Abstract
During the last years we have witnessed progressive evolution of preparation of acenes with length up to dodecacene by on-surface synthesis in ultra-high vacuum or generation of acenes up to decacene in solid matrices at low temperatures. While these protocols with very specific conditions produce the acenes in amount of few molecules, the strategies leading to the acenes in large quantities dawdle behind. Only recently and after 70 years of synthetic attempts, heptacene has been prepared in bulk phase. However, the preparative scale synthesis of higher homologues still remains a formidable challenge. Here we report the preparation and characterisation of nonacene and show its excellent thermal and in-time stability.
Subject terms: Synthetic chemistry methodology, Reactive precursors
Acenes, or linearly fused benzene rings, have both fundamental scientific interest and potential for electronic and material utility, but synthesis of acenes with more than six rings are difficult due to dimerization and degradation. Here the authors prepare nonacene and demonstrate that it is stable in inert conditions.
Introduction
Over the last decade, particular attention has been brought to long unsubstituted acenes (longer than pentacene) from both experimental and theoretical points of view1. The nature of their electronic structure, such as gap stabilization2–4 and open-shell singlet ground state for longer acenes is still actively discussed5–9. Furthermore, longer acenes can be seen as the narrowest zig-zag graphene nanoribbons (ZGNR) and could display spin-polarized edge-states of interest for carbon-based spin electronics10,11.
However, acenes longer than pentacene are challenging to prepare and to handle. First, intermolecular π−π stacking between these planar and rigid molecules rapidly limits their solubility as their sizes increase. Second, acenes possess only one Clar aromatic sextet spread over the whole skeleton, leading to a decrease of the HOMO-LUMO gap with an increasing number of benzene rings, and therefore to an increase of the chemical reactivity. Although photooxidation with molecular oxygen can be avoided by working under argon, rapid dimerization in solution even at low concentration might become a problematic limitation.
Several strategies have been pursued to prepare long acenes, all relying on the same concept masked stable and soluble precursors are prepared and purified by standard in-solution chemistry techniques and, in a final step, the masking groups are removed in the solid-state or at low temperature in very dilute conditions on surfaces or in matrices12. In particular Neckers, Bettinger and coworkers have explored the photogeneration, in stabilizing matrices, of hexacene13, heptacene14–17, octacene, nonacene18, and undecacene19 by photodecarbonylation of precursors comprising two bridging α-diketone groups, following Yamada’s concept20 using the Strating-Zwanenburg21 reaction. Long acenes can also be prepared by the on-surface synthesis in ultra-high vacuum (UHV) and observed at liquid helium temperature and their electronic structures mapped by scanning tunneling spectroscopy (STS). Higher acenes up to undecacene have been obtained by deoxygenation of epoxides4,22,23, dehydrogenation of partially saturated precursors24,25, or thermal or photo decarbonylation of diketone adducts26–28.
However, both types of generation, in stabilizing matrices or on surfaces in UHV only give minute amounts of materials and cannot be used for macroscopic amounts of materials needed for applications. Indeed, the preparation of acenes longer than pentacene in a pure state is very recent despite 70 years of claims29, and so far limited to hexacene and heptacene. In 2012, Chow and coworkers isolated hexacene by decarbonylation of a monoketone precursor in the solid-state and its structure was determined by X-ray diffraction30. They demonstrated that a field-effect transistor made with a single crystal of hexacene showed a hole mobility significantly higher than pentacene. In 2017, Bettinger et al. have reported the formation of heptacene in the solid-state by thermal cycloreversion from a mixture of diheptacenes obtained in solution16. More recently, we have also obtained heptacene and benzohexacene by cheletropic decarbonylation at moderate temperature, confirming the thermal stability of these higher acenes31. And lately, Miyazaki et al. prepared stable thin films of heptacene32. The preparation of even longer acenes in bulk form has been indeed an attracting challenge since Clar’s prediction in 1964 claiming that the synthesis of octacene (and beyond) was a remote target33. And in a recent review, C. Tönshoff and H. F. Bettinger conclude that “It is not even clear if acenes larger than that of heptacene can exist outside the special environment provided by matrix isolation or on-surface synthesis”34.
To answer this question, we present here the synthesis of nonacene 1 (Fig. 1) and demonstrate its surprising stability.
Fig. 1. Molecular structure of nonacene 1.
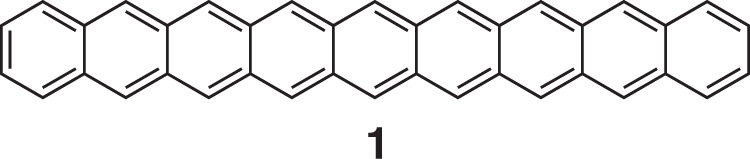
This topological representation shows one of the nine Clar’s structures with one single aromatic sextet.
Results and discussion
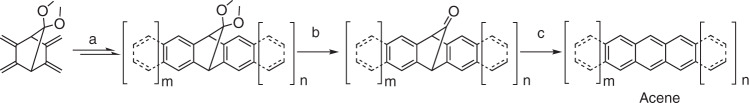
As nonacene was expected to be highly insoluble and reactive, we followed an alternative route: our strategy is based on pure soluble and chemically stable masked nonacene, that could be deprotected quantitatively by heating at medium temperatures in the solid-state. In previous contributions, we have shown31,35,36 that 7,7‐dimethoxy‐2,3,5,6‐tetramethylenebicyclo[2.2.1]-heptane (further in the text as tetraene) (Fig. 2), which can be prepared at the tens of grams scale, can undergo successive Diels-Alder reaction with arynes to provide non-planar, soluble, and not fully delocalized acene precursors. In these compounds, one of the benzene rings is bridged by a dimethyl ketal group.
Fig. 2. Schematic strategy of the preparation of various acenes starting from 7,7‐dimethoxy‐2,3,5,6‐tetramethylenebicyclo[2.2.1]-heptane.
Diels-Alder addition (a) with arynes, followed by aromatization gives a non-planar bridging dimethylketal, which can be deprotected (b) to yield a polyaromatic precursor bridged by a carbonyl group. (c) Solid-state thermal or photochemical decarbonylation gives the acene with only carbon monoxide as by-product.
Cleavage of this ketal yields the corresponding polyaromatic acene precursors comprising norbornadiene-7-one moieties, mentioned simply as ketone precursors in this report. These compounds are at least partially soluble, chemically stable and can be purified by standard in-solution techniques, such as chromatography or recrystallization. They are stable enough to be stored in the dark for long period of time. The final step is a thermal or photochemical cheletropic decarbonylation in the solid-state yielding the corresponding acenes in quantitative yields without any non-volatile by-products. It has been shown that this method of decarbonylation was very effective to prepare sensitive high-quality materials, such as pentacene and hexacene37, for optoelectronic devices.
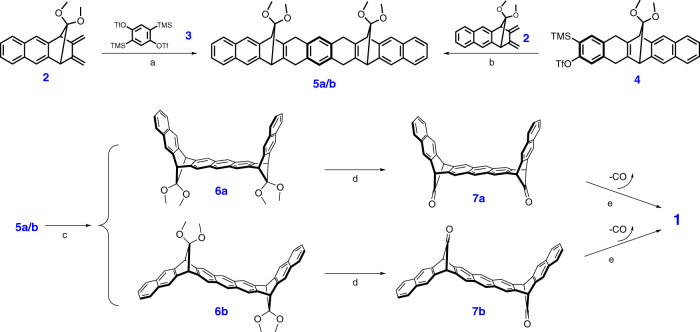
Our exploration of nonacene 1 commenced with the synthesis of such a carbonylated precursor 7a/7b which can be easily transformed in the solid-state form to the nonacene by simple heating.
The synthesis of the precursors 7a/7b leading to nonacene 1 follows the route shown in Fig. 3 with four synthetic steps starting from the diene 2, which can be prepared in two steps from the tetraene shown in Fig. 2 above35.
Fig. 3. Synthesis of nonacene 1.
a CsF, acetonitrile/THF (4:1), room temperature, 16 h., 62% (anti : syn 1:2); b CsF, acetonitrile/THF (5:1), room temperature, 16 h., 98% (anti : syn 1:2); c DDQ, toluene, room temperature, 4 h. 94%; d TMSI, room temperature, 24 h., 94% for 7a and 48 h., 95% for 7b; e neat 350 °C, 20 min., quant.
The key reaction of the synthetic sequence is the double Diels-Alder reaction of the diene 2 and the in situ generated bis(aryne) obtained by fluoride-induced decomposition of 2,5-bis(trimethylsilyl)-1,4-phenylene bis(trifluoromethanesulfonate) 338–40. Alternatively, the Diels-Alder reaction can be carried out from the aryne precursor 4 (synthesized from the same tetraene in three steps in 33% yield), with the diene 2. The formed mixture of these two isomers syn and anti 5a/5b can be easily separated on silica gel. As expected the NMR spectra of the two isomers are almost identical and owing to their symmetry and to the distance between the ketal groups, it is not possible to assign their structures by proton and carbon NMR spectroscopy. Fortunately, slow evaporation of a solution of the isomer 5b in a mixture of solvents (hexane/EtOAc) provided suitable crystals for X-ray analysis (Fig. 4).
Fig. 4. Ortep representation of isomer 5b.
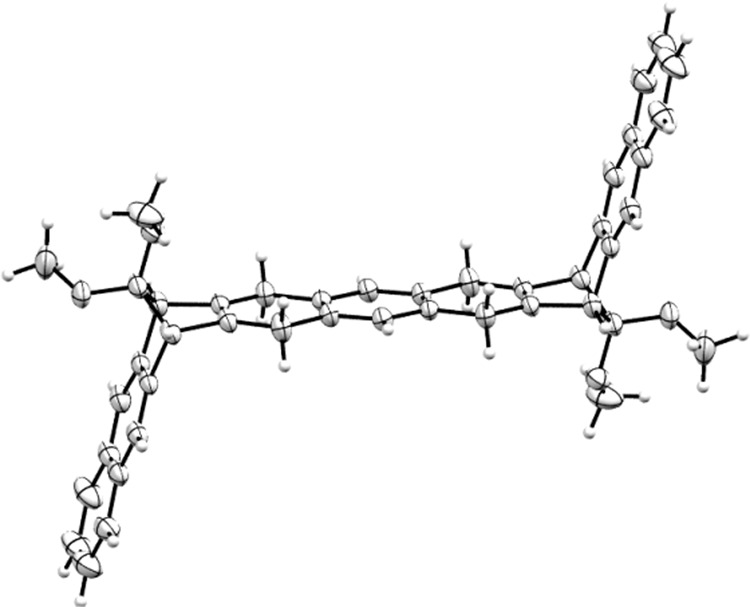
Thermal ellipsoids represent 50% probability level.
It shows that the two naphthalene ends of the anti-isomer 5b are perfectly parallel to each other and the angle between naphthalene and anthracene units is 105.5°.
Surprisingly, these products 5a and 5b were formed in the ratio 1:2 in favor of syn-isomer 5a. Indeed, considering the distance between the reactive sites, a statistical ratio 1:1 was initially expected. In order to investigate the stereoselectivity of the Diels-Alder reactions between the diene 2 and the benzyne compounds in acetonitrile, their transition states (TS) were determined by using density functional theory (DFT)41. By using Gaussian 16, Revision C.0141, the geometries of TS were optimized with QST3 method at the B3LYP/6-31 + G(d,p) level of theory with the polarizable continuum model (PCM) to include solvent effects (acetonitrile). Subsequently, single-point energy calculations of TS were calculated at the M06-2X/6-31 + G(d,p) level of theory with PCM (acetonitrile)42. Based on the activation energy determined by the calculations, anti/syn ratio was estimated to 1/1.64, which is consistent with the observed stereoselectivity in the experiment (anti/syn = 1/2) (See supporting information for details).
The isomers 5a and 5b underwent smooth aromatization by DDQ at room temperature in almost quantitative yield. Then, the two dimethylketal groups of 6a/6b were cleaved by trimethylsilyl iodide, which afforded the corresponding carbonyl isomers 7a and 7b in 94 and 95% yield, respectively. The anti-isomer 7b is less soluble in many organic solvents than its counterpart 7a, likely due to the ability to pack in quasi-one-dimensional chains with efficient π-π stacking, which is not the case for U-shape isomer 7a. Both isomers are colourless chemically stable compounds.
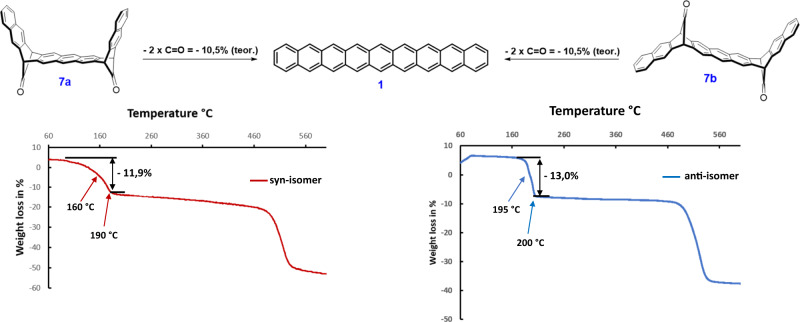
Decarbonylation of 7a/7b in the solid-state can be followed by thermal gravimetric analysis (TGA) as shown in Fig. 5.
Fig. 5. TGA thermograms of 7a/7b.
These curves show the weight loss of two CO groups (ca 12 and 13%; calcd. 10.5 %).
A weight loss of 11.9% for syn-isomer and 13.0% for antiisomer (calcd 10.5%) correspond to the loss of two carbonyl groups per molecule. The full decarbonylation occurred below 190 °C in a one-step process for both isomers. However, in the case of syn-isomer 7a, the TGA thermogram consists of a gradual weight loss starting at about 60 °C. This can be explained by a lower thermal stability compared to the anti-isomer (starting around 180 °C). The decarbonylation is accompanied by a colour change from white to anthracite and the formation of nonacene. Under these conditions, nonacene is surprisingly thermally stable up to almost 500 °C. The formation of nonacene 1 by loss of two carbonyl groups is also evident during the high-resolution DCI MS measurement of 7a/7b in which only a peak at m/z 478.1731 from 7a or 478.1727 from 7b corresponding to the formula C38H22 (calcd m/z: 478.1722) was recorded.
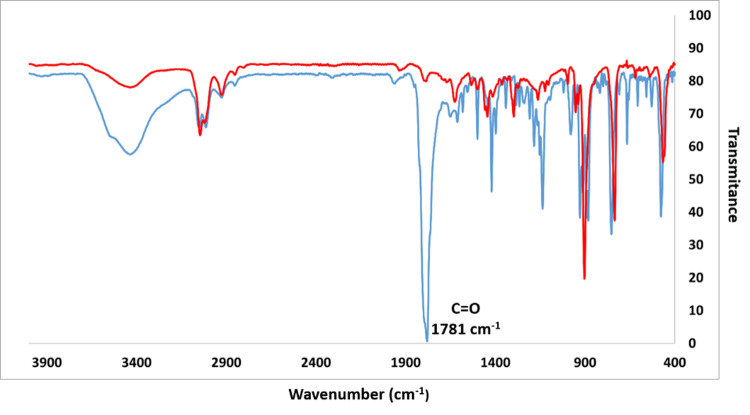
The decarbonylation process was also followed by FTIR experiments (Fig. 6), where the stretching vibration of carbonyl peak at 1780 cm−1 disappear after heating the carbonyl precursor in KBr pellet. This spectrum of nonacene is very similar to that of pentacene, hexacene, and heptacene with bands at 3042, 1298, 906, 735, and 463 cm−1 and in agreement with calculated spectra (see Supplementary Fig. 4).
Fig. 6. FTIR spectra (KBr pellets) of a carbonylated precursor and of nonacene 1.
The spectrum of the precursor 7b is in blue, with a strong CO peak at 1781 cm−1 and that one of the resulting nonacene 1 is in red, after 1 min. heating at 350 °C in glovebox.
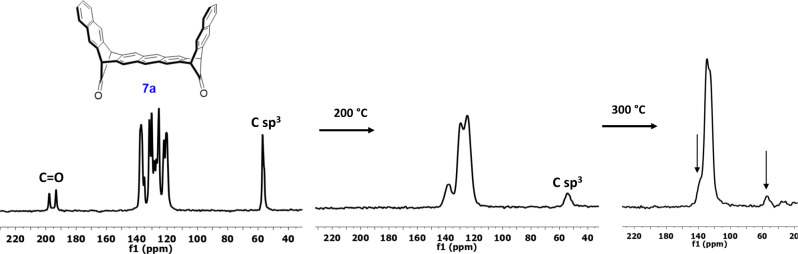
This transformation has been also followed by solid-state cross-polarization magic angle spinning (CP-MAS) NMR spectroscopy. The spectrum of the precursor 7a shows (Fig. 7) three groups of signals, one at 57 ppm (bridgehead sp3 carbons), a complex peak at 120–137 ppm (aromatic carbons) and the carbonyl carbons at 193 and 198 ppm. Despite the symmetry of the molecule, the different environments in the solid-state of the two carbonyl groups is a cause of these two signals. After heating the sample for 20 min at 200 °C under inert atmosphere, the carbonyl signals disappear and the aromatic region get narrower (Fig. 7). However, a smaller and broader sp3 signal at 54 ppm remains. We attribute this peak to partial dimerization/polymerization by of the decarbonylated compound. Upon heating at higher temperatures, this peak decreases whereas the peak attributed to the aromatic carbons gets narrower with a decrease of the shoulder at 137 ppm. The evolution of the CPMAS spectrum of the isomer 7b is very similar (See Supplementary Fig. 3).
Fig. 7. Thermal decarbonylation of 7a.
Evolution of the CPMAS 13C NMR spectra of 7a (left) in function of the temperature.
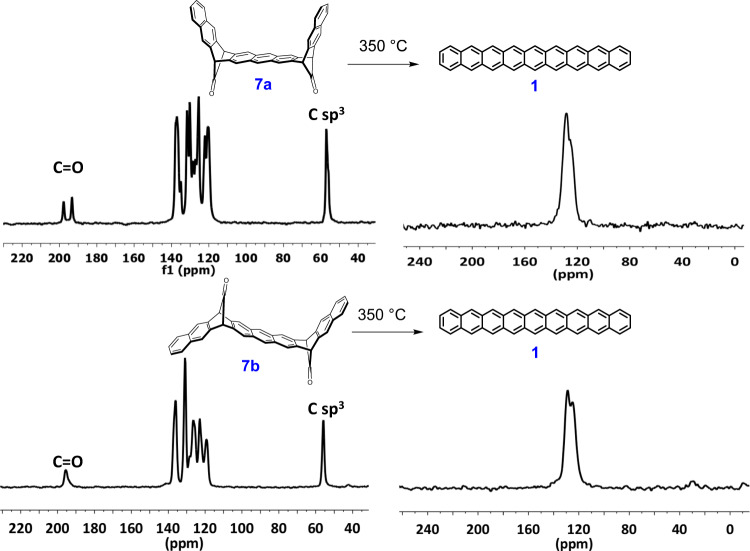
Based on the TGA experiment, the formed nonacene is surprisingly stable almost up to 500 °C, which allows to realize the extrusion of carbonyl groups at much higher temperature. In a new experiment, we carried out the decarbonylation of the precursors 7a/7b at 350 °C for 20 min (Fig. 8). Gratifyingly, the decarbonylation process was much cleaner with only a sharp doublet in the aromatic region without any signs of dimerization.
Fig. 8. Thermal decarbonylation of the precursors.
Evolution of the CPMAS spectra of 7a/7b by decarbonylation at 350 °C for 20 min to form the nonacene 1.
This behaviour is somehow reminiscent to that of heptacene as described by Bettinger et al.16. The reduction of 7,16-heptacenequinone in cyclooctanol produces a mixture of two diheptacene molecules. The diheptacenes undergo thermal cleavage to heptacene by retro [4 + 4] cyclization at high temperatures in the solid state, but some of the heptacene reacts back to the dimers. In this case, passing from diheptacene to heptacene only necessitates limited movements of the aromatic rings which are face to face in the crystal. In the case of nonacene, the planarization requires important changes in the geometries and it is likely that the orientations of the molecules are not in favour of a new [4 + 4] cyclization. This behaviour is very similar to that observed for the preparation of hexacenohexacene from its decarbonylated syn-isomer precursor36. In order to verify if we could detect a dimer, we have synthesized in solution dimers of heptacene by thermal decarbonylation of the heptacene carbonyl precursor (see Supplementary Methods). But these dimers are, as expected, not very stable and are cleaved during MS MALDI experiments using low energy lasers (see Supplementary Fig. 2). This experiment cannot be done in the case of nonacene which is too insoluble and precipitates before dimerizing (vide infra).
Keeping these samples in the NMR rotor at room temperature in a glove box for 2 months did not lead to any degradation or dimerization of nonacene 1 suggesting that the nonacene prepared under these conditions is stable (see Supplementary Fig. 3). This important result is comforted by the observation that bulk heptacene31 or even thin films of heptacene32 are also stable for months at RT when stored in a nitrogen-filled glove-box.
Alternatively, nonacene 1 can also be obtained by decarbonylation in a solvent at high temperature. For instance, the soluble precursor 7a was dissolved in chlorobenzene and the solution was thoroughly degassed. This solution was heated at 200 °C for 10 min and the formation of a dark violet precipitate was observed. This suspension was drop-casted on an indium-tin-oxide (ITO) glass slide without any matrix and dried. In a parallel experiment, the suspension was mixed with 2,5-dihydroxybenzoic acid as a matrix and then drop-casted on an ITO glass slide and dried. These ITO glass slides were introduced in a MALDI chamber, and the high-resolution mass spectra (HRMS) were recorded. Both spectra showed only the parent peak corresponding to the nonacene 1, and no trace of a dimer as was observed for heptacene16. These results suggest that, in contrast with heptacene, nonacene 1 is so highly insoluble that just after decarbonylation, immediate precipitation of the monomer prevents the dimerization in solution. In contrast, attempts to decarbonylate the precursors 7, thermally in drop-casted thin films or photochemically in dichloromethane solutions at low temperature (7 K) have been unsuccessful, showing a rapid decomposition of the compounds in these conditions.
In summary, pure nonacene can be prepared by thermal bis-decarbonylation of precursors either in the solid-state, or in high boiling point solvents. This long acene is surprisingly thermally stable up to 450 °C and does not decompose for months at room temperature under dry argon. Our preparation procedure could as well be applied for the construction of substituted nonacenes and to even longer acenes, opening the way to OFETs and molecular spintronics applications.
Methods
Direct synthesis of 6a/6b
A well-dried Schlenk flask was charged with diene 2 (200 mg, 0.72 mmol) and CsF (480 mg, 3.16 mmol, 4.0 equiv.) under argon and then anhydrous acetonitrile (16 mL) was added. The heterogenous mixture was cooled to 0 °C and then solution of aryne precursor bis(trimethylsilyl)-1,4-phenylene bis(trifluoromethanesulfonate) (298 mg, 0.58 mmol, 0.8 equiv.) in anhydrous THF (4 mL) was added dropwise. The reaction was allowed to warm to room temperature overnight. Progress of the reaction was controlled by TLC, eluent (hexane - EtOAc 3:2). Reaction time depending on a scale of the reaction (1–3 days). After the evaporation of the solvent, the residue was chromatographed on silica gel (hexane: acetone 3:1) to get the desired product as a mixture of two isomers as a colourless solid. This mixture (146 mg, 0.23 mmol) was dissolved in anhydrous toluene (15 mL) under argon. The solution was cooled to 0 °C and then DDQ (53 mg, 0.231 mmol, 2 equiv.) was added in one portion. The reaction mixture was stirred at 0 °C for 10 min. and then 6 h at room temperature. The volume of the reaction mixture was reduced to a half and the mixture was filtered over a frit S4. The solid was washed with toluene and finally with methanol to get the first isomer 6b (40 mg) as a white solid. The mother liquor was evaporated and the residue was purified by chromatography on silica gel (hexane: acetone 3:1) to get the second isomer 6a (82 mg) as a white solid. The ratio of isomers is 1:2 and combined yield is 54% after two synthetic steps.
6a
1H NMR (500 MHz, CD2Cl2): 3.23 (6H, s), 3.26 (6H, s), 4.71 (4H, s), 7.33–7.36 (4H, m), 7.68 – 7.71 (4H, m), 7.70 (4H, s), 7.77 (4H, s), 8.12 (2H, s) ppm.
13C NMR (126 MHz, CD2Cl2): 51.54, 51.58, 55.20, 120.43, 120.87, 124.77, 125.96, 126.04, 128.14, 131.39, 133.08, 142.94, 143.61 ppm.
DCI MS: 627 ([M + H] + ).
HR DCI MS: calcd for C44H35O4 627.2530; found 627.2511.
6b
1H NMR (300 MHz, CD2Cl2): 3.21 (6H, s), 3.22 (6H, s), 4.71 (4H, s), 7.37–7.40 (4H, m), 7.72–7.77 (4H, m), 7.73 (4H, s), 7.77 (4H, s), 8.12 (2H, s) ppm.
13C NMR (126 MHz, CD2Cl2): not measured due to the low solubility
DCI MS: 627 ([M + H]+).
HR DCI MS: calcd for C44H35O4 627.2530; found 627.2523.
Synthesis of 7a
In a well-dried Schlenk flask, 6a (160 mg, 0.255 mmol) was dissolved in anhydrous dichloromethane (10 mL) under argon. Then trimethylsilyl iodide (109 μL, 0.766 mmol, 3 equiv.) was added dropwise and the homogeneous reaction mixture was stirred overnight at room temperature. The next day the heterogeneous reaction mixture was stirred on-air at room temperature for 6 h to complete the hydrolysis of formed iodo-methoxy intermediate. The product was collected by filtration over a glass frit filter S4, washed with mixture of solvents (hexane: dichloromethane, 4:1) to afford the first portion of pure compound 7a as a white solid. The mother liquor was evaporated and the residue was purified by chromatography on silica gel (hexane: acetone 3:1) to get the second portion of the product as a white solid. The combined yield was (130 mg, 95%).
1H NMR (500 MHz, CD2Cl2): 4.99 (4H, s), 7.41–7.44 (4H, m), 7.78–7.81 (4H, m), 7.93 (4H, s), 8.04 (4H, s), 8.36 (2H, s).
13C NMR (126 MHz, CD2Cl2): 57.55, 120.88, 121.22, 126.57, 126.76, 128.34, 131.61, 133.31, 137.61, 137.93, 194.46 ppm.
CP MAS: 56.99 (bridgehead), 120.20–137.12 (aromatic), 193.26 and 197.73 (C=O) ppm.
DCI MS: 478 ([M – 2 x CO]+).
HR DCI MS: calcd for C38H22 478.1722 (M – 2xCO); found 478.1727.
Synthesis of 7b
In a well-dried Schlenk flask, 6b (25 mg, 39.89 μmol) was suspended in anhydrous dichloromethane (4 mL) under argon. Then trimethylsilyl iodide (23 μL, 159.6 μmol, 4 equiv.) was added dropwise and the heterogenous reaction mixture was stirred overnight at room temperature. Next day the heterogeneous reaction mixture was stirred on-air at room temperature for 6 h to complete the hydrolysis of the formed iodo-methoxy intermediate. The product was collected by filtration over a glass frit filter S4, washed with mixture of solvents (dichloromethane: acetone, 4:1) to afford pure compound 7b (20 mg, 94%) as a white solid.
CP MAS: 55.83 (bridgehead), 119.23–136.01 (aromatic), 195.55 (C = O) ppm.
DCI MS: 478 ([M – 2 x CO]+).
HR DCI MS: calcd for C38H22 478.1722 (M – 2xCO); found 478.1731.
Preparation of Nonacene 1
Nonacene was obtained by heating 7a or 7b in the solid state at 350 °C under vacuum for 15 min or as a suspension by heating a purged solution of 7a in chlorobenzene 10 min. at 200 °C
CP MAS: 125.5–128.8 ppm
MALDI MS: calcd for C38H22 478.1722; found:478.182 (without matrix); 478.179 (with DHB as a matrix). See SI 30 and SI31.
Supplementary information
Acknowledgements
The authors thank Yannick Coppel (LCC-Toulouse) for recording CP-MAS spectra, David Neumeyer (CEMES) for recording TGA spectra, Christian Bourgerette (CEMES), and Isabelle Seguy (LAAS) for help in spin-coating experiments. We also thank Sébastien Joulié (CEMES) for TEM experiments and Sonia Mallet-Ladeira (LCC) for XRD of powders of precursors. We gratefully acknowledge Nathalie Saffon, from the Institut de Chimie de Toulouse, who solved the X-ray structure. Vladimír Vrkoslav (IOCB, Prague) is gratefully accredited for the MALDI experiments. Colin Martin (NAIST-CEMES) is thanked for comments and corrections of the manuscript. A.J. acknowledges funding from the Foundation EXPERIENTIA and from ERDF/ESF “UOCHB MSCA Mobility” (No. CZ.02.2.69/0.0/ 0.0/17 050/0008490). This research has received funding from the EraNET Cofund Initiatives QuantERA under the European Union’s Horizon 2020 research and innovation programme grant agreement ORQUID. This project has received financial support from the CNRS through the MITI interdisciplinary programs and JST-ERATO (No. JPMJER1903) and JSPS-WPI.
Author contributions
A.J. and J.H. performed the syntheses and characterization of all products. M.S realized all the MALDI experiments. Y.N. did the simulations and calculations. A.J. and A.G. designed and supervised the project.
Peer review information
Nature Communications thanks Uwe Bunz, Tahsin Chow and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that all the important data to support the findings in this paper are available within the main text or in the supplementary information. CCDC-2071402 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Extra data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrej Jančařík, Email: andrej.jancarik@u-bordeaux.fr.
Andre Gourdon, Email: andre.gourdon@cemes.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-27809-0.
References
- 1.Dorel R, Echavarren AM. Strategies for the Synthesis of Higher Acenes. Eur. J. Org. Chem. 2016;2017:14–24. doi: 10.1002/ejoc.201601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korytár R, Xenioti D, Schmitteckert P, Alouani M, Evers F. Signature of the Dirac cone in the properties of linear oligoacenes. Nat. Commun. 2014;5:5000. doi: 10.1038/ncomms6000. [DOI] [PubMed] [Google Scholar]
- 3.Schmitteckert P, Thomale R, Korytár R, Evers F. Incommensurate quantum-size oscillations in acene-based molecular wires—Effects of quantum fluctuations. J. Chem. Phys. 2017;146:092320. doi: 10.1063/1.4975319. [DOI] [Google Scholar]
- 4.Krüger J, et al. Electronic Resonances and Gap Stabilization of Higher Acenes on a Gold Surface. ACS Nano. 2018;12:8506–8511. doi: 10.1021/acsnano.8b04046. [DOI] [PubMed] [Google Scholar]
- 5.Plasser F, et al. The Multiradical Character of One- and Two-Dimensional Graphene Nanoribbons. Angew. Chem. Int. Ed. 2013;52:2581–2584. doi: 10.1002/anie.201207671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachmann J, Dorando JJ, Avilés M, Chan GK-L. The radical character of the acenes: A density matrix renormalization group study. J. Chem. Phys. 2007;127:134309. doi: 10.1063/1.2768362. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Dai S. Electronic Ground State of Higher Acenes. J. Phys. Chem. A. 2008;112:332–335. doi: 10.1021/jp0765087. [DOI] [PubMed] [Google Scholar]
- 8.Malrieu J-P, Trinquier G. Can a Topological Approach Predict Spin-Symmetry Breaking in Conjugated Hydrocarbons? J. Phys. Chem. A. 2016;120:9564–9578. doi: 10.1021/acs.jpca.6b07597. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Davidson ER, Yang W. Nature of ground and electronic excited states of higher acenes. Proc. Natl Acad. Sci. USA. 2016;113:E5098. doi: 10.1073/pnas.1606021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita M, Wakabayashi K, Nakada K, Kusakabe K. Peculiar Localized State at Zigzag Graphite Edge. J. Phys. Soc. Jpn. 1996;65:1920–1923. doi: 10.1143/JPSJ.65.1920. [DOI] [Google Scholar]
- 11.Ruffieux P, et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature. 2016;531:489–492. doi: 10.1038/nature17151. [DOI] [PubMed] [Google Scholar]
- 12.Herwig PT, Müllen K. A Soluble Pentacene Precursor: Synthesis, Solid-State Conversion into Pentacene and Application in a Field-Effect Transistor. Adv. Mater. 1999;11:480–483. doi: 10.1002/(SICI)1521-4095(199904)11:6<480::AID-ADMA480>3.0.CO;2-U. [DOI] [Google Scholar]
- 13.Mondal R, Adhikari RM, Shah BK, Neckers DC. Revisiting the Stability of Hexacenes. Org. Lett. 2007;9:2505–2508. doi: 10.1021/ol0709376. [DOI] [PubMed] [Google Scholar]
- 14.Mondal R, Shah BK, Neckers DC. Photogeneration of Heptacene in a Polymer Matrix. J. Am. Chem. Soc. 2006;128:9612–9613. doi: 10.1021/ja063823i. [DOI] [PubMed] [Google Scholar]
- 15.Bettinger, H. F., Mondal, R. & Neckers, D. C. Stable photoinduced charge separation in heptacene. Chem. Commun. 48, 5209–5211 (2007). [DOI] [PubMed]
- 16.Einholz R, et al. Heptacene: Characterization in Solution, in the Solid State, and in Films. J. Am. Chem. Soc. 2017;139:4435–4442. doi: 10.1021/jacs.6b13212. [DOI] [PubMed] [Google Scholar]
- 17.Mondal R, Tönshoff C, Khon D, Neckers DC, Bettinger HF. Synthesis, Stability, and Photochemistry of Pentacene, Hexacene, and Heptacene: A Matrix Isolation Study. J. Am. Chem. Soc. 2009;131:14281–14289. doi: 10.1021/ja901841c. [DOI] [PubMed] [Google Scholar]
- 18.Tönshoff C, Bettinger HF. Photogeneration of Octacene and Nonacene. Angew. Chem. Int. Ed. 2010;49:4125–4128. doi: 10.1002/anie.200906355. [DOI] [PubMed] [Google Scholar]
- 19.Shen B, Tatchen J, Sanchez-Garcia E, Bettinger HF. Evolution of the Optical Gap in the Acene Series: Undecacene. Angew. Chem. Int. Ed. 2018;57:10506–10509. doi: 10.1002/anie.201802197. [DOI] [PubMed] [Google Scholar]
- 20.Yamada H, et al. Photochemical Synthesis of Pentacene and its Derivatives. Chem. - A Eur. J. 2005;11:6212–6220. doi: 10.1002/chem.200500564. [DOI] [PubMed] [Google Scholar]
- 21.Strating J, Zwanenburg B, Wagenaar A, Udding AC. Evidence for the expulsion of bis-CO from bridged α-diketones. Tetrahedron Lett. 1969;10:125–128. doi: 10.1016/S0040-4039(01)87489-X. [DOI] [Google Scholar]
- 22.Krüger J, et al. Imaging the electronic structure of on-surface generated hexacene. Chem. Commun. 2017;53:1583–1586. doi: 10.1039/C6CC09327B. [DOI] [PubMed] [Google Scholar]
- 23.Krüger J, et al. Decacene: On-Surface Generation. Angew. Chem. Int. Ed. 2017;56:11945–11948. doi: 10.1002/anie.201706156. [DOI] [PubMed] [Google Scholar]
- 24.Zuzak R, et al. Nonacene Generated by On-Surface Dehydrogenation. ACS Nano. 2017;11:9321–9329. doi: 10.1021/acsnano.7b04728. [DOI] [PubMed] [Google Scholar]
- 25.Zuzak R, et al. Higher Acenes by On-Surface Dehydrogenation: From Heptacene to Undecacene. Angew. Chem. Int. Ed. 2018;57:10500–10505. doi: 10.1002/anie.201802040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urgel JI, et al. On-Surface Synthesis of Heptacene Organometallic Complexes. J. Am. Chem. Soc. 2017;139:11658–11661. doi: 10.1021/jacs.7b05192. [DOI] [PubMed] [Google Scholar]
- 27.Zugermeier M, et al. On-surface synthesis of heptacene and its interaction with a metal surface. Nanoscale. 2017;9:12461–12469. doi: 10.1039/C7NR04157H. [DOI] [PubMed] [Google Scholar]
- 28.Urgel, J. I. et al. On-surface light-induced generation of higher acenes and elucidation of their open-shell character. Nat. Commun.10, 861 (2019). [DOI] [PMC free article] [PubMed]
- 29.Zade SS, Bendikov M. Heptacene and Beyond: The Longest Characterized Acenes. Angew. Chem. Int. Ed. 2010;49:4012–4015. doi: 10.1002/anie.200906002. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, et al. The synthesis, crystal structure and charge-transport properties of hexacene. Nat. Chem. 2012;4:574–578. doi: 10.1038/nchem.1381. [DOI] [PubMed] [Google Scholar]
- 31.Jancarik A, Levet G, Gourdon A. A Practical General Method for the Preparation of Long Acenes. Chem. - A Eur. J. 2019;25:2366–2374. doi: 10.1002/chem.201805975. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, et al. Heptacene: Synthesis and Its Hole‐Transfer Property in Stable Thin Films. Chem. Eur. J. 2021;27:10677–10684. doi: 10.1002/chem.202100936. [DOI] [PubMed] [Google Scholar]
- 33.Clar, E & Schoental, R. Polycyclic Hydrocarbons: Volume 1. (Springer, Heidelberg, 2013).
- 34.Tönshoff C, Bettinger HF. Pushing the Limits of Acene Chemistry: The Recent Surge of Large Acenes. Chem. Eur. J. 2021;27:3193–3212. doi: 10.1002/chem.202003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holec J, et al. A Large Starphene Comprising Pentacene Branches. Angew. Chem. Int. Ed. 2021;60:7752–7758. doi: 10.1002/anie.202016163. [DOI] [PubMed] [Google Scholar]
- 36.Jančařík, A. et al. Synthesis and Absorption Properties of Long Acenoacenes. Chem. Eur. J. Chem. 27, 202101577 (2021). [DOI] [PubMed]
- 37.Watanabe M, Chen K-Y, Chang YJ, Chow TJ. Acenes Generated from Precursors and Their Semiconducting Properties. Acc. Chem. Res. 2013;46:1606–1615. doi: 10.1021/ar400002y. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida S, Hosoya T. The Renaissance and Bright Future of Synthetic Aryne Chemistry. Chem. Lett. 2015;44:1450–1460. doi: 10.1246/cl.150839. [DOI] [Google Scholar]
- 39.Pena, D. Bottom-up Approaches to Nanographenes through Organic Synthesis. In Ideas in Chemistry and Molecular Sciences (ed. Pignataro, B.) 235–261 (Wiley-VCH Verlag GmbH & Co. KGaA, 2010).
- 40.Rodríguez-Lojo D, Peña D, Pérez D, Guitián E. Straightforward Synthesis of Novel Acene-Based Aryne Precursors. Synlett. 2015;26:1633–1637. doi: 10.1055/s-0034-1381005. [DOI] [Google Scholar]
- 41.Gaussian 16, Revision C.01, M. J. Frisch, et al, Gaussian, Inc., Wallingford CT, 2016.
- 42.Pieniazek SN, Clemente FR, Houk KN. Sources of Error in DFT Computations of C-C Bond Formation Thermochemistries: π→σ Transformations and Error Cancellation by DFT Methods. Angew. Chem. Int. Ed. 2008;47:7746–7749. doi: 10.1002/anie.200801843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the important data to support the findings in this paper are available within the main text or in the supplementary information. CCDC-2071402 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Extra data are available from the corresponding author upon request.