Abstract
Background
Chronic cough (CC) which is defined ≥8 weeks is a common condition in clinical practice. However, estimates of prevalence and associated comorbidities in German adults and key subgroups of age and gender are lacking.
Methods
Cross-sectional study based on a representative panel of 15 020 adult subjects of the general population who completed the German National Health and Wellness Survey, reporting CC and questions about comorbidities. Lifetime and 12-month prevalence are presented as unweighted estimates.
Results
The lifetime CC prevalence was 6.5% (range across age groups 5.1%–8.3%) and the 12-month prevalence was 4.9% (range 3.7–5.7%). The prevalence of diagnosed CC was 2.8% (range 0.9–4.1%) and the prevalence of persons currently on any prescription to treat CC was 0.6% (range 0.2–1.4%). Respondents who experienced CC were 52.0±17.0 years old, with a higher prevalence in those aged 50 years and older. Persons with CC had higher morbidity scores and were diagnosed with an increased number of comorbidities, most frequently diagnoses of the respiratory system (71.0%), followed by digestive tract disorders (34.0%) and sleep disorders (37.6%).
Conclusions
In a broadly representative sample of German adults, lifetime and 12-month prevalence of CC was greatest in current and former smokers and those older ≥50 years of age. Comorbidities are frequent and may complicate management of these patients.
Short abstract
Chronic cough is a prevalent condition in Germany among all age groups, regardless of sex, with highest rates in those 18–29 and ≥50 years, and more frequent among current or prior smokers. Comorbidities are common in this population. https://bit.ly/3uiYj4w
Introduction
Cough is one of the most common symptoms for patients seeking care from primary care specialists, allergists, otolaryngologists or pulmonologists [1]. While its exact characteristics are not always precisely defined [2, 3], the importance of cough as a global clinical problem has led multiple societies to publish guidelines on its diagnosis and management.
Similar to other societies, the German Respiratory Society guideline for chronic cough (CC) categorises cough based upon its duration [4–6]. Acute cough is present for less than 3 weeks and most often due to acute viral upper respiratory tract infection. A cough that has been present longer than 3 weeks is either sub-acute (up to 8 weeks) or chronic (CC; over 8 weeks) [7, 8].
CC negatively impacts quality of life directly due to the effect of cough on physical, social or psychological functioning or indirectly through associated comorbidities such as urinary incontinence, cough syncope and dysphonia leading to social isolation, depression, and difficulties in relationships [9].
CC often manifests in association with comorbidities, such as asthma, gastroesophageal reflux disease (GERD) and upper airway cough syndrome (previously called post-nasal drip syndrome) [10]. Zeiger et al. [11] reported that, among specialist-diagnosed CC patients, 44.1% exhibited GERD, 31.2% asthma, 24.3% obesity, 20.4% upper airway cough syndrome and 19.4% common cough complications.
Certain patients may continue to cough despite thorough investigation and treatment in cases where no cause can be determined (termed unexplained CC or idiopathic CC) or when cough persists despite treatment of existing underlying conditions (refractory CC) [12]. Increased exposure of the sensory nerve terminals to chemical irritants (e.g. prostaglandin-E2, ATP) or excess mucus may have a role in triggering cough [13]. Further, neuronal hypersensitivity of airway sensory nerves has been considered to cause cough, as many patients report singing, laughing, changes in temperature or noxious smells to trigger bouts of coughing [13].
There are currently no treatments approved by the European Medicines Agency for the treatment of refractory or unexplained CC. Given its prolonged nature, significant morbidity and lack of effective treatments, unexplained and refractory CC remains an unmet medical need.
Very little is known of the natural history of CC [5]. However, in recent years, substantial evidence on the epidemiology of CC has been collected [14]. The lack of reliable data on the prevalence of CC in Germany was noted as an important knowledge gap in the most recent German guideline [6]. Prevalence data has been particularly lacking in the general population, as many previously reported estimates of prevalence are derived from settings which require timely contact with a physician. A meta-analysis estimated that CC could impact as much as 12% of the European population [15]. There are some prevalence estimates as low as 4% in certain regions [16].
Furthermore, as there is no International Classification of Diseases-10 code for CC, the affected population is difficult to identify and has not been well studied. Therefore, in view of novel therapeutic approaches for CC, there is a need to better understand its prevalence as well as the characteristics, comorbidities and quality of life of these patients.
Thus, our study aimed to provide prevalence estimates for CC in Germany and to describe the characteristics, treatment patterns, healthcare resource use, and quality of life and health state patient-reported outcomes (PROs) of CC respondents in these countries relative to general population adults who have not experienced CC.
Methods
The National Health and Wellness Survey (NHWS; Kantar LLC, New York, USA) is a self-administered, internet-based questionnaire conducted annually in 12 regions in the world including Germany. This survey collects data regarding demographics, health and wellness history and healthcare utilisation, and incorporates validated patient-reported health outcome instruments measuring quality of life, anxiety, depression, work productivity and activity impairment. In Germany, roughly 15 000 adults (aged 18+) take part in the survey each year. Quota sampling, with strata by age and gender, is implemented to ensure that the demographic composition of the NHWS sample is representative of the adult German population. Comparisons between NHWS and other established country sources have been published previously [17–19]. All translations from US English to German were provided by GlobaLexicon (London, UK) and reviewed by Kantar Health.
The protocol and questionnaire for the NHWS was reviewed and approved by the Pearl Institutional Review Board. All respondents were informed of study details and provided their consent to participate. Each respondent was assigned a unique, anonymous code, and no identifiable personal information was collected.
Study sample
To be included in the German 2020 NHWS, respondents had to be ≥18 years, able to read and write in German, and state their age and gender. Potential respondents were recruited through an existing, general-purpose (i.e. not healthcare-specific) web-based survey panel. The 2020 NHWS, fielded in Germany between 30 December 2019 and 20 April 2020, utilised a targeted quota sampling of strata according to age and gender to ensure a demographic composition of the German adult population. A cross-sectional design was used to estimate disease prevalence and a case-control design for comparisons to describe respondents with CC. Respondents with and without CC were those answering, respectively, “yes” and “no” to the question: “Have you experienced daily CC for 8 weeks or longer within the past 12 months?” or “Have you ever experienced daily CC for 8 weeks or longer?”. Those respondents were further asked if they have ever been diagnosed with CC by a physician or are currently using a prescription medication to treat their CC. No medications nor therapeutic class lists were provided as prompts.
Study measures
To obtain a systematic overview of disease history, respondents were presented a comprehensive list of terms (partly with explanations). For example, the list comprised sleep conditions, with the terms “insomnia, narcolepsy, sleep apnoea, sleep difficulties (other)”. Respondents were presented with the terms and asked to select those they have ever experienced, those experienced in the past year, and those for which they have received a diagnosis. The full list of terms is presented with the results in table 1.
TABLE 1.
Prevalence estimates of chronic cough (CC) in the National Health and Wellness Survey (NHWS) sample: lifetime, in past 12 months, diagnosed, drug treated (unweighted estimated)
| NHWS sample size | Lifetime # | 12 months ¶ | Lifetime diagnosed + | Lifetime diagnosed and current user of a prescription medication to treat CC § | ||||||||||
| n | % | p-value* | n | % | p-value* | n | % | p-value* | n | % | p-value* | |||
| Total | 15 020 | 981 | 6.5 | 739 | 4.9 | 418 | 2.8 | 95 | 0.6 | |||||
| Gender | Female | 7741 | 518 | 6.7 | 0.412 | 397 | 5.1 | 0.223 | 248 | 3.2 | 0.001 | 45 | 0.6 | 0.415 |
| Male | 7279 | 463 | 6.4 | 342 | 4.7 | 170 | 2.3 | 50 | 0.7 | |||||
| Age group, years | 18–29 | 2357 | 195 | 8.3 | <0.001 | 113 | 4.8 | <0.001 | 40 | 1.7 | <0.001 | 8 | 0.3 | <0.001 |
| 30–39 | 2187 | 122 | 5.6 | 85 | 3.9 | 20 | 0.9 | 4 | 0.2 | |||||
| 40–49 | 2288 | 116 | 5.1 | 84 | 3.7 | 46 | 2.0 | 9 | 0.4 | |||||
| 50–64 | 4214 | 273 | 6.5 | 229 | 5.4 | 154 | 3.7 | 31 | 0.7 | |||||
| 65–74 | 3330 | 234 | 7.0 | 191 | 5.7 | 136 | 4.1 | 34 | 1.0 | |||||
| 75+ | 644 | 41 | 6.4 | 37 | 5.7 | 22 | 3.4 | 9 | 1.4 | |||||
| Smoking status | Current/Former smoker | 8187 | 655 | 8.0 | <0.001 | 526 | 6.4 | <0.001 | 293 | 3.6 | <0.001 | 68 | 0.8 | <0.001 |
| Never smoked | 6833 | 326 | 4.8 | 213 | 3.1 | 125 | 1.8 | 27 | 0.4 | |||||
| Region ƒ | East | 4960 | 310 | 6.3 | 0.008 | 232 | 4.7 | 0.004 | 130 | 2.6 | 0.327 | 27 | 0.5 | 0.424 |
| North | 1155 | 77 | 6.7 | 59 | 5.1 | 35 | 3.0 | 7 | 0.6 | |||||
| South | 3665 | 205 | 5.6 | 147 | 4.0 | 90 | 2.5 | 19 | 0.5 | |||||
| West | 5230 | 389 | 7.4 | 301 | 5.8 | 163 | 3.1 | 42 | 0.8 | |||||
*: comparison of subsample with total sample. #: prevalence estimates for ever being diagnosed with CC. ¶: prevalence estimates for experienced CC in past 12 months. +: prevalence estimates for ever being diagnosed with CC. §: prevalence estimates for ever being diagnosed and for currently using a prescription medication to treat their CC. ƒ: South: Baden-Wuerttemberg, Bavaria; East: Berlin, Brandenburg, Lower Saxony, Mecklenburg-Western Pomerania, Saxonia, Saxonia-Anhalt, Thuringia; North: Bremen, Hamburg, Schleswig-Holstein; West: Hessia, North Rhine-Westphalia, Rhineland-Palatinate, Saarland; not specified: 10 respondents chose not to answer that question but also did not report CC.
Health-related quality of life was measured, e.g. the Medical Outcomes Study 12-item Short Form Survey v2 (SF-12v2; Quality Metric, Lincoln, RI) [20, 21]. SF-12v2 physical and mental health component summary scores were calculated with the mean score set at 50 and the standard deviation (sd) at 10; higher scores represent better health. Anxiety was measured with the General Anxiety Disorder 7-item scale (GAD-7), which measures the severity of symptoms of generalised anxiety over the prior 2 weeks [22]. Respondents were asked to rate the frequency of anxiety symptoms on a Likert scale ranging from 0 (not at all) to 3 (nearly every day), with scores ranging from 0 to 21. Depression was assessed with the Patient Health Questionnaire 9-item scale (PHQ-9) [23]. Respondents were asked to rate the frequency of symptoms of depression in the last 2 weeks on a scale of 0 (not at all) to 3 (nearly every day).
Data analysis
CC prevalence was calculated as a proportion of the 15 020 German 2020 NHWS respondents and in demographic subgroups (male, female, age categories 18–29, 30–39, 40–49, 50–64, 65–74 and ≥75 years), and by smoking status (use of cigarettes or other tobacco products: never smoked, or current/former smoker).
CC prevalence estimates were defined as follows:
lifetime prevalence: report ever having had a CC (cough of ≥8 weeks); and
12-month prevalence: reporting of cough daily for ≥8 weeks in the past 12 months.
Total and subgroup prevalence estimates by age, gender, smoking status and geographic regions (South: Baden Wuerttemberg, Bavaria; East: Berlin, Brandenburg, Lower Saxony, Mecklenburg-Western Pomerania Saxonia, Saxonia-Anhalt, Thuringia; North: Bremen, Hamburg, Schleswig-Holstein; West: Hessen, North Rhine-Westphalia, Rhineland-Palatinate, Saarland) were calculated as unweighted and weighted estimates. As a sensitivity analysis, weighting was applied post-data collection using the Horvitz–Thompson method to align sample with age and gender composition of the German adult population using data reported in the 2019/2020 International Data Base of the US Census Bureau [24]. As weighted values did not relevantly differ from the unweighted values, only the latter are presented in this publication.
Propensity scores were used to match NHWS respondents with CC at a 1:3 ratio to respondents without CC. Propensity scores were calculated using age (as a continuous variable), gender, the NHWS Charlson Comorbidity Index modified to exclude chronic obstructive pulmonary disease (because it may contribute to CC), marital status, household income and an interaction term of marital status×household income. The interaction term was included as a reflection of socio-economic status, e.g. possible synergistic benefits from dual-income/partnered households, to control for the influence of an individual's ability to work irrespective of spousal income. Eligible controls were matched using a nearest neighbour approach on the logit with a caliper=0.25.
Results are presented as either N (%) or mean (sd). Between-group comparisons were conducted using two-tailed independent samples t-tests (Welch's t-test for unequal variances) for analysis of continuous variables, or the chi-quare tests for analysis of categorical variables. Respondents with CC in the past 12 months were compared to those without CC in terms of comorbidities and the named health-related quality of life scores.
Results were considered statistically significant at p<0.05. All analyses were performed by Kantar Health LLC in SPSS (v.23), R (v.4.0.2) and/or SAS (v.9.4).
Results
Prevalence estimates
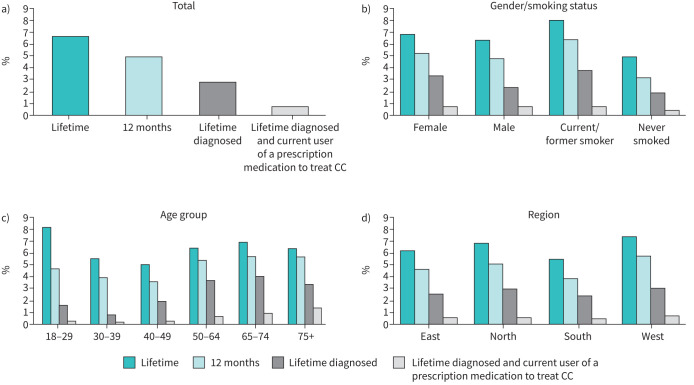
Out of the NHWS general population sample of 15 020 German respondents, 981 (6.5%) reported CC at any time during their lifetime. Across all (adult) age groups, the lifetime prevalence ranged from 5.1 to 8.3% (p<0.001) (table 1). Past/present smokers reported a higher prevalence than non-smokers (8.0 versus 4.8%, p<0.001). By the geographic region, prevalence rates ranged from 5.6 to 7.4% (p=0.008) with prevalence highest in western Germany. The diagnosed prevalence 2.8% (range 0.9–4.1%, p<0.001), and the current CC prescription medication user prevalence was 0.6% (range 0.2–1.4%, p<0.001). The 12-month prevalence of CC in this sample was 4.9% (ranging from 3.7% to 5.7% across age groups, p<0.001),
Respondent characteristics
Comparisons between respondents with and without CC in the past 12 months are presented in table 2. Matched respondents reporting CC were on average older than respondents without CC (52.1±17.0 versus 51.8±17.7; p=0.62). The proportion of females was similar across groups (53.8% with CC and 56.3% without, p=0.240). Differences between groups were noted for employment status (respondents with CC were less often employed), income (a similar proportion of respondents were in the middle income stratum but more respondents with CC were in the lower income stratum, p<0.001), region and health insurance (see table 2).
TABLE 2.
Characteristics in the subsample of respondents with chronic cough (CC) in the past 12 months versus the German adult general population subsample without CC
| Unmatched | Matched # | ||||||||||||||
| Total NHWS respondents | Respondents with CC in past 12 months | Respondents without CC in past 12 months | p-value | Total | Respondents with CC in past 12 months | Respondents without CC in past 12 months | p-value* | ||||||||
| n | 15 020 | 739 | 14 281 | 2930 | 736 | 2194 | |||||||||
| Age | Mean, sd | 50.1±16.8 | 52.0±17.0 | 50.0±16.7 | 0.001* | 51.8±16.7 | 52.1±17.0 | 51.8±17.7 | 0.62 | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Age group, years | 18–24 | 1276 | 8.5 | 66 | 8.9 | 1210 | 8.5 | 0.001* | 232 | 7.9 | 65 | 8.8 | 167 | 7.6 | 0.443 |
| 25–39 | 3268 | 21.8 | 132 | 17.9 | 3136 | 22 | 556 | 19 | 130 | 17.7 | 426 | 19.4 | |||
| 40–49 | 2288 | 15.2 | 84 | 11.4 | 2204 | 15.4 | 380 | 13 | 84 | 11.4 | 296 | 13.5 | |||
| 50–64 | 4214 | 28.1 | 229 | 31 | 3985 | 27.9 | 863 | 29.5 | 229 | 31.1 | 634 | 28.9 | |||
| 65–74 | 3330 | 22.2 | 191 | 25.8 | 3139 | 22 | 758 | 25.9 | 191 | 26.0 | 567 | 25.8 | |||
| 75+ | 644 | 4.3 | 37 | 5 | 607 | 4.3 | 141 | 4.8 | 37 | 5.0 | 104 | 4.7 | |||
| Gender | Male | 7279 | 48.5 | 342 | 46.3 | 6937 | 48.6 | 0.223 | 1299 | 44.3 | 340 | 46.2 | 959 | 43.7 | 0.24 |
| Female | 7741 | 51.5 | 397 | 53.7 | 7344 | 51.4 | 1631 | 55.7 | 396 | 53.8 | 1235 | 56.3 | |||
| Employment status | Employed full time/part time/self-employed | 8365 | 55.7 | 332 | 44.9 | 8033 | 56.2 | 0.000* | 1384 | 47.2 | 329 | 44.7 | 1055 | 48.1 | 0.049* |
| Homemaker/student/long-term disability/not employed and not looking for work | 1737 | 11.6 | 88 | 11.9 | 1649 | 11.5 | 374 | 12.8 | 88 | 12.0 | 286 | 13.0 | |||
| Retired | 4390 | 29.2 | 280 | 37.9 | 4110 | 28.8 | 1058 | 36.1 | 280 | 38.0 | 778 | 35.5 | |||
| Not employed. but looking for work/short-term disability | 528 | 3.5 | 39 | 5.3 | 489 | 3.4 | 114 | 3.9 | 39 | 5.3 | 75 | 3.4 | |||
| Marital status | Married or living with partner | 8329 | 55.5 | 390 | 52.8 | 7939 | 55.6 | 0.323 | 1556 | 53.1 | 389 | 52.9 | 1167 | 53.2 | 0.888 |
| Single/never married or divorced or separated or widowed | 6614 | 44 | 345 | 46.7 | 6269 | 43.9 | 1361 | 46.5 | 343 | 46.6 | 1018 | 46.4 | |||
| Decline to answer | 77 | 0.5 | 4 | 0.5 | 73 | 0.5 | 13 | 0.4 | 4 | 0.5 | 9 | 0.4 | |||
| Household income (categorical) | Low (< €20,000) | 3201 | 21.3 | 203 | 27.5 | 2998 | 21 | <0.001* | 824 | 28.1 | 202 | 27.4 | 622 | 28.4 | 0.53 |
| Medium (€20,000 - €49,999) | 6350 | 42.3 | 315 | 42.6 | 6035 | 42.3 | 1285 | 43.9 | 315 | 42.8 | 970 | 44.2 | |||
| High (≥ €50,000) | 4133 | 27.5 | 168 | 22.7 | 3965 | 27.8 | 639 | 21.8 | 166 | 22.6 | 473 | 21.6 | |||
| Decline to answer | 1336 | 8.9 | 53 | 7.2 | 1283 | 9 | 182 | 6.2 | 53 | 7.2 | 129 | 5.9 | |||
| Level of education | Did not attend school or declined to answer | 489 | 3.3 | 22 | 3 | 467 | 3.3 | 0.076 | 83 | 2.8 | 22 | 3.0 | 61 | 2.8 | 0.611 |
| Less than 4-year university degree | 10 456 | 69.6 | 542 | 73.3 | 9914 | 69.4 | 2115 | 72.2 | 540 | 73.4 | 1575 | 71.8 | |||
| 4-year university degree or higher | 4075 | 27.1 | 175 | 23.7 | 3900 | 27.3 | 732 | 25 | 174 | 23.6 | 558 | 25.4 | |||
| Smoking Status (cigarettes and other tobacco products) | Current smoker | 4566 | 30.4 | 374 | 50.6 | 4192 | 29.4 | <0.001* | 1010 | 34.5 | 371 | 50.4 | 639 | 29.1 | <0.001* |
| Former smoker | 3621 | 24.1 | 152 | 20.6 | 3469 | 24.3 | 737 | 25.2 | 152 | 20.7 | 585 | 26.7 | |||
| Never smoked | 6833 | 45.5 | 213 | 28.8 | 6620 | 46.4 | 1183 | 40.4 | 213 | 28.9 | 970 | 44.2 | |||
| Region | South | 3665 | 24.4 | 147 | 19.9 | 3518 | 24.6 | 0.004*,¶ | 675 | 23 | 147 | 20.0 | 528 | 24.1 | 0.032*,¶ |
| East | 4960 | 33 | 232 | 31.4 | 4728 | 33.1 | 961 | 32.8 | 231 | 31.4 | 730 | 33.3 | |||
| West | 5230 | 34.8 | 301 | 40.7 | 4929 | 34.5 | 1057 | 36.1 | 299 | 40.6 | 758 | 34.5 | |||
| North | 1155 | 7.7 | 59 | 8 | 1096 | 7.7 | 236 | 8.1 | 59 | 8.0 | 177 | 8.1 | |||
| Not specified | 10 | 0.1 | 0 | 0 | 10 | 0.1 | 1 | 0 | - | 0.01 | 1 | 0.0 | |||
| Health insurance type | Public, without additional private insurance | 10 246 | 68.2 | 489 | 66.2 | 9757 | 68.3 | 0.06 | 2054 | 70.1 | 487 | 66.2 | 1567 | 71.4 | 0.001* |
| Public, along with additional private insurance | 2393 | 15.9 | 120 | 16.2 | 2273 | 15.9 | 465 | 15.9 | 119 | 16.2 | 346 | 15.8 | |||
| Private insurance | 1199 | 8 | 70 | 9.5 | 1129 | 7.9 | 202 | 6.9 | 70 | 9.5 | 132 | 6.0 | |||
| Aid entitlement and additional private insurance | 688 | 4.6 | 42 | 5.7 | 646 | 4.5 | 127 | 4.3 | 42 | 5.7 | 85 | 3.9 | |||
| Other | 151 | 1 | 10 | 1.4 | 141 | 1 | 30 | 1 | 10 | 1.4 | 20 | 0.9 | |||
| None of the above | 343 | 2.3 | 8 | 1.1 | 335 | 2.3 | 52 | 1.8 | 8 | 1.1 | 44 | 2.0 | |||
Data are based on respondents who completed the 2020 Germany NHWS (National Health and Wellness Survey). *: t-test or chi-square statistic is significant at the 0.05 level. #: propensity score matched on age, gender, CCI modified to exclude COPD, marital status, household income, interaction term of marital status x household income; using 1:3 nearest neighbour on the logit, caliper=0.25. ¶: given low sample size, “undefined” region was not included in the statistical comparison.
Current medical conditions and comorbidities
Table 3 provides an overview on diseases and conditions in last 12 months as reported by respondents. In terms of organ systems, persons with CC reported most frequently medical diagnoses of the respiratory system (71.0% versus 29.9%, p<0.001), also diagnoses by the digestive tract, the heart and blood system and sleep disorders were more common than in persons without CC (table 3).
TABLE 3.
Matched respondent experienced and physician-diagnosed conditions
| Total NHWS respondents | Respondents with CC | Matched# respondents without CC | |||||
| n | 15 020 | 739 | 2194 | ||||
| n | % | n | % | n | % | p-value* | |
| Respondent-reported conditions experienced in prior 12 months | |||||||
| Migraine | 522 | 17.8 | 170 | 23.1 | 352 | 16.0 | 0.000* |
| Sleep conditions combined (at least one) | 1412 | 48.2 | 520 | 70.7 | 892 | 40.7 | 0.000* |
| Insomnia | 768 | 26.2 | 287 | 39.0 | 481 | 21.9 | 0.000* |
| Narcolepsy | 52 | 1.8 | 39 | 5.3 | 13 | 0.6 | 0.000* |
| Sleep apnoea | 252 | 8.6 | 114 | 15.5 | 138 | 6.3 | 0.000* |
| Other sleep difficulties | 711 | 24.3 | 257 | 34.9 | 454 | 20.7 | 0.000* |
| Respondent-reported being diagnosed with condition by a physician during lifetime | |||||||
| Cancer (any tumour, leukaemia, lymphoma, metastatic solid tumour) | 183 | 6.2 | 40 | 5.4 | 143 | 6.5 | 0.068 |
| Chronic pain due to rheumatoid arthritis | 159 | 54.0 | 45 | 6.1 | 114 | 5.2 | 0.047* |
| Digestive tract (gastroesophageal reflux, heartburn, irritable bowel syndrome, ulcerative colitis, ulcers) | 628 | 21.4 | 248 | 33.7 | 380 | 17.3 | 0.000* |
| Heart and blood (congestive heart failure, heart attack, mini-stroke/transient ischaemia attack, peripheral vascular disease, stroke, diabetes Type I/II, latent autoimmune diabetes) | 747 | 25.5 | 208 | 28.3 | 539 | 24.6 | 0.018* |
| Infectious diseases (AIDS, hepatitis B or C, HIV) | 91 | 3.1 | 36 | 4.9 | 55 | 2.5 | 0.001* |
| Liver (chronic liver disease or cirrhosis) | 63 | 2.2 | 28 | 3.8 | 35 | 1.6 | 0.000* |
| Neurological (hemiplegia, multiple sclerosis, Parkinson's disease) | 76 | 2.6 | 24 | 3.3 | 52 | 2.4 | 0.057 |
| Respiratory (allergies, asthma, hay fever, chronic bronchitis, CC COPD, emphysema) | 1178 | 40.2 | 522 | 70.9 | 656 | 29.9 | 0.000* |
| Sleep (insomnia, narcolepsy, sleep apnoea, other sleep difficulties) | 2828 | 18.8 | 278 | 37.6 | 2550 | 17.9 | 0.000* |
| Moderate or severe renal/kidney disease | 32 | 1.1 | 5 | 0.7 | 27 | 1.2 | 0.191 |
| Other (connective tissue disease or community-acquired pneumonia) | 86 | 2.9 | 50 | 6.8 | 36 | 1.6 | 0.000* |
CC: chronic cough; COPD: chronic obstructive pulmonary disease; NHWS: National Health and Wellness Survey. *: Chi-square statistic is significant at the 0.05 level. #: propensity score matched on age, gender, CCI modified to exclude COPD, marital status, household income, interaction term of marital status×household income; using 1:3 nearest neighbour on the logit, caliper=0.25
Body mass index and comorbidity scores
Mean BMI was slightly higher in respondents with CC compared to those without 27.9 versus 27.1 kg·m−2 (p=0.004). Also, the number of days with vigorous exercise was lower (5.0 versus 5.8 days, p= 0.023). Various patient-reported outcomes and scores are shown in table 4. In respondents with CC, the mean Charlson Comorbidity Score without COPD, the PHQ-9 score, the GAD-7 score and SF-12 physical component score and mental component score were significantly higher in persons with CC compared to those without (table 4).
TABLE 4.
Matched general health scores and measures and report of weight and exercise
| Total NHWS respondents | Respondents with CC | Matched* respondents without CC | p-value* | ||||
| n | 15 020 | 739 | 2194 | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| CCI | 0.87 | 1.31 | 1.16 | 1.39 | 0.77 | 1.27 | 0.000* |
| CCI–modified (without COPD) | 0.73 | 1.23 | 0.80 | 1.26 | 0.70 | 1.21 | 0.068 |
| PHQ-9 score¶ | 6.39 | 6.15 | 8.97 | 6.49 | 5.52 | 5.78 | 0.000* |
| GAD-7 score¶ | 4.73 | 4.72 | 6.75 | 5.15 | 4.06 | 4.36 | 0.000* |
| SF-12 physical component score+ | 46.33 | 10.11 | 42.75 | 9.52 | 47.53 | 10.02 | 0.000* |
| SF-12 mental component score+ | 46.27 | 10.77 | 42.75 | 10.45 | 47.53 | 10.59 | 0.000* |
| BMI§ | 27.29 | 6.51 | 27.93 | 7.03 | 27.08 | 6.31 | 0.004* |
| Numbers of days of vigorous exercise in the past monthƒ | 5.51 | 7.53 | 4.98 | 7.27 | 5.69 | 7.61 | 0.023* |
BMI: body mass index; CC: chronic cough; CCI: Charlson Comorbidity Index; COPD: chronic obstructive pulmonary disease; GAD-7: Generalized Anxiety Disorder questionnaire, 7-item; NHWS: National Health and Wellness Survey; PHQ-9: Patient Health Questionnaire, 9-item; sd: standard deviation; SF-12: Medical Outcomes Study 36-item Short Form Survey v2. *: t-test or chi-square statistic is significant at the 0.05 level. #: propensity score matched on age, gender, CCI modified to exclude COPD, marital status, household income, interaction term of marital status×household income; using 1:3 nearest neighbour on the logit, caliper=0.25. ¶: PHQ-9 and GAD-7 are validated instruments to assess depressive symptoms over the prior 2 weeks (score range 0–27) and severity of generalised anxiety symptoms over the prior 2 weeks (score range 0–21), respectively. +The physical and mental health component summary scores were calculated with a mean score set at 50 and a standard deviation at 10; higher scores represent better health. §: BMI was collected for a subset of respondents who reported their height and weight, respectively N=2824 out of 2930; 707 out of 736; and 2117 out of 2194. BMI between 25 and <30.0 kg·m−2 indicates overweight. ƒ: the question was “How many days in the past month did you exercise vigorously for at least 20 min for the purpose of improving of maintaining your health, with the purpose of losing weight, or for enjoyment?”.
Discussion
According to this cross-sectional study, the 12-month prevalence of CC among adult respondents from a representative sample in Germany is 4.9%. Of these, almost half have been diagnosed by a physician and one tenth were currently using prescription treatment. Typically, respondents with CC have comorbidities, most frequently of the respiratory system; approximately one third have sleep disorders.
The present survey adds to a substantial body of evidence on CC. However, studies differ in terms of setting, age group, and prevalence of smoking. We used a cut-off of 8 weeks or greater for the CC definition, which is largely consistent with the recommended CC definition of >8 weeks in current North American and European clinical guidelines [5, 25]. Other studies applied other periods such as ≥3 months, which might limit comparability of results.
Among the recently published studies on CC, the Rotterdam Study Group – a cohort of 9824 subjects aged ≥45 years – found a self-reported prevalence of CC (daily coughing for at least 3 months during the preceding 2 years) of 10.9% [26]. In adult patients in the UK who were enrolled in a Helicobacter pylori screening program, a prevalence of 12% was reported [27]. A Canadian study showed in subjects aged 45 to 85 years self-reported prevalence of CC (coughed most days in the previous 12 months) was 15.8% at baseline and 17.1% at 3-year follow-up [28]. In the US, based on 75 000 National Health and Wellness Survey adult (≥18 years) respondents, 5.0% had experienced CC (daily cough at least 8 weeks) in the previous 12 months [29].
Another study in Denmark (14 669 subjects, median age 58 years) found a point prevalence of 4% [16] and a study in Finland (mean age 51 years) of 7.2% [30]. The Respiratory Health in Northern Europe III cohort (n=13 500), a multi-centre study, reported non-productive CC in 7% and productive CC in 9% [31]. Based on a systematic review and meta-analysis, Song et al. [15] estimated the global CC prevalence at 10%, and the prevalence in Europe at 12%. However, there is no insight in the included data for the meta-analysis. As described above, there are notable differences in the design and population of mentioned studies that explain differences observed in CC prevalence.
When considering the age groups, we found the highest CC prevalence in the oldest (50+ years) and youngest (18–29 years) surveyed. Other studies have also reported higher rates of CC as persons age (Copenhagen [16], Rotterdam [26], Korea [32]). Interestingly, CC was more prevalent among 18–29-year-olds than among those aged 30–49 years. In other studies, the youngest adults have been reported in combination with persons in their thirties and sometimes with those up to age 49. For instance, Colak et al. [16] reported a nominally higher prevalence among Danish 20–39-year-olds than among those in their forties. Similarly, prior to adjusting for diagnostic proceures and other co-variables, a higher unadjusted prevalence was shown in Korea among 18–39-year-olds than those aged 40–64 years [32]. Combing the youngest adults with those in their third decade may temper the prevalence rate. Our study results were not stratified by age other than as reported for prevalence. It is therefore possible that owing to poorer economic circumstance and/or lower utilisation of healthcare services, this younger adult population has not addressed associated underlying conditions and/or that their behavioural choices, e.g., smoking, may impact the rate of reported CC. The reasons for our observed higher prevalence rate among 18–29-year-olds remain unclear and could be assessed in future studies.
It has been reported that the cough reflex is more sensitive in women [33]. Some studies such as the Rotterdam study found a higher prevalence of CC in women [26]. In contrast, we did not find a statistically significant difference in the prevalence of CC between men and women in Germany. The higher prevalence in women reported elsewhere could be due, in part, to women being more willing to seek care for CC and are less likely to smoke. Interpreted with appropriate caution, our data suggests that patients with CC, on average, have a higher BMI as well as, and possibly correlated, fewer days of self-reported “vigorous exercise” than the general population.
In our study, the leading comorbidities in subjects with CC were respiratory disorders, which is consistent with the known epidemiology of cough and guideline-based evaluation recommendations. Typical conditions associated with CC are chronic obstructive pulmonary disease (COPD; e.g. the most important risk factor found in the Rotterdam study [26]), asthma or bronchiectasis (both are among the three top risk factors in the Copenhagen study [16]). Among the digestive tract disorders, GERD has been identified as independent risk factor in the Rotterdam study [26], the Copenhagen study [16], and the Korean National Health Survey [32]. As listed in table 3, subjects which reported CC also suffered from a relatively large variety of other medical conditions and comorbidities which have not necessarily previously been associated clinically with cough. The reasons for these observations remain speculative and cannot be derived from the available data. While an increase in CC among people e.g. with “cancer”, “congestive heart failure”, “respiratory” conditions seems plausible, the increased prevalence in those with comorbid e.g. “migraine”, “chronic pain” or “liver diseases” could be investigated in future studies.
We also found a higher prevalence of sleep disorders in subjects with CC. CC can be the sole presenting symptom of obstructive sleep apnoea in about one third of affected patients [34]. Furthermore, the relationship of sleep complaints to respiratory symptoms is well established, and cough symptoms have been associated with daytime somnolence in general population studies [35].
As reported in a previous study, CC was accompanied by increased symptoms of depression [36]. A complex relationship between psychomorbidity and CC has been described and psychological issues may be an aetiological factor in the development of CC (previously termed “psychogenic cough”, now “somatic cough syndrome”) [37]. Alternatively, psychomorbidity may be a result of CC in some respondents.
One of the strengths of our survey is the attempt to have an age and gender-representative sample. However, a few limitations need to be taken into consideration when interpreting the findings of our survey. Given the observational nature of the survey, no causal relationships between, e.g., CC and the various comorbidities can be made [38]. Like other internet-based, patient-reported surveys, the NHWS sample likely under-represents people without access to or familiarity with electronic media, including the elderly, institutionalised patients, and those with severe comorbidities and disabilities which could make access to and ability to participate in online survey panels more difficult. The self-reported nature of the NHWS and cough module is also associated with potential corresponding biases, such as recall and self-presentation biases. Such biases could introduce additional measurement error. We analysed the general CC population which includes, among others, smokers and patients on angiotensin-converting enzyme inhibitors and did not confine the analysis to refractory or unexplained cough patients, which affects the reported prevalence estimates and gender ratios. Finally, as CC was defined by period prevalence (lifetime, 12 months) and not by point prevalence, there is the possibility of recall bias.
In conclusion, CC is a prevalent condition in Germany among all age groups without gender preference, with highest rates in those 18–29 and 50 years and older and more frequent among those current or prior smokers. Comorbidities are common in this population, typically of the respiratory system, sleep disorders and depressive symptoms.
FIGURE 1.
Prevalence estimates of chronic cough in the National Health and Wellness Survey (NHWS) sample: lifetime, in past 12 months, diagnosed, drug treated (unweighted estimated). CC: chronic cough.
Acknowledgements
Writing and editorial assistance were provided under the direction of the authors of 3P Consulting, Seefeld, Germany, with support from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Provenance: Submitted article, peer reviewed.
Data availability: Qualified researchers may request access to patient level data and related study documents including the study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymised and study documents will be redacted to protect the privacy of our trial participants.
Conflict of interest: J.C. Virchow has the following to disclose: lectured for and received honoraria from AstraZeneca, Avontec, Bayer, Bencard, Bionorica, Boehringer Ingelheim, Chiesi, Essex/Schering-Plough, GSK, Janssen-Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Altana, Pfizer, Revotar, Sandoz-Hexal, Stallergens, Teva, UCB/Schwarz-Pharma, Zydus/Cadila and possibly others; participated in advisory boards for: Avontec, Boehringer Ingelheim, Chiesi, Essex/Schering-Plough, GSK, Janssen-Cilag, MEDA, MSD, Mundipharma, Novartis, Regeneron, Revotar, Roche, Sanofi-Aventis, Sandoz-Hexal, Teva, UCB/Schwarz-Pharma and possibly others; and received research grants from: Deutsche Forschungsgesellschaft, Land Mecklenburg-Vorpommern, GSK and MSD. C. Jannowitz and H. Salmen are full-time employees of MSD Sharp & Dohme GmbH, Haar, Germany. E. Fonseca, J. Brady and J. Schelfhout are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and shareholders in Merck & Co., Inc., Kenilworth, NJ, USA. V.W. Li and A. Martin are employees of Kantar LLC.
Support statement: Kantar LLC conducted the National Health and Wellness Survey, and received funding for access to the data. This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician 2018; 64: 832–840. [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana GA, Widdicombe J. What is cough and what should be measured? Pulm Pharmacol Ther 2007; 20: 307–312. doi: 10.1016/j.pupt.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Widdicombe J, Fontana G. Cough: what's in a name? Eur Respir J 2006; 28: 10–15. doi: 10.1183/09031936.06.00096905 [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007; 29: 1256–1276. doi: 10.1183/09031936.00101006 [DOI] [PubMed] [Google Scholar]
- 5.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kardos P, Dinh QT, Fuchs KH, et al. German respiratory society guidelines for diagnosis and treatment of adults suffering from acute, subacute and chronic cough. Respir Med 2020; 170: 105939. doi: 10.1016/j.rmed.2020.105939 [DOI] [PubMed] [Google Scholar]
- 7.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 1S–23S. doi: 10.1378/chest.129.1_suppl.1S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 220S–221S. doi: 10.1378/chest.129.1_suppl.220S [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015; 193: 401–408. doi: 10.1007/s00408-015-9701-2 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan AG. Chronic cough in adults: make the diagnosis and make a difference. Pulm Ther 2019; 5: 11–21. doi: 10.1007/s41030-019-0089-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeiger RS, Schatz M, Butler RK, et al. Burden of specialist-diagnosed chronic cough in adults. J Allergy Clin Immunol 2020; 8: 1645–1657. doi: 10.1016/j.jaip.2020.01.054 [DOI] [PubMed] [Google Scholar]
- 12.McGarvey L, Gibson PG. What is chronic cough? Terminology. J Allergy Clin Immunol Pract 2019; 7: 1711–1714. doi: 10.1016/j.jaip.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 13.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014; 44: 1132–1148. doi: 10.1183/09031936.00218613 [DOI] [PubMed] [Google Scholar]
- 14.Song W-J, Chang Y-S, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 15.Song WJ, Morice AH, Kim MH, et al. Cough in the elderly population: relationships with multiple comorbidity. PLoS One 2013; 8: e78081. doi: 10.1371/journal.pone.0078081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colak Y, Nordestgaard BG, Laursen LC, et al. Risk factors for chronic cough among 14,669 individuals from the general population. Chest 2017; 152: 563–573. doi: 10.1016/j.chest.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 17.Bolge SDJ, Kannan H, Baran R. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res 2009; 18: 415–422. doi: 10.1007/s11136-009-9462-6 [DOI] [PubMed] [Google Scholar]
- 18.DiBonaventura MWJ-S, Yuan Y, L'Italien G, et al. Humanistic and economic impacts of hepatitis C infection in the United States. J Med Econ 2010; 13: 709–718. doi: 10.3111/13696998.2010.535576 [DOI] [PubMed] [Google Scholar]
- 19.Finkelstien EAB, DiBonaventura M, Burgess S. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med 2011; 53: 1025–1029. doi: 10.1097/JOM.0b013e318229aae4 [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Sherbourne CD. The MOS 36-item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21: 271–292. doi: 10.1016/S0167-6296(01)00130-8 [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166: 1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 23.Hinz A, Mehnert A, Kocalevent RD, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 2016; 16: 22. doi: 10.1186/s12888-016-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Census Bureau . 2019/2020 International Data Base (IDB) of the US Census Bureau. www.census.gov/programs-surveys/international-programs/about/idb.html. Date last Accessed: 24 February 2021.
- 25.Irwin RS, French CL, Chang AB, et al. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest 2018; 153: 196–209. doi: 10.1016/j.chest.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arinze JT, de Roos EW, Karimi L, et al. Prevalence and incidence of, and risk factors for chronic cough in the adult population: the Rotterdam Study. ERJ Open Res 2020; 6: 00300-2019. doi: 10.1183/23120541.00300-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006; 61: 975–979. doi: 10.1136/thx.2006.060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satia I, Mayhew AJ, Sohel N, et al. Prevalence, incidence and characteristics of chronic cough among adults from the Canadian Longitudinal Study on Aging. ERJ Open Res 2021; 7: 00160-2021. doi: 10.1183/23120541.00160-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meltzer EO, Zeiger RS, Dicpinigaitis P, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract 2021; 9: 4037–4044.e2. doi: 10.1016/j.jaip.2021.07.022 [DOI] [PubMed] [Google Scholar]
- 30.Latti AM, Pekkanen J, Koskela HO. Defining the risk factors for acute, subacute and chronic cough: a cross-sectional study in a Finnish adult employee population. BMJ Open 2018; 8: e022950. doi: 10.1136/bmjopen-2018-022950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson H, Johannessen A, Holm M, et al. Prevalence, progression and impact of chronic cough on employment in Northern Europe. Eur Resp J 2020; 57: 2003344. doi: 10.1183/13993003.03344-2020 [DOI] [PubMed] [Google Scholar]
- 32.Kang MG, Song WJ, Kim HJ, et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: The Korean national health and nutrition examination survey 2010–2012. Medicine 2017; 96: e6486. doi: 10.1097/MD.0000000000006486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastelik JA, Thompson RH, Aziz I, et al. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med 2002; 166: 961–964. doi: 10.1164/rccm.2109061 [DOI] [PubMed] [Google Scholar]
- 34.Chan KK, Ing AJ, Laks L, et al. Chronic cough in patients with sleep-disordered breathing. Eur Resp J 2010; 35: 368–372. doi: 10.1183/09031936.00110409 [DOI] [PubMed] [Google Scholar]
- 35.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest 1994; 105: 151–154. doi: 10.1378/chest.105.1.151 [DOI] [PubMed] [Google Scholar]
- 36.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006; 130: 1839–1843. doi: 10.1378/chest.130.6.1839 [DOI] [PubMed] [Google Scholar]
- 37.Vertigan AE, Murad MH, Pringsheim T, et al. Somatic cough syndrome (previously referred to as psychogenic cough) and tic cough (previously referred to as habit cough) in adults and children: chest guideline and expert panel report. Chest 2015; 148: 24–31. doi: 10.1378/chest.15-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado-Rodriguez M, Llorca J. Bias. J Epid Comm Health 2004; 58: 635–641. doi: 10.1136/jech.2003.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]