Abstract
The substantial advances attained by checkpoint blockade immunotherapies have driven an expansion in the approaches used to promote T cell access to the tumor microenvironment to provide targets for checkpoint immunotherapy. Inherent in any T cell response to a tumor antigen is the capacity of dendritic cells to initiate and support such responses. Here, the rationale and early immunobiology of CD40 as a master regulator of dendritic cell activation is reviewed, with further contextualization and appreciation for the role of CD40 stimulation not only in cancer vaccines but also in other contemporary immune-oncology approaches.
Keywords: CD40, vaccine, CD40L, T cell, dendritic cell
Subject terms: Tumour immunology, Immunization
Potential for cancer vaccines: days of future past
Undeniably, the treatment of cancer has been revolutionized by the introduction of novel immunotherapeutic agents that are administered as single agents or in combination with other cancer treatments. The clinical efficacy of checkpoint blockades and cellular therapies has prolonged lives in many cancer etiologies. However, it is also a hard reality that the majority of patients do not benefit from current immunotherapies, leaving us challenged to design and develop further iterations on successful therapies to expand their impact. In the case of solid tumors, the most sustained advances have been achieved with antibodies targeting checkpoint molecules that constrain immune cell function within the tumor microenvironment. However, the efficacy of these therapies, especially those targeting PD-1, generally correlates with the presence of T cells within the tumors. Thus, strategies to increase T cell presence, particularly cytotoxic CD8+ T cells, within tumors are an important focus. While cellular therapies have the potential to be immediately effective in this context, their activity in solid tumors has been modest. The basis for this observation is unclear, but some studies have suggested that immune cell trafficking and persistence within the tumor microenvironment (TME) is weak. These immune cells are potentially further compromised, particularly in the context of in vitro expanded cellular products with transgenic receptor targeting, by both their limited ability to form durable memory populations and the loss of the target antigen expression by the tumor. Thus, cancer vaccines, while initially promising in the tumor immunotherapy armamentarium, are now being strongly re-evaluated, as cancer vaccines can generate diversity in the immune response against target antigens and T cell differentiation states, both of which can promote trafficking and persistence at the tumor site. Further advances include the realization that a major source of antigens within tumors are inherent genetic aberrations and that the adjuvants used in combination with vaccines were initially underdeveloped. Here, we will focus on how targeting CD40, a TNF superfamily receptor expressed on a variety of immune cells, can be leveraged to improve cancer immunity in several vaccine-related settings, with the focus that most forms of vaccination will have limited therapeutic efficacy in the complex tumor microenvironment when the appropriate adjuvants are omitted from the vaccine regimen.
Weaknesses of current adjuvants
The opportunity to develop vaccines in the context of cancer was initiated following the identification of immunogenic targets in tumors. However, most vaccine technology was initially based on the use of adjuvants (e.g., alum) that had been tested in the context of generating an antibody response. There are two traditional settings for the delivery of cancer vaccines: postsurgery, in a state of no radiographically evident disease but the likely presence of micrometastatic burden; and prevalent disease where metastatic spread has made surgery or radiation unsuitable for the patient. In the former, prophylactic vaccines are aimed at generating durable memory T cells that will reactivate upon re-exposure to the tumor antigen. In the latter, large numbers of effector T cells that can home to multiple tumor deposits are required, almost akin to cellular therapy. The vaccine strategies needed for these two scenarios may be quite different. Some studies have shown that the location of vaccine delivery impacts the homing ability of the responding T cells [1], while others have shown that elements of vaccine composition can also limit the systemic availability of responding T cells [2]. Thus, understanding and “vaccineering” both the quantitative and qualitative effects of different adjuvants is required to develop T cell responses that exhibit broad recognition of antigens and represent a spectrum of differentiation states.
Critical to the expansion of T cell responses to pathogens and tumors [3] is the activation of dendritic cells (DCs). Normally, DCs are present in peripheral tissues and acquire antigens via a variety of engulfment processes [4]. Upon sensing the presence of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), DCs migrate to the lymph nodes and initiate encounters with naïve or memory T cell populations. On the basis of these interactions and a deepening of the understanding of innate pattern-recognition receptors such as Toll-like receptors (TLRs), NOD-like receptors, C-type receptors, RIG-1-like receptors and, more recently, the cGAS-STING pathway [5], innate sensors have begun to be exploited in cancer vaccines for their ability to promote the activation of antigen-presenting cells and the induction of T cell-supporting cytokines. However, few studies have consistently shown that targeting these innate sensors is sufficient to drive complete tumor control, perhaps because systemic induction of inflammation can perturb the chemokine gradients used by T cells to traffic to their target or that a high degree of toxicity occurs if they are delivered systemically. Thus, the alternative DC activation pathway of targeting CD40, the TNF superfamily at the nexus of innate and adaptive immunity, has the potential to serve this need.
Molecular structure and signals of CD40
Seminal studies demonstrated that a critical step in the licensing of DCs to induce productive CD8+ T cell responses is the engagement of CD40 by CD40 ligand-expressing CD4+ T cells. This is the fundamental basis of CD4+ T cell-mediated “help”, without which CD8+ T cell responses are muted and memory is not properly formed. In the context of normal responses to pathogens, CD40 stimulation with CD40 ligand (CD154) is either provided by recently activated CD4+ T cells or, in some instances, by natural killer/T (NK/NKT) cells. This activation, or licensing, of DCs serves as a temporal bridge between CD4+ T cell activation and their indirect support of CD8+ T cell expansion [6–8]. However, in the context of cancer vaccination, these precursor populations are relatively rare, so developing agonists of CD40 that can serve as adjuvants for vaccines is a promising pathway to promote both CD4+ and CD8 T cell responses following vaccination.
CD40 is a 48 kDa type 1 transmembrane protein consisting of 193 amino acids. It is structurally divided into extracellular, transmembrane and intracellular domains. Its ligand, CD154, is a type II transmembrane protein with extensive posttranslational modifications, resulting in variations in molecular weight between 32 and 39 kDa. The extracellular structure of CD40L favors the characteristic trimerization of TNF superfamily members, which presents implications and complications in the design and development of agonistic molecules. Expression of CD40L is primarily found on activated T cells, although in some instances, it can be found on B cells and platelets and can be induced by inflammatory conditions on a variety of myeloid-derived cells [9].
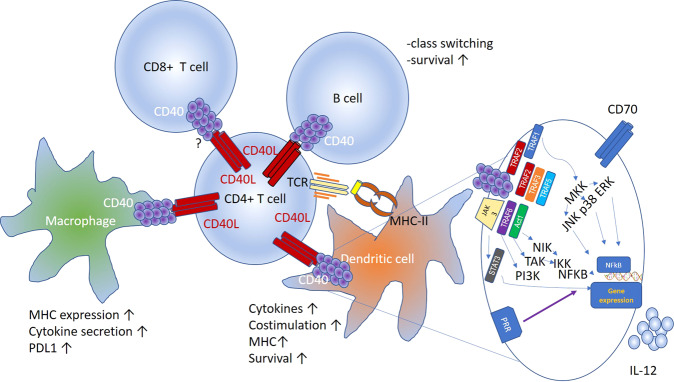
CD40 signaling primarily utilizes adapter proteins called TNF receptor-associated factors, resulting in the activation of both the canonical and noncanonical NFκB pathways, MAP kinase, PI3 kinase, and phospholipase-Cγ. Their activation leads to the characteristic downstream effects of these pathways, including transcriptional activation, cytoskeletal rearrangement and cell survival (Fig. 1). Other studies have indicated that CD40 can signal via JAK3-STAT5, and in the absence of this signaling, DCs induce T cell tolerance [10]. The extent to which these pathways contribute, individually or in combination, to the varied functional activities of DCs and DC differentiation has not been completely dissected. Furthermore, it is not yet known whether these pathways have different roles and outcomes in different cell types. Importantly, however, concerted and sustained CD40 signaling requires higher levels of oligomerization than those achieved through trimerization, which putatively supports more extensive engagement of the various signaling components. It is important to note that CD40 signaling pathways are quite distinct from those of pattern recognition receptors (PRRs), and while it is clear that the coactivation of these pathways has considerable functional consequences on DCs, how the two signaling pathways intersect has not been deeply studied.
Fig. 1.
Schematic of the interactions between CD40L expressed by activated CD4+ T cells and other cellular components of the tumor microenvironment. The inset shows signaling pathways activated by CD40 stimulation in dendritic cells, leading to the expression of CD70 and IL-12
Functional consequences of CD40 stimulation
Focusing on DCs, CD40 engagement has a variety of functional outcomes, some of which are shared with PRR stimulation (Fig. 1). Both pathways increase the expression of antigen-presenting MHC molecules and the costimulatory molecules CD80 and CD86. The capacity of CD40 stimulation to activate DCs in combination with the fact that conventional DCs are highly specialized in their ability to cross-present antigens on MHC class I molecules leads to the conclusion that CD40-activated DCs are a lynch pin in the initiation of CD8+ T cell immunity. The TH1/TC1-promoting cytokine IL-12 is also induced by both CD40 and PRR stimulation. Given the toxicity that occurs with systemic delivery of IL-12 in clinical settings, CD40 stimulation provides a method for local delivery in a physiologically relevant context. Divergence is seen between these pathways with respect to two functionally important elements. CD40 stimulation, along with TRANCE-R [11], promotes the expression of antiapoptotic molecules, including Bcl-XL [12, 13], that promote DC survival, likely allowing a longer duration of antigen presentation in the draining lymph node to surveiling naïve T cells. Second, CD40 stimulation appears uniquely capable of inducing the expression of TNF superfamily members, including CD70, CD134 (OX40 ligand) and CD137 (41BB ligand). These “signal 3-like” molecules provide critical post-TCR/CD28 signaling that supports the continued expansion of effector T cells and, in particular, contributes to their differentiation and survival as memory T cells. Thus, there are multiple aspects of CD40 stimulation that naturally align with the goals of cancer vaccines, and when our lab prepares CD40-stimulated DCs for cellular vaccines in murine models, we perform quality control by assessing their expression of IL-12 and CD70. It should be recognized, however, that considerable synergy has been observed when CD40 and PRR stimulation occurs concomitantly [14–17], suggesting that vaccines that target both components will likely have increased efficacy.
While the ability of CD40 stimulation to support T cell responses is generally considered to be dependent upon its action on DCs, it is important to recognize that CD40 stimulation can target other cells within the tumor microenvironment and the tumor-draining lymph nodes (Fig. 1). In some cancer models, CD40-mediated activation of macrophages, which possibly mimics the effects of CD4+ T cell stimulation, has been shown to drive tumor control in an IFNγ-dependent manner, resulting in substantial remodeling and collapse of the tumor’s fibrotic network. It is interesting to consider this effect of CD40 stimulation on tumor fibrosis in the context of increasing the tumor’s accessibility to other therapeutics, including chemotherapies [18–20] and cellular products [21]. B cells also express CD40, and while their contribution of antibodies against tumor control remains controversial, there are data suggesting that CD40-mediated activation of B cells increases their antigen-presenting capabilities and modulates cytokine production, possibly supporting effector CD4+ T cell responses within tumors. Indeed, CD40-stimulated B cells have been explored as a cellular vaccine in several cancer models [22]. Finally, some murine studies have shown that CD40 can stimulate activated CD8+ T cells, providing a critical signal for their survival [23]. Whether human T cells receive such support via CD40 signaling has not been decisively demonstrated, although CD40 endodomains are being explored for CAR-T signaling [24].
Preclinical integration of CD40 stimulation with tumor immunity
While a few studies have demonstrated the efficacy of CD40 stimulation as a monotherapy with agonists, these studies have generally occurred in the context of tumors that express strong antigens and have been shown to be generally responsive to many immunotherapeutic approaches [25–29].
More commonly, the ability of CD40 stimulation to control tumors has been performed with the addition of tumor-derived antigen. The earliest studies by Diehl et al. demonstrated that CD40 agonistic antibody stimulation could prevent the induction of tolerance to vaccines composed of peptides derived from shared tumor antigens [30]. Since this initial proof-of-principle study, numerous studies have shown that CD40-specific antibodies can provide a platform for peptide or whole protein-based cancer vaccines. Pertaining to the understanding that CD40 and PRR use discrete signaling molecules, seminal studies by Kedl and Seder showed that the addition of TLR agonists to proteins significantly boosted CD8+ T cell responses to protein vaccines, and directly conjugating TLR agonists was even more beneficial [31, 32]; this principle has been expanded to human studies with B cell-based vaccines [33]. Subsequent studies showed that expanded T cell responses were in part due to the elaboration of type 1 interferons (IFN-1) by activated DCs and the ability to target both the CD40 and PRR pathways to elicit CD70 expression on DCs [14]. Consequently, these data have led to the development of CD70-based agonists targeting CD27 as an alternative approach to targeting CD40 [34–37]. CD40-specific antibodies with TLR agonists, compared to either agent alone, significantly boosted the magnitude of CD8+ T cell responses to peptide-based vaccination in murine melanoma models [38–40]. Importantly, a recent study from our lab demonstrated that CD40 agonist vaccination with protein can elicit both CD4+ and CD8+ T cell responses to control murine melanoma with equivalent effectiveness [40]. Perhaps most striking, however, was the demonstration that blocking T cell egress from lymph nodes, the presumed site of CD40-mediated activation of DCs, did not impact either the expansion of intratumoral T cells or their ability to control tumor outgrowth. Thus, the tentative conclusion from this study is that CD40-based vaccinations may target intratumoral DCs and T cells. Given the recent studies that have demonstrated the critical importance of intratumoral DCs for tumor control [41], a logical next step will be to determine whether augmenting DC presence in tumors with DC mitogens such as FLT3 ligand [42] will further increase the activity of these vaccines. It also might suggest that some pre-existing T cell presence in tumors may be needed for CD40-based vaccines to be effective in the context of therapeutic cancer vaccines (as opposed to prophylactic vaccines).
While the efficacy of CD40-based cancer vaccines in preclinical models is clear, aside from the dependence on T cells, primarily but not absolutely CD8+ T cells, the mechanism of action of effective therapeutic CD40-based cancer vaccines is still under investigation. As mentioned above, CD40 stimulation mimics CD4+ T cell help and, as such, will convert tolerogenic protein vaccines to immunogenic vaccines [30]. In the previously mentioned study from our lab, within the tumor microenvironment, it was ascertained that CD40 stimulation promoted the activation of DCs by increasing CD86 expression and IL-12 secretion. At the level of T cells, several paradoxical changes in the frequency and function of T cells were observed. First, the massive increase in T cells seen with vaccination was primarily driven by a brief burst in the proliferation of phenotypically exhausted (PD1+IL2‒) T cells. Subsequently, the proliferative capacity and functional capability of these expanded T cells decreased, although the number of effector T cells remained higher than that of controls because of their vaccine-triggered expansion. Intriguingly, CD40 stimulation reduced PD1 expression on T cells but also decreased their expression of TCF-1, which has been associated with pluripotent T cells within the tumor microenvironment [43]. This may infer that CD40 stimulation drives further differentiation of T cells within the TME. However, a second round of vaccination to tumor-bearing mice with anti-CD40, polyIC and protein in the context of FTY720, which prevents T cells from leaving lymph nodes, resulted in additional tumor control, indicating that these intratumoral effector T cells were capable of a secondary response to vaccination. Going forward, understanding the relative contribution of pre-existing T cells compared to those that traffic from secondary lymphoid tissues will be important, as will whether immunization will synergize with checkpoint blockade therapies. Furthermore, understanding why the addition of an antigen promotes tumor immunity compared to adjuvants alone is worth understanding.
A criticism of cancer vaccines as an approach for tumor immunotherapy is that the tumor antigens that have been targeted to date are often (in the case of tumor-associated antigens, e.g., the melanocyte differentiation antigen gp100 or mesothelin) but not always (in the case of cancer-testes antigens, e.g., NY-ESO) derived from proteins expressed in the periphery by healthy cells. This could be expected to have induced a degree of self-tolerance, muting subsequent T cell responses to vaccination. The advent of genome sequencing aligned with algorithms that predict binding to MHC molecules has led to the development of next-generation cancer vaccines built on so-called “neoantigens” [44–47]. It is currently unclear whether neoantigen-based vaccines will need CD40 stimulation to overcome self-tolerance, but the cost-benefit between the toxicity of CD40 stimulation and the magnitude of CD40-driven T cell responses will need to be tested empirically in clinical trials.
Expanding on the theme that CD40 stimulation and PRR stimulation can be additive or synergistic but could be limited by cytokine-mediated toxicity, intratumoral delivery of a TLR7 agonist along with systemic CD40 stimulation has been shown to be effective in a murine model of mesothelioma [48, 49]. Furthermore, intratumoral TLR4 and CD40 stimulation, when combined with anti-PD1, has resulted in systemic rejection in a plethora of murine models [50]. In our recent study, a remarkably digital “responder” vs “nonresponder” phenotype was observed when melanoma-bearing mice were treated with anti-CD40 and polyIC without further tumor antigen treatment [40]. One possibility is that a threshold of antigens within targeted DCs needs to be met to support tumor-infiltrating DCs. Alternatively, differences in recruitment of immunosuppressive populations could account for variations in expanding T cell responses.
Analogous to protein-based vaccinations, CD40 stimulation has been deployed with DC-based vaccination [51]. Some initial efforts involved preactivating antigen-bearing DCs with CD40 stimulation to augment cytokine and costimulatory molecule expression [52, 53]. However, while these DCs have clearly ramped up immunogenicity, activated DCs often fail to traffic effectively to lymph nodes, instead staying at the injection site [54]. This can potentially be sidestepped by injecting the activated DCs directly into tumor-draining lymph nodes. Alternatively, CD40 stimulation can be provided after DC vaccination, allowing the activation of DCs once they have migrated to lymphoid tissues [55]. As mentioned above, promoting DC trafficking to tumors may also be a sensible strategy to consider prior to CD40 stimulation.
CD40 stimulation beyond extrinsic vaccinations
The recognition that CD40 is a potent activator of DCs has led to studies that interrogate its use in autovaccination settings. Autovaccination refers to the process of an antigen being introduced to the host from an external intervention, such as the induction of tumor cell death by chemotherapy or radiotherapy. In an extensive series of studies, Vonderheide and colleagues first demonstrated that CD40 stimulation in combination with gemcitabine induced T cell-independent remodeling of the tumor stroma in humans, in particular inducing tumor-infiltrating macrophages to become tumoricidal and deplete the tumor stroma, which is important in pancreatic cancer [18–20]. Similar tumor microenvironment remodeling as a function of CD40 stimulation has been reported by other groups in different models [56, 57], indicating a common theme that above and beyond DC priming, CD40 can promote a proimmunity landscape within tumors. Whether consistent mechanisms, such as IFNγ production or metalloproteinase elaboration from activated myeloid cells, are critical for this, or whether CD40 stimulation shuts off the profibrotic activity of macrophages, remains to be elucidated. Subsequent studies in murine models showed the ability of CD40 stimulation to achieve TME remodeling as a monotherapy, and increased tumor control was achieved when CD40 stimulation and anti-CTLA-4 and anti-PD1 were used in combination with chemotherapy. In this instance, T cell responses were strongly induced, and curative protection that resulted in immunological memory and resistance to subsequent tumor rechallenge was achieved [20]. Further investigations indicated that CD40 stimulation synergized with radiotherapy and checkpoint blockade, demonstrating the elusive abscopal effect of controlling unirradiated tumors, which is indicative of a substantial systemic immune response. These data show a potentially encouraging role for CD40 in remodeling distal, untreated tumors, making them more permissive for T cell trafficking and infiltration. Anti-CD40 has also shown activity in the novel approach of using focused ultrasound to introduce nonionizing damage to tumors [58].
From these studies that show the benefit of adding CD40 stimulation to standard of care therapies or viral delivery, it could be inferred that the degree of DAMP or PAMP release from either infected or dying cells achieved by the primary intervention is perhaps insufficient to fully activate T cells. Alternatively, these conventional approaches are not sufficiently targeting CD4 T cells or NK cells that would normally provide CD40 stimulation to DCs.
While it is clear that DCs provide a critical link between innate and adaptive immune responses within the tumor microenvironment and draining lymph nodes [59–61], it is worth noting that, as with other myeloid cells within the tumor microenvironment, activated conventional DCs express PDL1 and PDL2, which serve as ligands for the PD1 checkpoint molecule [62]. The expression of high levels of PD1 on T cells is associated with dampened activity (reviewed extensively elsewhere). Host expression of PDL1 and PDL2 can significantly contribute to limitations in the T cell responses to tumor antigens [63, 64]. Some studies have indicated that CD40 stimulation can augment PDL1 expression on DCs and macrophages [65, 66], which may explain the need for anti-PD1 in the aforementioned chemotherapy and radiotherapy studies with anti-CD40. The addition of TLR stimulation in combination with CD40 stimulation can further increase PDL1 expression on DCs [67]. Not surprisingly, CD40-based immunotherapies have benefitted from the inclusion of checkpoint inhibitor blockade, commonly in the form of anti-PD1 [68–70]. It should be noted that there is a relative dearth in knowledge as to whether PDL1 and other checkpoint molecules are similarly regulated on cDC1 and cDC2 by CD40 stimulation and whether the location of these DCs within tumors or lymph nodes influences the expression of these molecules.
CD40 stimulation and cellular therapies
Adoptive transfer of in vitro expanded TILs, TCR-transgenic T cells or CAR-T cells has been proposed as an approach to overcome a paucity of pre-existing T cells within the TME. While these approaches have shown consistent efficacy against hematopoietic tumors, their activity (particularly that of CAR-T cells) in solid tumors needs improvement. Given the ability of CD40-stimulating antibodies to promote DC function and the association of intratumoral DCs with T cell infiltration, it is encouraging that anti-CD40 infusion, in combination with IL-2 infusion, supports the antitumor activity of melanoma-specific TCR transgenic T cells in an IL-12- and CD80/CD86-dependent manner [71] and potentially involves CD70 [72]. Whether pretreatment with anti-CD40 can broaden the repertoire of T cells that can be expanded from patient tumor explants has not yet been studied. The aforementioned ability of CD40 to reprogram the pancreatic cancer tumor microenvironment has shown promising ability to increase the frequency and absolute number of TCR-engineered cellular products in the context of pancreatic cancer models. Anti-CD40 stimulation showed better results than anti-CSF1R on the promotion of the accumulation of transferred T cells [21]. Interestingly, IFNγ production by the engineered T cells was not enhanced with CD40 stimulation in vivo, suggesting that additional interventions will be necessary to sustain full functional activity. However, this study suggested that cellular therapies are compromised in the context of solid tumors by the ability of myeloid cells to shield tumors from T cell infiltration or limit their persistence or survival once in the tumor microenvironment. It will be important to dissect these alternatives, as it may be possible to achieve further enhancement if the results indicate that remodeling does not influence the expression of homing receptor ligands or chemokines on the tumor vasculature, for example. The activity of infused anti-CD40 in the context of CAR-T cell transfer has not been extensively addressed at this point. Rather, engineering approaches that confer the ability of CAR-T cells to express CD40L [73] or secrete agonistic CD40 antibodies [74] have shown some promise and may have the capacity to limit CD40 stimulation to the local environment of CAR-T cells, as CD40L is not expressed until CAR-T cells are activated, possibly lowering toxicity. Intriguingly, the capacity of the CD40 intracellular domains to provide costimulation of CAR-T cells has recently shown a promising ability to activate NF-κB and the subsequent expression of T cell costimulatory molecules in a manner that is discrete from CD137 (4-1BB) signaling [75] and T cells were found to be in a less differentiated state when combined with MyD88 endodomains [24].
Delivery formats for CD40
Several studies have expanded the scope of the use of CD40 stimulation to promote antitumor immunity beyond the realm of traditional peptide/protein vaccine settings (Table 1). First, CD40 stimulation, via viral delivery of CD40L, can support the ability of viruses to promote tumor immunity in murine models and cancer patients [50, 76–80]. While not explicitly oncolytic viruses, presumably the efficacy of these approaches engages some element of antigen release by viral lytic activity and the additional innate sensing pathways activated by viral infection of cancer cells, in combination with myeloid cell activation by CD40 stimulation. Further formulations to achieve CD40 stimulation include recombinant proteins with natural trimer conformation [81], or in some instances hexamer construction [82], with the goal of more naturally and potently inducing CD40 signaling without accompanying cytokine storm toxicity that has been evident with antibody-mediated stimulation. It should be noted that some discussion is ongoing about the relative effectiveness of different CD40 cross-linking antibodies being dependent upon Fc receptor-mediated binding. On the one hand, some studies have argued that engagement of the inhibitory FcγRIIB in vivo is necessary for the functional activity of some anti-CD40 antibodies [83–86], although others have argued for FcR independence [87]. Clearly, if tumors have minimal infiltration by myeloid, NK and B cells, the opportunity to engage FcR will be limited, potentially constraining the activity of anti-CD40 to its roles in the lymph node. CD40L-based strategies, in recombinant protein form, delivered by a virus, or even as antibodies or ScFV delivered by CAR-T cells [74], may be able to circumvent this potential limitation. However, initial clinical testing of sCD40L as an agonist did not produce strong outcomes [88], prompting the development of multivalent versions of this protein. More recent studies taking advantage of aligning the antibody epitope with functional activity have suggested that improvements in antibody activity can be achieved by targeting membrane-proximal regions of CD40 [85]. Taking a different approach, CD40 oligodinucleotide aptamers have shown encouraging preclinical activity [89] with direct targeting capability to B cell lymphomas. To date, this agent has not been tested for its ability to stimulate functional activity in DCs. Notably, in the brave new world of synthetic biology, multiformat antibodies, such as bispecifics, are being used to either deliver antigenic or stimulatory payloads to CD40-expressing cells such as DCs or are being used to bring target cells close to DCs to improve their interactivity [90, 91].
Table 1.
Developments in CD40-associated agonism
Clinical outcomes and developments
The preclinical activity of anti-CD40 has made clinical investigation a high priority, especially when considering juxtaposing CD40-mediated immune “acceleration” with the success of anti-PD1 relieving immune “brakes”. Thus far, the deployment of anti-CD40 antibodies in hematological malignancies has been to either drive differentiation of the targeted B cells or induce antibody-dependent cellular cytotoxicity. For solid tumors, initial phase I testing revealed that there is a degree of toxicity for systemically delivered anti-CD40, which is not surprising given its mode of action. Nevertheless, anti-CD40 has been deployed in clinical trials either as a monotherapy where modest activity has been observed [92, 93], or more commonly, as part of a treatment regimen composed of chemotherapy [18] or with a checkpoint blockade [94]. Some significant differences have been observed in the cytokines and cellular responses between different anti-CD40 clones, which has raised speculation about the importance of the Fc domain and its affinity for FcR for the purposes of cross-linking. On the basis of these observations, second-generation engineered anti-CD40 antibodies are being tested, again generally as part of combination therapy [95, 96] with some encouraging results but also noted toxicity [95] (Table 2). There ultimately needs to be a balance between toxicity and patient outcomes, such as that seen with the enhanced activity observed with the combination of anti-PD1 and anti-CTLA4 compared to either agent alone, accompanied by increased high-grade immune-related adverse events and mortality. Further studies will need to be performed to ascertain the basis of the toxicity caused by anti-CD40 antibody therapy, as it is possible to ameliorate some elements of cytokine storms, such as blocking IL-6, which is commonly used during CAR-T therapy infusion. Alternatively, it may be preferential to deliver anti-CD40 at a lower dose via subcutaneous injection to target specific tumor deposits, draining lymph nodes, or vaccine-specific sites, which is an approach we are currently exploring in a melanoma clinical trial at our institution.
Table 2.
Examples of active and completed trials based on varied formats of CD40 stimulation. Information from ClinicalTrials.gov
| TRIAL NUMBER | AGENT | DISEASE | STATUS |
|---|---|---|---|
| NCT04491084 | FLT3L; anti-CD40; stereotactic radiation | NSCLC | Recruiting |
| NCT01433172 | GM.CD40L transfected K562 cellular vaccine with CCL21 | Lung adenocarcinoma | Completed |
| NCT04635995 | Agonistic anti-CD40+/‒ anti-PD1+/‒ anti-CD137 | Advanced/metastatic malignancies | Recruiting |
| NCT02482168 | Agonistic anti-CD40 | Varied solid tumors | Completed |
| NCT00058799 | CD40L and IL-2 transfected fibroblasts | Leukemia | Completed |
| NCT04364230 | Agonistic anti-CD40 + peptides+polyICLC | Melanoma | Recruiting |
| NCT04406623 | SIRPα-Fc-CD40L | Ovarian | Recruiting |
| NCT01561911 | Agonistic Anti-CD40 | Advanced malignancies | Completed |
| NCT01103635 | Anti-CD40 + anti-CTLA4 | Metastatic melanoma | Completed |
| NCT00020540 | Flt3L and soluble CD40L | Metastatic melanoma or renal cancer | Completed |
| NCT03719430 | Anti-CD40 and doxorubicin | Advanced sarcoma | Recruiting |
| NCT03329950 | Anti-CD40+/‒ Ftl3L or anti-PD1 or chemotherapy | Varied malignancies | Recruiting |
| NCT03852511 | Oncolytic adenovirus expressing anti-CD40 ab | Metastatic cancer and epithelial tumors | Recruiting |
The ability to extrinsically introduce CD40L expression into the tumor by transducing with nonreplicating adenoviral infection has been tested in several small clinical trials and has shown some intriguing clinical improvements in patients with advanced malignancies [80, 94, 97]. Interrogation of local and systemic alterations that are consistent with antitumoral immunity as opposed to induced resistance mechanisms will be critical for advancing these approaches. For example, in some of the AdCD40L trials, intratumoral IL-8 and systemic IL-8 were observed to be modulated in some patients [80, 98]. IL-8 is a well-described chemoattractant for neutrophils/granulocytic MDSCs that can have both tumor- and immune-constraining influences.
Further considerations in the development of CD40 stimulation
Aside from the toxicity observed with targeting CD40 stimulation for immune-oncology purposes, our knowledge of the immunobiology of CD40 stimulation should provide some opportunities for optimization in the clinical setting. One of the most important considerations is the sequencing of CD40 stimulation, which will be context dependent when delivered as part of either conventional therapy or vaccination. Given that CD40 stimulation rapidly promotes the maturation of DCs, which initiates their migration to lymph nodes and increases the expression of costimulatory molecules and cytokines and that mature DCs have a reduced capacity to acquire antigens, providing CD40 stimulation prior to or coincident with conventional therapies may not be optimal. However, if CD40 stimulation is used to remodel the tumor microenvironment to allow more chemotherapy access, then sequencing CD40 prior to chemotherapy may be logical. However, increased toxicity has been reported when CD40 stimulation is introduced prior to chemotherapy [99]. Similar to cancer vaccines, the format of the antigen will be an important consideration for the timing of CD40 stimulation. Preprocessed MHC class I- or MHC class II-restricted peptides will be less dependent upon engulfment by DCs or macrophages, while recombinant proteins or tumor lysates will likely be best deployed in the context of relatively immature DCs that are subsequently activated via CD40 stimulation. Clearly, clinical trials will be needed to determine the relative efficacy with an empirical approach. It should also be noted that CD40 stimulation has been shown to have some antagonistic activity on T cells in animal models. In one report, infusion of agonistic CD40 as a monotherapy in tumor-bearing mice resulted in functional deletion of tumor-specific CD8 + T cells [100]. This situation was alleviated by vaccination with a recombinant virus expressing the targeted tumor antigen, suggesting that either virally induced inflammation or providing additional antigen was needed, as seen with the benefits of cotargeting PRR with CD40 stimulation. In unpublished studies from our lab intended to understand how long CD70 can be induced on DCs, it was observed that prevaccination infusion of anti-CD40 instilled a period of tolerance lasting 2–3 weeks that limited T cell expansion to peptide/protein vaccination or T cell responses to tumors (McClintic, H, Francica, B and Bullock, TNJ; manuscript in preparation). Thus, while these studies were performed in mice with transplantable tumors, they suggest that the sequencing of CD40 stimulation may be very important with respect to its impact on T cell responses.
Concluding remarks
Considerable attention has been given to the beneficial effects of removing the inherent “brakes” on antitumor immunity, either in the context of promoting preexisting T cell responses or supporting vaccination approaches. CD40 stimulation provides an opportunity to concurrently modulate the state of primarily myeloid cells within the TME, allowing for increased T cell priming, infiltration and functional activity. Advances in our knowledge of CD40 biology combined with results and immune correlate material from early-stage clinical trials will inevitably provide opportunities to promote the outcomes of CD40 stimulation whether in combination with traditional cancer vaccines or with interventions that result in autovaccination. Considerable opportunities exist for further development of rationally designed bispecifics or conjugates and incorporation into cellular therapies. This knowledge will likely result in the improved design, execution and outcomes of second-generation clinical trials and the development of novel treatment modalities that will improve clinical outcomes in patients.
Competing interests
The author declares no competing interests.
References
- 1.Mullins DW, Sheasley SL, Ream RM, Bullock TNJ, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003;198:1023–34. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013;19:465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature. 2020;584:624–629. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007;219:143–56. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/S0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 6.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD4O signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 7.Ridge JP, Di Rosa F, et al. A conditioned dendritic cell can be a temporal bridge between a CD4 + T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 8.Schoenberger SP, Toes REM, Van Dervoort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD4OL interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 9.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L) Crit. Rev. Immunol. 2017;37:371–420. doi: 10.1615/CritRevImmunol.v37.i2-6.100. [DOI] [PubMed] [Google Scholar]
- 10.Säemann MD, Kelemen P, Zeyda M, Böhmig G, Staffler G, and Zlabinger GJ. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. In Transplant. Proc. 2002;34:1407–8. [DOI] [PubMed]
- 11.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (Tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in t cells, is a dendritic cell-specific survival factor. J. Exp. Med. 1997;186:2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-κB subunits. Immunity. 2002;16:257–70. doi: 10.1016/S1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 13.Miga AJ, Masters SR, Durell BG, Gonzalez M, Jenkins MK, Maliszewski C, et al. Dendritic cell longevity and T cell persistence is controlled by CD154-CD40 interactions. Eur. J. Immunol. 2001;31:959–65. doi: 10.1002/1521-4141(200103)31:3<959::AID-IMMU959>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Ahonen CL, Gibson SJ, Smith RM, Pederson LK, Lindh JM, Tomai MA, et al. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell. Immunol. 1999;197:62–72. doi: 10.1006/cimm.1999.1555. [DOI] [PubMed] [Google Scholar]
- 15.Bullock TNJ, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8 + T cell responses in the absence of CD4 + T cells. J. Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 16.Van Deusen KE, Rajapakse R, Bullock TNJ. CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell-dependent, licensed CD8+ T cell responses. J. Leukoc. Biol. 2010;87:477–85. doi: 10.1189/jlb.0809535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H-I, Jung S-H, Sohn H-J, Celis E, Kim T-G. An optimized peptide vaccine strategy capable of inducing multivalent CD8 + T cell responses with potent antitumor effects. Oncoimmunology. 2015;4:e1043504. doi: 10.1080/2162402X.2015.1043504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne KT, Vonderheide RH. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 2016;15:2719–32. doi: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc. Natl Acad. Sci. U.S.A. 2020;117:8022–31. doi: 10.1073/pnas.1918971117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stromnes IM, Burrack AL, Hulbert A, Bonson P, Black C, Brockenbrough JS, et al. Differential effects of depleting versus programming tumor-associated macrophages on engineered T cells in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 2019;7:977–89. doi: 10.1158/2326-6066.CIR-18-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu M, Odagiu L, Gaudot L, Daudelin JF, Melichar HJ, Lapointe R, et al. Inflammation enhances the vaccination potential of CD40-activated B cells in mice. Eur. J. Immunol. 2017;47:269–79. doi: 10.1002/eji.201646568. [DOI] [PubMed] [Google Scholar]
- 23.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 24.Prinzing B, Schreiner P, Bell M, Fan Y, Krenciute G, and Gottschalk S. MyD88/CD40 signaling retains CAR T cells in a less differentiated state. JCI Insight. 2020;5:e136093. [DOI] [PMC free article] [PubMed]
- 25.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J. Clin. Invest. 2013;123:1999–2010. doi: 10.1172/JCI63980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Peter W, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 cos. Blood. 2013;109:4810–5. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 27.Schmielau J, Finn OJ, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 28.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of cd40. Nat. Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 29.van Mierlo GJD, den Boer AT, Medema JP, van der Voort EIH, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl Acad. Sci. U. S. A. 2002;99:5561–6. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehl L, Den Boer AT, Schoenberger SP, Van Der Voort EIH, Schumacher TNM, Melief CJM, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 31.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl Acad. Sci. U.S.A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter EL, Mick R, Rüter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J. Transl. Med. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 2010;33:769–79. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrends T, Baba A N, Xiao Y, Yagita H, van Eenennaam H, Borst J. CD27 agonism plus PD-1 blockade recapitulates CD4+ T-cell help in therapeutic anticancer vaccination. Cancer Res. 2016;76:2921–31. doi: 10.1158/0008-5472.CAN-15-3130. [DOI] [PubMed] [Google Scholar]
- 36.Riccione KA, He L-Z, Fecci PE, Norberg PK, Suryadevara CM, Swartz A, et al. CD27 stimulation unveils the efficacy of linked class I/II peptide vaccines in poorly immunogenic tumors by orchestrating a coordinated CD4/CD8 T cell response. Oncoimmunology. 2018;7:e1502904. doi: 10.1080/2162402X.2018.1502904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burris HA, Infante JR, Ansell SM, Nemunaitis JJ, Weiss GR, Villalobos VM, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J. Clin. Oncol. 2017;35:2028–36. doi: 10.1200/JCO.2016.70.1508. [DOI] [PubMed] [Google Scholar]
- 38.Kumai T, Lee S, Il Cho H, Sultan H, Kobayashi H, Harabuchi Y, et al. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol. Res. 2017;5:72–83. doi: 10.1158/2326-6066.CIR-16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borrás-Cuesta F, et al. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer Immunol. Immunother. 2008;57:19–29. doi: 10.1007/s00262-007-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens AD, Bullock TNJ. Therapeutic vaccination targeting CD40 and TLR3 controls melanoma growth through existing intratumoral CD8 T cells without new T cell infiltration. Cancer Immunol. Immunother. 2021. 10.1007/s00262-020-02841-z. [DOI] [PMC free article] [PubMed]
- 41.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat. Med. 2013;19:730–8. doi: 10.1038/nm.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1 + PD-1 + CD8 + T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50:195–211.e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016;534:396–401. [DOI] [PubMed]
- 45.Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019;565;234–9. [DOI] [PMC free article] [PubMed]
- 47.Hu Z, Leet DE, Allesøe RL, Oliveira G, Li S, Luoma AM, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021;27:515–25. doi: 10.1038/s41591-020-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J. Immunol. 2009;182:5217–24. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 49.Khong A, Cleaver AL, Fahmi Alatas M, Wylie BC, Connor T, Fisher SA, et al. The efficacy of tumor debulking surgery is improved by adjuvant immunotherapy using imiquimod and anti-CD40. BMC Cancer 2014;14:969–77. [DOI] [PMC free article] [PubMed]
- 50.Khalil DN, Suek N, Campesato LF, Budhu S, Redmond D, Samstein RM, et al. In situ vaccination with defined factors overcomes T cell exhaustion in distant tumors. J. Clin. Invest. 2019;129:3435–47. doi: 10.1172/JCI128562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nimanong S, Ostroumov D, Wingerath J, Knocke S, Woller N, Gürlevik E, et al. CD40 signaling drives potent cellular immune responses in heterologous cancer vaccinations. Cancer Res. 2017;77:1918–26. doi: 10.1158/0008-5472.CAN-16-2089. [DOI] [PubMed] [Google Scholar]
- 52.Hermans IF, Ritchie DS, Daish A, Yang J, Kehry MR, Ronchese F. Impaired ability of MHC class II(-/-) dendritic cells to provide tumor protection is rescued by CD40 ligation. J. Immunol. 1999;163:77–81. [PubMed] [Google Scholar]
- 53.Ribas A, Butterfield LH, Amarnani SN, Dissette VB, Kim D, Meng WS, et al. CD40 cross-linking bypasses the absolute requirement for CD4 T cells during immunization with melanoma antigen gene-modified dendritic cells. Cancer Res. 2001;61:8787–93. [PubMed] [Google Scholar]
- 54.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J. Immunol. 2003;171:6275–82. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 55.Lau SP, van Montfoort N, Kinderman P, Lukkes M, Klaase L, van Nimwegen M, et al. Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model. J. Immunother. Cancer. 2020;8:772. doi: 10.1136/jitc-2020-000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luheshi NM, Coates-Ulrichsen J, Harper J, Mullins S, Sulikowski MG, Martin P, et al. Transformation of the tumour microenvironment by a CD40 agonist antibody correlates with improved responses to PD-L1 blockade in a mouse orthotopic pancreatic tumour model. Oncotarget. 2016;7:18508–20. doi: 10.18632/oncotarget.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma HS, Poudel B, Torres ER, Sidhom J-W, Robinson TM, Christmas B, et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of nonimmunogenic tumors to allow T-cell–mediated anticancer activity. Cancer Immunol. Res. 2019;7:428–42. doi: 10.1158/2326-6066.CIR-18-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh MP, Sethuraman SN, Ritchey J, Fiering S, Guha C, Malayer J, et al. In-situ vaccination using focused ultrasound heating and anti-CD-40 agonistic antibody enhances T-cell mediated local and abscopal effects in murine melanoma. Int. J. Hyperth. 2019;36:64–73. doi: 10.1080/02656736.2019.1663280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4+T cells to induce anti-tumour immunity. Nature. 2020;584:624–9. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell. 2019;177:556–571.e16. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 63.Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade–mediated tumor regression. J. Clin. Invest. 2018;128:805–15. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, et al. PD-L1 on host cells is essential for PD-L1 blockade–mediated tumor regression. J. Clin. Invest. 2018;128:580–588. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zippelius A, Schreiner J, Herzig P, Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol. Res. 2015;3:236–44. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]
- 66.Gu T, Zhu Y, Chen C, Li M, Chen Y, Yu G, et al. Fine-tuned expression of programmed death 1 ligands in mature dendritic cells stimulated by CD40 ligand is critical for the induction of an efficient tumor specific immune response. Cell. Mol. Immunol. 2008;5:33–39. doi: 10.1038/cmi.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varthaman A, Moreau HD, Maurin M, Benaroch P. TLR3-Induced maturation of murine dendritic cells regulates CTL responses by modulating PD-L1 trafficking. PLoS One 2016;11:e0167057. [DOI] [PMC free article] [PubMed]
- 68.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013;19:1035–43. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev. Anticancer Ther. 2017;17:175–86. doi: 10.1080/14737140.2017.1270208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djureinovic D, Wang M, Kluger HM. Agonistic cd40 antibodies in cancer treatment. Cancers. 2021;13:1–18. doi: 10.3390/cancers13061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu C, Lewis CM, Lou Y, Xu C, Peng W, Yang Y, et al. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J. Immunother. 2012;35:276–82. doi: 10.1097/CJI.0b013e31824e7f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oba T, Hoki T, Yamauchi T, Keler T, Marsh HC, Cao X, et al. A critical role of CD40 and CD70 signaling in conventional type 1 dendritic cells in expansion and antitumor efficacy of adoptively transferred tumor-specific T cells. J. Immunol. 2020;205:1867–77. doi: 10.4049/jimmunol.2000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuhn NF, Lopez AV, Li X, Cai W, Daniyan AF, Brentjens RJ. CD103+ cDC1 and endogenous CD8+ T cells are necessary for improved CD40L-overexpressing CAR T cell antitumor function. Nat. Commun. 2020;11:6171. [DOI] [PMC free article] [PubMed]
- 74.Zhang Y, Wang P, Wang T, Fang Y, Ding Y, Qian Q. Chimeric antigen receptor T cells engineered to secrete CD40 agonist antibodies enhance antitumor efficacy. J. Transl. Med. 2021;19:82. doi: 10.1186/s12967-021-02750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleinberg LR. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: Implication for patient management. Cancer Invest. 2004;2004/04/09:1–9. doi: 10.1081/CNV-120027575. [DOI] [PubMed] [Google Scholar]
- 76.Kikuchi T, Miyazawa N, Moore MAS, Crystal RG. Tumor regression induced by intratumor administration of adenovirus vector expressing CD40 ligand and naive dendritic cells. Cancer Res. 2000;60:6391–5. [PubMed] [Google Scholar]
- 77.Sorensen MR, Holst PJ, Steffensen MA, Christensen JP, Thomsen AR. Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine. 2010;28:6757–64. doi: 10.1016/j.vaccine.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 78.Ylösmäki E, Ylösmäki L, Fusciello M, Martins B, Ahokas P, Cojoc H, et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. - Oncolytics. 2021;20:459–69. doi: 10.1016/j.omto.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zafar S, Basnet S, Launonen IM, Quixabeira DCA, Santos J, Hemminki O, et al. Oncolytic adenovirus type 3 coding for CD40L facilitates dendritic cell therapy of prostate cancer in humanized mice and patient samples. Hum. Gene Ther. 2021;32:192–202. doi: 10.1089/hum.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irenaeus S, Hellström V, Wenthe J, Krause J, Sundin A, Ahlström H, et al. Intratumoral immunostimulatory AdCD40L gene therapy in patients with advanced solid tumors. Cancer Gene Ther. 2020;1–10. 10.1038/s41417-020-00271-8. [DOI] [PubMed]
- 81.Gupta S, Termini JM, Rivas Y, Otero M, Raffa FN, Bhat V, et al. A multi-trimeric fusion of CD40L and gp100 tumor antigen activates dendritic cells and enhances survival in a B16-F10 melanoma DNA vaccine model. Vaccine. 2015;33:4798–806. doi: 10.1016/j.vaccine.2015.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miconnet I, Pantaleo G. A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. Vaccine. 2008;26:4006–14. doi: 10.1016/j.vaccine.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 83.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White AL, Chan HTC, Roghanian A, French RR, Mockridge CI, Tutt AL, et al. Interaction with FcγRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J. Immunol. 2011;187:1754–63. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 85.Filbert EL, Björck PK, Srivastava MK, Bahjat FR, and Yang X. APX005M, a CD40 agonist antibody with unique epitope specificity and Fc receptor binding profile for optimal therapeutic application. Cancer Immunol. Immunother. 2021;70:1853–65. [DOI] [PMC free article] [PubMed]
- 86.Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer Immunol. Res. 2014;2:19–26. doi: 10.1158/2326-6066.CIR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richman L, Vonderheide R. Anti-human CD40 monoclonal antibody therapy is potent without FcR crosslinking. Oncoimmunology. 2014;3:e28610. doi: 10.4161/onci.28610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001;19:3280–7. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 89.Soldevilla MM, Villanueva H, Bendandi M, Inoges S, López-Díaz de Cerio A, Pastor F. 2-fluoro-RNA oligonucleotide CD40 targeted aptamers for the control of B lymphoma and bone-marrow aplasia. Biomaterials. 2015;67:274–85. doi: 10.1016/j.biomaterials.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 90.de Weerdt I, Lameris R, Scheffer GL, Vree J, de Boer R, Stam AG, et al. A bispecific antibody antagonizes prosurvival CD40 signaling and promotes Vγ9Vδ2 T cell-mediated antitumor responses in human B-cell malignancies. Cancer Immunol. Res. 2021;9:50–61. doi: 10.1158/2326-6066.CIR-20-0138. [DOI] [PubMed] [Google Scholar]
- 91.Luke JJ, Barlesi F, Chung K, Tolcher AW, Kelly K, Hollebecque A, et al. Phase I study of ABBV-428, a mesothelin-CD40 bispecific, in patients with advanced solid tumors. J. Immunother. Cancer 2021;9:e002015. [DOI] [PMC free article] [PubMed]
- 92.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 93.Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018;7:e1468956. doi: 10.1080/2162402X.2018.1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh M, Vianden C, Cantwell MJ, Dai Z, Xiao Z, Sharma M, et al. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat. Commun. 2017;8:1447. [DOI] [PMC free article] [PubMed]
- 95.O’Hara MH, O’Reilly EM, Varadhachary G, Wolff RA, Wainberg ZA, Ko AH, et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. 2021;22:118–31. doi: 10.1016/S1470-2045(20)30532-5. [DOI] [PubMed] [Google Scholar]
- 96.Yu X, Chan HTC, Fisher H, Penfold CA, Kim J, Inzhelevskaya T, et al. Isotype switching converts anti-CD40 antagonism to agonism to elicit potent antitumor activity. Cancer Cell. 2020;37:850–866.e7. doi: 10.1016/j.ccell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loskog A, Maleka A, Mangsbo S, Svensson E, Lundberg C, Nilsson A, et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br. J. Cancer. 2016;114:872–80. doi: 10.1038/bjc.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schiza A, Wenthe J, Mangsbo S, Eriksson E, Nilsson A, Tötterman TH, et al. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J. Transl. Med. 2017;15:79. doi: 10.1186/s12967-017-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byrne KT, Leisenring NH, Bajor DL, Vonderheide RH. CSF-1R–dependent lethal hepatotoxicity when agonistic CD40 antibody is given before but not after chemotherapy. J. Immunol. 2016;197:179–87. doi: 10.4049/jimmunol.1600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8+ T cells in the absence of tumor-antigen vaccination. Proc. Natl Acad. Sci. U.S.A. 2001;98:10811–6. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]