Abstract
Background:
Evidence to date supports continued human epidermal growth factor receptor 2 (HER2) suppression beyond progression on HER2-directed therapy for advanced HER2-positive breast cancer. Data from several phase II and III trials evaluating HER2-directed therapy following second-line T-DM1 have recently become available.
Methods:
We performed a systematic search of the published and presented literature to identify phase II and phase III trials assessing novel HER2-targeted agents as third-line therapy or beyond for HER2-positive advanced breast cancer using search terms ‘breast cancer’ AND ‘HER2’ AND ‘advanced’ AND (‘phase II’ OR ‘phase III’).
Results:
Eight clinical trials reporting efficacy outcomes on third-line or greater HER2-directed therapy for HER2-positive advanced breast cancer were identified. In phase III trials, margetuximab and neratinib combinations demonstrated significant 1.3-month (hazard ratio, HR = 0.71, p < 0.001) and 0.1-month (HR = 0.76, p = 0.006) net improvements in median progression-free survival (PFS), respectively, with no significant improvements in overall survival (OS). Tucatinib added to trastuzumab and capecitabine demonstrated a significant 2.7-month improvement in median PFS (HR = 0.57, p < 0.00001) and a 5.5-month improvement in median OS (HR = 0.73, p = 0.004) in a randomized phase II trial, including significant clinical benefit for patients with brain metastases. Finally, trastuzumab-deruxtecan, zenocutuzumab, and poziotinib demonstrated benefit in phase II trials with the most robust overall response rate (62.0%) and median duration of response (18.2 months) observed for trastuzumab-deruxtecan among heavily pretreated patients.
Conclusion:
Tucatinib plus trastuzumab and capecitabine significantly prolongs OS, and promising preliminary response outcomes for trastuzumab-deruxtecan suggest that sequencing of these regimens following second-line therapy is reasonable.
Keywords: advanced disease, breast cancer, HER2-positive, neratinib, pertuzumab, T-DM1, T-DXd, trastuzumab, tucatinib
Introduction
Globally, breast cancer (BC) remains one of the leading causes of morbidity and mortality among women. 1 Approximately 15% to 20% of invasive BCs are characterized by human epidermal growth factor receptor 2 (HER2) gene amplification and/or HER2 protein overexpression, translating to an estimated global incidence of 340,000 to 450,000 new cases annually.1–3 Although often detected at an early stage, approximately one-third of HER2-positive BC patients present with or develop regional or distant metastatic disease. 4
HER2-directed therapies for advanced HER2-positive disease have evolved dramatically over the past two decades with the advent of monoclonal antibodies (MoAbs),5–7 small molecule tyrosine kinase inhibitors (TKIs),8–11 and antibody drug conjugates (ADCs).12,13 Improved systemic control has led to an increased prevalence of brain metastases, possibly due to variable penetrance of MoAbs across the blood–brain barrier. 14 Patients with hormone receptor negative, HER2-positive BC are nearly three times more likely to present with brain metastases, 15 and approximately half of patients with metastatic HER2-positive BC are expected to develop brain metastases over the course of their illness.16–18
Currently, the recommended first-line treatment for most patients with advanced HER2-positive BC is trastuzumab plus pertuzumab and a taxane based on results from CLEOPATRA,19–21 with the ADC trastuzumab emtansine (T-DM1) consisting of the humanized MoAb trastuzumab covalently linked to the cytotoxic agent DM1, a standard second-line option based on the EMILIA trial. 12 The novel ADC trastuzumab-deruxtecan (T-DXd), consisting of trastuzumab and a cleavable linker to a potent topoisomerase I inhibitor payload, has also demonstrated substantial activity in the second-line setting. 22 Although evidence supports continued HER2 suppression after progression on HER2-directed therapy,11,23,24 no standardized treatment strategies have been established following T-DM1.20,21,25 Historically, candidate third-line and beyond regimens have included lapatinib with capecitabine, trastuzumab with capecitabine, or other chemotherapeutics with continued trastuzumab.10,11
More recently, data from several trials assessing novel MoAbs, TKIs, and ADCs in the third-line and beyond setting for HER2-positive advanced BC have become available. The next generation HER2-specific MoAb margetuximab is a fragment crystallizable (Fc) engineered monoclonal anti-HER2 antibody which binds with greater affinity than trastuzumab to FcγRIIIa (CD16A) expressed on immune effector cells, thereby increasing antibody-dependent cellular cytotoxicity, with demonstrated activity in a phase III trial.26–29 Dual targeted bi-specific HER2 antibodies are also in development, including those targeting multiple HER2 epitopes, anti-HER2/CD3, and anti-HER2/human epidermal growth factor receptor 3 (HER3) MoAbs. 29 While many of these are still in early phase studies, the anti-HER2/HER3 MoAb zenocutuzumab (MCLA-128) in addition to the ADC T-Dxd have demonstrated efficacy in phase II studies among heavily pretreated patients.25,30 Three novel HER2-targeted TKIs have also shown clinical benefit in heavily pretreated patients, including the irreversible pan-HER inhibitor neratinib in a phase III trial, 31 the highly selective HER2-specific reversible inhibitor tucatinib in a randomized phase II study, 32 and the potent epidermal growth factor receptor and HER2 exon 20 insertion inhibitor poziotinib in a phase II study. 33 Biosimilars are also being developed that mimic existing biologic drugs to provide similarly active and potentially more cost-effective HER2-directed MoAb therapeutic options. 34 This review will summarize the efficacy and safety outcomes of phase II and III trials evaluating novel third-line and beyond HER2-directed therapies for advanced HER2-positive BC and suggest guidance on treatment selection and sequencing.
Methods
A systematic search of published and presented literature was performed to identify phase II or III trials assessing novel third-line or beyond HER2-targeted therapy for HER2-positive advanced BC. PubMed (all time to February 10, 2021) and proceedings from the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the San Antonio Breast Cancer Symposium (SABCS) 2019 and 2020 annual meetings were searched for phase II or III trials using the search terms ‘breast cancer’ AND ‘HER2’ AND ‘advanced’ AND (‘phase II’ OR ‘phase III’) OR respective aliases or using the respective filters when appropriate [Figure 1 – Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), Supplemental File S1]. A supplemental bibliographic search of review articles and pooled/meta-analyses was also conducted, in addition to directed searches after the database search cutoff date to ensure that the most up-to-date reports of eligible studies were considered.
Figure 1.
PRISMA diagram of eligible studies.
ASCO, American Society of Clinical Oncology; BC, breast cancer; ESMO, European Society for Medical Oncology; HER2, human epidermal growth factor receptor 2; n, number; SABCS, San Antonio Breast Cancer Symposium; T-DM1, trastuzumab emtansine.
aPrimary reports of eligible studies that were not identified through database.
bIncluding current standards of treatment, trastuzumab, pertuzumab, and T-DM1.
English language records were vetted at abstract level and confirmed at full text as needed. Non-original research, preclinical, correlative science, modeling or simulation, those in earlier stages of disease or without BC patients, case reports, retrospective, prospective studies of undefined design, phase I trials, and those that did not assess or report outcomes for HER2-directed therapies in HER2-positive BC were excluded (PRISMA, Figure 1). Trials with less than two prior lines of HER2-directed treatment, those restricted to low HER2 expression levels, those with exclusively hormone receptor-positive populations, or those with fewer than 30 patients per cohort were also excluded. Phase II trials were only eligible if the majority of the patients were pretreated with two or more HER2-directed agents.
Findings beyond second-line HER2-directed therapy
The literature search identified a total of 1348 records. Selection criteria revealed eight phase II or III trials reporting efficacy outcomes on third-line or greater HER2-directed therapy for HER2-positive advanced BC, which excluded the phase I/II trial of ruxolitinib plus trastuzumab trial due to size (n = 26, PRISMA; Figure 1).
T-DM1
The phase III TH3RESA study randomized 602 patients treated with at least two prior lines of HER2-directed therapy consisting of trastuzumab and lapatinib [median 4 prior systemic therapies in experimental arm (range = 1–19) and no prior pertuzumab or T-DM1] 2:1 to receive either T-DM1 or treatment of physician’s choice (TPC; Figure 2). The co-primary endpoints were investigator-assessed, progression-free survival (PFS) and overall survival (OS). At a median follow-up of 7.2 months in the T-DM1 arm and 6.5 months in the TPC arm, significant improvements for T-DM1 versus TPC were observed in the co-primary endpoints of PFS – median 6.2 versus 3.3 months, hazard ratio (HR) = 0.53, 95% confidence interval (CI) = [0.42–0.66], p < 0.0001 – and OS – median 22.7 versus 15.8 months, HR = 0.68, 95% CI = [0.54–0.85], p = 0.0007 (Table 1).35,36 Among patients with measurable disease at baseline (n = 508), objective response rates (ORRs) were 31.3% versus 8.6% favoring T-DM1, with a median duration of response (DoR) of 9.7 months versus not yet reached (NYR). 35 Adverse events (AEs) led to treatment withdrawal in 14.6% of patients receiving T-DM1 and 10.9% of TPC patients (Table 2). 36 Grade ⩾ 3 AEs of any cause occurred in 40.0% of patients receiving T-DM1 versus 47.3% on TPC, with thrombocytopenia (6.0%), anemia (3.5%), and both dyspnea and aspartate aminotransferase elevation (2.5% each) most commonly reported with T-DM1. Treatment-related deaths were reported in 2.2% and 1.6% of patients in the T-DM1 and TPC groups, respectively.
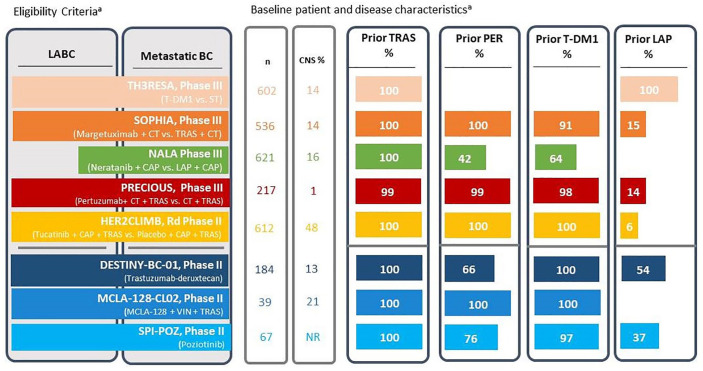
Figure 2.
Clinical trial overview for HER2-directed therapy in third-line and beyond HER2-positive advanced breast cancer.
BC, breast cancer; CAP, capecitabine; CNS, central nervous system; CT, chemotherapy; LABC, locally advanced breast cancer; LAP, lapatinib; MCLA-128, zenocutuzumab; n, number of patients; NR, not reported; PER, pertuzumab; ST, systemic therapy (physician’s choice); T-DM1, trastuzumab emtansine; TRAS, trastuzumab; VIN, vinorelbine.
aLength of bars give an approximation of the proportion of patients with each treatment or disease characteristic.
Table 1.
Clinical trials assessing efficacy of later lines of therapy of HER2-directed therapy in HER2-positive breast cancer.
| Trial name (NCT number) |
Study type Line of therapy Prior HER2-directed therapy |
Regimen(s) | n | Median follow-up (months) [range] |
Overall response rate, % (95% CI) |
Median DoR, months (95% CI) [range] |
Median progression-free survival,
months HR (95% CI) |
Median overall survival, months HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trial | ||||||||
| TH3RESA35,36
(NCT01419197) |
Phase III ⩽4th line (35%) >4th line (65%) Prior Trastuzumab (100%) Prior lapatinib (100%) |
Trastuzumab emtansine 3.6 mg/kg q3w | 404 | 7.2
a
[5.0–10.1] |
31.3 (16.2–29.2) p < 0.0001 |
9.7 (6.6–10.5) |
6.2
HR = 0.53 (0.42–0.66) p < 0.0001 |
22.7
a
HR = 0.68 (0.54–0.85) p = 0.0007 |
| Physician’s choice – systemic therapy (CT or HT or HER2i(s) or CT + HER2i) | 198 | 6.5
a
[4.1–9.7] |
8.6 | NYR | 3.3 | 15.8 a | ||
| SOPHIA
26
(NCT02492711) |
Phase III ⩽3rd line (66%) >3rd line (34%) Prior Trastuzumab (100%) Prior Pertuzumab (100%) Prior T-DM1 (91%) Prior Lapatinib (15%) |
Margetuximab 15 mg/kg q3w plus chemotherapy q3w | 266 | 15.6 | 25.2 (20.1–30.9) p = 0.0006 |
6.9 (5.45–7.49) |
5.7
b
HR = 0.71 (0.58–0.86) p < 0.001 |
21.6
HR = 0.89 (0.69–1.13) p = 0.33 |
| Trastuzumab 8 mg/kg loading then 6 mg/kg q3w plus chemotherapy q3w | 270 | 13.7 (9.8–18.4) |
7.0 (5.55–8.15) p = 0.74 |
4.4 | 19.8 | |||
| NALA
31
(NCT01808573) |
Phase III ⩽3rd line (69%) >3rd line (31%) Prior trastuzumab (100%) Prior pertuzumab (42%) Prior T-DM1 (64%) |
Neratinib 240 mg, QD, q3w plus capecitabine 1500 mg/m2 BID D1-14, q3w | 307 | 29.9 [21.9–40.6] |
32.8 (27.1–38.9) p = 0.12 |
8.5 HR = 0.50 (0.33–0.74) p = 0.0004 |
5.6
HR = 0.76 (0.63–0.93) p = 0.006 c |
21.0
HR = 0.88 d (0.72–1.07) p = 0.21 d |
| Lapatinib 1250 mg QD, plus capecitabine 2000 mg/m2 BID D1-14, q3w | 314 | 26.7 (21.5–32.4) |
5.6 | 5.5 | 18.7 | |||
| PRECIOUS
37
(NCT02514681) |
Phase III Median 3rd-line therapy Pertuzumab (99%) Trastuzumab (99%) T-DM1 (98%) Lapatinib (14%) Others (8%) |
Pertuzumab 840 mg loading then 420 mg IV q3w + Trastuzumab 8 mg/kg loading then 6 mg/kg q3w + Physician’s choice chemotherapy e | 108 | 14.2 | 18.9 | NR |
5.3
HR = 0.76 (NR–0.97) p = 0.022 |
28.8 HR = 0.71 (NR–1.03) p = 0.062 |
| Trastuzumab 8 mg/kg loading then 6 mg/kg q3w + Physician’s choice chemotherapy e | 109 | 19.6 | NR | 4.2 | 23.4 | |||
| HER2CLIMB32,38
(NCT02614794) |
Randomized phase II Median 4th-line therapy Trastuzumab (100%) T-DM1 (100%) Pertuzumab (100%) Lapatinib (7%) |
Tucatinib 300 mg BID plus trastuzumab 6 mg/kg q3w + capecitabine 100 mg/m2 BID D1-14, q3w | 410 | 29.6 | 40.6
f
(35.3–46.0) p < .001 |
NR |
7.6
HR = 0.57 (0.47–0.70) p < 0.00001 |
24.7
HR = 0.73 (0.59–0.90) p = 0.004 |
| Placebo BID plus trastuzumab 6 mg/kg q3w + capecitabine 100 mg/m2 BID D1-14, q3w | 202 | 22.8
f
(16.7–29.8) |
NR | 4.9 | 19.2 | |||
| Non-randomized cohort | ||||||||
| DESTINY-Breast01
39
(NCT03248492) |
Phase II Median 6th-line therapy Trastuzumab (100%) T-DM1 (100%) Pertuzumab (66%) Other anti-HER2 therapy (54%) |
Trastuzumab-deruxtecan 5.4 mg/kg q3w | 184 | 26.5 |
62.0
(54.5–69.0) |
18.2 (15.0–NE) |
19.4 (14.1–25.0) |
29.1 (24.6–36.1) |
| MCLA-128-CL02
30
(NCT03321981) |
Phase II (cohort 1) Median 6th-line therapy Median 4th-line HER2-directed Trastuzumab (100%) Pertuzumab (100%) T-DM1 (100%) |
Zenocutuzumab 750 mg plus trastuzumab 8 mg/kg loading then 6 mg/kg and vinorelbine 25 mg/m², D1,8, q3w | 37 | NR | 18.9
g
(9.2–32.6) |
NR | NR | NR |
| SPI-POZ-201
33
(NCT02659514) |
Phase II Median 5th- to 8th-line therapy Trastuzumab (100%) T-DM1 (97%) Lapatinib (37%) Pertuzumab (76%) Neratinib (3%) |
Cohort 1: Poziotinib 24 mg QD D1–14 q3w | 33 | NR | 23.3 | 5.6 [range: 3.0–9.6] |
3.0 [range: 0.9–10.8] |
NR |
| Cohort 2: Poziotinib 16 mg QD q3w | 34 | 22.2 | 13.8 [range: 4.4–18.7] |
4.9 [range: 0.1–19.8] |
NR | |||
BID, twice daily; CI, confidence interval; CT, chemotherapy; DoR, duration of response; Dx, day X; HER2, human epidermal growth factor 2; HER2i, HER2-inhibitor; HR, hazard ratio; HT, hormone therapy; IV, intravenous, mg/kg, milligrams/kilogram, n, number of patients; NE, not estimable; NR, not reported; NYR, not yet reached; QD, daily; qxw, every x weeks; T-DM1, trastuzumab emtansine.
Efficacy outcomes of phase II and III trials of later lines of HER2-directed therapy in advanced BC ordered by study type, then chronologically by release of primary analyses. Primary endpoints are in bold text.
Final OS analysis at a median follow-up of 30.5 months.
Primary endpoint was centrally assessed PFS at 2.8 months – HR = 0.76, 95% CI = 0.59–0.98, p = 0.03.
Restricted analysis at 24 months.
Restricted analysis at 48 months.
Chemotherapy was chosen from docetaxel, paclitaxel, nab-paclitaxel, vinorelbine, eribulin, capecitabine, or gemcitabine.
At an earlier median follow-up of 14.0 months.
Primary endpoint investigator-assessed clinical benefit rate at 24 weeks.
Table 2.
Safety outcomes from clinical trials assessing later lines of HER2-directed therapy in HER2-positive BC.
| Trial phase | TH3RESA35,36 Phase III |
SOPHIA26 Phase III |
NALA31 Phase III |
PRECIOUS37 Phase III |
HER2CLIMB32 Rd Phase II |
MCLA-128-CL0230 Phase II |
DESTINY-Breast0125 Phase II |
SPI-POZ-20133 Phase II |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arms | T-DM1 | Physician’s choice (HER2i + CT) | Margetuximab + CT | Trastuzumab + CT | Neratinib + Capecitabine | Lapatinib + Capecitabine | Pertuzumab + Trastuzumab +Physician’s choice CT | Trastuzumab + Physician’s choice CT | Tucatinib + Capecitabine + Trastuzumab | Placebo + Capecitabine + Trastuzumab | Zenocutuzumab + Trastuzumab + Vinorelbine | Trastuzumab-deruxtecan | Poziotinib 24 mg | Poziotinib 16 mg |
| Safety population (n) | 403 | 184 | 264 | 266 | 303 | 311 | 105 | 108 | 404 | 197 | 28 | 184 | 33 | 34 |
| Overall | ||||||||||||||
| Any grade AE | 377 (93.5) a | 163 (88.6) a | 260 (98.5) | 261 (98.1) | 302 (99.7) | 309 (99.4) | 91 (86.7) | 95 (88.0) | 401 (99.3) | 191 (97.0) | 25 (89.3) | 183 (99.5) | 33 (100.0) | 33 (97.1) |
| Grade ⩾ 3 AEs | 161 (40.0) | 87 (47.3) | 142 (53.8) | 140 (52.6) | 184 (60.7) | 188 (60.5) | 65 (61.9) | 75 (69.4) | 223 (55.2) | 96 (48.7) | 15 (53.6) | 105 (57.1) | 22 (66.7) | 25 (73.5) |
| AEs leading to discontinuation of any
treatment n (%) |
59 (14.6) | 20 (10.9) | 8 (3.0) | 7 (2.6) | 42 (13.9) | 56 (18.0) | NR | NR | 23 (5.7) | 6 (3.0) | 2 (7.1) b | 28 (15.2) | 6 (18.2) | 10 (29.4) |
| AE- or treatment-associated
deaths n (%) |
9 (2.2) | 3 (1.6) | 3 (1.1) | 2 (0.8) | 8 (2.6) | 10 (3.2) | 1 (1.0) | 0 (0.0) | NR | NR | 1 (3.6) b | 9 (4.9) | 3 (9.1) | 1 (2.9) |
| Select grade ⩾ 3 AEs | ||||||||||||||
| Most common grade 3/4 AEs (%) | Thrombocytopenia (6.0) Anemia (3.5) Increased AST (2.5) Dyspnea (2.5) Fatigue (2.2) |
Neutropenia (15.8) Diarrhea (4.4) Febrile neutropenia (3.8) Dyspnea (3.8) Asthenia (3.3) Abdominal pain (2.7) |
Neutropenia (19.7) Decreased neutrophil count (8.7) Anemia (4.9) Fatigue (4.9) |
Neutropenia (12.4) Decreased neutrophil count (10.5) Anemia (6.4) Febrile neutropenia (4.9) |
Diarrhea (24.4) PPE Syndrome (9.6) Hypokalemia (4.6) Nausea (4.3) |
Diarrhea (12.5) PPE Syndrome (11.3) Hypokalemia (6.4) Anemia (3.5) |
Febrile neutropenia (15.2)
c
Anemia (13.3) c Infection (5.7) c Diarrhea (2.9) c Stomatitis (1.9) c |
Febrile neutropenia (16.7)
c
Anemia (6.5) c Infection (1.9) c Stomatitis (1.9) c Diarrhea (0.9) c |
PPE Syndrome (13.1) Diarrhea (12.9) ALT increase (5.4) Fatigue (4.7) |
PPE Syndrome (9.1) Diarrhea (8.6) Fatigue (4.1) Vomiting (3.6) |
Neutropenia (53.6) Diarrhea (3.6) Febrile neutropenia (3.6) Peripheral motor neuropathy (3.6) |
Decreased neutrophil count (23.7) Anemia (11.1) Decreased white blood cell count (8.7) Decreased platelet count (6.3) Decreased lymphocyte count (6.3) Interstitial lung disease (0.5) |
Diarrhea (30.3) Rash (18.2) Stomatitis (6.1) Nausea (6.1) Mucosal inflammation (6.1) Dermatitis acneiform (6.1) |
Diarrhea (29.4) Rash (11.8) Rash maculo-papular (11.8) Dermatitis acneiform (6.1) Somatitis (2.9) Nausea (2.9) |
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate transaminase; BC, breast cancer; CT, chemotherapy; HER2i, human epidermal growth factor receptor 2 inhibitor; n, number of patients; NR, not reported; PPE, palmar-plantar erythrodysesthesia; T-DM1, trastuzumab emtansine.
Safety outcomes of phase II and III trials of later lines of HER2-directed therapy ordered chronologically by release of primary analyses. Treatment-related AEs were summarized when available.
From the earlier progression-free survival analysis.
Considered related to vinorelbine.
Most common AEs among reported ‘AEs of special interest’.
Margetuximab
The phase III SOPHIA trial randomized 536 patients with disease progression after at least two prior lines of HER2-directed therapy (34% with ⩾3 lines) including pertuzumab (all patients except 1) and T-DM1 (91.2%) to receive margetuximab plus TPC or trastuzumab plus TPC (Figure 2). 26 Preliminary findings at a median follow-up of 2.8 months showed a significant improvement in the co-primary endpoint of centrally assessed PFS for margetuximab plus TPC (median 5.8 versus 4.9 months, HR = 0.76, 95% CI = 0.59–0.98, p = 0.03) which did not translate into improved OS at the second planned interim analysis (median 21.6 versus 19.8 months, HR = 0.89, 95% CI = 0.69–1.13, p = 0.33) (Table 1), despite a lack of cross-over and longer follow-up (median 15.6 months). Investigator assessed ORRs were significantly greater for margetuximab versus trastuzumab (25.2% versus 13.7%, p = 0.0006), although median DoRs were comparable (6.9 versus 7.0 months, p = 0.74). Although data suggested that presence of a CD16A-158F allele predicted margetuximab benefit over trastuzumab, patients homozygous for the CD16A-158VV allele saw no benefit. AEs leading to treatment withdrawal (3.0% versus 2.6%) and grade ⩾ 3 AEs of any cause (53.8% versus 52.6%) were similar (Table 2), with the most commonly reported grade ⩾ 3 AEs for margetuximab versus trastuzumab being neutropenia (19.7% versus 12.4%), neutrophil count decrease (8.7% versus 10.5%), anemia (4.9% versus 6.4%), and fatigue (4.9% versus 3.0%). Any grade infusion-related reactions were more common in the margetuximab arm (13.3% versus 3.4%). No treatment-related deaths were reported.
Neratinib
The open label phase III NALA study randomized 621 patients previously treated with at least two prior lines of HER2-directed therapy (31% with ⩾3 prior lines, 42% and 54% had received prior pertuzumab and T-DM1, respectively) to neratinib or lapatinib, both administered with capecitabine (Figure 2). 31 The co-primary endpoints were centrally assessed PFS and OS. At a median follow-up of 29.9 months, PFS was significantly improved for patients receiving neratinib (median 5.6 versus 5.5 months, HR = 0.76, 95% CI = 0.63–0.93, p = 0.006) although no significant differences in OS were observed (median 21.0 versus 18.7 months, HR = 0.88, 95% CI = 0.72–1.07, p = 0.21) (Table 1). Median DoR was 8.5 versus 5.6 months, significantly favoring neratinib (HR = 0.50, 95% CI = 0.33–0.74, p = 0.004) and ORR was 32.8% versus 26.7% (p = 0.12). Treatment-emergent AEs (TEAEs) led to treatment withdrawal in 13.9% of patients receiving neratinib versus 18.0% in the lapatinib arm (Table 2). Grade ⩾ 3 AEs of any cause occurred in 60.7% versus 60.5% of patients overall, with the most common grade 3/4 TEAEs in the neratinib versus lapatinib arms being diarrhea (24.4% versus 12.5%), palmar-plantar erythrodysesthesia (PPE, 9.6% versus 11.3%), hypokalemia (4.6% versus 6.4%), and nausea (4.3% versus 2.9%). Deaths due to TEAEs were reported in 2.6% and 3.2% of patients in the neratinib and lapatinib combination arms, respectively.
Pertuzumab retreatment
The phase III PRECIOUS trial randomized 217 patients previously treated with pertuzumab plus trastuzumab (99%) and T-DM1 (98%) 1:1 to receive retreatment with pertuzumab plus trastuzumab and TPC or trastuzumab plus TPC. 37 With a median follow-up of 14.2 months, the primary endpoint of investigator-assessed PFS was significantly improved for the addition of pertuzumab to trastuzumab plus chemotherapy (median 5.3 versus 4.2 months, HR = 0.76, 95% CI = not reported, NR–0.97, p = 0.022). Median OS was 28.8 versus 23.4 months with the addition of pertuzumab versus the doublet (HR = 0.71, 95% CI = NR–1.03, p = 0.062) and ORRs were 18.9% versus 19.6%, respectively. AEs leading to treatment discontinuation were NR, with grade ⩾ 3 AEs of any cause occurring in 61.9% versus 69.4% of patients (pertuzumab plus trastuzumab and TPC or trastuzumab plus TPC), and the most common in the pertuzumab arm were febrile neutropenia (15.2% versus 16.7%), anemia (13.3% versus 6.5%), infection (5.7% versus 1.9%), and diarrhea (2.9% versus 0.9%). Deaths due to AEs were reported in one patient receiving pertuzumab (1.0%) with none in the control arm.
Tucatinib
The phase II HER2CLIMB study involved 612 patients previously treated with both pertuzumab (99.7%) and T-DM1 (100%), including patients with brain metastases (47.5%). 32 Patients were randomized in a 2:1 ratio to receive tucatinib (n = 410) or placebo (n = 202), in combination with trastuzumab and capecitabine (Figure 2). With a median follow-up of 14.0 months in the total population, the primary endpoint analysis conducted for the first 480 randomized patients observed a significant improvement in centrally assessed PFS favoring tucatinib over placebo (median PFS 7.8 versus 5.6 months, HR = 0.54, 95% CI = 0.42–0.71, p < 0.001) as well as a doubling of ORR (40.6% versus 22.8%, p < 0.001). At a longer follow-up of 29.6 months, tucatinib also demonstrated a statistically significant improvement in investigator-assessed PFS (median 7.6 versus 4.9 months, HR = 0.57, 95% CI = 0.47–0.70, p < 0.00001; Table 1) and OS (median 24.7 versus 19.2 months, HR = 0.73, 95% CI = 0.59–0.90, p = 0.004) compared with placebo. 38 AEs led to discontinuation of tucatinib versus placebo in 5.7% and 3.0% of patients, respectively (Table 2). Grade ⩾ 3 AEs of any cause occurred in 55.2% versus 48.7% (tucatinib versus placebo) with the most common grade ⩾ 3 AEs in the tucatinib arm being PPE (13.1% versus 9.1%), diarrhea (12.9% versus 8.6%), alanine transaminase increase (5.4% versus 0.5%), and fatigue (4.7% versus 4.1%). 32 Deaths due to AEs were reported in 1.5% and 2.5% of patients in the tucatinib and placebo arms, respectively.
T-DXd
The single-arm phase II DESTINY-Breast01 trial assessed T-DXd in 184 heavily pretreated patients (100% prior T-DM1 and 66% prior pertuzumab; Figure 2). 25 The primary endpoint was ORR by independent central review. Updated results at a median follow-up of 26.5 months observed an ORR of 62.0% (95% CI = 54.5%–69.0%) with a median DoR of 18.2 months (95% CI = 15.0–not estimable, NE) (Table 1). 39 Median PFS was 19.4 months (95% CI = 14.1–25.0) and at a median follow-up of 31.1 months, and median OS was 29.1 months (95% CI = 24.6–36.1). TEAEs led to T-DXd discontinuation in 15.2% of patients with grade ⩾ 3 TEAEs occurring in 57.1% (Table 2). 25 The most common grade 3/4 TEAEs were decreased neutrophil count (23.7%), anemia (11.1%), and decreased white blood cell (8.7%) and platelet (6.3%) counts. Deaths due to TEAEs were reported in nine patients (4.9%). Overall, 15.2% of patients developed T-DXd-related interstitial lung disease (ILD), with one grade 3 event (0.5%) and five ILD-related deaths (2.7%). 40
Zenocutuzumab and poziotinib
Zenocutuzumab and poziotinib were assessed in two phase II trials (MCLA-128-CL02 and SPI-POZ-201), both enrolling heavily pretreated patients (100% prior T-DM1 and pertuzumab in MCLA-128-CL02 and 76% and 97%, respectively, in SPI-POZ-201).30,33 The single-arm MCLA-128-CL02 study evaluated zenocutuzumab plus trastuzumab and vinorelbine in 37 evaluable patients, observing an ORR of 18.9% (secondary endpoint) 30 and SPI-POZ-201 observed an ORR of 22.2% to 23.3% (primary endpoint) among 57 evaluable patients at the two different doses of poziotinib (Table 1). 33 DoRs were not reported in MCLA-128-CL02 and were 5.6 to 13.8 months in SPI-POZ-201. Median PFS was not reported in MCLA-128-CL02 and were similar in both SPI-POZ-201 cohorts (3.0–4.9 months).30,33 Discontinuation due to vinorelbine-related AEs occurred in 7.1% of patients receiving the zenocutuzumab combination, which was more common for those receiving poziotinib (18.2%–29.4%; Table 2).
Discussion
Two lines of HER2-directed therapy are currently approved in many jurisdictions for HER2-positive advanced BC. Data on novel HER2-directed agents in the third-line and beyond setting highlight the importance of continuing HER2-targeting beyond second-line treatment. Although additional research is needed to determine optimal sequencing of these agents, a proposed approach informed by current data is outlined.
What is the clinical impact of HER2-directed therapy for third-line line treatment and beyond in HER2-positive advanced BC?
The phase III EMILIA study led to the US Food and Drug Administration (FDA) approval of T-DM1 as second-line therapy following trastuzumab and a taxane in February 2013 (Table 3).12,41 Continued HER2 targeting beyond second-line therapy is standard of care for HER2-positive advanced BC, with multiple lines of HER2-directed agents common.20,21,42 Available data, however, suggest that outcomes vary between HER2-targeted strategies and optimal options are beginning to evolve for third-line treatment and beyond.
Table 3.
Regulatory status of later lines and beyond HER2-directed therapy for HER2-positive advanced breast cancer.
| Regulatory agency (search date) | Indication | Level of data (primary outcome) | Type of approval | Date of approval |
|---|---|---|---|---|
| Trastuzumab-emtansine monotherapy | FDA – HER2-positive metastatic breast cancer following trastuzumab and a taxane used separately or in combination | Phase III (PFS and OS) | Approved | February 2013 |
| EMA – HER2-positive advanced breast cancer following trastuzumab and a taxane used separately or in combination | Phase III (PFS and OS) | Approved | September 2013 | |
| Margetuximab plus chemotherapy | FDA – HER2-positive metastatic breast cancer following two or more prior HER2-directed therapies, with at least one for metastatic disease | Phase III (PFS and OS) | Approved | December 2020 |
| EMA – Not approved | ||||
| Neratinib plus capecitabine | FDA – HER2-positive advanced breast cancer following two or more prior anti-HER2 regimens for metastatic disease | Phase III (PFS and OS) | Approved | February 2020 |
| EMA – Not approved | ||||
| Tucatinib plus trastuzumab and capecitabine | FDA – HER2-positive advanced breast cancer following one or more prior anti-HER2 regimens for metastatic disease | Rd Phase II (PFS) | Approved | April 2020 |
| EMA – In combination with trastuzumab and capecitabine for HER2-positive advanced breast cancer following at least two prior anti-HER2 regimens | Phase III (PFS) | Approved | December 2020 | |
| Trastuzumab-deruxtecan monotherapy | FDA – HER2-positive advanced breast cancer following two or more anti-HER2-based regimens in metastatic setting | Phase II (ORR) | Accelerated approval | December 2019 |
| EMA – HER2-positive advanced breast cancer following two or more prior anti-HER2 regimens | Phase II (ORR) | Approved with conditions | December 2020 |
EMA, European Medicines Agency; FDA, US Food and Drug Administration; HER2, human epidermal growth factor 2; NA, not approved; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Rd, randomized.
Regulatory data were collected through review of FDA and EMA news bulletins and product monographs.
Studies addressing HER2-directed therapy in the third-line setting and beyond include TH3RESA, which reported a 32% reduced risk of death 36 and 47% reduced risk of progression 35 for T-DM1 among patients with prior trastuzumab, lapatinib, and taxane exposure (Table 1). A second trial, PRECIOUS, observed limited clinical benefit from doublet re-challenge using pertuzumab added to trastuzumab and chemotherapy for patients with prior pertuzumab and trastuzumab exposure (Table 1). 37 The open label SOPHIA and NALA trials assessed the benefit of replacing established agents with novel HER2-directed therapies, both combined with chemotherapy.26,31 The anti-HER2 MoAb margetuximab replaced trastuzumab in SOPHIA and the pan-HER TKI neratinib replaced lapatinib in NALA. These trials enrolled more than 500 and 600 patients, respectively, and included similar proportions of patients with baseline central nervous system (CNS) metastases (13%–16%) and with Eastern Cooperative Oncology Group Performance Status 1 (42%–46%) (Figure 2), although patients in SOPHIA were more heavily pretreated. 26 Both agents demonstrated statistically significant PFS improvements versus established comparators, with discontinuation rates due to toxicities of 3.0% for margetuximab and 13.9% for neratinib.26,31 Substitution of margetuximab for trastuzumab resulted in a 1.3-month improvement in median PFS (29% reduced risk of progression, p < 0.001) with a 0.1-month median PFS improvement observed for neratinib when substituted for lapatinib (24% reduced risk of progression, p = 0.006) (Table 1)26. Neratinib significantly reduced the cumulative incidence of CNS interventions compared with lapatinib (n = 621, 22.8% versus 29.2%, p = 0.043), 31 consistent with prior neratinib trial data.43,44 Neither agent resulted in a statistically significant OS benefit (HR = 0.89, p = 0.33 and HR = 0.88, p = 0.21) at median follow-ups of 15.6 and 29.9 months, respectively,26,31 remaining non-significant for margetuximab at the final OS analysis (HR = 0.95, p = 0.62). 45 The phase III landscape is rapidly changing with data on new agents continually emerging, including recently presented results from the TULIP trial which demonstrated a PFS benefit for the novel ADC SYD985 compared with physician’s choice of therapy in heavily pretreated patients (87.6% prior T-DM1, HR = 0.64, 95% CI = 0.49–0.84, p = 0.002). 46
Four phase II trials assessed novel HER2-directed therapies beyond second line,25,30,32,33 with notable benefits seen for tucatinib and T-DXd (Table 1).38,39 The randomized phase II placebo-controlled HER2CLIMB study evaluating tucatinib added to trastuzumab and capecitabine showed a statistically significant 46% reduced risk of progression in the primary endpoint population (n = 480, p < 0.001) 32 and a 27% reduction in risk of death in the overall trial population with longer follow-up (n = 612, p = 0.004). 38 Tucatinib and its metabolites have been shown to effectively distribute to the cerebrospinal fluid 47 and resulted in a 68% reduced risk of intracranial progression or death (p < 0.0001), a 42% reduced risk of death (p = 0.005), and a 47.3% confirmed intracranial ORR in patients with measurable active brain metastases at baseline (n = 55). 48 The phase II DESTINY-Breast01 study (n = 184) reported a high ORR (62.0%), an estimated 85% 1-year OS rate, and an 18.2-month median DoR for T-DXd in a heavily pretreated patient population. 39 An encouraging ORR of 58.3% was observed among the 13.0% of patients with CNS disease with a 42.1% CNS response for patients with brain metastases at baseline (n = 17).25,49 T-DXd continues to be assessed in pretreated patients in the ongoing phase III DESTINY-Breast02 trial (NCT03523585). In addition, T-DXd is under study in patients with pretreated HER2-low BC (DESTINY-Breast04, NCT03734029) and in patients with HER2-positive or HER2-low BC or leptomeningeal carcinomatosis and brain metastases (DEBBRAH, NCT04420598). T-DXd received accelerated FDA approval in December 2019 and the tucatinib combination was approved in April 2020 following at least one prior anti-HER2 regimen for metastatic disease (Table 3).50,51
Although both tucatinib and T-DXd were evaluated in phase II trials,32,39 HER2CLIMB was a large, placebo-controlled, randomized study showing significant OS benefits for tucatinib and clinically relevant CNS outcomes, including for active CNS disease.32,48 This study was novel for enrolling these patients and paves the way for their inclusion in future trials. DESTINY-Breast01, on the contrary, was a single-arm study of T-DXd enrolling very heavily pretreated patients (Table 1). 39 Both agents have a role in metastatic HER2-positive BC sequential therapy, with further data awaited from the phase III HER2CLIMB-02 (NCT03975647) and CompassHER2 RD (NCT04457596) trials evaluating the efficacy of tucatinib in various settings (Table 4). These findings and data from upcoming trials will be critical to adjudicate the ultimate benefit of both agents.
Table 4.
Ongoing phase III clinical trials of HER2-directed therapy in advanced HER2-positive breast cancer.
| Experimental agent(s) | Trial ID (NCT#) |
Key eligibility criteria | Experimental regimen | Comparator | Primary end point(s) | Estimated PCD |
|---|---|---|---|---|---|---|
| Early breast cancer | ||||||
| Neoadjuvant | ||||||
| QL1209 | QL1209-301 (NCT04629846) |
Early or locally advanced ER/PR negative disease | QL1209 + trastuzumab + docetaxel | Trastuzumab + pertuzumab + docetaxel | tpCR | October 2022 |
| Pyrotinib | HRHB-CB001 (NCT04290793) |
Early breast cancer | Pyrotinib + epirubicin + cyclophosphamide → taxanes + trastuzumab | Epirubicin + cyclophosphamide → taxanes + trastuzumab | pCR | March 2022 |
| HS627/Pertuzumab | HS627-III (NCT04514419) |
Early or locally advanced disease | HS627 + trastuzumab + docetaxel | Trastuzumab + pertuzumab + docetaxel | pCR | November 2021 |
| SIBP-01 | SIBP-01-3 (NCT03989037) |
Early or locally advanced disease | SIBP-01 + docetaxel + carboplatin | Herceptin + docetaxel + carboplatin | tpCR | May 2021 |
| TX05 | TX05-03 (NCT03556358) |
Early breast cancer | TX05 | Herceptin | pCR | November 2020 |
| EG12014 | EGC002 (NCT03433313) |
Early or locally advanced disease | EG12014 | Herceptin | pCR | November 2020 |
| Apatinib | HebeiMUFH (NCT03580395) |
Stage IIb–IIIc breast cancer | Apatinib + paclitaxel + cisplatin | Paclitaxel + cisplatin | pCR | May 2020 |
| Residual disease post-NAC/adjuvant | ||||||
| Tucatinib, T-DM1 | CompassHER2 RD (NCT04457596) |
High-risk residual disease after HER2-directed NAT | T-DM1 + tucatinib | T-DM1 + placebo | iDFS | January 2028 |
| T-DXd | DESTINY-Breast05 (NCT04622319) |
High-risk residual invasive disease after NAT | T-DXd | T-DM1 | iDFS | December 2025 |
| Pyrotinib | ATP (NCT04254263) |
Residual invasive disease after NAT | Pyrotinib | Observation | iDFS | August 2023 |
| Pertuzumab | BOLD-1 (NCT02625441) |
Moderate/high risk early breast cancer | Pertuzumab + trastuzumab + docetaxel | Trastuzumab + docetaxel | iDFS | December 2022 |
| Adjuvant | ||||||
| Trastuzumab | TMH Project-982 (NCT01785420) |
Resectable early breast cancer | Trastuzumab | Placebo | DFS | April 2025 |
| TX05 | TX 05-03 E (NCT04109391) |
Early disease following NAT and SR in protocol TX05-03 | TX05 | Herceptin | RAE, IA, DFS, OS | January 2022 |
| Pyrotinib | HR-BLTN-III-EBC (NCT03980054) |
Early breast cancer | Pyrotinib | Placebo | iDFS | July 2022 |
| Advanced breast cancer | ||||||
| HER2-naïve | ||||||
| T-DXd | DESTINY-Breast09 (NCT04784715) |
Metastatic disease | T-DXd + pertuzumab or placebo | SOC taxane + trastuzumab + pertuzumab | PFS | July 2025 |
| Pyrotinib | HR-BLTN-III-MBC-C (NCT03863223) |
Metastatic disease | Pyrotinib + trastuzumab + docetaxel | Placebo + trastuzumab + docetaxel | PFS | July 2022 |
| TQ-B211 | TQB211-III-01 (NCT04385563) |
Metastatic disease | TQB211 + docetaxel | Herceptin + docetaxel | ORR | February 2021 |
| GB221 | GENOR GB221-003 (NCT04164615) |
Advanced disease with at least one measurable target lesion | GB221 + capecitabine | Placebo + capecitabine | PFS | July 2020 |
| HER2-pretreated ⩾1 prior regimen | ||||||
| Tucatinib | HER2CLIMB-02 (NCT03975647) |
Prior trastuzumab-based and taxane-based therapy | Tucatinib + T-DM1 | Placebo + T-DM1 | PFS | April 2024 |
| Inetetamab | IR-1.1 (NCT04736589) |
Abnormal activation of PI3K/Akt/mTOR pathway after progression on trastuzumab | Inetetamab + rapamycin + chemotherapy | Pyrotinib + chemotherapy | PFS | February 2024 |
| T-Dxd | DESTINY-Breast03 (NCT03529110) |
Prior trastuzumab-based and taxane-based therapy | T-Dxd | T-DM1 | PFS | February 2022 |
| T-DM1 | BO29919 (NCT03084939) |
Prior trastuzumab-based and taxane-based therapy | T-DM1 | Lapatinib + capecitabine | PFS | September 2021 |
| BAT8001 | BAT-8001-002-CR (NCT04185649) |
Prior trastuzumab-based and taxane-based therapy | BAT8001 | Lapatinib + capecitabine | PFS | July 2020 |
| Third line and beyond | ||||||
| T-Dxd | DESTINY-Breast02 (NCT03523585) |
Prior T-DM1 therapy | T-Dxd | Trastuzumab + capecitabine/lapatinib + capecitabine | PFS | February 2022 |
CT, chemotherapy; DFS, disease-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IA, immunogenicity assessments; iDFS, invasive disease-free survival; NAC, neoadjuvant chemotherapy; NAT, neoadjuvant therapy, ORR; objective response rate; OS, overall survival; PCD, primary completion date; pCR, pathologic complete response; PFS, progression-free survival; PR, progesterone receptor; RAE, rate of adverse events; SR, surgical resection; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab-deruxtecan; tpCR, total pathologic complete response.
Ongoing (trials that are actively recruiting for which efficacy outcomes are not yet available) phase III trials of HER2-directed therapy for treatment HER2-positive BC listed at CT.gov on February 22, 2021, ordered by treatment setting and estimated primary completion date (PCD). Brand names used to distinguish between original trastuzumab and biosimilar products or memetics.
What is the safety of novel HER2-targeted agents for the third-line line treatment of HER2-positive advanced BC and beyond?
HER2-directed therapies in current usage are generally well tolerated, with predictable and manageable safety profiles. Anti-HER2 MoAbs have been associated with a risk of generally reversible left ventricular ejection fraction (LVEF) decline, although event rates are low for trastuzumab in the absence of anthracyclines, with no additional risk when pertuzumab is added and low event rates for margetuximab.19,26,52,53 LVEF dysfunction was not observed in the tucatinib arm of HER2CLIMB, although two patients died of cardiac arrest or failure, 32 and LVEF toxicity rate was low for T-DXd (1.6% overall), with no symptomatic events or LVEF-related cardiac failure. 25 Diarrhea and PPE or rash are often associated with TKIs, with diarrhea and PPE being the most common AEs in both arms of HER2CLIMB (Table 2). 32 Any grade diarrhea and PPE occurred in well over half of patients in the experimental arm, with grade 3/4 events, reported approximately 1.5 times more frequently in the tucatinib versus control arm for both toxicities (Table 2). Importantly, the chemotherapy backbone in both arms was capecitabine which may also have contributed to the higher rates of these two clinically relevant toxicities. Grade ⩾ 3 diarrhea was less frequent for tucatinib compared with neratinib across the relevant trials (12.9% versus 24.4%) even with more active mandatory primary prophylaxis for neratinib in NALA, with both agents requiring proactive patient education and early intervention to optimize quality of life (QoL).31,32 T-DXd was associated with substantial hair loss in DESTINY-Breast01, 25 with nearly half of patients experiencing alopecia of any grade. ILD is a rare but potentially life-threatening treatment complication of T-DXd, which was associated with any grade ILD in 13.6% of patients including four with grade 5 events (2.2%). Subsequent analyses on ILD time course have observed a median time of onset of 5.6 months, with 97% of events occurring within the first year.54–56 Potential risk factors may include dose >5.4 mg/kg and Japanese ethnicity. 57 Patient education, close monitoring, multidisciplinary collaboration, and prompt intervention with glucocorticoids are essential to avoid poor outcomes. Optimized treatment algorithms are needed, and further refinement of risk factors is awaited for further elucidation.
What is the optimal place in therapy of novel HER2-directed agents?
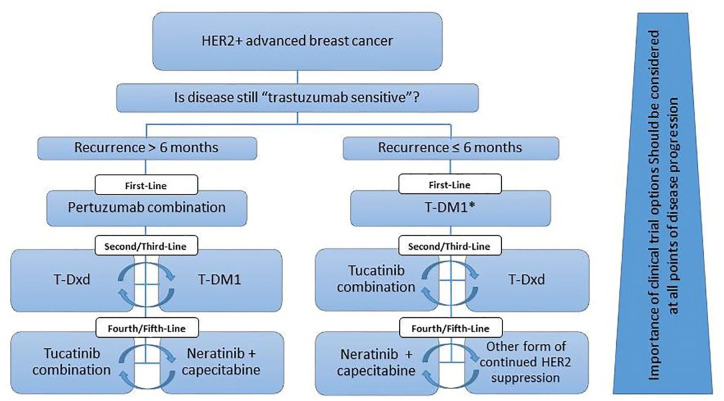
First-line pertuzumab plus trastuzumab and chemotherapy and second-line T-DM1 have been standard of care for metastatic disease since 2013,12,58–60 and are now being considered as (neo)adjuvant therapies based on results of the randomized phase II TRYPHAENA and NeoSphere studies as well as the phase III APHINITY (BIG 4-11) and KATHERINE trials.61–64 Rapidly evolving data are quickly changing standards of care, making treatment comparisons difficult, and highlighting the importance of clinical insight to navigate treatment selection for advanced disease. Based on the eligibility criteria for the phase III CLEOPATRA trial, 60 appropriate patients with a disease-free interval beyond 6 to 12 months following adjuvant HER2-directed therapy should be offered trastuzumab plus pertuzumab and a taxane (Figure 3). T-DM1 was established as second-line therapy, although recent results from the phase III DESTINY-Breast03 trial (49% ⩽1 prior line of therapy) have shown an unprecedented statistically significant improvement in PFS for T-DXd versus T-DM1 (median NYR versus 6.8 months, HR = 0.28, 95% CI = 0.22–0.37, p < 0.000001), 22 supporting it as a new standard of second-line therapy. The tucatinib combination is a good option following second-line T-DM1 based on improved survival outcomes, favorable toxicity profile, and CNS activity.32,48 There are currently no data to inform optimal third-line therapy following second-line T-Dxd. Following progression on the tucatinib combination, neratinib plus capecitabine or other forms of continued HER2 targeting could be considered.
Figure 3.
Proposed sequencing of HER2-directed therapy for HER2-positive advanced breast cancer.
HER2, human epidermal growth factor 2; HER2+, HER2-positive; Pertuzumab combination, pertuzumab plus trastuzumab and a taxane; T-DM1, trastuzumab emtansine; T-Dxd, trastuzumab-deruxtecan; Tucatinib combination, tucatinib plus trastuzumab and capecitabine.
*Especially if the patient has progressed on the adjuvant pertuzumab combination. If patient has progressed on adjuvant TDM-1, suggest pertuzumab combination first line.
For patients that progress within 6 months of completing standard adjuvant HER2-directed therapy, T-DM1 remains a reasonable option based on EMELIA entry criteria. 12 Patients with relapsed disease following adjuvant TDM-1 as a strategy indicated by results from the KATHERINE trial, 61 as well as patients with disease progression on first-line TDM-1, could be candidates for the tucatinib combination or T-Dxd. Results from the QoL components of these trials and formal cost-utility analyses have not yet been completed and will be important to adjudicate optimal treatment decisions.
What upcoming trials will further our understanding of novel HER2-directed therapy in BC?
There are many exciting areas of ongoing HER2-directed research, including novel ADCs (ARX788 and RC48),65,66 bi-specific antibodies,67,68 and chimeric antigen receptor T-cells.69–72 A number of phase III trials exploring the role of new HER2-directed agents for advanced and earlier stage BC are also underway (Table 4). In the advanced setting, a number of novel agents are being assessed for patients with progressive disease on prior HER2-directed therapy, including tucatinib combined with T-DM1 (HER2CLIMB-02, NCT03975647), T-DXd (DESTINY-Breast02, NCT03523585), and the trastuzumab ADC BAT8001 (NCT04185649). For HER2-therapy naïve advanced disease, T-DXd with or without pertuzumab (DESTINY-Breast09, NCT04784715), trastuzumab biosimilars [TQ-B211 (NCT04385563) and GB221 (NCT04164615)], and pyrotinib (HR-BLTN-III-MBC-C, NCT03863223) are also being evaluated. The rapidity of early drug and clinical development in this area suggests that promising agents like bi-specific antibodies and chimeric antigen receptor T-cells may become clinically relevant in the near future.
In the (neo)adjuvant setting, pyrotinib, HER2 biosimilars, and a number of novel HER2-directed agents are being assessed alone or in combination with chemotherapy with or without trastuzumab (Table 4). Neoadjuvant pyrotinib (NCT04290793) and the highly selective vascular endothelial growth factor receptor 2 TKI apatinib (NCT03580395) are being assessed and HER2-directed biosimilars under development in this setting include those of trastuzumab (EG12014, NCT03433313 and SIBP-01, NCT03989037) and pertuzumab (HS627, NCT04514419 and QL1209, NCT04629846). Agents being evaluated in patients with residual invasive disease following neoadjuvant treatment include T-DXd (DESTINY-Breast05, NCT04622319), pyrotinib (ATP, NCT04254263), and tucatinib added to T-DM1 following HER2-directed neoadjuvant therapy (CompassHER2 RD, NCT04457596). Neoadjuvant HER2-directed therapy in combination with immune checkpoint blockade is also being explored in a phase II trial (neoHIP, NCT03747120).
Finally, we must acknowledge that there is substantial heterogeneity of BC which may include variable HER2 expression within metastatic deposits and possible changes in HER2 expression over time. 73 Studies are underway to explore treatment options for patients with advanced BC and low HER2 expression with or without co-expression of hormone receptors (NCT03734029, NCT04494425, and NCT04400695).
Summary
The development of efficacious and generally well-tolerated HER2-directed therapies has led to clinically meaningful benefits for patients with advanced HER2-positive BC and evidence continues to support continued HER2 suppression beyond disease progression. Based on the OS benefit in favor of the tucatinib plus trastuzumab and capecitabine regimen in HER2CLIMB and the magnitude of response observed in the DESTINY-Breast01 study of T-DXd, either regimen is an appropriate consideration for third- and/or fourth-line treatment, with important consideration for proactive toxicity management. Further information on QoL and cost-effectiveness, as well as optimal sequencing and toxicity management strategies are awaited. Ongoing randomized trials and real-world evidence will further clarify the role of these agents in this rapidly evolving field.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211066677 for Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: redrawing the lines by Christine Simmons, Daniel Rayson, Anil Abraham Joy, Jan-Willem Henning, Julie Lemieux, Heather McArthur, Paul B. Card, Rebecca Dent and Christine Brezden-Masley in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Ilidio Martins and Deanna McLeod from Kaleidoscope Strategic, Inc., AstraZeneca Global, Inc., Hoffmann La-Roche Canada, Knight Therapeutics, Inc., Viatris, Inc. (Mylan Pharmaceuticals), and Pfizer Canada, Inc., for their support.
Footnotes
Author contributions: Christine Simmons: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Resources; Supervision; Validation; Writing – review & editing
Daniel Rayson: Conceptualization; Data curation; Resources; Supervision; Validation; Writing – review & editing
Anil Abraham Joy: Resources; Supervision; Validation; Writing – review & editing
Jan-Willem Henning: Resources; Validation; Writing – review & editing
Julie Lemieux: Resources; Validation; Writing – review & editing
Heather McArthur: Resources; Validation; Writing – review & editing
Paul B Card: Data curation; Formal analysis; Project administration; Resources; Validation; Writing – original draft; Writing – review & editing
Rebecca Dent: Resources; Validation: Writing – review & editing
Christine Brezden-Masley: Conceptualization; Data curation; Methodology; Project administration; Resources; Supervision; Validation; Writing – review & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christine Simmons has served in a consultancy or advisory role and received honorarium from Pfizer, Eli Lilly, Roche, and Mylan, and has received research funding from AstraZeneca Global, Roche, Knight Therapeutics, Viatris, and Pfizer. Daniel Rayson has served in a consultancy or advisory role and received honorarium from AstraZeneca, Pfizer, Eli Lilly, Merck, Gilead, Novartis, and Seagen. Anil Abraham Joy has served in a consultancy or advisory role and received honorarium from AstraZeneca, BMS, Eli Lilly, Knight Therapeutics, Gilead, Roche, Novartis, Pfizer, Mylan, and Teva. Jan-Willem Henning has served in a consultancy or advisory role and received honorarium from AstraZeneca, Pfizer, Novartis, Eli Lilly, Roche, Knight Therapeutics, Seagen, and Mylan. Julie Lemieux has served in a consultancy or advisory role and received honorarium from Novartis, Eli Lilly, Gilead, Pfizer, and AstraZeneca, and has received research funding from Celgene, Genentech, GlaxoSmithKline, Roche, Millennium, Novartis, Merck Gilead, Abbvie, Acerta, Bayer, Pfizer, BMS, Esai, Sanofi, Janssen, Ozmosys, Sierra Astrazeneca, and Takeda. Heather McArthur has served in a consultancy or advisory role and received honorarium from Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Puma Biotechnology, Daiichi-Sankyo, Seattle Genetics, Merck, and Lilly, and has received research funding from Bristol-Myers Squibb, MedImmune, LLC/AstraZenica, BTG, and Merck. Paul B. Card has nothing to declare. Rebecca Dent has served in a consultancy or advisory role and received honorarium from AstraZeneca, Viatris, Pfizer, Eisai, Merck, Eli Lilly, Novartis, and Roche, and has received research funding from AstraZeneca. Christine Brezden-Masley has served in a consultancy or advisory role and received honorarium from AstraZeneca, Eli Lilly, Knight Therapeutics, Mylan, Gilead, Roche, Amgen, Seagen, and Novartis, and has received research funding from Eli Lilly and AstraZeneca.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this review was provided through unrestricted educational grants from AstraZeneca Global, Inc., Hoffmann La-Roche Canada, Knight Therapeutics, Inc., Viatris Inc. (Mylan Pharmaceuticals), and Pfizer Canada, Inc. No discussion or viewing of review content was permitted with sponsors at any stage of review development.
Disclaimer: This review was prepared according to ICMJE standards with editorial assistance from Kaleidoscope Strategic, Inc.
ORCID iDs: Christine Simmons  https://orcid.org/0000-0002-6571-4587
https://orcid.org/0000-0002-6571-4587
Anil Abraham Joy  https://orcid.org/0000-0003-4201-8930
https://orcid.org/0000-0003-4201-8930
Paul B. Card  https://orcid.org/0000-0003-0612-0380
https://orcid.org/0000-0003-0612-0380
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Christine Simmons, Medical Oncology, British Columbia Cancer Agency – Vancouver Centre, University of British Columbia, 600 West 10th Avenue, Vancouver, BC V5Z 4E6, Canada.
Daniel Rayson, Queen Elizabeth II Health Sciences Centre, Dalhousie University, Halifax, NS, Canada.
Anil Abraham Joy, Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada.
Jan-Willem Henning, Tom Baker Cancer Center, University of Calgary, Calgary, AB, Canada.
Julie Lemieux, Centre hospitalier universitaire de Québec, Université Laval, Quebec, QC, Canada.
Heather McArthur, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Paul B. Card, Kaleidoscope Strategic, Inc., Toronto, ON, Canada
Rebecca Dent, National Cancer Centre Singapore, Duke-NUS Medical School, Singapore.
Christine Brezden-Masley, Sinai Health, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Bredin P, Walshe J, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol 2020; 47: 259–269. [DOI] [PubMed] [Google Scholar]
- 3. Chan W-L, Lam TC, Lam KO, et al. Local and systemic treatment for HER2-positive breast cancer with brain metastases: a comprehensive review. Ther Adv Med Oncol 2020; 12: 1758835920953729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69: 438–451. [DOI] [PubMed] [Google Scholar]
- 5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256: 495–497. [DOI] [PubMed] [Google Scholar]
- 6. Hudziak RM, Lewis GD, Winget M, et al. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol 1989; 9: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 8. Chien AJ, Rugo HS. Tyrosine kinase inhibitors for human epidermal growth factor receptor 2-positive metastatic breast cancer: is personalizing therapy within reach? J Clin Oncol 2017; 35: 3089–3091. [DOI] [PubMed] [Google Scholar]
- 9. Spector N, Xia W, El-Hariry I, et al. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Res 2007; 9: 205. [Google Scholar]
- 10. Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in women with HER-2–positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 2010; 15: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733–2743. [DOI] [PubMed] [Google Scholar]
- 12. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012; 367: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barroso-Sousa R, Tolaney SM. Clinical development of new antibody–drug conjugates in breast cancer: to infinity and beyond. Biodrugs 2021; 35: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen SC, Krogh BO, Jensen A, et al. Characterization of basigin monoclonal antibodies for receptor-mediated drug delivery to the brain. Sci Rep 2020; 10: 14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devanaboyina M, Bailey MM, Hamouda DM. A retrospective study of characteristics and survival in patients with breast cancer brain metastases classified by subtype using NCI SEER registry. J Clin Oncol 2021; 39: 1031. [Google Scholar]
- 16. Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast 2014; 23: 623–628. [DOI] [PubMed] [Google Scholar]
- 17. Kim JS, Kim IA. Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther Adv Med Oncol 2020; 12: 1758835920936117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci 2016; 17: 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013; 14: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31: 1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol 2018; 36: 2736–2740. [DOI] [PubMed] [Google Scholar]
- 22. Cortés J, Kim S, Chung W, et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol 2021; 32: S1287–S1288. [Google Scholar]
- 23. Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008; 112: 533–543. [DOI] [PubMed] [Google Scholar]
- 24. von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol 2009; 27: 1999–2006. [DOI] [PubMed] [Google Scholar]
- 25. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020; 382: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rugo HS, Im S-A, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bang Y-J, Giaccone G, Im S, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 2017; 28: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mezni E, Vicier C, Guerin M, et al. New therapeutics in HER2-positive advanced breast cancer: towards a change in clinical practices? Cancers 2020; 12: 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019; 11: 1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamilton EP, Petit T, Pistilli B, et al. Clinical activity of MCLA-128 (zenocutuzumab), trastuzumab, and vinorelbine in HER2 amplified metastatic breast cancer (MBC) patients (pts) who had progressed on anti-HER2 ADCs. J Clin Oncol 2020; 38: 3093. [Google Scholar]
- 31. Saura C, Oliveira M, Feng Y-H, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ⩾2 HER2-directed regimens: phase III NALA trial. J Clin Oncol 2020; 38: 3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 2020; 382: 597–609. [DOI] [PubMed] [Google Scholar]
- 33. Brufsky A, Zulfiqar M, Peguero J, et al. A phase 2 study of poziotinib in patients with HER2-positive metastatic breast cancer heavily pre-treated with HER2-targeted therapy. Philadelphia, PA: American Association Cancer Research, 2021. [Google Scholar]
- 34. Tesch ME, Gelmon KA. Targeting HER2 in breast cancer: latest developments on treatment sequencing and the introduction of biosimilars. Drugs 2020; 80: 1811. [DOI] [PubMed] [Google Scholar]
- 35. Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 689–699. [DOI] [PubMed] [Google Scholar]
- 36. Krop IE, Kim S-B, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017; 18: 743–754. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto Y, Iwata H, Naruto T, et al. Abstract PD3-11: a randomized, open-label, phase III trial of pertuzumab re-treatment in HER2-positive, locally advanced/metastatic breast cancer patients previously treated with pertuzumab, trastuzumab, and chemotherapy: the Japan Breast Cancer Research Group-M05 (PRECIOUS) study, 2021, https://cancerres.aacrjournals.org/content/81/4_Supplement/PD3-11.short [DOI] [PubMed]
- 38. Curigliano G, Mueller V, Borges VF, et al. Updated results of tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB). Philadelphia, PA: Wolters Kluwer Health, 2021. [DOI] [PubMed] [Google Scholar]
- 39. Manich C, Modi S, Krop I, et al. 279P trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol 2021; 32: S485–S486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modi S, Saura C, Yamashita T, et al. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer. In: San Antonio Breast Cancer Symposium®, 8–11 December 2020, https://www.physiciansweekly.com/wp-content/uploads/2021/01/Modi_PD3-06_DESTINY-Breast01.pdf
- 41. CancerNetwork.com. FDA approves T-DM1 (Kadcyla) for HER2-positive breast cancer, https://www.cancernetwork.com/view/fda-approves-t-dm1-kadcyla-her2-positive-breast-cancer (accessed 18 May 2021).
- 42. Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020; 18: 452–478. [DOI] [PubMed] [Google Scholar]
- 43. Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol 2019; 37: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol 2016; 2: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 45. Globenewswire.com. MacroGenics announces final overall survival results from SOPHIA study of MARGENZA™ in patients with HER2-positive metastatic breast cancer, https://www.globenewswire.com/news-release/2021/09/07/2292944/0/en/MacroGenics-Announces-Final-Overall-Survival-Results-from-SOPHIA-Study-of-MARGENZA-in-Patients-with-HER2-Positive-Metastatic-Breast-Cancer.html (accessed 7 October 2021).
- 46. Manich C, O’Shaughnessy J, Aftimos P, et al. LBA15 Primary outcome of the phase III SYD985. 002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol 2021; 32: S1288. [Google Scholar]
- 47. Stringer-Reasor EM, O’Brien BJ, Topletz-Erickson A, et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 48. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial, 2020, https://air.unimi.it/handle/2434/824756#.YatA5dBByUk [DOI] [PMC free article] [PubMed]
- 49. Jerusalem GHM, Park YH, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol 2021; 39: 526–526. [Google Scholar]
- 50. FDA.gov. ENHERTU® (fam-trastuzumab deruxtecan-nxki), https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761139s000lbl.pdf (accessed 1 June 2021).
- 51. FDA.gov. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer (accessed 23 June 2021).
- 52. Copeland-Halperin RS, Liu JE, Anthony FY. Cardiotoxicity of HER2-targeted therapies. Curr Opin Cardiol 2019; 34: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bouwer NI, Jager A, Liesting C, et al. Cardiac monitoring in HER2-positive patients on trastuzumab treatment: a review and implications for clinical practice. Breast 2020; 52: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Powell C, Camidge D, Modi S, et al. Risk factors for interstitial lung disease in patients treated with trastuzumab deruxtecan from two interventional studies. Ann Oncol 2020; 31: S357–S358. [Google Scholar]
- 55. Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease (ILD) in 8 single-arm trastuzumab deruxtecan (T-DXd) studies. In: Presented at the AACR Annual Meeting 2021, Philadelphia, PA, 10–15 April 2021 (Virtual: Abstract CT167). [Google Scholar]
- 56. Powell C, Modi S, Iwata H, et al. 92O Analysis of study drug-related interstitial lung disease (ILD) in patients (pts) with HER2+ metastatic breast cancer (mBC) treated with trastuzumab deruxtecan (T-DXd). Ann Oncol 2021; 32: S61–S62. [Google Scholar]
- 57. Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. Epub ahead of print 14 October 2021. DOI: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 58. Hardy-Werbin M, Quiroga V, Cirauqui B, et al. Real-world data on T-DM1 efficacy–results of a single-center retrospective study of HER2-positive breast cancer patients. Sci Rep 2019; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Danese MD, Masaquel A, Santos E, et al. Estimated life-years saved in women with HER2-positive metastatic breast cancer receiving first-line trastuzumab and pertuzumab in the United States. Value Health 2015; 18: 876–883. [DOI] [PubMed] [Google Scholar]
- 60. Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012; 366: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380: 617–628. [DOI] [PubMed] [Google Scholar]
- 62. Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol 2021; 39: 1448–1457. [DOI] [PubMed] [Google Scholar]
- 63. Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018; 89: 27–35. [DOI] [PubMed] [Google Scholar]
- 64. Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016; 17: 791–800. [DOI] [PubMed] [Google Scholar]
- 65. Wang J, Liu Y, Zhang Q, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 66. Hurvitz SA, Park H, Frentzas S, et al. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpressing solid tumors: results from two phase 1 clinical trials. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 67. Gu C-l, Zhu H-x, Deng L, et al. Bispecific antibody simultaneously targeting PD1 and HER2 inhibits tumor growth via direct tumor cell killing in combination with PD1/PDL1 blockade and HER2 inhibition. Acta Pharmacol Sin. Epub ahead of print 14 May 2021. DOI: 10.1038/s41401-021-00683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ji D, Zhang J, Shen W, et al. Preliminary safety, efficacy and pharmacokinetics (PK) results of KN026, a HER2 bispecific antibody in patients (pts) with HER2-positive metastatic breast cancer. J Clin Oncol 2020; 38: 1041.32031899 [Google Scholar]
- 69. Li H, Yuan W, Bin S, et al. Overcome trastuzumab resistance of breast cancer using anti-HER2 chimeric antigen receptor T cells and PD1 blockade. Am J Cancer Res 2020; 10: 688. [PMC free article] [PubMed] [Google Scholar]
- 70. Li P, Yang L, Li T, et al. The third generation anti-HER2 chimeric antigen receptor mouse T cells alone or together with anti-PD1 antibody inhibits the growth of mouse breast tumor cells expressing HER2 in vitro and in immune competent mice. Front Oncol 2020; 10: 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cao YJ, Wang X, Wang Z, et al. Switchable CAR-T cells outperformed traditional antibody-redirected therapeutics targeting breast cancers. ACS Synth Biol 2021; 10: 1176–1183. [DOI] [PubMed] [Google Scholar]
- 72. Szöőr Á, Tóth G, Zsebik B, et al. Trastuzumab derived HER2-specific CARs for the treatment of trastuzumab-resistant breast cancer: CAR T cells penetrate and eradicate tumors that are not accessible to antibodies. Cancer Lett 2020; 484: 1–8. [DOI] [PubMed] [Google Scholar]
- 73. Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol 2009; 20: 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211066677 for Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: redrawing the lines by Christine Simmons, Daniel Rayson, Anil Abraham Joy, Jan-Willem Henning, Julie Lemieux, Heather McArthur, Paul B. Card, Rebecca Dent and Christine Brezden-Masley in Therapeutic Advances in Medical Oncology