Abstract
Background
The Hospital Frailty Risk Score (HFRS) has made it possible internationally to identify subgroups of patients with characteristics of frailty from routinely collected hospital data.
Objective
To externally validate the HFRS in France.
Design
A retrospective analysis of the French medical information database.
Setting
743 hospitals in Metropolitan France.
Subjects
All patients aged 75 years or older hospitalised as an emergency in 2017 (n = 1,042,234).
Methods
The HFRS was calculated for each patient based on the index stay and hospitalisations over the preceding 2 years. Main outcome measures were 30-day in-patient mortality, length of stay (LOS) >10 days and 30-day readmissions. Mixed logistic regression models were used to investigate the association between outcomes and HFRS score.
Results
Patients with high HFRS risk were associated with increased risk of mortality and prolonged LOS (adjusted odds ratio [aOR] = 1.38 [1.35–1.42] and 3.27 [3.22–3.32], c-statistics = 0.676 and 0.684, respectively), while it appeared less predictive of readmissions (aOR = 1.00 [0.98–1.02], c-statistic = 0.600). Model calibration was excellent. Restricting the score to data prior to index admission reduced discrimination of HFRS substantially.
Conclusions
HFRS can be used in France to determine risks of 30-day in-patient mortality and prolonged LOS, but not 30-day readmissions. Trial registration: Reference ID on clinicaltrials.gov: ID: NCT03905629.
Keywords: hospitalisation, statistics and numerical data, frailty, risk assessment, length of stay, mortality, older people
Key Points
The HFRS predicts mortality and extended length of stay risks among French hospitalised older adults, but not readmissions.
It is mainly applicable for service planning, but not sufficient for individual clinical decision-making.
The HFRS has the potential be used as a frailty risk marker in epidemiological studies using administrative data worldwide.
Introduction
Hospitals are increasingly faced with older patients with many comorbidities, requiring multi-professional assessment and management [1–3]. In France in 2017, over half (56%) of patients aged 75 years and over presenting to emergency departments (EDs) were admitted for acute hospital care [4]. Frail older people admitted to hospital have increased risk of adverse events, have long hospital stays and high rates of long-term care use [1, 5–7]. Frailty can generally be defined as a state of increased vulnerability of older patients towards adverse outcomes when exposed to a stressor event [8]. Given the complex nature of the frailty construct, no existing frailty tool has emerged as a gold standard method to predict individual patient outcomes [9, 10].
Multiple tools have been developed to identify frailty in both clinical and research settings, typically requiring face-to-face assessment by trained observers, some requiring specific clinical measurements, such as hand-grip strength [10]. However, the implementation of such tools remains difficult in busy urgent care settings, in which screening can be achieved in 52% of patients at best [11]. Thus, there is widespread interest in using electronic health records and administrative datasets to mine Big Data, derive frailty prediction models and help direct holistic care to patients most likely to benefit [12–14]. Other key applications include service planning, benchmarking, evaluating health policies and developing markers for epidemiological research. Frailty, as a multi-dimensional construct, is not routinely documented in electronic health records and has not yet been captured by a single diagnosis code [15]. Accumulated deficits and high resource use are commonly recognised by clinicians as key indicators of frailty in acute care settings [16].
The Hospital Frailty Risk Score (HFRS) [17] was recently developed and validated in England using national hospital episode statistics data, building on the results of a preliminary cluster analysis to identify a subgroup of older patients displaying characteristics of frailty (of whom 48% had died over the 2-year follow-up period). Intermediate and high levels of frailty were significantly associated with increased risks of 30-day mortality, prolonged hospital length of stay (LOS) and 30-day readmission [17]. A key strength of this score is that it has the potential to be implemented in administrative datasets using International Classification of Diseases, 10th revision (ICD-10) codes internationally. However, given the risk of potential discrepancies in terms of routine coding practices, this requires prior validation to confirm that the model achieves satisfactory discrimination and calibration in the specific context of application [18, 19].
The main objective of this study was to determine if HFRS can be used in French hospitals to discriminate outcomes in patients aged 75 years and older admitted to acute care, with satisfactory prediction accuracy across settings.
Methods
Study design and participants
We conducted a retrospective analysis of routinely collected secondary care data from the French nationwide Medical Information System database (Programme de Médicalisation des Systèmes d’Information [PMSI], source: ATIH). Inclusion criteria were replicated from the initial HFRS validation study [17]. We included all patients aged 75 years and older (on the day of the index admission) hospitalised as an emergency in Metropolitan France over a period of 1 year between 1 January and 31 December 2017. In case of multiple admissions of the same patient, only the first (index) admission was used for the analysis, in order to maintain population homogeneity. Considering their low frequency, patients with missing data for city of residence median income or medical accessibility were also excluded from the study population as described in the flowchart (Figure 1). The predictive ability of the HFRS was estimated on three binary outcomes: 30-day in-patient mortality, LOS > 10 days and 30-day emergency readmissions. Odds ratios (ORs) and c-statistics were calculated and compared to the original English results in order to validate the use of the HFRS in France.
Figure 1 .

Flowchart.
Data source and outcomes
We used anonymised data from the French PMSI database, which contains routinely collected data from all public and private hospitals in France. Each in-patient stay is allocated to a single Diagnosis-Related Group based on standard discharge abstracts containing compulsory information about the patient, primary and secondary diagnoses using the ICD-10 codes [20].
The main outcome was in-hospital mortality within 30 days from the beginning of the index admission. Other outcomes included long LOS (>10 days) and emergency readmissions up to 30 days from discharge from the index admission (excluding patients who had died in hospital).
Patient covariates considered included age, sex, Charlson comorbidity index categories (0, 1, 2 or 3+, as previously described in Gilbert et al.) [17, 21, 22] and admission history (i.e. number of hospital bed-days during the past 2 years from the index admission as a continuous variable and, for descriptive purposes only, the number of previous admissions in this period). Patients’ socioeconomic status (median household income in the city of residence, continuous) and medical accessibility (mean number of family medicine consultation/year/inhabitant in the city of residence, continuous) were associated with patients’ city of residence postcode and provided by the National Institute of Statistics and Economic Studies and Department of Research, Studies, Evaluation and Statistics respectively. Those two variables were not available for 0.8% of patients that were consequently excluded.
The hospital status (university hospital, regional or local hospital, private clinic) was retrieved for each index hospitalisation, the aim being to identify and describe possible specificities in terms of case-mix and level of frailty according to different types of settings.
Hospital Frailty Risk Score
The HFRS was calculated for each included patient [17], based on the ICD-10 diagnoses documented in their index emergency admission and hospital records and retrieved from anonymised discharge summaries going back 2 years. The ICD-10 codes considered corresponded to the list used to create the HFRS in England [17]. These included principal as well as secondary diagnoses, in the same way restricted to the third figure (for example ‘R26’, and not ‘R26.2’). Patients were allocated to one of three categories based on their HFRS score, applying the same three thresholds used in the original study: low risk <5, intermediate risk 5–15 and high risk >15.
Statistical analysis
Patient characteristics were described using mean and standard deviation for continuous variables, frequencies and percentages for qualitative variables.
To estimate the association of the HFRS categories with outcomes, we fitted mixed logistic regression models with random effects to capture hospital variation. Models were first estimated without adjustment and then with adjustment on age, sex, socioeconomic data, admission history and the Charlson comorbidity index categories. Associations between HFRS categories and each outcome were evaluated with ORs, and discrimination with C-statistics, accompanied by their 95% confidence intervals. Model calibration was assessed by plotting the observed frequency of events per tenths of predicted risks [23]. Models assessing readmissions excluded patients who died during the index admission.
We considered diagnostic information from the patient’s index admission and those occurring in the previous 2 years to build ‘standard’ HFRS. As sensitivity analysis we excluded information from the index admission to build a ‘historic’ score.
We performed two other sensitivity analyses. First, we considered HFRS as a continuous variable using splines and functional forms to model its association with outcomes. Then, we considered a survival approach using Cox’s proportional hazards model to estimate hazard ratios (HRs) for mortality and readmissions. To take into account the influence of the competing risk of in-patient mortality on the association of HFRS with time to readmission, we also ran a proportional hazards model for the sub-distribution of a competing risk (i.e. the ‘Fine-Gray’ model [24]) with robust variance estimator to account for clustering within hospitals.
Data manipulation and analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Results
A total of 1,042,234 patients aged 75 years and older hospitalised as an emergency in 743 hospitals were included (Figure 1). Patient characteristics are presented in Table 1. Patients with higher risks of frailty were generally older and more likely to be female. Number of past admissions was also greater among these patients. Finally, university and public hospitals cared for patients with higher risks of frailty than private clinics. Comorbidity scores and outcomes are presented in Table 2.
Table 1 .
Characteristics of patients by HFRS category—mean (SD) and frequencies (%)
| HFRS | |||||
|---|---|---|---|---|---|
| Overall | <5 | 5–15 | >15 | ||
| Variable | Value | N = 1,042,234 | N = 472,816 (45.4%) | N = 386,894 (37.1%) | N = 182,524 (17.5%) |
| Male, N (%) | 417,487 (40.1%) | 200,594 (42.4%) | 149,736 (38.7%) | 67,157 (36.8%) | |
| Age, mean (SD) | 84.9 (5.8) | 83.9 (5.8) | 85.5 (5.8) | 86.2 (5.6) | |
| Living in low-income areas (5th quintile of the city of residence median incomea) | 208,703 (20.0%) | 92,508 (19.6%) | 78,306 (20.2%) | 37,889 (20.8%) | |
| Medical accessibility, mean (SD) | 4.1 (1.1) | 4.1 (1.1) | 4.1 (1.1) | 4.1 (1.2) | |
| Number of past hospital bed-days, mean (SD) | 10.7 (18.2) | 3.9 (7.8) | 11.4 (16.4) | 26.9 (28.0) | |
| Nb past admissions (including ambulatory), mean (SD) | 1.6 (2.2) | 0.9 (1.6) | 1.7 (2.2) | 3.1 (2.9) | |
| Nb past admissions (including ambulatory), N (%) | 0 | 411,438 (39.5%) | 253,594 (53.6%) | 132,666 (34.3%) | 25,178 (13.8%) |
| 1 | 238,835 (22.9%) | 106,960 (22.6%) | 96,169 (24.9%) | 35,706 (19.6%) | |
| 2 | 157,070 (15.1%) | 59,028 (12.5%) | 63,626 (16.4%) | 34,416 (18.9%) | |
| ≥3 | 234,891 (22.5%) | 53,234 (11.3%) | 94,433 (24.4%) | 87,224 (47.8%) | |
| Index stay duration (nights), mean (SD) | 8.6 (9.1) | 6.2 (6.7) | 10.0 (9.3) | 11.9 (11.8) | |
| Hospital status, N (%) | University Hospital | 202,632 (19.4%) | 89,631 (19.0%) | 74,383 (19.2%) | 38,618 (21.2%) |
| Private | 97,140 (9.3%) | 59,025 (12.5%) | 28,636 (7.4%) | 9,479 (5.2%) | |
| Public | 742,462 (71.2%) | 324,160 (68.6%) | 283,875 (73.4%) | 134,427 (73.6%) | |
| Charlson, mean (SD) | 1.5 (1.4) | 0.9 (1.1) | 1.6 (1.4) | 2.5 (1.6) |
aPatients’ city of residence median income was considered continuously in the final models.
Table 2 .
Patients’ outcome by HFRS category—mean (SD) and frequencies (%)
| HFRS | Low risk | Intermediate risk | High risk | ||
|---|---|---|---|---|---|
| Overall | <5 | 5–15 | >15 | ||
| Variable | N = 1,042,234 | N = 472,816 (45.4%) | N = 386,894 (37.1%) | N = 182,524 (17.5%) | |
| Mortality within 30 days, N (%) | 74,929 (7.2%) | 23,160 (4.9%) | 32,743 (8.5%) | 19,026 (10.4%) | |
| LOS > 10 days, N (%) | 304,303 (29.2%) | 85,189 (18.0%) | 139,161 (36.0%) | 79,953 (43.8%) | |
| Emergency readmissions within 30 days, N (%) | 95,171 (9.1%) | 38,536 (8.2%) | 36,722 (9.5%) | 19,913 (10.9%) |
In-patient 30-day mortality was significantly higher in intermediate and high-risk HFRS groups compared to low risk (Table 3, HFRS crude OR = 1.79 [1.76–1.82]/2.29 [2.25–2.34], c-statistic = 0.617). Likewise, there was an association of increased levels of HFRS with prolonged hospital LOS (OR = 2.56 [2.54–2.59]/3.59 [3.55–3.64], c-statistic = 0.669). These trends were similar after adjustment on covariates (adjusted ORs = 1.34 [1.32–1.37]/1.38 [1.35–1.42] for mortality and 2.34 [2.32–2.37]/3.27 [3.22–3.32] for prolonged LOS, with c-statistics = 0.676 and 0.684 respectively). Even though higher HFRS levels were associated with increased risks of readmission in the unadjusted model (OR = 1.24 [1.22–1.26]/1.48 [1.45–1.51], c-statistic = 0.565), we found no clear association after adjustment on covariates, and discrimination for this outcome was poor (Table 3, adjusted OR = 1.04 [1.02–1.05]/1.00 [0.98–1.02], c-statistic = 0.600). Model calibration was excellent, with a calibration slope around 1 for all models and a calibration line close to the diagonal line (Figure 2).
Table 3 .
Relationship between HFRS frailty risk category and outcomes (n = 1,042,234)
| Outcome, HFRS frailty risk | % | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| 30-day in-patient mortality | |||
| Low risk (<5) | 4.9% | 1.00 | 1.00 |
| Intermediate risk (5–15) | 8.5% | 1.79 (1.76–1.82) | 1.34 (1.32–1.37) |
| High risk (>15) | 10.4% | 2.29 (2.25–2.34) | 1.38 (1.35–1.42) |
| C-statistic | 0.617 (0.615–0.619) | 0.676 (0.674–0.678) | |
| LOS > 10 days | |||
| Low risk (<5) | 18.0% | 1.00 | 1.00 |
| Intermediate risk (5–15) | 36.0% | 2.56 (2.54–2.59) | 2.34 (2.32–2.37) |
| High risk (>15) | 43.8% | 3.59 (3.55–3.64) | 3.27 (3.22–3.32) |
| C-statistic | 0.669 (0.668–0.670) | 0.684 (0.683–0.685) | |
| Emergency readmission within 30 daysa | |||
| Low risk (<5) | 8.2% | 1.00 | 1.00 |
| Intermediate risk (5–15) | 9.5% | 1.24 (1.22–1.26) | 1.04 (1.02–1.05) |
| High risk (>15) | 10.9% | 1.48 (1.45–1.51) | 1.00 (0.98–1.02) |
| C-statistic | 0.565 (0.563–0.567) | 0.600 (0.598–0.602) | |
Models were fitted with random effects to capture hospital variation, first without any adjustment and then adjusted for age, gender, hospital bed-days, primary care access, city median income and Charlson’s index categories. Results displayed are ORs with their 95% CIs. CI, confidence interval.
aDeaths censored for readmissions: 68,875.
Figure 2 .
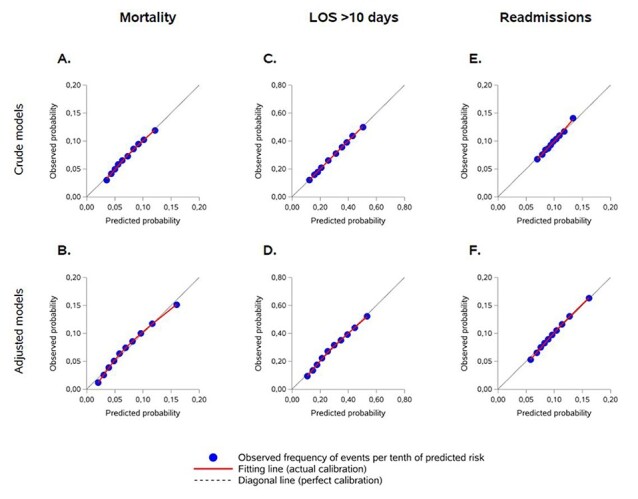
Calibration assessment for mixed logistic regression models. Crude (A, C, E) and adjusted models (B, D, F) for 30 day in-patient mortality, prolonged LOS > 10 days and 30-day readmissions, respectively.
Using only data prior to the index admission reduced predictive abilities of the HFRS for all outcomes significantly: adjusted OR for in-patient 30-day mortality were 1.02 (1.00–1.05) for intermediate risk and 1.05 (1.01–1.08) for high risk (Appendix 1 is available in Age and Ageing online), with lower discriminations (c-statistics = 0.648 for ‘historic’ HFRS versus 0.676 for ‘standard’ HFRS). Furthermore, we observed decreased odds of prolonged LOS for high-risk patients on the ‘historic’ HFRS (Appendix 1 is available in Age and Ageing online, adjusted OR = 0.97 [0.95–0.99], c-statistic = 0.603).
Substituting the HFRS categories by using the HFRS score as a continuous variable did not improve model discrimination (Appendix 2 is available in Age and Ageing online). Using a survival approach to assess in-hospital mortality and 30-day re-admission (Appendix 3 is available in Age and Ageing online), Cox’s HRs were consistent with the results of logistic regression analyses. Furthermore, considering the competing risk of mortality did not affect the results for readmissions (Appendix 3 is available in Age and Ageing online).
Discussion
Principal findings
Our study confirms that HFRS can be used in France to predict 30-day in-patient mortality and prolonged LOS, but not 30-day readmissions. Patients displayed similar characteristics to the English validation cohort, with comparable distributions of low, intermediate and high risk of frailty and similar numbers of past admissions. The thresholds of 5, 5–15 and >15 used to define these categories seemed adequate, and there was no added value in constructing the score as a continuous variable. Model calibration was excellent for all outcomes.
Another key finding of our study was that the ‘historic’ HFRS score (i.e. suppressing data from the index admission) performed no better than the model with adjustment factors alone to predict these outcomes. This compares to the original validation cohort, in which the ‘historic’ HFRS also had weaker amplitude of effect and discrimination (30-day mortality adjusted OR = 1.31 (1.28–1.34) for intermediate and 1.35 (1.31–1.39) for high risk; c-statistic = 0.59) [17], and suggests that the HFRS would be of limited use to direct clinical care of older patients upon arrival to EDs but could still be useful as a frailty mapping tool at the system level.
Comparison with other studies
Since its publication in May 2018, the use of HFRS has been successfully validated in various other countries ([25–34], Appendix 4 is available in Age and Ageing online). Our results were similar to previous studies for mortality prediction and LOS, with similar c-statistics (0.55–0.76 for in-patient mortality, 0.64–0.73 for prolonged LOS). The discrimination of our adjusted model for these two outcomes (both c-statistics around 0.68) was just below the threshold of 0.70 defining ‘good’ discrimination but remains insufficient for directly guiding clinical care. By way of comparison, the HFRS seemed to achieve good discrimination (i.e. 0.70 or above) for these outcomes in specific contexts such as acute heart failure or myocardial infarction [29, 30]. However, results for unplanned readmission risk are much less consistent across studies (Appendix 4 is available in Age and Ageing online).
Patients with higher levels of HFRS tended to have longer LOS in France compared to England. This could highlight differences in the way health systems and community services are organised in the different countries, for example it might be that England’s focus on rapid discharge might be associated with increased readmissions [35, 36].
Strengths and limitations of this study
This study was based on a very large population of patients admitted in any public or private hospital in metropolitan France, for a whole year, taking into account hospital variation and seasonality. The population is representative of all older subjects admitted to EDs in France or other comparable countries. Both discrimination and calibration were assessed in the validation process, and results were robust to several sensitivity analyses. This brings a good level of confidence in the applicability of the score to all French hospitals. However, a few limitations need to be highlighted. First, due to issues regarding authorisation to access and linking of database to national mortality registries, only in-patient mortality data were directly available from the PMSI database, which may impact validity and generalisability of our results. In particular, the model built to predict readmissions could only exclude patients who had died in hospital, and new nursing home admissions were not captured in our study [37]. Second, as all proxy measures based on collected diagnoses, the HFRS score and results of our study might be subject to coding bias. In addition, patients with more frequent admissions and longer hospital stays may have, consequently, more coded information and might be more likely to be labelled as ‘frail’. More generally, hospital administrative data are not usually collected for research purposes, and diagnosis for instance can be inaccurately coded, leading to missing data bias. We cannot rule out the possibility that other residual confounders may have influenced our findings, owing to potential inaccuracies inherent to these kinds of data.
Finally, one of the main limitations of HFRS is that the score does not take into account the severity of the presenting condition [38–41]. A previous study has highlighted the limited value of HFRS for risk prediction in intensive care units, where prognosis is essentially guided by the severity of the acute condition [27]. Further iterations of the score should aim to take this into account, and we will also follow-up with great interest the results of the HAVEN risk score construct (Hospital alerting via electronic noticeboard), which will compile ‘static’ variables such as age, HFRS and comorbidity indices, with a set of ‘dynamic’ variables reflecting disease acuity [42].
Conclusions and implications
This study confirms the validity of HFRS for use on French national data in relation to mortality and prolonged LOS. This is consistent with the results observed in other countries and paves the way for the conduct of international studies using equivalent datasets in populations of older subjects admitted from EDs. The HFRS is mainly applicable as a marker of frailty risk for service planning and epidemiological studies using hospital data, but in keeping with other frailty scores, not suitable for individual patient decision-making.
Supplementary Material
Contributor Information
Thomas Gilbert, Service de médecine gériatrique, Hospices Civils de Lyon, Groupement Hospitalier Sud, 69495 Pierre-Bénite, France; Research on Healthcare professionals and Performance (RESHAPE, inserm U1290), université Claude Bernard Lyon1, Lyon, France.
Quentin Cordier, Health Data Department, Hospices Civils de Lyon, Lyon, France.
Stéphanie Polazzi, Research on Healthcare professionals and Performance (RESHAPE, inserm U1290), université Claude Bernard Lyon1, Lyon, France; Health Data Department, Hospices Civils de Lyon, Lyon, France.
Marc Bonnefoy, Service de médecine gériatrique, Hospices Civils de Lyon, Groupement Hospitalier Sud, 69495 Pierre-Bénite, France; U1060 INSERM, CarMeN, 69921 Oullins, France.
Eilìs Keeble, The Nuffield Trust, London W1G 7LP, UK.
Andrew Street, Department of Health Policy, London School of Economics, London WC2A 2AE, UK.
Simon Conroy, Department of Health Sciences, College of Life Sciences, University of Leicester, Leicester LE1 7HA, UK.
Antoine Duclos, Research on Healthcare professionals and Performance (RESHAPE, inserm U1290), université Claude Bernard Lyon1, Lyon, France; Health Data Department, Hospices Civils de Lyon, Lyon, France.
Acknowledgements
The authors would like to thank Léa Pascal for her contribution to this work.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was supported by a grant from Hospices Civils de Lyon (direction de la recherche clinique et de l’innovation), France. The funding source had no influence in the design, data collection, interpretation of data, writing of the report or decision to submit the article for publication.
References
- 1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394: 1365–75. [DOI] [PubMed] [Google Scholar]
- 2. Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res 2015; 50: 305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394: 1376–86. [DOI] [PubMed] [Google Scholar]
- 4. Boisguérin B, Mauro L. Les personnes âgées aux urgences: une patientèle au profil particulier. https://drees.solidarites-sante.gouv.fr/sites/default/files/er1008.pdf (28 May 2021, date last accessed).
- 5. Kahlon S, Pederson J, Majumdar SR et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ 2015; 187: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vermeiren S, Vella-Azzopardi R, Beckwée D et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc 2016; 17: 1163.e1–17. [DOI] [PubMed] [Google Scholar]
- 7. Buurman BM, Hoogerduijn JG, de Haan RJ et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS ONE 2011; 6: e26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpenter CR, Shelton E, Fowler S et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta-analysis. Acad Emerg Med 2015; 22: 1–21. [DOI] [PubMed] [Google Scholar]
- 10. Clegg A, Rogers L, Young J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age Ageing 2015; 44: 148–52. [DOI] [PubMed] [Google Scholar]
- 11. Elliott A, Phelps K, Regen E, Conroy SP. Identifying frailty in the Emergency Department-feasibility study. Age Ageing 2017; 46: 840–5. [DOI] [PubMed] [Google Scholar]
- 12. Todd OM, Burton JK, Dodds RM et al. New Horizons in the use of routine data for ageing research. Age Ageing 2020; 49: 716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nghiem S, Sajeewani D, Henderson K et al. Development of frailty measurement tools using administrative health data: A systematic review. Arch Gerontol Geriatr 2020; 89: 104102. [DOI] [PubMed] [Google Scholar]
- 14. Kim DH. Measuring Frailty in Health Care Databases for Clinical Care and Research. Ann Geriatr Med Res 2020; 24: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muscedere J. The Need to Implement Frailty in the International Classification of Disease (ICD). J Frailty Aging 2020; 9: 2–3. [DOI] [PubMed] [Google Scholar]
- 16. Soong JTY, Poots AJ, Bell D. Finding consensus on frailty assessment in acute care through Delphi method. BMJ Open 2016; 6: e012904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert T, Neuburger J, Kraindler J et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018; 391: 1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riley RD, Ensor J, Snell KIE et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ 2016; 353: i3140. doi: 10.1136/bmj.i3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 2013; 13: 33. doi: 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Busse R, ed. Diagnosis-related groups in Europe: moving towards transparency, efficiency and quality in hospitals. BMJ 2013; 346. doi: 10.1136/bmj.f3197. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 22. Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 23. Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ 2009; 338: b605. [DOI] [PubMed] [Google Scholar]
- 24. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 25. McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf 2019; 28: 284–8. [DOI] [PubMed] [Google Scholar]
- 26. Eckart A, Hauser SI, Haubitz S et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open 2019; 9: e026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruno RR, Wernly B, Flaatten H, Schölzel F, Kelm M, Jung C. The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care 2019; 23: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwok CS, Zieroth S, Van Spall HGC et al. The Hospital Frailty Risk Score and its association with in-hospital mortality, cost, length of stay and discharge location in patients with heart failure short running title: Frailty and outcomes in heart failure. Int J Cardiol 2020; 300: 184–90. [DOI] [PubMed] [Google Scholar]
- 29. Kwok CS, Lundberg G, Al-Faleh H et al. Relation of Frailty to Outcomes in Patients With Acute Coronary Syndromes. Am J Cardiol 2019; 124: 1002–11. [DOI] [PubMed] [Google Scholar]
- 30. McAlister FA, Savu A, Ezekowitz JA, Armstrong PW, Kaul P. The hospital frailty risk score in patients with heart failure is strongly associated with outcomes but less so with pharmacotherapy. J Intern Med 2020; 287: 322–32. [DOI] [PubMed] [Google Scholar]
- 31. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med 2019; 14: 407–10. [DOI] [PubMed] [Google Scholar]
- 32. Marshall D, Salciccioli J, Hatch M, Rowland M. EP.305: Validation of the Hospital Frailty Risk Score in the ICU. Journal of the Intensive Care Society 2019; 20 Supplement 1–253: 230–1. [Google Scholar]
- 33. Hannah TC, Neifert SN, Caridi JM et al. Utility of the Hospital Frailty Risk Score for Predicting Adverse Outcomes in Degenerative Spine Surgery Cohorts. Neurosurgery 2020; nyaa248. doi: 10.1093/neuros/nyaa248. [DOI] [PubMed] [Google Scholar]
- 34. Shebeshi DS, Dolja-Gore X, Byles J. Validation of hospital frailty risk score to predict hospital use in older people: Evidence from the Australian Longitudinal Study on Women’s Health. Arch Gerontol Geriatr 2021; 92: 104282. [DOI] [PubMed] [Google Scholar]
- 35. Keeble E, Roberts HC, Williams CD, Van Oppen J, Conroy SP. Outcomes of hospital admissions among frail older people: a 2-year cohort study. Br J Gen Pract 2019; 69: e555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Craven E, Conroy S. Hospital readmissions in frail older people. Rev Clin Gerontol 2015; 25: 107. [Google Scholar]
- 37. Hollinghurst J, Housley G, Watkins A, Clegg A, Gilbert T, Conroy SP. A comparison of two national frailty scoring systems. Age Ageing 2020; afaa252. doi: 10.1093/ageing/afaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: An observational study. Eur J Intern Med 2016; 35: 24–34. [DOI] [PubMed] [Google Scholar]
- 39. Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 2020; 49: 1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dynesen J, Skov MJ, Mackenhauer J et al. The 7-day mortality associated with an early warning score varies between age groups in a cohort of adult Danish emergency department patients. Eur J Emerg Med 2019; 26: 453–7. [DOI] [PubMed] [Google Scholar]
- 41. Elliott A, Taub N, Banerjee J et al. Does the Clinical Frailty Scale at triage predict outcomes from emergency care for older people? Ann Emerg Med 2021; 77: 620–7. [DOI] [PubMed] [Google Scholar]
- 42. Malycha J, Redfern OC, Ludbrook G, Young D, Watkinson PJ. Testing a digital system that ranks the risk of unplanned intensive care unit admission in all ward patients: protocol for a prospective observational cohort study. BMJ Open 2019; 9: e032429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


