Abstract
Radiation-induced lung injury is a common complication of radiotherapy for lung cancer, breast cancer, esophageal cancer, and thymoma. This study aims to illustrate biomarkers of radiation-induced lung injury and its potential mechanism through the study of metabolomic alterations in serum of Sprague-Dawley rats with different radiation doses. Serum from 0, 10, or 20 Gy irradiated rats were collected and subjected to gas chromatography-mass spectrometry. The result showed that there were 23 dysregulated metabolites between the 10 Gy irradiation group and the 0 Gy control group, whereas 36 preferential metabolites were found between the 20 Gy irradiated rat serum and the control groups. Among them, there were 19 common differential metabolites in the 2 irradiation groups, including 3 downregulated (benzyl thiocyanate, carbazole, and N-formyl-L-methionine) and 16 upregulated metabolites. We further analyzed the metabolic pathways of different metabolites; the results showed that there were 3 significant enrichment pathways in the 10 Gy vs 0 Gy group and 7 significant enrichment pathways in the 20 Gy vs 0 Gy group. Among them, taurine and hypotaurine metabolism, riboflavin metabolism, and glyoxylate and dicarboxylate metabolism were the common metabolic enrichment pathways of the 10 Gy vs 0 Gy group and the 20 Gy vs 0 Gy group.
Keywords: ionizing radiation, radiation-induced lung injury, serum, metabolomic, biomarker
Radiation-induced lung injury remains a common complication in thoracic tumor radiotherapy. 1 Metabolites are the final downstream products of gene expression, which are directly related to phenotype. 2 The progress of radiation-induced lung injury is accompanied by the changes of metabolism-related genes and metabolites. Therefore, metabolomics can better reflect the overall information of organisms and reveal the physiological and biochemical functional states of biological systems than other omics. 3 In previous studies, we explored the metabolic changes of lung tissues in the rat model of radiation-induced lung injury, and revealed the possible molecular mechanism of metabolites in the progression of radiation-induced lung injury. 4 Ionizing radiation has been shown to modulate serum metabolites. This study aims to explore the alterations of serum metabolites with radiation-induced lung injury in rats to seek early warning markers in the progression of radiation-induced lung injury, so as to provide a new basis for the early diagnosis and treatment of radiation-induced lung injury.
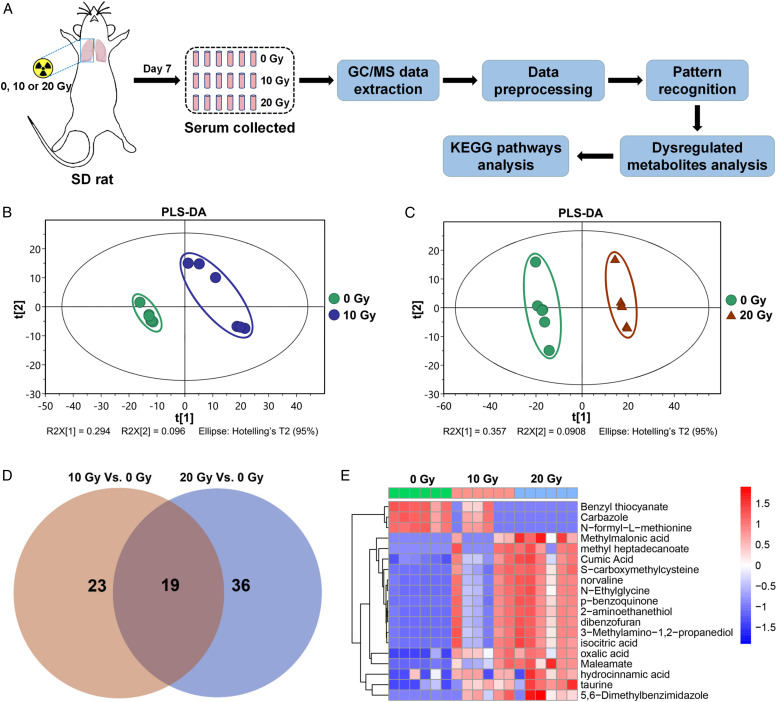
To this aim, we constructed the radiation-induced lung injury model of Sprague-Dawley rats with different radiation doses (0, 10, or 20 Gy) in the right lung at a dose rate of 200 cGy/min with 6-MeV X-ray radiation (Clinac 2100EX, Varian Medical Systems, Inc, Palo Alto, CA) as reported previously. 4 The protocols for experiments involving animals were approved by the Animal Experimentation Ethics Committee at Soochow University (Suzhou, China). Male Sprague-Dawley (SD) rats (4 weeks of age) were purchased from the Shanghai SLAC Laboratory Animal Ltd (Shanghai, China). These animals were housed in a pathogen-free environment at the Medical School facility of Soochow University. Sprague-Dawley rats were randomly divided into the control group, and 10, and 20 Gy irradiation groups (n = 6). After the rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium (Sigma-Aldrich, St Louis, MO, USA) at a dose of 45 mg/kg, the radiation site (right lung) was located by computed tomography–guided precise positioning system. The radiation doses (10 and 20 Gy) selected in this study were to adapt to large fractional dose delivery, such as stereotactic body radiation therapy. 5 One week after irradiation, the blood of rat abdominal aorta was collected, and the serum was collected after centrifugation (3000 × g, 15 minutes). The changes of serum metabolite were analyzed by a non-targeted metabolomic method based on gas chromatography-mass spectrometry (GC/MS). The serum samples were analyzed on an Agilent 7890B gas chromatography system coupled with an Agilent 5977A MSD system (Agilent Technologies Inc, Folsom, California). After extracting the GC/MS data, metabolites were annotated through the Fiehn or NIST database, then the GC/MS data were processed with Simca-P 13.0 (Umetrics AB, Umea, Sweden) to perform the partial least squares discriminant analysis (PLS-DA) to discriminate the metabolic differences between the groups of the study. In this study, the method combining multidimensional and one-dimensional analysis was used for inter-group screening of differential metabolites; the VIP value greater than 1.0 and P value less than .05 were regarded as differential metabolites. Finally, the differential metabolites were mapped to the Kyoto Encyclopedia of Genes and Genomes database to obtain the enrichment results of their metabolic pathways (Figure 1(a)). The PLS-DA scores of the 2 groups (10 and 20 Gy) were significantly different from those of the 0 Gy control group (Figure 1(b) and 1(c)). We found that there were 23 different metabolites between the 10 Gy irradiation group and the 0 Gy control group, and there were 36 different metabolites between the 20 Gy irradiation group and the 0 Gy control groups, and there were 19 common differential metabolites in the 2 irradiation groups (Figure 1(d)). Comparing the 19 common differential metabolites, it was found that benzyl thiocyanate, carbazole, and N-formyl-L-methionine were downregulated, and 16 metabolites such as taurine, 2-aminoethanethiol, 5,6-dimethylbenzimidazole, and methyl heptadecanoate were upregulated (Figure 1(e) and Supplementary Table 1). In order to understand the metabolic mechanism in the progression of radiation-induced lung injury, we mapped the serum-dysregulated metabolites to metabolic pathways to explore which pathways were significantly regulated. The results showed that there were 3 significant enrichment pathways in the 10 Gy vs 0 Gy group and 7 significant enrichment pathways in the 20 Gy vs 0 Gy group (Supplementary Table 2). Among them, taurine and hypotaurine metabolism, riboflavin metabolism, and glyoxylate and dicarboxylate metabolism were the common metabolic enrichment pathways of the 10 Gy vs 0 Gy group and the 20 Gy vs 0 Gy group. Among these differential metabolites, we found that taurine was upregulated in the irradiated group, and a variety of common differential metabolites such as 2-aminoethanethiol, 5,6-dimethylbenzimidazole, and methyl heptadecanoate were enriched in the taurine and hypotaurine metabolism pathway, which may predict that the metabolism of taurine was closely related to the occurrence of radiation injury.6,7 In addition, in the clinical radiotherapy of lung cancer, the heart may also be irradiated, and taurine is a biomarker of heart injury. 8 In this study, it is likely that part of the heart was irradiated, and a portion of taurine in the serum may come from the heart. Taurine may be an important biomarker in the progression of radiation-induced lung injury. Through the study of serum metabolite of acute lung injury in rats, we found a variety of metabolites and metabolic pathways that changed significantly after different doses of irradiation, which can not only be used to verify the efficacy of experimental drugs in the treatment of acute lung injury in rats, but also provide further evidence for the pathogenesis of radiation-induced lung injury and provide a basis for early diagnosis based on serum markers.
Figure 1.
(a) Schematic diagram of radiation-induced lung injury models in SD rats and the procedures for serum metabolomics analysis using gas chromatographymass spectrometry. Rats were irradiated with 0, 10, or 20 Gy thoracic radiation. The degree of separation among the 10 Gy vs 0 Gy group (b) and the 20 Gy vs 0 Gy group (c) of serum samples in the partial least squares discriminant analysis (PLS-DA) score maps. (d) Venn diagram of differential metabolites between the 10 Gy vs 0 Gy group and the 20 Gy vs 0 Gy group. (e) Heatmap plot of dose-dependent metabolites among the 3 groups.
Supplemental Material
Supplemental Material, sj-pdf-1-dos-10.1177_15593258211067060 for Serum Metabolomic Analysis of Radiation-Induced Lung Injury in Rats by Yahui Feng, Yiying Gao, Wenling Tu, Yang Feng, Jianping Cao and Shuyu Zhang in Dose-Response
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China (U1967220, 82103773, 82073477 and 82003390), Natural Science Foundation of Chengdu Medical College (CYZ19-31) and Young Talent Program of China National Nuclear Corporation.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Shuyu Zhang https://orcid.org/0000-0003-1419-3635
References
- 1.Li X, Zhuang X, Qiao T. Role of ferroptosis in the process of acute radiation-induced lung injury in mice. Biochem Biophys Res Commun. 2019;519(2):240-245. [DOI] [PubMed] [Google Scholar]
- 2.Cui L, Zheng D, Lee YH, et al. Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci Rep. 2016;6:26076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones JW, Jackson IL, Vujaskovic Z, Kaytor MD, Kane MA. Targeted metabolomics identifies pharmacodynamic biomarkers for BIO 300 mitigation of radiation-induced lung injury. Pharm Res (N Y). 2017;34(12):2698-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Li X, Gao J, et al. Metabolomic analysis of radiation-induced lung injury in rats: The potential radioprotective role of taurine. Dose Response. 2019;17(4):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amini A, Yeh N, Gaspar LE, Kavanagh B, Karam SD. Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy: A review. Radiat Oncol. 2014;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita T, Kato T, Isogai T, Gu Y, Ma N. Protective effects of taurine on the radiation exposure induced cellular damages in the mouse intestine. Adv Exp Med Biol. 2019;1155:443-450. [DOI] [PubMed] [Google Scholar]
- 7.Ma N, Kato T, Isogai T, Gu Y, Yamashita T. The potential effects of taurine in mitigation of radiation nephropathy. Adv Exp Med Biol. 2019;1155:497-505. [DOI] [PubMed] [Google Scholar]
- 8.Mousavi K, Niknahad H, Ghalamfarsa A, et al. Taurine mitigates cirrhosis-associated heart injury through mitochondrial-dependent and antioxidative mechanisms. Clin Exp Hepatol. 2020;6(3):207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-dos-10.1177_15593258211067060 for Serum Metabolomic Analysis of Radiation-Induced Lung Injury in Rats by Yahui Feng, Yiying Gao, Wenling Tu, Yang Feng, Jianping Cao and Shuyu Zhang in Dose-Response