Abstract
Background
Increases in lipids have been observed in people with HIV (PWH) switching from tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF). We assessed changes in low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) following a switch from TDF to TAF.
Methods
Adults with ≥1 lipid measure before and after switch from TDF to TAF were identified in the OPERA cohort. Multivariable linear regression using generalized estimating equations was used to estimate predicted changes in lipids over time on TAF, modeled flexibly with linear splines.
Results
A total of 6451 PWH switched from TDF to TAF, of whom 4328 maintained all other agents. LDL-C increased significantly by 1.40 mg/dL/mo over the first 3 months on TAF, by 0.33 mg/dL/mo between 3 and 9 months and then plateauing beyond 9 months. TG increased significantly by 3.52 mg/dL/mo over the first 3 months of TAF, by 0.91 mg/mL/mo between 3 and 9 months and by 0.72 mg/mL/mo between 9 and 16 months, but decreased thereafter. Similar patterns were observed in analyses restricted to PWH who switched from TDF to TAF but maintained all other agents.
Conclusions
TDF-to-TAF switch was associated with LDL-C and TG increases over the first 9 to 16 months on TAF. The dynamic patterns observed cannot be attributed to changes in other agents.
Keywords: cohort, lipids, low-density lipoprotein cholesterol, tenofovir alafenamide, tenofovir disoproxil fumarate, triglyceride
Tenofovir disoproxil fumarate (TDF) is a commonly used backbone agent in antiretroviral therapy for people with HIV (PWH). Tenofovir alafenamide (TAF), a pro-drug of tenofovir, has been gaining popularity since its approval in the United States. Compared with TDF, TAF has been associated with lower risk of bone [1–5] and renal [6–9] toxicity, leading many virologically suppressed PWH to switch from TDF to TAF.
However, several studies have reported an association between TDF use and a reduction in lipid levels [10–16]. What is more, there have recently been several reports of weight gain associated with TAF use [17–25]. Whether such weight gain also translates into other metabolic complications has yet to be established, although a worsening of lipid profiles following a switch from TDF to TAF has been demonstrated in preliminary work in the OPERA cohort among virologically suppressed PWH [26]. In clinical trials, TDF use appeared preferable to TAF when considering lipid profiles [6, 27–30]. However, the long-term impact on lipids of removing TDF from a stable regimen to replace it with TAF remains unclear in real-world clinical practice, as most previous observational studies were small, had short follow-up times, did not look at TDF-to-TAF switch directly, did not evaluate the impact of boosting agents, or did not control for potential confounders [31–40].
The objective of this study was to assess changes in low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels over time following a switch from TDF to TAF among virologically suppressed PWH receiving routine clinical care in the United States.
METHODS
Study Population
Data from the Observational Pharmaco-Epidemiology Research and Analysis (OPERA) cohort were utilized. The OPERA cohort is a database of prospectively captured clinical data from the electronic health records of 93 170 PWH at 84 clinics across the United States (18 states and 1 US territory) at the time of this study. The OPERA database obtains annual institutional review board (IRB) approval from Advarra IRB, including a waiver of informed consent and authorization for the use of protected health information.
All PWH at least 18 years of age were included if they had at least 4 weeks of TDF use and switched directly from TDF to TAF between November 5, 2015, and March 31, 2018. All included PWH had at least 1 lipid panel while on TDF within 6 months before switch as well as at least 1 lipid panel at any time while on TAF. Follow-up started at the date of switch from TDF to TAF, until (1) discontinuation of TAF, (2) cessation of continuous clinical activity (patients censored 12 months after their last contact), (3) death, or (4) study end (June 30, 2018), whichever occurred first.
Measurements
The results of every lipid panel captured in the electronic health record (EHR) during the study period were used. Repeated measures of LDL-C and TG were considered continuously. Direct and calculated LDL-C values provided by the lab were used. If no LDL-C value was provided with a lipid panel and TG was ≤400 mg/dL, then LDL-C was calculated as LDL-C = total cholesterol – high-density lipoprotein cholesterol – (TG/5); LDL-C was otherwise set to missing.
Severity of dyslipidemia was defined using the NCEP ATP III Dyslipidemia Categorization [41]. For LDL-C, dyslipidemia was defined as levels ≥3.3–<4.1 mmol/L (≥130–<160 mg/dL), and severe to very severe dyslipidemia was defined as levels ≥4.1 mmol/L (≥160 mg/dL). For TG, dyslipidemia was defined as levels ≥2.25–<5.64 mmol/L (≥200–<500 mg/dL), and severe dyslipidemia was defined as levels ≥5.64 mmol/L (≥500 mg/dL). Only the last lipid panel while on TDF and the first lipid panel at least 7 days after switch to TAF were considered for dyslipidemia categorization, although all lipid panels on TAF were included for modeling.
Baseline characteristics were measured at the time of switch. Preswitch characteristics were measured at the time of the last lipid panel on TDF, and postswitch characteristics were measured at the time of the first lipid panel on TAF.
Statistical Analyses
Linear regression was used to assess changes in LDL-C or TG values associated with a 1-month increase in TAF use after switch. Time on TAF was modeled using linear splines with knots at 3, 9, and 16 months to flexibly assess the relationship between time on TAF and lipid changes without making a strong assumption of linearity. Linear splines were selected because they provide easily interpretable estimates while relaxing the assumption of linearity. The position of knots was selected based on data distribution (first quartile, median, third quartile). Repeated lipid measures were used as the outcome, thus making use of all the data available and allowing a more precise estimate of changing slopes over time. Models were fit with generalized estimating equations (GEEs) with an autoregressive correlation structure to account for the correlated nature of the data. Models were adjusted for baseline lipid level (LDL-C or TG, as appropriate), age, sex, and months on TDF, as well as time-updated HIV viral load ≥50 copies/mL, hormones, statin use, nonstatin lipid-lowering agent use, boosting agent use, and protease inhibitor (PI) use. Continuous variables were modeled with cubic splines.
Sensitivity Analyses
A sensitivity analysis was conducted to assess changes in LDL-C and TG values over time in a population restricted to PWH who maintained all other ART regimen components when they switched from TDF to TAF. The same model specifications detailed above were used for this sensitivity analysis.
Another sensitivity analysis was conducted to assess if changes in lipids followed a pattern similar to changes in weight in the main study population. Repeated measures of weight were modeled over time on TAF using a univariate linear regression model fit with GEE (autoregressive correlation structure), with knots at 3, 9, and 16 months.
RESULTS
Study Populations
The main study population included all PWH who switched from TDF to TAF, regardless of other antiretroviral (ARV) changes (Table 1). It consisted of 6451 PWH followed for a median (interquartile range [IQR]; max) of 9 (3–16; 32) months. The median duration of TDF-containing regimens was 29 months. The majority were men (84%) and had an undetectable viral load at switch (83%). Most did not have a prescription for a medication that may affect lipid levels at the time of switch. The subset of PWH who maintained all other ARVs when they switched from TDF to TAF included 4328 individuals followed for a median (IQR; max) of 10 (4–16; 32) months. Their characteristics at switch were very similar to those of the overall study population including all switches (Table 1).
Table 1.
Demographic and Clinical Characteristics at the Time of TDF-to-TAF Switch
| All Switches (n = 6451) | Maintained Other ARVs (n = 4328) | |
|---|---|---|
| Months on TDF preswitch, median (IQR) | 29 (14–52) | 28 (14–46) |
| Age, median (IQR), y | 48 (38–55) | 47 (37–54) |
| Female, No. (%) | 1010 (16) | 674 (16) |
| HIV viral load ≥50 copies/mL, No. (%) | 1103 (17) | 620 (14) |
| Hormone use, No. (%) | 554 (8) | 372 (9) |
| Statin use, No. (%) | 1112 (17) | 732 (17) |
| Nonstatin lipid-lowering agenta use, No. (%) | 425 (7) | 267 (6) |
Abbreviations: ARV, antiretroviral; IQR, interquartile range; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Cholestyramine, ezetimibe, fibrate, niacin, omega-3.
Table 2 summarizes ART regimens prescribed at the time of the lipid panel on TDF or TAF (all switches) or at the time of switch (maintained other ARVs). When considering all switches, the proportion of PWH on a pharmaco-enhanced regimen increased from 51% to 62%. Of those, the proportion using cobicistat increased from 69% to 90%. Protease inhibitor use decreased over time from 24% to 19%, while integrase inhibitor use increased from 47% to 65%. When considering only those who maintained all other ARVs at TDF to TAF switch, of the 58% using a pharmaco-enhancer, 86% were prescribed cobicistat. Integrase inhibitors were the most frequently used anchor class at 61%.
Table 2.
ART Regimens Pre- and Postswitch (All Switches) or at the Time of Switch From TDF to TAF (Maintained Other ARVs)
| All Switches (n = 6451) | Maintained Other ARVs (n = 4328) | ||
|---|---|---|---|
| Preswitch, No. (%) | Postswitch, No. (%) | No. (%) | |
| Pharmaco-enhancer | |||
| Any | 3289 (51) | 3998 (62) | 2494 (58) |
| Ritonavir | 1032 (31) | 405 (10) | 350 (14) |
| Cobicistat | 2269 (69) | 3582 (90) | 2144 (86) |
| Anchor agent | |||
| Protease inhibitor | 1566 (24) | 1228 (19) | 790 (18) |
| Non-nucleoside reverse transcriptase inhibitor | 2319 (36) | 1546 (24) | 1151 (27) |
| Integrase strand transfer inhibitor | 3007 (47) | 4185 (65) | 2657 (61) |
| Other class | 46 (1) | 41 (1) | 30 (1) |
Abbreviations: ARV, antiretroviral; ART, antiretroviral therapy; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Changes in LDL Cholesterol
Based on the NCEP ATP III Dyslipidemia Categorization for LDL-C, the proportion of PWH with dyslipidemia increased from the last lipid panel on TDF to the first lipid panel on TAF, from 12% to 15%. The proportion with severe dyslipidemia increased from 3% to 6%. Similarly, among the subgroup who maintained all other ARVs, the proportion of PWH with dyslipidemia increased from 11% to 15%, and the proportion with severe dyslipidemia increased from 3% to 6%, from the last lipid panel on TDF to the first lipid panel on TAF, respectively.
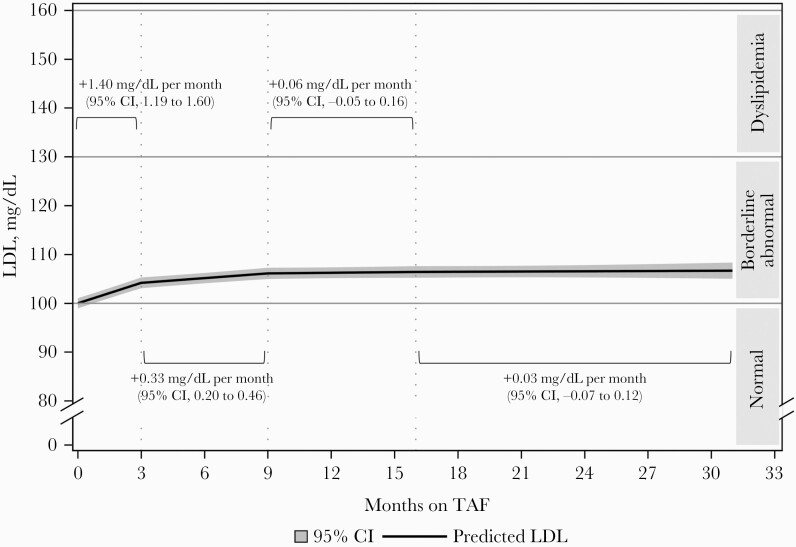
Adjusted rates of change in LDL-C among all PWH who switched from TDF to TAF were estimated as average predicted LDL-C values over time (Figure 1, Table 3). LDL-C increased statistically significantly over the first 3 months after switch, at a rate of +1.40 mg/dL per month (95% CI, 1.19 to 1.60). LDL-C values continued to increase over the next 6 months, albeit at a slower rate of +0.33 mg/dL per month (95% CI, 0.20 to 0.46) between 3 and 9 months after switch. Beyond 9 months after switching from TDF to TAF, no statistically significant change in LDL-C cholesterol was detected over time (9–16 months: +0.06 mg/dL per month; 95% CI, –0.05 to 0.16; 16+ months: +0.03 mg/dL per month; 95% CI, –0.07 to 0.12).
Figure 1.
Adjusted predicted LDL cholesterol over time after TDF-to-TAF switch, estimated from a linear regression model with linear splines, all switches (n = 6451).a aEstimated from a linear regression model fit with generalized estimated equations, with linear splines on time (knots at 3, 9, 16 months); reference covariate pattern: male aged 45, on TDF for 24 months and with an LDL value of 98 mg/dL at the last lipid panel before switch, a viral load <50 copies/mL throughout follow-up, and no hormone, statin, nonstatin lipid-lowering agent, pharmaco-enhancer, or PI use at any point during follow-up. Abbreviations: LDL, low-density lipoprotein; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 3.
Rates of Change in LDL Over Time After a Switch From TDF to TAFa
| All Switches (n = 6451) | Maintained Other ARVs (n = 4328) | |
|---|---|---|
| Ratesa of LDL changes, mg/dL/mo (95% CI) | ||
| 0–3 mo | 1.40 (1.19 to 1.60) | 1.72 (1.47 to 1.96) |
| 3–9 mo | 0.33 (0.20 to 0.46) | 0.27 (0.11 to 0.42) |
| 9–16 mo | 0.06 (–0.05 to 0.16) | 0.10 (–0.03 to 0.23) |
| 16+ mo | 0.03 (–0.07 to 0.12) | –0.00 (–0.11 to 0.11) |
Abbreviations: ARV, antiretroviral; LDL, low-density lipoprotein; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Estimated from a linear regression model fit with generalized estimated equations, with linear splines on time (knots at 3, 9, 16 months), adjusted for baseline LDL, age, months on TDF, sex, and use of a PI and/or a boosting agent, as well as time-updated HIV viral load and use of hormones, statins, and/or nonstatin lipid-lowering agents.
Similar rates of LDL-C changes over time on TAF were observed among those who maintained all other ARVs (Table 3). Indeed, the rates of LDL-C increases were statistically significant initially (0–3 months: +1.72 mg/dL per month; 95% CI, 1.47 to 1.96; 3–9 months: +0.27 mg/dL per month; 95% CI, 0.11 to 0.42), before reaching a plateau beyond 9 months after the switch from TDF to TAF (9–16 months: +0.10 mg/dL per month; 95% CI, –0.03 to 0.23; 16+ months: –0.00 mg/dL per month; 95% CI, –0.11 to 0.11).
Changes in Triglycerides
Among all PWH who switched from TDF to TAF, the proportion with TG levels corresponding to dyslipidemia increased from 20% at the last lipid panel on TDF to 24% at the first lipid panel on TAF. The proportion with severe dyslipidemia increased from 2% to 5%. Similarly, in the subset of PWH who maintained all other ARVs, the proportion with TG dyslipidemia increased from 19% on TDF to 24% on TAF and the proportion with severe dyslipidemia increased from 2% to 3%.
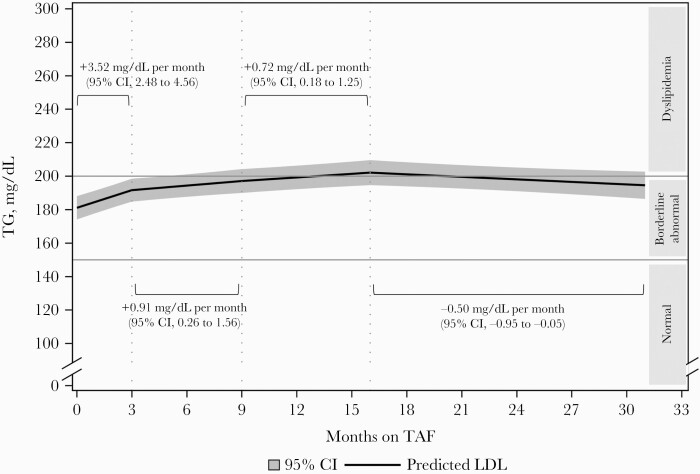
Figure 2 and Table 4 present changes in TG levels over time after a switch from TDF to TAF, including all switches. Statistically significant increases in TG were observed early after the switch. Indeed, the rate of TG change was estimated at +3.52 mg/dL per month (95% CI, 2.48 to 4.56) over the first 3 months of TAF. Increases in TG then slowed down to a rate of +0.91 mg/mL per month (95% CI, 0.26 to 1.56) between 3 and 9 months after switch and to 0.72 mg/mL per month (95% CI, 0.18 to 1.25) between 9 and 16 months after switch. However, predicted TG levels decreased statistically significantly beyond 16 months, at a rate of –0.50 mg/mL per month (95% CI, –0.95 to –0.05).
Figure 2.
Adjusted predicted triglyceride levels over time after TDF-to-TAF switch, estimated from a linear regression model with linear splines, maintained other ARVs (n = 4328).a aEstimated from a linear regression model fit with generalized estimated equations, with linear splines on time (knots at 3, 9, 16 months); reference covariate pattern: male aged 45, on TDF for 24 months and with a triglyceride value of 163 mg/dL at the last lipid panel before switch, a viral load <50 copies/mL throughout follow-up, and no hormone, statin, nonstatin lipid-lowering agent, pharmaco-enhancer, or PI use at any point during follow-up. Abbreviations: LDL, low-density lipoprotein; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TG, triglycerides.
Table 4.
Rates of Change in Triglycerides Over Time After a Switch From TDF to TAFa
| All Switches (n = 6451) | Maintained Other ARVs (n = 4328) | |
|---|---|---|
| Ratesa of triglyceride changes, mg/dL/mo (95% CI) | ||
| 0–3 mo | 3.52 (2.48 to 4.56) | 4.58 (3.25 to 5.92) |
| 3–9 mo | 0.91 (0.26 to 1.56) | 1.18 (0.32 to 2.04) |
| 9–16 mo | 0.72 (0.18 to 1.25) | 0.79 (0.11 to 1.47) |
| 16+ mo | –0.50 (–0.95 to –0.05) | –0.61 (–1.18 to –0.03) |
Abbreviations: ARV, antiretroviral; LDL, low-density lipoprotein; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Estimated from a linear regression model fit with generalized estimated equations, with linear splines on time (knots at 3, 9, 16 months), adjusted for baseline LDL, age, months on TDF, sex, and use of a PI and/or a boosting agent, as well as time-updated HIV viral load and use of hormones, statins, and/or nonstatin lipid-lowering agents.
Similar patterns of TG changes over time on TAF were observed in the subset of PWH who maintained all other ARVs (Table 4). Statistically significant increases in TG were detected in the first 3 months (+4.58 mg/dL per month; 95% CI, 3.25 to 5.92), from 3 to 9 months after switch (+1.18 mg/dL per month; 95% CI, 0.32 to 2.04), and from 9 to 16 months after switch (+0.79 mg/dL per month; 95% CI, 0.11 to 1.47). A statistically significant decrease in TG levels was then observed beyond 16 months (–0.61 mg/dL per month; 95% CI, –1.18 to –0.03).
Rates of Body Weight Changes
In an unadjusted sensitivity analysis, rates of weight gain followed a similar pattern as rates of lipid changes. Indeed, the fastest weight gain occurred immediately after switch, with rates of 0.25 kg per month (95% CI, 0.21 to 0.29) over the first 3 months and 0.10 kg per month (95% CI, 0.07 to 0.13) from 3 to 9 months after switch. Weight gain then slowed down to 0.02 kg per month (95% CI, –0.01 to 0.05) from 9 to 16 months and 0.04 kg per month (95% CI, 0.01 to 0.07) after 16 months.
DISCUSSION
In the OPERA cohort, switching from TDF to TAF was associated with an initial worsening of LDL-C and TG levels that was reflected in increasing proportions with dyslipidemia after switch. The magnitude of lipid increases observed in this OPERA study was clinically meaningful. Indeed, the proportion of PWH with dyslipidemia or severe dyslipidemia increased by 6% (LDL-C) to 7% (TG) at the first lipid panel after switch, corresponding to 451 additional PWH with concerning levels of dyslipidemia that may contribute to increased risk of atherosclerotic cardiovascular disease (ASCVD) [41, 42]. In addition, average predicted LDL-C levels increased over time after a switch from TDF to TAF. Such increases were rapid and statistically significant in the first 3 months on TAF (+1.40 mg/dL per month), but persisted, albeit at a slower but still statistically significant rate of increase, after 3–9 months on TAF (+0.33 mg/dL per month), followed by a plateau of LDL-C levels beyond 9 months. Similarly, average TG levels also increased rapidly and statistically significantly following the switch from TDF to TAF in the first 3 months (+3.52 mg/dL per month) and continued to increase at a slower rate after 3 to 9 months (+0.91 mg/dL per month) and 9 to 16 months on TAF (+0.72 mg/dL per month). However, mean TG levels decreased statistically significantly beyond 16 months of TAF use (–0.50 mg/dL per month). Similar LDL-C and TG patterns were observed in the subset of the population that maintained all other ARVs, suggesting that the observed increases cannot be attributed to changes in other ARVs. Of note, despite an eventual plateau of LDL-C and decrease of TG levels, neither returned to baseline levels over 32 months of follow-up on TAF.
These results from the OPERA cohort are consistent with previous TDF-to-TAF switch studies, which have shown a worsening of lipids after switch. In trials of virologically suppressed PWH, lipid levels increased slightly in groups randomized to switch to TAF, while they remained stable in groups maintaining or switching to TDF [27–30]. Interestingly, in a meta-analysis, among ART-naïve PWH in 3 trials, although point estimates were aligned with the OPERA study findings, no statistically significant difference was detected in either LDL-C changes (TAF: +20.0 mg/dL; TDF: +4.5 mg/dL; P = .124) or TG changes (TAF: +24.0 mg/dL; TDF: +1.5 mg/dL; P = .111) [6]. Moreover, in the same meta-analysis, among ART-experienced PWH from 2 trials, the mean change in TG was statistically greater with a switch to TAF (+10.5 mg/dL) compared with maintaining TDF (–2 mg/dL; P = .002), although no statistically significant difference was observed in LDL-C changes (TAF: +11 mg/dL; TDF: +1 mg/dL; P = .109) [6].
Several observational studies have investigated changes in lipid levels after a switch from TDF to TAF, with findings aligning with this OPERA study. In studies with postswitch lipid measurements taken a median of 1.5–12 months after switch, statistically significant increases were observed in LDL-C (range, 6–25 mg/dL) and TG (range, 12–21 mg/dL) [31, 32, 35, 39, 40]. Only 1 small observational study of 48 PWH switching from TDF to TAF found no significant difference in LDL-C and TG levels over at least 12 months on TAF, although the magnitude of change estimated was comparable to other studies [33]. These studies were all considerably smaller in size, each including <600 PWH, as opposed to the 6451 PWH included in this OPERA study. Moreover, these studies only compared 2 lipid measurements, 1 at baseline and 1 during follow-up, and could not introduce any flexibility in the patterns of lipid changes.
It appears the other ARVs taken in combination with TAF may have affected the magnitude of lipid changes observed after a switch from TDF to TAF. Indeed, in 1 study of PWH switching from TDF to TAF who maintained their anchor agent, increases in lipids were observed after switch, regardless of the regimen. However, the magnitude of change differed by regimen, and when cardiovascular risk was assessed, those using cobicistat were 2.5 times more likely to have an LDL-C level above the cardiovascular target compared with PWH not using cobicistat [40]. In this OPERA study, while changes in lipids were not estimated for different regimens, the use of PIs and boosting agents was nonetheless controlled for in statistical models. Moreover, a sensitivity analysis restricted to only PWH who maintained all other ARVs in the regimen confirmed that changes in other ARV agents could not explain away the changes in lipids observed.
Whether increases in LDL-C and TG after a switch from TDF to TAF are associated with TAF initiation or the loss of the lipid-lowering effect of TDF remains to be answered, although the persistent rises in lipids up to and beyond 9 months after cessation of TDF observed in this analysis suggest that the changes are unlikely to be solely related to loss of lipid-lowering effect from TDF. While there is evidence of higher total cholesterol, LDL-C, and high-density lipoprotein cholesterol levels with TAF compared with any other backbones [31], it has been suggested that increases in total cholesterol and LDL-C levels after a switch to TAF may only be observed when switching from TDF, but not when switching from any other backbone [43]. However, a study comparing PWH starting a regimen of elvitegravir/cobicistat/emtricitabine and either TDF or TAF showed stable LDL-C levels in those taking TDF, but statistically significant increases after 48 weeks among those on TAF [36]. Moreover, increases in lipid levels observed after a switch from TDF to TAF have been shown to come back down after switching back to TDF in an observational study in Germany [34].
The patterns of lipid changes described in this study also correspond to changes in body weight, with more rapid unadjusted weight gain within the first 9 months after switch in an unadjusted sensitivity analysis. Such weight gain was also observed in a more comprehensive study of weight changes before and after a switch from TDF to TAF in OPERA [25]. In that study, depending on the core agents used, only modest adjusted weight gain was observed while on TDF (0.24 to 0.71 kg/y), but adjusted mean increases in weight over the first 9 months after switch to TAF ranged from 1.8 to 4.5 kg/y, followed by a deceleration or plateau in mean weights [25]. Such parallels suggest that beyond the TAF-associated weight gains reported in recent studies [18–25], a switch from TDF to TAF may have additional negative metabolic effects accompanying weight gain.
This study is not without limitations. While the OPERA cohort is representative of HIV care in the United States, these results may not be representative of the risk in other populations. Residual confounding remains possible, as behavioral factors such as diet are generally poorly recorded in EHRs. While the use of linear splines relaxed the assumption of linearity, it may not have provided the best fit to the data. However, estimates generated with linear splines are easy to interpret and provide meaningful estimates of rates of change over different periods of time. Another limitation of this study is the absence of a control group. Indeed, either the inclusion of a group of PWH who maintained TDF throughout follow-up or the assessment of lipid changes before the TDF-to-TAF switch could have provided a contrast against which to compare changes in lipids after a switch to TAF. In addition, the impact of different anchor agents on lipids was not evaluated. Therefore, an interaction between TAF and other ARVs cannot be ruled out. However, the sensitivity analysis restricted to those who maintained all other ARVs suggested that switching from TDF to TAF may result in lipid increases regardless of the anchor agent used. Finally, information on fasting status can be limited in EHR data, and the inclusion of both fasting and nonfasting lipid measurements cannot be ruled out, although this concern is alleviated by the fact that either random or fasting lipid profiles are currently recommended in HIV treatment guidelines [42, 44].
Importantly, this study has several strengths. The OPERA cohort is comprised of a diverse population of PWH in the United States and represented ~8% of PWH in care in the United States at the time of this study. Moreover, the demographic characteristics of the OPERA population are representative of the HIV epidemic in the US population [45]. This rich database yielded a very large study population of 6451 PWH who switched directly from TDF to TAF. Statistical models were adjusted for both baseline and time-varying covariates that could confound the relationship between time on TAF and lipid levels, including lipid-lowering agent prescriptions. Linear splines on time since switch to TAF introduced some flexibility and uncovered dynamic patterns of change in LDL-C and TG over time on TAF. Finally, the results generated were robust to a sensitivity analysis restricted to PWH who maintained all other ARVs. Therefore, it is unlikely that the observed increases in LDL-C and TG could be attributed to changes in other ARVs.
In conclusion, to the best of our knowledge, this is the first study to show the dynamic patterns of LDL-C and TG fluctuations over time after a switch from TDF to TAF. Both lipids investigated tended to increase rapidly over the first 3 months of TAF use and persist up to and beyond 9 months after switch to TAF before reaching a plateau or a decline. These patterns were robust to a sensitivity analysis in which all other ARVs remained the same, suggesting that a switch from TDF to TAF may indeed be associated with a rise in LDL-C and TG, regardless of anchor or boosting agents used. Thus, clinical judgment must be used when considering a switch from TDF to TAF, weighing the risks of dyslipidemia and weight gain against the benefits to kidney and bone health.
Acknowledgments
This research would not be possible without the people with HIV in the OPERA Observational Database and the health care providers who care for them. Additionally, we are grateful for the following individuals: Robin Beckerman (SAS programming), Jeff Briney (SAS programming), Bernie Stooks (Database Mgmt), Judy Johnson (Med Terminology Classification), and Rodney Mood (Site Support).
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Potential conflicts of interest. P.W.G.M. and/or his institution have received research grants, honoraria, and/or speaker fees from Gilead Sciences, Janssen Cilag, ViiV, and MSD. L.B., J.S.F., and G.P.F. are employed by Epividian, Inc.; Epividian has had research funded by AIDS Healthcare Foundation, ViiV Healthcare, Merck & Co., Janssen Scientific Affairs, LLC, Gilead Sciences, and EMD-Serono. A.B. and G.P. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. M.B.W. has participated in postconference advisory boards for the Conference on Retroviruses and Opportunistic Infections (CROI) and International AIDS Conference (IAC) and also serves as a principal investigator on ViiV Healthcare clinical trials but does not receive personal compensation for this work, which goes directly to the AIDS Healthcare Foundation. M.B.W. is also a member of the Epidemiology and Clinical Advisory Board for Epividian. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. OPERA complies with all HIPAA and HITECH requirements, which expand upon the ethical principles detailed in the 1964 Declaration of Helsinki and has received annual institutional review board (IRB) approval by Advarra IRB, including a waiver of informed consent and authorization for use of protected health information.
Prior presentation. This work was presented in part at the 17th European AIDS Conference (EACS 2019), Basel, Switzerland, 6–9 November 2019 (PE2/44).
References
- 1. Grant PM, Cotter AG.. Tenofovir and bone health. Curr Opin HIV AIDS 2016; 11:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Mansky KC.. Tenofovir-associated bone density loss. Ther Clin Risk Manag 2010; 6:41–7. [PMC free article] [PubMed] [Google Scholar]
- 3. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–15. [DOI] [PubMed] [Google Scholar]
- 4. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. [DOI] [PubMed] [Google Scholar]
- 5. Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 2016; 3:e158–65. [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Lu X, Yang X, Xu N.. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine 2016; 95:e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012; 26:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper R, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M.. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010; 51:496–505. [DOI] [PubMed] [Google Scholar]
- 9. Winston J, Chonchol M, Gallant J, et al. Discontinuation of tenofovir disoproxil fumarate for presumed renal adverse events in treatment-naive HIV-1 patients: meta-analysis of randomized clinical studies. HIV Clin Trials 2014; 15:231–45. [DOI] [PubMed] [Google Scholar]
- 10. Randell PA, Jackson AG, Zhong L, Yale K, Moyle GJ.. The effect of tenofovir disoproxil fumarate on whole-body insulin sensitivity, lipids and adipokines in healthy volunteers. Antivir Ther 2010; 15:227–33. [DOI] [PubMed] [Google Scholar]
- 11. Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS 2010; 24:1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 2011; 25:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behrens G, Maserati R, Rieger A, et al. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther 2012; 17:1011–20. [DOI] [PubMed] [Google Scholar]
- 14. Souza SJ, Luzia LA, Santos SS, Carvalho R.. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: a review. Rev Assoc Med Bras 2013; 59:186–98. [DOI] [PubMed] [Google Scholar]
- 15. Santos JR, Saumoy M, Curran A, et al. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:403–8. [DOI] [PubMed] [Google Scholar]
- 16. Shaheen AA, AlMattooq M, Yazdanfar S, et al. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther 2017; 46:599–604. [DOI] [PubMed] [Google Scholar]
- 17. Venter WF, Moorhouse M, Sokhela S, et al. The ADVANCE trial: phase 3, randomized comparison of TAF/FTC/DTG, TDF/FTC/DTG or TDF/FTC/EFV for first-line treatment of HIV-1 infection [WEAB0405LB]. Paper presented at: 10th IAS Conference on HIV Science; 21–24 July 2019; Mexico City, Mexico. [Google Scholar]
- 18. Hill A, Venter F, Delaporte E, et al. Progressive rises in weight and clinical obesity for TAF/FTC/DTG and TDF/FTC/DTG versus TDF/FTC/EFV: ADVANCE and NAMSAL trials [MOAX0102LB]. Paper presented at: 10th IAS Conference on HIV Science; 21–24 July 2019; Mexico City, Mexico. [Google Scholar]
- 19. Schafer JJ, Sassa K, O’Connor J, Shimada A, Keith S, DeSimone JA Jr. BMI and ASCVD Risk Score changes in virologically suppressed patients with HIV infection switching from TDF to TAF containing ART [abstract 979]. Paper presented at: ID Week; 2–6 October 2019; Washington DC, USA. [Google Scholar]
- 20. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 21. Gomez M, Seybold U, Roider J, Härter G, Bogner JR.. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection 2019; 47:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo P-H, Sun H-Y, Chuang Y-C, Wu P-Y, Liu W-C, Hung C-C.. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis 2020; 92:71–7. [DOI] [PubMed] [Google Scholar]
- 23. Taramasso L, Berruti M, Briano F, Di Biagio A.. The switch from tenofovir disoproxil fumarate to tenofovir alafenamide determines weight gain in patients on rilpivirine-based regimen. AIDS 2020; 34:877–81. [DOI] [PubMed] [Google Scholar]
- 24. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc 2021; 24:e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunet L, Mallon P, Fusco JS, et al. Switch from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: lipid changes and statin underutilization. Clin Drug Invest 2021; 41:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raffi F, Orkin C, Clarke A, et al. Long-term (96-week) efficacy and safety after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-infected, virologically suppressed adults. J Acquir Immune Defic Syndr (1999) 2017; 75:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orkin C, Castelli F, Yazdanpanah Y, et al. PEB104. Cardiovascular disease risk assessments and fasting lipid changes in virologically suppressed patients randomized to switch to tenofovir alafenamide versus continuing tenofovir disoproxil fumarate. Paper presented at: 22nd International AIDS Conference; 23‒27 July 2018; Amsterdam, the Netherlands. [Google Scholar]
- 29. Orkin C, DeJesus E, Ramgopal M, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV 2017; 4:e195–204. [DOI] [PubMed] [Google Scholar]
- 30. Arribas JR, Thompson M, Sax PE, et al. Randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment week 144 results. J Acquir Immune Defic Syndr 2017; 75:211–8. [DOI] [PubMed] [Google Scholar]
- 31. Lacey A, Savinelli S, Barco EA, et al. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS 2020; 34:1161–70. [DOI] [PubMed] [Google Scholar]
- 32. Taramasso L, Di Biagio A, Riccardi N, et al. Lipid profile changings after switching from rilpivirine/tenofovir disoproxil fumarate/emtricitabine to rilpivirine/tenofovir alafenamide/emtricitabine: different effects in patients with or without baseline hypercholesterolemia. PLoS One 2019; 14:e0223181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taberner Bonastre P, Vallez Valero L, Cano Marrón S, et al. 5PSQ-042 modification on fasting lipid and renal parameters in patients switching from tenofovir disoproxil to tenofovir alafenamide. Eur J Hosp Pharm 2019; 26:A221. [Google Scholar]
- 34. Milinkovic A, Berger F, Arenas-Pinto A, Mauss S.. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS 2019; 33:2387–91. [DOI] [PubMed] [Google Scholar]
- 35. Kauppinen KJ, Kivelä P, Sutinen J.. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide significantly worsens the lipid profile in a real-world setting. AIDS Patient Care STDS 2019; 33:500–6. [DOI] [PubMed] [Google Scholar]
- 36. Cid-Silva P, Fernández-Bargiela N, Margusino-Framiñán L, et al. Treatment with tenofovir alafenamide fumarate worsens the lipid profile of HIV-infected patients versus treatment with tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine. Basic Clin Pharmacol Toxicol 2019; 124:479–90. [DOI] [PubMed] [Google Scholar]
- 37. Winston A, Wohl D, Stellbrink HJ, et al. PEB103. Changes in cardiovascular risk estimation and fasting lipids in virologically suppressed patients randomized to switch to tenofovir alafenamide versus continuing abacavir. Paper presented at: 22nd International AIDS Conference; 23–27 July 2018; Amsterdam, the Netherlands. [Google Scholar]
- 38. Raffi F, Antinori A, Ramgopal M, et al. PEB098. Switching from an abacavir (ABC)/lamivudine (3TC)-based regimen to a single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF): cardiovascular disease risk profile analysis from a randomized controlled study in virologically-suppressed adults. Paper presented at: 22nd International AIDS Conference; 23–27 July 2018; Amsterdam, the Netherlands. [Google Scholar]
- 39. Ewers E, Won S, Okulicz J, et al. 2248. Changes in lipid profiles for patients to tenofovir alafenamide (TAF)-containing regimens: perspectives from a military HIV-positive cohort. Open Forum Infect Dis 2018; 5:S665. [Google Scholar]
- 40. Gazzola L, Tagliaferri G, De Bona A, et al. Dyslipidaemia after switch to tenofovir alafenamide (TAF)-based cART regimens in a cohort of HIV-positive patients: what clinical relevance? HIV Med 2021; 22:140–5. [DOI] [PubMed] [Google Scholar]
- 41. US Department of Health and Human Services. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2001. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/atp3xsum.pdf. Accessed 5 June 2019.
- 42. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73:3168–209. [DOI] [PubMed] [Google Scholar]
- 43. Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr (1999) 2016; 71:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2021. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed 4 June 2021.
- 45. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2019. 2021. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 26 July 2021.