Abstract
Allergic contact dermatitis (ACD) and atopic dermatitis (AD) are inflammatory eczematous skin diseases caused by various factors. Here, we report that topical application of the dipeptide, L-glutamic acid-L-tryptophan (L-Glu-L-Trp), improved symptoms in both ACD and AD in mice. Using a mouse model of ACD induced by repeated application of 2,4-dinitorofluorbenzene (DNFB), we demonstrated that L-Glu-L-Trp attenuated DNFB-induced skin thickening. In addition, quantification of cytokines in serum revealed that L-Glu-L-Trp suppressed the DNFB-induced increase in the interleukin (IL)-22 level. Moreover, L-Glu-L-Trp attenuated mite antigen extract-induced AD model symptoms such as the increase of skin thickening and elevation of serum IL-22. We also confirmed that the dipeptide structure rather than the individual amino acid components was important for the therapeutic effects of L-Glu-L-Trp. Furthermore, we showed that IL-22 decreased the expression level of filaggrin mRNA in human epidermal keratinocytes, and L-Glu-L-Trp attenuated that effect. These results suggested that the topical application of the dipeptide, L-Glu-L-Trp, to the skin may be useful for treating ACD and AD.
Keywords: Allergic contact dermatitis, Atopic dermatitis, Interleukin-22, Dipeptide, L-Glu-L-Trp
Allergic contact dermatitis, Atopic dermatitis, Interleukin-22, Dipeptide, L-Glu-L-Trp.
1. Introduction
Contact dermatitis is a disease in which the skin is irritated by direct contact with specific substances. It is classified into irritant contact dermatitis and allergic contact dermatitis (ACD). During irritant contact dermatitis, the skin is damaged nonspecifically by external factors, and an inflammatory response in the epidermis is elicited by mediators [1]. ACD is a delayed type IV hypersensitivity reaction induced by an immune reaction for specific proteins modified by haptens such as dicyclohexylcarbodiimide and oxazolone [2]. 2,4-Dinitorofluorbenzene (DNFB), a strong skin sensitizer, is also known to give rise to ACD by repeated topical application to mice skin. These ACD experimental models are often used for drug evaluation [3].
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by severe itching and dryness. It is estimated that up to 20% of children, and up to 3% of adults worldwide, have AD, and that the number of patients is increasing. Thus, preventing and/or treating AD is an important global issue [4]. Human patients with AD are highly sensitive to house dust mite antigen. NC/Nga mice are a unique strain that can develop the characteristics of AD. Specifically, the application of a house dust mite (Dermatophagoides farina body, Dfb) antigen extract to the skin of NC/Nga mice can induce AD symptoms [5, 6].
The dipeptide, L-glutamic acid-L-tryptophan (L-Glu-L-Trp), was isolated originally from the native calf thymus peptide complex [7]. It has been reported that L-Glu-L-Trp exhibits immunomodulatory, anti-stress, and anti-tumor effects [8]. However, its detailed mechanism of action remains unclear. Furthermore, the effects of the dipeptide on dermatitis haven not been validated using established experimental models. In the present study, we report that topical application of L-Glu-L-Trp ameliorates the symptoms of ACD and AD in mice.
2. Materials and methods
2.1. Reagents
The disodium salt of the synthetically manufactured dipeptide, L-Glu-L-Trp, was provided by Implicit Biosciences (Woolloongabba, Australia) or synthesized by Biologica (Nagoya, Japan).
2.2. Animal experiments
All animal experiments in this study were conducted with the consent of the Animal Research Committee of Rohto Pharmaceutical Co., Ltd., Japan.
2.3. Induction of ACD in mice
Six-week-old male BALB/c mice were purchased from CLEA Japan (Tokyo, Japan). ACD was induced at 7 weeks of age. DNFB (Nacalai Tesque, Kyoto, Japan; lot.no. MIA5731) was applied twice, the day after hair shaving and two days later at 0.5% to the shaved abdomen for sensitization. Four days after the first sensitization, 20 μL of 0.2% DNFB was applied to the right ear. This was done once a week for three weeks. L-Glu-L-Trp (0.05 and 0.5%) was applied at 20 uL daily on weekdays over 3 weeks. Measurements of the ear thickness were performed using a micrometer.
2.4. Induction of AD-like lesions in NC/Nga mice
Nine-week-old male NC/Nga mice were purchased from Japan SLC (Hamamatsu, Japan). The Biostir AD cream was applied twice a week for 5 weeks (total 10 times) from 10 weeks to 14 weeks of age to induce dermatitis (Figure 2A). Hair of the dorsal skin of NC/Nga mice was shaved off using an electric razor. A 4% aqueous sodium dodecyl sulfate solution (150 μL) was applied to the back of all mice except the control (no treatment group) prior to 100 mg of Biostir AD cream (a Dfb extract) (Biostir, Osaka, Japan).
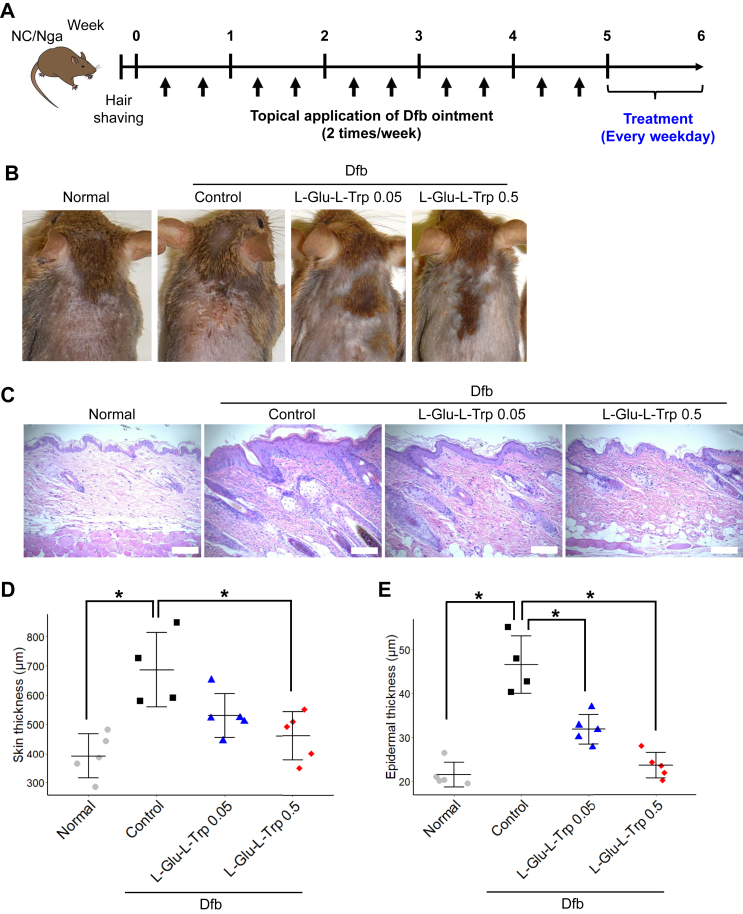
Figure 2.
L-Glutamic acid-L-tryptophan (L-Glu-L-Trp) treatment of Dermatophagoides farinae body (Dfb)-induced atopic dermatitis in mice. (A) Schematic of the experimental protocol for Dfb-induced atopic dermatitis in mice. (B) Representative images of mouse back skin. (C) Representative histological images of mouse skin. Hematoxylin and eosin staining. Scale bar, 10 μm. (D) Quantitation of skin thickness. Data are presented as means ± SD. Individual data points are shown. n = 4 or 5, ∗p < 0.05, ∗∗p < 0.01. (E) Quantitation of epidermis thickness. Data are presented as means ± SD. Individual data points are shown. n = 4 or 5, ∗p < 0.05.
2.5. Measurements of cytokine and chemokine levels in serum
Blood samples were collected from mice after treatment and serum was isolated by centrifugation at 1700 x g for 10 min. Samples were stored at -80 °C until use. Serum was analyzed simultaneously for multiple T helper (Th)17 cell cytokines and chemokines with Th17 Bead-Based Multiplex Assays using the Luminex 100 with xPONENT software (v3.1) system (Luminex, Austin, TX, USA). For analyzing serum interleukin (IL)-22 levels, a mouse IL-22 Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA; #MTLP00) was used according to the manufacturer's instructions.
2.6. Histological analysis
Skin samples were isolated at week six and fixed with 10% formalin and embedded in paraffin. Sections of paraffin embedded samples were stained with hematoxylin and eosin (H&E). Skin samples were observed and measured with a digital VHX-1000 microscope (Keyence, Osaka, Japan). Skin thickness was evaluated in H&E-stained sections as the distance from the bottom of the stratum corneum to the deepest portion of the reticular dermis where the adipose tissue or the muscle was reached. The epidermal thickness in H&E-stained sections was also evaluated by the distance between the top of the basement membrane and the bottom of the stratum corneum. Three randomly selected fields from each section were measured.
2.7. Cell culture
Normal human epidermal keratinocytes (NHEKs) were purchased from Kurabo (Kurashiki, Japan). NHEKs were cultured in KG-2 medium (Kurabo) at 37 °C under 5% CO2.
2.8. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from NHEKs and purified using an RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA). RNA (500 μg) was subject to the reverse transcription reaction using a SuperScript II system (Invitrogen, Carlsbad, CA, USA). qRT-PCR was carried out on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The Taqman probes used in this study were FLG (Hs00856927_g1) and housekeeping 18SrRNA (Hs99999901_s1).
2.9. Statistical analyses
Statistical analyses were performed using StatLight 2000 software (Yukms, Kawasaki, Japan) or BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Two-tailed student's t-test was used for comparison of the two groups after checking the normal distribution by Shapiro-Wilk test. For multiple comparisons to the control, after homoscedasticity was tested with Bartlett's test, Dunnett's test (for homoscedastic data) or Steel's test (for heteroscedastic data) were used. In all analyses, p < 0.05 was regarded as statistically significant.
3. Results
3.1. L-Glu-L-Trp attenuated the increased ear thickness and IL-22 serum levels in DFNB-induced contact hypersensitivity
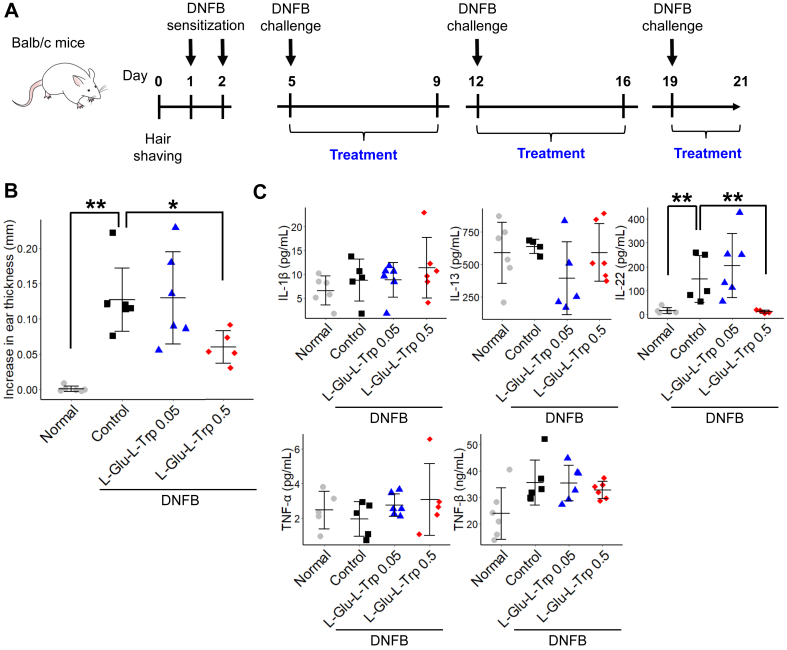
To evaluate the effects of the dipeptide, L-Glu-L-Trp, on ACD, we used a DNFB-induced contact hypersensitivity model in mice. After hair shaving, DNFB was applied twice onto the abdominal skin for elicitation. Thereafter, DNFB was applied to the pinna of the ear once a week for three weeks. At day 21, we measured the ear thickness (Figure 1A). We found that the DNFB challenges induced an increase in ear thickness that was attenuated by topical application of 0.5% but not 0.05% L-Glu-L-Trp (Figure 1B). Because Th17 cells are involved in the pathology of ACD [9], we measured the levels of Th17 cell-related cytokines and chemokines in mouse serum using the Milliplex assay. Figure 1C shows the cytokines that could be detected. DNFB challenges led to an increase of IL-22 in serum. Interestingly, L-Glu-L-Trp treatment significantly reduced the DNFB-induced IL-22 level (Figure 1C). These results indicated that the application of L-Glu-L-Trp was useful for treating skin in a model of contact dermatitis.
Figure 1.
L-Glutamic acid-L-tryptophan (L-Glu-L-Trp) treatment of 2,4-dinitorofluorbenzene (DNFB)-induced contact dermatitis in mice. (A) Schematic of the experimental protocol for DNFB-induced contact dermatitis in mice. (B) Increase in ear thickness. Data are presented as means ± SD. Individual data points are shown. n = 5 or 6. ∗p < 0.05. (C) Quantitation of detectable cytokines in serum. Data are presented as means ± SD. Individual data points are shown. n = 5 or 6. ∗p < 0.05.
3.2. L-Glu-L-Trp improved Dfb-induced AD in mice
We next examined whether L-Glu-L-Trp was effective for treating a model of AD elicited in mice by repeated application of Dfb cream to the shaved-dorsal skin of NC/Nga mice. After inducing skin inflammation, we applied L-Glu-L-Trp topically to the skin daily (Figure 2A). Dermatitis was clearly evident in the control mice, and treatment with L-Glu-L-Trp reduced the severity of symptoms (Figure 2B). We also analyzed the skin histologically. Dfb application induced infiltration of inflammatory cells and thickening of the skin, especially the epidermis. These phenotypes induced by Dfb were suppressed by L-Glu-L-Trp application (Figure 2C). Quantitative analysis showed that both 0.05 and 0.5% L-Glu-L-Trp treatment suppressed the increased thickness of the skin and epidermis compared with control mice. Application of 0.5% L-Glu-L-Trp almost completely suppressed epidermal hyperplasia (Figure 2D). Therefore, application of L-Glu-L-Trp to the skin was also effective for treating an AD model.
3.3. The dipeptide structure but not the individual amino acids was important for attenuation of dermatitis
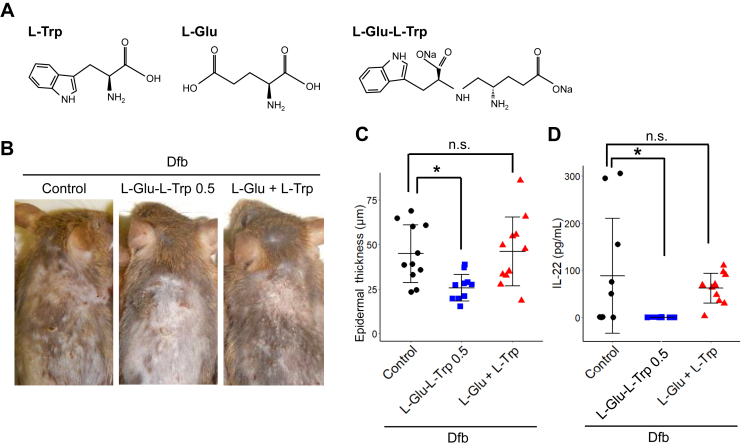
Next, we sought to determine whether the effectiveness of L-Glu-L-Trp was due to its constituent amino acids. L-Glu-L-Trp or a mixture of L-Trp and L-Glu was applied to the AD model induced in the same manner as in Figure 2 (Figure 3A). The mixture of amino acids had no inhibitory effect on skin thickening; only application of the dipeptide, L-Glu-L-Trp, suppressed Dfb-induced skin thickening (Figure 3B, C).
Figure 3.
Effects of L-glutamic acid-L-tryptophan (L-Glu-L-Trp) and a mixture of L-Glu and L-Trp on atopic dermatitis. (A) Structure of L-Glu-L-Trp, L-glutamic acid, and L-tryptophan. (B) Representative images of mouse back skin. (C) Quantitation of skin thickness. Data are presented as means ± SD. Individual data points are shown. n = 11, ∗p < 0.05. (D) Quantitation of IL-22 in serum. Data are presented as means ± SD. Individual data points are shown. n = 11, ∗p < 0.05. NA, non-significant (p > 0.05).
Similarly, the increased amount of IL-22 in the blood caused by Dfb application was suppressed by L-Glu-L-Trp, but not by the amino acid mixture (Figure 3D). Therefore, it appears that the dipeptide structure, but not the amino acid components, is important in the efficacy of L-Glu-L-Trp for dermatitis.
3.4. L-Glu-L-Trp mitigates IL-22-induced filaggrin gene downregulation
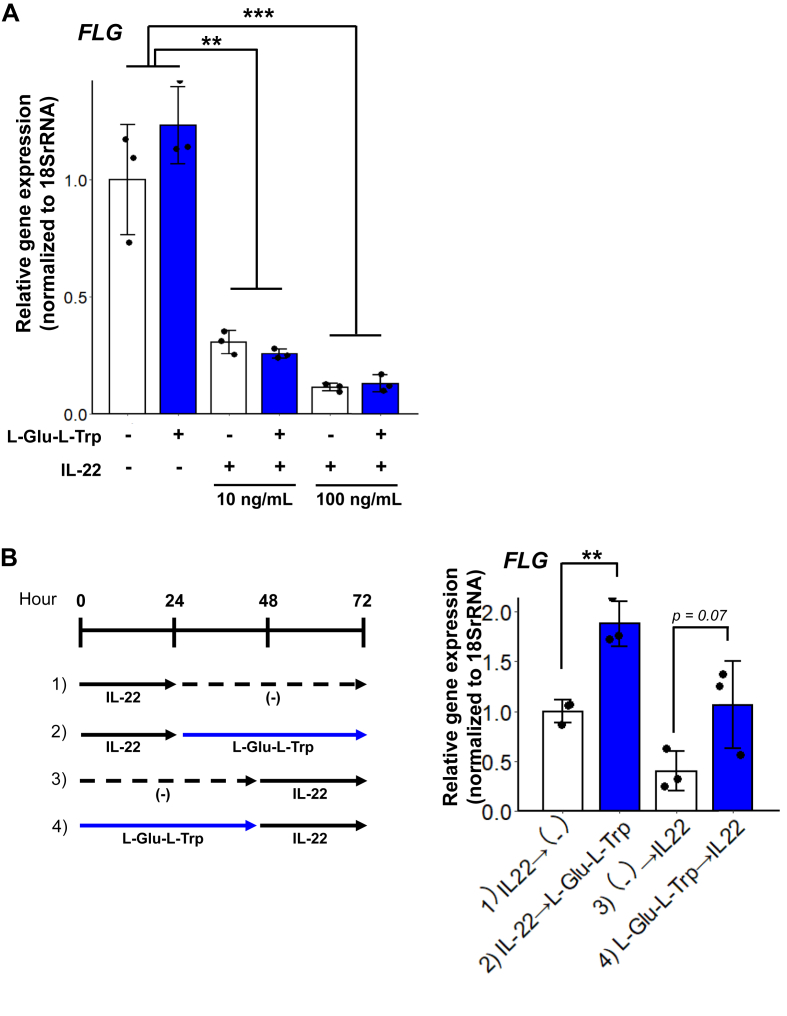
We next examined the effect of L-Glu-L-Trp and IL-22 on epidermal cells. L-Glu-L-Trp and recombinant IL-22 were added to NHEKs at concentrations of 10 or 100 ng/mL. Expression of the filaggrin gene, which is important for epidermal barrier function, was examined using qRT-PCR. Filaggrin mRNA levels in keratinocytes was decreased by IL-22 concentration-dependently (Figure 4A).
Figure 4.
Expression of the filaggrin (FLG) gene in normal human epidermal keratinocytes (NHEKs) treated with interleukin (IL)-22 and L-glutamic acid-L-tryptophan (L-Glu-L-Trp). (A) Relative gene expression levels of FLG in NHEKs treated with IL-22 (10 or 100 ng/mL) and L-Glu-L-Trp (0.004%). Data are presented as means ± SD. n = 3. (B) Schematic of the experiments for pre- or post-treatment with L-Glu-L-Trp. (left). Relative gene expression levels of FLG in NHEKs treated with IL-22 (10 ng/mL) or L-Glu-L-Trp (0.004%) (right). Data are presented as means ± SD. n = 3. ∗∗p < 0.01.
Next, we examined the effect of L-Glu-L-Trp on filaggrin gene expression in NHEKs supplemented with IL-22 (Figure 4B). L-Glu-L-Trp did not affect filaggrin mRNA levels when treated for 72 h with IL-22. However, when L-Glu-L-Trp was administered for 48 h before and after a 24 h IL-22 treatment, expression of the filaggrin gene was increased significantly in NHEKs (Figure 4C). These results show that IL-22 decreased expression of the filaggrin gene in NHEKs, and the decrease was reduced by pre- and post-treatment with L-Glu-L-Trp.
4. Discussion
L-Glu-L-Trp is a natural dipeptide that can be isolated from the calf thymic peptide complex and possesses immunomodulatory properties [8, 10]. Because it is isolated from the thymus, the dipeptide may have some functions related to immunity. ACD and AD are common allergic and inflammatory skin diseases caused by a combination of eczema, scratching, and skin sensitization with allergens. In this study, we show efficacy of L-Glu-L-Trp on mouse models of ACD and AD. Specifically, topical application of L-Glu-L-Trp reduced ear swelling in a DNFB-induced ACD model, and skin thickness in a Dfb-induced AD model (Figures 1, 2).
ACD develops following two phases of hypersensitivity: sensitization and elicitation. In the elicitation phase, the allergen interacts with a hapten in the skin to activate keratinocytes, Langerhans cells, and skin dendritic cells. Chemokines and cytokines produced by these activated cells cause the infiltration of antigen-specific T cells into the skin. Activated T cells cause additional attraction of T cells into skin areas and cytokine production, which leads to further skin inflammation [11, 12].
AD is a form of dermatitis with an impaired barrier function and dryness, and chronic or recurrent eczema and severe itching. AD is also a skin disease closely related to the immune response, and is considered to be a T cell-mediated disease. Immune (T, mast, and Langerhans cells) and skin (keratinocytes and fibroblasts) cells are important players in both ACD and AD pathologies.
We found that L-GLu-L-Trp suppressed the amount of IL-22 in serum in both the ACD and AD mouse models. IL-22 belongs to the IL-10 cytokine family, and is secreted mainly from Th17 and Th22 cells. Several studies have reported higher levels of IL-22 in sera from patients with psoriasis [13] or nickel-induced contact dermatitis [14]. Even in AD skin tissue, the frequency of IL-22-producing CD4+ and CD8+ cells is high, and the latter correlates with the severity of AD disease [15]. Therefore, IL-22 may be involved in the pathogenesis of various dermatitides caused by inflammation and the immune response.
Aryl hydrocarbon receptors (AhRs) are factors controlling IL-22 production. AhRs are ligand-dependent transcription factors that mediate the toxicity of chemicals such as dioxin [16]. Th17 cells are a major source of IL-22, and AhRs are required for IL-22 production by these cells [17]. Trp metabolites are known ligands for AhR [18]. Because L-Glu-L-Trp has Trp as a component, L-Glu-L-Trp might act on AhR to regulate IL-22 production. In the present study, we tested a mixture of L-Glu and L-Trp, the component amino acids of the dipeptide, in the AD model but found no efficacy against dermatitis (Figure 3). This suggests that the dipeptide structure is important for the efficacy of L-Glu-L-Trp.
We showed that IL-22 reduced filaggrin gene expression in NHEKs. This is consistent with findings reported in a study using HaCaT cells, immortalized keratinocytes [19]. Filaggrin is a key molecule for homeostasis and barrier functions in skin. Inherent or acquired filaggrin deficiency is involved in progression of the pathology of chronic dermatitis such as AD [20]. In AD skin lesions, IL-22-producing cells, such as Th17 cells, infiltrate into the skin [21]. This suggests that IL-22 released by Th17 cells homed to the skin acts on the epidermis to reduce the expression of filaggrin. Interestingly, IL-22-induced downregulation of the filaggrin gene in NHEKs was ameliorated by pre- and post-treatment with L-Glu-L-Trp (Figure 4). This suggests that L-Glu-L-Trp not only has the effect of lowering the amount of IL-22 in blood, but also benefit keratinocytes whose filaggrin was reduced by IL-22. The enhancement of filaggrin expression by the use of AhR ligands is expected to be useful for treating AD [22, 23, 24]. Because the structure of L-Glu-L-Trp is similar to an AhR ligand, it might have some influence on the activity of the AhR. Further studies are needed to elucidate the mechanism of L-Glu-L-Trp.
Conventional treatment of ACD and AD often involves the topical or systemic use of immunosuppressants such as corticosteroids, cyclosporine, and tacrolimus. However, these drugs can have side effects with prolonged use [25, 26]. Because peptide drugs are composed of endogenous amino acids, they are considered to have high potential therapeutic activity and low toxicity. In addition, dipeptides have higher cell permeability than polypeptides [27].
In conclusion, the results of this study show that the dipeptide, L-Glu-L-Trp, is effective for treating in vivo mouse models of ACD and AD. The application of this dipeptide is expected to lead to the establishment of new therapeutic agents for chronic and inflammatory skin disease including AD and ACD.
Declarations
Author contribution statement
Shun Shibata: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Akiko Kuwahara: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Masayo Sakaki-Yumoto: Conceived and designed the experiments.
Yoichi Honma: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Tsuyoshi Ishii and Makoto Kawaguchi: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare the following conflict of interests: This work was performed as part of a collaborative research project with Implicit Bioscience. The L-Glu-L-Trp used in this study was provided by Implicit Bioscience.
Additional information
No additional information is available for this paper.
References
- 1.Litchman G., Nair P.A., Atwater A.R., Gossman W.G. StatPearls Publishing; 2020. Contact Dermatitis.http://www.ncbi.nlm.nih.gov/pubmed/29083649 [PubMed] [Google Scholar]
- 2.Novak-Bilić G., Vučić M., Japundžić I., Meštrović-Štefekov J., Stanić-Duktaj S., Lugović-Mihić L. Irritant and allergic contact dermatitis - skin lesion characteristics., Acta Clin. Croatica. 2018;57:713–720. doi: 10.20471/acc.2018.57.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M., Qu S., Ma J., Wang X., Yang Y. Metformin suppresses LPS-induced inflammatory responses in macrophage and ameliorates allergic contact dermatitis in mice via autophagy. Biol. Pharm. Bull. 2020;43:129–137. doi: 10.1248/bpb.b19-00689. [DOI] [PubMed] [Google Scholar]
- 4.Avena-Woods C. Overview of atopic dermatitis., Am. J. Manag. Care. 2017;23:S115–S123. http://www.ncbi.nlm.nih.gov/pubmed/28978208 [PubMed] [Google Scholar]
- 5.Yamamoto M., Haruna T., Yasui K., Takahashi H., Iduhara M., Takaki S., Deguchi M., Arimura A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007;56:139–148. doi: 10.2332/allergolint.O-06-458. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka H., Maki N., Yoshida S., Arai M., Wang J., Oikawa Y., Ikeda T., Hirota N., Nakagawa H., Ishii A. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003;58:139–145. doi: 10.1034/j.1398-9995.2003.23790.x. [DOI] [PubMed] [Google Scholar]
- 7.Morozov V.G., Khavinson V.K. Natural and synthetic thymic peptides as therapeutics for immune dysfunction. Int. J. Immunopharm. 1997;19:501–505. doi: 10.1016/s0192-0561(97)00058-1. [DOI] [PubMed] [Google Scholar]
- 8.Anisimov V.N., Khavinson V.K., Morozov V.G. Immunomodulatory synthetic dipeptide L-Glu-L-Trp slows down aging and inhibits spontaneous carcinogenesis in rats. Biogerontology. 2000;1:55–59. doi: 10.1023/a:1010042008969. [DOI] [PubMed] [Google Scholar]
- 9.Peiser M. Role of Th17 cells in skin inflammation of allergic contact dermatitis. Clin. Dev. Immunol. 2013;2013:261037. doi: 10.1155/2013/261037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergeon J.A., Chan Y.-N., Charles B.G., Toth I. Oral absorption enhancement of dipeptide L-Glu-L-Trp-OH by lipid and glycosyl conjugation. Biopolymers. 2008;90:633–643. doi: 10.1002/bip.21003. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K., Meguro K., Nakagomi D., Nakajima H. Roles of alternatively activated M2 macrophages in allergic contact dermatitis. Allergol. Int. 2017;66:392–397. doi: 10.1016/j.alit.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Tan C.H., Rasool S., Johnston G.A. Contact dermatitis: allergic and irritant. Clin. Dermatol. 2014;32:116–124. doi: 10.1016/j.clindermatol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Boniface K., Guignouard E., Pedretti N., Garcia M., Delwail A., Bernard F.-X., Nau F., Guillet G., Dagregorio G., Yssel H., Lecron J.-C., Morel F. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin. Exp. Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricciardi L., Minciullo P.L., Saitta S., Trombetta D., Saija A., Gangemi S. Increased serum levels of IL-22 in patients with nickel contact dermatitis. Contact Dermatitis. 2009;60:57–58. doi: 10.1111/j.1600-0536.2008.01454.x. [DOI] [PubMed] [Google Scholar]
- 15.Nograles K.E., Zaba L.C., Shemer A., Fuentes-Duculan J., Cardinale I., Kikuchi T., Ramon M., Bergman R., Krueger J.G., Guttman-Yassky E. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009;123:1244–1252.e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denison M.S., Nagy S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.-C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 18.Schiering C., Wincent E., Metidji A., Iseppon A., Li Y., Potocnik A.J., Omenetti S., Henderson C.J., Wolf C.R., Nebert D.W., Stockinger B. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Taylor S., Ogg G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011;165:492–498. doi: 10.1111/j.1365-2133.2011.10400.x. [DOI] [PubMed] [Google Scholar]
- 20.Cabanillas B., Novak N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016;42:1–8. doi: 10.1016/j.coi.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Kabashima-Kubo R., Nakamura M., Sakabe J., Sugita K., Hino R., Mori T., Kobayashi M., Bito T., Kabashima K., Ogasawara K., Nomura Y., Nomura T., Akiyama M., Shimizu H., Tokura Y. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: possible immunological state of the intrinsic type. J. Dermatol. Sci. 2012;67:37–43. doi: 10.1016/j.jdermsci.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Furue M., Chiba T., Tsuji G., Ulzii D., Kido-Nakahara M., Nakahara T., Kadono T. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017;66:398–403. doi: 10.1016/j.alit.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Furue M., Tsuji G., Mitoma C., Nakahara T., Chiba T., Morino-Koga S., Uchi H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015;80:83–88. doi: 10.1016/j.jdermsci.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Napolitano M., Patruno C. Aryl hydrocarbon receptor (AhR) a possible target for the treatment of skin disease. Med. Hypotheses. 2018;116:96–100. doi: 10.1016/j.mehy.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Boguniewicz M., Alexis A.F., Beck L.A., Block J., Eichenfield L.F., Fonacier L., Guttman-Yassky E., Paller A.S., Pariser D., Silverberg J.I., Lebwohl M. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J. Allergy Clin. Immunol. Pract. 2017;5:1519–1531. doi: 10.1016/j.jaip.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen C., Liu X., Li Y., Liang H., Li K., Li J., Cheng C., Liu X., Zhong S., Li L., Wang Y. Effects of acupuncture on 1-chloro-2,4-dinitrochlorobenzene-induced allergic contact dermatitis in mice. J. Acupunct. Meridian Stud. 2017;10:252–260. doi: 10.1016/j.jams.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Gudasheva T.A., Ostrovskaya R.U., Seredenin S.B. Novel technologies for dipeptide drugs design and their implantation. Curr. Pharmaceut. Des. 2018;24:3020–3027. doi: 10.2174/1381612824666181008105641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.