Abstract
Cutaneous squamous cell carcinoma (CSCC) is the second most common skin malignancy in white-skinned populations. Only a minority of patients (<5%) develop advanced disease, but this is often difficult to treat and characterised by a poor prognosis. Cemiplimab, a fully human IgG4 monoclonal antibody against programmed cell death-1 receptor, is indicated for advanced (i.e. locally advanced or metastatic) CSCC. Although the definition of metastatic CSCC is clear, there is currently no agreed definition of locally advanced CSCC. In recent guidelines, locally advanced CSCC was described as non-metastatic CSCC that is unlikely to be cured with surgery, radiotherapy or combination treatment. A multi-disciplinary advisory group of Italian CSCC experts was convened to develop criteria to assist in identifying appropriate candidates for cemiplimab therapy in advanced CSCC, based on the literature and clinical experience. In locally advanced CSCC, absolute, or mandatory, criteria for the use of cemiplimab are deep invasion, multiple lesions without defined margins, inadequate surgical excision margins and multiple recurrences, whereas relative criteria include large lesions, in critical or functionally significant areas and that are surgically complex. In addition, physicians should consider patient willingness/preferences (an absolute criterion), and their age and health status/comorbidities (relative criteria). It is hoped that these proposed absolute and relative criteria will help guide rational identification of patients who will receive maximum benefit from immunotherapy, while more clinical data accumulate.
Keywords: cemiplimab, cutaneous squamous cell carcinoma, immunotherapy, skin malignancy
Introduction
Cutaneous squamous cell carcinoma (CSCC) is the second most common skin malignancy in white-skinned populations, in whom its incidence is increasing globally. 1 It is also increasingly recognised in people of African-American ancestry where it may manifest in inflamed areas or in sites with minimal sun exposure. 2
When treated early, CSCC has a good prognosis, with a 5-year cure rate of > 90% after surgery. 1 However, while not common, CSCC can develop to an advanced stage when the initial tumour is inadequately treated or is at a high risk of recurrence. 1 Advanced CSCC is a disease that is either locally advanced (more frequent) or metastatic (in < 5% of patients).1,3–7 Metastatic CSCC is defined by the presence of metastasis, either distant or locoregional (in transit to or within regional lymph nodes). While the definition of metastatic CSCC is clear, 8 there is currently no agreed definition of locally advanced CSCC. In recent guidelines, locally advanced CSCC was described as non-metastatic CSCC that is unlikely to be cured with surgery, radiotherapy or combination treatment.1,7
Once at an advanced stage, many patients are considered inoperable given the location/invasive nature/recurrence of their lesion(s). Advanced CSCC, which typically affects elderly or immunosuppressed individuals, is thus often difficult to treat and is characterised by a poor prognosis.1,3 The UK National Cancer Registration and Analysis Service has recently estimated the risk of metastasis in CSCC and reported that advanced age, male gender, immunosuppression, deprivation, and ear/lip location of CSCC were associated with increased metastatic risk. 9
At present, four main systems for classifying and staging CSCC are described in the literature, but none is universally accepted. 2 The system developed by the American Joint Committee on Cancer (AJCC) 10 is limited to CSCC of the head and neck, and addresses nodal involvement as well as regional and distant metastases. The Union for International Cancer Control (UICC) system is similar to the AJCC system, but also includes CSCC of the trunk and limbs. 11 The risk factor–based system developed at Brigham and Women’s Hospital (BWH) 12 classifies primary tumours only, not locoregional or metastatic disease; however, it has been validated retrospectively and offers better prognostication. 2 Consequently, many dermato-oncologists and oncologists consider it to be the best system in current use. The fourth system, developed by the US National Comprehensive Cancer Network (NCCN), defines low-risk, high-risk and very high–risk disease, and is intended to guide treatment decision-making rather than provide prognostic information. 2
The rationale for the use of immunotherapy in CSCC comes from well-established concepts and a substantial body of evidence. Studies performed in immunosuppressed patients, in particular organ transplant recipients, have considerably contributed to our knowledge of the role of the immune system in driving CSCC carcinogenesis. 13 Supporting the rationale of an immunotherapeutic approach is also the fact that CSCC, along with other skin cancers caused by UV radiation, carries a high rate of somatic mutations leading to mutated proteins that can be targeted by the immune system as neoantigens. 14 Immune checkpoints, including the programmed cell death-1 (PD-1) receptor and its principal ligand PD-L1, have been found to be active in a variety of tumours.15,16 PD-L1 expression has been detected in around 26% of primary CSCC and up to 50% of metastatic CSCC. 1 Furthermore, the expression of PD-1 receptor and the PD-L1 ligand appears to be increased in CSCC lesions compared to healthy skin. 17 Cemiplimab (Libtayo®, Regeneron Pharmaceuticals/Sanofi) is a fully human IgG4 monoclonal antibody to the PD-1 receptor approved by the US Food and Drug Administration (FDA) in September 2018, and by the European Medicines Agency (EMA) in June 2019, ‘for the treatment of adult patients with metastatic or locally advanced cutaneous squamous cell carcinoma who are not candidates for curative surgery or curative radiation’.18,19 Cemiplimab was the first drug to be granted approval for the systemic treatment of CSCC and is recommended by the 2020 European interdisciplinary guidelines (issued by the EDF, EADO and EORTC) as first-line treatment for patients with advanced disease who cannot be treated with curative surgery or RT (grade of recommendation A; level of evidence 2). 20 Furthermore, the UK National Institute of Health and Care Excellence has recommended cemiplimab for use as an option for treating locally advanced or metastatic CSCC when curative surgery or curative RT is not appropriate, 21 which is the recommendation made also by the NCCN. 22
Given the subjective nature of the FDA and EMA approved indication for cemiplimab, it is relevant to define the clinical and patient characteristics that would identify appropriate candidates for cemiplimab therapy. To this end, a multi-disciplinary panel of experts in the management of advanced CSCC in Italy was identified and two meetings were convened. The current article describes the methodology and the outcomes of these meetings and the eligibility criteria defined by these experts for cancer immunotherapy with cemiplimab, along with the place of this agent in the current treatment paradigm. Although other immunotherapies have been studied in the treatment of CSCC, the scope of our discussion was limited to cemiplimab.
Expert panel
The advisory group consisted of 10 Italian medical practitioners (4 dermatologists, 2 oncologists, 3 dermato-surgeons, and 1 radiation oncologist) with extensive experience in managing patients with advanced CSCC. Advisory group members were selected by Sanofi in collaboration with the coordinator (G.A.) based on their proven expertise in dermato-oncology.
The first meeting was held in Rome on December 10, 2019, and the second meeting was conducted via videoconference on March 23, 2020. At each meeting, the advisory group discussed clinical cases considered to be candidates for immunotherapy, and the clinical features that made them appropriate for such treatment. The criteria for optimal use of such therapy were formalised as a result of these multidisciplinary clinical discussions. All patients presented in this article provided written informed consent for their details to be published, including their photographs.
Clinical use of cemiplimab
As described earlier, cemiplimab is indicated for advanced CSCC. Unfortunately, there is currently no agreed definition of advanced CSCC, which includes locally advanced and metastatic disease. 23 While metastatic CSCC is easily defined as Stage III, IVA or IVB tumours on the tumour-node-metastasis (TNM) staging system, 24 the definition of locally advanced CSCC is less clear. Typically, the term ‘locally advanced CSCC’ is applied to a tumour that is no longer amenable to curative surgery or RT. 23 A locally advanced CSCC may therefore meet TNM criteria for stage I to III, as long as it is inoperable and there are no nodal or distant metastases. 23 Clinical trials have used a definition for advanced CSCC in which curative surgery is not possible or where surgery would result in significant morbidity or deformity, or the tumour has recurred multiple times after surgery and is not suitable for further surgical intervention.14,25
Advanced CSCC treatment includes several therapeutic options and a multidisciplinary approach is required.2,7,20 The first choice for patients with primary CSCC who are not eligible for curative surgery is usually RT. RT can be administered as external beam radiotherapy (photons or electrons) with different delivery techniques (3D-conformational, static or volumetric modulated intensity) or as brachytherapy. Different doses and fractions can be chosen to achieve disease control. Contraindications to RT may be related to patient (connective tissue disease, comorbidity), disease (localisation, previous irradiation) and treatment (inability to maintain the treatment position, not satisfactory treatment plan) characteristics. 26
Systemic treatments include immunotherapy, chemotherapy (platinum-based), and epidermal growth factor receptor (EGFR) inhibitors (cetuximab). 20 Cemiplimab is currently indicated as monotherapy. 18 Platinum-based chemotherapy is still used as systemic therapy for CSCC, even though the evidence to support it comes from small, retrospective studies rather than prospective clinical trials. 27 With the advent of immunotherapy, platinum-based therapy is no longer considered the standard of care; additionally, adverse events may limit its use in many older people, who constitute the largest proportion of CSCC patients. 28 The use of cetuximab in CSCC is supported by the results of a prospective phase II trial 29 and a real-world study. 28 In addition, it is generally well-tolerated, which is an important consideration in a predominantly older patient population. However, its effects may be short-lived. 30 Overall, the results achieved with platinum-based therapy or with cetuximab, in terms of duration of response and survival, are poor.27,29,30
The anti-PD-1 monoclonal antibody cemiplimab is the first systemic therapy to have been evaluated in well-designed, prospective trials involving patients with advanced CSCC (ClinicalTrials.gov numbers, NCT02383212 (phase 1, duration of treatment of up to 48 weeks, n = 26) and NCT02760498 (phase 2, duration of treatment of up to 96 weeks, n = 78)).31,32 Patients were eligible for the studies if they were not candidates for surgery; if curative resection was considered unlikely or surgery was anticipated to result in substantial complications or deformity (phase 1 study); 14 or because of substantial local invasion that precluded complete resection; CSCC that was technically amenable to surgery, but clinically inappropriate because the lesion was in an anatomically challenging location for which surgery may result in severe disfigurement or dysfunction; CSCC that was technically amenable to surgery, but clinically inappropriate because the lesion was in the same location as two or more surgical procedures and curative resection was considered unlikely; or other conditions that contraindicated surgery (phase 2 study). 25 In these trials, at median follow-up of approximately 9–11 months, cemiplimab was associated with response rates as assessed by independent central review of 44%–50%, with a median time to response of approximately 2 months. Further, a duration of response that exceeded 6 months was seen in 54%–68% of patients, and durable disease control in 63%–65%.14,25
Longer-term follow-up from the phase 2 study demonstrated an objective response rate of 46.1% and a complete response rate of 16.1% at a median duration of follow-up of 15.7 months (n = 193). 33 Median time to first tumour response was 2.1 months and to complete response was 11.2 months. In patients who responded to treatment, the estimated proportion of patients with an ongoing response at 24 months was 69.4%. Estimated median progression-free survival was 18.4 months for all patients. Median overall survival had not been reached, but estimated probability of overall survival at 2 years was 73.3%.
How to identify potential responders to immunotherapy among CSCC patients is currently the subject of intense research.34–36 To address this issue in relation to cemiplimab specifically, the phase 2 trial also analysed the association between potential biomarkers (tumour mutational burden (TMB) and PD-L1 levels assessed by immunohistochemistry) and the clinical activity of cemiplimab.25,37 Although median TMB was found to be higher in responders versus non-responders,25,37 low values can be found in responders and, conversely, high values in non-responders, suggesting that TMB may have limited predictive value in clinical practice. A post hoc analysis evaluated the response rates in subgroups of patients according to the reasons why they were not considered eligible for conventional treatment with surgery or RT. 25 Patients who had received two or more surgeries were less likely to respond than those who had undergone none or one surgery: objective response was seen in 24% of patients who had received two or more surgeries versus 50% in patients with none or one surgery.
In the interim analysis of the phase 2 study, the most common treatment-emergent adverse events (TEAEs) were fatigue (42% of patients), diarrhoea (27%), pruritus (27%), nausea (22%), and cough (19%), similarly to others immune checkpoint inhibitors. The vast majority of the TEAEs (99%) were National Cancer Institute Common Terminology Criteria for Adverse Events grades 1–2. No new safety issues were reported despite the advanced age of the study cohorts. The discontinuation rate for TEAEs was 8%. 25 Similarly, the 3-year results of the study reported the most common TEAE to be fatigue (35% of patients), diarrhoea (28%), nausea (24%) and pruritus (22%). 38
Proposed disease and clinical features for identification of patients with locally advanced CSCC
As noted previously, the definition of metastatic CSCC is not disputed. We therefore limited our discussion to locally advanced disease, because there is an urgent need to improve the selection of patients with locally advanced CSCC who are likely to benefit from treatment with systemic cancer immunotherapy. After an extensive literature review and analysis, the advisory group defined the features of the lesion and the patient’s characteristics that could be considered to address the course of treatment for locally advanced CSCC. Lesion-related factors are the size of the lesion, invasiveness, the number of lesions (i.e. multiple primary CSCC lesions in a cancerisation field), lesion margins (defined or not), possibility of achieving adequate excision margins, anatomically critical/functionally significant location, and previous relapse. Relevant patient characteristics are patient age, health status/comorbidities, patient willingness/preferences, and the level of surgical complexity. The group has defined absolute and relative criteria for the use of cemiplimab (Table 1). An absolute criterion is one that, when present, makes that patient a candidate for cemiplimab. Relative criteria are not by themselves an indication to use cemiplimab, but when present, can tip the balance in favour of using cemiplimab treatment.
Table 1.
Proposed absolute and relative criteria for the identification of patients with locally advanced cutaneous squamous cell carcinoma eligible for systemic therapy with cemiplimab.
| Absolute criteria | Relative criteria |
|---|---|
| ● Deep invasion ● Multiple/agminated lesions without defined margins ● Inadequate excision margins ● Multiple recurrences ● Patient willingness |
Large size ● Critical or functionally significant areas ● Surgical complexity (large size, site of lesion) ● Patient’s health status/comorbidities a ● Patient age a |
On occasion, patients may not be eligible for surgery because of comorbidities or very advanced age. These patients may be candidates for immunotherapy with cemiplimab.
Lesion-related features that suggest cemiplimab is the preferred option are a large lesion, deep invasion, multiple lesions, lesions without defined margins, lesions that will not allow an adequate excision margin to be achieved, lesions in critical or functionally significant areas, and lesions at the site of previous relapse or recurrence. Of these, the board considers the following to be absolute criteria for the use of cemiplimab: deep invasion, multiple/agminated lesions without defined margins, lesions that will not allow an adequate excision margin to be achieved, and multiple recurrences (Table 1). A large lesion or one in a critical or functionally significant area is a relative criterion.
Patient willingness is considered an absolute criterion for cemiplimab treatment. Other patient characteristics (age, health status/comorbidities, surgical complexity) are relative criteria because they may tip the balance in favour of cemiplimab based on surgical/anaesthetic risks (Table 1). A collaborative approach is recommended, because input from a multidisciplinary panel of specialists should be obtained. This is important partly because of the subjective nature of some of the criteria, but also because it harnesses the expertise and skills of surgeons from different specialities (otorhinolaryngologists, maxillofacial surgeons, and general surgeons), as well as radiotherapists and oncologists, to enhance decision-making and, ultimately, improve the quality of patient care.
Illustrative examples
Table 2 provides illustrative examples of patients who are candidates for cemiplimab based on the current criteria.
Table 2.
Examples of patients diagnosed with locally advanced cutaneous squamous cell carcinoma eligible for systemic therapy with cemiplimab.
| CT case | Presentation | Demographics | Criteria for cemiplimab |
|---|---|---|---|
| 1. |
|
Male 80 years old |
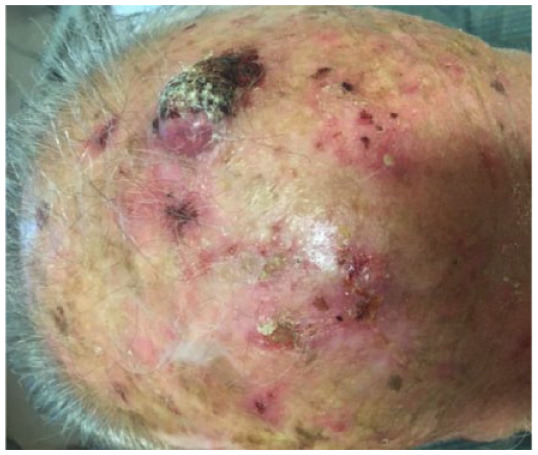
Absolute: ● Multiple lesions with no defined margins |
| 2. |
|
Male 82 years old |
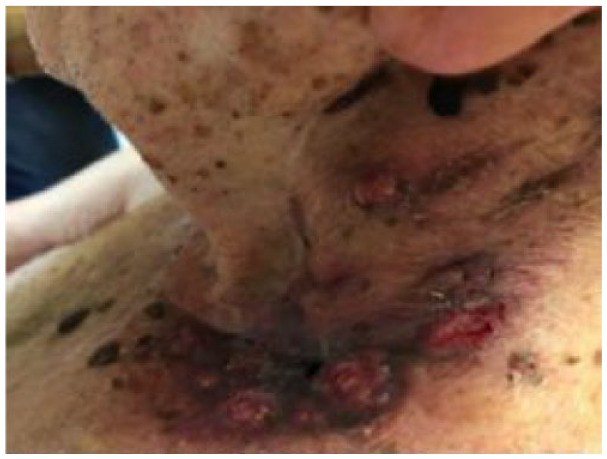
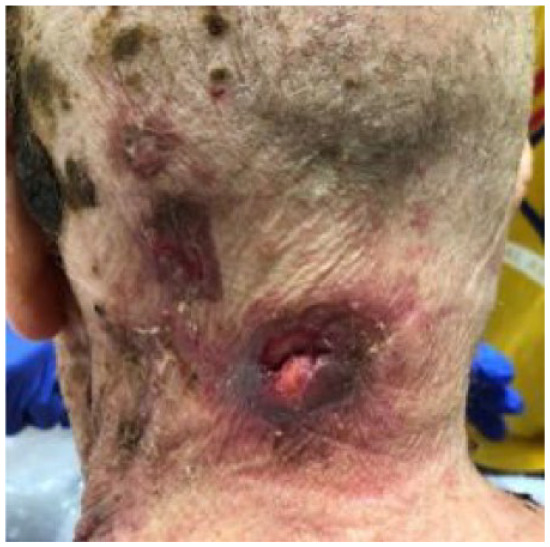
Absolute: ● Multiple, agminated lesions with no defined margins ● Multiple recurrences |
| 3. |
|
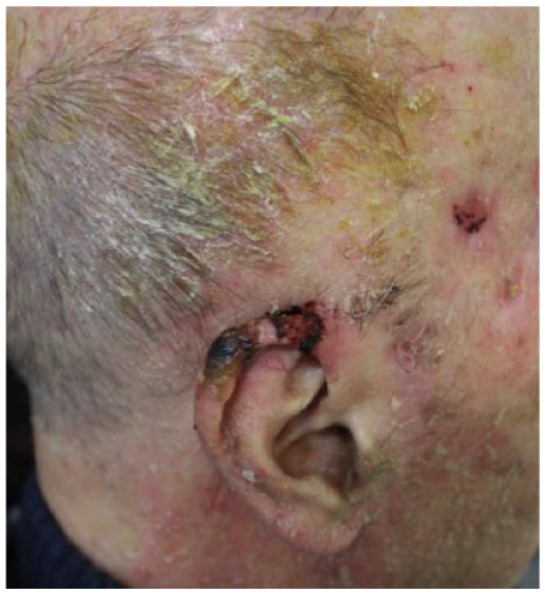
Male 79 years old |
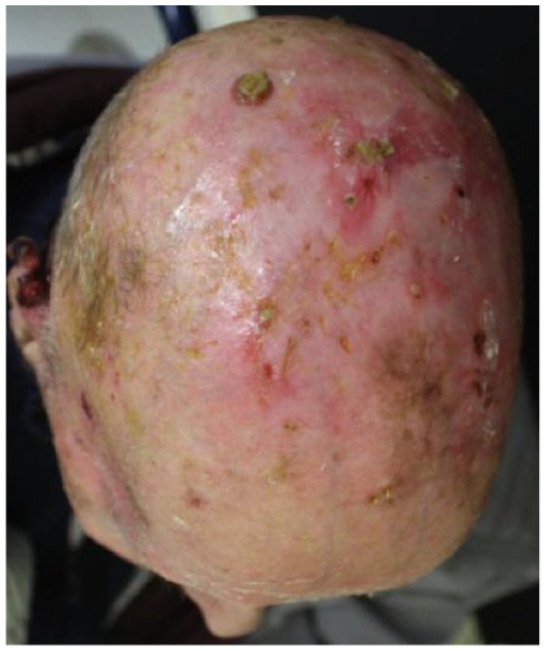
Absolute: ● Multiple lesions with no defined margins ● Relative: ● Comorbidities (Parkinson’s disease, diabetes, hypertension) ● Older age |
| 4. |
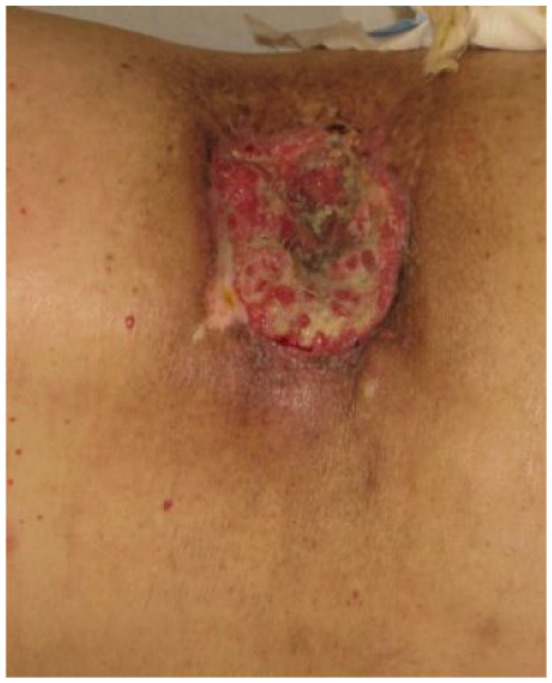
|
Female 70 years old |
Absolute: ● Multiple recurrences |
| 5. |
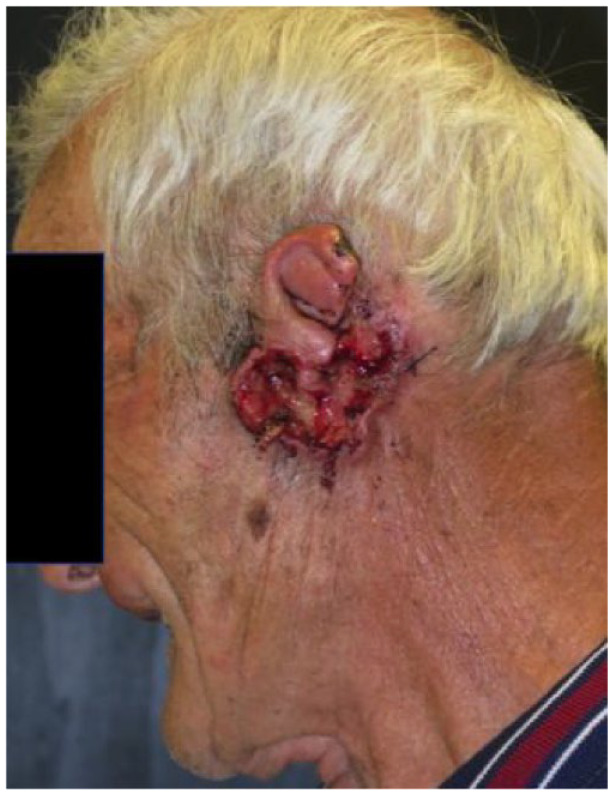
|
Male 82 years old |
Absolute: ● Multiple recurrences ● Deep invasion ● Relative: ● Surgical complexity |
| 6. |
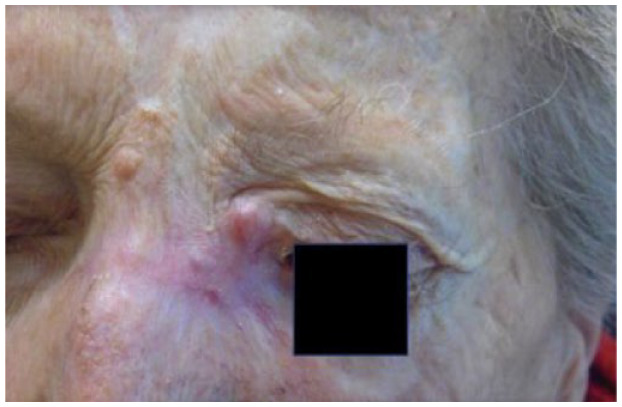
|
Female 78 years old |
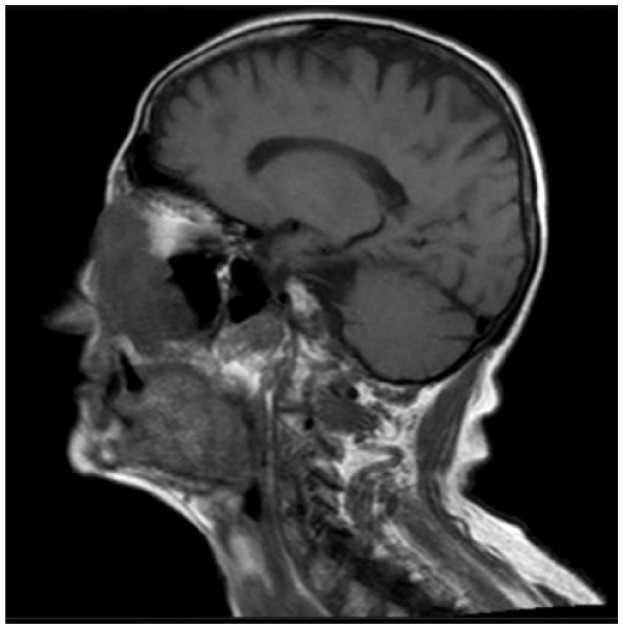
Absolute: ● Deep invasion (as demonstrated by MRI) ● Recurrence ● Relative: ● Age ● Critical or functionally significant area |
| 7. |
|
Male 74 years old |
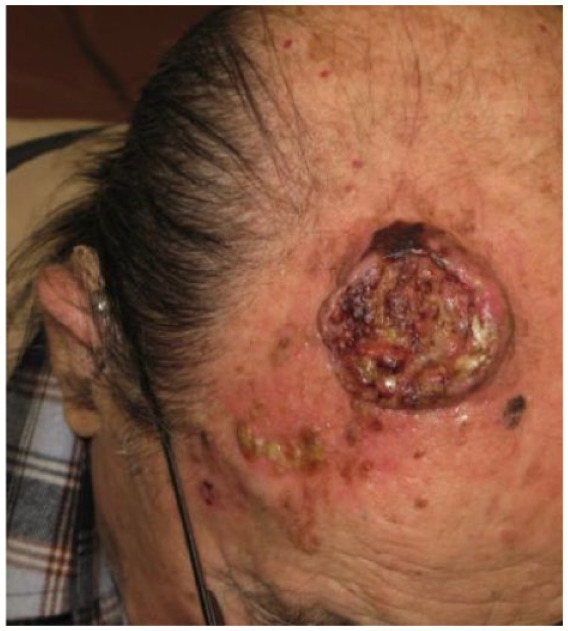
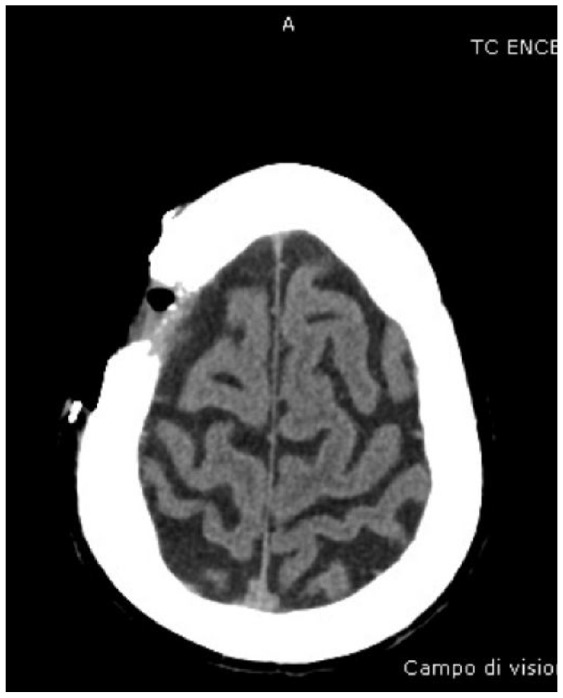
Absolute: ● Deep invasion (as demonstrated by CT scans) |
| 8. |
|
Female 87 years old |
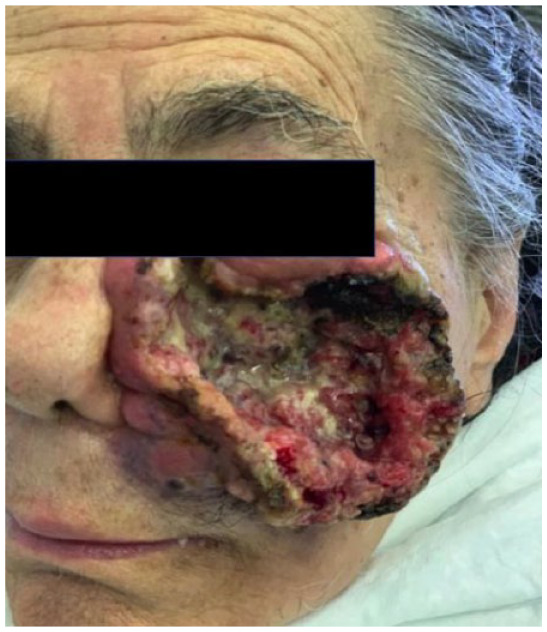
Absolute: ● Deep invasion ● Relative: ● Surgical complexity |
| 9. |
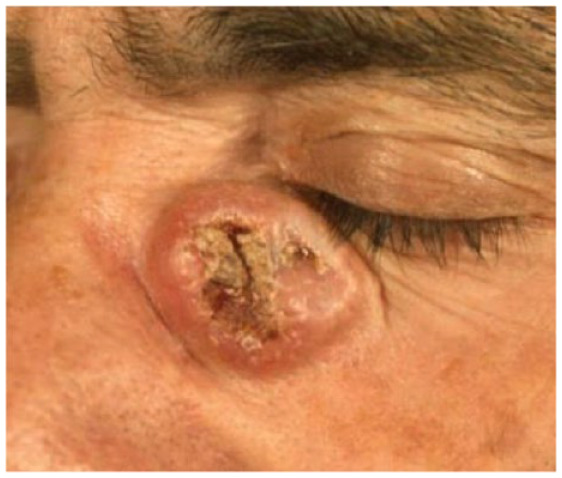
|
Male 40 years old |
Absolute: ● Lack of adequate excision margin ● Relative: ● Large size ● Critical site |
CT, computerised tomography; MRI, magnetic resonance imaging.
Patients 1, 2, and 3 are examples of patients with multiple lesions without defined margins, an absolute criterion. Similarly, the lesions of patients 2, 4 and 5 represent absolute criteria for cemiplimab, since they are multiple recurrences at the same site.
Patient 6 has a single recurrence, which is not an absolute criterion, but it is in a critical site, a relative criterion. However, patient 6 has deep invasion apparent on magnetic resonance imaging (MRI), and deep invasion is an absolute criterion. Patient 6’s CSCC occupies part of the left ethmoidal sinus and the entire nasal cavity on the left side, as well as the ipsilateral maxilla, and has infiltrated the orbital fat on the left, displacing the eyeball. The criterion of deep invasion is also illustrated by patients 5, 7 and 8; the choice of cemiplimab is reinforced by the complexity of surgical resection in patients 5 and 8.
Patient 9 shows a large tumour in a critical site, which presents surgical challenges because of the lack of optimal surgical margins. This patient was young (~40 years old), so age and health status were not considerations. One the lesion’s features is an absolute criterion for cemiplimab (lack of an adequate surgical margin), and together with the presence of two relative criteria (large size, critical site), this suggests a patient who may benefit, particularly since surgery would be complex and put the patient’s vision at risk.
Patient factors that may increase the risk of surgery are relative criteria for cemiplimab. Among these cases, patients 1, 2, 3, 5, 6, 7 and 8 were all elderly, and patient 3 also had significant comorbidities including Parkinson’s disease, diabetes and arterial hypertension.
Discussion
CSCC is a common skin malignancy, the incidence of which is increasing globally. 1 As a result, adequate assessment and treatment are imperative, particularly for those patients with advanced disease. However, risk assessment and identification of prognostic factors have proven difficult in CSCC due to the lack of data correlating potential prognostic factors to outcomes.3,39 The 2020 European interdisciplinary guidelines (issued by the EDF, EADO and EORTC) propose a list of ‘indicative prognostic high-risk factors for recurrence’ including (1) clinical features (tumour diameter, location, symptomatic perineural invasion); (2) histological features (thickness or deep invasion, poor differentiation, desmoplasia, perineural invasion (PNI)); (3) radiological features (radiological PNI, bone erosion); and (4) immunosuppression. 1 One study found that, in descending order of relative risk, tumour diameter, location on the temple, and poor differentiation were associated with local recurrence, while tumour diameter > 20 mm, poor differentiation, PNI, location on the temple, ear or lip, and immunosuppression were associated with metastasis. 39 This study also found that the precise nature of immunosuppression (i.e. whether caused by HIV, chronic lymphocytic leukaemia or prevention of organ transplant rejection) is needed to accurately predict metastasis. 39
In a study of real-world treatment patterns, conducted before immune checkpoint inhibitors were available, 59% of patients with locally advanced CSCC did not receive any therapy. 40 Clinicians were often reluctant to administer systemic therapies to patients with locally advanced CSCC, who are usually older and affected by multiple comorbidities, because of fear of adverse events, short duration of response, and likely also because of a lack of certainty regarding therapeutic benefit. 40 Furthermore, advanced CSCC is rarely treated in a medical oncology or dermato-oncology setting, meaning that patients are often not optimally treated or followed up.
Cemiplimab was the first systemic therapy approved specifically for the treatment of advanced CSCC. 41 The most recent (3-year) data from the phase 2 study have demonstrated that cemiplimab is associated with an objective response rate of approximately 46% and an estimated probability of overall survival at 2 years of approximately 73%. 38 However, there are as yet no predictive biomarkers that will help clinicians to select the most appropriate patients for treatment, 41 so guidance is needed to select the patients most likely to benefit. Our recommendations expand on the EMA/FDA indications for cemiplimab, by providing more detail to help clinicians identify suitable candidates for such treatment. It should be noted, however, that we recommend undertaking an in-depth discussion with the patient on the potential risks and benefits of all available treatment options before a decision is made. This is particularly important if surgery is potentially curative but likely to result in unacceptable complications, morbidity or disfigurement, especially if the patient is young. Furthermore, as previously stated, it is important to obtain the input of multidisciplinary team due to the subjective nature of some of the absolute eligibility criteria.
Importantly, cemiplimab is undergoing clinical trials in the adjuvant (NCT03969004), neoadjuvant (NCT03916627; NCT04315701), and combined adjuvant/neoadjuvant (NCT4428671; NCT04632433) settings in patients with resectable or potentially resectable CSCC, and the results of these trials are awaited with interest. Preliminary data on its presurgical use have been promising: in a single-centre, phase 2 trial of neoadjuvant cemiplimab in 20 patients with stage III or IV head and neck CSCC (NCT03565783), 42 the overall response rate was 30% and the pathological complete response rate was 55%.
Conclusions
This advisory group proposes to select patients with locally advanced CSCC for immunotherapy with cemiplimab according to the following features: large lesion, deep invasion, multiple lesions without defined margins, lesions that will not allow an adequate excision margin, lesions in critical or functionally significant areas, and lesions at the site of previous relapse or recurrence. Patient age, health status/comorbidities and the complexity of performing surgery should also be taken into consideration. Further research is needed to define the optimal candidates for cemiplimab treatment, but until this work is completed, the adoption of absolute and relative criteria as outlined in these recommendations provides a rational basis for selecting which patients should receive this novel therapy.
Acknowledgments
We would like to thank Marion Barnett who wrote the first draft of the manuscript on behalf of Springer Healthcare Communications, and Kate Palmer of Springer Healthcare Communications who provided additional medical writing assistance and manuscript styling prior to submission. This medical writing assistance was funded by Sanofi-Genzyme.
Footnotes
Author contributions: All authors participated in the advisory group meeting. Giuseppe Argenziano designed the advisory board path. Giuseppe Argenziano and Maria Concetta Fargnoli prepared the first draft of the manuscript and made subsequent revisions. All other authors contributed substantial revisions to the first draft of the manuscript. All authors approved the final draft.
Giuseppe Argenziano: Conceptualization; Project administration; Writing-original draft; Writing-review & editing
Maria Fargnoli: Conceptualization; Project administration; Writing-original draft; Writing-review & editing
Fabrizio Fantini: Writing-review & editing
Massimo Gattoni: Writing-review & editing
Giulio Gualdi: Writing-review & editing
Francesco Pastore: Writing-review & editing
Giovanni Pellicani: Writing-review & editing
Pietro Quaglino: Writing-review & editing
Paola queirolo: Writing-review & editing
Teresa Troiani: Writing-review & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Giuseppe Argenziano, Maria Concetta Fargnoli, Fabrizio Fantini, Massimo Gattoni, Giulio Gualdi, Giovanni Pellacani, Pietro Quaglino, Paola Queirolo and Teresa Troiani received a fee for their attendance and contribution to the advisory board meetings. Giuseppe Argenziano has no conflict of interest. Maria Concetta Fargnoli has served on advisory boards and received honoraria for lectures and research grants from Mylan, Medac Pharma, Pierre Fabre, Sanofi-Genzyme, Sunpharma and Merck Sharp & Dohme (MSD). Fabrizio Fantini, Massimo Gattoni, Giulio Gualdi and Francesco Pastore have no conflicts of interest. Pietro Quaglino has received speaker fees and contributed to advisory boards for Sanofi, Sun-Pharma, Roche, Igea, BMS, Novartis, MSD and Pierre Fabre. Paola Queirolo has received speaker fees and contributed to advisory boards for Roche, Novartis, BMS, MSD, Merck, Sanofi, SunPharma and Pierre Fabre. Teresa Troiani has served on advisory boards from Novartis, BMS Roche, Servier and Amgen Bayer.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sanofi Genzyme Italy organised and funded the two advisory boards, and funded the development of this manuscript. None of the authors received financial support for the writing of the article.
Consent for publication: All patients presented in this article provided written informed consent for their details to be published, including their photographs.
Contributor Information
Giuseppe Argenziano, Dermatology Unit, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Maria Concetta Fargnoli, Dermatology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, Via Vetoio, Coppito 2, 67100 L’Aquila, Italy.
Fabrizio Fantini, Dermatology Unit, ‘A. Manzoni’ Hospital, Lecco, Italy.
Massimo Gattoni, Dermatologia, Ospedale S. Andrea, Vercelli, Italy.
Giulio Gualdi, Dermatologic Clinic, Department of Medicine and Aging Science, University G. D’Annunzio Chieti-Pescara, Italy.
Francesco Pastore, Radiation Oncology, Emicenter, Naples, Italy.
Giovanni Pellacani, Dermatology Clinic, La Sapienza, University of Rome, Rome, Italy.
Pietro Quaglino, Clinica Dermatologica, AOU Città della Salute e della Scienza, Università degli Studi di Torino, Torino, Italy.
Paola Queirolo, Melanoma, Sarcoma and Rare Tumors Oncology Department, IEO, European Institute of Oncology IRCCS, Milan, Italy.
Teresa Troiani, Oncology Unit, Department of Precision Medicine, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
References
- 1. Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 1. epidemiology, diagnostics and prevention. Eur J Cancer 2020; 128: 60–82. [DOI] [PubMed] [Google Scholar]
- 2. Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol 2018; 78: 560–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amaral T, Osewold M, Presser D, et al. Advanced cutaneous squamous cell carcinoma: real world data of patient profiles and treatment patterns. J Eur Acad Dermatol Venereol 2019; 33(Suppl. 8): 44–51. [DOI] [PubMed] [Google Scholar]
- 4. Nelson TG, Ashton RE. Low incidence of metastasis and recurrence from cutaneous squamous cell carcinoma found in a UK population: do we need to adjust our thinking on this rare but potentially fatal event? J Surg Oncol 2017; 116: 783–788. [DOI] [PubMed] [Google Scholar]
- 5. Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008; 9: 713–720. [DOI] [PubMed] [Google Scholar]
- 6. Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 2013; 149: 541–547. [DOI] [PubMed] [Google Scholar]
- 7. Peris K, Alaibac M, Argenziano G, et al. Cutaneous squamous cell carcinoma. Italian guidelines by SIDeMaST adapted to and updating EADO/EDF/EORTC guidelines. G Ital Dermatol Venereol 2018; 153: 747–762. [DOI] [PubMed] [Google Scholar]
- 8. Brancaccio G, Fargnoli MC, Briatico G, et al. Risk factors and diagnosis of advanced cutaneous squamous cell carcinoma. Dermatol Pract Concept 2021; 11: e2021166S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Venables ZC, Autier P, Nijsten T, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol 2019; 155: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. Cham: Springer, 2017. [Google Scholar]
- 11. Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken, NJ: Wiley-Blackwell, 2017. [Google Scholar]
- 12. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol 2013; 149: 402–410. [DOI] [PubMed] [Google Scholar]
- 13. Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci 2019; 20: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341–351. [DOI] [PubMed] [Google Scholar]
- 15. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevenson ML, Wang CQ, Abikhair M, et al. Expression of programmed cell death ligand in cutaneous squamous cell carcinoma and treatment of locally advanced disease with pembrolizumab. JAMA Dermatol 2017; 153: 299–303. [DOI] [PubMed] [Google Scholar]
- 18. European Medicines Agency. Libtayo (cemiplimab): EPAR – product information, https://www.ema.europa.eu/en/medicines/human/EPAR/libtayo#product-information-section (2019, accessed 27 July 2020).
- 19. US Food and Drug Administration. Libtayo (cemiplimab-rwlc) injection, for intravenous use: highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf (2018, accessed 27 July 2020).
- 20. Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 2. Eur J Cancer 2020; 128: 83–102. [DOI] [PubMed] [Google Scholar]
- 21. National Institute of Health and Care Excellence. Cemiplimab for treating metastatic or locally advanced cutaneous squamous cell carcinoma. Technology appraisal guidance [TA592], https://www.nice.org.uk/guidance/TA592 (2019, accessed 11 August 2020).
- 22. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): squamous cell skin cancer, version 2.2021, https://www.nccn.org (2021, accessed 17 February 2021).
- 23. Soura E, Gagari E, Stratigos A. Advanced cutaneous squamous cell carcinoma: how is it defined and what new therapeutic approaches are available? Curr Opin Oncol 2019; 31: 461–468. [DOI] [PubMed] [Google Scholar]
- 24. Skin tumours. In: Brierley JD, Gospodarowicz MK, Eittekind C. (eds) TNM classification of malignant tumours. 8th ed. Hoboken, NJ: John Wiley & Sons Inc., 2017, pp. 131–149. [Google Scholar]
- 25. Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020; 21: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): squamous cell skin cancer NCCN evidence blocks, version 2.2020, https://www.nccn.org/professionals/physician_gls/pdf/squamous_blocks.pdf (2020, accessed 21 October 2020).
- 27. Jarkowski A, 3rd, Hare R, Loud P, et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): the Roswell Park experience and a review of the literature. Am J Clin Oncol 2016; 39: 545–548. [DOI] [PubMed] [Google Scholar]
- 28. Montaudié H, Viotti J, Combemale P, et al. Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: a retrospective, multicentre study. Oncotarget 2020; 11: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol 2011; 29: 3419–3426. [DOI] [PubMed] [Google Scholar]
- 30. Dereure O, Missan H, Girard C, et al. Efficacy and tolerance of cetuximab alone or combine with chemotherapy in locally advanced or metastatic cutaneous squamous cell carcinoma: an open study of 14 patients. Dermatology 2016; 232: 721–730. [DOI] [PubMed] [Google Scholar]
- 31. Regeneron Pharmaceuticals. A first-in-human study of repeat dosing with REGN2810, a monoclonal, fully human antibody to programmed death-1 (PD-1), as single therapy and in combination with other anti-cancer therapies in patients with advanced malignancies (ClinicalTrials.gov record: NCT02383212), https://clinicaltrials.gov/ct2/show/NCT02383212 (2015, accessed 27 July 2020).
- 32. Regeneron Pharmaceuticals. A phase 2 study of REGN2810, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with advanced cutaneous squamous cell carcinoma (ClinicalTrial.gov record: NCT02760498), https://clinicaltrials.gov/ct2/show/NCT02760498 (2016, accessed 27 July 2020).
- 33. Rischin D, Khushalani NI, Schmults CD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer 2021; 9: e002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Büttner R, Longshore JW, López-Ríos F, et al. Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open 2019; 4: e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16: 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rischin D, Migden MR, Lim AM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer 2020; 8: e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rischin D, Khushalani NI, Schmults CD, et al. Phase 2 study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (CSCC): longer follow-up. J Clin Oncol 2020; 38: 10018. [Google Scholar]
- 39. Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol 2016; 152: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hillen U, Leiter U, Haase S, et al. Advanced cutaneous squamous cell carcinoma: a retrospective analysis of patient profiles and treatment patterns – results of a non-interventional study of the DeCOG. Eur J Cancer 2018; 96: 34–43. [DOI] [PubMed] [Google Scholar]
- 41. Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs 2020; 80: 813–819. [DOI] [PubMed] [Google Scholar]
- 42. Gross N, Ferrarotto R, Nagarajan P, et al. LBA74 – phase II study of neoadjuvant cemiplimab prior to surgery in patients with stage III/IV (M0) cutaneous squamous cell carcinoma of the head and neck (CSCC-HN). Ann Oncol 2019; 30: v910. [Google Scholar]


