Abstract
The assays are used for the diagnosis of hydatid disease are still imperfect. The reported diagnostic sensitivity and specificity vary greatly depending on the panel of sera used, the laboratory conducting the assay, and, more critically, the antigen used. To contribute to its standardization, we have recently ranked the diagnostic performances of the major parasite antigens and the available synthetic peptides using a large collection of serum samples. That work showed that antigen B (AgB) possesses the highest diagnostic value among these antigens. In the present work we further dissected its antigenicity by analyzing the reactivity of the same panel of sera against a set of synthetic peptides spanning the sequence of both AgB subunits. The N-terminal extension of these subunits appeared to be immunodominant in human infections. A 38-mer peptide (p176) delineated from the N-terminal extension of the AgB/1 subunit performed in an enzyme-linked immunosorbent assay with a higher diagnostic sensitivity (80%) and specificity (94%) than native AgB, Ag5, or any other peptide antigen tested against this collection of serum samples. In view of its high diagnostic value and its nature as a well-defined reproducible antigen, p176 could conveniently be used as a reference standard antigen in the diagnosis of hydatid disease.
Cystic hydatidosis, caused by infection with larval Echinococcus granulosus, affects both humans and domestic animals and is recognized as one of the world's major zoonoses (15). Clinical diagnosis of the disease is based on symptomatology, epidemiological data, imaging techniques, and immunodiagnosis (1). However, clinical symptoms do not appear until the larval cyst structure of the parasite has reached a certain size, which normally requires years after the primary infection. During this time, immunodiagnosis has also proved effective and can conveniently be used to diagnose the disease in asymptomatic high-risk populations (2, 5). This is particularly important for early diagnosis, which is of great significance, because surgery and chemotherapy are poorly effective if they are applied to patients with advanced infections.
The need for a reliable immunodiagnostic test has prompted abundant research in the field; various tests have been used for detection of specific antibodies, but the key component of the different systems remains the antigen. From the beginning it was evident that the transition from crude preparations to purified fractions improved the sensitivity and specificity of the assays. In this process, two major antigens of hydatid cyst fluid, namely, antigen B (AgB) and Ag5, were identified (17). AgB is a 120-kDa oligomeric lipoprotein composed of multiples of two subunits of 8 kDa (9), namely, AgB8/1 (8) and AgB8/2 (7). Ag5 is a high-molecular-mass lipoprotein complex composed of 57- and 67-kDa components that under reducing conditions dissociate into 38- and 22- to 24-kDa subunits (13). Although both antigens proved to be diagnostically valuable, there are difficulties related to their lack of sensitivity and specificity and problems with the standardization of their use (3). In order to overcome these difficulties, efforts have been made to define discrete epitopes of these antigens that could be mimicked by synthetic peptides. The first application of such peptides was reported by Chamekh et al. (4), who used a 34-mer peptide (p89-122), which was suggested to be a major epitope of Ag5 but which was recently shown to be a fragment of a 29-kDa protoscolex component of E. granulosus (10). This was followed by the work of Leggatt and McManus (11), who explored the antigenicity of AgB with three peptides and found that p65, a 27-mer peptide corresponding to residues 12 to 39 of AgB8/1, had potential for use as a diagnostic reagent. In an effort to contribute to the standardization of the immunodiagnosis of hydatid disease, we recently compared the diagnostic value of p89-122, p65, native AgB and Ag5, and Gu4, a 34-mer synthetic peptide corresponding to the C-terminal end of the AgB8/2 subunit (3). This work stressed the relevance of an internal comparison of the different antigens in one laboratory in order to rank their diagnostic performance in a reliable manner and showed that (i) individually considered, native AgB has the highest diagnostic value among these antigens and (ii) the available AgB-derived peptides do not reproduce all the major epitopes of AgB.
In the present work we complete the characterization of the antigenicities of both subunits of AgB by introducing three additional synthetic peptides. The study allowed the identification of a highly antigenic region of AgB residing in the N-terminal extension of the AgB8/1 subunit. An enzyme-linked immunosorbent assay (ELISA) based on the use of a single peptide representing this region exhibited a diagnostic performance that was superior to that obtained by the use of native AgB, and the peptide constitutes a promising candidate for standardization of the serodiagnosis of human cystic echinococcosis.
MATERIALS AND METHODS
Human serum sample collection.
Sera from 90 patients with surgically confirmed hydatid disease were tested. The samples were not preselected on the basis of previous serologic information and were collected before surgery. Sixty-five of them had records of cyst location, which were as follows: liver (n = 40), lungs (n = 11), bones (n = 8), and multiple sites (n = 6). In order to evaluate the specificities of the various antigens, 86 serum samples from patients with the following diseases were included in the study: alveolar hydatid disease (n = 27), Taenia solium cysticercosis (n = 22), toxocariasis (n = 10), schistosomiasis (n = 6), rheumatoid arthritis (n = 5), Chagas' disease (n = 4), toxoplasmosis (n = 4), filariasis (n = 4), syphilis (n = 2), and cancer (n = 2). Negative controls comprised 28 serum samples from healthy donors. The sera were stored at −20°C until they were tested.
Antigens.
AgB was purified to homogeneity from hydatid cyst fluid as described by González et al. (9). The following AgB-derived peptides were used in this study: p65 (LKMFGEVKYFFERDPLGQKVVDLLKEL) (11); p176 (DDGLTSTSRSVMKMFGEVKYFFERDPLGQKVVDLLKEL), a 38-mer corresponding to the N-terminal extension of AgB8/1; p175 (KDEPLAHMGQVVLLRWGELRDFFRNDPLGQRLVALG), a 36-mer corresponding to the N-terminal extension of AgB8/2; and p177 (FFRNDPLGQRLVALGNDLTAICQKL), a 25-mer corresponding to the central region of the AgB8/2 sequence. These peptides were synthesized, purified by reverse-phase high-performance liquid chromatography, and analyzed by mass spectrometry at The Molecular Biology Unit, University of Newcastle Upon Tyne (Newcastle Upon Tyne, United Kingdom).
ELISA.
The ELISAs were performed essentially as described by Barbieri et al. (3). Briefly, microtitration plates were coated by incubation with 100 μl of antigen solution (5 μg/ml) per well in 100 mM sodium bicarbonate (pH 9.2) overnight at 4°C. The plates were then blocked with phosphate-buffered saline (PBS; pH 7.2)–1% bovine serum albumin (BSA) for 1 h at room temperature and washed with PBS–0.05% Tween 20 (PBS-T). Serum samples were diluted 1/400 in PBS-T containing 1% BSA, and 100 μl was dispensed into each well. After 2 h of incubation, the plates were washed three times with PBS-T, 100 μl of peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG) appropriately diluted in PBS–1% BSA was added, and the mixture was incubated for 3 h at room temperature and washed three times with PBS-T and once with PBS. A substrate solution containing H2O2, 3-methyl-2-benzothiazolinone hydrazone hydrochloride, and 2-dimethylaminobenzoic acid (200 μl/well) was added, and the plates were incubated for 20 min at room temperature with shaking. The optical densities at 600 nm were measured with an ELISA reader (Labsystems Multiscan MS, Helsinki, Finland).
Data analysis.
The cutoff for positive scores was calculated for each test from the mean absorbance value obtained for the 28 healthy donors plus 3 standard deviations. The following definitions were used to calculate the corresponding diagnostic parameters: true-positive values (tp), sera from patients with surgically confirmed cystic hydatidosis showing positive readings; false-negative values (fn), sera from patients with surgically confirmed cystic hydatidosis showing negative readings; false-positive values (fp), sera from healthy donors or patients without cystic hydatidosis showing positive readings; true-negative values (tn), sera from healthy donors or patients without cystic hydatidosis showing positive readings; sensitivity, tp × 100/(tp + fn); specificity, tn × 100/(tn + fp); diagnostic efficiency, (tn + tp) × 100/(tp + fp + tn + fn).
RESULTS
The amino acid sequences of the peptides introduced in this study, the amino acid sequences of the previously described peptides, and their alignments with the subunits of AgB are depicted in Fig. 1. The peptides were designed in order to complete the available information on the antigenicity of AgB. p176 covers the N-terminal extension of AgB8/1 (not included in the study of Leggatt and McManus [11]), while p175 and p177 represent the N-terminal and central regions of AgB8/2, respectively, and are therefore complementary to Gu4.
FIG. 1.
Amino acid sequences of the two AgB 8-kDa subunits and related peptides. Peptides p65, p66, and p67 (11) and Gu4 (3) have been described previously and are underlined. The peptides introduced in this study (p175, p176, and p177) were designed in order to complete the analysis of the antigenicities of the AgB subunits. Note that p176 comprised the sequence of p65 plus the 12 N-terminal residues of AgB8/1, which were unknown at the time that p65 was first reported.
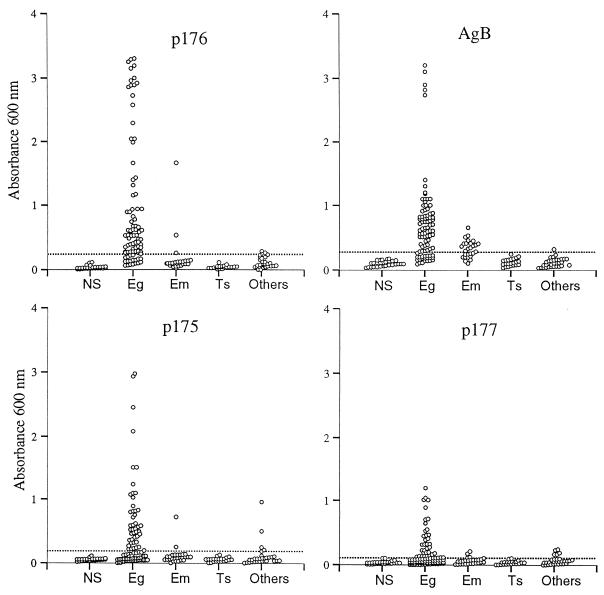
The reactivity of our panel of sera with these peptides and AgB was analyzed by ELISA (total IgG) and is shown in Fig. 2 and Table 1. The peptides exhibited important differences in their diagnostic sensitivities. While peptides p175 and p177 detected only a limited number of confirmed cases of the disease (diagnostic sensitivities of 49 and 38%, respectively), the diagnostic sensitivity accomplished with p176 was 80%, similar to that of AgB (77%). The number of serum samples classified as positive by using p176 was also markedly superior to that obtained with the previously described peptides p65 and Gu4. The correlation between location and seropositivity was similar to what has been reported before for other antigens (3).
FIG. 2.
Reactivity of our panel of sera against AgB and peptides p175, p176, and p177 as assessed by ELISA. The sera were grouped as follows: NS, sera from healthy donors; Eg, sera from patients with cystic hydatidosis; Em, sera from patients with alveolar hydatidosis; Ts, sera from patients with cysticercosis; Others, other sera used in this study. The cutoff for each assay is display as a horizontal line.
TABLE 1.
Diagnostic performances of the synthetic peptides and AgB in the immunodiagnosis of hydatid disease by ELISA
| Antigen | Sensitivity (%) | Specificity (%) | Diagnostic efficiency (%) |
|---|---|---|---|
| p176 | 80 | 93 | 87 |
| p175 | 49 | 94 | 74 |
| p177 | 38 | 92 | 68 |
| AgB | 77 | 85 | 81 |
| p65 | 44 | 96 | 73 |
| Gu4 | 18 | 98 | 63 |
All peptides showed a high diagnostic specificity, which, in all cases, was superior to that of AgB. Detailed information about the disease groups that gave place to the limited cases of cross-reactivity is displayed in Table 2. While the cross-reactivity observed with AgB (which has previously been identified as genus specific) was found among the serum samples from patients with alveolar hydatidosis, the few cases of cross-reactivity observed with the peptides covered a wider spectrum of disease groups, and, in general, the cross-reactivity was marginal. The overall diagnostic values of the various antigens, which are best represented by their diagnostic efficiencies, indicate that p176 (diagnostic efficiency, 87%) is superior to AgB (diagnostic efficiency, 81%).
TABLE 2.
Number of positive serum samples from a group of healthy individuals and patients with different diseases tested by ELISA on plates coated with various peptides or AgB
| Disease | No. of positive serum samples
|
||||||
|---|---|---|---|---|---|---|---|
| Total | p176 | p175 | p177 | p65 | Gu4 | AgB | |
| Cystic hydatidosis | 90 | 72 | 44 | 34 | 40 | 16 | 69 |
| Alveolar hydatidosis | 27 | 3 | 2 | 4 | 3 | 2 | 16 |
| Cysticercosis | 22 | 0 | 0 | 0 | 0 | 0 | 0 |
| Schistosomiasis | 6 | 1 | 2 | 0 | 1 | 0 | 0 |
| Toxocariasis | 10 | 2 | 3 | 3 | 1 | 0 | 0 |
| Filariasis | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toxoplasmosis | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Syphilis | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| Chagas' disease | 4 | 1 | 0 | 1 | 0 | 0 | 0 |
| Rheumatoid arthritis | 5 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cancer | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Healthy | 28 | 0 | 0 | 0 | 0 | 0 | 0 |
DISCUSSION
Native or recombinant antigens are powerful reagents for serology, because they contain a large spectrum of epitopes, which cover variations in the individual responses among patients. However, they represent complex structures, and there is always a balance between diagnostically relevant epitopes versus cross-reactive ones, which sometimes compromises the specificity of the system. For this reason, efforts have been made to find alternatives to biological antigens by using synthetic peptides (14, 16). Synthetic peptides mimicking relevant B-cell epitopes are potentially ideal tools for dissecting the antigenicities of the native antigens, making it possible to measure antibodies directed against very specific antigenic determinants. Additionally, synthetic peptides, in contrast to biological products, are easily standardized and can be readily produced in large amounts. Consequently, an increasing number of diagnostic assays based on synthetic peptides are being developed or are already commercially available.
In a previous study we performed an internal comparison of the diagnostic value of the available synthetic peptides and the major antigens of E. granulosus (3). That study showed that the diagnostic performance of AgB in ELISA was notoriously superior to that of other native antigens, such as Ag5 or crude hydatid cyst fluid, and also demonstrated that the peptides at hand constituted an unsatisfactory representation of the major epitopes of AgB. In order to find alternative peptide antigens that could mimic these epitopes, we examined new regions of both AgB subunits by using peptides p176, p175, and p177. Since these peptides were analyzed against the same panel of serum samples against which AgB and the other AgB-related peptides were analyzed, it provided an overall view of the antigenicity of AgB, which indicates that the dominant epitopes for humans are localized in the N-terminal extension of the 8-kDa subunits. In effect, those peptides delineated from the middle or C-terminal region of the AgB subunits (p177, Gu4, or p67 and p66 [11]) exhibited comparatively much lower sensitivities than p176, p175, or p65. Among these, p176 emerged, undoubtedly, as the most valuable antigen. Compared to p65, p176 has an additional 12-mer N-terminal fragment which appears to be crucial for recovery of supplementary immunodominant epitopes. As a consequence, the diagnostic sensitivity was augmented from 44% (p65) to 80% (p176) without a significant effect on the diagnostic specificity of p176.
In general, the use of a combination of two or more peptides as probes in ELISA improves the performance of the peptide-based immunoassay (14). Therefore, we contemplated the possibility of complementarity among our antigens, particularly between p176 and p175. However, examination of individual E. granulosus-specific sera showed that all serum samples negative by ELISA against p176 did not react with any other peptide antigen, including p89-122 (data not shown), which has been shown to be complementary to p65 (3). This suggests that the antigenicity of the AgB molecule is concentrated in the N-terminal extension of the AgB8/1 subunit, which explains why p176 exhibited a better diagnostic performance than native AgB. Indeed, the fact that both the diagnostic sensitivity and the specificity of p176 were superior to those of AgB was a striking finding and provides remarkable experimental support for the peptide approach.
This improvement in the diagnostic efficiency of p176 was more evident in connection with the diagnostic specificity of the peptide (93 and 80% for p176 and AgB, respectively) and was mostly due to a lower level of cross-reactivity with sera from patients with alveolar echinococcosis. E. granulosus and E. multilocularis AgB8/1 subunits have a high degree of identity, and they differ at only five amino acid residues (8). Four of them are located in the stretch represented by p176, which may explain the fine specificity of p176.
Finally, examination of the individual sera did show in this case that a limited number of E. granulosus-specific serum samples reacted with AgB but not with p176, and vice versa. Therefore, there seem to be additional AgB epitopes which could be valuable in increasing the sensitivity of the system. These epitopes cannot be mimicked by linear peptides, as shown in this study. They appear to be discontinuous in nature, and therefore, their identification would require a combinatorial approach (6). We are carrying out that type of an analysis by using different phage display peptide libraries.
There have been numerous reports of diagnostically relevant native, recombinant, or peptide antigens for diagnosis of human E. granulosus hydatid disease. The reported diagnostic sensitivity and specificity vary greatly among the different reports, even for similar antigen preparations (12). Since these parameters have been determined with different panels of sera and under different laboratory conditions, there is no consensus on the most suitable antigen. Regarding this, we believe that this work represents a significant contribution to the standardization of the serodiagnosis of hydatid disease, because we have developed an improved immunoassay based on the use of p176, a 38-mer synthetic peptide with excellent diagnostic efficiency. This parameter was established in a reliable manner through an internal comparison performed against the same panel of sera, including the major and more frequently used antigens of the parasite (AgB and Ag5), as well as previously described and novel synthetic peptides derived from these antigens. Furthermore, due to its nature, p176 constitutes a highly standardized reagent that can be obtained by chemical synthesis in any laboratory, and it can readily be used in ELISA by passive adsorption on the plastic surface without the need for conjugation to a carrier protein.
ACKNOWLEDGMENTS
This work was supported by The Swedish Agency for Research Cooperation with Developing Countries, the Consejo Nacional de Investigaciones Científicas y Técnicas, the Comisión Sectorial de Investigación Científica, Universidad de la República, Montevideo, Uruguay, and the Zaffaroni Foundation.
REFERENCES
- 1.Ammann R, Eckert J. Clinical diagnosis and treatment of echinococcosis in humans. In: Thompson R C A, Lymbery A J, editors. Echinococcus and hydatid disease. Wallingord, United Kingdom: CAB International; 1995. pp. 411–463. [Google Scholar]
- 2.Barbieri M, Severi M, Pírez M, Battistoni J, Nieto A. Use of specific antibody and circulating antigen serum levels in the hydatid immunodiagnosis of asymptomatic population. Int J Parasitol. 1994;24:937–942. doi: 10.1016/0020-7519(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri M, Fernández V, González G, Martinez Luaces V, Nieto A. Diagnostic evaluation of a synthetic peptide derived from a novel antigen B subunit as related to other available peptides and native antigens used for serology of cystic hydatidosis. Parasite Immunol. 1998;20:51–61. doi: 10.1046/j.1365-3024.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 4.Chamekh M, Gras-Masse H, Bossus M, Facon B, Dissous C, Tartar A, Capron A. Diagnostic value of a synthetic peptide derived from Echinococcus granulosus recombinant protein. J Clin Investig. 1992;89:458–464. doi: 10.1172/JCI115606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coltorti E, Guarnera E, Larrieru E, Santillan G, Aquino A. Seroepidemiology of human hydatidosis: use of dried blood samples on filter paper. Trans R Soc Trop Med Hyg. 1988;82:607–610. doi: 10.1016/0035-9203(88)90527-5. [DOI] [PubMed] [Google Scholar]
- 6.Cortese R, Felici F, Galfre G, Luzzago A, Moanci P, Nicosia A. Epitope discovery using peptide libraries displayed on phage. Trends Biotechnol. 1994;12:262–267. doi: 10.1016/0167-7799(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 7.Fernández V, Ferreira B, Fernánde C, Zaha A, Nieto A. Molecular characterization of a novel 8 kDa subunit of Echinococcus granulosus antigen. Mol Biochem Parasitol. 1955;77:247–250. doi: 10.1016/0166-6851(96)02602-3. [DOI] [PubMed] [Google Scholar]
- 8.Frosch P, Hartmann M, Mühlschlegel F, Frosch M. Sequence heterogeneity of the echinococcal antigen B. Mol Biochem Parasitol. 1994;64:171–175. doi: 10.1016/0166-6851(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 9.González G, Nieto A, Fernández C, Örn A, Wernstedt C, Hellman U. Two different 8 kDa monomers are involved in the oligomeric organization of the native Echinococcus granulosus antigen B. Parasite Immunol. 1996;18:587–596. doi: 10.1046/j.1365-3024.1996.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 10.González G, Spinelli P, Lorenzo C, Hellman U, Nieto A, Willis A, Salinas G. Molecular characterization of P-29, a metacestode-specific component of Echinococcus granulosus which is immunologically related to, but distinct from antigen 5. Mol Biochem Parasitol. 2000;105:177–184. doi: 10.1016/s0166-6851(99)00166-8. [DOI] [PubMed] [Google Scholar]
- 11.Leggatt G, McManus D. Identification and diagnostic value of a major antibody epitope on the 12 kDa antigen from Echinococcus granulosus (hydatid disease) cyst fluid. Parasite Immunol. 1994;16:87–96. doi: 10.1111/j.1365-3024.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 12.Lightowlers M, Gottstein B. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis. In: Thompson R C A, Lymbery A J, editors. Echinococcus and hydatid disease. Wallingord, United Kingdom: CAB International; 1995. pp. 355–410. [Google Scholar]
- 13.Lightowlers M, Liu D, Haralambous A, Rickard M. Subunit composition and specificity of the major cyst fluid antigens of Echinococcus granulosus. Mol Biochem Parasitol. 1989;37:171–182. doi: 10.1016/0166-6851(89)90149-7. [DOI] [PubMed] [Google Scholar]
- 14.Marc H, Van Regenmortel V. Synthetic peptides help in diagnosing viral infections. ASM News. 1998;64:332–338. [Google Scholar]
- 15.McManus D, Smyth J. Hydatidosis: changing concepts in epidemiology and speciation. Parsitol Today. 1986;2:163–167. doi: 10.1016/0169-4758(86)90147-x. [DOI] [PubMed] [Google Scholar]
- 16.Meloen R H, Langedijk J, Langeveld J P. Synthetic peptides for diagnostic use. Vet Q. 1997;19:122–126. doi: 10.1080/01652176.1997.9694755. [DOI] [PubMed] [Google Scholar]
- 17.Oriol R, Williams J F, Perez M V, Oriol C. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg. 1971;20:569–574. doi: 10.4269/ajtmh.1971.20.569. [DOI] [PubMed] [Google Scholar]