Abstract
Chronic obstructive pulmonary disease (COPD) is understood as a complex, heterogeneous and multisystem airway obstructive disease. The association of deterioration in health-related quality of life (HRQoL) with mortality and hospitalisation for COPD exacerbation has been explored in general terms. The specific objectives of this study were to determine whether a change in HRQoL is related, over time, to mortality and hospitalisation.
Overall, 543 patients were recruited through Galdakao Hospital's five outpatient respiratory clinics. Patients were assessed at baseline, and the end of the first and second year, and were followed up for 3 years. At each assessment, measurements were made of several variables, including HRQoL using the St George's Respiratory Questionnaire (SGRQ).
The cohort had moderate obstruction (forced expiratory volume in 1 s 55% of the predicted value). SGRQ total, symptoms, activity and impact scores at baseline were 39.2, 44.5, 48.7 and 32.0, respectively. Every 4-point increase in the SGRQ was associated with an increase in the likelihood of death: “symptoms” domain odds ratio 1.04 (95% CI 1.00–1.08); “activity” domain OR 1.12 (95% CI 1.08–1.17) and “impacts” domain OR 1.11 (95% CI 1.06–1.15). The rate of hospitalisations per year was 5% (95% CI 3–8%) to 7% (95% CI 5–10%) higher for each 4-point increase in the separate domains of the SGRQ.
Deterioration in HRQoL by 4 points in SGRQ domain scores over 1 year was associated with an increased likelihood of death and hospitalisation.
Short abstract
In the short term, HRQoL changes in the evaluation of COPD patients can be used as a warning tool for prognosis of hospitalisations and mortality https://bit.ly/3m3kqtc
Introduction
Nowadays, chronic obstructive pulmonary disease (COPD) is understood not only as an airway obstruction disease but also as a complex, heterogeneous and multisystem disease [1, 2]. Given that, there is a clear need to have tools that enable us to evaluate all aspects of the disease in a broad and integrative approach. Exacerbations and mortality are two of the key outcomes in COPD. Several factors and models have been described and developed for predicting mortality in COPD patients [2, 3]. In recent years, multidimensional instruments have been proposed seeking to capture as many of the aspects of the condition as possible and improve the accuracy of prognosis regarding certain outcomes of the disease [4–7].
Several studies have focused on the impact of exacerbations and COPD exacerbation-related hospitalisation (hCOPD) as factors associated with impairment of health-related quality of life (HRQoL) in COPD patients [8–10]. In fact, hCOPD is the factor with the greatest influence on the deterioration of HRQoL in COPD patients [11]. However, conversely, how changes in HRQoL impacts in suffering a hCOPD has been less studied.
Furthermore, HRQoL is considered an important outcome itself and a predictor of other outcomes, including mortality, in COPD patients [12, 13]. HRQoL is very often assessed in clinical trials, but its use in and impact on routine clinical practice has yet to be properly or thoroughly investigated, especially considering that HRQoL provides a broad picture of how the disease affects patients.
It is also well recognised that, as well as hCOPD, mortality is related to HRQoL. In this context, our hypothesis was that HRQoL could be a useful prognostic instrument in the follow-up of COPD patients. The aim of this study was to investigate these relationships in more detail, the specific objectives being to explore whether these outcomes (mortality and hCOPD) are correlated with changes in HRQoL over time and, hence, whether such changes might be useful as a warning sign in a cohort of COPD patients.
Methods
Participants and data collection
Patients were recruited after being treated for COPD in five outpatient respiratory clinics run by Galdakao Hospital's Dept of Respiratory Medicine. Patients were consecutively included in the study if they had been diagnosed with COPD for ≥6 months and had been stable for ≥6 weeks. The inclusion of patients started in 1999. Other inclusion criteria were forced expiratory volume in 1 s (FEV1) <80% of the predicted value and FEV1/forced vital capacity ratio <70%.
Patients were not eligible for the study if they had been diagnosed with asthma, extensive pulmonary tuberculosis, bronchiectasis, diffuse interstitial lung disease, cancer, or psychiatric or neurological problems that might hinder effective collaboration. The protocol was approved by the Ethics and Research Committees of the hospital (030906005). All candidate patients were given detailed information about the study and all those included provided written informed consent.
Study protocol
Data were recorded on participants’ sociodemographic characteristics and smoking habits, as well as several clinical variables (table 1). Comorbidities were identified by reviewing the patients' entire electronic health record and summarised using the Charlson comorbidity index [14]. HRQoL was assessed using the validated Spanish version of the St George's Respiratory Questionnaire (SGRQ) [15, 16]. The SGRQ comprises three dimensions (symptoms, impact and activity) and a global score. Each dimension receives a score between 0 and 100, with 0 representing a complete lack of deterioration.
TABLE 1.
Sociodemographic and clinical characteristics of the cohort at the time of each assessment
| Baseline | 1 year | 2 years | p-value# | ||
| Patients | 543 | 480 (88%) | 428 (79%) | ||
| SGRQ score | |||||
| Symptoms | 44.5±22.2 | 42.5±22.4 | 43.1±23.5 | 0.363 | |
| Activity | 48.7±24.9 | 45.9±25.0 | 46.6±25.0 | 0.111 | |
| Impacts | 32.0±20.9 | 30.4±21.1 | 30.1±20.4 | 0.243 | |
| Total | 39.2±20.1 | 37.1±20.5 | 37.2±20.4 | 0.188 | |
| Sex | |||||
| Male | 522 (96.1%) | 459 (95.6%) | 408 (95.3%) | 0.820 | |
| Female | 21 (3.9%) | 21 (4.4%) | 20 (4.7%) | ||
| Age, years | 68.3±8.3 | 67.6±8.4 | 67.4±8.3 | 0.042 | |
| Body mass index, kg·m−2 | 28.3±4.4 | 28.3±5.2 | 28.1±4.4 | 0.659 | |
| mMRC dyspnoea score | |||||
| 0 | 69 (12.7%) | 85 (17.7%) | 75 (17.5%) | 0.005 | |
| 1 | 264 (48.6%) | 248 (51.7%) | 188 (43.9%) | ||
| 2 | 166 (30.6%) | 127 (26.5%) | 142 (33.2%) | ||
| 3–4 | 44 (8.1%) | 20 (4.3%) | 23 (5.4%) | ||
| FEV1, mL | 1464±441 | 1470±501 | 1513±470 | 0.304 | |
| FEV1, % predicted | 55.0±13.3 | 55.2±16.0 | 57.7±14.7 | 0.027 | |
| <30% predicted | 18 (3.3%) | 22 (4.6%) | 13 (3.0%) | 0.253 | |
| 30–50% predicted | 167 (30.8%) | 131 (27.3%) | 111 (25.9%) | ||
| ≥50% predicted | 358 (65.9%) | 327 (68.1%) | 304 (71.0%) | ||
| Smoking history | |||||
| Exposure, pack-years | 46.8±27.3 | 46.3±26.4 | 46.5±26.0 | 0.989 | |
| Smokers | 114 (23.0%) | 91 (19.0%) | 79 (18.5%) | 0.622 | |
| Former smokers | 414 (76.2%) | 375 (78.1%) | 338 (79.0%) | ||
| Never-smokers | 15 (2.8%) | 14 (2.9%) | 11 (2.6%) | ||
| 6MWD, m | 409±92 | 421±118 | 412±116 | 0.002 | |
| Charlson comorbidity index score | 2.41±1.42 | 2.46±1.42 | 2.47±1.41 | 0.630 | |
| <2 | 172 (31.7%) | 145 (30.2%) | 130 (30.4%) | 0.765 | |
| 2–3 | 264 (48.6%) | 233 (48.5%) | 208 (48.6%) | ||
| >3 | 107 (19.7%) | 102 (21.3%) | 90 (21.0%) | ||
| Previous hospitalisations | |||||
| 0 | 427 (78.6%) | 406 (84.6%) | 377 (88.1%) | 0.033 | |
| 1 | 80 (14.7%) | 51 (10.6%) | 30 (7.0%) | ||
| 2 | 16 (3.0%) | 14 (2.9%) | 16 (3.7%) | ||
| 3 | 9 (1.7%) | 6 (1.3%) | 4 (0.9%) | ||
| >3 | 11 (2.0%) | 3 (0.6%) | 1 (0.2%) | ||
| Treatment¶ | |||||
| 0–1 | 73 (13.4%) | 57 (11.9%) | 48 (11.2%) | 0.204 | |
| 2 | 121 (22.2%) | 91 (19.0%) | 87 (20.3%) | ||
| 3 | 349 (64.3%) | 330 (68.8%) | 293 (68.5%) | ||
| COPD group | |||||
| A | 284 (56.0%) | 260 (62.4%) | 205 (56.9%) | 0.206 | |
| B | 143 (28.2%) | 89 (21.3%) | 90 (25.0%) | ||
| C | 35 (6.9%) | 39 (9.3%) | 33 (9.2%) | ||
| D | 45 (8.9%) | 29 (7.0%) | 32 (8.9%) | ||
| Comorbidities | |||||
| Coronary artery disease | 32 (5.9%) | 27 (5.6%) | 23 (5.4%) | 0.941 | |
| Angina | 40 (7.4%) | 34 (7.1%) | 33 (7.7%) | 0.937 | |
| Congestive heart failure | 79 (14.6%) | 73 (15.2%) | 64 (15.0%) | 0.956 | |
| Arrhythmia | 73 (13.4%) | 68 (14.2%) | 61 (14.3%) | 0.920 | |
| Hypertension | 211 (38.9%) | 200 (41.7%) | 179 (41.8%) | 0.557 | |
| Peripheral artery disease | 49 (9.0%) | 45 (9.4%) | 38 (8.9%) | 0.964 | |
| Cerebrovascular accident | 39 (7.2%) | 40 (8.3%) | 40 (9.4%) | 0.472 | |
| Diabetes | 88 (16.2%) | 85 (17.7%) | 79 (18.5%) | 0.637 |
Data are presented as mean±sd or n (%), unless otherwise stated. SGRQ: St George's Respiratory Questionnaire; mMRC: modified Medical Research Council; FEV1: forced expiratory volume in 1 s; 6MWD: 6-min walking distance. #: significant difference of probability distribution at the three time points calculated for each variable using nonparametric Kruskal–Wallis test; ¶: classified as 0–1 long-acting β2-agonist (LABA) or long-acting muscarinic antagonist (LAMA), 2 out of LABA, LAMA and inhaled corticosteroid (ICS), or all 3 of them (LABA+LAMA+ICS).
Complete pulmonary function tests included forced spirometry, bronchodilator testing and body plethysmography, as well as measurements of carbon monoxide diffusing capacity and respiratory muscle strength. These tests were performed in accordance with the standards of the Spanish Society of Respiratory Medicine and Thoracic Surgery (SEPAR) [17]. For reference values, we considered those of the European Community for Steel and Coal [18].
Follow up
Patients were assessed at baseline and the end of the first and second years, and were followed up for 3 years. Specifically, once a year during the study period, survivors were interviewed and measurements were made of several variables, including HRQoL (using the SGRQ) and pulmonary function. Furthermore, up to 3 years after the baseline assessment, patient health records and the hospital database on hCOPD were reviewed. Vital status was established by reviewing health records, the hospital database and public death registries. Deaths from any cause were considered confirmed if the name, sex and date of birth on the record matched those of the participants. No interventions were performed in relation to this study and the research team did not take part in patients' routine treatment or the treatment of any exacerbations.
Statistical analysis
Disease-specific quality of life was measured using the SGRQ. SGRQ scores are presented as mean±sd. The outcome variables were: 1) vital status of the patient, recorded as alive or dead, for 1-year periods; and 2) hCOPD rate for 1-year periods. Data at baseline and the three immediately consecutive 1-year periods were considered in the analysis. Generalised linear mixed models for repeated measurements were built with SGRQ score as the covariate and the outcome in the following 1-year period for each domain [19]. A logistic model was fitted for vital status, whereas a generalised Poisson model accounting for overdispersion was fitted for hospitalisations [20]. Results were expressed as the odds ratio of a 4-point increase in SGRQ score, which is considered a clinically significant change, along with the 95% confidence interval.
Results
The cohort included 543 patients (mean age 68 years), mostly men (96%) with a moderate level of obstruction (FEV1 55% of the predicted value), and 23% were active smokers. Using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification [21], 56% of the cohort was classified as GOLD group A and 28% as GOLD B. Regarding the HRQoL, the SGRQ total, symptoms, activity and impact scores at baseline were 39.2, 44.5, 48.7 and 32.0, respectively. Table 1 summarises other characteristics of the cohort at the three assessments.
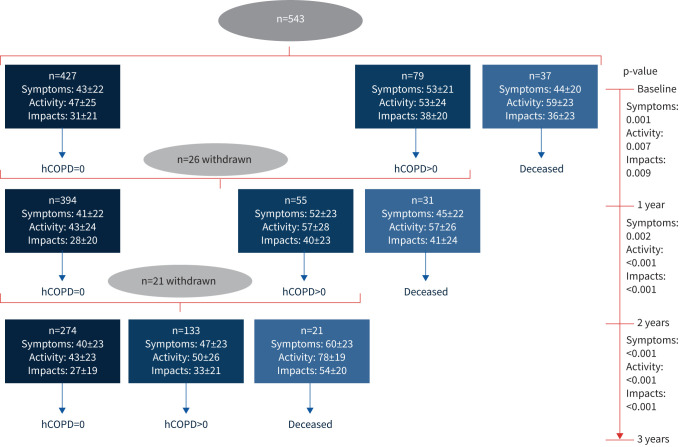
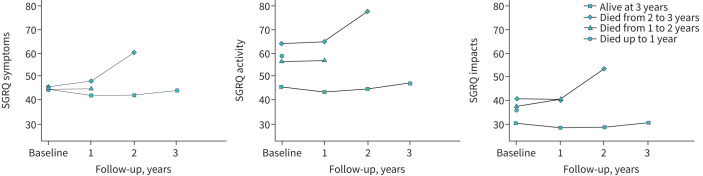
During the follow-up, 37 (6.8%) patients died in the first year, 31 (6.4%) in the second year and 21 (4.9%) in the third year (figure 1). The SGRQ symptoms, activity and impacts mean scores at the start of the study in those who survived to the end of the follow-up (n=407) were 44.5, 45.9 and 30.5, respectively, and during the follow-up, these values did not significantly increase (which would indicate worsening) (figure 2). Conversely, the patients who died at any point during the 3 years of follow-up (n=89) had SGRQ symptoms, activity and impacts mean scores at the start of the study of 45.5, 61.9 and 40.1, respectively (figure 1). A similar pattern was observed in HRQoL in relation to hCOPD during follow-up.
FIGURE 1.
Follow-up of patients and St Georges’ Respiratory Questionnaire scores (mean±sd) before death and hospitalisation due to COPD (hCOPD) (considering events during the year following assessment of health-related quality of life).
FIGURE 2.
Changes in scores in the three domains of the St Georges’ Respiratory Questionnaire (SGRQ) by study group depending on vital status during the follow-up period.
Notably, a clinically relevant deterioration in quality of life as measured by SGRQ score at the annual follow-up was seen in at least one of the domains in patients who died before the next assessment (figure 2). In the generalised linear mixed model for 1-year mortality over the 3-year follow-up based on the HRQoL measurement before death (last measurement), it was shown that every 4-point increase in the SGRQ was associated with a higher likelihood of death: “symptoms” domain odds ratio 1.04 (95% CI 1.00–1.08); “activity” domain OR 1.12 (95% CI 1.08–1.17); and “impacts” domain OR 1.11 (95% CI 1.06–1.15) (table 2 and figure 2).
TABLE 2.
Results of the generalised linear mixed model for 1-year mortality over 3 years of follow-up based on health-related quality of life (HRQoL) score before death (considering the most recent measurement, at the start of the 1-year period) in a longitudinal study
| HRQoL | β±sd | p-value | OR (95% CI) |
| SGRQ symptoms | 0.0386±0.0200 | 0.054 | 1.04 (1.00–1.08) |
| SGRQ activity | 0.1135±0.0199 | <0.001 | 1.12 (1.08–1.17) |
| SGRQ impacts | 0.1000±0.0209 | <0.001 | 1.11 (1.06–1.15) |
| SGRQ total | 0.1113±0.0224 | <0.001 | 1.12 (1.07–1.17) |
Modelled on a 4-point increase in St George's Respiratory Questionnaire (SGRQ) score.
Furthermore, higher values in the three SGRQ domains were significantly related to a higher hCOPD rate in the following 1-year period (p<0.001). The rate of hospitalisation per year was, depending on the domain, 5% (95% CI 3–8%) to 7% (95% CI 5–10%) higher for each 4-point increase in SGRQ score (table 3). Estimated values of the mean±sd overdispersion parameter of the generalised Poisson distribution were 0.381±0.046, 0.365±0.045 and 0.362±0.045 for the symptoms, impacts and activity domains respectively; all of them statistically significant (p<0.001).
TABLE 3.
Results of the generalised Poisson mixed model for 1-year chronic obstructive pulmonary disease exacerbation-related hospitalisations over 3-years of follow-up based on health-related quality of life (HRQoL) score before hospitalisation (considering the most recent measurement, at the start of the 1-year period) in a longitudinal study
| HRQoL | β±sd | p-value | eβ (95%CI) | τ±sd |
| SGRQ symptoms | 0.0513±0.0113 | <0.001 | 1.05 (1.03–1.08) | 0.381±0.046 |
| SGRQ activity | 0.0594±0.0107 | <0.001 | 1.06 (1.03–1.08) | 0.365±0.045 |
| SGRQ impacts | 0.0694±0.0120 | <0.001 | 1.07 (1.05–1.10) | 0.362±0.045 |
| SGRQ total | 0.0774±0.0127 | <0.001 | 1.08 (1.05–1.11) | 0.365±0.045 |
Modelled on a 4-point increase in St George's Respiratory Questionnaire (SGRQ) score.
Discussion
The main finding of our study was that maintaining HRQoL (keeping the same score) was related to patient survival, while deterioration from one assessment to the next, that is, over a 1-year period, corresponding to a more than 4-point decrease in score for any domains of the SGRQ, was associated with a higher likelihood of death and hCOPD. Notably, this pattern was seen for all the domains of the questionnaire, though especially in the activity and impacts domains for the mortality outcome (figure 2).
It is known from previous studies that there is an independent association between HRQoL measured by SGRQ and mortality, this being applicable not only to all-cause mortality but also to respiratory mortality [12, 22–25]. These results were mainly obtained in cross-sectional studies. In our study, when performing separate analysis for those in the cohort who survived or died during follow-up, the latter had higher scores at the start of the study in all the domains of the SGRQ, especially activity and impacts. In this respect, our results are in line with those of the aforementioned studies.
One recent observational study went a step further and demonstrated an association between 1-year change in SGRQ and mortality over a 10-year follow-up [26]. What is novel about our study is that the annual change (deterioration) in SGRQ score was predictive of the risk of death during the next year of follow-up. It was not the SGRQ domain scores, but the annual change therein that was associated with the higher likelihood of death (specifically, 12% and 11% for the activity and impacts domains respectively for every 4-point increase in the score). Nonetheless, not all the domains of the SGRQ (the most widely used specific questionnaire in clinical research in COPD) seem to be equally predictive of mortality. In our patients, the activity and impacts domains showed the highest predictive capacity.
In line with this, Antonelli-Incalzi et al. [23] showed that, among the SGRQ scores, it was the impact domain that was the best predictor of mortality and this performed better than FEV1 during the 53-month follow-up of their cohort: hazard ratio 1.13 (95% CI 1.06–1.22). In our study, this domain had a similar power as a predictor of mortality to that seen in the aforementioned study and the same power as that of the activity domain, both of them being more valuable than the symptom domain. The impacts domain covers issues related to social functioning but also aspects of psychological disturbances. However, the activity score measures disturbances to daily physical activity (activities that cause or are limited by breathlessness). The impacts score is the broadest component of the SGRQ. It correlates with the whole range of disturbances that respiratory patients experience in their lives, from dyspnoea to anxiety and depression, including limitations in exercise capacity. All these aspects are crucial as strong predictors of mortality [27–29].
In a study by Domingo-Salvany et al. [12], in their final model for all-cause and respiratory mortality, only the activity domain reached statistical significance. In our study, activity was the domain that was the strongest predictor of mortality, probably because it reflects dyspnoea of pulmonary and/or cardiac origin, and our cohort had a high level of comorbidities, including cardiovascular conditions. As noted above, dyspnoea has shown to be a strong predictor of mortality [27], and these two domains of the SGRQ capture not only the importance of the symptom itself but also of its relationship with physical and psychological limitations, which seem to be the most important issue [30, 31].
These findings are noteworthy bearing in mind that symptoms in COPD patients are important in several aspects of disease burden [32], and moreover, productive cough is a prognostic marker of outcomes in COPD patients, including respiratory mortality [33]. The SGRQ symptoms domain is focused on the frequency and severity of respiratory symptoms. Hence, a higher predictive capacity of the symptoms domain could have been expected in the mortality of our cohort.
Although less widely reported than the association of mortality with HRQoL, a relationship between hospitalisation and quality of life has also been described in patients with COPD. For example, Fan et al. [22] showed that the score on a specific HRQoL instrument (the Seattle Obstructive Lung Disease Questionnaire) [34] was an independent predictor of hospitalisation and death, the physical function scale of the questionnaire being the best predictor of hospitalisation. These findings are partially in agreement with ours, but we observed no differences between the three domains of the SGRQ in this respect. Similar findings were reported in another study which showed that all SGRQ domains were independent predictors of hospitalisation [25].
The main limitation of this study is the uncertainty in determining the cause of death. For this reason, we have analysed only general mortality. Furthermore, our cohort was mainly composed of men but this has been a common finding in Spanish COPD cohorts to date because, in our country, women started to smoke relatively late in the 20th century. We cannot currently translate our results to other HRQoL questionnaires, such as the COPD Assessment Test (CAT), which would give us a more practical point of view of the usefulness of the tool.
No other confounders, although recorded, have been included in the analysis. The reason is the goal of the study, where the effect of a patient-reported outcome, such as HRQoL, is being tested as a surrogate indicator of prognosis as regards to mortality and hospitalisations. Summarising, the purpose was to show the usefulness of the HRQoL in the prediction of prognosis apart from other factors.
The use of generalised linear mixed models for the statistical analysis of repeated measurements over time, in this particular case using binary and count outcomes, accounting for overdispersion, which is quite common in this kind of data, adds robustness to the results and conclusions.
Our results have two main implications. First, we can interpret deterioration by 4 points in SGRQ scores, particularly those for certain domains (activity and impacts), from one year to the next, as a possible warning of hCOPD and/or death. Hence, if any clinically significant deterioration in HRQoL is detected in the follow-up of COPD patients (especially in the activity domain of the SGRQ), this change should be considered a warning concerning the clinical status of the patient, and efforts should be made to identify the cause of the deterioration and take measures to reverse the situation.
Second, there is a need for further research to investigate whether our findings with the SGRQ are replicated with other measures, in particular, with specific HRQoL questionnaires that are easier to administer in routine clinical practice, such as the CAT or the Clinical COPD Questionnaire. If similar patterns were found with simpler tools, routinely measuring HRQoL would be not only useful but feasible. To sum up, our findings emphasise the importance of quantifying HRQoL as a prognosis tool in COPD and allow a new perspective on the measurement of HRQoL in that it may provide a tool for general prognosis in the routine clinical assessment of COPD patients.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: C. Esteban and J.M. Quintana designed the study; I. Arostegui performed the statistical analyses; and C. Esteban, I. Arostegui and J.M. Quintana wrote the report with input from the other authors. All authors contributed to the interpretation of the data, made critical revisions of the manuscript and approved the final version of the manuscript.
Conflict of interest: C. Esteban has nothing to disclose.
Conflict of interest: I. Arostegui has nothing to disclose.
Conflict of interest: A. Aramburu has nothing to disclose.
Conflict of interest: J. Moraza has nothing to disclose.
Conflict of interest: L. Chasco has nothing to disclose.
Conflict of interest: M. Aburto has nothing to disclose.
Conflict of interest: S. Aizpiri has nothing to disclose.
Conflict of interest: J.M. Quintana has nothing to disclose.
Support statement: This work was supported by the Spanish Health Research Fund (FIS grant number PI020510), and by funding from the Dept of Health of the Basque Government (grant number 200111002), Spanish Ministry of Economy and Competitiveness (MTM2016–74931-P and BCAM Severo Ochoa excellence accreditation SEV-2017-0718), Dept of Education, Linguistic Policy and Culture of the Basque Government (IT1294-19 and BERC 2018-2021), and the University of the Basque Country (COLAB20/01).
References
- 1.Vanfleteren LE, Spruit MA, Wouters EF, et al. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016; 4: 911–924. doi: 10.1016/S2213-2600(16)00097-7 [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11; 122. doi: 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteban C, Quintana JM, Aburto M, et al. Predictors of mortality in patients with stable COPD. J Gen Intern Med 2008; 23: 1829–1834. doi: 10.1007/s11606-008-0783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli BC, Cote CG, Marín JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 5.Boeck L, Soriano JB, Brusse-Keizer M, et al. Prognostic assessment in COPD without lung function: the B-AE-D indices. Eur Respir J 2016; 47: 1635–1644. doi: 10.1183/13993003.01485-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almagro P, Martinez-Camblor P, Miravitlles M, et al. External validation and recalculation of the CODEX index in COPD patients. A 3CIAplus cohort study. COPD 2019; 16: 8–17. doi: 10.1080/15412555.2018.1484440 [DOI] [PubMed] [Google Scholar]
- 7.Aramburu A, Arostegui I, Moraza J, et al. COPD classification models and mortality prediction capacity. Int J Chron Obstruct Pulmon Dis 2019; 14: 605–613. doi: 10.2147/COPD.S184695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004; 59: 387–395. doi: 10.1136/thx.2003.008730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteban C, Quintana JM, Moraza J, et al. Impact of hospitalisations for exacerbations of COPD on health related quality of life. Respir Med 2009; 103: 1201–1208. doi: 10.1016/j.rmed.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Chen Y, Zhang W, et al. Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: a meta-analysis. Int J Surg 2020; 78: 28–35. doi: 10.1016/j.ijsu.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 11.Esteban C, Arostegui I, Aramburu A, et al. Predictive factors over time of health-related quality of life in COPD patients. Respir Res 2020; 21: 138. doi: 10.1186/s12931-020-01395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 680–685. doi: 10.1164/rccm.2112043 [DOI] [PubMed] [Google Scholar]
- 13.Marin JM, Cote CG, Diaz O, et al. Prognostic assessment in COPD: health related quality of life and the BODE index. Respir Med 2011; 105: 916–921. doi: 10.1016/j.rmed.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85: Suppl. B, 25–31. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 16.Ferrer M, Alonso J, Prieto L, et al. Validity and reliability of the St George's Respiratory Questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J 1996; 9: 1160–1166. doi: 10.1183/09031936.96.09061160 [DOI] [PubMed] [Google Scholar]
- 17.García-Río F, Calle M, Burgos F, et al. Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol 2013; 49: 388–401. doi: 10.1016/j.arbres.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party standardization of lung function test, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J 1993; 6: Suppl. 16, 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 19.McCulloch CE, Searle SR. Generalized, Linear and Mixed Models. Hoboken, John Wiley & Sons, Inc., 2001. [Google Scholar]
- 20.Joe H, Zhu R. Generalized Poisson distribution: the property of mixture of Poisson and comparison with negative binomial distribution. Biom J 2005; 47: 219–229. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 22.Fan VS, Curtis JR, Tu SP, et al. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest 2002; 122: 429–436. doi: 10.1378/chest.122.2.429 [DOI] [PubMed] [Google Scholar]
- 23.Antonelli-Incalzi R, Pedone C, Scarlata S, et al. Correlates of mortality in elderly COPD patients: focus on health-related quality of life. Respirology 2009; 14: 98–104. doi: 10.1111/j.1440-1843.2008.01441.x [DOI] [PubMed] [Google Scholar]
- 24.Oga T, Nishimura K, Tsukino M, et al. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167: 544–549. doi: 10.1164/rccm.200206-583OC [DOI] [PubMed] [Google Scholar]
- 25.Yorgancioglu A, Havlucu Y, Celik P, et al. Relation between quality of life and morbidity and mortality in COPD patients: two-year follow-up study. COPD 2010; 7: 248–253. doi: 10.3109/15412555.2010.496816 [DOI] [PubMed] [Google Scholar]
- 26.Havlucu Y, Yorgancıoglu A, Sakar Coskun A, et al. Does one year change in quality of life predict the mortality in patients with chronic obstructive pulmonary disease? Prospective cohort study. J Thorac Dis 2019; 11: 3626–3632. doi: 10.21037/jtd.2019.07.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura K, Takateru I, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440. doi: 10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- 28.Atlantis E, Fahey P, Cochrane B, et al. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest 2013; 144: 766–777. doi: 10.1378/chest.12-1911 [DOI] [PubMed] [Google Scholar]
- 29.Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23: 28–33. doi: 10.1183/09031936.03.00034603 [DOI] [PubMed] [Google Scholar]
- 30.Jones PW, Quirk FH, Baveystock CM, et al. A self complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 31.Jones PW, Quirk FH, Baveystock CM. The St. George's Respiratory Questionnaire. Resp Med 1991, 85: Suppl., 25–31. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 32.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res 2017; 18: 67. doi: 10.1186/s12931-017-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J 2017; 50: 1602470. doi: 10.1183/13993003.02470-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu SP, McDonell MB, Spertus JA, et al. A new self-administered questionnaire to monitor health-related quality of life in patients with COPD. Ambulatory Care Quality Improvement Project (ACQUIP) Investigators. Chest 1997; 112: 614–622. doi: 10.1378/chest.112.3.614 [DOI] [PubMed] [Google Scholar]