Abstract
A cardiac cavernous hemangioma is a rare, primary, benign tumor that is usually diagnosed in young or middle-aged patients. In this article, we report the case of a 71-year-old male patient whose doctors incidentally discovered a heart tumor on his transthoracic echocardiography. Triple-phase computed tomography (CT) (pre-contrast, arterial and portal venous) missed the lesion, and magnetic resonance imaging (MRI) revealed a small, oval tumor attached to the wall of the right ventricle. The tumor was successfully removed surgically, and the patient recovered after 2 weeks. A histopathological examination resulted in the diagnosis of a benign cavernous hemangioma.
Keywords: Cardiac tumor, Cavernous hemangioma, Echocardiography, Computed tomography (CT), Magnetic resonance imaging (MRI)
Introduction
According to the literature, primary cardiac tumors are uncommon, accounting for about 0.02% at autopsy. When they are still small in size, most of them are asymptomatic and are often discovered incidentally during physical examination. The cardiac primary hemangioma was first described in 1893, and is a rare, benign, primary tumor, accounting for only 2.8% of cardiac tumors. Histologically, hemangiomas form from the abnormal proliferation of excess blood vessels and endothelial cells. Based on pathological features, hemangiomas can be divided into three types: cavernous, capillary, or anastomosing hemangiomas [1]. Histopathological features of cavernomas, which are manifested by abnormally dilated vessels and separated by septa of fibrous stroma, are similar in various parts of the body. Microscopically, the intratumoral vascular channels are lined by endothelial cells, mostly flat cells. There are no features of mitosis and atypical nuclei. Sometimes, the moderately diverse structures of vessels have cytologic atypia forming the focal vascular tufts.
Advances in diagnostic imaging such as transthoracic echocardiography, transesophageal echocardiography, contrast-enhanced ultrasound, cardiac computed tomography (CT), and magnetic resonance imaging (MRI) have provided valuable imaging information, although pathology is still considered the gold standard. Being a benign tumor, a cardiac cavernous hemangioma usually grows more slowly than other malignancies [1]. However, in some cases, due to not being detected early, the tumor grows large or in a place that affects the functioning of the heart; then the tumor can be a cause of fainting, cyanosis or sudden death. Early detection of a heart tumor when it is small in size is not an easy job because the heart is always moving, affecting the image acquisition; and it will be more difficult to diagnose, assess the nature of the tumor, and differentiate it from other types of malignancies; in general, it is important to have an accurate diagnosis for continued monitoring, treatment and prognosis [1]. Here we present a case of cardiac cavernous hemangioma, primary, and small in size, which was incidentally discovered on transthoracic echocardiography.
Case report
The patient was a 71-year-old man, with a history of stomach disease. He came to our hospital with symptoms of dull abdominal pain in the epigastrium, sometimes- sharp pain, spreading to the left chest and back, for about 1 month. He had no history of cough, cyanosis, or syncope. 3-4 days before hospital admission, due to pain, the patient self-administered Nexium at home with a dose of 40mg x 1 tablet/day and Gastropulgite at 3 packs/day before each meal, but the pain symptom did not decrease. At the hospital, the clinician initially diagnosed gastritis, possibly with reflux, and indicated gastroscopy with blood routine tests such as hematology, biochemistry, immunology, coagulation, urinalysis, electrocardiogram (ECG), chest X-ray, and abdominal ultrasound; the results were nothing out of the ordinary. Incidentally, transthoracic echocardiography revealed a small tumor adhered to the right ventricular wall, measuring 1.3 × 1.7 × 1.4 cm, and changing shape with the heart contraction (Fig. 1). Cardiac function was normal; no pericardial effusion was observed. Cardiologists examined the tumor and assumed it was small in size and therefore unrelated to the clinical symptom (pain).
Fig. 1.
Transthoracic echocardiographic images. A, B: 2-chamber views showed an oval isoechoic mass (arrow) attached to the right ventricular lateral wall and changed shape according to the heart contraction. C: 4-chamber view showed the mass protruding into the right ventricular chamber. RV-Right ventricle, LV-Left ventricle, RA-Right atrium, LA-Left atrium.
Indication for a CT scan of the chest and abdomen was made to find out if there were other causes for the pain. A CT scan was performed on an Aquilion one 320-slice CT scanner (Toshiba, Japan) with a tube voltage of 120kV, tube current of 50mA, tube rotation time of 0.35 seconds, FOV (LL) of 400, and slice thickness of 0.5 mm, breath-holding. An 18-gauge needle was inserted into the antecubital vein in order to administer a nonionic contrast agent bolus (Xenetic vial 300 mg/100ml) (Guerbet, France) with a dose of 1.5 ml/kg, at a speed of 5 ml/sec, by a Medrad Stellant Injector (Bayer, USA), followed by a 50 ml saline flush at the same speed. The delayed time for the scanning after the contrast injection was based on the maximum appearance of the contrast agent at the abdominal aorta just above the renal arteries (test bolus), usually 20 and 60 seconds for the arterial and portal venous phases, respectively. However, in both arterial and portal venous phases, no abnormalities were detected (Fig. 2), so the radiographer did not carry out the late phase.
Fig. 2.
Chest CT axial images at the arterial (A1, A2, A3) and portal venous phases (V1, V2, V3) showed no mass in the right ventricular chamber. RV-Right ventricle, LV-Left ventricle, RA-Right atrium, LA-Left atrium.
With the above examination results, the symptom of epigastric pain was explained by the stomach's contraction according to the nervous mechanism, without any physical damage. The patient was prescribed the antispasmodic drug Buscopan 10mg x 4 tablets/day, the sedative Rotunda 30mg x 2 tablets/day before going to bed, resting for a few days, pain symptom relieved. After 1 week, the patient returned to our hospital with the desire to re-assess the heart tumor, so a MRI was indicated.
The cardiac MRI scan was conducted on a 3 Tesla MR Revolution (GE, USA), with gadolinium and following imaging protocol: (1) Routine functional views of the heart including SA, 2CB, 3CB, 4CB, and LVOT using the FIESTA CINE sequence, (2) Cardiac structure signal intensity characterization assessed by 2 sequences: SA, 2CB, 4CB T1W double-IR FSE and SA, 2CB, 4CB T2W triple-IR FSE, (3) First-pass perfusion imaging, using a FGRE time course rest perfusion sequence on SA and 4CB planes, with 0.05–0.1 mmol/kg of gadolinium, (4) Myocardial early enhancement: Performed on SA and 4CB planes, following 0.1–0.2 mmol/kg of gadolinium, within the first 2 minutes of contrast agent administration, (5) Myocardial delayed enhancement: Started acquiring CINE IR - the test TI images on SA and 4CB planes, at 8-10 minutes after contrast agent administration.
After image acquisition, we could not detect the tumor on 2, 3, or 4CB longitudinal axial views, or the longitudinal axial white blood CINE. The tumor was only clearly seen on the SA and 2CB perfusion images when it was enhanced in the early stages and in the late phase. After finding it, we again carefully reviewed the images of all sequences and could also notice the tumor.
The mass was relatively small in size, about 17 × 14 × 13 mm, with regular margins and well-defined boundaries, attached to the right ventricular wall by a narrow leg in the form of a pedicle but did not invade the ventricular wall or adhere to the superior valve leaflet. It was isointensive or slightly hypointensive on T1W, T2W as compared with cardiac muscle, and hyperintensive on STIR images. After gadolinium injection, the mass was enhanced from the early stages of the perfusion sequence and more enhanced in the late phase as compared with a normal myocardium (Figs. 3,4). These images suggested a benign tumor (myxoma, papillary fibroelastoma, hemangioma) that should be followed up.
Fig. 3.
Cardiac magnetic resonance images. T2W: Two-chamber view showed a mass (arrow) which was isointensive as compared with the myocardium and attached to the right ventricular wall (A); STIR: The mass was hyperintensive on the short-axis views, 17 × 14 × 13mm in size (B); Perfusion and LGE: Diffused enhancement at the first-pass perfusion and LGE images (C, D). Ao-Aorta, RV-Right ventricle, LV-Left ventricle, STIR-Short tau inversion recovery, LGE-Late gadolinium enhancement.
Fig. 4.
Images of the perfusion sequence, including plain and the early stage images of the same level showed the contrast agent was gradually and diffusely absorbed into the mass (arrow).
After that, despite being counseled and having the benign nature of the tumor very carefully explained, the patient still wished to have it surgically removed. In surgery, we applied a median sternotomy to approach the mediastinum with the support of a cardiopulmonary bypass (CPB) system. Next, we opened the pericardium and the right atrium; through the tricuspid valve, the tumor was seen adhering to the free wall of the right ventricle and involving the chordae tendineae of the tricuspid valve. The tumor was oval in shape, about 16 × 14 × 15mm in size, with a rather smooth boundary, and a narrow and non-invasive root (Fig. 5).
Fig. 5.
Image of an oval mass, attached to the right ventricle wall, revealed during open heart surgery, measuring 16 × 14 × 15 mm and partially covered by a thin capsule.
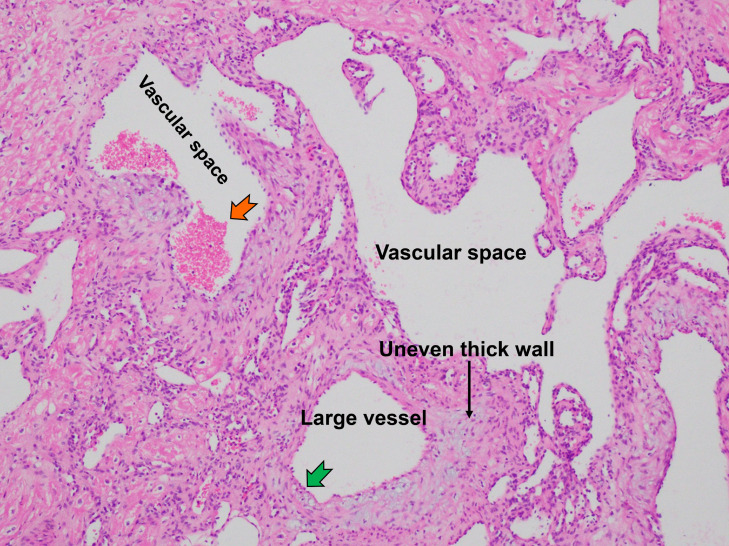
The histopathological images showed the presence of multiple vessels in the fibrous tissue. The tortuous vascular spaces, lined by a layer of flattened endothelium were separated by fibrous septa. Some of the vessel walls were additionally thickened by foci of scattered myxoid matrices (Fig. 6). The histological diagnosis confirmed the cavernous hemangioma.
Fig. 6.
This histological image (HE x 200) showed multiple dilated and tortuous vessels intervening fibrous stroma, the dilated vascular spaces lined with a single layer of flat endothelial cells (green arrow), clusters of red blood cells inside the vascular lumen (orange arrow) (Color version of figure is available online).
The patient has been postoperatively hospitalized for two weeks and recovered quickly.
Discussion
Primary cardiac cavernous hemangiomas are benign and extremely rare tumors, originating from vascular endothelial cells [2]. Kojima et al. [1] studied on 56 patients and found out that the tumors are most commonly located in the ventricles (right - 35%, left - 34%), followed by the right atrium (23%), internal septum (11%), interventricular septum (11%), and the left atrium (7%). Most cardiac hemangiomas have no symptoms. However, they can cause pericardial effusion, asymptomatic murmurs, arrhythmia, hemopericardium or tamponade, complete block bundle branch, or even sudden death.
Echocardiography is often the first choice applied to diagnose cardiac hemangiomas [3], helping to accurately determine the location, size, and number of tumors, even when their diameters are smaller than 1cm, and assess the cardiac valves and pericardial structures; in addition, ultrasound is easy to perform, an inexpensive diagnostic modality, and the patient is not affected by radiation. In fact, our patient had the tumor discovered by chance during transthoracic echocardiography. Furthermore, echocardiography also clearly showed the tumor morphology, the tumor's attachment to the right ventricular wall, and the motion and changes in morphology according to the contraction rhythm of the heart. In order to assess the tumor angiogenesis for this case, the contrast-enhanced ultrasound was also considered, but in our hospital, there are no experienced specialists in this field. Therefore, it was our hope that a chest and abdomen CT scan with contrast would provide more information about the nature of the tumor, the heart wall, blood vessels, lung parenchyma, and abdominal organs. However, the CT showed the opposite result, that is we could not observe the tumor at all in all three phases (pre-contrast, arterial, and portal venous). The reason was thought to be that, in the pre-contrast phase, the tumor was isodense or nearly isodense with blood, so it could not be observed. During the phases with contrast, if we did not voluntarily choose the moment when the contrast agent was fully filled into the right ventricle or the moment when the tumor was maximally enhanced for imaging (late phase), the tumor would not be detected because it was small in size, and often confused with blood and flow; in addition, the spatial resolution and tissue contrast of CT for the soft tissue were inferior to those of MRI.
On MRI, hemangiomas are often seen as a heterogeneous mass with moderate to high signal intensity on T1-weighted images because of the slow flow and diffuse high signal on T2-weighted images. Contrast agent is usually absorbed unevenly, has high signal intensity, and is prolonged [4]. Regardless of the imaging method used, the final diagnosis still depends on the pathological findings. However, it is not always possible to do this with the cardiac tumors, especially for those small in size, with no indication for biopsy or surgery [5]. Therefore, differential diagnosis of benign or malignant cardiac tumors by imaging plays an important role in the assessment, prognosis and management of tumors.
In fact, it is not easy to identify a small tumor on MRI, especially when the tumor is located close to the wall of the right ventricle, which has a thinner wall than that of the left ventricle: it moves much with contraction rhythm of the heart, and is easily confused with papillary muscle and flow signals. The flexible application of different section views, with thin slice thickness and more focus on the tumor, will greatly help in diagnosis. In our patient, when observed on the longitudinal, 2-, 3-, and 4-chamber views and also on the longitudinal white blood CINE, at first no lesions were detected; only on the two-chamber short axis, after contrast injection and application of perfusion sequence we were able to locate the tumor. Then we had to review all images of the remaining sequences and section views to look for the tumor. For an inexperienced doctor, we think it is likely that the tumor can also be missed on MRI.
On MR images combined with ultrasound, we determined this to be a benign tumor, with early enhancement on perfusion sequences, and strong enhancement in the late (equilibrium) phase after 10 minutes, which helped us completely rule out the tumor as a thrombosis, but suggested a papillary fibroelastoma, myxoma or carvenous hemagioma [6]. However, the tumor was attached to the lateral wall of the right ventricle rather than the tricuspid valve, which helped rule out papillary fibroelastoma because this kind of tumor rarely presents independently at the right ventricular wall [7]. Based on the location and heterogeneous enhancement, as well as the late phase of the enhancement, we did not think a myxoma likely. Finally, we found that the diagnosis of cavernous hemangioma was the most suitable choice [8].
In most cases, surgical removal of the tumor is the first choice of treatment, often with good results. However, surgery is not always necessary, especially when the tumor is small or widespread but does not cause symptoms, while performing surgery is quite complicated, and can cause dangerous complications [9].
Conclusion
In summary, a cardiac cavernous hemangioma is a rare, benign tumor; when it is small in size, it usually causes no symptoms. The initial imaging diagnosis is usually based on echocardiography; the detailed diagnosis is based on MRI with gadolinium; the definitive diagnosis is based on the pathological results. CT scanning can also be used, but the lesion must be localized on echocardiography in advance, and then it is necessary to choose an appropriate imaging protocol for contrast injection.
Ethical approval
This study was approved by the institutional review board of the Hospital 108 in Vietnam. Informed consent was obtained from the subject included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
Funding
This study did not receive any funding or financial support.
References
- 1.Kojima S, Sumiyoshi M, Suwa S, Tamura H, Sasaki A, Kojima T, et al. Cardiac hemangioma: a report of two cases and review of the literature. Heart Vessels. 2003;18(3):153–156. doi: 10.1007/s00380-003-0699-7. [DOI] [PubMed] [Google Scholar]
- 2.Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. 2013;61(8):435–447. doi: 10.1007/s11748-013-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng JJ, Chou MM, Lee YH, Ho ES. In utero diagnosis of cardiac hemangioma. Ultrasound Obstet Gynecol. 1999;13(5):363–365. doi: 10.1046/j.1469-0705.1999.13050363.x. [DOI] [PubMed] [Google Scholar]
- 4.Luna A, Ribes R, Caro P, Vida J, Erasmus JJ. Evaluation of cardiac tumors with magnetic resonance imaging. Eur Radiol. 2005;15(7):1446–1455. doi: 10.1007/s00330-004-2603-y. [DOI] [PubMed] [Google Scholar]
- 5.Oshima H, Hara M, Kono T, Shibamoto Y, Mishima A, Akita S. Cardiac hemangioma of the left atrial appendage: CT and MR findings. J Thorac Imaging. 2003;18(3):204–206. doi: 10.1097/00005382-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Perchinsky MJ, Lichtenstein SV, Tyers GF. Primary cardiac tumors: forty years' experience with 71 patients. Cancer. 1997;79(9):1809–1815. doi: 10.1002/(sici)1097-0142(19970501)79:9<1809:aid-cncr25>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Teng P, Xu H, Ma L, Ni Y. Cardiac hemangioma: a comprehensive analysis of 200 cases. Ann Thorac Surg. 2015;99(6):2246–2252. doi: 10.1016/j.athoracsur.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Chen Y, Liu J, Xu L, Li Y, Liu D, Sun Z, Wen Z. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant Imaging Med Surg. 2020;10(1):294–313. doi: 10.21037/qims.2019.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MW, Goo JM, Chung MJ, Park CM, Hyun Ju Lee HJ, Im JG. CT Appearance of cardiac hemangioma: case report. J Korean Radiol Soc. 2003;49(4):281–284. doi: 10.3348/jkrs.2003.49.4.281. [DOI] [Google Scholar]