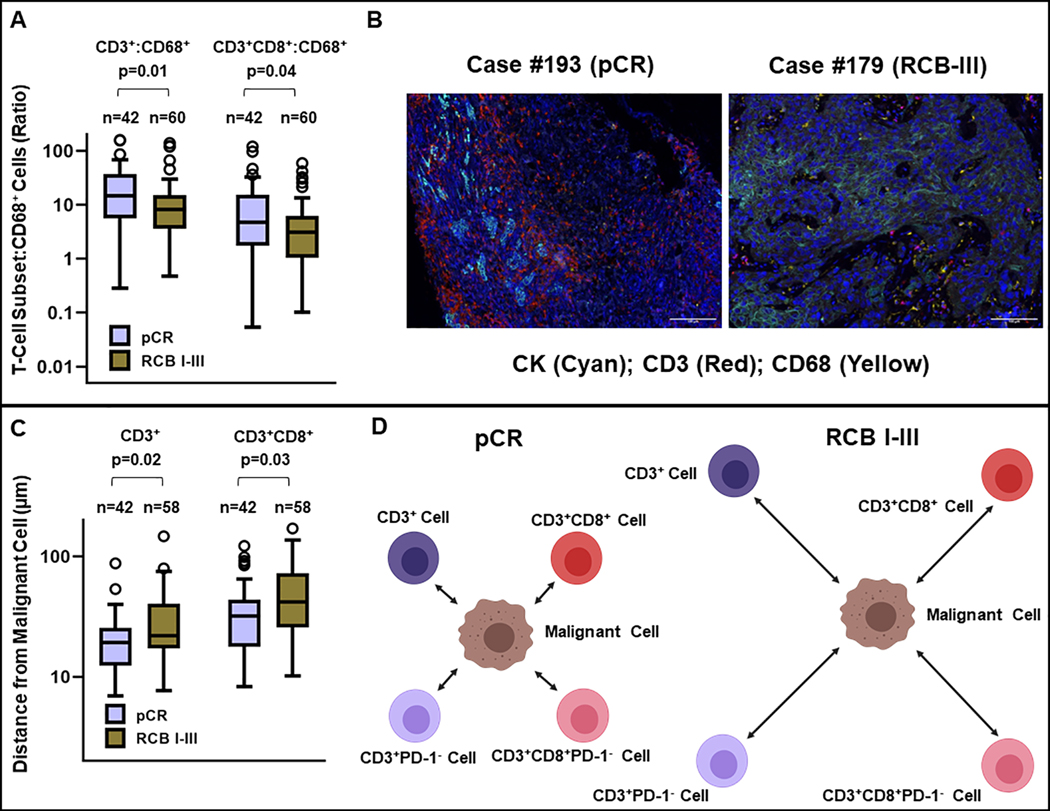
Relative abundance of CD3+ to CD68+ cells in the tumor-immune microenvironment is associated with pCR
Although the cell densities of CD3+, CD3+CD8+, and CD68+ cells in the TIME were not significantly associated with pCR when considered in isolation (Supplementary Table S2), pre-treatment tumors from patients who experienced a pCR after NAT had a higher ratio of CD3+:CD68+ cells and CD3+CD8+:CD68+ cells (Figure 2A–2B), suggesting that the relative abundance of T-cells to macrophages may be important in modulating response to NAT. This is in line with other reports in breast cancer that an immune response characterized by CD8low/CD4high/CD68high represents a population of patients at risk for poor outcomes (33).
FIGURE 2. Response to neoadjuvant therapy is associated with a higher ratio of CD3+:CD68+ and CD3+CD8+:CD68+ cells as well as increased spatial proximity of T-cell subsets to tumor cells in the tumor-immune microenvironment.
(A) Ratio of CD3+:CD68+ and CD3+CD8+:CD68+ cells in the tumor-immune microenvironment in patients with NAT-sensitive disease (pCR, n=42) and NAT-resistant disease (RCB I-III, n=60). (B) Representative multiplexed images (Cyan: CK, Red: CD3, Yellow: CD68) of the tumor-immune microenvironment from a patient with NAT-sensitive disease (pCR) and another with NAT-resistant disease (RCB-III). (C) Increased proximity (reduced distance) between tumor cells and CD3+ and CD3+CD8+ T-cells. (D) Schematic diagram illustrating that increased spatial proximity of T-cell subsets to tumor cells in the tumor-immune microenvironment is associated with response to neoadjuvant therapy.
Spatial analysis of tumor-immune microenvironment
Next, we sought to determine if tumor-immune cell proximity was different in pre-treatment tumors from patients who experienced a pCR compared to those from patients who did not experience a pCR. We calculated nearest neighbor distances between various tumor-immune cell pairs identified through mIF. Notably, pCR was associated with reduced spatial separation between tumor (CK+) cells and CD3+ cells (median: 19.26μm vs 21.94μm, p=0.02) (Figure 2C–2D) and CD3+CD8+ cells (median: 31.96μm vs 41.93 μm, p=0.03) (Figure 2C–2D). Although increased spatial separation between tumor cells and CD3+PD-1+ cells was observed in pre-treatment tumors samples obtained from patients experiencing pCR (median: 130.84μm vs 121.28μm, p=NS) (Figure 3) as well as CD3+CD8+PD-1+ cells (median: 153.67μm vs 124.80μm, p=NS), these differences were not statistically significant (Figure 3). Interestingly, spatial separation between tumor cells and CD3+(CD8+)PD-L1+ was not significantly different in tumors from patients experiencing pCR (n=24, median: 121.40μm) compared to tumors from patients with non-pCR (n=25, median: 125.79μm).
FIGURE 3. Spatial proximity of T-cell subsets to tumor cells in the tumor-immune microenvironment and response to neoadjuvant therapy (NAT).
Tumor cells were further away from CD3+PD-1+ and CD3+CD8+PD-1+ T-cells in NAT-sensitive (pCR) tumors (p=NS). Spatial proximity of tumor cells and CD3+(CD8+)PD-L1+ T-cells were similar in NAT-sensitive (pCR) and NAT-resistant (RCB I-III) tumors.
Phenotypic and spatial features of the tumor-immune microenvironment
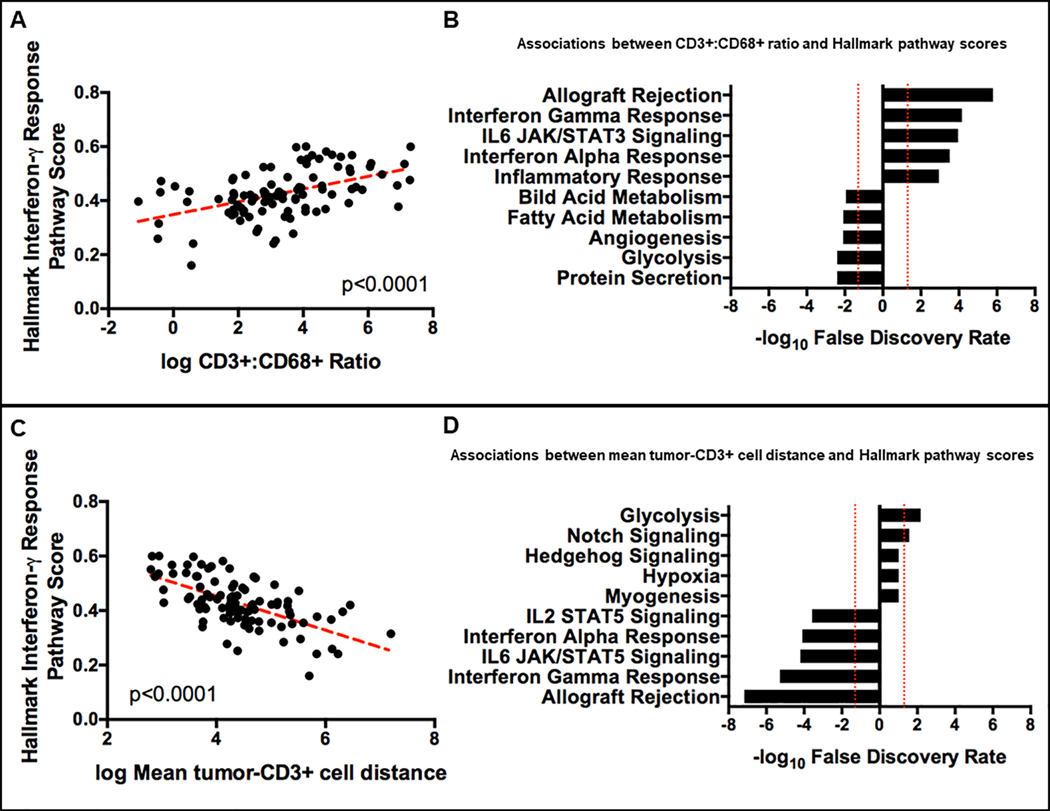
We next investigated if the increased ratio of CD3+:CD68+ cells in the TIME and/or reduced spatial separation of tumor cells and CD3+ cells were associated with evidence of increased T-cell activity. An analysis of gene expression profiles derived from whole transcriptomic sequencing showed that increased ratio of CD3+:CD68+ cells in the TIME correlated positively with the Hallmark interferon-γ response pathway scores (R= 0.45, p<0.0001, Figure 4A). Further, the CD3+:CD68+ ratio was positively correlated with several Hallmark immune-associated pathway scores but negatively correlated with Hallmark tumor-associated pathway scores (Figure 4B). Together, these data suggest that a high CD3+:CD68+ ratio in the TIME is reflective of increased T-cell anti-tumor activity. Similarly, reduced spatial separation of tumor (CK+) and CD3+ cells was associated with higher Hallmark interferon-γ response pathway scores (R= −0.49, p<0.0001, Figure 4C). In addition, reduced spatial separation of tumor (CK+) and CD3+ cells were associated with increased scores in several Hallmark immune-associated pathways but lower scores in several Hallmark tumor-associated pathways (Figure 4D), suggesting that reduced spatial separation of tumor (CK+) and CD3+ cells reflect higher T-cell anti-tumor activity.
FIGURE 4. A higher ratio of CD3+:CD68+ cells and increased proximity of T-cells to tumor cells in the tumor-immune microenvironment is associated with increased expression of interferon-γ pathway associated genes, increased transcriptional activity in immune related pathways and reduced transcriptional activity in tumor-associated pathways.
(A) A higher ratio of CD3+:CD68+ cells in the tumor-immune microenvironment is correlated positively with increased expression of interferon-γ pathway associated genes. (B) A higher ratio of CD3+:CD68+ cells in the tumor-immune microenvironment is associated with increased transcriptional activity in immune related pathways but reduced transcriptional activity in tumor-associated pathways. (C) Increased proximity (reduced distance) of T-cells to tumor cells in the tumor-immune microenvironment is associated with increased expression of interferon-γ pathway associated genes. (D) Increased proximity (reduced distance) of T-cells to tumor cells in the tumor-immune microenvironment is associated with increased transcriptional activity in immune related pathways but reduced transcriptional activity in tumor-associated pathways.
Multivariable analysis of clinical and immune predictors of pCR
Starting with a univariable logistic regression model with clinical TNM stage as the independent variable and pathological response (pCR vs non-pCR) as the dependent variable, we assessed the impact of the following variables on model fit in a turn-based manner (using data from patients with non-missing values in all 10 variables considered for multivariable modeling): Ki-67 (continuous); Ki-67 (categorical: ≤35% vs >35%); stromal TIL (continuous); stromal TIL (ordinal: <10% vs 10–29% vs ≥30%); PD-L1 by immune cell staining criteria (categorical: <1% vs ≥1%); PD-L1 by CPS score (categorical: <10 vs ≥10); TCR clonality (continuous); Hallmark interferon gamma response pathway score (continuous); CD3+:CD68+ ratio (continuous); and tumor-CD3+ cell distance. Akaike Information Criterion and Bayesian Information Criterion values for each model are summarized in Supplementary Table S3. The three variables demonstrating the greatest improvement in model fit when integrated with clinical TNM stage, as assessed by both the Akaike and Bayesian Information Criterion, were stromal TIL (continuous), PD-L1 by immune cell staining criteria, and tumor-CD3+ cell distance. Starting with clinical TNM stage, we fit all possible models by including various permutations of the three additional variables described above in the cohort of patients with non-missing values for all three variables (n=100). Based on the Akaike and Bayesian Information Criterion, Area Under the Receiver Operating Curve (AUC) (Supplementary Table S4), and considerations of model parsimony, the final features included in our multivariable model were clinical TNM stage, PD-L1 by immune cell staining criteria, and tumor-CD3+ cell distance. ROC analyses of each iteration of the model demonstrated improved model characteristics (higher AUC) with each additional variable (Figure 5). Of note, adding phenotypic (PD-L1 expression) and spatial features (tumor-CD3+ cell distance) of the TIME improves the model significantly over using clinical features alone (Figure 5).
FIGURE 5. Immune parameters enhance response prediction in patients receiving neoadjuvant therapy when considered in parallel with traditional clinical predictors.
(A) Receiver operating characteristic (ROC) curve demonstrating predictive accuracy of clinical stage alone (Area Under the Curve [AUC]=63%). (B) ROC curve demonstrating predictive accuracy of a multivariable model based on clinical stage and PD-L1 (AUC=68%). (C) ROC curve demonstrating predictive accuracy of a multivariable model based on clinical stage, and tumor-CD3+ cell distance (AUC=72%). (D) ROC curve demonstrating predictive accuracy of a multivariable model based on clinical stage, PD-L1, and tumor-CD3+ cell distance (AUC=74%).
Effect of baseline clinical, pathological, and immune features on survival
To understand the effect of the three selected baseline features (clinical TNM stage, PD-L1 by immune cell staining criteria, and tumor-CD3+ cell distance) on survival, we performed univariable and multivariable analyses of these three features on EFS, MFS, and OS (Supplementary Table S5). Higher clinical TNM stage was independently with shorter EFS (aHR: 4.48; 95% CI: 1.56–12.90; p=0.0055), MFS (aHR: 5.21; 95% CI: 1.76–15.47; p=0.003), and OS (aHR 4.9; 95% CI: 1.09–22.32; p=0.04). Larger tumor-CD3+ cell distance (increased separation) was independently associated with shorter EFS aHR: 1.03; 95% CI: 1.01–1.06; p=0.0037) and MFS (aHR: 1.03; 95% CI: 1.01–1.06, p=0.008) but had no effect on OS, likely due to the small number of OS events in our cohort.
DISCUSSION
In this study, we have more fully characterized the endogenous T-cell response and TIME in TNBC and identified associations with response to NAT. Specifically, we found that a more clonal T-cell population was associated with pCR following NAT in TNBC. In addition, higher TCR clonality was associated with PD-L1 positivity as well as higher densities of CD3+ and CD3+CD8+ cells but lower CD3+PD-1+ and CD3+CD8+PD-1+ cells. PD-L1 positivity was associated with a greater likelihood of pCR following NAT, but cell densities of various immune cell populations did not differ significantly between NAT-sensitive and NAT-resistant tumors. Interestingly, although cell densities of immune cell populations were not associated with pCR when considered in isolation, there was a positive association between the ratio of T-cells to macrophages (CD3+:CD68+ and CD3+CD8+:CD68+) and pCR. Notably, through spatial analysis, we found that compared to tumor (CK+) cells in NAT-resistant tumors, tumor cells in NAT-sensitive tumors were in closer proximity to CD3+ and CD3+CD8+T-cells. From a functional standpoint, a higher ratio of T-cells to macrophages and closer proximity of tumor cells to T-cells were associated with higher expression of IFN-γ response pathway genes, suggesting higher T-cell activity.
Since clonal expansion of TILs suggest anti-tumor reactivity (34), our finding that NAT-sensitive tumors have higher TCR clonality compared to NAT-resistant tumors supports the hypothesis that the anti-tumor immune response plays a significant role in modulating NAT response. The observed positive correlation between TCR clonality and CD3+ as well as CD3+CD8+ cell densities along with the corresponding negative correlation between TCR clonality and CD3+PD-1+ as well as CD3+CD8+PD-1+ cell densities suggest a clonal expansion of TILs without evidence of T-cell exhaustion in tumors with higher TCR clonality. Interestingly, higher TCR clonality was associated with PD-L1 positivity, presumably due to the induction of PD-L1 expression through increased interferon-gamma production from activated T-cells (35). We hypothesize that PD-L1 positivity in pre-treatment tumor samples is a surrogate marker for increased T-cell activation, explaining, in part, the enhanced sensitivity to NAT and higher rates of pCR observed in PD-L1-positive breast cancers in this and other studies (36).
Our finding that cell densities of various immune cell populations were not significantly different between NAT-sensitive and NAT-resistant tumors appears inconsistent with earlier studies reporting positive correlations between higher CD3+ infiltration (37) as well as CD8+ infiltration (38) with increased rates of pCR in patients receiving NAT for breast cancer. However, most of these studies were not restricted to patients with TNBC, and there remains limited data on the relationship between immune cell subsets and response to NAT in TNBC. Despite the lack of observed associations between various immune cell densities and pCR in our study, we found that a higher ratio of T-cells to macrophages and closer proximity of tumor cells to T-cells was associated with pCR to NAT, suggesting that these measures may reflect underlying anti-tumor activity and be an important determinant of response to NAT. Indeed, both of these measures correlated significantly with the expression of IFN-γ response pathway genes.
Interestingly, the finding that NAT-sensitive tumors have a higher T-cell to macrophage ratio compared to NAT-resistant tumors is consistent with preclinical studies showing that tumor-associated macrophages (TAMs) mediate resistance to chemotherapeutic agents (33,39,40) and blocking macrophage recruitment pathways (33) or converting pro-tumor macrophages to an anti-tumor phenotype (41) in combination with chemotherapy improves outcomes through CD8+ T-cell-dependent mechanisms.
Through multivariable modeling, we demonstrated that integrating phenotypic (PD-L1 expression) and spatial features (tumor-CD3+ cell distance) of the TIME significantly improved the model fit and operating characteristics compared to clinical stage alone. Notably, larger tumor-CD3+ T-cell distance (increased separation) was also associated with worse EFS and MFS in univariable and multivariable analyses. To the best of our knowledge, this is the largest study reporting on the relationship between spatial separation of tumor cells and CD3+ T-cells in the TIME and outcomes in patients with TNBC receiving NAT. Collectively, these data further support our hypothesis that deep characterization of the TIME will enhance prediction of response and long-term outcomes in patients with TNBC receiving NAT over using clinical features alone.
In addition to providing a deeper understanding of the relationship between specific features of the TIME and response to NAT in TNBC, our study provides a framework for dissecting the TIME in the context of emerging neoadjuvant therapies. Specifically, although neoadjuvant PD-(L)1 blockade in combination with chemotherapy improves the rate of pCR compared with chemotherapy alone in unselected TNBC (11–13), receipt of anti-PD-(L)1 therapy is associated with a definite risk of immune-related adverse events which has the potential to cause long-term morbidity (42–44). Thus, optimizing the risk-benefit ratio for anti-PD-(L)1 therapy is especially critical in patients with potentially curable, stage I-III TNBC. Data presented in this study demonstrate that an immune-enriched TIME, characterized by features suggestive of increased T-cell mediated anti-tumor immunity, is associated with pCR to NAT, bringing to question the extent to which adding neoadjuvant PD-(L)1 blockade to chemotherapy improves responses in patients with immune-enriched TNBCs. Thus, further studies are needed to better define the degree of benefit from adding anti-PD-(L)1 therapy to neoadjuvant chemotherapy in patients with immune-enriched TNBC.
Our study has a few limitations. First, the relatively modest sample size and limited follow up period precluded a robust analysis of associations between immune phenotype and time-to-event endpoints at the present time. Despite this limitation, we were able to demonstrate that spatial features of the TIME, specifically tumor-CD3+ cell distance was associated with worse EFS and MFS. Second, there was a certain degree of heterogeneity with regards to the type of NAT received. This arose due to the use of non-standard targeted agents as part of therapeutic escalation offered to patients enrolled in ARTEMIS (NCT02276443) with suboptimal responses to doxorubicin and cyclophosphamide. However, since the primary goal of this study is to identify phenotypic and spatial features of the TIME that would enhance response prediction in all patients with stage I-III TNBC receiving NAT, it was important to include patients with suboptimal responses to doxorubicin and cyclophosphamide in our study because these patients were also the least likely to experience pCR with standard NAT. In other words, excluding them would have introduced significant bias into our study. Despite this heterogeneity, all patients received a uniform anthracycline-based chemotherapy backbone during the initial phase of NAT and an overwhelming majority (94%) received taxane-based therapy in the second phase of NAT. Further, the aggregate rate of pCR (data not shown) in this limited sub-cohort of patients who were enrolled on clinical trials after suboptimal response to standard NAT was similar to that of historical controls (3,45). This observation provides orthogonal evidence suggesting that the relationship between baseline features and response to NAT was not significantly altered by the use of non-standard agents in the second phase of NAT in this limited sub-cohort of patients. Third, while CD68 has been used as a pan-macrophage marker in this and other studies (33,46–49), there are data suggesting that CD68 is not a macrophage-specific marker in human breast cancer (50) and more precise markers are needed to accurately distinguish TAMs from other CD68+ immune cells, as well as distinguish pro- from anti-tumor macrophages (51). Thus, as technological advances in multiplexed protein-based assays allow for simultaneous evaluation of an increasing number of markers, given our data suggesting that tumor-infiltrating CD68+ may influence response to NAT, future studies in this clinical context should consider the use of assays which include markers that enable accurate categorization of macrophage populations based on functional states. Fourth, because the primary objective of this study was to identify baseline phenotypic and spatial features of the TIME that would help predict response to NAT, we did not interrogate the TIME in residual disease following NAT or characterize circulating immune cells using paired peripheral blood samples. This limited our ability to identify (1) longitudinal changes in the TIME following NAT, and (2) differential representation of immune cell populations and T-cell clones in the TIME, relative to peripheral blood. Given the potential for identifying novel therapeutic strategies through deep characterization of longitudinal changes in the TIME and paired analysis of peripheral blood and tumor biospecimens, future studies incorporating longitudinal tissue and paired blood collection should be undertaken to help inform the development of innovative clinical trials in this setting.
In conclusion, our data suggest that deep immune profiling can further refine the predictive value of TILs in patients with TNBC receiving NAT through detailed molecular characterization, spatial analysis, and examining the relative abundance of various immune cell populations. Associations identified in this study should be further explored in larger cohorts, particularly in patients receiving combination immunotherapy and chemotherapy in the neoadjuvant setting, to facilitate greater individualization of therapeutic options for patients with TNBC.
Supplementary Material
Statement of Translational Relevance.
The presence of tumor-infiltrating lymphocytes (TILs) in the tumor-immune microenvironment (TIME) is associated with response to neoadjuvant therapy (NAT) in triple-negative breast cancer (TNBC). This suggests a critical role for the immune system in modulating that response. Therefore, deep characterization of the immune cell response in TNBC represents an approach that may improve NAT response-prediction. In this study, we use T-cell receptor sequencing and multiplex immunofluorescence to comprehensively examine the TIME in prospectively collected pretreatment tumor tissue obtained from patients with stage I-III TNBC enrolled in ARTEMIS (NCT02276443). Our data demonstrate that deep immune profiling through detailed phenotypic characterization and spatial analysis of the TIME can improve response-prediction in patients receiving NAT for TNBC when considered together with traditional clinical predictors of response.
ACKNOWLEDGMENTS
This work was funded by a 2020 Conquer Cancer Career Development Award, supported by Fleur Fairman (2020CDABC-5423266503, to C. Yam), the 2018 Gianni Bonadonna Breast Cancer Research Fellowship (Conquer Cancer Foundation, 12266 to C. Yam), the Winterhoff fund (to S.L. Moulder), the Pink Ribbons Project (to E.A. Mittendorf), the Nancy Owens Memorial Foundation (to E.A. Mittendorf) and generous philanthropic contributions to the Moon Shots Program, The University of Texas MD Anderson Cancer Center. Pre-sequencing processing work was completed by the Cancer Genomics Laboratory Moon Shots Platform and the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy at The University of Texas MD Anderson Cancer Center. Sequencing, data generation, and data analysis (including use of the Biostatistics Resource Group) was supported in part by the NIH/NCI Cancer Center Support Grant (award number P30 CA016672). Funding and drug support were provided (in part) by Amgen Inc.; Astellas Pharma Global Development, Inc.; Genentech, USA Inc.; Novartis AG; Pfizer Inc. C. Yam was additionally supported by the Allison and Brian Grove Endowed Fellowship for Breast Medical Oncology and the Susan Papizan Dolan Fellowship in Breast Oncology. J.T. Chang was supported by the Cancer Prevention Research Institute of Texas (RP170668, RP160710) and the National Institutes of Health’s National Cancer Institute (U54CA209978). E.A. Mittendorf is also supported by the Rob and Karen Hale Distinguished Chair in Surgical Oncology and the Ludwig Center at Harvard. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology, Conquer Cancer, Fleur Fairman, or other listed sponsors.
The authors thank Kaitlyn T. Bifolck, BA, for editorial support.
Conflicts of interest: J.L.G. is a consultant for Glaxo-Smith Kline (GSK), Array BioPharma, Codagenix, Verseau and Kymera and received sponsored research support from GSK, Eli Lilly and Array BioPharma. J.K.L. has received a grant or research support from Novartis, Medivation/Pfizer, Genentech, GSK, EMD-Serono, Astra-Zeneca, Medimmune, Zenith, Jounce; is on the data and safety monitoring board for Paxman Scalp Cooler; has participated in Speaker’s Bureau for MedLearning, Physician’s Education Resource, Prime Oncology, Medscape, Clinical Care Options; has received honoraria from UpToDate; has served on advisory committees or review panels for Astra-Zeneca, Ayala, Pfizer (all uncompensated), NCCN, ASCO, NIH, PDQ, SITC Breast Committee, SWOG Breast Committee. E.A.M. serves on scientific advisory boards for Merck, Genomic Health, and Roche/Genentech and has a sponsored research agreement with GSK. All other authors have no relevant conflict of interest disclosures.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author (E.A.M.). The data are not publicly available due to IRB restrictions of data containing information that could compromise research participant privacy and/or consent.
REFERENCES
- 1.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer 2009;9 Suppl 2:S73–81 doi 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol 2017;35(10):1049–60 doi 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010;124(1):133–40 doi 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 4.Schmid P, Salgado R, Park YH, Munoz-Couselo E, Kim SB, Sohn J, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol 2020;31(5):569–81 doi 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28(1):105–13 doi 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 6.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32(27):2959–66 doi 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33(9):983–91 doi 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 2012;43(10):1688–94 doi 10.1016/j.humpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 2012;132(3):793–805 doi 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 2013;16(1):32–9 doi 10.4048/jbc.2013.16.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382(9):810–21 doi 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 12.Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol 2020. doi 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396(10257):1090–100 doi 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 14.Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 2019;25(1):89–94 doi 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31(31):3997–4013 doi 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 16.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19(5):403–10 doi 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 17.AJCC cancer staging manual. Amin MB, Edge SB, editors. Switzerland: Springer; 2017. [Google Scholar]
- 18.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26(2):259–71 doi 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinder SE, Wencyk P, Sibbering DM, Bell JA, Elston CW, Nicholson R, et al. Assessment of the new proliferation marker MIB1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer 1995;71(1):146–9 doi 10.1038/bjc.1995.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V, Yu J, Phan V, Tudor IC, Peterson A, Uppal H. Androgen Receptor Immunohistochemistry as a Companion Diagnostic Approach to Predict Clinical Response to Enzalutamide in Triple-Negative Breast Cancer. JCO Precision Oncology 2017(1):1–19 doi 10.1200/PO.17.00075. [DOI] [PubMed] [Google Scholar]
- 21.Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 2018;6(1):48 doi 10.1186/s40425-018-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, et al. Evolution of Metastases in Space and Time under Immune Selection. Cell 2018;175(3):751–65 e16 doi 10.1016/j.cell.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung MW, Parsons JM, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun 2013;4:2680 doi 10.1038/ncomms3680. [DOI] [PubMed] [Google Scholar]
- 24.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 2009;114(19):4099–107 doi 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21 doi 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014;30(7):923–30 doi 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 2010;26(4):493–500 doi 10.1093/bioinformatics/btp692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1(6):417–25 doi 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013;14:7 doi 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Chang JT. Planning bioinformatics workflows using an expert system. Bioinformatics 2017;33(8):1210–5 doi 10.1093/bioinformatics/btw817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25(28):4414–22 doi 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 33.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011;1(1):54–67 doi 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuben A, Gittelman R, Gao J, Zhang J, Yusko EC, Wu CJ, et al. TCR Repertoire Intratumor Heterogeneity in Localized Lung Adenocarcinomas: An Association with Predicted Neoantigen Heterogeneity and Postsurgical Recurrence. Cancer Discov 2017;7(10):1088–97 doi 10.1158/2159-8290.CD-17-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov 2015;5(9):915–9 doi 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Q, Che J, Jiang X, Li L, Luo X, Li Q. PD-L1 Acts as a Promising Immune Marker to Predict the Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. Clin Breast Cancer 2020;20(1):e99–e111 doi 10.1016/j.clbc.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest 2008;26(10):1024–31 doi 10.1080/07357900802098165. [DOI] [PubMed] [Google Scholar]
- 38.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 2013;109(10):2705–13 doi 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson OC, Kim H, Quail DF, Foley EA, Joyce JA. Tumor-Associated Macrophages Suppress the Cytotoxic Activity of Antimitotic Agents. Cell Rep 2017;19(1):101–13 doi 10.1016/j.celrep.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res 2006;66(8):4349–56 doi 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 41.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017;543(7645):428–32 doi 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6(1):38 doi 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Holstein Y, Kapiteijn E, Bastiaannet E, van den Bos F, Portielje J, de Glas NA. Efficacy and Adverse Events of Immunotherapy with Checkpoint Inhibitors in Older Patients with Cancer. Drugs Aging 2019;36(10):927–38 doi 10.1007/s40266-019-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naing A, Hajjar J, Gulley JL, Atkins MB, Ciliberto G, Meric-Bernstam F, et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer 2020;8(2) doi 10.1136/jitc-2020-001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutcheon AW, Heys SD, Sarkar TK, Aberdeen Breast G. Neoadjuvant docetaxel in locally advanced breast cancer. Breast Cancer Res Treat 2003;79 Suppl 1:S19–24 doi 10.1023/a:1024333725148. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer 2012;107(5):864–73 doi 10.1038/bjc.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 2005;14(2):425–31. [PubMed] [Google Scholar]
- 48.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996;56(20):4625–9. [PubMed] [Google Scholar]
- 49.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat 2011;128(3):703–11 doi 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 2012;109(8):2796–801 doi 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med 2018;24(5):472–89 doi 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.