Abstract
Repeated serial in vitro passage of Histomonas meleagridis, the etiological agent of histomoniasis (blackhead) of turkeys, was demonstrated to markedly achieve attenuation and reduction of virulence as compared to the original wild-type isolate. Four experiments were performed to evaluate the route (oral vs. intracloacal) and age (day-of-hatch vs. d 14) for administration of attenuated H. meleagridis isolates as vaccine candidates against homologous or heterologous wild-type challenge. Attenuated H. meleagridis were developed from 2 different strains (Buford strain originating in Georgia; PHL2017 strain originating in Northwest Arkansas). Buford P80a (passage 80, assigned as isolate lineage “a” following repeated passage) was selected as the primary vaccine candidate and was evaluated in Experiments 1–3. Experiment 4 evaluated selected candidates of attenuated PHL2017 (P67, P129) and Buford (P80a, P200a, P138b, P198c) strains against Buford wild-type challenge. As has been demonstrated previously, wild-type H. meleagridis cultures administered orally after 1 day of age were not infective in the current studies, but infection with wild-type cultures could be induced orally at day-of-hatch. Infection was effectively achieved via the intracloacal route at day-of-hatch and in older turkeys (d 21, d 28–29, d 35). Intracloacal inoculation of turkeys with the attenuated passaged isolates as vaccine candidates at d 14 was shown to produce significant (P < 0.05) protection from mortality, reduction in body weight gain, as well as reduction in hepatic and cecal lesions in these experiments following challenge with either the homologous wild-type isolate or from a wild-type strain obtained years later from a geographically disparate area of the United States. Inoculation with the attenuated H. meleagridis isolates at day-of-hatch, either orally or cloacally, did not produce significant protection against subsequent wild-type challenge. While offering significant protection with minimal vaccine-related negative effects, the protection from cloacal vaccine administration was neither significantly robust nor encouraging for industry application using the methods evaluated in the present manuscript since mortalities and lesions were not completely reduced which could thereby potentially allow transmission from residual infection and shedding within a flock.
Key words: histomoniasis, Histomonas meleagridis, live-attenuated, turkey, vaccination
INTRODUCTION
Histomoniasis (synonyms: blackhead disease, infectious enterohepatitis, and histomonosis) is an intestinal protozoal disease of gallinaceous birds with particularly deleterious impact to turkeys (Clarkson, 1963; Hess et al., 2015). Initial infection with the trichomonad parasite Histomonas meleagridis, the etiological agent of histomoniasis, is thought to occur via infected Heterakis gallinarum cecal worms (Tyzzer, 1934; Lund et al., 1966b; Cupo and Beckstead, 2019). Disease transmission can occur rapidly in turkeys via cloacal drinking, whereby reverse peristalsis quickly uptakes materials into the cloaca and transfers to the cecae (Sorvari et al., 1977; Hu and McDougald, 2003; Hu et al., 2004; McDougald and Fuller, 2005). H. meleagridis has been characterized as an extracellular parasite that reproduces through binary fission and can exhibit either amoeboid or flagellated morphology (Tyzzer, 1920; Bayon and Bishop, 1937; Cuckler, 1970). Turkeys should be reared separately from chickens due to the propensity of chickens to serve as reservoirs of histomonads and heterakids (Joyner et al., 1963; Lund et al., 1966b; McDougald, 1998). Since the voluntary removal of nitarsone in 2015, no approved therapeutics or prophylactics are available to treat histomoniasis, leaving turkey flocks to suffer morbidities and mortalities often reaching 80 to 100% (McDougald, 2005; Hess and McDougald, 2013; Regmi et al., 2016). In vitro and in vivo studies have yielded inconsistent results for antihistomonal candidate compounds, and no effective alternatives have been introduced to replace the previously used nitroimidazoles, nitrofurans, and arsenical compounds (Grabensteiner et al., 2008; van der Heijden and Landman, 2008a,b; Thøfner et al., 2012). Further complicating this problem, H. meleagridis isolates may vary in susceptibility to chemotherapeutics in vitro and in vivo (Berks and Neal, 1952; Grabensteiner et al., 2007; van der Heijden and Landman, 2008a,b). Without viable substitute treatment options, producers are suffering losses of turkeys and decreased performance of broiler breeders and layer pullets (Hu and McDougald, 2004; Popp et al., 2011). Although vaccinations are important for the induction of a host-immune response to protect against disease, early immunological studies were discouraging toward successful vaccine development for histomoniasis (Tyzzer, 1934, 1936; Clarkson, 1963; Lund et al., 1966a). Some success with immunization of histomoniasis has been reported experimentally in recent years, but a vaccine has not yet been developed for industry application (McAllister, 2014; Liebhart et al., 2017; Mitra et al., 2018).
Cloacal administration of infected liver or cecal tissue or suspensions of H. meleagridis culture has reproduced histomoniasis within experimental settings; however, direct oral ingestion of unprotected histomonads has not reliably induced disease presumably due to adverse acidity and mechanical action within the crop and ventriculus (Berks and Neal, 1952; Hu et al., 2004). Liebhart and Hess (2009) induced histomoniasis with in vitro cultivated clonal H. meleagridis administered orally to 1-day-old turkeys followed by 5-h feed withdrawal. A putative cyst-like structure of H. meleagridis was identified in vitro; therefore, the oral transmission route for poultry in contact with large amounts of contaminated excreta or litter in the absence of H. gallinarum should not be disregarded (Munsch et al., 2009a,b; Zaragatzki et al., 2010a,b). Turkeys recovered from histomoniasis had a semblance of protection upon maturity, but reinfection occurred when birds were kept on infected soil, suggesting only temporary immunity or overload of pathogen exposure (Tyzzer and Fabyan, 1922). Dimetridazole treatment of H. meleagridis-infected turkeys resulted in resistance to subsequent infection in the recovered turkeys, further suggesting that acquired protective immunity is possible (Joyner, 1963). Additionally, Cuckler (1970) reported turkeys recovered from histomoniasis were resistant to subsequent challenge, even with maintained presence of histomonads within the cecae, further supporting the idea of protective immune response development that prevented migration of the protozoa to hepatic tissue.
Tyzzer (1932, 1934, 1936) observed inconsistent reduction in virulence of H. meleagridis serially passaged for extended periods of time in vitro, and immunization results yielded conflicting success. Following 2 yr of in vitro propagation, an isolate originally pathogenic to chickens had lost pathogenicity but was able to induce protection against virulent strains only when allowed to propagate within the chicken's cecae (Tyzzer, 1932). Attenuated isolates were later found to confer immunity against virulent challenge only when the histomonads were administered cloacally and not incorporated into heterakid eggs (Lund, 1959; Lund et al., 1966a). In vitro passaging more than 1,000 times over a period of 7 yr resulted in loss of pathogenicity and efficacy as an immunizing strain for protection against pathogenic H. meleagridis (Lund et al., 1967). Prolonged in vitro passaging has been reported to decrease vaccination efficacy, and the attenuated histomonads could be restored to original virulence with serial passage in poultry (Dwyer and Honigberg, 1970). In vitro attenuation is variable, and susceptibility of chickens and turkeys to histomoniasis varies based on breed (Al-Khateeb et al., 1974; Lotfi et al., 2014). In experimental settings, the oral or cloacal administration of clonal in vitro attenuated H. meleagridis and subsequent challenge with a virulent isolate has conferred some protection (Hess et al., 2008; Liebhart et al., 2010, 2013; Sulejmanovic et al., 2016). Liver and cecal lesions were reduced in chickens and turkeys following intracloacal administration of attenuated histomonads utilized as a vaccine strain (Hess et al., 2008; Liebhart et al., 2013). Liebhart et al. (2010) demonstrated a protective effect of in vitro attenuated H. meleagridis administered orally to 1-day-old turkeys, suggesting the necessity to further evaluate this age and route. With prolonged in vitro passaging, H. meleagridis adapt to cell culture and lose the ability to invade host tissue as seen in a recent study where an attenuated isolate was observed only in cecal tissue and presumably unable to parasitize other regions (Liebhart et al., 2011). In vitro attenuated histomonads have induced protection against virulent challenge without reducing performance (Liebhart et al., 2010, 2013). Pullets vaccinated at 18-wk-of-age with an attenuated isolate exhibited reduced pathology and prevented the severe drop in egg production observed in unvaccinated pullets (Liebhart et al., 2013). Furthermore, cross-protection against heterologous virulent isolates was demonstrated by vaccinating with an attenuated clonal strain of H. meleagridis developed through prolonged in vitro propagation (Sulejmanovic et al., 2016). Although known to rely on bacteria for cultivation, the in vitro attenuation of H. meleagridis occurs independently of culture media bacterial load (Ganas et al., 2012). A low virulent isolate obtained after intracloacal serial back-passaging in turkeys protected against subsequent virulent challenge (Pham et al., 2013). Stable attenuation of H. meleagridis with no reversion to virulence was demonstrated after 295 serial passages in vitro and 5 subsequent back-passages in vivo (Sulejmanovic et al., 2013).
Intravenous injection of H. meleagridis-infected liver tissue did not protect turkeys from subsequent subcutaneous challenge (Tyzzer et al., 1921). Attempts with inactivated vaccine for inducing a humoral response either passively (by intraperitoneal injection of antisera from immune into naïve poultry) or actively (by intramuscular injection of lysed H. meleagridis fragments) have also failed to confer protection against virulent challenge (Clarkson, 1963; Hess et al., 2008; Bleyen et al., 2009). IgG increased following administration of attenuated H. meleagridis and subsequent virulent challenge, although antibodies do not seem to serve a substantial role in development of protective immunity (Windisch and Hess, 2009, 2010). T and B cell subset deviations were reduced with an attenuated isolate in chickens and turkeys, while histomoniasis-related mortality in turkeys was associated with higher cellular immune response as compared to chickens (Mitra et al., 2017). An increase of CD4+ T cells occurred in chickens that were challenged with a virulent monoxenic culture of H. meleagridis (Lagler et al., 2019). Acquired immunity for histomoniasis may be primarily cell-mediated rather than antibody-based humoral.
Although the feasibility of administering live-attenuated H. meleagridis to confer immunity is questionable for meeting industry demand, attenuated histomonads appear to be somewhat efficacious for initiating an immune response to subsequent wild-type challenge (Hess and McDougald, 2013; Hess et al., 2015). Taken together, these data suggest protective immune response against histomoniasis may be possible with administration of live-attenuated H. meleagridis isolates. Development of a histomoniasis vaccine would be beneficial to the poultry industry and is encouraged by these immunological research advances. The objectives of this study were to evaluate highly in vitro-passaged H. meleagridis isolates for protection of turkeys in an experimental challenge model and to further elucidate the possible routes and age for administration of vaccine candidates.
MATERIALS AND METHODS
Animal Source
On day-of-hatch, female poults were obtained from a local commercial hatchery (Cargill, Gentry, AR), individually tagged, and randomly allocated to floor pens at the University of Arkansas Poultry Health Laboratory. All animal handling procedures complied with regulations of the University of Arkansas Institutional Animal Care and Use Committee (IACUC protocols #18113 and #19032). A corn-soy based starter feed meeting the nutrient requirements of poultry (NRC, 1994) and water were provided ad libitum. Mortalities unrelated to histomoniasis were recorded and the altered group numbers are reported.
Histomonas Meleagridis Isolates and Culture
Two field isolates of H. meleagridis were obtained from histomoniasis outbreaks in Buford, Georgia (Buford strain; isolated from infected chickens) and Northwest Arkansas (cultured at the University of Arkansas Poultry Health Laboratory and subsequently labeled as PHL2017 strain; isolated from infected turkeys). These wild-type field isolates were serially passaged up to 200 times in vitro and selected for evaluation as live-attenuated H. meleagridis vaccine candidates (Vacc). The strain and passage indicator of Vacc isolates are listed below within corresponding experiments. Challenge in all experiments occurred with wild-type H. meleagridis (WTH) consisting of low passaged (<10 serial passages) Buford strain. According to previously published methods, histomonads were grown in 25 cm2 tissue culture flasks (Product #10062-874, VWR International, Radnor, PA) containing Modified Dwyer's Media (MDM) comprised of Medium 199 (Product #12-118F, Lonza, Basel, Switzerland) supplemented with 10% heat-inactivated horse serum (Product #26050-088, Gibco, Life Technologies Corporation, Waltham, MA), 1.6mg/mL organic white rice flour (Arrowhead Mills, Boulder, CO), and an undefined bacterial population from the original field cecal isolate (van der Heijden and Landman, 2005, 2007; Beer et al., 2020). Culture flasks were incubated anaerobically at 40°C for 48 to 72h before 1 mL was subcultured into 12.5 mL of fresh, supplemented MDM. For long-term preservation of H. meleagridis, 10% dimethylsulfoxide (OmniSolv, MilliporeSigma, Burlington, MA) was added as a cryoprotectant, and aliquots were cryogenically stored. Viable H. meleagridis cells/mL was enumerated using Trypan blue dye exclusion (Product #15250-061, Gibco) and a hemocytometer. MDM was utilized as the diluent to prepare the proper H. meleagridis dosage concentration within all experiments.
Lesion Scoring System
According to previously described methods, liver and cecal lesions were separately scored on a scale of “0” to “3”, with “3” indicating the most severe lesion (Beer et al., 2020). Described briefly, healthy liver or cecae received a score of “0”; detectible yet not clinically relevant lesions received a score of “1”; intermediate lesions related to H. meleagridis-infection received a score of “2”; and classically confluent lesions related to H. meleagridis-infection received a score of “3”. Individuals assigning lesion scores (LS) were blinded to the treatment groups. All mortalities were evaluated for liver and cecal LS pertaining to histomoniasis.
Experiment 1
The objective of Experiment 1 was to evaluate the efficacy of Vacc administered intracloacally at d 14 for protection against subsequent cloacal WTH-challenge.
Vaccination Phase (d 14–29)
Groups included a non-challenged control (NC; n = 59), Vacc (n = 39), and positive-challenged control (V-PC; n = 20). On d 14, the Vacc group received a total dose of 2 × 105 Vacc Buford P80a (cell passage number 80, “a” differentiates the isolate lineage assigned following repeated passage) cells/turkey, and the V-PC group received a total dose of 2 × 105 Buford WTH cells/turkey (Figure 1A). Intracloacal administration occurred with an animal gavage needle and occurred twice at half dosage with 1 h between each inoculation. On d 27, the V-PC group was humanely euthanized to evaluate characteristic disease lesions and compared against the Vacc group. On d 28, a subset of n = 5 turkeys/group was sampled from the NC and Vacc groups to evaluate for lesions. Individual body weights were recorded from the Vacc and NC groups on d 14.
Figure 1.
Experimental timelines for administration of live-attenuated vaccine candidate (Vacc) Histomonas meleagridis and subsequent challenge with Buford strain wild-type H. meleagridis (WTH). The Buford strain was isolated from infected chickens in Georgia; PHL2017 strain was isolated from infected turkeys in Arkansas. Vacc passage number and isolate indicator are included in each group name, where applicable. Abbreviations: C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V-PC, Vaccination Phase positive-challenged control.
Challenge Phase (d 29–40)
On d 29, individual body weights were recorded from all groups, and all groups except for the NC were intracloacally challenged with 2 × 105 Buford WTH cells/turkey using the procedure described above. A newly introduced C-PC group (n = 27) was created from a subset of the NC group (n = 27 remaining) to serve as concurrent reference against the Vacc group (n = 34 remaining). On d 40, individual body weights were recorded, and all remaining poults were humanely euthanized and subsequently evaluated for liver and cecal LS.
Experiment 2
The objective of Experiment 2 was to evaluate different doses and routes of Vacc administered at day-of-hatch to protect against subsequent cloacal WTH-challenge.
Vaccination Phase (d 0–21)
Groups included NC (n = 34), Vacc Oral 2 × 103 (n = 36; Vacc Oral 2k), Vacc Oral 2 × 104 (n = 38; Vacc Oral 20k), Vacc Cloacal 2 × 103 (n = 36; Vacc Cloacal 2k), Vacc Cloacal 2 × 104 (n = 37; Vacc Cloacal 20k), Vacc Cloacal 2 × 105 (n = 30; Vacc Cloacal 200k), and V-PC Cloacal 2 × 105 (n = 36; V-PC Cloacal 200k). On day-of-hatch and prior to feeding, Vacc group turkeys were either orally or intracloacally administered with respective dose of Vacc Buford P80a cells/turkey (Figure 1B). The V-PC Cloacal received 2 × 105 Buford WTH cells/turkey administered intracloacally. A total of 30 day-of-hatch poults were humanely euthanized prior to feeding, and the pH of the combined proventriculus-ventriculus region was measured of each poult using pH indicator strips (Sigma-Aldrich, St. Louis, MO). On d 15, a subset from each group was evaluated for liver and cecal LS to compare the Vacc groups to the V-PC Cloacal group, leaving a remaining subset of n = 20 from each Vacc group for the Challenge Phase. Individual body weights were recorded on d 0, d 7, d 14, and d 21, except for the V-PC Cloacal group which was terminated on d 15 for liver and cecal LS.
Challenge Phase (d 21–35)
On d 21, the NC poults were reallocated into new groups consisting of PC Oral (n = 14) and C-PC Cloacal (n = 20). On d 21, all groups received intracloacal challenge of 2 × 105 total Buford WTH cells/turkey in a pair of inoculations, except for the C-PC Oral group which received this dose orally in a single administration. On d 35, individual body weights were recorded, and all remaining poults were humanely euthanized and evaluated for liver and cecal LS.
Experiment 3
The objective of Experiment 3 was to further evaluate the Vacc administered at day-of-hatch or d 14 to protect against subsequent cloacal WTH-challenge. Additionally, the oral and cloacal administration routes at day-of-hatch were compared between Vacc and WTH isolates.
Vaccination Phase 1 (d 0–14)
Groups included NC (n = 216), d 0 Vacc Oral (n = 53), d 0 Vacc Cloacal (n = 52), d 0 V1-PC Oral (n = 60), and d 0 V1-PC Cloacal (n = 60). On day-of-hatch and prior to feeding, turkeys were either orally or intracloacally administered 2 × 105 cells/turkey of either Vacc Buford P80a or Buford WTH (Figure 1C). A total of 60 day-of-hatch poults were humanely euthanized prior to feeding, and the pH of the combined proventriculus-ventriculus region was measured of each poult using pH indicator strips (Sigma-Aldrich). On d 14, all turkeys from the V1-PC Oral and V1-PC Cloacal groups were humanely euthanized and evaluated for liver and cecal LS. A total of n = 10 turkeys/group were likewise evaluated from the NC, d 0 Vacc Oral, and d 0 Vacc Cloacal groups to compare to the V1-PC groups. Individual body weights were recorded on d 0 and d 14.
Vaccination Phase 2 (d 14–28)
On d 14, the newly introduced V2-PC Cloacal (n = 43) and Vacc Cloacal (n = 55) groups were created from subsets of the NC group (n = 108 remaining). The V2-PC Cloacal and d 14 Vacc Cloacal groups were intracloacally administered 2 × 105 cells/turkey of either Buford WTH or Vacc Buford P80a, respectively. On d 28, all poults from the V2-PC Cloacal were humanely euthanized and evaluated for liver and cecal LS. A total of n = 10 turkeys/group were likewise evaluated from the NC and d 14 Vacc Cloacal groups; n = 5 turkeys/group were evaluated from the d 0 Vacc Oral and d 0 Vacc Cloacal groups. Individual body weights were recorded on d 28.
Challenge Phase (d 28–42)
On d 28, the newly introduced C-PC Cloacal (n = 45) was created from a subset of the NC group (n = 53 remaining). On d 28, all turkeys except for the NC group were intracloacally challenged with 2 × 105 Buford WTH cells/turkey. On d 42, individual body weights were recorded, and all remaining turkeys were humanely euthanized and evaluated for liver and cecal LS.
Experiment 4
The objective of Experiment 4 was to evaluate Buford and PHL Vacc isolates intracloacally administered at d 14 to protect against subsequent cloacal Buford WTH-challenge; therefore, to compare efficacy of Vacc isolates to homologous and heterologous WTH-challenge.
Vaccination Phase (d 14–35)
Groups included NC (n = 69), V-PC Buford (n = 60), Vacc PHL P67 (n = 59), Vacc PHL P129 (n = 60), Vacc Buford P80a (n = 60), Vacc Buford P200a (n = 60), Vacc Buford P138b (n = 59), and Vacc Buford P198c (n = 60). Cell passage numbers are indicated with respective isolate of either PHL2017 or Buford strain (where “a”, “b”, and “c” further differentiate lineage of highly passaged isolates when applicable). On d14, the Vacc groups were intracloacally administered 2 × 105 cells/turkey of the respective Vacc isolate, and the V-PC Buford group received a total dose of 2 × 105 Buford WTH cells/turkey (Figure 1D). On d 28, subsets of n = 10 turkeys/group were evaluated from the Vacc groups to compare liver and cecal LS to subsets of the NC (n = 5) and V-PC Buford (n = 34). On d 35, subsets of n = 10 turkeys/group were likewise evaluated from the Vacc groups to compare LS to subsets of the NC (n = 5) and the remainder of V-PC Buford (n = 26). Individual body weights were recorded on d 14, d 28, and d 34.
Challenge Phase (d 35–49)
On d 35, the newly introduced C-PC Buford group (n = 39) was created from a subset of the NC group (n = 20 remaining). On d 35, all groups except for the NC group were intracloacally challenged with a total of 2 × 105 Buford WTH cells/turkey. On d 49, individual body weights were recorded, and all remaining turkeys were humanely euthanized and evaluated for liver and cecal LS.
Statistical Analysis
Differences in mortalities and each subgrouping of positive liver or positive cecal LS were compared to the PC group using the chi-square test. As a further consideration of differences of Vacc and WTH impact to liver and cecal tissues, additional subgroupings of turkeys with either a positive liver or cecal LS of “1–3” were also analyzed as follows: positive liver LS and positive cecal LS; negative liver LS and positive cecal LS; positive liver LS and negative cecal LS. Pre-challenge and post-challenge BWG data were compiled from each group based on surviving poult weights during each phase and were analyzed using JMP Pro 16 software (SAS Institute Inc., Cary, NC) with significant differences between treatment groups determined using ANOVA. Tukey's multiple range test was used to further separate the means, where applicable. LS data were analyzed using the Proc Mixed Procedure in SAS 9.4 software (SAS Institute). Statistical significance for all analyses was set at P < 0.05.
RESULTS
Experiment 1: d 14 Vacc Administration
Vaccination Phase (d 14–29)
Histomoniasis-related mortalities in the V-PC, Vacc, and NC groups were 30.0, 0.00, and 0.00%, respectively (Table 1). The Vacc and NC group mortalities were significantly different (P < 0.05) as compared to the V-PC group. No difference (P > 0.05) in d 14–29 BWG was observed between the Vacc group as compared to the NC group. On d 27, cumulative lesions of the V-PC group revealed 75% liver and 80% cecal lesions characteristic of histomoniasis (data not shown). On d 28 among the Vacc subset (n = 5) examined, one turkey exhibited normal liver and cecae under macroscopic examination. Two turkeys exhibited normal livers and relatively normal cecae except for small, button-like lesions. One turkey had target-like liver lesions with the cecae feeling hard, thickened, and exhibiting larger bumps and scalloping. The fifth turkey exhibited pale liver edges with narrow and thin margins and was possibly beginning to develop liver lesions; in addition, the cecae were large, with the presence of thickened walls and scalloping. No histomoniasis-related lesions were observed in the NC group at any time.
Table 1.
Body weight gain (BWG) and histomoniasis-related mortalities during Vaccination and Challenge Phases (Experiment 1).1
| Vaccination phase |
Challenge phase |
|||
|---|---|---|---|---|
| Group2 | Mortality | D 14–29 BWG (g)3 | Mortality | D 29–40 BWG (g) |
| PC | 6/20 (30.0%) | - | 6/27 (22.2%) | 230 ± 41.4c |
| Vacc | 0/39 (0.00%)* | 621 ± 16.5a | 1/34 (2.94%)* | 435 ± 45.8b |
| NC | 0/59 (0.00%)* | 630 ± 12.4a | 0/27 (0.00%)* | 732 ± 18.7a |
BWG data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). Data were analyzed using JMP Pro 16 ANOVA, further separated by Tukey's HSD.
Vaccination Phase consisted of intracloacal administration of either 2 × 105 Vacc Buford P80a or Buford WTH cells/turkey on d14; Challenge Phase began on d 29 when the Vacc and a newly introduced PC group (formed from a subset of NC) were intracloacally challenged with 2 × 105 Buford WTH cells/turkey.
NC, non-challenged control; PC, positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
The PC was terminated on d 27 due to mortality percentage; No BWG data were collected for PC at this time-point.
Indicates significant difference in mortalities (P < 0.05) as compared to the respective Vaccination or Challenge Phase PC group with chi-square test.
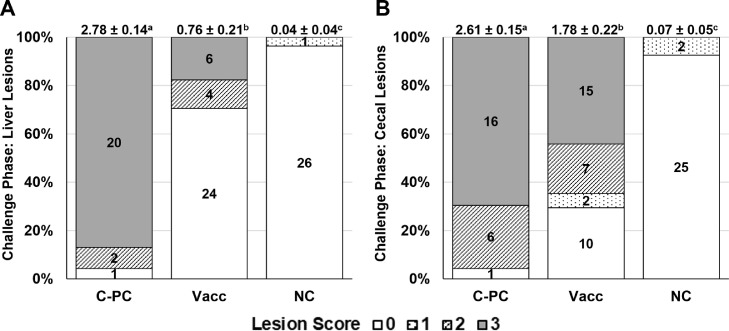
Challenge Phase (d 29–40)
Histomoniasis-related mortalities in the C-PC, Vacc, and NC groups were 22.2, 2.94, and 0.00%, respectively (Table 1). The Vacc and NC group mortalities were different (P < 0.05) as compared to the C-PC group. The d 29–40 BWG for Vacc and C-PC groups were significantly lower (P < 0.05) as compared to the NC group; however, the Vacc group BWG was significantly higher (P < 0.05) than the C-PC group. The Vacc group resulted in lowered (P < 0.05) mean liver and cecal LS as compared to the C-PC group (Table 2). From all turkeys evaluated, those with a positive liver LS for the C-PC, Vacc, and NC groups were 95.7, 29.4, and 3.70% while those with a positive cecal LS were 95.7, 70.6, and 7.41%, respectively. From all turkeys evaluated, those with a positive LS of “1-3” were further considered with the breakdown as follows: positive liver LS and positive cecal LS for the C-PC, Vacc, and NC groups were 100, 41.7, and 0.00%; negative liver LS and positive cecal LS were 0.00, 58.3, and 66.7%; positive liver LS and negative cecal LS were 0.00, 0.00, and 33.3%, respectively. Within the NC group, LS were only a score of “1” and were not considered to be related to histomoniasis according to the scoring system. Frequencies of liver and cecal LS for each group are shown in Figures 2A and 2B.
Table 2.
Liver and cecal lesion scores (LS) for histomoniasis during Challenge Phase (Experiment 1).1
| Out of total n scored2,3 |
Out of positive for n w/1-3 LS5 |
||||||
|---|---|---|---|---|---|---|---|
| Group4 | Mean Liver LS | Mean Cecal LS | +Liver LS | +Cecal LS | +Liver LS; +Cecal LS | - Liver LS; +Cecal LS | +Liver LS; - Cecal LS |
| C-PC | 2.78 ± 0.14a | 2.61 ± 0.15a | 22/23 (95.7%) | 22/23 (95.7%) | 22/22 (100%) | 0/22 (0.00%) | 0/22 (0.00%) |
| Vacc | 0.76 ± 0.21b | 1.78 ± 0.22b | 10/34 (29.4%)* | 24/34 (70.6%)* | 10/24 (41.7%)* | 14/24 (58.3%)* | 0/24 (0.00%) |
| NC | 0.04 ± 0.04c | 0.07 ± 0.05c | 1/27 (3.70%)* | 2/27 (7.41%)* | 0/3 (0.00%)* | 2/3 (66.7%)* | 1/3 (33.3%)* |
LS data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). LS were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software.
Vaccination Phase consisted of intracloacal administration of either 2 × 105 Buford P80a Vacc or Buford WTH cells/turkey on d 14; Challenge Phase began on d 29 when the Vacc and a newly introduced C-PC group (formed from a subset of NC) were intracloacally challenged with 2 × 105 Buford WTH cells/turkey.
Scores in the NC were only “1” on the LS scale of “0–3”.
Experimental error resulted in 4 of the C-PC turkeys not being evaluated for LS; hence, the difference in total n for Challenge Phase mortality and LS values in Table 1.
C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Subgroupings of total n of turkeys with either a positive liver or cecal LS of “1–3” were further considered to compare differences of Vacc and WTH impact to liver and cecal tissues.
Indicates significant difference of categorical LS classifications (P < 0.05) compared to C-PC with chi-square test.
Figure 2.
Experiment 1 frequency of lesion scores during Challenge Phase for (A) liver and (B) cecae. Numbers within columns indicate the number of turkeys per evaluated lesion score. Numbers at the top of each column indicate the lesion score mean ± SE for that group with different superscripts denoting significance (P < 0.05). Lesion scores were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software. Challenge Phase began on d 29 when the Vacc and C-PC group were intracloacally challenged with 2 × 105 Buford WTH cells/turkey. Abbreviations: C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Experiment 2: Day-of-Hatch Vacc Administration
Vaccination Phase (d 0–21)
A mean pH of 4.4 was determined from the proventriculus-ventriculus region from the day-of-hatch poult subset prior to feeding. Histomoniasis-related mortalities in the V-PC Cloacal 200k, Vacc Oral 2k, Vacc Oral 20k, Vacc Cloacal 2k, Vacc Cloacal 20k, Vacc Cloacal 200k, and NC groups were 22.2, 2.78, 2.63, 0.00, 5.41, 3.33, and 0.00%, respectively (Table 3). The Vacc and NC group mortalities were lower (P < 0.05) as compared to the V-PC Cloacal 200k group. The Vacc Cloacal 20k group had higher (P < 0.05) d 0–7 BWG as compared to the V-PC Cloacal 200k and was not different (P > 0.05) from the NC group. The Vacc 200k group had lower (P < 0.05) d0-7 BWG as compared to the NC group, but the groups were similar (P > 0.05) for d 7–14, d 14–21, and d 0–21 BWG. The d 7–14 BWG for Vacc Oral 20k and Cloacal 20k groups were higher (P < 0.05) as compared to the V-PC Cloacal 200k and were not different from the NC group. The d 14–21 BWG and d 0–21 BWG for the Vacc Cloacal 20k group were higher (P < 0.05) as compared to the Vacc Cloacal 200k and NC groups. On d 15, the mean liver and cecal LS were lower in all Vacc groups as compared to the V-PC Cloacal 200k (Table 4). From all turkeys evaluated, those with a positive liver LS were lower (P < 0.05) in all Vacc groups as compared to the V-PC Cloacal 200k; those with a positive cecal LS were lower (P < 0.05) in all Vacc groups, except for the Vacc Oral 20k group, as compared to the V-PC Cloacal 200k. Further comparisons of turkeys with positive LS of “1–3” are shown in Table 4; frequencies of liver and cecal LS for each group are shown in Figures 3A and 3B.
Table 3.
Body weight gain (BWG) and histomoniasis-related mortalities during Vaccination Phase (Experiment 2).1,2
| Group3 | Mortality | BWG (g) |
|||
|---|---|---|---|---|---|
| d 0–7 | d 7–14 | d 14–21 | d 0–21 | ||
| V-PC Cloacal 200k | 8/36 (22.2%) | 67 ± 3.94bc | 99 ± 7.17b | - | - |
| Vacc Oral 2k | 1/36 (2.78%)* | 70 ± 3.61abc | 112 ± 6.09ab | 202 ± 6.60abc | 401 ± 14.7abc |
| Vacc Oral 20k | 1/38 (2.63%)* | 81 ± 2.61ab | 133 ± 4.05a | 210 ± 6.86ab | 430 ± 7.73ab |
| Vacc Cloacal 2k | 0/36 (0.00%)* | 81 ± 2.38ab | 116 ± 5.51ab | 195 ± 7.27abc | 397 ± 14.6abc |
| Vacc Cloacal 20k | 2/37 (5.41%)* | 84 ± 3.73a | 133 ± 4.26a | 213 ± 6.05a | 440 ± 11.4a |
| Vacc Cloacal 200k | 1/30 (3.33%)* | 58 ± 3.61c | 119 ± 5.76ab | 181 ± 7.35bc | 373 ± 14.1bc |
| NC | 0/34 (0.00%)* | 76 ± 3.80ab | 110 ± 7.27ab | 181 ± 6.77c | 366 ± 14.6c |
BWG data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). Data were analyzed using JMP Pro 16 ANOVA, further separated by Tukey's HSD.
Vaccination Phase began on d0 with administration of respective dose and route of either Vacc Buford P80a or Buford WTH cells/turkey.
The PC was terminated on d15 for lesion scores.
V-PC = Vaccination Phase positive-challenged control; Vacc = live-attenuated Histomonas meleagridis; NC = non-challenged control; WTH = wild-type H. meleagridis.
Indicates significant difference in mortalities (P < 0.05) as compared to V-PC Cloacal 200k with chi-square test.
Table 4.
Liver and cecal lesion scores (LS) for histomoniasis during Vaccination and Challenge Phases (Experiment 2).1,2
| Group3 | Out of total n4 |
Out of positive for n w/1-3 LS5 |
|||||
|---|---|---|---|---|---|---|---|
| Vaccination phase (d 15) | Mean Liver LS | Mean Cecal LS | +Liver LS | +Cecal LS | +Liver LS; +Cecal LS | - Liver LS; +Cecal LS | +Liver LS; - Cecal LS |
| V-PC Cloacal 200k | 1.17 ± 0.20a | 1.67 ± 0.21a | 18/30 (60.0%) | 26/30 (86.7%) | 17/26 (65.4%) | 9/26 (34.6%) | 1/26 (3.85%) |
| Vacc Oral 2k | 0.00 ± 0.00b | 0.00 ± 0.00b | 0/15 (0.00%)* | 0/15 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
| Vacc Oral 20k | 0.00 ± 0.00b | 0.75 ± 0.11b | 0/16 (0.00%)* | 12/16 (75.0%) | 0/12 (0.00%)* | 12/12 (100%)* | 0/12 (0.00%) |
| Vacc Cloacal 2k | 0.00 ± 0.00b | 0.50 ± 0.13b | 0/16 (0.00%)* | 8/16 (50.0%)* | 0/8 (0.00%)* | 8/8 (100%)* | 0/8 (0.00%) |
| Vacc Cloacal 20k | 0.00 ± 0.00b | 0.27 ± 0.15b | 0/15 (0.00%)* | 3/15 (20.0%)* | 0/3 (0.00%)* | 3/3 (100%)* | 0/3 (0.00%) |
| Vacc Cloacal 200k | 0.22 ± 0.22b | 0.56 ± 0.34b | 1/9 (11.1%)* | 3/9 (33.3%)* | 1/3 (33.3%) | 2/3 (66.7%) | 0/3 (0.00%) |
| Challenge phase (d 35) | |||||||
| C-PC Cloacal 200k | 2.00 ± 0.31ab | 1.93 ± 0.28a | 14/20 (70.0%) | 15/20 (75.0%) | 14/15 (93.3%) | 1/15 (6.67%) | 0/15 (0.00%) |
| Vacc Oral 2k | 1.90 ± 0.30ab | 1.88 ± 0.25a | 14/20 (70.0%) | 16/20 (80.0%) | 14/16 (87.5%) | 2/16 (12.5%) | 0/16 (0.00%) |
| Vacc Oral 20k | 2.05 ± 0.27ab | 2.15 ± 0.24a | 16/20 (80.0%) | 17/20 (85.0%) | 16/17 (94.1%) | 1/17 (5.88%) | 0/17 (0.00%) |
| Vacc Cloacal 2k | 1.90 ± 0.30ab | 1.85 ± 0.26a | 14/20 (70.0%) | 16/20 (80.0%) | 14/16 (87.5%) | 2/16 (12.5%) | 0/16 (0.00%) |
| Vacc Cloacal 20k | 2.25 ± 0.27a | 2.30 ± 0.25a | 16/20 (80.0%) | 17/20 (85.0%) | 16/17 (94.1%) | 1/17 (5.88%) | 0/17 (0.00%) |
| Vacc Cloacal 200k | 1.45 ± 0.30b | 1.80 ± 0.30a | 12/20 (60.0%) | 14/20 (70.0%) | 12/14 (85.7%) | 2/14 (14.3%) | 0/14 (0.00%) |
| C-PC Oral 200k | 0.00 ± 0.00c | 0.00 ± 0.00b | 0/14 (0.00%)* | 0/14 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
LS data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). LS were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software.
Vaccination Phase began on d 0 with administration of respective dose and route of either Vacc Buford P80a or Buford WTH cells/turkey; Challenge Phase began on d 21 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey to all groups, except for C-PC Oral 200k which received the dose orally. Turkeys from the NC group were redistributed to form the new C-PC groups for the Challenge Phase.
Scores in the NC were only “1” on the LS scale of “0–3”.
C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V-PC, Vaccination Phase positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Experimental error resulted in the LS not being recorded from turkey subsets during the Vaccination Phase as follows: V-PC Cloacal 200k (n = 6), Vacc Oral 2k (n = 1), Vacc Oral 20k (n = 2), Vacc Cloacal 20k (n = 2), and Vacc Cloacal 200k (n = 1); hence, the difference in total n between Vaccination Phase mortality and LS values in Table 3.
Subgroupings of total n of turkeys with either a positive liver or cecal LS of “1–3” were further considered to compare differences of Vacc and WTH impact to liver and cecal tissues.
Indicates significant difference of categorical LS classifications (P < 0.05) compared to the respective Vaccination or Challenge Phase PC Cloacal 200k group with chi-square test.
Figure 3.
Experiment 2 frequency of lesion scores during Vaccination Phase for (A) liver and (B) cecae and during Challenge Phase for (C) liver and (D) cecae. Numbers within columns indicate the number of turkeys per evaluated lesion score. Numbers at the top of each column indicate the lesion score mean ± SE for that group with different superscripts denoting significance (P < 0.05). Lesion scores were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software. Vaccination Phase began on d 0 with administration of respective dose and route of either Vacc Buford P80a or Buford WTH cells/turkey; Challenge Phase began on d 21 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey to all groups, except for C-PC Oral 200k which received the dose orally. Turkeys from the NC group were redistributed to form the new C-PC groups for the Challenge Phase. Abbreviations: C-PC, Challenge Phase positive-challenged control; V-PC, Vaccination Phase positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Challenge Phase (d 21–35)
Histomoniasis-related mortalities in the C-PC Cloacal 200k, Vacc Oral 2k, Vacc Oral 20k, Vacc Cloacal 2k, Vacc Cloacal 20k, Vacc Cloacal 200k, and C-PC Oral 200k were 55.0, 50.0, 50.0, 60.0, 55.0, 35.0, and 0.00% respectively (Table 5). The C-PC Oral 200k group mortalities were lower (P < 0.05) as compared to the C-PC Cloacal 200k group. Mortalities in the Vacc groups were not different (P > 0.05) as compared to the C-PC Cloacal 200k group. The d21-35 BWG was similar (P > 0.05) for all groups. On d35, the mean liver and cecal LS were similar (P > 0.05) for all Vacc groups as compared to the C-PC Cloacal 200k group (Table 4). The C-PC Oral group received LS of only “0”, indicating no detectable lesions associated with histomoniasis. From all turkeys evaluated, those with a positive liver or cecal LS were similar (P > 0.05) in all Vacc groups as compared to the C-PC Cloacal 200k. Further comparisons of turkeys with positive LS of “1–3” are shown in Table 4; frequencies of liver and cecal LS for each group are shown in Figures 3C and 3D.
Table 5.
Body weight gain (BWG) and histomoniasis-related mortalities during Challenge Phase (Experiment 2).1
| Group2 | Mortality | d 21–35 BWG (g) |
|---|---|---|
| C-PC Cloacal 200k | 11/20 (55.0%) | 531 ± 61.9a |
| Vacc Oral 2k | 10/20 (50.0%) | 546 ± 84.1a |
| Vacc Oral 20k | 10/20 (50.0%) | 497 ± 74.2a |
| Vacc Cloacal 2k | 12/20 (60.0%) | 511 ± 71.4a |
| Vacc Cloacal 20k | 11/20 (55.0%) | 487 ± 68.2a |
| Vacc Cloacal 200k | 7/20 (35.0%) | 527 ± 51.0a |
| C-PC Oral 200k | 0/14 (0.00%)* | 596 ± 34.7a |
BWG data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). BWG data were analyzed using JMP Pro 16 ANOVA, with no difference detected.
Challenge Phase began on d 21 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey to all groups, except for C-PC Oral 200k which received the dose orally. Turkeys from the NC group were redistributed to form the new C-PC groups for the Challenge Phase.
C-PC, Challenge Phase positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; NC, non-challenged control; WTH, wild-type H. meleagridis.
Indicates significant difference in mortalities (P < 0.05) as compared to C-PC Cloacal 200k with chi-square test.
Experiment 3: Day-of-Hatch vs. d 14 Vacc Administration
Vaccination Phase 1 (d 0–14)
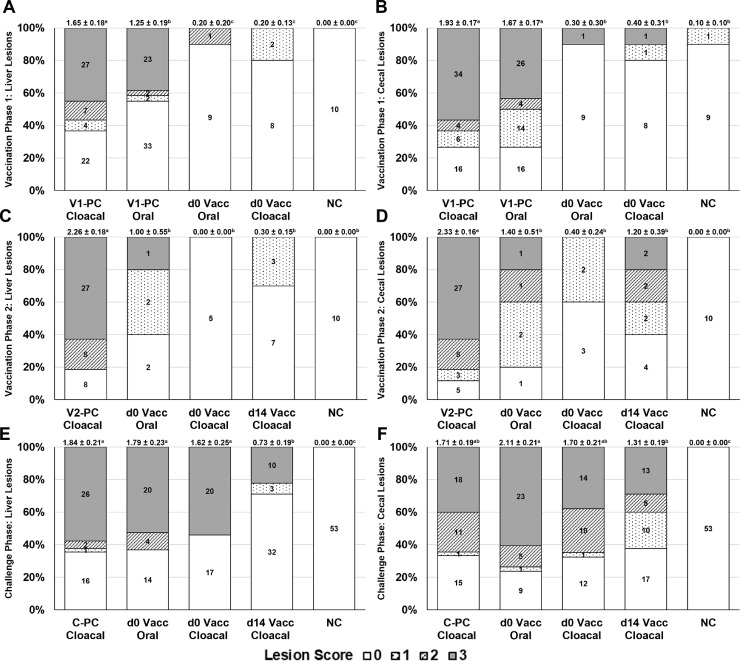
A mean pH of 5.0 was determined from the proventriculus-ventriculus region from the day-of-hatch poult subset prior to feeding. Histomoniasis-related mortalities in the V1-PC Cloacal, V1-PC Oral, d 0 Vacc Oral, d 0 Vacc Cloacal, and NC groups were 15.0, 16.7, 0.00, 0.00, and 0.00%, respectively (Table 6). The Vacc and NC groups mortalities were lower (P < 0.05) as compared to the V1-PC Cloacal group. The d0 Vacc Cloacal group had higher (P < 0.05) d 0–14 BWG than the V1-PC Cloacal and V1-PC Oral groups and was not different (P > 0.05) from the NC group. The mean liver and cecal LS were lower (P < 0.05) in the Vacc and NC groups as compared to the V1-PC Cloacal and V1-PC Oral groups (Table 7). The V1-PC Oral group had lower (P < 0.05) mean liver LS as compared to the V1-PC Cloacal group. From all turkeys evaluated, those with a positive liver LS were lower (P < 0.05) in all groups as compared to the V1-PC Cloacal; those with a positive cecal LS were lower (P < 0.05) in the Vacc and NC groups as compared to the V1-PC Cloacal. Further comparisons of turkeys with positive LS of “1–3” are shown in Table 7; frequency of liver and cecal LS for each group is shown in Figures 4A and 4B.
Table 6.
Body weight gain (BWG) and histomoniasis-related mortalities during Vaccination and Challenge Phases (Experiment 3).1,2
| Vaccination phase 1 | Mortality | d 0–14 BWG (g) |
|---|---|---|
| V1-PC Cloacal | 9/60 (15.0%) | 136 ± 5.87c |
| PC Oral | 10/60 (16.7%) | 146 ± 6.81c |
| d0 Vacc Oral | 0/53 (0.00%)* | 154 ± 4.39bc |
| d0 Vacc Cloacal | 0/52 (0.00%)* | 166 ± 3.96ab |
| NC | 0/216 (0.00%)* | 172 ± 2.41a |
| Vaccination phase 2 | Mortality | d 14-28 BWG (g) |
| V2-PC Cloacal | 21/43 (48.8%) | 250 ± 26.4c |
| d0 Vacc Oral | 0/43 (0.00%)* | 400 ± 8.48ab |
| d0 Vacc Cloacal | 0/42 (0.00%)* | 423 ± 8.86a |
| d14 Vacc Cloacal | 0/55 (0.00%)* | 380 ± 8.00b |
| NC | 0/108 (0.00%)* | 393 ± 5.05ab |
| Challenge phase | Mortality | d 28–42 BWG (g) |
| C-PC Cloacal | 19/45 (42.2%) | 521 ± 40bc |
| d0 Vacc Oral | 17/38 (44.7%) | 431 ± 58c |
| d0 Vacc Cloacal | 12/37 (32.4%) | 548 ± 42bc |
| d14 Vacc Cloacal | 10/45 (22.2%)* | 642 ± 28ab |
| NC | 0/53 (0.00%)* | 719 ± 16a |
BWG data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). Data were analyzed using JMP Pro 16 ANOVA, further separated by Tukey's HSD.
Vaccination Phase 1 began on d 0 with administration of 2 × 105 cells/turkey of either Vacc Buford P80a or Buford WTH cells/turkey via respective route; Vaccination Phase 2 began on d 14 with the introduction of a d 14 Vacc group and new PC (formed from subsets of the NC) which received intracloacal administration of 2 × 105 either Vacc Buford P80a or Buford WTH cells/turkey, respectively; Challenge Phase began on d 28 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey.
C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V1-PC, Vaccination Phase 1 positive-challenged control; V2-PC, Vaccination Phase 2 positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Indicates significant difference in mortalities (P < 0.05) as compared to the respective Vaccination or Challenge Phase PC Cloacal group with chi-square test.
Table 7.
Liver and cecal lesion scores (LS) for histomoniasis during Vaccination and Challenge Phases (Experiment 3).1,2
| Group3 | Out of total n |
Out of positive for n w/1-3 LS4 |
|||||
|---|---|---|---|---|---|---|---|
| Vaccination phase 1 (d 14) | Mean Liver LS | Mean Cecal LS | +Liver LS | +Cecal LS | +Liver LS; +Cecal LS | - Liver LS; +Cecal LS | +Liver LS; -Cecal LS |
| V-PC Cloacal | 1.65 ± 0.18a | 1.93 ± 0.17a | 38/60 (63.3%) | 44/60 (73.3%) | 38/44 (86.4%) | 6/44 (13.6%) | 0/44 (0.00%) |
| PC Oral | 1.25 ± 0.19b | 1.67 ± 0.17a | 27/60 (45.0%)* | 44/60 (73.3%) | 27/44 (61.4%)* | 17/44 (38.6%)* | 0/44 (0.00%) |
| d0 Vacc Oral | 0.20 ± 0.20c | 0.30 ± 0.30b | 1/10 (10.0%)* | 1/10 (10.0%)* | 1/1 (100%) | 0/1 (0.00%) | 0/1 (0.00%) |
| d0 Vacc Cloacal | 0.20 ± 0.13c | 0.40 ± 0.31b | 2/10 (20.0%)* | 2/10 (20.0%)* | 2/2 (100%) | 0/2 (0.00%) | 0/2 (0.00%) |
| NC | 0.00 ± 0.00c | 0.10 ± 0.10b | 0/10 (0.00%)* | 1/10 (10.0%)* | 0/1 (0.00%)* | 1/1 (100%)* | 0/1 (0.00%) |
| Vaccination phase 2 (d 28) | |||||||
| V-PC Cloacal | 2.26 ± 0.18a | 2.33 ± 0.16a | 35/43 (81.4%) | 38/43 (88.4%) | 35/38 (92.1%) | 3/38 (7.89%) | 0/38 (0.00%) |
| d0 Vacc Oral | 1.00 ± 0.55b | 1.40 ± 0.51b | 3/5 (60.0%) | 4/5 (80.0%) | 3/4 (75.0%) | 1/4 (25.0%) | 0/4 (0.00%) |
| d0 Vacc Cloacal | 0.00 ± 0.00b | 0.40 ± 0.24b | 0/5 (0.00%)* | 2/5 (40.0%)* | 0/2 (0.00%)* | 2/2 (100%)* | 0/2 (0.00%) |
| d14 Vacc Cloacal | 0.30 ± 0.15b | 1.20 ± 0.39b | 3/10 (30.0%)* | 6/10 (60.0%)* | 3/6 (50.0%)* | 3/6 (50.0%)* | 0/6 (0.00%) |
| NC | 0.00 ± 0.00b | 0.00 ± 0.00b | 0/10 (0.00%)* | 0/10 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
| Challenge phase (d 42) | |||||||
| C-PC Cloacal | 1.84 ± 0.21a | 1.71 ± 0.19ab | 29/45 (64.4%) | 30/45 (66.7%) | 29/30 (96.7%) | 1/30 (3.33%) | 0/30 (0.00%) |
| d0 Vacc Oral | 1.79 ± 0.23a | 2.11 ± 0.21a | 24/38 (63.2%) | 29/38 (76.3%) | 24/29 (82.8%) | 5/29 (17.2%) | 0/29 (0.00%) |
| d0 Vacc Cloacal | 1.62 ± 0.25a | 1.70 ± 0.21ab | 20/37 (54.1%) | 25/37 (67.6%) | 20/25 (80.0%)* | 5/25 (20.0%)* | 0/25 (0.00%) |
| d14 Vacc Cloacal | 0.73 ± 0.19b | 1.31 ± 0.19b | 13/45 (28.9%)* | 28/45 (62.2%) | 13/28 (46.4%)* | 15/28 (53.6%)* | 0/28 (0.00%) |
| NC | 0.00 ± 0.00c | 0.00 ± 0.00c | 0/53 (0.00%)* | 0/53 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
LS data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). LS were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software.
Vaccination Phase 1 began on d 0 with administration of 2 × 105 cells/turkey of either Vacc Buford P80a or Buford WTH cells/turkey via respective route; Vaccination Phase 2 began on d 14 with the introduction of a d 14 Vacc group and new PC (formed from subsets of the NC) which received intracloacal administration of 2 × 105 either Vacc Buford P80a or Buford WTH cells/turkey, respectively; Challenge Phase began on d 28 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey.
Scores in the NC were only “1” on the LS scale of “0–3”.
C-PC, Challenge Phase positive-challenged control; NC = non-challenged control; V1-PC, Vaccination Phase 1 positive-challenged control; V2-PC, Vaccination Phase 2 positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Subgroupings of total n of turkeys with either a positive liver or cecal LS of “1–3” were further considered to compare differences of Vacc and WTH impact to liver and cecal tissues.
Indicates significant difference of categorical LS classifications (P < 0.05) compared to the respective Vaccination or Challenge Phase PC group with chi-square test.
Figure 4.
Experiment 3 frequency of lesion scores during Vaccination Phase 1 for (A) liver and (B) cecae; Vaccination Phase 2 for (C) liver and (D) cecae; Challenge Phase for (E) liver and (F) cecae. Numbers within columns indicate the number of turkeys per evaluated lesion score. Numbers at the top of each column indicate the lesion score mean ± SE for that group with different superscripts denoting significance (P < 0.05). Lesion scores were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software. Vaccination Phase 1 began on d 0 with administration of 2 × 105 cells/turkey of either Vacc Buford P80a or Buford WTH cells/turkey via respective route; Vaccination Phase 2 began on d 14 with the introduction of a d 14 Vacc group and new PC (formed from subsets of the NC) which received intracloacal administration of 2 × 105 either Vacc Buford P80a or Buford WTH cells/turkey, respectively; Challenge Phase began on d 28 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey. Abbreviations: C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V1-PC, Vaccination Phase 1 positive-challenged control; V2-PC = Vaccination Phase 2 positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Vaccination Phase 2 (d 14–28)
No histomoniasis-related mortalities occurred in the d 0 Vacc Oral, d 0 Vacc Cloacal, d14 Vacc Cloacal, or NC groups by d 28, whereas the V2-PC Cloacal group reached 48.8% (Table 6). All Vacc groups had higher (P < 0.05) d 14–28 BWG than the V2-PC Cloacal group and were not different (P > 0.05) from the NC group. The d 0 Vacc Oral, d 0 Vacc Cloacal, d 14 Vacc Cloacal, and NC groups were lower in mean liver and cecal LS as compared to the V2-PC Cloacal group (Table 7). From all turkeys evaluated, those with a positive liver or cecal LS were lower (P < 0.05) in the Vacc Cloacal and NC groups as compared to the V2-PC Cloacal. Further comparisons of turkeys with positive LS of “1–3” are shown in Table 7; frequencies of liver and cecal LS for each group are shown in Figures 4C and 4D.
Challenge Phase (d 28–42)
Histomoniasis-related mortalities in the C-PC Cloacal, d0 Vacc Oral, d0 Vacc Cloacal, d 14 Vacc Cloacal, and NC groups were 42.2, 44.7, 32.4, 22.2, and 0.00%, respectively (Table 6). The Vacc groups were similar (P > 0.05) for d 28–42 BWG as compared to the C-PC Cloacal group; however, the d 14 Vacc Cloacal group was also not different (P > 0.05) for d 28–42 BWG as compared to the NC group. The d 14 Vacc Cloacal group had lower (P < 0.05) mean liver LS than the C-PC Cloacal group (Table 7). From all turkeys evaluated, those with a positive liver LS were lower (P < 0.05) in d 14 Vacc Cloacal and NC groups as compared to the C-PC Cloacal group. Further comparisons of turkeys with a positive LS of “1–3” are shown in Table 7; frequencies of liver and cecal LS for each group are shown in Figures 4E and 4F.
Experiment 4: d 14 Vacc Administration With Homologous or Heterologous WTH-Challenge
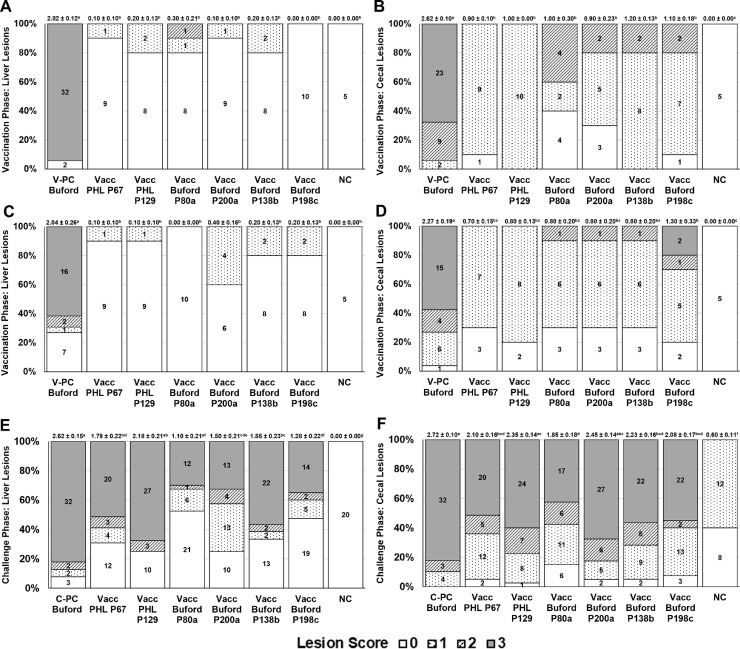
Vaccination Phase (d 14–35)
No histomoniasis-related mortalities occurred in the Vacc PHL P67, Vacc PHL P129, Vacc Buford P80a, Vacc Buford P200a, Vacc Buford P138b, Vacc Buford P198c, or NC groups whereas the V-PC Buford group reached 41.7% by d 28 (Table 8). All Vacc groups had higher (P < 0.05) d 13–34 BWG than the V-PC Buford group and were not different (P > 0.05) than the NC group. All Vacc groups had lower (P < 0.05) liver and cecal LS for d 28 and d35 as compared to the V-PC Buford group (Table 9). From all turkeys evaluated on d 28, those with a positive liver LS were lower (P < 0.05) in the Vacc and NC groups as compared to the V-PC Buford group; those with a positive cecal LS were lower (P < 0.05) in the Vacc Buford P80a, Vacc Buford P200a, and NC groups as compared to the V-PC Buford group. From all turkeys evaluated on d 35, those with a positive liver LS were lower (P < 0.05) in all Vacc and NC groups, except for the Vacc Buford P200a group, as compared to the V- PC Buford group; those with a positive cecal LS were lower (P < 0.05) in all Vacc and NC groups, except for the Vacc PHL P129 and Vacc Buford P198c, as compared to the V- PC Buford group. Further comparisons of turkeys with positive LS of “1-3” are shown in Table 9; frequencies of liver and cecal LS for each group are shown in Figures 5A–5D.
Table 8.
Body weight gain (BWG) and histomoniasis-related mortalities during Vaccination and Challenge Phases (Experiment 4).1
| Vaccination phase |
Challenge phase |
||||
|---|---|---|---|---|---|
| Group2,3 | d 14–28 Mortality | d 28–35 Mortality4 | d 13–34 BWG (g) | d 35–49 Mortality | d 34–49 BWG (g) |
| PC Buford | 25/60 (41.7%) | 14/35 (40.0%) | 682 ± 86.4b | 24/39 (61.5%) | 647 ± 112c |
| Vacc PHL P67 | 0/59 (0.00%)* | 0/49 (0.00%)* | 901 ± 15.6a | 8/39 (20.5%)* | 1090 ± 76.7ab |
| Vacc PHL P129 | 0/60 (0.00%)* | 0/50 (0.00%)* | 917 ± 16.8a | 9/40 (22.5%)* | 893 ± 85.6bc |
| Vacc Buford P80a | 0/60 (0.00%)* | 0/50 (0.00%)* | 861 ± 16.0a | 7/40 (17.5%)* | 937 ± 88.4bc |
| Vacc Buford P200a | 0/60 (0.00%)* | 0/50 (0.00%)* | 898 ± 15.1a | 3/40 (7.50%)* | 931 ± 62.0bc |
| Vacc Buford P138b | 0/59 (0.00%)* | 0/49 (0.00%)* | 909 ± 13.4a | 12/39 (30.8%)* | 899 ± 68.5bc |
| Vacc Buford P198c | 0/60 (0.00%)* | 0/50 (0.00%)* | 874 ± 18.0a | 7/40 (17.5%)* | 1000 ± 76.5abc |
| NC | 0/69 (0.00%)* | 0/59 (0.00%)* | 888 ± 13.8a | 0/20 (0.00%)* | 1359 ± 33.5a |
BWG data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). Data were analyzed using JMP Pro 16 ANOVA, further separated by Tukey's HSD.
Vaccination Phase consisted of intracloacal administration of 2 × 105 cells/turkey of either Vacc (PHL2017 or Buford isolates of passage indicated) or Buford WTH cells/turkey on d 14; Challenge Phase began on d 35 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey to the Vacc and PC groups (new PC Buford formed from subset of the NC).
NC, non-challenged control; PC, positive-challenged control, Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Isolates of H. meleagridis: Buford strain (isolated from infected chickens in Georgia); PHL2017 strain (isolated from infected turkeys in Arkansas); Passage number and isolate indicator follow each group name.
Adjusted for remaining n/group after d 28 lesion score subset.
Indicates significant difference in mortalities (P < 0.05) as compared to the respective Vaccination or Challenge Phase PC Buford group with chi-square test.
Table 9.
Liver and cecal lesion scores (LS) for histomoniasis during Vaccination and Challenge Phases (Experiment 4).1,2
| Group3,4 | Out of total n |
Out of positive for n w/1-3 LS5 |
|||||
|---|---|---|---|---|---|---|---|
| Vaccination phase (d 28) | Mean Liver LS | Mean Cecal LS | +Liver LS | +Cecal LS | +Liver LS; +Cecal LS | - Liver LS; +Cecal LS | +Liver LS; - Cecal LS |
| V-PC Buford | 2.82 ± 0.12a | 2.62 ± 0.10a | 32/34 (94.1%) | 34/34 (100%) | 32/34 (94.1%) | 2/34 (5.88%) | 0/34 (0.00%) |
| Vacc PHL P67 | 0.10 ± 0.10b | 0.90 ± 0.10b | 1/10 (10.0%)* | 9/10 (90.0%) | 1/9 (11.1%)* | 8/9 (88.9%)* | 0/9 (0.00%) |
| Vacc PHL P129 | 0.20 ± 0.13b | 1.00 ± 0.00b | 2/10 (20.0%)* | 10/10 (100%) | 2/10 (20.0%)* | 8/10 (80.0%)* | 0/10 (0.00%) |
| Vacc Buford P80a | 0.30 ± 0.21b | 1.00 ± 0.30b | 2/10 (20.0%)* | 6/10 (60.0%)* | 2/6 (33.3%)* | 4/6 (66.7%)* | 0/6 (0.00%) |
| Vacc Buford P200a | 0.10 ± 0.10b | 0.90 ± 0.23b | 1/10 (10.0%)* | 7/10 (70.0%)* | 1/7 (14.3%)* | 6/7 (85.7%)* | 0/7 (0.00%) |
| Vacc Buford P138b | 0.20 ± 0.13b | 1.20 ± 0.13b | 2/10 (20.0%)* | 10/10 (100%) | 2/10 (20.0%)* | 8/10 (80.0%)* | 0/10 (0.00%) |
| Vacc Buford P198c | 0.00 ± 0.00b | 1.10 ± 0.18b | 0/10 (0.00%)* | 9/10 (90.0%) | 0/9 (0.00%)* | 9/9 (100%)* | 0/9 (0.00%) |
| NC | 0.00 ± 0.00b | 0.00 ± 0.00c | 0/5 (0.00%)* | 0/5 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
| Vaccination phase (d 35) | |||||||
| V-PC Buford | 2.04 ± 0.26a | 2.27 ± 0.19a | 19/26 (73.1%) | 25/26 (96.2%) | 19/25 (76.0%) | 6/25 (24.0%) | 0/25 (0.00%) |
| Vacc PHL P67 | 0.10 ± 0.10b | 0.70 ± 0.15bc | 1/10 (10.0%)* | 7/10 (70.0%)* | 1/7 (14.3%)* | 6/7 (85.7%)* | 0/7 (0.00%) |
| Vacc PHL P129 | 0.10 ± 0.10b | 0.80 ± 0.13bc | 1/10 (10.0%)* | 8/10 (80.0%) | 1/8 (12.5%)* | 7/8 (87.5%)* | 0/8 (0.00%) |
| Vacc Buford P80a | 0.00 ± 0.00b | 0.80 ± 0.20bc | 0/10 (0.00%)* | 7/10 (70.0%)* | 0/7 (0.00%)* | 7/7 (100%)* | 0/7 (0.00%) |
| Vacc Buford P200a | 0.40 ± 0.16b | 0.80 ± 0.20bc | 4/10 (40.0%) | 7/10 (70.0%)* | 4/7 (57.1%) | 3/7 (42.9%) | 0/7 (0.00%) |
| Vacc Buford P138b | 0.20 ± 0.13b | 0.80 ± 0.20bc | 2/10 (20.0%)* | 7/10 (70.0%)* | 2/7 (28.6%)* | 5/7 (71.4%)* | 0/7 (0.00%) |
| Vacc Buford P198c | 0.20 ± 0.13b | 1.30 ± 0.33b | 2/10 (20.0%)* | 8/10 (80.0%) | 2/8 (25.0%)* | 6/8 (75.0%)* | 0/8 (0.00%) |
| NC | 0.00 ± 0.00b | 0.00 ± 0.00c | 0/5 (0.00%)* | 0/5 (0.00%)* | 0/0 (0.00%) | 0/0 (0.00%) | 0/0 (0.00%) |
| Challenge phase (d 49) | |||||||
| C-PC Buford | 2.62 ± 0.15a | 2.72 ± 0.10a | 36/39 (92.3%) | 39/39 (100%) | 36/39 (92.3%) | 3/39 (7.69%) | 0/39 (0.00%) |
| Vacc PHL P67 | 1.79 ± 0.22bd | 2.10 ± 0.16bcd | 27/39 (69.2%)* | 37/39 (94.9%) | 27/37 (73.0%)* | 10/37 (27.0%)* | 0/37 (0.00%) |
| Vacc PHL P129 | 2.18 ± 0.21ab | 2.35 ± 0.14ac | 30/40 (75.0%)* | 39/40 (97.5%) | 30/39 (76.9%) | 9/39 (23.1%) | 0/39 (0.00%) |
| Vacc Buford P80a | 1.10 ± 0.21ef | 1.85 ± 0.18d | 19/40 (47.5%)* | 34/40 (85.0%)* | 19/34 (55.9%)* | 15/34 (44.1%)* | 0/34 (0.00%) |
| Vacc Buford P200a | 1.50 ± 0.19cde | 2.45 ± 0.14abc | 30/40 (75.0%)* | 38/40 (95.0%) | 30/38 (78.9%) | 8/38 (21.1%) | 0/38 (0.00%) |
| Vacc Buford P138b | 1.85 ± 0.23bc | 2.23 ± 0.16bcd | 26/39 (66.7%)* | 37/39 (94.9%) | 26/37 (70.3%)* | 11/37 (29.7%)* | 0/37 (0.00%) |
| Vacc Buford P198c | 1.28 ± 0.22df | 2.08 ± 0.17bcd | 21/40 (52.5%)* | 37/40 (92.5%) | 21/37 (56.8%)* | 16/37 (43.2%)* | 0/37 (0.00%) |
| NC | 0.00 ± 0.00g | 0.60 ± 0.11f | 0/20 (0.00%)* | 12/20 (60.0%)* | 0/12 (0.00%)* | 12/12 (100%)* | 0/12 (0.00%) |
LS data are expressed as mean ± SE; Values within a column with no common superscript differ significantly (P < 0.05). LS were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software.
Vaccination Phase consisted of intracloacal administration of 2 × 105 cells/turkey of either Vacc (PHL2017 or Buford isolates of passage indicated) or Buford WTH cells/turkey on d 14; Challenge Phase began on d 35 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey to the Vacc and C-PC groups (new PC Buford formed from subset of the NC).
Scores in the NC were only “1” on the LS scale of “0–3”.
C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V-PC, Vaccination Phase positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Isolates of H. meleagridis: Buford strain (isolated from infected chickens in Georgia); PHL2017 strain (isolated from infected turkeys in Arkansas); Passage number and isolate indicator follow each group name.
Subgroupings of total n of turkeys with either a positive liver or cecal LS of “1–3” were further considered to compare differences of Vacc and WTH impact to liver and cecal tissues.
Indicates significant difference of categorical LS classifications (P < 0.05) compared to the respective Vaccination or Challenge Phase PC Buford group with chi-square test.
Figure 5.
Experiment 4 frequency of lesion scores during Vaccination Phase for (A) liver and (B) cecae on d 28; Vaccination Phase for (C) liver and (D) cecae on d 35; Challenge Phase for (E) liver and (F) cecae. Numbers within columns indicate the number of turkeys per evaluated lesion score. Numbers at the top of each column indicate the lesion score mean ± SE for that group with different superscripts denoting significance (P < 0.05). Lesion scores were based on a scale of “0” to “3” and were analyzed using the Proc Mixed Procedure in SAS 9.4 software. Vaccination Phase began on d 14 with the intracloacal administration of 2 × 105 cells/turkey of the respective Vacc isolate while the PC Buford received the same dose of Buford WTH; Challenge Phase began on d 35 with the intracloacal administration of 2 × 105 Buford WTH cells/turkey. Abbreviations: C-PC, Challenge Phase positive-challenged control; NC, non-challenged control; V-PC, Vaccination Phase positive-challenged control; Vacc, live-attenuated Histomonas meleagridis; WTH, wild-type H. meleagridis.
Challenge Phase (d 35–49)
Histomoniasis-related mortalities in the C-PC Buford, Vacc PHL P67, Vacc PHL P129, Vacc Buford P80a, Vacc Buford P200a, Vacc Buford P138b, Vacc Buford P198c, and NC groups were 61.5, 20.5, 22.5, 17.5, 7.50, 30.8, 17.5, and 0.00%, respectively (Table 8). The Vacc PHL P67 group had higher (P < 0.05) d34-49 BWG as compared to the C-PC Buford group and was not different (P > 0.05) than the NC group. All Vacc groups, except for the Vacc PHL P129 group, had significantly lower (P < 0.05) mean liver LS as compared to the C-PC Buford group (Table 9). The Vacc PHL P67, Vacc Buford P80a, Vacc Buford P138b, and Vacc Buford P198c groups had significantly lower (P < 0.05) mean cecal LS as compared to the C-PC Buford group. From all turkeys evaluated, positive liver LS were lower (P < 0.05) in all the Vacc groups and the NC group as compared to the C-PC Buford group; positive cecal LS were lower in the Vacc Buford P80a and NC groups as compared to the C-PC Buford group. Further comparisons of turkeys with positive LS of “1–3” are shown in Table 9; frequencies of liver and cecal LS for each group are shown in Figures 5E and 5F.
DISCUSSION
During the Vaccination Phase of all experiments, mortalities and mean LS were lower (P < 0.05) in the Vacc groups regardless of dose, route, or attenuated isolate (Vacc Buford or Vacc PHL) when compared to the WTH V-PC Cloacal group. Additionally, there was no difference (P > 0.05) in BWG with d 14 cloacal administration of the Vacc Buford P80a isolate as compared to the NC group, indicating that the Vacc administered alone did not harm performance. Moreover, BWG was improved (P < 0.05) with d 14 cloacal administration of the Vacc Buford P80a isolate as compared to the WTH V-PC Cloacal group during the Vaccination Phases (Experiments 1, 3, and 4). These results are consistent with previous research indicating the safety of attenuated H. meleagridis administration (Hess et al., 2008; Liebhart et al., 2010, 2011, 2013). During the Challenge Phase, the d 14 cloacally administered Vacc Buford P80a group resulted in lowered mortalities and liver LS (P < 0.05) than the WTH C-PC Cloacal group (Experiments 1, 3, and 4), suggesting that this might be an efficacious option to prevent histomoniasis.
Long-term in vitro passaging of H. meleagridis can eventually reduce the ability to parasitize host tissue or to confer an immune response; however, studies have reported stable attenuation of histomonads without reversion to virulence upon serial back-passage in the bird (Tyzzer, 1936; Lund et al., 1966a, 1967; Sulejmanovic et al., 2013). Meanwhile, successful vaccination with in vitro attenuated (passage 295) clonal H. meleagridis induced protection in turkeys subsequently challenged with a virulent isolate (passage 21); the attenuated histomonads were restricted to the cecae with reduced pathogenicity (Liebhart et al., 2011). Although histomoniasis was not completely prevented in our study, the lowered LS and decreased mortalities during the Challenge Phase suggest the Vacc Buford P80a isolate is sufficiently attenuated to stimulate the turkey's immune response without resulting in Vacc-related lethality or rampant disease (Experiments 1, 3, and 4). A similar response was observed with the Vacc PHL P67 isolate (Experiment 4). Taken together, these data suggest intracloacal administration of live-attenuated H. meleagridis at d 14 to turkeys appears to induce acquired immunity, which aligns with previous research (Lund et al., 1967; Hess et al., 2008; Pham et al., 2013). Incidence of cecal LS of “2” and “3” occurring in the Vacc Buford P80a group following Buford WTH-challenge (Figures 2B, 4F, and 5F) suggests robust immunity did not occur; therefore, the case reproductive rate is unlikely to decrease, which potentially allows horizontal transmission to occur due to residual cecal infection and shedding. This lack of robust immunity to completely protect against LS or mortalities following WTH-challenge suggests more research is necessary before live-attenuated H. meleagridis can be recommended as an industry relevant vaccination option for histomoniasis. Complete protection against disease was not conferred and intracloacal administration of live-attenuated H. meleagridis would be both labor-intensive and economically unfeasible for commercial application at a large industry level. Tyzzer and Fabyan (1922) reported that turkeys recovered from infection with H. meleagridis displayed transient immunity with histomoniasis symptoms reappearing after several months possibly due to repeated exposure to infectious materials. Lund (1959) experimented with heterakid eggs to deliver attenuated histomonads, but immunization via this method was not protective. Even if it were possible to incorporate the Vacc isolates into a heterakid delivery system to provide a possible method of mass-scale administration, the variation in protection and inconsistency of response to WTH-challenge is concerning.
Liebhart et al. (2010) reported that oral vaccination of turkeys at day-of-hatch with clonal live-attenuated H. meleagridis effectively protected against subsequent intracloacal challenge with clonal WTH. Conversely, Experiments 2 and 3 indicated day-of-hatch administration of the Buford Vacc P80a isolate either orally or cloacally was not effective (P > 0.05) in reducing mortalities or LS upon subsequent challenge with Buford WTH, and BWG was not improved (P > 0.05) as compared to the C-PC Cloacal group. Within the current experiments, only the cloacal route at d 14 appeared to be efficacious for inducing protection with H. meleagridis Vacc as compared to the d 0 oral administration route. Previous research by Sulejmanovic et al. (2016) suggested cloacal booster administrations would be needed at d 14 if attenuated H. meleagridis are administered orally as a vaccine at day-of-hatch, which is further discouraging for relevance and practicality to the turkey industry. The optimum vaccination window was possibly missed with the day-of-hatch vaccination in our experiments; but if so, the method of boosters would still not seem promising as a commercial-scale application for industry.
In the Vaccination Phase of Experiment 2, low Vacc-related mortalities and cecal LS occurred in the Vacc Oral (2k and 20k doses) and Vacc Cloacal (20k and 200k doses) groups. The d 0 Vacc Oral (200k dose) group in Experiment 3 also exhibited low Vacc-related liver and cecal LS during Vaccination Phases 1 and 2. Since the H. meleagridis Vacc isolate was not an established clonal population, low levels of virulent histomonads potentially remaining in the culture could have contributed to low LS and mortalities. Alternatively, turkeys could have greater susceptibility to infection at day-of-hatch prior to feeding, even with apparently live-attenuated H. meleagridis. The more likely hypothesis would be that the variation in mortalities and LS frequency could be a result of population differences within the Vacc isolate. The high levels of in vitro propagation and replication by binary fission could lead towards a consistent population of histomonads adapted for an in vitro environment and thereby a relatively homogenous live-attenuated culture with low virulent properties (Lund et al., 1966a, 1967). The Vacc isolates in these experiments remain a potential mixture of genotypes since they were not single-cell cloned; the Buford WTH-challenge also originated from a field outbreak potentially containing multiple genotypes and better simulating realistic challenge conditions. The lack of complete protection against either homologous or heterologous WTH-challenge would suggest that the use of Vacc isolates may not be efficacious for conferring robust immune protection. Although the Vacc isolates used in these experiments are not conclusively clonal populations and potentially contain a greater diversity of genotypes, this diversity would be expected to better mimic a real-world scenario where turkeys are not exposed to a single isolate at any given time. The possible incidence of different genotypes remaining in either the WTH or Vacc isolates could arguably be considered more efficacious for inducing broad protection against re-infection, but regardless, only partial immunity seemed to be imparted with this methodology.
In Experiment 4, the Vacc PHL P67 offered some protection against heterologous challenge with Buford WTH, as indicated by lowered liver and cecal LS (P < 0.05) similar to the Vacc Buford P80a group response (Experiments 1, 3, and 4) as compared to the WTH C-PC Buford. The Vacc Buford P138b and Vacc Buford P198c groups also resulted in lowered lesions (P < 0.05) following challenge with Buford WTH. Since H. meleagridis reproduces by binary fission, the Buford and PHL isolates are likely to be genetically different from each other due to the temporal and geographical differences from when these isolates were obtained. Recent research indicates variation in virulence factors and pathogenicity of H. meleagridis isolates obtained from different geographical sources (Wei et al., 2020). Interestingly, the similar efficacy of protection of the Vacc PHL (particularly P67) and Vacc Buford (particularly P80a) isolates following challenge with Buford WTH in Experiment 4 suggest no serotype differences between isolates. The similarity in response of Vacc PHL P67 as the Vacc Buford P80a isolate for immunoprophylaxis against heterologous Buford WTH-challenge is encouraging and consistent with previous research showing that attenuated H. meleagridis can induce cross-protective immunity to heterologous isolates (Sulejmanovic et al., 2016).
Absence of detectable lesions, as indicated by LS of “0” within a subset of the PC groups, could have resulted from a difference of susceptibility to Buford WTH-challenge, variation in cecal retrograde of the inoculum, or expulsion of the inoculum before cloacal uptake in some turkeys (Hu and McDougald, 2003; Hu et al., 2004; McDougald and Fuller, 2005; Wei et al., 2020). Although passages of the Buford WTH isolate were reduced to prevent in vitro attenuation, changes in virulence or population are possible with each propagation depending on spontaneous mutation occurrences and media adaptation. Nevertheless, since field isolates of H. meleagridis are certainly of varied genotype and virulence, turkeys would be exposed to more than one strain in an industry setting (Bilic et al., 2014). The consideration remains that the non-clonal yet attenuated Vacc strains reported above did not induce vigorous protection to histomoniasis; therefore, immunization with live-attenuated H. meleagridis remains doubtful for industry purposes. Although if future studies were to be conducted, WTH and Vacc isolates should be single-cell cloned to ensure the same genetic population is being evaluated in subsequent experimental situations. Efficacy of clonal vaccination for non-clonal field challenge conditions remains to be evaluated.
Interestingly, the C-PC Oral 200k group (Experiment 2) resulted in no mortalities or LS following oral challenge with Buford WTH on d 21, which is consistent with the prevailing understanding that unprotected H. meleagridis do not survive the low pH within the proventriculus-ventriculus region. Conversely, the V1-PC Oral group (Experiment 3) challenged with Buford WTH on d 0 was similar in mortalities and cecal LS as compared to the V1-PC Cloacal group, indicating susceptibility of turkeys at early age to H. meleagridis-infection. Previous studies with chickens have demonstrated that feed deprivation and an alkaline pH prior to oral challenge resulted in the development of lesions characteristic with histomoniasis; therefore, potential oral transfer of H. meleagridis should not be discounted (Cuckler, 1970). An average pH of 3.5 has been reported in the proventriculus-ventriculus region of broiler chickens following feed ingestion with variability between a pH of 1.9 and 4.5 (Svihus, 2011). The susceptibility of day-of-hatch turkeys to oral H. meleagridis-infection prior to feeding could be potentially explained by pH closer to neutral (measured as 4.4 and 5.0 in Experiments 2 and 3, respectively) within the proventriculus-ventriculus region. Environmental pH could have allowed the histomonads to survive long enough to reach the cecae and parasitize the tissue. If repeated in future studies, pH should also be measured in a subset of turkeys post-feeding to compare to pre-feeding measurements. Recently, a purported cyst-like stage of H. meleagridis has been observed in vitro which could function in oral transmission but has not been elucidated in vivo (Munsch et al., 2009a,b; Zaragatzki et al, 2010a,b; Gruber et al., 2017).
Intracloacal administration of attenuated histomonads has previously provided some immunoprophylaxis against virulent isolates, but further research is warranted to elucidate the most efficacious administration route, dose, and age for the inoculation procedure since the current methods do not induce robust immunity and are not an applicable industry solution (Pham et al., 2013; Sulejmanovic et al., 2016). Furthermore, although male and female turkeys have similar susceptibility to infection with H. meleagridis, variation occurs between genetic lines (van der Heijden and Landman, 2008b; Liebhart et al., 2008; Abdul-Rahman and Hafez, 2009). A major limiting factor to large-scale production of Vacc isolates is the requirement for cell culture which is impractical for mass production because histomonads grow at varied rates and culture media is relatively costly. Efficient methods to feasibly propagate H. meleagridis to meet commercial production needs would be challenging, and the d 14 intracloacal administration would not be practical for large-scale application to the turkey industry. Vaccine administration at day-of-hatch via the oral route would be ideal for incorporation within the hatchery but results are conflicting. Our data indicate only the cloacal route at d 14 to be an effective administration method for Vacc but with only partial protection to subsequent WTH-challenge. Within these experiments, the NC group did not exhibit mortalities or LS from histomoniasis, further confirming that management and absence of exposure are crucial to preventing this disease. In conclusion, considering the research completed previously and as reported above, vaccination seems possible yet impractical for industry application with the current methods. Acquired immunity appears achievable but is not robust using the current methodology.
DISCLOSURES
The authors have no conflicts of interest to report.
REFERENCES
- Abdul-Rahman L., Hafez H.M. Susceptibility of different turkey lines to Histomonas meleagridis after experimental infection. Parasitol. Res. 2009;105:113–116. doi: 10.1007/s00436-009-1369-1. [DOI] [PubMed] [Google Scholar]
- Al-Khateeb G.H., Hansen M.F. Plasma enzymes as a measure of susceptibility of chickens and turkeys to infection with Histomonas meleagridis. Avian Dis. 1974;18:507–514. [PubMed] [Google Scholar]
- Bayon H.P., Bishop A. Cultivation of Histomonas meleagridis from the liver lesions of a hen. Nature. 1937;139:370–371. [Google Scholar]
- Beer L.C., Vuong C.N., Barros T.L., Latorre J.D., Tellez G., Fuller A.L., Hargis B.M. Research note: evaluation of boric acid as a chemoprophylaxis candidate to prevent histomoniasis. Poult. Sci. 2020;99:1978–1982. doi: 10.1016/j.psj.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks G., Neal R. The effect of some drugs upon Histomonas meleagridis in vitro. Ann. Trop. Med. Parasitol. 1952;46:68–71. doi: 10.1080/00034983.1952.11685507. [DOI] [PubMed] [Google Scholar]
- Bilic I., Jaskulska B., Souillard R., Liebhart D., Hess M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLoS One. 2014;9:e92438. doi: 10.1371/journal.pone.0092438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyen N., Ons E., De Gussem M., Goddeeris B.M. Passive immunization against Histomonas meleagridis does not protect turkeys from an experimental infection. Avian Pathol. 2009;38:71–76. doi: 10.1080/03079450802641255. [DOI] [PubMed] [Google Scholar]
- Clarkson M. Immunological responses to Histomonas meleagridis in the turkey and fowl. Immunology. 1963;6:156–168. [PMC free article] [PubMed] [Google Scholar]
- Cuckler A. In: Pages 371-397 in Immunity to Parasitic Animals. Jackson G.J., Herman R., Singer I., editors. Appleton-Century-Crofts, Meredith Corporation; 1970. Coccidiosis and histomoniasis in avian hosts. New York, NY. [Google Scholar]
- Cupo K.L., Beckstead R.B. Heterakis gallinarum, the cecal nematode of gallinaceous birds: a critical review. Avian Dis. 2019;63:381–388. doi: 10.1637/0005-2086-63.3.381. [DOI] [PubMed] [Google Scholar]
- Dwyer D.M., Honigberg B.M. Effect of certain laboratory procedures on the virulence of Histomonas meleagridis for turkeys and chickens. J. Parasitol. 1970;56:694–700. [Google Scholar]
- Ganas P., Liebhart D., Glösmann M., Hess C., Hess M. Escherichia coli strongly supports the growth of Histomonas meleagridis, in a monoxenic culture, without influence on its pathogenicity. Int. J. Parasitol. 2012;42:893–901. doi: 10.1016/j.ijpara.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Grabensteiner E., Arshad N., Hess M. Differences in the in vitro susceptibility of mono-eukaryotic cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. to natural organic compounds. Parasitol. Res. 2007;101:193–199. doi: 10.1007/s00436-007-0459-1. [DOI] [PubMed] [Google Scholar]
- Grabensteiner E., Liebhart D., Arshad N., Hess M. Antiprotozoal activities determined in vitro and in vivo of certain plant extracts against Histomonas meleagridis, Tetratrichomonas gallinarum, and Blastocystis sp. Parasitol Res. 2008;103:1257–1264. doi: 10.1007/s00436-008-1122-1. [DOI] [PubMed] [Google Scholar]
- Gruber J., Ganas P., Hess M. Long-term in vitro cultivation of Histomonas meleagridis coincides with the dominance of a very distinct phenotype of the parasite exhibiting increased tenacity and improved cell yields. Parasitology. 2017;144:1253–1263. doi: 10.1017/S0031182017000646. [DOI] [PubMed] [Google Scholar]
- van der Heijden H.M.J.F., Landman W.J.M. Improved culture of Histomonas meleagridis in a modification of Dwyer medium. Avian Dis. 2007;51:986–988. doi: 10.1637/8018-051007-REVIEWR.1. [DOI] [PubMed] [Google Scholar]
- van der Heijden H.M.J.F., Landman W.J.M. In vitro effect of herbal products against Histomonas meleagridis in turkeys. Vet. Parasitol. 2008;154:1–7. doi: 10.1016/j.vetpar.2008.02.033. [DOI] [PubMed] [Google Scholar]
- van der Heijden H.M.J.F., Landman W.J.M. In vivo effect of herbal products against Histomonas meleagridis in turkeys. Avian Pathol. 2008;37:45–50. doi: 10.1080/03079450701784883. [DOI] [PubMed] [Google Scholar]
- van der Heijden H.M.J.F., McDougald L.R., Landman W.J.M. High yield of parasites and prolonged in vitro culture of Histomonas meleagridis. Avian Pathol. 2005;34:505–508. doi: 10.1080/03079450500368474. [DOI] [PubMed] [Google Scholar]
- Hess M., Liebhart D., Bilic I., Ganas P. Histomonas meleagridis—new insights into an old pathogen. Vet. Parasitol. 2015;208:67–76. doi: 10.1016/j.vetpar.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Hess M., Liebhart D., Grabensteiner E., Singh A. Cloned Histomonas meleagridis passaged in vitro resulted in reduced pathogenicity and is capable of protecting turkeys from histomonosis. Vaccine. 2008;26:4187–4193. doi: 10.1016/j.vaccine.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Hess M., McDougald L. In: Pages 1172-1178 in Diseases of Poultry. 13th ed. Swayne E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V.L., editors. Wiley-Blackwell; Ames, IA: 2013. Histomoniasis (blackhead) and other protozoan diseases of the intestinal tract. [Google Scholar]
- Hu J., Fuller L., McDougald L.R. Infection of turkeys with Histomonas meleagridis by the cloacal drop method. Avian Dis. 2004;48:746–750. doi: 10.1637/7152. [DOI] [PubMed] [Google Scholar]
- Hu J., McDougald L.R. Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis. 2003;47:489–492. doi: 10.1637/0005-2086(2003)047[0489:DLTOHM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hu J., McDougald L.R. The efficacy of some drugs with known antiprotozoal activity against Histomonas meleagridis in chickens. Vet. Parasitol. 2004;121:233–238. doi: 10.1016/j.vetpar.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Joyner L.P. Immunity to histomoniasis in turkeys following treatment with dimetridazole. J. Comp. Pathol. Ther. 1963;73:201–207. doi: 10.1016/s0368-1742(63)80023-5. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Davies S.F.M., Kendall S.B. Pages 333-349 in Experimental Chemotherapy. Vol. 1. Academic Press; New York, NY: 1963. Chemotherapy of histomoniasis. [Google Scholar]
- Lagler J., Mitra T., Schmidt S., Pierron A., Vatzia E., Stadler M., Hammer S.E., Mair K.H., Grafl B., Wernsdorf P., Rauw F., Lambrecht B., Liebhart D., Gerner W. Cytokine production and phenotype of Histomonas meleagridis-specific T cells in the chicken. Vet. Res. 2019;50:107. doi: 10.1186/s13567-019-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhart D., Ganas P., Sulejmanovic T., Hess M. Histomonosis in poultry: previous and current strategies for prevention and therapy. Avian Pathol. 2017;46:1–18. doi: 10.1080/03079457.2016.1229458. [DOI] [PubMed] [Google Scholar]
- Liebhart D., Grabensteiner E., Hess M. A virulent mono-eukaryotic culture of Histomonas meleagridis is capable of inducing fatal histomonosis in different aged turkeys of both sexes, regardless of the infective dose. Avian Dis. 2008;52:168–172. doi: 10.1637/8107-090707-ResNote. [DOI] [PubMed] [Google Scholar]
- Liebhart D., Hess M. Oral infection of turkeys with in vitro-cultured Histomonas meleagridis results in high mortality. Avian Pathol. 2009;38:223–227. doi: 10.1080/03079450902912192. [DOI] [PubMed] [Google Scholar]
- Liebhart D., Sulejmanovic T., Grafl B., Tichy A., Hess M. Vaccination against histomonosis prevents a drop in egg production in layers following challenge. Avian Pathol. 2013;42:79–84. doi: 10.1080/03079457.2012.760841. [DOI] [PubMed] [Google Scholar]
- Liebhart D., Windisch M., Hess M. Oral vaccination of 1-day-old turkeys with in vitro attenuated Histomonas meleagridis protects against histomonosis and has no negative effect on performance. Avian Pathol. 2010;39:399–403. doi: 10.1080/03079457.2010.506906. [DOI] [PubMed] [Google Scholar]
- Liebhart D., Zahoor M., Prokofieva I., Hess M. Safety of avirulent histomonads to be used as a vaccine determined in turkeys and chickens. Poult. Sci. 2011;90:996–1003. doi: 10.3382/ps.2010-01255. [DOI] [PubMed] [Google Scholar]
- Lotfi A., Hauck R., Olias P., Hafez H.M. Pathogenesis of histomonosis in experimentally infected specific-pathogen-free (SPF) layer-type chickens and SPF meat-type chickens. Avian Dis. 2014;58:427–432. doi: 10.1637/10782-012814-Reg.1. [DOI] [PubMed] [Google Scholar]
- Lund E.E. Immunizing action of a nonpathogenic strain of Histomonas against blackhead in turkeys. J. Protozool. 1959;6:182–185. [Google Scholar]
- Lund E.E., Augustine P.C., Chute A.M. Histomonas meleagridis after one thousand in vitro passages. J. Eukaryot. Microbiol. 1967;14:349–351. doi: 10.1111/j.1550-7408.1967.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Lund E.E., Augustine P.C., Ellis D.J. Immunizing action of in vitro-attenuated Histomonas meleagridis in chickens and turkeys. Exp. Parasitol. 1966;18:403–407. doi: 10.1016/0014-4894(66)90041-5. [DOI] [PubMed] [Google Scholar]
- Lund E.E., Wehr E.E., Ellis D.J. Earthworm transmission of Heterakis and Histomonas to turkeys and chickens. J. Parasitol. 1966;52:899–902. [PubMed] [Google Scholar]
- McAllister M.M. Successful vaccines for naturally occurring protozoal diseases of animals should guide human vaccine research: a review of protozoal vaccines and their designs. Parasitology. 2014;141:624–640. doi: 10.1017/S0031182013002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougald L.R. Intestinal protozoa important to poultry. Poult. Sci. 1998;77:1156–1158. doi: 10.1093/ps/77.8.1156. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Dis. 2005;49:462–476. doi: 10.1637/7420-081005R.1. [DOI] [PubMed] [Google Scholar]
- McDougald L., Fuller L. Blackhead disease in turkeys: Direct transmission of Histomonas meleagridis from bird to bird in a laboratory model. Avian Dis. 2005;49:328–331. doi: 10.1637/7257-081004R.1. [DOI] [PubMed] [Google Scholar]
- Mitra T., Gerner W., Kidane F.A., Wernsdorf P., Hess M., Saalmüller A., Liebhart D. Vaccination against histomonosis limits pronounced changes of B cells and T-cell subsets in turkeys and chickens. Vaccine. 2017;35:4184–4196. doi: 10.1016/j.vaccine.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra T., Kidane F.A., Hess M., Liebhart D. Unravelling the immunity of poultry against the extracellular protozoan parasite Histomonas meleagridis is a cornerstone for vaccine development: a review. Front. Immunol. 2018;9:2518. doi: 10.3389/fimmu.2018.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch M., Lotfi A., Hafez H.M., Al-Quraishy S., Mehlhorn H. Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitol. Res. 2009;104:683–689. doi: 10.1007/s00436-008-1246-3. [DOI] [PubMed] [Google Scholar]
- Munsch M., Mehlhorn H., Al-Quraishy S., Lotfi A.R., Hafez H.M. Molecular biological features of strains of Histomonas meleagridis. Parasitol. Res. 2009;104:1137–1140. doi: 10.1007/s00436-008-1299-3. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pham A.D.N., De Gussem J.K., Goddeeris B.M. Intracloacally passaged low-virulent Histomonas meleagridis protects turkeys from histomonosis. Vet. Parasitol. 2013;196:307–313. doi: 10.1016/j.vetpar.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Popp C., Hauck R., Balczulat S., Hafez H.M. Recurring histomonosis on an organic farm. Avian Dis. 2011;55:328–330. doi: 10.1637/9596-110810-Case.1. [DOI] [PubMed] [Google Scholar]
- Regmi P.R., Shaw A.L., Hungerford L.L., Messenheimer J.R., Zhou T., Pillai P., Omer A., Gilbert J.M. Regulatory considerations for the approval of drugs against histomoniasis (blackhead disease) in turkeys, chickens, and game birds in the United States. Avian Dis. 2016;60:725–730. doi: 10.1637/11451-061516-Review.1. [DOI] [PubMed] [Google Scholar]
- Sorvari R., Naukkarinen A., Sorvari T.E. Anal sucking-like movements in the chicken and chick embryo followed by the transportation of environmental material to the bursa of Fabricius, caeca, and caecal tonsils. Poult. Sci. 1977;56:1426–1429. doi: 10.3382/ps.0561426. [DOI] [PubMed] [Google Scholar]
- Sulejmanovic T., Bilic I., Hess M., Liebhart D. An in vitro attenuated strain of Histomonas meleagridis provides cross-protective immunity in turkeys against heterologous virulent isolates. Avian Pathol. 2016;45:46–53. doi: 10.1080/03079457.2015.1117057. [DOI] [PubMed] [Google Scholar]
- Sulejmanovic T., Liebhart D., Hess M. In vitro attenuated Histomonas meleagridis does not revert to virulence, following serial in vivo passages in turkeys or chickens. Vaccine. 2013;31:5443–5450. doi: 10.1016/j.vaccine.2013.08.098. [DOI] [PubMed] [Google Scholar]
- Svihus B. The gizzard: function, influence of diet structure and effects on nutrient availability. Worlds Poult. Sci. J. 2011;67:207–224. [Google Scholar]
- Thøfner I.C.N., Liebhart D., Hess M., Schou T.W., Hess C., Ivarsen E., Fretté X., Christensen L.P., Grevsen K., Engberg R.M., Christensen J.P. Antihistomonal effects of artemisinin and Artemisia annua extracts in vitro could not be confirmed by in vivo experiments in turkeys and chickens. Avian Pathol. 2012;41:487–496. doi: 10.1080/03079457.2012.714459. [DOI] [PubMed] [Google Scholar]
- Tyzzer E.E. The flagellate character and reclassification of the parasite producing “Blackhead” in turkeys: Histomonas (gen. nov.) meleagridis (Smith) J. Parasitol. 1920;6:124–131. [Google Scholar]
- Tyzzer E.E. Problems and observations concerning the transmission of blackhead infection in turkeys. Proc. Am. Philos. Soc. 1932;71:407–410. [Google Scholar]
- Tyzzer E.E. Studies on histomoniasis, or “blackhead” infection, in the chicken and the turkey. Daedalus. 1934;69:189–264. [Google Scholar]
- Tyzzer E.E. A study of immunity produced by infection with attenuated culture-strains of Histomonas meleagridis. J. Comp. Patho. Ther. 1936;49:285–303. [Google Scholar]
- Tyzzer E.E., Fabyan M. A further inquiry into the source of the virus in blackhead of turkeys, together with observations on the administration of ipecac and of sulfur. J. Exp. Med. 1922;35:791–812. doi: 10.1084/jem.35.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzzer E.E., Fabyan M., Foot N.C. Further observations on “blackhead” in turkeys. J. Infect. Dis. 1921;29:268–286. [Google Scholar]
- Wei Z., Abraham M., Chadwick E.V., Beckstead R.B. Histomonas meleagridis isolates compared by virulence and gene expression. Vet. Parasitol. 2020;286 doi: 10.1016/j.vetpar.2020.109233. [DOI] [PubMed] [Google Scholar]
- Windisch M., Hess M. Establishing an indirect sandwich enzyme-linked-immunosorbent-assay (ELISA) for the detection of antibodies against Histomonas meleagridis from experimentally infected specific pathogen-free chickens and turkeys. Vet. Parasitol. 2009;161:25–30. doi: 10.1016/j.vetpar.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch M., Hess M. Experimental infection of chickens with Histomonas meleagridis confirms the presence of antibodies in different parts of the intestine. Parasite Immunol. 2010;32:29–35. doi: 10.1111/j.1365-3024.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- Zaragatzki E., Hess M., Grabensteiner E., Abdel-Ghaffar F., Al-Rasheid K.A.S., Mehlhorn H. Light transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol. Res. 2010;106:977–983. doi: 10.1007/s00436-010-1777-2. [DOI] [PubMed] [Google Scholar]
- Zaragatzki E., Mehlhorn H., Abdel-Ghaffar F., Al-Rasheid K.A.S., Grabensteiner E., Hess M. Experiments to produce cysts in cultures of Histomonas meleagridis – The agent of histomonosis in poultry. Parasitol. Res. 2010;106:1005–1007. doi: 10.1007/s00436-010-1776-3. [DOI] [PubMed] [Google Scholar]