Abstract
Objective
Diabetes is a risk factor for dementia but little is known about the impact of diabetes duration on the risk of dementia. We investigated the effect of type 2 diabetes duration on the risk of dementia.
Design
Prospective cohort study using health claims data representative for the older German population. The data contain information about diagnoses and medical prescriptions from the in- and outpatient sector.
Methods
We performed piecewise exponential models with a linear and a quadratic term for time since first type 2 diabetes diagnosis to predict the dementia risk in a sample of 13,761 subjects (2,558 dementia cases) older than 65 years. We controlled for severity of diabetes using the Adopted Diabetes Complications Severity Index.
Results
We found a U-shaped dementia risk over time. After type 2 diabetes diagnosis the dementia risk decreased (26% after 1 year) and reached a minimum at 4.75 years, followed by an increase through the end of follow-up. The pattern was consistent over different treatment groups, with the strongest U-shape for insulin treatment and for those with diabetes complications at the time of diabetes diagnosis.
Conclusions
We identified a non-linear association of type 2 diabetes duration and the risk of dementia. Physicians should closely monitor cognitive function in diabetic patients beyond the first few years after diagnosis, because the later increase in dementia occurred in all treatment groups.
Keywords: Type 2 diabetes, dementia, risk factors, health claims data, older people
Key Points
The dementia risk over time since first type 2 diabetes diagnosis was U-shaped.
The U-shaped pattern was consistent over different diabetes treatment groups.
Less severe cases with no diabetes complications at baseline showed a stronger U-shape than more severe cases.
Introduction
The prevalence of dementia and diabetes has increased in recent decades [1]. The WHO forecasts an increase in the all-cause dementia prevalence to 152 million by 2050 [2].
Type 2 diabetes (T2D) is a well-known risk factor for dementia [3–8]. The prevalence of T2D was estimated to be 6.28% (462 million people) worldwide, and 22% among people aged 70 years and older [9]. Even pre-diabetes has been shown to be a risk factor for all-cause dementia, Alzheimer’s disease (AD) and vascular dementia (VD). Changes of diabetes-related biochemical indicators such as fasting plasma insulin were associated with an increased dementia risk [8]. While cardiovascular risk factors are the main drivers for an increased dementia risk in midlife, diabetes seems to be the strongest predictor among the cardiovascular risk factors in later-life [10].
There are various mechanisms by which T2D and dementia are connected [11]. First, there are atherosclerotic consequences of T2D that favour VD. T2D is strongly associated with micro- and macrovascular diseases. This association is essentially determined by diabetes duration [12, 13].
However, little is known about the association of diabetes duration and the risk of dementia.
Differences in the mean age of dementia onset with respect to diabetes duration attenuated in older age groups [14]. Other studies found an increased risk of cognitive decline in prevalent diabetes rather than in incident diabetes [15] or with longer duration [16]. Studies emphasising the role of age at diabetes onset also provided evidence of increasing dementia risk with longer diabetes duration [17, 18].
In addition to duration and earlier onset of diabetes, the risk of mild cognitive impairment (MCI) increases with the severity of diabetes [19], and diabetes is associated with a higher risk for the progression from MCI to dementia [20]. Baseline HemoglobinA1c (HbA1c) was negatively associated with cognition, supporting the assumption that severity of T2D play a crucial role in the development of dementia [21]. Severe hypoglycaemia also promotes the occurrence of dementia [22]. Peripheral insulin resistance appears to be linked to cerebral insulin resistance, and lower glucose utilisation in the brain may drive ad development [22, 23], and T2D severity and progression are associated with the risk for dementia [24].
Due to dementia subtypes and the role T2D can play in the development of VD and ad, the influence of different treatments for T2D on dementia is complex. It was recently reported that distinct treatments of T2D result in different incident rates of dementia, with the lowest risk in oral anti-diabetic medications (ADM), followed by diabetes patients without ADM and those dependent on insulin [7, 25]. However, some studies suggest a potential benefit from diabetes treatment to the risk of AD [26] and all-cause dementia [27, 28]. There is evidence for a reduced dementia risk from metformin [27–29].
The aim of this study was to explore the association between diabetes duration and dementia incidence, taking severity and treatment form of T2D into account.
We hypothesised that the dementia risk increases with longer T2D duration independent of T2D severity. This duration effect should be moderated by the different diabetes treatment strategies.
Methods
Study design and sample
A random sample of 250,000 individuals was drawn in 2004 by the largest German health insurance, the ‘Allgemeine Ortskrankenkasse’ (AOK). The sample is representative of the German population aged 65 and older, as measured in terms of mortality (Supplementary Figure S1). The claims data were anonymized (by the data provider) and we did not have access to the primary data, so no ethical review or patient consent was required. The sample included insured people born before 1955 with a follow-up to 2015 and contained information about demographic data, diagnosis from in- and outpatient sector based on International Classification of Diseases (ICD-10, [30]), as well as all medical prescriptions according to the Anatomical Therapeutic Chemical Classification (ATC) System [31]. The data were structured on a quarterly basis. We included people with incident T2D diagnosis who were born before 1940 and excluded people with dementia diagnoses or T2D diagnoses before 2006, or people with any type 1 diabetes diagnosis during the follow-up (excluded in this specific order). Our sample consisted of 13,761 insured people older than 64 years with newly diagnosed T2D and at least one quarter of follow-up. We observed 2,558 dementia cases through the end of 2014, 2,845 people died within this period and 8,544 reached the end of follow-up (Supplementary Figure S2), 107 people dropped out of the data for other reasons (e.g. change of insurance company). Data from the year 2015 were used for validation only.
Diabetes and Dementia
T2D and dementia were identified by ICD-10 codes. To reduce the problem of false positive diagnoses, we used internal validation strategies (Supplementary Text S1).
Diabetes treatment
We defined three groups of ADM, coded by ATC-codes, as well as a group without any ADM prescription. Insulin users received prescriptions with ATC-code A10A. The group of non-insulin ADM had prescriptions with ATC-code A10B. We assigned concurrent prescriptions of insulin and non-insulin ADM to the group of mixed ADM.
Covariates
To approximate the severity of diabetes, we implemented the Adopted Diabetes Complications Severity Index (aDCSI) [32–34]. The aDCSI score ranged from 0 to 13. For our analysis we categorised the aDCSI into five levels (0, 1, 2, 3 and 4+).
Further covariates were age, sex, hypertensive diseases, depression, cerebrovascular diseases, ischemic heart diseases, atrial fibrillation and flutter, obesity and disorders of lipoprotein metabolism (ICD-10 codes: Supplementary Text S2).
Statistical analysis
We assessed the risk of dementia depending on the time since first T2D diagnosis. We measured dementia incidence from the quarter of first T2D diagnosis through the last quarter of 2014. In the multivariable analysis we performed piecewise exponential regression models, which permitted us to explicitly model the baseline hazard over the analysis time. We split the baseline hazard into quarters and defined T2D duration as a second-degree polynomial in terms of time since T2D diagnosis (d) and a quadratic term (d2) (Table 2: Model 1). More details on methods and formulas are provided in the online supplement (Supplementary Text S3).
Table 2.
Results of regression models, hazard ratios and 95% confidence intervals, risk of dementia dependent on the duration of diabetes
| Variable | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
|---|---|---|---|
| Model 1 (all) | Model 2 (65–84 years) | Model 3 (85+) | |
| Time since T2D (d) | 0.918*** (0.904–0.932) |
0.924*** (0.909–0.940) |
0.899*** (0.864–0.935) |
| Time since T2D 2 (d2) | 1.002*** | 1.002*** | 1.003*** |
| (1.002–1.003) | (1.001–1.002) | (1.001–1.004) | |
| Model 4 (all) | Model 5 (65–84 years) | Model 6 (85+ years) | |
| Time since T2D (d) | 0.926*** (0.910–0.943) |
0.933*** (0.914–0.952) |
0.908*** (0.870–0.948) |
| Time since T2D 2 (d2) | 1.002*** (1.001–1.003) |
1.002*** (1.001–1.002) |
1.003*** (1.001–1.004) |
| Treatment (tg i ) (ref. no ADM tg 0 ) | |||
| Insulin (tg1) | 3.609** (2.305–5.651) |
3.819*** (2.289–6.372) |
4.347*** (1.634–11.565) |
| Non-insulin ADM (tg2) | 1.168 (0.926–1.471) |
1.164 (0.893–1.519) |
1.119 (0.674–1.858) |
| Mixed ADM (tg3) | 2.186* (1.000–4.775) |
2.030 (0.811–5.077) |
2.937 (0.657–13.122) |
| Treatment (tg i ) × Time since T2D (d) | |||
| Insulin (d, tg1) | 0.862*** (0.794–0.937) |
0.883*** (0.807–0.966) |
0.660*** (0.495–0.879) |
| Non-insulin ADM (d, tg2) | 0.981 (0.945–1.018) |
0.979 (0.940–1.019) |
1.002 (0.901–1.115) |
| Mixed ADM (d, tg3) | 0.966 (0.849–1.100) |
0.981 (0.849–1.134) |
0.883 (0.631–1.237) |
| Treatment (tg i ) × Time since T2D 2 (d2) | |||
| Insulin (d2, tg1) | 1.004*** (1.001–1.006) |
1.003** (1.00–1.006) |
1.014** (1.003–1.024) |
| Non-insulin ADM (d2, tg2) | 1.000 (0.999–1.002) |
1.001 (0.999–1.002) |
0.999 (0.995–1.004) |
| Mixed ADM (d2, tg3) | 0.999 (0.995–1.004) |
0.999 (0.994–1.003) |
1.002 (0.990–1.014) |
95% CI: 95% Confidence interval.
* P value <0.10.
** P value <0.05.
*** P value <0.01.
All models controlled for: age, sex, comorbidity and aDCSI.
Models 1, 2 and 3 explore the total duration effect. Models 4, 5 and 6 explore the duration effect by treatment groups.
Treatment strategies were included as a time-varying predictor, thus, individuals were able to change the treatment groups. To avoid biases we followed Hernan et al. [35, 36] (Supplementary Text S4).
The diabetes complications (aDCSI) was measured as a time-varying variable, and a time dummy controlled for a structural changes in the billing system for physicians [37] (Supplementary Text S3).
To examine how the duration effect of T2D differs between treatment strategies of T2D, we included interaction effects between the treatment group (tgi) and the baseline hazard function (model 4). Formulas are provided in the online supplement (Supplementary Text S3).
In a sensitivity analysis, we performed models separately for age groups (65–84 years and older than 84 years; models 2 and 3, and 5 and 6), and for the diabetes severity at the time of T2D incidence in terms of less severe cases (aDCSI = 0) vs. more severe (aDCSI>0).
We performed Cox regression models to statistically confirm that the effects of treatment strategies and T2D severity on dementia incidence were independent of our model strategy (results upon request).
All analyses were performed using Stata 16.0 (College Station, TX).
Results
Descriptive results
The analysis sample comprised 57,613 person-years. The mean follow-up time per subject was 4.18 years, and the mean age at first T2D diagnosis 76.9 years (sd = 5.8). The mean of the aDCSI at the time of T2D incidence was 2.34 (sd = 1.46) with 2,278 people without any diabetes complications.
The incidence of dementia decreased after the first year, remained nearly constant for the next 3 years (Table 1), and increased thereafter. More severe T2D cases were more likely to receive a new diagnosis of dementia than less severe cases. Dementia incidence was significantly higher in women than in men and increased with age and with almost all the comorbidities considered. Incidence differed significantly among treatment groups, with those treated with insulin having the highest incidence (Table 1).
Table 1.
Characteristics of the study population and dementia incidence rate per 1,000 person-years with 95% confidence intervals
| Dementia incidence rate per 1,000 person-years | |||||
|---|---|---|---|---|---|
| Variable | Person-years | Cases with dementia | Rate | 95% Confidence interval | |
| Time since T2D diagnosis | |||||
| Up to 1 year | 15,913 | 816 | 51.28 | 47.88 | 54.92 |
| Up to 2 years | 10,672 | 408 | 38.23 | 34.70 | 42.13 |
| Up to 3 years | 8,966 | 352 | 39.26 | 35.37 | 43.58 |
| Up to 4 years | 7,335 | 270 | 36.81 | 32.67 | 41.47 |
| Up to 5 years | 5,713 | 246 | 43.06 | 38.00 | 48.79 |
| Up to 6 years | 4,216 | 194 | 46.01 | 39.97 | 52.96 |
| Up to 7 years | 2,815 | 153 | 54.35 | 46.39 | 63.69 |
| Up to 8 years | 1,574 | 95 | 60.35 | 49.36 | 73.79 |
| More than 8 years | 407 | 24 | 58.91 | 39.48 | 87.89 |
| Diabetes severity at T2D incidence | |||||
| Less severe cases | 11,407 | 323 | 28.32 | 25.39 | 31.58 |
| more severe | 46,206 | 2,235 | 48.37 | 46.41 | 50.42 |
| Sex | |||||
| Man | 22,568 | 883 | 39.13 | 36.63 | 41.79 |
| Woman | 35,044 | 1,675 | 47.80 | 45.56 | 50.14 |
| Age group at T2D incidence | |||||
| 65–84 | 53,500 | 2,054 | 38.39 | 36.77 | 40.09 |
| 85+ | 4,112 | 504 | 122.56 | 112.31 | 133.74 |
| Treatment groups | |||||
| No ADM | 40,772 | 1,864 | 45.72 | 43.69 | 47.84 |
| Insulin | 1,071 | 83 | 77.47 | 62.47 | 96.07 |
| Non-insulin ADM | 15,044 | 577 | 38.35 | 35.35 | 41.61 |
| Mixed ADM | 725 | 34 | 46.88 | 33.50 | 65.61 |
| Hypertensive diseases | |||||
| No | 3,129 | 126 | 40.27 | 33.82 | 47.95 |
| Yes | 54,484 | 2,432 | 44.64 | 42.90 | 46.45 |
| Depression | |||||
| No | 39,047 | 1,444 | 36.98 | 35.12 | 38.94 |
| Yes | 18,566 | 1,114 | 60.00 | 56.58 | 63.63 |
| Cerebrovascular diseases | |||||
| No | 38,850 | 1,192 | 30.68 | 28.99 | 32.47 |
| Yes | 18,763 | 1,366 | 72.80 | 69.04 | 76.77 |
| Ischemic heart diseases | |||||
| No | 26,453 | 953 | 36.03 | 33.81 | 38.39 |
| Yes | 31,160 | 1,605 | 51.51 | 49.05 | 54.09 |
| Atrial fibrillation and flutter | |||||
| No | 43,528 | 1,615 | 37.10 | 35.34 | 38.96 |
| Yes | 14,085 | 943 | 66.95 | 62.81 | 71.36 |
| Obesity | |||||
| No | 35,852 | 1,742 | 48.59 | 46.36 | 50.93 |
| Yes | 21,761 | 816 | 37.50 | 35.01 | 40.16 |
| Disorders of lipoprotein metabolism | |||||
| No | 17,761 | 880 | 49.55 | 46.38 | 52.93 |
| Yes | 39,852 | 1,678 | 42.11 | 40.14 | 44.17 |
| Total | 57,613 | 2,558 | 44.40 | 42.71 | 46.15 |
Model results
Table 2 shows the estimated hazard ratios (HR) of the piecewise exponential models to assess the dementia risk since T2D incidence. In models 1–3 the linear term d of time since T2D incidence revealed a decreasing risk (model 1: HR(d) = 0.92; 95%CI = 0.90–0.93; model 2: HR(d) = 0.92; 95%CI = 0.91–0.94, model 3: HR(d) = 0.90; 95%CI = 0.86–0.93). Furthermore the significant quadratic terms indicated an increase in dementia risk for longer time-spans (model 1: HR(d2) = 1.002; 95%CI = 1.002–1.003; model 2: HR(d2) = 1.002; 95%CI = 1.001–1.002, model 3: HR(d2) = 1.003; 95%CI = 1.001–1.004). This combination resulted in a U-shaped risk pattern for dementia over time. After1year the dementia risk decreased by 26% (predicted HR(d,d2) = 0.74; 95%CI = 0.70–0.78), and reached its minimum 4.75 years after T2D incidence (predicted HR(d,d2) = 0.44; 95%CI = 0.39–0.50, Figure 1). Model 4 included an interaction term between diabetes duration and the treatment groups. We observed a U-shaped risk pattern for diabetic people without any ADM (model reference group: HR(d) = 0.93; 95%CI = 0.91–0.94, and HR(d2) = 1.002; 95%CI = 1.001–1.003), and an increased dementia risk for the group of insulin users within the quarter of T2D incidence (model 4: HR(d,d2,tg1) = 3.61; 95%CI = 2.30–5.65), with a stronger U-shape thereafter (HR(d,tg1) = 0.86; 95%CI = 0.79–0.94, HR(d2,tg1) = 1.004; 95%CI = 1.001–1.006). Thus, after 1 year the predicted dementia risk in the group of insulin users was about 60% higher (HR(d,d2,tg1) = 1.61; 95%CI = 1.21–2.14) than for individuals without ADM. Both other treatment groups did not differ significantly from the group without any ADM. The U-shaped pattern consisted in both age groups as well as in all treatment groups, with the exception of the mixed ADM group (Figure 2). Stratification for diabetes complications at T2D incidence shifted the U-shape upwards for individuals with complications, which we consider to be more severe cases (Figure 3). The initial decrease in the dementia risk in subsequence of incident T2D is stronger for less severe diabetes cases, while the U-shaped pattern for the more severe cases is less pronounced.
Figure 1.
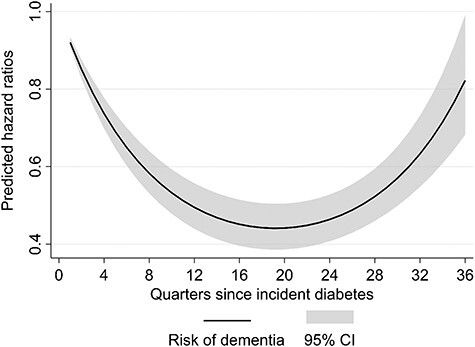
Predicted risk of dementia over time since incident T2D diagnosis. CI = Confidence intervals. Source: AOK data 2004–2015, authors’ calculations.
Figure 2.
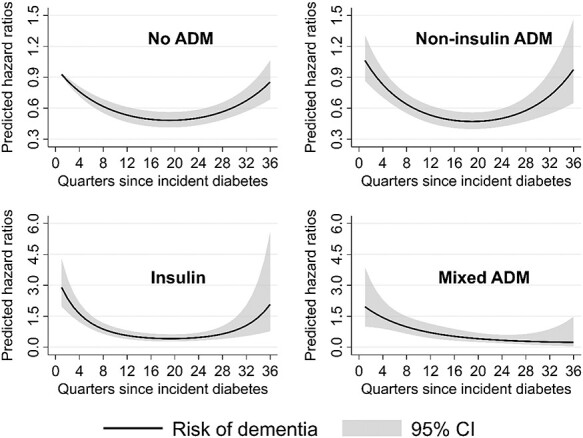
Predicted risk of dementia over time since incident T2D diagnosis for treatment groups. CI = Confidence intervals. Note different y-axis scales. Source: AOK data 2004–2015, authors’ calculations.
Figure 3.
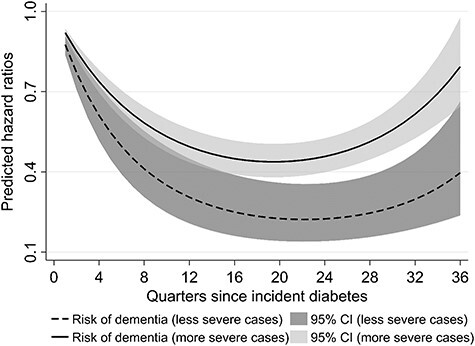
Predicted risk of dementia over time since T2D by diabetes severity at T2D incidence. Source: AOK data 2004–2015, authors’ calculations.
Discussion
This study provides evidence for a U-shaped association between T2D duration and the dementia risk for individuals older than 64 years with newly diagnosed T2D. The identified U-shaped pattern was independent of the severity of diabetes. After the initial T2D diagnosis, the dementia risk continued to decrease for 5 years, followed by an increase thereafter. However, even over longer durations the risk did not surpass levels observed immediately after T2D diagnosis. This pattern held true for both age groups. Interaction between the duration of T2D and different treatment strategies did not change the U-pattern for three of four treatment strategies and disclosed the strongest U-shape for insulin treatment. Only the group with mixed treatment strategy did not reveal a clear U-pattern. To consider different stages of T2D progression at the time of first diagnosis, we used stratified models by diabetes-severity. We found a stronger U-shape for less severe cases at baseline than for more severe cases.
Our results support previous findings from Chiu et al. [24], who identified diabetes progression significantly associated with dementia risk. Contrary to these findings we also identified the T2D duration as a risk factor, independent of the diabetes severity and unrelated to the progression of T2D. Our results contrast with other findings from Wu et al. [16], who did not indicate a higher risk for cognitive decline in incident diabetes patients.
In general, a worsening of the cerebrovascular blood supply can promote loss of cognitive function. Mild chronic inflammatory constellations in the context of insulin resistance favour this process [38]. The U-shape may be caused by several factors. In Germany, exposure to medical services increases in advanced age, particularly among men [39]. This can generally contribute to an increased detection rate of dementia during check-up or other examinations, so it may be a monitoring effect that need not be limited to diabetes. Moreover, the German Diabetes Association recommends close-meshed tests for dementia in T2D subjects [40], which may explain the initial high dementia risk. Pre-diabetes is associated with an increased risk of impaired cognitive function [8], which could contribute to the high dementia prevalence at T2D diagnosis. However, when glycaemic control has not yet been established, a diagnosis of dementia cannot be made without restrictions. A newly diagnosed T2D is usually accompanied by start of a therapy, which consists of medical treatment and/or changes in lifestyle. One aspect for the initial decrease in dementia diagnosis might be that a formal diagnosis of dementia in subjects with poorly controlled T2D will be delayed until good glycaemic control has been established. This should, however, only affect a small number of patients and a diagnosis of dementia after drug or lifestyle interventions is usually made before the increase observed here. The start of diabetes therapy may reduce or delay cognitive decline [41] in the prodromal phase [26] and might be partially responsible for a delayed development/diagnosis of dementia. Notably, patient compliance with diabetes self-management decreases over time [42] and reduces this effect. Also, loss of beta cell function continues contributing to the need for more medication [43]. Expanding treatment complexity, not limited to diabetes, may play a role here. [44–46]. It is also conceivable that there is decreased motivation to adhere to treatment recommendations due to an abatement of perceived treatment efficacy. It appears that if the diagnosis of dementia is not made within the first year after T2D diagnosis, this happens hesitantly for a longer time. Diagnosis of dementia can be challenging as early dementia symptoms may not be apparent during time-limited physician–patient interactions. Furthermore, there might be a low awareness for cognitive decline in younger subjects. Accordingly, early dementia symptoms often remain undetected by physicians and patients resulting in delayed diagnosis. Cognitive decline may worsen self-management of T2D therapy, even in early and undetected stages of dementia. This is a vulnerable phase, as cognitive changes may be aggravated by incorrect self-management of T2D as a consequence of hypo- or hyperglycaemia [47, 48].
Over time micro- and macrovascular complications develop in T2D and may outweigh the therapy effect. This suspicion is supported by the weaker shaped U-form for the severe diabetes cases at baseline (Figure 3). The incidence of dementia diagnoses starting five or more years after T2D diagnosis could thus express the long-term consequences of T2D on cognition.
The lack of a clear U-shaped pattern in the mixed group may be caused by heterogeneous composition in terms of T2D severity and treatment, and needs further investigation.
Strengths and limitations
Unlike several smaller previous studies [14, 16, 19] and similar to Chiu et al. [24] we analysed a large longitudinal sample with up to 9 years of follow-up.
As these health claims data are not based on interviews and recruiting, recall and selection bias as well as panel attrition can be ruled out. Patients were included regardless of their cognitive and functional status, which is particularly important for the oldest ones who are living in nursing homes.
We used a validated measure for the diabetes severity (aDCSI) as a time-varying covariate. This allowed us to consider the severity and progression of T2D.
Our study is not without limitations. We observed the time of diagnoses rather than the time of onset. T2D and dementia are both slowly progressive diseases and the lag time between onset and first diagnoses can vary considerably [49, 50], as can the level of diabetes complications [51].
Diagnoses are made not only through physician services, which can lead to underdiagnosis, but also through a higher rate of dementia detection through the use of health care services not limited to diabetes. These diagnoses tools are neither standardised nor always specific, which may lead to inaccurately encoded diagnoses. To reduce this problem, we applied established internal validation strategies for diabetes and dementia. In case of dementia more than 50% of diagnoses were coded as ‘unspecific’ (ICD-F03), hence we cannot distinguish between dementia subtypes, although T2D might affect VD in a different way than ad [11].
Information about drug use is restricted to prescriptions filled in a pharmacy and we have no information on whether medication was taken. A significant proportion of T2D patients might actually be affected by latent autoimmune diabetes in adults (LADA), a type of diabetes that could not be distinguished in the current study. The prevalence of LADA is estimated between 2 and 14% [52], and it cannot be ruled out that this affected the results. Finally, the data do not contain information about socio-economic background, lifestyle factors and health behaviour. While changes in lifestyle are usually part of diabetes treatment strategies, we do not know whether these were followed. We cannot draw conclusions about glycaemic control as we have no information on blood glucose levels.
Conclusion
The main finding of the current study is the U-shaped risk of dementia over time from the time of diabetes diagnosis. Possible explanations for the initially high incidence of dementia include a better screening in T2D patients, a consequence of a deteriorated metabolic situation even before T2D diagnosis and a higher exposure to medical services in this population. The following decrease in dementia incidence 2–5 years after diagnosis of T2D could be due to lifestyle and drug intervention but might also depend on higher awareness for dementia in T2D subjects. It might be assumed that after a longer exposure to T2D and diabetic complications the incidence of dementia raises over time starting approximately 5–8 years after the initial diagnosis of T2D. The aspects discussed above certainly cannot fully explain the U-shaped dementia risk over time in patients following a diabetes diagnosis, and further research is needed to obtain a more comprehensive picture and derive practical implications for the prevention and treatment of cognitive impairment in T2D patients. Our data suggest that physicians should be encouraged to continue close monitoring of the development of cognitive function in diabetic patients even if diabetes was diagnosed more than 2 years ago. In this context, treatment outcome and adherence should also be considered. Future research should investigate whether primary prevention, and detection and treatment of T2D might be beneficial not only regarding the development of T2D, but also regarding the development of dementia.
Supplementary Material
Acknowledgment
We are grateful to the Scientific Research Institute of the AOK, WIdO, for providing the data. The Scientific Research Institute of the AOK (WIdO) imposes strict rules on sharing health claims data as these are classified according to ethical restrictions due to privacy concerns. Anonymised data are available to researchers and institutions upon request. In order to request access to the health claims data of the AOK, please contact the WIdO directly (http://www.wido.de/, mail: wido@wido.bv.aok.de).
Contributor Information
Constantin Reinke, Institute for Sociology and Demography, University of Rostock, 18057 Rostock, Germany.
Nikolaus Buchmann, Charité–Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Endocrinology and Metabolic Diseases (including Division of Lipid Metabolism), Biology of Aging working group, 13353 Berlin, Germany.
Anne Fink, German Center for Neurodegenerative Diseases, 53127 Bonn, Germany.
Christina Tegeler, Charité–Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Endocrinology and Metabolic Diseases (including Division of Lipid Metabolism), Biology of Aging working group, 13353 Berlin, Germany; MSB Medical School Berlin, Department of Psychology, 14197 Berlin, Germany.
Ilja Demuth, Charité–Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Endocrinology and Metabolic Diseases (including Division of Lipid Metabolism), Biology of Aging working group, 13353 Berlin, Germany; Berlin Institute of Health at Charité – Universitätsmedizin Berlin, BCRT - Berlin Institute of Health Center for Regenerative Therapies, Berlin, Germany.
Gabriele Doblhammer, Institute for Sociology and Demography, University of Rostock, 18057 Rostock, Germany; German Center for Neurodegenerative Diseases, 53127 Bonn, Germany.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
C.R. and G.D. were part of the project ‘Multi-state, multi-time, multi-level analysis of health-related demographic events: Statistical aspects and applications’, which was funded by the German Research Foundation (Funding program ‘Research Grants’ https://gepris.dfg.de/ project number 386913674). The funders did not have any role in study design, collection, analysis and interpretation of data, in writing of the report or in the decision to submit the article for publication.
References
- 1. Nichols E, Szoeke CEI, Vollset SE et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurology 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Dementia. Key facts. World Health Organization, 2021. https://www.who.int/en/news-room/fact-sheets/detail/dementia (5 March 2021, date last accessed). [Google Scholar]
- 3. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig 2013; 4: 640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatterjee S, Peters SAE, Woodward M et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016; 39: 300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012; 42: 484–91. [DOI] [PubMed] [Google Scholar]
- 6. Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, Booth GL. Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care 2015; 38: 1868–75. [DOI] [PubMed] [Google Scholar]
- 7. Buchmann N, Fink A, Tegeler C, Demuth I, Doblhammer G, Steinhagen-Thiessen E. Different treatment forms of type II diabetes and the risk of dementia in German health claims data. Acta Diabetol 2019; 56: 995–1003. [DOI] [PubMed] [Google Scholar]
- 8. Xue M, Xu W, Ou Y-N et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev 2019; 55: 100944. [DOI] [PubMed] [Google Scholar]
- 9. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health 2020; 10: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol 2008; 585: 97–108. [DOI] [PubMed] [Google Scholar]
- 11. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zoungas S, Woodward M, Li Q et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014; 57: 2465–74. [DOI] [PubMed] [Google Scholar]
- 13. Nanayakkara N, Ranasinha S, Gadowski A et al. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J Diabetes Complications 2018; 32: 279–90. [DOI] [PubMed] [Google Scholar]
- 14. Zilkens RR, Davis WA, Spilsbury K, Semmens JB, Bruce DG. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am J Epidemiol 2013; 177: 1246–54. [DOI] [PubMed] [Google Scholar]
- 15. Croxson SC, Jagger C. Diabetes and cognitive impairment: a community-based study of elderly subjects. Age Ageing 1995; 24: 421–4. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Tchetgen Tchetgen EJ, Osypuk T et al. Estimating the cognitive effects of prevalent diabetes, recent onset diabetes, and the duration of diabetes among older adults. Dement Geriatr Cogn Disord 2015; 39: 239–49. [DOI] [PubMed] [Google Scholar]
- 17. Barbiellini Amidei C, Fayosse A, Dumurgier J et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA 2021; 325: 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 2009; 58: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts RO, Geda YE, Knopman DS et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol 2008; 65: 1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Velayudhan L, Poppe M, Archer N, Proitsi P, Brown RG, Lovestone S. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br J Psychiatry 2010; 196: 36–40. [DOI] [PubMed] [Google Scholar]
- 21. Cukierman-Yaffe T, Gerstein HC, Williamson JD et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009; 32: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee AK, Rawlings AM, Lee CJ et al. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the atherosclerosis risk in communities (ARIC) cohort study. Diabetologia 2018; 61: 1956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GEHM, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. The Lancet Neurology 2015; 14: 329–40. [DOI] [PubMed] [Google Scholar]
- 24. Chiu W-C, Ho W-C, Liao D-L et al. Progress of diabetic severity and risk of dementia. J Clin Endocrinol Metab 2015; 100: 2899–908. [DOI] [PubMed] [Google Scholar]
- 25. McMillan JM, Mele BS, Hogan DB, Leung AA. Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res Care 2018; 6: e000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McIntosh EC, Nation DA. Importance of treatment status in links between type 2 diabetes and Alzheimer's disease. Diabetes Care 2019; 42: 972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chin-Hsiao T. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis 2019; 10: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017; 89: 1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 2018; 65: 1225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . International statistical classification of diseases and related health problems. Geneva: World Health Organization, 2004. [Google Scholar]
- 31. World Health Organization Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2018. In: Guidelines for ATC classification and DDD assignment 2018. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology, 2017. [Google Scholar]
- 32. Young BA, Lin E, von Korff M et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008; 14: 15–23. [PMC free article] [PubMed] [Google Scholar]
- 33. Chang H-Y, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care 2012; 18: 721–6. [PubMed] [Google Scholar]
- 34. Wicke FS, Glushan A, Schubert I et al. Performance of the adapted diabetes complications severity index translated to ICD-10. Am J Manag Care 2019; 25: e45–9. [PubMed] [Google Scholar]
- 35. Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol 2017; 4: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016; 79: 70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulz M, Goffrier B, Stillfried DV et al. Entwicklung der vertragsärztlichen Leistungsdichte bei Diagnostik und Therapie der Demenz – Update 2010 bis 2014. Berlin, Germany: Zentralinstitut für die kassenärztliche Versorgung in Deutschland, 2017. [Google Scholar]
- 38. Ninomiya T. Diabetes mellitus and dementia. Curr Diab Rep 2014; 14: 487. [DOI] [PubMed] [Google Scholar]
- 39. Robert Koch-Institut . Daten und Fakten: Ergebnisse der Studie "Gesundheit in Deutschland aktuell 2010". Berlin: Robert-Koch-Inst, 2012. [Google Scholar]
- 40. Kulzer B, Albus C, Herpertz S et al. Psychosoziales und diabetes (Teil 1). Diabetologie und Stoffwechsel 2013; 8: 198–242. [Google Scholar]
- 41. Yaffe K, Falvey C, Hamilton N et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012; 69: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elder DA, Hornung LN, Khoury JC, D'Alessio DA. β-Cell function over time in adolescents with new type 2 diabetes and obese adolescents without diabetes. J Adolesc Health 2017; 61: 703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coleman CI, Limone B, Sobieraj DM et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm 2012; 18: 527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016; 10: 1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chapman CD, Schiöth HB, Grillo CA, Benedict C. Intranasal insulin in Alzheimer's disease: food for thought. Neuropharmacology 2018; 136: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hopkins R, Shaver K, Weinstock RS. Management of Adults with diabetes and cognitive problems. Diabetes Spectr 2016; 29: 224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puttanna A, Padinjakara NK. Management of diabetes and dementia. Br J Diabetes 2017; 17: 93. [Google Scholar]
- 49. Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care 1992; 15: 815–9. [DOI] [PubMed] [Google Scholar]
- 50. Karr JE, Graham RB, Hofer SM, Muniz-Terrera G. When does cognitive decline begin? A systematic review of change point studies on accelerated decline in cognitive and neurological outcomes preceding mild cognitive impairment, dementia, and death. Psychol Aging 2018; 33: 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Porta M, Curletto G, Cipullo D et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014; 37: 1668–74. [DOI] [PubMed] [Google Scholar]
- 52. Hernández M, Mauricio D. Latent autoimmune diabetes in adults: a review of clinically relevant issues. Adv Exp Med Biol 2021; 1307: 29–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


