Abstract
Background
Cell-derived influenza vaccines are not subject to egg-adaptive mutations that have potential to decrease vaccine effectiveness. This retrospective analysis estimated the relative vaccine effectiveness (rVE) of cell-derived quadrivalent influenza vaccine (IIV4c) compared to standard egg-derived quadrivalent influenza vaccines (IIV4e) among recipients aged 4–64 years in the United States during the 2019–2020 influenza season.
Methods
The IQVIA PharMetrics Plus administrative claims database was utilized. Study outcomes were assessed postvaccination through the end of the study period (7 March 2020). Inverse probability of treatment weighting (IPTW) was implemented to adjust for covariate imbalance. Adjusted rVE against influenza-related hospitalizations/emergency room (ER) visits and other clinical outcomes was estimated through IPTW-weighted Poisson regression models for the IIV4c and IIV4e cohorts and for the subgroup with ≥1 high-risk condition. Sensitivity analyses modifying the outcome assessment period as well as a doubly-robust analysis were also conducted. IPTW-weighted generalized linear models were used to estimate predicted annualized all-cause costs.
Results
The final sample comprised 1 150 134 IIV4c and 3 924 819 IIV4e recipients following IPTW adjustment. IIV4c was more effective in preventing influenza-related hospitalizations/ER visits as well as respiratory-related hospitalizations/ER visits compared to IIV4e. IIV4c was also more effective for the high-risk subgroup and across the sensitivity analyses. IIV4c was also associated with significantly lower annualized all-cause total costs compared to IIV4e (–$467), driven by lower costs for outpatient medical services and inpatient hospitalizations.
Conclusions
IIV4c was significantly more effective in preventing influenza-related hospitalizations/ER visits compared to IIV4e and was associated with significantly lower all-cause costs.
Keywords: cell-derived influenza vaccine, egg-derived influenza vaccine, healthcare costs, influenza, relative vaccine effectiveness
During the 2019–2020 influenza season, cell-derived quadrivalent influenza vaccines were significantly more effective in preventing hospitalizations and emergency room visits related to influenza and respiratory events than were egg-derived quadrivalent influenza vaccines, among individuals 4–64 years old and a high-risk subgroup.
Annual influenza vaccination is the most effective way to protect against influenza and its potentially severe complications [1]. In the United States (US), the Centers for Disease Control and Prevention (CDC) recommends seasonal influenza vaccination for all individuals ≥6 months of age with rare exceptions [2]. Most influenza vaccines in the US are manufactured using a traditional egg-based process in which influenza viruses are grown in chicken eggs [3, 4]. However, this can lead to egg adaptation in which mutations can accumulate and alter viral antigenicity. This can result in reduced vaccine effectiveness, particularly for influenza A(H3N2) viruses [5, 6]. In addition, antigenic drift can lead to alterations in some influenza viruses, which inhibit them from replicating efficiently in chicken eggs [7].
The cell culture–derived, inactivated quadrivalent influenza vaccine (IIV4c; Flucelvax Quadrivalent, Seqirus) is the only cell-based inactivated influenza vaccine licensed for use in the US. For the 2019–2020 influenza season, IIV4c was approved for use among individuals at least 4 years of age, and the cell-based candidate vaccine viruses for all 4 influenza strains recommended by the World Health Organization (WHO) were grown in cultured cells of mammalian origin [8]. Because cell-derived influenza vaccines are not subject to egg-adapted mutations, they have the potential to be more effective than traditional egg-based influenza vaccines. The viruses used in cell-derived vaccines may be more similar to the starting candidate virus than the viruses used in egg-derived vaccines. Several recent real-world studies evaluating the relative vaccine effectiveness (rVE) of IIV4c vs standard egg-derived quadrivalent influenza vaccines (IIV4e) showed a trend favoring IIV4c across multiple populations [9–13].
Influenza activity in the US during the 2019–2020 influenza season began to increase in November 2019, was consistently high through January and February 2020, and began to decline in March 2020 [14]. In November and December 2019, B/Victoria viruses were dominant, whereas the A(H1N1)pdm09 virus became dominant from January until the end of the season (which was shortened by the coronavirus disease 2019 [COVID-19] pandemic) [14, 15]. The 2019–2020 influenza season is described as having moderate severity overall and predominantly H1N1; however, the 2019–2020 influenza season was atypical in that it was severe for age groups 0–4 years and 18–49 years (related to influenza B/Victoria viruses), compared to influenza-associated hospitalization and death rates among these age groups for other influenza seasons [14, 15]. According to the WHO, there was significant antigenic drift for H1N1 viruses that was even more pronounced for egg-based vs cell-based vaccines [16]. Any assessment of influenza-related burden during the 2019–2020 influenza season must also consider the impact of the COVID-19 pandemic [17].
It is important to assess rVE of available vaccines during each influenza season due to annual changes in epidemiologic patterns of influenza. The current study adds data from a new influenza season, 2019–2020, for a representative, commercially insured population 4–64 years of age in the US. The overall objective of the study was to estimate the rVE of IIV4c compared to IIV4e in preventing influenza-related hospitalizations/emergency room (ER) visits and respiratory-related hospitalizations/ER visits during the 2019–2020 influenza season. Additionally, annualized all-cause healthcare costs were compared between IIV4c and IIV4e recipients in an economic analysis.
METHODS
Study Design
This retrospective claims-based analysis evaluated subjects 4–64 years of age in the US vaccinated with either IIV4c or IIV4e during the 2019–2020 influenza season using the IQVIA PharMetrics Plus database.
Data Source
PharMetrics Plus is one of the largest US commercial health plan claims databases, comprising adjudicated claims for >190 million unique patients across the US, and considered representative of the national, commercially insured population in terms of age (<65 years) and gender. The data are de-identified and compliant with the Health Insurance Portability and Accountability Act.
Study Population and Time Period
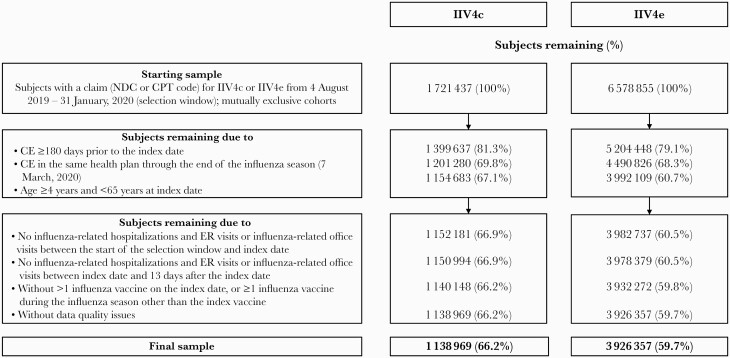
Subjects with ≥1 medical or pharmacy claim for IIV4c or IIV4e during the selection window (4 August 2019–31 January 2020 [18]) were assigned to 2 mutually exclusive cohorts (Figure 1). The study eligibility criteria are detailed in Figure 1. Definitions followed similar published methods [9, 10].
Figure 1.
Patient selection. Abbreviations: CE, continuous enrollment; CPT, Current Procedural Terminology; ER, emergency room; IIV4c, cell-derived quadrivalent influenza vaccine; IIV4e, standard egg-derived quadrivalent influenza vaccine; NDC, National Drug Code.
For the purpose of this analysis, the 2019–2020 influenza season was defined as beginning 4 August 2019 [18] and ending 7 March 2020. The study period began 4 February 2019 to allow for a 6-month preindex or baseline period. While the actual 2019–2020 influenza season extended past 7 March 2020, the 2019–2020 study period ended on 7 March 2020 to minimize any outcome misclassification that might be caused by the COVID-19 pandemic. The date 7 March 2020 was selected due to escalation of the COVID-19 pandemic, as more widespread community transmission began around 15 March 2020 (week 12) [19]. Continuous health plan enrollment was required from the start of the 6-month preindex period through 7 March 2020. Patients were followed over a variable follow-up period for the assessment of study outcomes, starting from 14 days after the index date (allowing for the development of vaccine-specific immunity) to the end of the influenza season (7 March 2020). However, any hospitalizations with admission date before or on 7 March 2020, but with discharge date past 7 March 2020, continued to be considered for outcome assessment. As part of the main analysis, the observation period was also restricted to the high influenza activity period (HIAP). Outcomes were assessed from (last of: [index date + 14] or [8 December 2019 {week 50}]) to 7 March 2020 (week 10). The HIAP was determined through a moving epidemic method (MEM) algorithm, which was applied to CDC surveillance data in order to establish epidemic thresholds for the start and end of the influenza season [20, 21]. The proportion of general practitioner visits due to laboratory-confirmed influenza was evaluated, and the proportions from week 50 through week 10 during the 2019–2020 influenza season were determined to be above epidemic thresholds.
Study Outcomes
Study outcomes included hospitalizations/ER visits for events of interest that were identified based on a hospitalization or ER visit with a diagnosis code for the event in any position. Influenza-related hospitalizations/ER visits were identified based on diagnosis codes for influenza (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 487.x, 488.x; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]: J09.x, J10.x, J11.x [22]). Hospitalizations/ER visits related to any respiratory event (ICD-9-CM: 460.x–519.x; ICD-10-CM: Jxx]), pneumonia, and asthma/chronic obstructive pulmonary disease (COPD)/bronchial events were also assessed. These definitions followed previously published methods [9, 10, 23]. Urinary tract infection (UTI)–related hospitalizations were evaluated as a negative control outcome [23, 24]. For each outcome of interest, the first occurring event at the subject level was identified. A subject could contribute an event to >1 outcome of interest. Number and rates (events per 1000 vaccinated patients) for each outcome were evaluated. Influenza-related hospitalization/ER visits and respiratory hospitalization/ER visits were evaluated over the HIAP.
Covariates
Baseline demographic characteristics (Supplementary Table 1) were assessed at the index date. Clinical characteristics (Supplementary Table 2) were measured over the 6-month preindex period (not including the index date, unless otherwise specified). In addition, 7 potential indicators of health-seeking behavior [18] were reported over the 6-month preindex period to determine whether unmeasured confounders could still have affected the study results (Supplementary Table 3).
Statistical Methods
Descriptive statistics include mean, standard deviation (SD), and median for continuous variables and frequencies and percentages for categorical variables. Standardized mean difference (SMD) was used to evaluate baseline covariate balance between the 2 cohorts. An SMD (absolute) of ≥0.10 between cohorts was considered a sign of imbalance [25].
Due to the potential for bias in the selection or receipt of an influenza vaccine, inverse probability of treatment weighting (IPTW) was used to adjust for imbalances in measured confounders between the cohorts. Weights were constructed in a logistic regression model that included baseline variables considered clinically relevant or that were imbalanced in the unadjusted sample: age group, gender, payer type, US Department of Health and Human Services region, Charlson Comorbidity Index score, preindex hospitalization, and preindex pharmacy cost. Further details of the IPTW method can be found in a previous publication [9].
IPTW-weighted univariate Poisson regression models were developed to allow for a more robust regression adjustment and to reduce any residual confounding and associated bias. The Poisson regression models were used to estimate adjusted rate ratios (RRs) along with corresponding 95% confidence intervals (CIs) for IIV4c compared to IIV4e. Adjusted rVE was calculated as ([1 – RR] × 100%).
All analyses for this study were performed using SAS software release 9.4 (SAS Institute, Cary, North Carolina).
Missing Data
Baseline categorical variables with missing values were classified as “unknown.” However, patients were required to have data recorded on age, gender, geographic region, and payer type to be considered eligible for the study sample. Study outcomes were determined based on observed records for healthcare activity and associated diagnosis codes.
Additional Analyses
Subgroup Analysis
A high-risk subgroup was evaluated for the rVE assessment. Patients in this subgroup were identified based on having ≥1 claim during the 6-month preindex period with a diagnosis code, drug code, or procedure code associating them with clinical conditions for which influenza vaccination is indicated due to higher risk for influenza complications: chronic liver, neurological, respiratory, heart, or kidney disease; diabetes; immunosuppression; morbid obesity; pregnancy; and asplenia or dysfunction of the spleen. These clinical risk groups were derived following review of international guidelines from countries where risk-based vaccination is recommended (eg, United Kingdom, Germany, Taiwan, Brazil [26–32]). IPTW was conducted separately for the high-risk subgroup.
Sensitivity Analysis
Two sensitivity analyses were conducted among the overall cohort and the high-risk subgroup for the assessment of rVE against influenza-related hospitalization/ER visits and respiratory hospitalization/ER visits.
The first sensitivity analysis was conducted for a shortened influenza period, truncated at 15 February 2020 (end of week 7), to help eliminate the potential impact of earlier-than-expected community transmission of COVID-19 from the analysis [33].
The second sensitivity analysis was a doubly-robust analysis following the methodology of recent studies [12, 18] to test the robustness of the findings from the main analysis. Doubly-robust analysis included the IPTW weight and all variables from the IPTW logistic regression model as covariates in the outcome regression model. Doubly-robust adjustment is used to account for any residual confounding from measured covariates [34].
Economic Analysis
Annualized all-cause healthcare costs were evaluated over the variable follow-up period (starting 14 days after the index date) for the overall IIV4c and IIV4e cohorts. Weighted generalized linear models (GLMs) with log link functions and gamma distribution were developed to estimate predicted mean annualized all-cause costs for the first 3 outcomes: total healthcare costs, outpatient medical costs, and outpatient pharmacy costs. Outliers were adjusted for by capping the respective postindex annualized cost at the 99th percentile [35]. Two-part GLM models were developed for the last 2 outcomes: inpatient costs and ER visit costs because hospitalizations and ER visits were infrequent. The first GLM had a binomial distribution and logit link to estimate odds of having a non-zero cost for the outcome of interest. The second GLM had a gamma distribution and log link to estimate the cost of the outcome of interest, among patients with the outcome of interest. Adjustment for outliers was made by capping cost at the 99th percentile among patients with at least 1 such outcome. Parameter estimates of the GLMs were used to derive the predicted recycled means, and 95% CIs were obtained through bootstrapping (500 replications).
RESULTS
Study Sample
We initially identified 1 721 437 IIV4c and 6 578 855 IIV4e recipients during the 2019–2020 influenza season selection window. After applying the eligibility criteria, the final unadjusted sample comprised 1 138 969 IIV4c and 3 926 357 IIV4e recipients, or 66.2% and 59.7% of the initial sample, respectively (Figure 1). Following IPTW adjustment, the final adjusted sample comprised 1 150 134 IIV4c and 3 924 819 IIV4e recipients. Of these, 21.5% and 19.6%, respectively, were included in the high-risk subgroup due to baseline presence of a high-risk condition.
Patient Characteristics
Baseline patient characteristics prior to IPTW adjustment are presented in Supplementary Tables 1 and 2. Baseline indicators of health-seeking behavior are presented in Supplementary Table 3. A few demographic characteristics were imbalanced prior to adjustment. IIV4c recipients were older than IIV4e recipients, with a mean age of 42.6 (SD, 16.1) years and 35.5 (SD, 19.6) years, respectively. There was variation in geographic region between the 2 cohorts with 53.4% of IIV4c recipients located in the South compared to 35.9% of IIV4e recipients. The proportion of Medicaid enrollees was lower among the IIV4c cohort (0.1% and 0.7%, respectively). IIV4c recipients also had higher mean outpatient pharmacy costs than IIV4e patients ($1503 and $1341) during the 6-month baseline period. Post-IPTW, following adjustment for potential confounders, both cohorts were well-balanced across all measured baseline covariates with SMDs (absolute) ≤0.06 (Supplementary Tables 3–5).
Clinical Outcomes
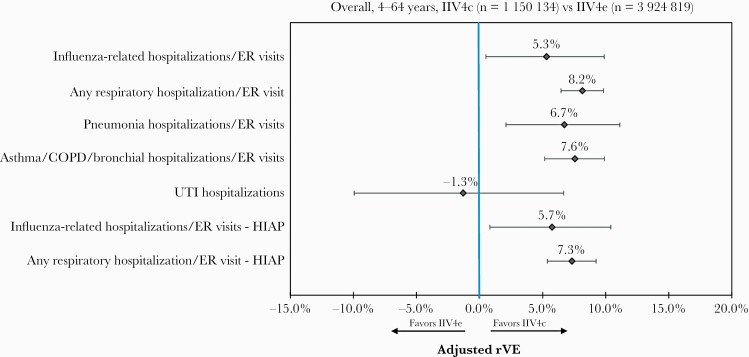
Event rates post-IPTW are presented in Supplementary Figure 1. Unadjusted rVE is presented in Supplementary Table 6, and adjusted rVEs following IPTW and Poisson regression adjustment are presented in Figure 2 for the overall cohort. The unadjusted rVE against influenza-related hospitalizations/ER visits for IIV4c vs IIV4e was 22.7% (95% CI, 18.6%–26.7%), whereas the adjusted rVE was 5.3% (95% CI, 0.5%–9.9%). Adjusted rVE was also significantly higher for IIV4c in preventing hospitalizations/ER visits related to any respiratory event (8.2% [95% CI, 6.5%–9.8%]), pneumonia (6.7% [95% CI, 2.1%–11.1%]), or asthma/COPD/bronchial events (7.6% [95% CI, 5.2%–9.9%]). The rVE for UTI-related hospitalizations was not significant, further suggesting that the 2 cohorts were well-balanced. IIV4c was associated with significantly higher rVE in preventing influenza-related hospitalization/ER visits (5.7% [95% CI, .8%–10.4%]) and any respiratory hospitalization/ER visit (7.3% [95% CI, 5.4%–9.2%]) during the HIAP.
Figure 2.
Adjusted relative vaccine effectiveness, overall (4–64 years old), post–inverse probability of treatment weighting and Poisson regression. Abbreviations: COPD, chronic obstructive pulmonary disease; ER, emergency room; HIAP, high influenza activity period; IIV4c, cell-derived quadrivalent influenza vaccine; IIV4e, standard egg-derived quadrivalent influenza vaccine; rVE, relative vaccine effectiveness; UTI, urinary tract infection.
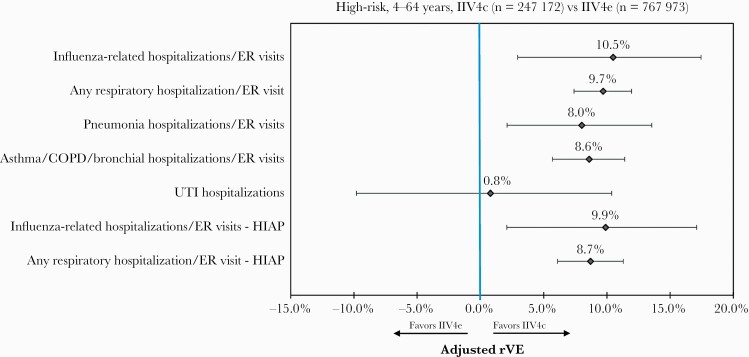
Among the high-risk subgroup, similar trends were observed. IIV4c was significantly more effective than IIV4e against hospitalizations/ER visits related to influenza, any respiratory event, pneumonia, and asthma/COPD/bronchial events (Figure 3). Notably, rVE against influenza-related hospitalizations/ER visits for IIV4c vs IIV4e increased from 5.3% (95% CI, .5%–9.9%) for the overall cohort to 10.5% (95% CI, 2.9%–17.5%) for the high-risk subgroup. IIV4c was associated with significantly higher rVE in preventing influenza-related hospitalization/ER visits and any respiratory hospitalization/ER visit during the HIAP for the high-risk subgroup.
Figure 3.
Adjusted relative vaccine effectiveness for the high-risk subgroup (4–64 years old), post–inverse probability of treatment weighting and Poisson regression. Abbreviations: COPD, chronic obstructive pulmonary disease; ER, emergency room; HIAP, high influenza activity period; IIV4c, cell-derived quadrivalent influenza vaccine; IIV4e, standard egg-derived quadrivalent influenza vaccine; rVE, relative vaccine effectiveness; UTI, urinary tract infection.
Sensitivity Analyses
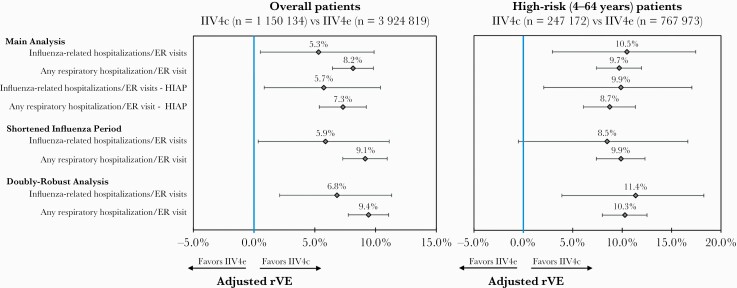
Results from the sensitivity analyses were consistent with the main analysis for the overall cohort and the high-risk subgroup (Figure 4). Following IPTW and Poisson regression adjustment, IIV4c was associated with significantly higher rVE in preventing influenza-related hospitalization/ER visits and any respiratory hospitalization/ER visit during the shortened influenza period, as well as in the doubly-robust analysis. The one exception was for rVE against influenza-related hospitalization/ER visits for the shortened influenza period, which was similar in magnitude and direction, but no longer significant among the high-risk subgroup.
Figure 4.
Sensitivity analysis. Abbreviations: ER, emergency room; HIAP, high influenza activity period; IIV4c, cell-derived quadrivalent influenza vaccine; IIV4e, standard egg-derived quadrivalent influenza vaccine; rVE, relative vaccine effectiveness.
Economic Analysis
Following IPTW and GLM adjustment, IIV4c was associated with significantly lower predicted mean annualized all-cause total costs per patient compared to IIV4e ($6769 vs $7236, P < .0001). This difference in costs (–$467) was primarily driven by significantly lower outpatient medical and inpatient costs (Table 1).
Table 1.
Economic Outcomes After Inverse Probability of Treatment Weighting and Generalized Linear Model Adjustment
| Predicted Mean Annualized All-Cause Cost | IIV4c | IIV4e | Incremental Mean | ||
|---|---|---|---|---|---|
| (n = 1 150 134) | (n = 3 924 819) | ||||
| Mean | (95% CI) | Mean | (95% CI) | ||
| Totala | $6769 | ($6760–$6778) | $7236 | ($7231–$7241) | $467 |
| Inpatient | $1241 | ($1235–$1249) | $1400 | ($1395–$1405) | $158 |
| Outpatient medical | $3376 | ($3370–$3382) | $3658 | ($3655–$3660) | $282 |
| Emergency room | $227 | ($226–$229) | $252 | ($251–$253) | $25 |
| Outpatient pharmacy | $1779 | ($1777–$1779) | $1806 | ($1806–$1807) | $28 |
Nonoverlapping CIs indicate statistical significance. Incremental mean = IIV4e – IIV4c.
Abbreviations: CI, confidence interval; IIV4c, cell-derived quadrivalent influenza vaccine; IIV4e, standard egg-derived quadrivalent influenza vaccine.
Total = outpatient pharmacy + inpatient + outpatient medical + emergency room.
DISCUSSION
This retrospective analysis of individuals aged 4–64 years during the 2019–2020 influenza season found that IIV4c was significantly more effective than IIV4e in preventing influenza-related hospitalizations/ER visits and respiratory-related hospitalizations/ER visits. Findings were very similar in terms of both magnitude and direction between the full outcome assessment period (4 August 2019–7 March 2020) and the HIAP (8 December 2019–7 March 2020), suggesting that IIV4c was significantly more effective than IIV4e in preventing influenza-related and respiratory-related hospitalizations/ER visits during both periods. For example, among the overall sample, adjusted rVE was significantly higher for IIV4c against influenza-related hospitalizations/ER visits over the full outcome assessment period (5.3% [95% CI, .5%–9.9%]) as well as the HIAP (5.7% [95% CI, .8%–10.4%]). The HIAP is a critical assessment that helps to improve specificity given the insufficient use of laboratory confirmation of influenza in clinical practice. The data were robust across the high-risk subgroup of patients and the sensitivity analyses were consistent with the main analysis. There was one exception for the high-risk subgroup during the shortened influenza period in which rVE against influenza-related hospitalizations/ER visits was similar to the main analysis in terms of magnitude and direction, but was nonsignificant. Of note, rVE against influenza-related hospitalizations/ER visits almost doubled in the high-risk subgroup compared to the overall cohort. Our study findings for the current 2019–2020 influenza season corroborate findings from real-world studies of the 2017–2018 and 2018–2019 influenza seasons and support the trend favoring IIV4c relative to IIV4e [9–13] across several distinct influenza seasons with varying epidemiologic characteristics. For instance, the study authors found that IIV4c was associated with an rVE against influenza-related hospitalizations/ER visits of 14.4% in 2017–2018 (a high severity season where A[H3N2] was predominant) and 6.5% in 2018–2019 (a moderate-severity season where A[H1N1]pdm09 was predominant from October to mid-February, followed by A[H3N2] compared to IIV4e) (both P < .05) [9, 10].
This is the only study to compare rVE of IIV4c vs IIV4e during the 2019–2020 influenza season among a population aged 4–64 years in the US. One study similarly compared rVE of IIV4c vs IIV4e during the 2019–2020 influenza season, but it evaluated a Medicare Fee-for-Service population ≥65 years old [18]. In that study, a positive rVE was reported for IIV4c in preventing influenza-related hospital encounters, but this was nonsignificant (2.8% [95% CI, –2.8% to 8.2%]). The difference in study findings may be related to the underlying differences in the study population, notably age (eg, 40.9%–41.6% were 18- to 49-year-olds in our study, and 50.4%–51.1% were 65- to 74-year-olds in the other study) and type of health insurance, limiting any direct comparisons. For example, frailty is associated with reduced vaccine effectiveness, and increased age is associated with reductions in humoral immunity and certain aspects of cell-mediated immunity [36]. The difference in study findings may also be related to circulating strains. During the 2019–2020 influenza season, there was no significant circulation of influenza A(H3N2) [14]. The influenza A(H3N2) virus is responsible for the majority of influenza morbidity and mortality among the elderly, and the greatest impact in the elderly occurs during years when A(H3N2) is the predominant circulating strain [36]. It is also important to note that the other study included the assessment of recombinant quadrivalent hemagglutinin vaccine, which is produced in insect cells (RIV4; Flublok, Sanofi Pasteur). RIV4 is indicated in individuals at least 18 years of age and would be relevant to the 18- to 64-year-old population in our study. We initially considered the inclusion of RIV4 in the current analysis. However, among recipients of IIV4c, IIV4e, or RIV4 during the 2019–2020 season in the PharMetrics Plus database, only 4.8% received RIV4. Therefore, we considered an analysis including a RIV4 cohort to be underpowered. Any future analyses will continue to consider RIV4.
Our study also found that IIV4c was associated with significantly lower predicted mean annualized all-cause total healthcare costs postvaccination (–$467 per subject) compared to IIV4e. We have not identified any other studies comparing economic outcomes between cell-derived and egg-derived vaccines during the 2019–2020 influenza season, although prior real-world studies evaluating the 2017–2018 and 2018–2019 influenza seasons similarly showed IIV4c to be associated with significant cost savings vs IIV4e [9, 10].
Our study has limitations related to the retrospective study design as well as the utilized data source. Results from retrospective studies must be interpreted with caution as they can only establish associations. Both vaccine cohorts were well-balanced post-IPTW, but it is possible that imbalances remained due to potential unmeasured confounders. Administrative claims data are collected primarily for the purposes of payment and do not provide as much clinical detail as medical records. Therefore, the potential for miscoding or misclassification exists. Because diagnostic test results were unavailable in the data, we relied on diagnosis codes to identify laboratory-confirmed influenza. However, we anticipate the sensitivity of having influenza to be high when an ICD code for influenza is recorded in the hospital/ER setting following clinical practice guidelines [37]. Finally, since the study sample employed was largely commercially insured or self-insured, these findings may not be representative of the uninsured or Medicaid populations. Despite these limitations, our study also has important strengths. We used robust methodology to adjust for imbalances in measured confounders and to account for residual confounding in our estimation of adjusted clinical and economic outcomes. We evaluated a large sample overall as well as a relevant high-risk population. The findings from the main analysis were also robust across several sensitivity analyses.
CONCLUSIONS
In our adjusted analysis of individuals aged 4–64 years during the 2019–2020 influenza season, IIV4c was significantly more effective in preventing influenza-related hospitalizations/ER visits and respiratory-related hospitalizations/ER visits compared to IIV4e. Similar trends were observed among the high-risk subgroup. The results were robust across 3 sensitivity analyses. IIV4c was also associated with significantly lower annualized all-cause total healthcare costs. Further research is needed to validate these findings during future influenza seasons.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. V. D., V. R. A., M. D., J. M.-Q., S. I. P., M. J. L., and M. J. P. were involved in study conception and design. V. D., V. R. A., and M. D. were involved in the analysis. All named authors were involved in the interpretation of data. V. D. and V. R. A. were involved in drafting the manuscript. All authors critically revised the manuscript and approved the final version.
Patient consent statement. This was a retrospective analysis of de-identified data and as such this study does not include factors necessitating patient consent.
Data availability. The original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf).
Financial support. This work was supported by Seqirus USA Inc, Summit, New Jersey.
Potential conflicts of interest. V. D., V. R. A., and M. D. are employees of IQVIA, which received funding for this study from Seqirus. J. M.-Q. is an employee of Seqirus USA Inc and a shareholder of CSL Ltd. S. I. P., M. J. L., and M. J. P. received financial support for time and effort from Seqirus for this study. M. J. L. also reports research funds from GSK, adjudication committee participation for GSK, and advisory board participation for AstraZeneca.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, and hospitalizations averted by vaccination in the United States—2019–2020 influenza season. https://www.cdc.gov/flu/about/burden-averted/2019-2020.htm. Accessed 26 July 2021.
- 2. Centers for Disease Control and Prevention. Who needs a flu vaccine. https://www.cdc.gov/flu/prevent/vaccinations.htm. Accessed 26 July 2021.
- 3. Harding AT, Heaton NS.. Efforts to improve the seasonal influenza vaccine. Vaccines 2018; 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajaram S, Boikos C, Gelone DK, Gandhi A.. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother 2020; 8:2515135520908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajaram S, Suphaphiphat P, van Boxmeer J, et al. Retrospective assessment of the antigenic similarity of egg-propagated and cell culture-propagated reference influenza viruses as compared with circulating viruses across influenza seasons 2002–2003 to 2017–2018. Int J Environ Res Public Health 2020; 17:5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Cell-based flu vaccines. https://www.cdc.gov/flu/prevent/cell-based.htm. Accessed 26 July 2021.
- 9. Divino V, Krishnarajah G, Pelton SI, et al. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine 2020; 38:6334–43. [DOI] [PubMed] [Google Scholar]
- 10. Krishnarajah G, Divino V, Postma MJ, et al. Clinical and economic outcomes associated with cell-based quadrivalent influenza vaccine vs. standard-dose egg-based quadrivalent influenza vaccines during the 2018–19 influenza season in the United States. Vaccines 2021; 9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis 2019; 220:1255–64. [DOI] [PubMed] [Google Scholar]
- 12. Boikos C, Sylvester GC, Sampalis JS, Mansi JA.. Relative effectiveness of the cell-derived quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017-2018. Clin Infect Dis 2020; 71:e665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boikos C, Fischer L, O’Brien D, et al. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018-2019 influenza season in the United States. Clin Infect Dis 2021; 73:e692–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States—2019–2020 influenza season. https://www.cdc.gov/flu/about/burden/2019-2020.html. Accessed 26 July 2021.
- 15. Centers for Disease Control and Prevention. US virologic surveillance weekly. Week 10. https://www.cdc.gov/flu/weekly/weeklyarchives2019-2020/Week10.htm. Accessed 26 July 2021.
- 16. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2020–2021 northern hemisphere influenza season. https://www.who.int/influenza/vaccines/virus/recommendations/202002_recommendation.pdf?ua=1. Accessed 26 July 2021.
- 17. Jernigan DB; CDC COVID-19 Response Team. Update: public health response to the coronavirus disease 2019 outbreak—United States, February 24, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019–20 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed]
- 19. Dong E, Du H, Lauren Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lozano JE;, et al. lozalojo/mem: second release of the MEM R library.https://zenodo.org/record/165983. Accessed 26 July 2021.
- 21. Biggerstaff M, Kniss K, Jernigan DB, et al. Systematic assessment of multiple routine and near real-time indicators to classify the severity of influenza seasons and pandemics in the United States, 2003–2004 through 2015–2016. Am J Epidemiol 2018; 187:1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Armed Forces Health Surveillance Center’s Standard Case Definitions. Influenza-like illness (ILI). https://health.mil/Reference-Center/Publications/2015/10/01/Influenza-Like-Illness. Accessed 26 July 2021.
- 23. van Aalst R, Gravenstein S, Mor V, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: a retrospective cohort study. Vaccine 2020; 38:372–79. [DOI] [PubMed] [Google Scholar]
- 24. Young-Xu Y, Snider JT, van Aalst R, et al. Analysis of relative effectiveness of high-dose versus standard-dose influenza vaccines using an instrumental variable method. Vaccine 2019; 37:1484–90. [DOI] [PubMed] [Google Scholar]
- 25. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health England. The Green Book. Chapter 19: influenza. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/931139/Green_book_chapter_19_influenza_V7_OCT_2020.pdf. Accessed 26 July 2021.
- 27. Robert Koch Institut. Epidemiologisches bulletin (34 2020; 20 August 2020) [in German]. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2020/Ausgaben/34_20.pdf?__blob=publicationFile. Accessed 26 July 2021.
- 28. Robert Koch Institut. Epidemiologisches bulletin (1 2021; 7 January 2020) [in German]. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/01_21.pdf?__blob=publicationFile. Accessed 26 July 2021.
- 29. Federal Ministry of Health, Germany. Regulation on the right to vaccination against influenza and measles [in German]. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/S/Schutzimpfung_gegen_Influenza_und_Masern_VO_BAnz_AT_11.03.2021_V2.pdf. Accessed 26 July 2021.
- 30. Taiwan Centers for Disease Control. Influenza. https://www.cdc.gov.tw/En/Category/ListContent/bg0g_VU_Ysrgkes_KRUDgQ?uaid=Zvnt3Ff941PorUmUD0-leA. Accessed 26 July 2021.
- 31. Taiwan Centers for Disease Control. Influenza complicated by severe illness. https://www.cdc.gov.tw/Disease/SubIndex/x7jzGIMMuIeuLM5izvwg_g. Accessed 26 July 2021.
- 32. Ministério da Saúde, Brazil. Informe técnico: 22ª campanha nacional de vacinação contra A influenza [in Portuguese]. https://www.saude.go.gov.br/files/imunizacao/influenza/InformeTecnicoInfluenza.2020.pdf. Accessed 26 July 2021.
- 33. Jorden MA, Rudman SL, et al. ; CDC COVID-19 Response Team. Evidence for limited early spread of COVID-19 within the United States, January–February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:680–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghosh D, Vogt A.. Outliers: an evaluation of methodologies. Proceedings of the Survey Research Methods [abstract 304068]. In: Section on Survey Research Methods. San Diego, CA: Joint Statistical Meetings; 2012:3455–60. [Google Scholar]
- 36. Andrew MK, Bowles SK, Pawelec G, et al. Influenza vaccination in older adults: recent innovations and practical applications. Drugs Aging 2019; 36:29–37. [DOI] [PubMed] [Google Scholar]
- 37. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68:e1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.